Abstract
IgA, the major class of Ig in secretions, classically functions by interfering with microbial attachment to host tissues. Many mucosal pathogens, including Streptococcus pneumoniae, express an IgA1 protease that may circumvent the protective effects of this Ig subclass. Because these proteases are specific for human IgA1, we generated human mAbs to the major surface antigen of the pneumococcus, its capsular polysaccharide, and tested their effect in a colonization model of bacterial adherence to respiratory epithelial cells in culture. Rather than inhibiting adherence, type-specific IgA1 markedly enhanced bacterial attachment to host cells, but only when cleaved by IgA1 protease. Neither antibodies of protease-insensitive subclasses (IgA2 and IgG) nor those directed against heterologous capsules had such activity. The adherence-promoting properties of cleaved antibodies correlated with the cationic characteristics of their variable segments, suggesting that bound Fab fragments may neutralize the inhibitory effect of negatively charged capsules on adhesive interaction with host cells. Coating of pneumococci with anticapsular polysaccharide antibody unmasked the bacterial phosphorylcholine ligand, allowing for increased adherence mediated by binding to the platelet activating factor receptor on epithelial cells. In addition, our findings provide evidence for a novel function of bacterial IgA1 proteases. These enzymes may enable pathogens to subvert the antigen specificity of the humoral immune response to facilitate adhesive interactions and persistence on the mucosal surface.
IgA is the most abundant class of Igs on mucosal surfaces. The contribution of IgA to host defense is thought to result from its ability to block colonization-initiating adhesive interactions of microbes with host tissues (1). Nasopharyngeal carriage of pathogens such as Streptococcus pneumoniae is common, despite the prevalence of mucosal IgA to the capsular polysaccharide (PnPS), which is the immunodominant surface antigen of the pneumococcus (2, 3). Because of the age-dependent response to polysaccharide antigens, naturally acquired anti-PnPS IgA is particularly prominent among young children, the age group with the highest rates of pneumococcal colonization and disease (4). Type-specific IgA (and IgG) has been shown to initiate dose-dependent killing of S. pneumoniae with complement and phagocytes (5). The reduction in carriage after immunization with PnPS has been associated with induction of a type-specific IgG response (6). The effects of IgA on the mucosal surface and on pneumococcal colonization have not been examined.
An additional factor in considering the protective effect of IgA is that human (h)IgA1 is uniquely susceptible to bacterial IgA1 proteases (7). IgA1 comprises >90% of IgA in upper respiratory secretions and most of the naturally acquired antibody to bacterial polysaccharides (3, 8, 9). IgA1 proteases, expressed by many common respiratory pathogens including the pneumococcus, are highly specific for prolyl-threonyl or prolyl-seryl bonds in the hinge region of hIgA1 (10). These bonds are absent in hIgA2, other classes of hIg, and in IgA from species other than higher primates (7). This family of enzymes removes the Fcα domain needed for secondary effector function and has been noted to reduce the inhibition of adherence by human secretory IgA (S-IgA) (11). The residual Fabα fragment retains antigen-binding properties but no longer possesses the antimicrobial agglutinating capacity of multimeric forms of IgA (12). In this report, we used human anti-PnPS IgA1 to show that IgA1 protease modifies IgA1 antibody so that it promotes rather than inhibits pneumococcal adherence to epithelial cells in a model of colonization.
Methods
PnPS-Specific Human mAbs (hmAbs).
Seven days after immunization of three healthy adults with 23-valent pneumococcal vaccine, purified B cells were fused with K6H6/B5 heteromyeloma fusion partner (1:2) with 38% wt/vol polyethylene glycol (Sigma) and serially incubated with RPMI medium 1640/20% FBS (Life Technologies, Carlsbad, CA) containing hypoxanthine/aminopterin/thymidine (Sigma) for 2–4 weeks, then RPMI medium 1640 with 10% FBS alone (13). Cells with supernatants reactive with PnPS serotypes 2, 6B, and 8 (American Type Culture Collection) by ELISA were plated at 0.6 cells per well with irradiated (2,500 rad) feeder peripheral blood mononuclear cells, rescreened at 2–3 weeks, and replated at 0.6 cells per well, yielding a 99% clonal probability (5, 14). The heavy chain subclass and light chain utilization was determined by ELISA (15). The specificity of each mAb was established with binding and competitive inhibition assays by using 12 pneumococcal polysaccharides [types 1, 2, 3, 4, 6B, 8, 9V, 12F, 14, 19A, and 19F (American Type Culture Collection), and C polysaccharide (Statens Serum Institut, Copenhagen)] and six unrelated antigens in controls as ELISA captures (16). IgG was purified from culture supernatants with a protein G Hi-Trap column (Amersham Biotech) and IgA with affinity columns prepared with goat anti-hIgA (Southern Biotechnology Associates) coupled to CNBr-activated Sepharose 4B (Amersham Pharmacia; ref. 5). The purity of all fractions was >98.5%. Other antibodies included type-specific pneumococcal antisera raised in rabbits (Statens Serum Institut) and murine IgG and IgM mAbs to various PnPS types (a gift of Uffe Sørensen, Aarhus University, Aarhus, Denmark) and total S-IgA purified from pooled human colostrum (Sigma).
Treatment of Antibodies.
Fab fragments were generated by using immobilized papain according to the manufacturer's instructions (Pierce) and analyzed on 12.5% SDS/PAGE gels before use in adherence assays. Protease digestion of hmAbs (2 μg) with pneumococci (107 colony-forming units) or culture supernatant (10 μl) from IgA1 protease-producing Haemophilus influenzae grown in supplemented brain–heart infusion broth to mid-logarithmic phase was performed for 1–3 h at 37°C. The effect of protease treatment of antibodies was analyzed in Western blots of 10% SDS/PAGE gels by using an anti-hIgA antibody conjugated to alkaline phosphatase.
V Region Gene Sequencing and Analysis.
Total RNA extracted from hybridomas with Trizol (Life Technologies) was treated with RNase-free DNase I and converted to cDNA as described (17). cDNA (0.5 μl) was amplified with VH and VL leader, heavy (IgA or IgG) and light chain (κ or λ) primers (International ImMunoGeneTics database, http://imgt.cines.fr; initiator and coordinator, Marie-Paule Lefranc, Montpellier, France). The PCR band of expected size was excised and purified with the Bio101 GeneClean Kit (Qbiogene, Carlsbad, CA) and directly sequenced in both directions by using the original sense and antisense PCR primers. The variable region framework and complementarity-determining regions were analyzed by comparison with germ-line V region sequences in two online databases (V BASE, www.mrc-cpe.cam.ac.uk/vbase; International ImMunoGeneTics database, http://imgt.cines.fr) and aligned by using dnaplot software.
Adherence Assays.
Clinical isolates of S. pneumoniae (type 2, D39; type 6A, P384; type 8, P407) were grown in semisynthetic medium (C+Y, pH 6.8) to mid-logarithmic phase (OD620 = 0.3–0.4) unless otherwise specified (18). Minimally passaged (n < 5) Detroit 562 pharyngeal epithelial cells (D562, ATCC CCL-138) were grown to confluence as previously described (19). Monolayers in 12-well plates were washed three times with PBS (pH 7.2) and maintained in tissue culture medium (TCM//MEM with l-glutamine/1 mM sodium pyruvate/10% FBS; GIBCO/BRL) without antibiotics until ready to infect. Where indicated, a receptor for platelet-activating factor (rPAF)-binding antagonist (1-hexadecyl-2-acetyl-sn-glycerol-3-phospho-[N,N,N-trimethyl]-hexanolamine; Calbiochem) or mAb to the rPAF receptor (Alexis Biochemicals, Lausen, Switzerland) were used at 1 μM and 2.5 μg/ml, respectively, to treat confluent D562 cells for 30 min before and during adherence assays. A 10-μl aliquot of bacterial culture was added to an equal volume of antibody in PBS and incubated with agitation at 4°C for 30 min. When specified, the antibody was pretreated with a source of IgA1 protease or control. A total of 480 μl of TCM without antibiotics was added to this mixture and incubated for an additional 30 min at 4°C. The mixture was then divided among the wells and applied to the monolayer by centrifugation (1,200 × g for 5 min) to give a multiplicity of infection of 10:1, bacteria to D562 cells. After allowing for adherence at 37°C for 60 min, the wells were washed five times with PBS, and the adherent bacteria and cells were lifted off by treatment with 200 μl per well of a solution containing 0.25% trypsin and 1 mM EDTA, vortexed, and maintained at 4°C. Serial dilutions of the inoculum or adherent bacteria were quantified by plating on tryptic soy medium supplemented with catalase in duplicate. The percent adherence was calculated as the portion of the untreated inoculum that was adherent and was expressed as the mean of at least three wells per condition. Data were analyzed for significance by Student's t test for at least three independent determinations. For microscopy, the adherence assay was modified by using D562 cells grown on glass coverslips incubated for 60 min at 37°C with bacteria labeled with fluorescein as previously described, washed five times in PBS, inverted and mounted by using VECTASHIELD (Vector Laboratories), and viewed under fluorescence (19). hIgA was detected by immunocytochemistry with FITC-conjugated anti-hIgA or light chain antibody as described (19).
C-Reactive Protein (CRP) Binding.
Accessibility of cell-surface phosphorylcholine (ChoP) was determined by incubation of pneumococci grown to midlogarithmic phase with a 1 in 10 dilution of pooled human serum as a source of CRP (20). After incubation with agitation for 15 min at 37°C in serum, the cells were washed twice in PBS to remove unbound CRP. A whole-cell lysate obtained by treatment of cells at 100°C for 5 min was adjusted for equal loading based on total protein content and separated on 12.5% SDS/PAGE gels before transfer to nylon membranes and immunoblotting with a mAb to human CRP (Sigma). The specificity of this reactivity was confirmed with controls in which the serum was pretreated with ChoP linked to agarose beads (Pierce) to selectively remove CRP (data not shown). Where indicated, the bacteria were incubated with human mAb (2 μg/ml) for 30 min at 37°C before the addition of serum.
Results and Discussion
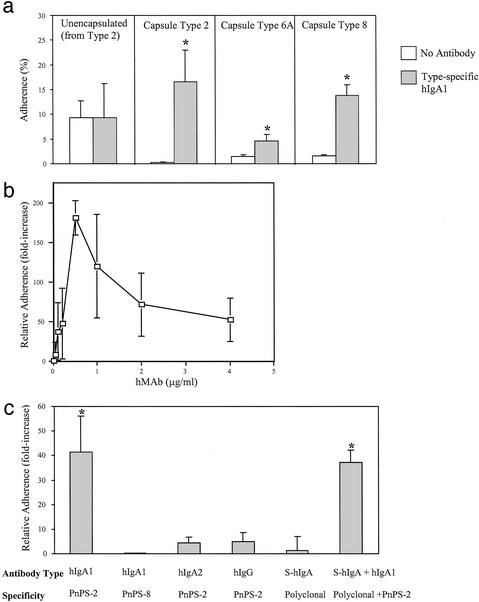
A series of hmAbs specific for PnPS of serotypes 2, 6A, and 8 was generated by fusing and cloning capsule-specific B cells from healthy individuals immunized with a 23-valent pneumococcal vaccine. These hmAbs were examined for their effect on adherence of pneumococci to human pharyngeal epithelial cell monolayers in culture (D562 cells). Expression of the pneumococcal capsule interfered with adherence (type 2 encapsulated D39 vs. its unencapsulated mutant R6), confirming previous reports (Fig. 1a, open bars, Left and Center Left; ref. 21). Rather than further reducing adherence, preincubation of encapsulated strains with IgA1 hmAb specific for each type (2 μg/ml) significantly enhanced adherence (Fig. 1a, Center Left, Center Right, and Right). This increased adherence to epithelial cells required expression of the capsule (Fig. 1a, Left and Center Left) and was present at all concentrations of specific IgA1 hmAb tested (Fig. 1b; range 9- to 180-fold increase compared with values in the absence of antibody at final concentrations of 0.05–4.0 μg/ml). This effect was antigen specific, because an IgA1 hmAb to type 8 PnPS supported no increased adherence of type 2 strain D39 (Fig. 1c). Furthermore, this effect was restricted to the IgA class and IgA1 subclass, because equivalent concentrations of IgG or IgA2 hmAbs with the same antigen specificity showed limited activity. The antigen specificity of the effect was further supported by observations that addition of a 10-fold excess of non-PnPS-specific S-IgA neither enhanced adherence alone nor diminished, in combination, the effect of type-specific IgA1 hmAb. Moreover, the effect was observed only with human antibody; neither polyclonal rabbit antisera nor murine IgG and IgM mAbs to the homotypic PnPS increased adherence (data not shown). Together these results suggested that antibody-mediated enhancement of attachment requires type-specific antibody and is restricted to hIgA1.
Figure 1.
(a) Clinical isolates of the serotype indicated or R6 (Left), an unencapsulated mutant of strain D39 (Center Left), were preincubated with or without type-specific IgA1 hmAb (2 μg/ml) and the effect on attachment to D562 epithelial cells expressed as the percent of the inoculum bound (mean ± SD). *, P ≤ 0.05 compared with the same strain without antibody. (b) Dose–response showing the effect of a type-specific IgA1 hmAb on the relative adherence (ratio of the percentage of adherence with and without antibody ±SD) of strain D39. (c) Effect of the hmAbs (2 μg/ml) indicated on the relative adherence of strain D39. Human S-IgA was used at 20 μg/ml. *, P ≤ 0.05 compared with groups without type-specific IgA1.
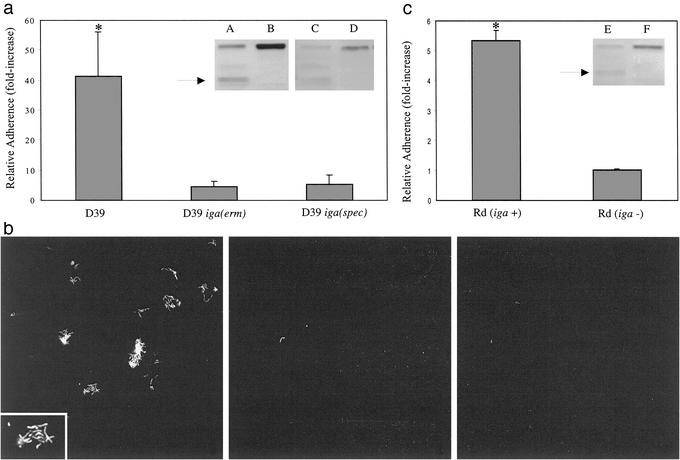
The ability of the organism to produce IgA1 protease was required for the full enhancement of adherence to respiratory epithelial cells by type-specific hIgA1. The IgA1-associated increase in adherence observed with the IgA1 protease-producing wild-type D39 strain was absent with two independently derived, otherwise isogenic, nonproducing mutants [D39iga(erm) and D39iga(spec); Fig. 2a]. Unlike the parent strain, mutants in which the IgA1 protease gene was interrupted failed to cleave type-specific IgA1 hmAb into Fcα and Fabα fragments as previously demonstrated for human S-IgA (Fig. 2a Inset and ref. 22). Microscopy confirmed these results, showing increased adherence of wild-type bacteria, which formed small clusters, or microcolonies, upon treatment with type-specific hIgA (Fig. 2b Left). Type-specific hIgA1, whether cleaved (with wild-type bacteria) or uncleaved (with the IgA1 protease mutant), was detected on adherent bacteria but not on the epithelial cells (data not shown). In contrast, adherent bacteria and microcolonies were rarely seen on the epithelial monolayer when the bacteria were not treated with type-specific hIgA1 (Fig. 2b Center) or when organisms lacking the IgA1 protease were treated with type-specific hIgA1 (Fig. 2b Right). Further evidence for the requirement of IgA1 protease activity in the adherence-enhancing modification of type-specific hIgA1 was obtained through functional complementation of the iga-mutant. Because the 1,964-aa pneumococcal protease has not yet been purified as an active enzyme, this enhancement was confirmed by using Haemophilus influenzae as a source of secreted IgA1 protease. Cleavage of hIgA1to generate a truncated Fcα was seen after treatment with culture supernatant from H. influenzae strain Rd, but not with culture supernatant from the same strain in which IgA1 protease production was inactivated (Fig. 2c Inset and ref. 23). Only antibody treated with the protease-expressing H. influenzae culture supernatant partially corrected the inability of D39 iga(erm) to increase its adherence in response to type-specific hIgA1 (Fig. 2c). This observation confirmed that the effect of the antibody on adherence requires digestion by an IgA1 protease. In addition, this experiment demonstrated that one member of the oropharyngeal flora (H. influenzae) could be modulating the interaction of another species (S. pneumoniae) with its host, because the oropharynx is colonized with a number of species that secrete IgA1 proteases. In contrast to the secreted H. influenzae protease, the enzymatic activity of the pneumococcal protease is predominately cell associated (22, 24). This characteristic could account for the requirement for bound or type-specific antibody substrate to induce an effect on pneumococcal adherence.
Figure 2.
(a) Effect of type-specific IgA1 hmAb (2 μg/ml) on the relative adherence (ratio of the percentage of adherence with and without antibody ±SD) of strain D39 compared with IgA1 protease gene mutants constructed by deletion–insertion with antibiotic resistance cassettes, D39 iga(erm) and D39 iga(spec). (Inset) Western analysis of type-specific IgA1 hmAb showing the cleaved product of the heavy chain (arrow) upon incubation of IgA with the IgA1 protease-producing D39 (lanes A and C) and the intact heavy chain upon incubation with its IgA1 protease-deficient mutants [lane B, D39 iga(erm); and lane D, D39 iga(spec)]. (b) Strain D39 (Left and Center) or isogenic IgA1 protease mutant (Right), D39 iga(erm), adherent to a D562 epithelial cell monolayer with (Left and Right) or without (2) pretreatment with type-specific IgA1 hmAb (×200; Inset, ×400). (c) Effect of treatment of type-specific IgA1 hmAb with culture supernatant from isogenic Haemophilus influenzae IgA1-protease-expressing (Rd iga +) or -nonexpressing strains (Rd iga −) on adherence of D39 iga(erm). Adherence is expressed relative to controls consisting of culture medium alone. (Inset) Western analysis showing the effect on the heavy chain of treatment of IgA1 hmAb with culture supernatant from isogenic H. influenzae IgA1 protease expressing (lane E) or nonexpressing strains (lane F). Arrows indicate Fcα fragment. *, P ≤ 0.05 compared with other groups.
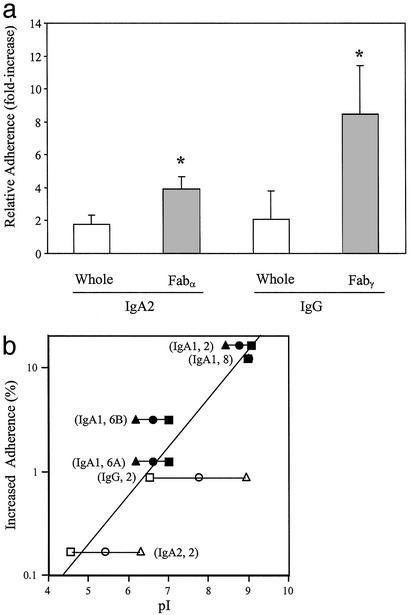
Thus, the species and subclass specificity of hIgA1-mediated enhancement of adherence appeared to be related to the restricted susceptibility of this Ig subclass to cleavage by IgA1 protease. Extending these observations, Fab fragments generated from type-specific IgA2 and IgG hmAbs by digestion with papain also significantly enhanced adherence. The magnitude of this effect, however, was less than that of the aforementioned IgA1 protease-sensitive antibodies against the same PnPS type (Fig. 3a). These data suggest that the deposition of Fab fragments on the bacteria is responsible for facilitating attachment to epithelial cells. These results also confirm that the class and subclass restriction described above likely derived from the selective susceptibility of hIgA1 to the protease rather than any other intrinsic characteristic of this Ig subclass.
Figure 3.
(a) Effect of type 2-specific IgA2 or IgG hmAbs (2 μg/ml) on relative adherence (ratio of the percentage of adherence with and without antibody ±SD) of type 2 strain D39 (open bars). Fab fragments to these antibodies were generated by papain digestion and used at the equivalent concentration (filled bars). *, P ≤ 0.05 compared with the corresponding whole hmAb. (b) The relationship between enhanced adherence and the characteristics of hmAbs. The calculated charge (pI) is based on the deduced amino acid sequence. The variable region pI of the heavy chain, VH (rectangles), and light chain, VL (triangles), of each IgA1 Fab (filled symbols) or papain-generated Fab fragment (open symbols) tested is plotted in comparison to the net contribution of each homotypic antibody to adherence (difference in the percentage of adherence with and without antibody). VH and VL for the same antibody are connected by horizontal lines and labeled by the class or subclass of Ig and its type specificity. For the type 8 hIgA1, values for VH and VL are overlapping. The best-fit line is based on the mean of the pIs (circle) of the VH and VL for each antibody (correlation coefficient = 0.89).
We next considered whether Fab fragments augmented bacterial adherence by modulating the organisms' surface characteristics. The ability of encapsulated organisms such as the pneumococcus to adhere to host cells is limited by the highly hydrophilic and anionic properties of almost all known PnPS structures (25). As a result, many encapsulated pathogens must vary expression of their surface polysaccharide to meet the differing requirements for adherence during colonization (by decreasing capsule size) and evasion of humoral clearance mechanisms during an inflammatory response (by increasing capsule size; refs. 26 and 27). An alternative strategy for improving the adhesive characteristics of bacteria with negatively charged, hydrophilic surfaces may be the binding of hydrophobic, cationic molecules such as the Fab fragments described in this study. In this regard, the binding of cationic neutrophil defensins has been shown to increase adherence of H. influenzae to epithelial cells (28). Protease cleavage of hIgA1 separates the extensively N- and O-glycosylated and sialylated hydrophilic Fcα domain from the relatively hydrophobic Fabα fragment (29, 30). We demonstrate that the efficiency of type-specific Fab fragments of hmAbs (whether generated naturally with the IgA1 protease of S. pneumoniae or artificially with papain) on adherence was proportional to the predicted cationic nature of the variable regions, VH and VL, that distinguish each antibody (Fig. 3b). Those Fab fragments with the most basic variable segments had the greatest impact on pneumococcal adherence. A similar association was observed when the calculated charge was based on the amino acid sequence of entire Fab fragments (data not shown). Thus, bound antigen-specific Fab fragments generated by removal of Fc fragments by bacterial IgA1 protease may modulate the physical properties of the bacterial cell surface and negate the antiadhesive effects of surface molecules such as PnPS. In this regard, the increased bacterial–bacterial association seen in the presence of cleaved type-specific antibody was consistent with an effect mediated by altered physical characteristics of the pneumococcal surface (Fig. 2b Inset).
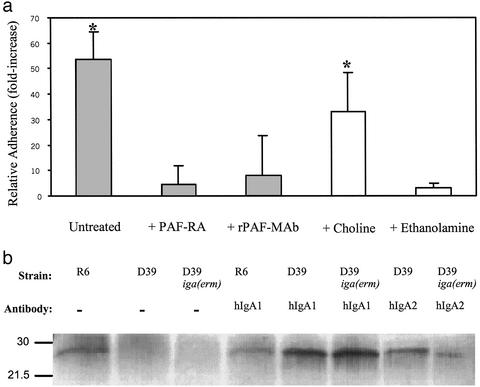
This model for antibody-enhanced pneumococcal adherence led us to consider the identity of the epithelial cell receptor interacting with the modified bacterial surface. A major mechanism for pneumococcal attachment to epithelial cells is binding of ChoP, a canonical bacterial surface structure shared by many species residing in the respiratory tract, to the rPAF (31). rPAF is expressed on human nasal epithelium as well as on D562 cells (19). Addition of either a rPAF-binding antagonist or mAb to the rPAF substantially abrogated the increased attachment resulting from incubation with type-specific hIgA1 (Fig. 4a). As further evidence that antibody-enhanced adherence involves ChoP binding to rPAF, ethanolamine was substituted for choline in a chemically defined growth medium for the pneumococcus. Only pneumococci grown in the presence of choline demonstrated enhanced adherence in the presence of type-specific hIgA1. These data suggest that binding of type-specific Fab fragments may not lead to a novel receptor-ligand interaction but, rather, to an increased efficiency of ChoP–rPAF-mediated adherence.
Figure 4.
(a) Relative adherence (ratio of the percentage of adherence with and without antibody ±SD) in the presence of IgA1 hmAb to type 2 capsular polysaccharide (2 μg/ml) for type 2 strain D39. Where indicated, the D562 cells were treated with a rPAF binding antagonist or mAb to the rPAF. Bacteria were grown in standard conditions (filled bars) or a chemically defined medium (Cden) containing choline or with ethanolamine substituted for choline as indicated (open bars; ref. 36). *, P ≤ 0.05 compared with other groups. (b) Western analysis showing the binding of CRP to IgA1 protease-producing strain D39 or its nonproducing mutant [D39iga(erm)] or its unencapsulated mutant (R6), as indicated. Before treatment with human serum as a source of CRP, bacteria were pretreated with 2 μg/ml type-specific IgA1 or IgA2 and compared with controls without antibody (−) as indicated. Size markers are in kilodaltons.
ChoP, a zwitterionic molecule with a surface-oriented positively charged quaternary amine, is attached to the pneumococcal teichoic acid, which is intercalated in the negatively charged capsule and is typically poorly accessible for binding to its epithelial cell receptor (19, 32). The pneumococcal capsule, for example, blocks interaction with CRP, a component of the innate immune response that specifically binds to ChoP (20, 33). CRP binding to pneumococcal cells was used to determine the effect of antibody on the accessibility of the ChoP ligand (Fig. 4b). Encapsulated pneumococci bound CRP only when treated with type-specific hmAbs, whereas antibody had no effect on CRP binding to unencapsulated pneumococci. The greatest impact on CRP binding was noted for hIgA1, the antibody demonstrating the greatest enhancement of ChoP-mediated adherence. These data provide direct evidence that binding of anti-PnPS antibody may act to increase the exposure of the ChoP ligand. A mechanism involving unmasking surface receptors by antibody has not been described previously in the pathogenesis of bacterial infection. A similar paradigm has recently been described for the encapsulated yeast Cryptococcus neoformans, in which antibody to the negatively charged capsular glucuronoxylomannan capsule leads to a structural change that exposes a binding domain for CD18 (34). For C. neoformans, however, antibody-enhanced adherence facilitates an interaction with macrophages that promotes clearance through phagocytosis. This contrasts with observations in our study in which the effect of antibody on the encapsulated pneumococci is predicted to result in increased persistence on the mucosal surface. The events described in our study are also distinct from antibody-dependent enhancement of infection proposed for some viruses, such as dengue, in which different receptors for interacting with host cells are used in the presence and absence of antibody (35).
Unlike the protease-dependent effects of IgA1 on adherence, the antibody-dependent increase in binding of CRP to the ChoP ligand did not require the presence of functional IgA1 protease (Fig. 4b). Exposure of ChoP on the organisms may be necessary but not sufficient to support binding to rPAF. Rather, changes in charge, and, perhaps, increased physical proximity by removal of the Fc component to limit steric hindrance may also be required.
In summary, we propose that IgA1-mediated enhancement of adherence of S. pneumoniae to epithelial cells in the presence of IgA1 protease represents a novel function for this enzyme. The mechanisms described reveal a heretofore unrecognized strategy by which mucosal pathogens may appropriate antigen-specific components of the acquired immune response to promote colonization.
Acknowledgments
We thank Dr. A. Plaut (Tufts University, Boston) for providing strains. This study was supported by the National Institute of Allergy and Infectious Disease (Grants AI44231 and AI38446 to J.N.W. and AI48796 to E.N.J.), National Institute of Dental and Craniofacial Research Grant DE72621 (to E.N.J.), the Pediatric Infectious Disease Society–St. Jude Children's Research Hospital Fellowship Program (A.J.R.), the Veterans Affairs Research Service, and the Mucosal and Vaccine Research Center.
Abbreviations
- PnPS
capsular polysaccharide
- S-IgA
secretory IgA
- h
human
- rPAF
receptor for platelet-activating factor
- CRP
C-reactive protein
- ChoP
phosphorylcholine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Russell M, Kilian M, Lamm M. In: Mucosal Immunology. Ogra P, Mestecky J, Lamm M, Strober W, Bienenstock J, editors. New York: Academic; 1999. pp. 225–240. [Google Scholar]
- 2.Virolainen A, Jero J, Kayhty H, Karma P, Leinonen M, Eskola J. J Infect Dis. 1995;172:1115–1118. doi: 10.1093/infdis/172.4.1115. [DOI] [PubMed] [Google Scholar]
- 3.Opstad N, Daley C, Thurn J, Rubins J, Merrifield C, Hopewell P, Janoff E. J Infect Dis. 1995;172:566–570. doi: 10.1093/infdis/172.2.566. [DOI] [PubMed] [Google Scholar]
- 4.Simell B, Kilpi T, Kayhty H. J Infect Dis. 2002;186:1106–1114. doi: 10.1086/344235. [DOI] [PubMed] [Google Scholar]
- 5.Janoff E, Fasching C, Orenstein J, Rubins J, Opstad N, Dalmasso A. J Clin Invest. 1999;104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbelle N, Huebner R, Wasas A, Kimura A, Chang I, Klugman K. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 7.Qiu J, Brackee G P, Plaut A G. Infect Immun. 1996;64:933–937. doi: 10.1128/iai.64.3.933-937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkeby L, Rasmussen T, Reinholdt J, Kilian M. Clin Diagn Lab Immunol. 2000;7:31–39. doi: 10.1128/cdli.7.1.31-39.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engstrom P, Norhagen G, Bottaro A, Carbonara A, Lefranc G, Steinitz M, Soder P, Smith C, Hammarstrom L. J Immunol. 1990;145:109–116. [PubMed] [Google Scholar]
- 10.Kilian M, Mestecky J, Kulhavy R, Tomana M, Butler W. J Immunol. 1980;124:2596–2600. [PubMed] [Google Scholar]
- 11.Reinholdt J, Kilian M. J Dent Res. 1987;66:492–497. doi: 10.1177/00220345870660021801. [DOI] [PubMed] [Google Scholar]
- 12.Tyler B, Cole M. Microbiol Immunol. 1998;42:503–508. doi: 10.1111/j.1348-0421.1998.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 13.Carroll W, Thielemans K, Dilley J, Levy R. J Immunol Methods. 1986;89:61–72. doi: 10.1016/0022-1759(86)90032-3. [DOI] [PubMed] [Google Scholar]
- 14.Lefkovits I, Waldmann H. Immunol Today. 1984;5:265–268. doi: 10.1016/0167-5699(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 15.Carson P, Schut R, Simpson M, O'Brien J, Janoff E. J Infect Dis. 1995;172:340–345. doi: 10.1093/infdis/172.2.340. [DOI] [PubMed] [Google Scholar]
- 16.Janoff E, Hardy W, Smith P, Wahl S. J Immunol. 1991;147:2130–2135. [PubMed] [Google Scholar]
- 17.Scamurra R, Miller D, Dahl L, Abrahamsen M, Kapur V, Wahl S, Milner E, Janoff E. J Immunol. 2000;164:5482–5491. doi: 10.4049/jimmunol.164.10.5482. [DOI] [PubMed] [Google Scholar]
- 18.Lacks S, Hotchkiss R D. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 19.Gould J, Weiser J. J Infect Dis. 2002;186:361–371. doi: 10.1086/341658. [DOI] [PubMed] [Google Scholar]
- 20.Kim J O, Romero-Steiner S, Sørensen U, Blom J, Carvalho M, Barnardi S, Carlone G, Weiser J N. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ring A, Weiser J N, Tuomanen E I. J Clin Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wani J, Gilbert J, Plaut A, Weiser J. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy F, Plaut A, Wright A. J Bacteriol. 1987;169:4442–4450. doi: 10.1128/jb.169.10.4442-4450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinholdt J, Kilian M. Infect Immun. 1997;65:4452–4459. doi: 10.1128/iai.65.11.4452-4459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dam J E G, Fleer A, Snippe H. Antonie Leeuwenhoek. 1990;58:1–47. doi: 10.1007/BF02388078. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Weiser J. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 27.Weiser J, Bae D, Epino H, Gordon S, Kapoor M, Zenewicz L, Shchepetov M. Infect Immun. 2001;69:5430–5439. doi: 10.1128/IAI.69.9.5430-5439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorter A D, Eijk P P, van Wetering S, Hiemstra P S, Dankert J, van Alphen L. J Infect Dis. 1998;178:1067–1074. doi: 10.1086/515667. [DOI] [PubMed] [Google Scholar]
- 29.Mattu T, Pleass R, Willis A, Kilian M, Wormald M, Lellouch A, Rudd P, Woof J, Dwek R. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 30.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 31.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 32.Fischer W, Behr T, Hartmann R, Peter K C J, Egge H. Eur J Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taborda C, Casadevall A. Immunity. 2002;16:791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 35.Halstead S, O'Rourke E. J Exp Med. 1977;146:201–207. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomasz A. Bacteriol Proc. 1964;64:29. [Google Scholar]