Abstract
Group A streptococci control expression of key virulence determinants via the two-component sensor/regulator system CsrR/CsrS. The membrane-bound sensor CsrS is thought to respond to previously unknown environmental signal(s) by controlling phosphorylation of its cognate regulator component CsrR. Phosphorylation of CsrR increases its affinity for binding to the promoter regions of Csr-regulated genes to repress transcription. Here we show that environmental Mg2+ concentration is a potent and specific stimulus for CsrR/CsrS-mediated regulation. We studied the effect of divalent cations on expression of the Csr-regulated hyaluronic acid capsule genes (hasABC) by measuring chloramphenicol acetyltransferase (CAT) activity in a reporter strain of group A Streptococcus carrying a has operon promoter-cat fusion. Addition of Mg2+, but not of Ca2+, Mn2+, or Zn2+, repressed capsule gene expression by up to 80% in a dose-dependent fashion. The decrease in capsule gene transcription was associated with a marked reduction in cell-associated capsular polysaccharide. RNA hybridization analysis demonstrated reduced expression of the Csr-regulated hasABC operon, streptokinase (ska), and streptolysin S (sagA) during growth in the presence of 15 mM Mg2+ for the wild-type strain 003CAT but not for an isogenic csrS mutant. We propose that Mg2+ binds to CsrS to induce phosphorylation of CsrR and subsequent repression of virulence gene expression. The low concentration of Mg2+ in extracellular body fluids predicts that the CsrR/CsrS system is maintained in the inactive state during infection, thereby allowing maximal expression of critical virulence determinants in the human host.
Pathogenic bacteria are able to adapt rapidly in the host by up-regulating expression of gene products necessary for survival in the specific environmental niche defined by the site of infection. In the human host, environments encountered by bacteria can vary widely, for example, from the external skin to mucosal surfaces of the airway or the intestine, or deeper tissues and the bloodstream. Therefore, adaptation and survival of the bacteria hinge on their ability to probe the environment and respond appropriately. Group A Streptococcus (GAS or Streptococcus pyogenes) can produce a spectrum of clinical syndromes in humans that range from superficial infection of the pharyngeal mucosa to invasive infection of deep tissues or the bloodstream. GAS elaborate a repertoire of cell-associated (capsular polysaccharide, M protein, extracellular matrix binding proteins) and secreted products (pyrogenic exotoxins, enzymes, cytotoxins) that contribute to the pathogenesis of infection (reviewed in ref. 1). Regulated expression of these products may augment the organism's survival and its virulence in specific niches within the infected host.
Expression of several GAS virulence determinants is controlled by the CsrR/CsrS two-component regulatory system (also called CovR/CovS) (2–5). First reported as a regulator of the has operon that directs synthesis of the hyaluronic acid capsule, the Csr system also controls expression of secreted and cell-associated GAS proteins including streptokinase (ska), the integrin-like protein/IgG protease, Mac or IdeS (mac or ideS), streptolysin S (sag operon), and streptodornase (speMF) (4–7). Csr-mediated regulation of cysteine protease (speB) has been observed in some strains, but not in others (2, 4, 5). DNA microarray analysis suggests the system influences, directly or indirectly, expression of 15% of chromosomal genes (8).
Of 13 putative two-component systems identified in the GAS genome, the CsrR/CsrS system is the best characterized to date and, together with the FasBCA system, one of only two such systems linked to virulence (9, 10). By analogy to well characterized two-component sensor/regulator bacterial systems, the predicted extracellular domain of the membrane-bound sensor component CsrS is expected to bind an extracellular ligand or to sense an environmental condition. This interaction is predicted to trigger autophosphorylation of the cytoplasmic domain of the CsrS protein. The phosphate group is then transferred on to the receiver domain of the cognate regulator CsrR. The regulator component CsrR, when phosphorylated, shows increased binding affinity for the promoters of several genes proposed to be regulated by the system, including the has operon promoter (3, 6, 11). Both CsrR and CsrS mutants exhibit increased transcription of Csr-regulated genes, a phenotype that indicates the system acts to repress target gene expression through its regulator component, CsrR.
A critical issue in understanding the potential function of the Csr system in virulence gene regulation in vivo is the identification of the environmental signal(s) sensed by CsrS. As is the case with many bacterial two-component systems, the identity of such signal(s) for the Csr system has, until now, been completely unknown. In this investigation we used a GAS reporter strain that carries a fusion of the has operon promoter to the gene encoding chloramphenicol acetyltransferase (cat) to identify the environmental signal(s) that interact with the sensor component CsrS. The results of these studies, together with experiments measuring transcription of other Csr-regulated genes, support a model in which environmental Mg2+ binds to CsrS to trigger the phosphorelay that results in repression of Csr-regulated genes.
Materials and Methods
Bacterial Strains and Growth Conditions.
GAS strains used in this study are listed in Table 1. DLS003 is an encapsulated M-type 3 strain isolated from a patient with necrotizing fasciitis (12). Derivative strain 003CAT carries on its chromosome a fusion of a 688-bp fragment, encompassing its autologous has operon promoter sequences with the 5′ portion of hasA, to the cat sequence (13). GAS was grown at 37°C in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY), or on THY agar supplemented with 5% defibrinated sheep blood. For cloning experiments, Escherichia coli DH5α was grown in Luria–Bertani (LB) broth (Difco) or on LB agar. When appropriate, antibiotics were added at the following concentrations: ampicillin, 50 μg/ml; kanamycin, 50 μg/ml for E. coli, 300 μg/ml for GAS; erythromycin, 200 μg/ml for E. coli, 1 μg/ml for GAS; chloramphenicol, 20 μg/ml for E. coli, 5 μg/ml for GAS.
Table 1.
GAS strains used in this study
CAT Assays.
GAS cytoplasmic extracts were prepared as described (13) with slight modifications. Briefly, bacteria grown overnight on THY-blood agar were inoculated in THY broth and collected by centrifugation (1,250 × g; 8 min) at mid-exponential phase growth (A600 = 0.3–0.4) unless otherwise stated. Pellets were washed in 1 ml Tris (10 mM, pH 7.8) and resuspended in 180 μl of the same buffer, to which 20 μl (≈15 units) of mutanolysin was added. Bacteria were treated for 1 h at 37°C and then centrifuged at 18,000 × g for 5 min, and the supernatant carrying cytoplasmic extracts was collected. Determination of CAT activities in 3–5 μg of total protein extract was performed by using the spectrophotometric assay described by Shaw (14). To control for the effects of the different chemicals used on both mutanolysin and CAT activity, control bacterial cultures received the chemical studied immediately before collection of samples. Cytoplasmic extracts from the non-CAT expressing wild-type GAS strains DLS003 and 87-282 did not produce any background CAT activity, nor did the CAT-expressing strains 003CAT and 282CAT when chloramphenicol, the acetyl acceptor in the enzymatic reaction, was omitted from the reaction mix. Results were recorded as milliunits of CAT per mg total cell protein per min (mu/mg/min).
Measurement of GAS Cell-Associated Capsular Polysaccharide.
Cell-associated hyaluronic acid was measured as described (15) with slight modifications. Briefly, 6 ml of GAS culture grown in THY to mid-exponential phase (A600 = 0.35–0.4) were harvested by centrifugation, washed in 10 mM Tris buffer (pH 7.5), resuspended in 300 μl of the same buffer, and mixed on a vortex mixer in an equal volume of chloroform. The hyaluronic acid content of the aqueous phase was then measured spectrophotometrically at 640 nm by using 1-ethyl-2-[3-(1-ethylnaptho-[1,2-d]thiazolin-2-ylidene)-2-methylpropenyl]-naptho-[1,2-d]- thiazolium bromide (Stains-All, Sigma). The values shown represent the values obtained for each strain tested minus the background values obtained by using the acapsular mutant strain DLS003hasA∷ΩKm−2 (12).
Measurement of Total Magnesium and Calcium Concentrations in Growth Media.
Magnesium and calcium concentration measurements in THY broth were performed at the Brigham and Women's Hospital Chemistry Laboratory (Boston) by using an Olympus AU640 analyzer according to the manufacturer's instructions. Mg2+ bound to xylidyl blue at pH 11. 4 was measured bichromatically at 520/800 nm, whereas Ca2+ bound to Arsenazo III (2,2-[1,8-dihydroxy-3,6-disulphonaphthylene-2,7-bisazo]-bisbenzenearsonic acid) at pH 6.5 was measured bichromatically at 660/800 nm. To control for the accuracy of the measurements, magnesium and calcium concentration measurements in chemically defined medium were determined in parallel.
DNA Techniques.
Plasmid pCR2.1 is a linearized E. coli vector used for direct cloning of PCR products (Invitrogen); pUC19 is a high copy number cloning vector (16); pBRΩKm−2 is a pBR322 derivative carrying the ΩKm−2 cassette (17); and pJRS233 is a temperature sensitive E. coli-Gram-positive shuttle vector provided by June Scott (18). Plasmid DNA was obtained from E. coli by using the Qiagen miniprep or maxiprep kit according to manufacturer's instructions. GAS chromosomal DNA was prepared as described (19). Restriction endonuclease digestions, DNA ligations, transformations of chemically competent E. coli, and Southern hybridizations (ECL, Pharmacia) were performed by using standard protocols (20). GAS electrocompetent cells were prepared and transformed by using Gene Pulsar II (Bio-Rad) as described (13).
PCR.
PCR was performed with Taq DNA polymerase (Life Technologies) on a GeneAmp PCR 2400 System (Perkin–Elmer) for 32 cycles at the following conditions: 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min. Primers used in PCR are shown in Table 3, which is published as supporting information on the PNAS web site, www.pnas.org.
Construction of a csrS Mutant.
The sensor component gene csrS of GAS strain DLS003 was inactivated by inserting in its 5′ end the kanamycin resistance cassette ΩKm−2. In brief, a 1.1-kb DNA fragment encompassing the 3′ end of csrR and the 5′ end of csrS was amplified from DLS003 chromosomal DNA by using primers csrR-F1 and csrS-R1 (Table 3). The amplicon was cloned into vector pCR2.1 (Invitrogen), released by EcoRI digestion and ligated into pUC19 to give plasmid pUCcsrS. The ΩKm−2 cassette obtained from vector pBRΩKm−2 (17) by SmaI digestion was then inserted into the EcoRV site 310 bp downstream of the csrS start codon. The resulting csrSΩKm−2 construct was released by PvuII digestion and cloned into SmaI-digested pJRS233 (18) to give plasmid pJRcsrSΩKm−2. The fidelity of the final construct containing the 5′ end of csrS disrupted with ΩKm−2 was verified by DNA sequencing. pJRcsrSΩKm−2 was introduced into DLS003 by electroporation and the csrS mutant obtained as described (2). Replacement of the wild-type csrS copy with csrSΩKm−2 and loss of pJRS233 sequences was verified by Southern hybridization.
DNA Sequencing.
Sequencing of plasmid DNA was performed at the Brigham and Women's Hospital Automated Sequencing and Genotyping Facility. Primers used for sequencing are listed in Table 3.
RNA Dot Hybridizations.
Total bacterial RNA was isolated by using the Rneasy mini kit (Qiagen) according to manufacturer's instructions, except that bacteria were lysed by shaking with glass beads on a dental amalgamator (Patterson Brand). RNA samples were treated with 5 units of DNase I (Invitrogen) for 30 min at room temperature to remove any contaminating DNA. The final concentrations of the RNA obtained were adjusted to 100 ng/μl, and the samples were divided into aliquots and frozen at −80°C until needed. RNA was blotted onto Hybond N+ membranes (Pharmacia) by using the Bio-Dot Microfiltration apparatus (Bio-Rad). DNA probes were generated by PCR using 003CAT chromosomal DNA as template, and were radiolabeled with [α-32P]dATP by random priming using the RadPrime DNA Labeling System (Invitrogen). The primer pairs used to generate probes for rpsL, recA, emm3, csrR, csrS, hasB, ska, sagBCD, and speB are listed in Table 3. Hybridizations were carried out in Church buffer at 60°C for 16 h followed by one wash in 1× SSC − 0.1% SDS at room temperature and three washes in 0.5× SSC − 0.1% SDS at 60°C (20). Negative controls consisted of 2 μg total E. coli RNA; positive controls carried 0.3 ng of unlabeled denatured probe DNA in addition to 2 μg total E. coli RNA.
Results
GAS Capsule Gene Expression Is Repressed at High Mg2+ Concentrations.
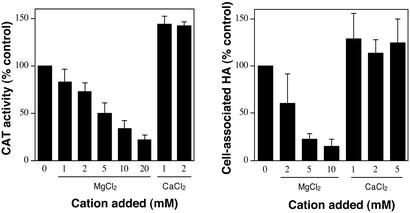
In an effort to identify the signal(s) that regulate virulence gene expression in GAS through the two-component system CsrR/CsrS, we studied the effect of divalent cations on expression of the csr-regulated capsule gene operon (hasABC). Divalent cations such as Mg2+ and Ca2+ are required for fundamental biochemical reactions in bacteria, but also function as environmental stimuli to which two-component systems of certain pathogenic species respond by altering expression of genes involved in virulence (21–23). Capsule gene expression levels in GAS were quantified by measuring CAT activity in cell extracts of strain 003CAT that carries on its chromosome a fusion of its autologous has operon promoter to the gene encoding chloramphenicol acetyltransferase (13). To determine whether the divalent cation concentration influenced capsule gene expression, we compared the CAT activity in extracts of 003CAT grown in unsupplemented broth with that from the same strain grown in broth supplemented with additional amounts of one of four divalent cations: Mg2+, Ca2+, Mn2+, or Zn2+. During growth in THY broth, GAS capsule gene expression is maximal at mid-exponential phase after which it declines to a very low level in stationary phase. Maximum CAT activity consistent with high capsule gene expression was observed in cell extracts of 003CAT obtained at mid-exponential phase growth (see below). However, when 003CAT was grown in broth supplemented with MgCl2 (or MgSO4), capsule gene expression was reduced in a dose-dependent fashion. CAT activities for bacteria grown in 5 and 20 mM additional MgCl2 were reduced by 50% and 78%, respectively, compared with those for bacteria grown in THY broth without additional MgCl2 (Fig. 1). Growth rate, viability, and final cell density reached at stationary phase remained unaffected. Capsule gene expression did not increase when the Mg2+ concentration in THY broth was lowered by the addition of EDTA. Instead, capsule gene expression decreased, as reflected by a 3-fold reduction in CAT activity in extracts of bacteria grown in THY-0.5 mM EDTA. This reduction in capsule gene expression was not attributable to Mg2+ depletion, however, because wild-type CAT activity was restored during growth in THY-0.5 mM EDTA supplemented with 0.5 mM CaCl2 (data not shown). Therefore, capsule gene expression levels were maximal in THY broth without additional Mg2+. The total Mg2+ concentration in THY broth was measured at 0.97 ± 0.02 mM, a level that is similar to the physiological Mg2+ concentrations encountered by GAS in human serum and extracellular body fluids (0.7–1 mM) (24).
Figure 1.
Effect of Mg2+ or Ca2+ on GAS capsule gene expression. GAS strain 003CAT was grown to mid-exponential phase with or without supplemental MgCl2 or CaCl2. Capsule gene expression levels were measured by CAT assay (Left) and cell-associated hyaluronic acid was quantified (Right). Data are represented as percent of the values obtained in the absence of added Mg2+ or Ca2+. Data points represent mean ± SD from at least three independent experiments.
The reduced CAT activity of 003CAT at high MgCl2 concentrations correlated with reduced capsule production: the amount of cell-associated capsular polysaccharide measured on cells grown in 5 and 10 mM additional MgCl2 was 22% and 15%, respectively, of the amount on cells grown in THY broth without additional MgCl2 (Fig. 1). Repression of capsule expression was also evident during growth of 003CAT on THY-blood agar supplemented with MgCl2. Colonies on medium supplemented with 10 mM MgCl2 were small and dry, an appearance indistinguishable from that of colonies of an isogenic acapsular mutant strain DLS003hasA∷ΩKm−2 (12).
Down-regulation of capsule gene expression was specific to Mg2+ as no repression was evident when bacteria were grown in THY broth supplemented with 5 mM or 10 mM additional CaCl2. On the contrary, capsule gene expression increased by ≈40% when an additional 1 or 2 mM CaCl2 was provided (Fig. 1). Because the total concentration of calcium in unsupplemented THY broth was measured at 0.36 ± 0.02 mM, addition of 1 mM CaCl2 produced a total Ca2+ concentration near the physiological range found in human serum and extracellular body fluids (1–1.3 mM) (25). Addition of Mn2+ up to 0.5 mM or Zn2+ up to 0.1 mM did not alter capsule gene expression levels or the amount of cell-associated hyaluronic acid. Addition of either of the two cations at higher concentrations reduced bacterial viability as determined by counts of colony-forming units, growth rates, and final cell densities reached in culture at stationary phase.
Csr-Regulated Genes Are Repressed by High Mg2+ Concentrations in Strain 003CAT.
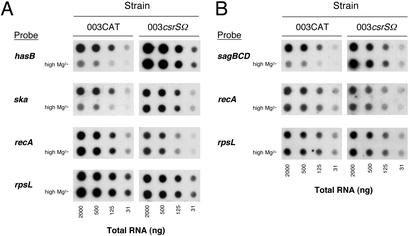
To investigate whether expression of other Csr-regulated genes was repressed at high Mg2+ concentrations, we studied expression not only of the hyaluronic acid synthesis genes (hasABC), but also of streptokinase (ska) during exponential phase and streptolysin S (sagA) during stationary phase. Expression of both Csr components, CsrS and CsrR, was also monitored at both growth phases. In addition, the effect of Mg2+ on expression of M-type 3 protein (emm3) that is regulated by a Csr-independent mechanism was studied (4, 5, 26). GAS strain 003CAT was grown in THY broth with or without supplemental MgCl2 (15 mM), and total RNA was collected at mid-exponential (A600 = 0.3) and stationary (A600 = 0.8) phase. Four-fold serial dilutions of total RNA were blotted onto nylon membranes and hybridized to a radiolabeled DNA probe specific for each gene or operon of interest. The probe for the capsule gene cluster was to the middle gene (hasB) of the has operon (27). The probe for streptolysin S was to the gene cluster sagBCD located downstream of the streptolysin S structural gene (sagA) in the sagABCDEFGHI operon. Streptolysin S activity requires expression of the genes downstream of sagA; therefore, transcript levels for sagBCD reflect streptolysin activity more accurately than sagA transcript itself (28). As a control, the RNA was also probed for two housekeeping genes, rpsL and recA, neither of which is known to be regulated by Csr. As shown in Fig. 2, expression of rpsL and recA was not altered during either exponential or stationary phase in the presence of 15 mM additional MgCl2. By contrast, growth in supplemental Mg2+ was associated with dramatic repression of the Csr-regulated capsule and streptokinase genes at exponential phase and of streptolysin S at stationary phase. Based on the relative intensity of the hybridization signals, the amount of mRNA produced under high Mg2+ conditions was estimated to be reduced 4- to 16-fold for each of the three genes compared with that from cells grown in unsupplemented broth. As expected, expression of streptokinase and capsular polysaccharide was maximal at exponential phase and very low or absent in stationary phase, whereas the converse was true for streptolysin S. The finding that Mg2+ repressed not only capsule gene expression, but also expression of streptokinase and streptolysin S suggests strongly that the effect was mediated by the Csr system.
Figure 2.
Effect of Mg2+ on expression of Csr-regulated genes. Dot blots of total GAS RNA were hybridized to radiolabeled DNA probes specific to the indicated genes, then exposed to x-ray film. GAS wild-type strain 003CAT and the isogenic CsrS mutant strain 003csrSΩ were grown in unsupplemented THY or in THY supplemented with 15 mM MgCl2 (high Mg2+). (A) RNA from GAS collected at exponential phase (A600 = 0.3). (B) RNA collected at stationary phase (A600 = 0.8).
Conflicting data have been reported regarding Csr regulation of speB and of the csrRS locus itself. Whereas speB expression is repressed by Csr in some strains, it appears not to be affected by Csr in others, including DLS003 (2, 4). CsrR was previously reported to repress its own expression, as increased csrRS transcript levels were observed in a csrR mutant (4). However, a subsequent study indicated that phosphorylated CsrR did not bind to its own promoter and therefore did not repress its own expression directly (6). In the present investigation, we found the transcription levels of speB and of the csrRS operon, as well as expression of the Csr-independent emm3, remained unaffected at high Mg2+ concentrations (data not shown).
Expression of Csr-Regulated Genes Is Not Repressed by High Mg2+ Concentrations in a csrS Mutant.
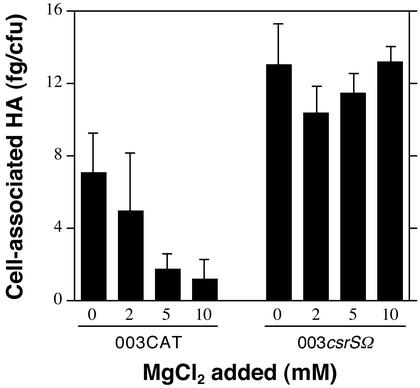
If the hypothesis is correct that Mg2+ down-regulates gene expression by signaling through the CsrR/CsrS system, Csr-regulated genes that are repressed by Mg2+ in the wild-type strain 003CAT should be unaffected by high Mg2+ in a mutant strain that lacks the CsrS sensor. To test this prediction, we constructed an isogenic sensor component mutant (003csrSΩ) by inserting the ΩKm−2 cassette (17) within the coding sequence of csrS near the 5′ end of the gene. In contrast to wild-type strain 003CAT, the sensor component mutant strain showed no decrease in production of capsular polysaccharide at Mg2+ concentrations up to 10 mM (Fig. 3). In further experiments, we examined the expression of Csr-regulated genes in 003csrSΩ grown in the presence or absence of 15 mM MgCl2. As shown previously for csr mutants, expression of hasABC, ska, and sagABCDEFGHI during growth in unsupplemented medium was increased relative to that for wild-type strain 003CAT. However, in contrast to the results obtained with 003CAT, growth of the csrS mutant in 15 mM Mg2+ had no effect on expression of the Csr-regulated genes, hasABC, ska, or sagABCDEFGHI (Fig. 2). The fact that inactivation of csrS abrogated Mg2+ regulation provides strong evidence that Mg2+ signals repression of Csr-regulated gene expression through its interaction with the membrane-bound sensor protein, CsrS.
Figure 3.
Effect of Mg2+ on capsular polysaccharide production by wild-type strain 003CAT and the isogenic CsrS mutant strain, 003csrSΩ. Data represent the amount of cell-associated hyaluronic acid produced by GAS cells grown to mid-exponential phase in THY containing the indicated concentrations of supplemental MgCl2. Values represent mean ± SD of at least three independent experiments.
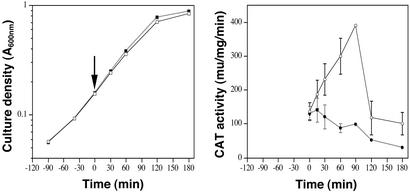
Kinetics of Mg2+-Mediated Capsule Gene Repression.
To study the kinetics of transcriptional regulation in response to a change in Mg2+ concentration, we grew duplicate cultures of 003CAT to early exponential phase (A600 = 0.15) at which point one culture was exposed to 15 mM MgCl2. Sequential bacterial samples were collected from both Mg2+-treated and untreated cultures and CAT activity was determined at each time point. As shown in Fig. 4, the rate of growth was similar with or without supplemental MgCl2. CAT activity for bacteria grown in THY with no additional Mg2+ increased from early exponential phase to reach a maximum 90 min later and then dropped sharply at early stationary phase, a pattern that is also reflected in the amount of cell-associated hyaluronic acid produced (Fig. 4). In contrast, CAT activity in bacterial samples collected after addition of MgCl2 remained relatively unchanged for the first 15 min and then declined over time. At 60 min and 90 min after addition of MgCl2, CAT activities were reduced by 71% and 75%, respectively, compared with those recorded at the same time points for bacteria grown without additional MgCl2. These results indicate that repression of Csr-regulated gene transcription occurs within minutes of a change in environmental Mg2+.
Figure 4.
Kinetics of Mg2+-mediated capsule gene repression in GAS. Duplicate cultures of strain 003CAT were grown in THY to early exponential phase (arrow, Time 0), then one culture was supplemented with 15 mM MgCl2 (filled symbols) and the other was not (open symbols). Growth curves for the two cultures were similar (Left). CAT activity, reflecting capsule gene expression, is shown for GAS cell extracts of the two cultures collected at the indicated time points (Right). Data points represent mean ± range for two independent experiments.
High Mg2+ Concentrations Repress Capsular Polysaccharide Expression in Diverse GAS Isolates.
Because the csr system is highly conserved among GAS strains, we reasoned that Mg2+-dependent repression of capsule synthesis should be demonstrable in other GAS strains in addition to 003CAT. To test this hypothesis, we examined capsule production during growth in THY supplemented with 10–20 mM MgCl2 in a series of GAS isolates of various M protein types. We tested capsule gene expression in strain 282CAT, derived from the heavily encapsulated strain 87-282 (M type 18), that also carries a fusion of its autologous has operon promoter to cat (13). CAT activity in 282CAT extracts from exponential phase cells grown in THY were 2- to 3-fold higher than those for 003CAT extracts, consistent with the higher amounts of cell-associated hyaluronic acid produced by the former strain (13) (see below). Similar to the results obtained for M type 3 strain 003CAT, capsule gene expression in 282CAT decreased dramatically during growth in broth supplemented with MgCl2. Somewhat higher concentrations of MgCl2 were required to achieve maximum repression of capsule gene expression in 282CAT compared with 003CAT: 20 mM MgCl2 was required for a 3-fold decrease in CAT activity for 282CAT, whereas 10 mM MgCl2 produced a similar decrease in the less encapsulated strain 003CAT. Because the has operon is transcribed more actively in 282CAT than in 003CAT, it is possible that higher MgCl2 concentrations are necessary to produce sufficient amounts of phosphorylated CsrR for maximal repression of gene expression in 282CAT. Reduced capsule gene expression by 282CAT during growth in high Mg2+ correlated with markedly reduced production of cell-associated hyaluronic acid (Table 2). Similar Mg2+-dependent repression of capsule synthesis was also observed for three additional GAS strains of M types 1 and 3 (Table 2).
Table 2.
Cell-associated hyaluronic acid expression by wild-type GAS strains grown in THY with or without additional Mg2+
| Strain | M type | Cell-associated HA (fg/cfu)
|
% Reduction | |
|---|---|---|---|---|
| THY | THY + MgCl2 | |||
| 003CAT | 3 | 7.0 ± 0.7 | 0.97 ± 0.2 | 86 |
| 87-764 | 3 | 22 ± 1.3 | 7.8 ± 4.3 | 64 |
| 950802 | 3 | 2.6 ± 1.5 | 0 | 100 |
| DLS048 | 1 | 4.0 ± 0.7 | 0.82 ± 0.3 | 80 |
| 282CAT | 18 | 200 ± 21 | 58 ± 14 | 71 |
cfu, colony-forming unit.
Discussion
The evidence presented here suggests that environmental magnesium regulates virulence gene expression by GAS through the CsrR/CsrS two-component system. The fact that high Mg2+ concentrations stimulated repression of Csr-dependent virulence genes in the wild-type GAS strain but not in an isogenic CsrS mutant points to a direct interaction of Mg2+ with the putative sensor component protein. The interaction is likely to involve the extracellular domain of CsrS in a fashion analogous to that described for the periplasmic domain of the PhoQ Mg2+ sensor of Salmonella enterica serovar Typhimurium (21, 29). Both CsrS and PhoQ belong to the EnvZ family of sensor kinases, and CsrS is similar to PhoQ in several ways: the two sensor proteins are of similar size; each has two predicted membrane spanning domains near the N terminus; both have a predicted extracellular or periplasmic domain of similar size (146 and 151 aa for PhoQ and CsrS, respectively). The two proteins share 43% similarity over 300 aa by blastp analysis (30). Within the predicted extracellular domains of the two proteins, there is particularly striking similarity in acidic residues, which have been implicated in cation binding. Ten of 20 acidic amino acids within the predicted extracellular domain of CsrS are similar or identical in PhoQ (Fig. 5, which is published as supporting information on the PNAS web site). PhoQ of E. coli has been proposed to bind Mg2+ on its periplasmic domain via a cluster of seven amino acids, six of which are acidic (31). Such a cluster is also found in the PhoQ periplasmic domain of S. enterica serovar Typhimurium, but not in some other PhoQ homologues that are also able to respond to Mg2+ (23, 32). Thus, a specific motif of acidic residues required for Mg2+ binding has not been defined.
Although there are striking parallels between CsrR/CsrS and the PhoP/PhoQ system, there are also important differences that may reflect the specific adaptive functions of these regulatory systems for pathogens that employ different strategies for survival within their animal or human hosts. In S. enterica serovar Typhimurium, binding of Mg2+ to PhoQ promotes dephosphorylation of PhoP, which renders it inactive as a transcriptional activator of PhoP-induced genes (23). By contrast, binding of Mg2+ to CsrS results in repression of CsrR-regulated genes, presumably by increasing phosphorylation of CsrR. So, although the net result in both cases is repression of virulence gene expression by high Mg2+, repression is achieved through opposite effects on phosphorylation of the respective regulator proteins. Another important difference between the two systems is the range of magnesium concentrations to which they respond: maximal repression of PhoP-induced genes in Salmonella occurs during growth in Mg2+ concentrations of ≈1 mM or greater, whereas the Csr system is fully active in repressing gene expression only at Mg2+ concentrations ≥10 mM. It has been suggested that responsiveness of the PhoP/PhoQ system is optimized for survival of Salmonella within the phagosome where Mg2+ concentrations are likely to be in the micromolar range (33). Although GAS may enter a variety of eukaryotic cells, they fail to proliferate intracellularly and their intracellular survival is limited (15, 34). The Mg2+ concentration at which the Csr-regulated genes are maximally expressed (≈1 mM) is approximately that of human extracellular fluids, as might be expected for an extracellular pathogen. Furthermore, whereas one of the functions of the PhoP/PhoQ system is induction of expression of two magnesium importer systems (MgtA, MgtCB) to permit Salmonella survival in very low Mg2+ concentrations, no such system for scavenging magnesium has been identified in GAS. Rather, the CsrR/CsrS system appears to be optimized to ensure maximum expression of virulence factors under conditions encountered by GAS in mucosal secretions and extracellular fluids and not under conditions of higher Mg2+ concentrations such as the natural environment outside the body.
Expression of the GAS genes that direct synthesis of capsular polysaccharide, streptokinase, and streptolysin S was strikingly repressed in the presence of high Mg2+ concentrations. However, expression of the two Csr components in the same conditions was not affected. Although some studies have suggested autoregulation of the csr system, the CsrR protein appears not to interact directly with the csr operon promoter (6). The apparent lack of Mg2+ regulation of the csr locus itself suggests that the repressive activity of the system depends primarily on the phosphorylation state of CsrS which governs phosphorylation of CsrR, rather than on the relative abundance of the CsrS and CsrR proteins. Repression of capsule gene expression in GAS subjected to high Mg2+ concentrations was evident within minutes. Such rapid repression is consistent with the proposed model of Mg2+ interacting with CsrS already present on the cell membrane and initiation of the phosphorelay cascade. Magnesium is abundant in the natural environment and may signal GAS to adapt for survival in the external environment. Signaling through the Csr system may permit GAS to rapidly down-regulate production of host-specific virulence factors and divert energy to alternative metabolic pathways important for survival outside the human host.
The csr system response appears to be specific to Mg2+, because other divalent cations tested did not affect capsule gene expression at nontoxic concentrations. Whereas high Mg2+ resulted in down-regulation of Csr-regulated genes, we observed a modest increase in capsule gene expression as the Ca2+ concentration was increased to a level similar to those found in human extracellular fluids. It remains to be determined whether the GAS response to Ca2+ is Csr-dependent. If so, both Mg2+ and Ca2+ concentrations encountered by GAS in the human host are ideal for maximum Csr-regulated gene expression.
Although the experiments presented here were performed in vitro, Mg2+-dependent regulation of virulence gene expression by GAS is expected to operate also in vivo during infection. Expression of Csr-regulated virulence genes was maximal, hence, the csr system was minimally induced, at ≈1 mM Mg2+, a concentration that is similar to the physiologic Mg2+ levels in body fluids such as mucosal secretions and the blood (24). A previous study demonstrated that expression of the Csr-regulated has operon is rapidly induced after introduction of GAS into the mouse peritoneum or the baboon pharynx (35). Although the precise stimulus for up-regulation of capsule gene expression was not defined, the results are consistent with those of the current investigation. In recent experiments, we also observed Mg2+-dependent repression of capsule gene expression in GAS grown in 10% or 50% human plasma (data not shown). Thus, it appears unlikely that other factor(s) in plasma inhibit the down-regulatory effects of high magnesium on Csr-regulated genes. Rather, the low concentrations of Mg2+ at both deep tissue and mucosal sites ensure that CsrR will remain in the unphosphorylated state that has low affinity for Csr-regulated promoters, thereby permitting high-level expression of the has operon and other Csr-regulated virulence genes.
Identification of magnesium as a specific environmental stimulus that controls Csr-mediated regulation of GAS virulence determinants expands our understanding of the complex interactions between GAS and the human host. Successful adaptation of a pathogen requires dynamic modulation of metabolic activities and expression of critical virulence determinants in a manner that optimizes bacterial survival in the various environmental conditions the organism confronts. Our data implicate environmental Mg2+ as a stimulus that signals GAS to up-regulate expression of Csr-regulated virulence factors under conditions encountered during human infection. To our knowledge, this is the first specific signal demonstrated to interact with any two-component regulatory system in this important human pathogen.
Supplementary Material
Acknowledgments
We thank D. Wessels for technical assistance and R. Ross and J. Cocchiaro for helpful discussions. This work was supported by National Institutes of Health Grant AI29952 and by a grant-in-aid from the American Heart Association.
Abbreviations
- GAS
group A Streptococcus
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cunningham M W. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin J C, Wessels M R. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernish B, van de Rijn I. J Biol Chem. 1999;274:4786–4793. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- 4.Federle M J, McIver K S, Scott J R. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath A, DiRita V J, Barg N L, Engleberg N C. Infect Immun. 1999;67:5298–5305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller A A, Engleberg N C, Dirita V J. Mol Microbiol. 2001;40:976–990. doi: 10.1046/j.1365-2958.2001.02441.x. [DOI] [PubMed] [Google Scholar]
- 7.Lei B, DeLeo F R, Hoe N P, Graham M R, Mackie S M, Cole R L, Liu M, Hill H R, Low D E, Federle M J, et al. Nat Med. 2001;7:1298–1305. doi: 10.1038/nm1201-1298. [DOI] [PubMed] [Google Scholar]
- 8.Graham M R, Smoot L M, Lux Migliaccio C A, Virtaneva K, Sturdevant D E, Porcella S F, Federle M J, Adams G J, Scott J R, Musser J M. Proc Natl Acad Sci USA. 2002;99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferretti J J, McShan W M, Ajdic D, Savic D J, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov A N, Kenton S, et al. Proc Natl Acad Sci USA. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreikemeyer B, Boyle M D, Buttaro B A, Heinemann M, Podbielski A. Mol Microbiol. 2001;39:392–406. doi: 10.1046/j.1365-2958.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 11.Federle M J, Scott J R. Mol Microbiol. 2002;43:1161–1172. doi: 10.1046/j.1365-2958.2002.02810.x. [DOI] [PubMed] [Google Scholar]
- 12.Schrager H M, Albertí S, Cywes C, Dougherty G J, Wessels M R. J Clin Invest. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albertí S, Ashbaugh C D, Wessels M R. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 14.Shaw W V. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 15.Schrager H M, Rheinwald J G, Wessels M R. J Clin Invest. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanisch-Perron C, Viera J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Casal J, Caparon M G, Scott J R. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Casal J, Price J A, Maguin E, Scott J R. Mol Microbiol. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor S P, Cleary P P. J Infect Dis. 1987;156:495–504. doi: 10.1093/infdis/156.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Russell D W. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 21.Garcia Vescovi E, Soncini F C, Groisman E A. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 22.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 23.Groisman E A. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinhart R A. Arch Intern Med. 1988;148:2415–2420. doi: 10.1001/archinte.148.11.2415. [DOI] [PubMed] [Google Scholar]
- 25.Brown E M. In: The Parathyroids. Bilezikian J P, Marcus R, Levine M A, editors. New York: Raven; 2001. pp. 167–181. [Google Scholar]
- 26.Caparon M, Scott J. Proc Natl Acad Sci USA. 1987;84:8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougherty B A, van de Rijn I. J Biol Chem. 1993;10:7118–7124. [PubMed] [Google Scholar]
- 28.Nizet V, Beall B, Bast D J, Datta V, Kilburn L, Low D E, De Azavedo J C. Infect Immun. 2000;68:4245–4254. doi: 10.1128/iai.68.7.4245-4254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vescovi E G, Ayala Y M, Di Cera E, Groisman E A. J Biol Chem. 1997;272:1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 30.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 31.Waldburger C D, Sauer R T. J Biol Chem. 1996;271:26630–26636. doi: 10.1074/jbc.271.43.26630. [DOI] [PubMed] [Google Scholar]
- 32.Lesley J A, Waldburger C D. J Biol Chem. 2001;276:30827–30833. doi: 10.1074/jbc.M104262200. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 34.Greco R, De Martino L, Donnarumma G, Conte M P, Seganti L, Valenti P. Res Microbiol. 1995;146:551–560. doi: 10.1016/0923-2508(96)80561-4. [DOI] [PubMed] [Google Scholar]
- 35.Gryllos I, Cywes C, Shearer M H, Cary M, Kennedy R C, Wessels M R. Mol Microbiol. 2001;42:61–74. doi: 10.1046/j.1365-2958.2001.02635.x. [DOI] [PubMed] [Google Scholar]
- 36.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. J Clin Invest. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.