Abstract
Dopaminergic (DA) neurons of substantia nigra in the midbrain control voluntary movement, and their degeneration is the cause of Parkinson's disease. The complete set of genes required to specifically determine the development of midbrain DA subgroups is not known yet. We report here that mice lacking the bicoid-related homeoprotein Pitx3 fail to develop DA neurons of the substantia nigra. Other mesencephalic DA neurons of the ventral tegmental area and retrorubral field are unaltered in their dopamine expression and histological organization. These data suggest that Pitx3-dependent gene expression is specifically required for the differentiation of DA progenitors within the mesencephalic DA system.
Midbrain dopaminergic (DA) cells contribute to the control of voluntary movement, cognition, and emotional behavior. Degeneration of these cells results in Parkinson's disease, and aberrant dopamine neurotransmitter signaling is implicated in schizophrenia and addictive behavioral disorders (1–3). Within the midbrain there are three subgroups of DA neurons, the ventral tegmental area (VTA/A10), substantia nigra (SN/A9), and retrorubral field (RRF/A8), that together constitute the mesencephalic (mes) system (4). Those DA cells in the SN that innervate the striatum preferentially degenerate in Parkinson's disease, whereas other DA subgroups regulate emotional and reward behaviors.
Transcription factors regulate the differentiation of midbrain DA neuron precursors. Two transcription factors have been shown to contribute to different aspects of mesDA neuronal differentiation. One of these, the orphan nuclear hormone receptor Nurr1, is required for maturation of mesDA neuron precursors. Mice harboring null alleles of Nurr1 do not express tyrosine hydroxylase (TH), which catalyzes the initial step of dopamine neurotransmitter biosynthesis (5–11). The other factor, LIM homeodomain transcription factor Lmx1b, contributes partially to the specification of mesDA neuronal progenitors beginning on embryonic day 12.5 in the mouse but is not essential for TH gene expression (12).
A third transcription factor, the bicoid-related homeodomain-containing transcription factor Pitx3, which is also known as Ptx3, has been implicated in the development of DA neurons. Pitx3 gene expression is restricted to the developing eye and DA progenitor cells from embryonic day 11 throughout adult life in mice (13, 14). In the brain, Pitx3 mRNA localizes specifically to the SN and VTA (14). A reduction in Pitx3 mRNA levels is observed in the ventral midbrain of Lmx1b knockout mice, 6-hydroxydopamine-lesioned rats, and Parkinson's patients (8, 11, 12, 14). Yet, Pitx3 expression is maintained in the ventral midbrain of Nurr1 null mutant embryos (12). These data have been interpreted to suggest that Pitx3 contributes to the combinatorial code defined by multiple transcription factors that establish specification and differentiation of midbrain DA progenitors (12). Here we report that Pitx3 is essential for the development of neurons specific to the SN. The DA cell population in VTA and A8 of Pitx3-deficient newborn mice is intact, whereas these cells in SN are absent. This is the first described factor that is required for the development of a specific DA neuronal population within the mesDA system.
Materials and Methods
Animals.
A mating pair of +/aphakia (ak) heterozygote mice (C57BL/6 × C57BLKS hybrid strain) was a generous gift from Ron DePinho (Harvard Medical School, Boston, MA). ak heterozygotes were intercrossed to generate wild-type (wt) and homozygous animals for experiments. Animal housing and husbandry was in accordance with institutional guidelines. Adult mice (6–8 weeks of age) and postnatal day (P)2 newborns were used for experiments.
Immunohistochemistry.
Adult and newborn mice were perfused intracardially with PBS followed by 4% formaldehyde/PBS. Dissected brains were postfixed in 4% formaldehyde/PBS for 2–5 days and cryoprotected in 20% sucrose/PBS overnight before freezing in 2-methylbutane. Serial coronal sections (30 μm) of the midbrain and striatum were cut on a freezing microtome and immediately processed for TH immunoreactivity (IR) as described elsewhere (15). In brief, floating sections were blocked in PBS/0.5% BSA, permeabilized in PBS/0.5% BSA/0.1% Triton X-100, and incubated in rabbit anti-TH antibodies 1:1,000 (Calbiochem)/PBS/0.5% BSA for 48 h. Sections were washed in PBS/0.5% BSA before incubating with the secondary antibody biotinylated goat anti-rabbit antiserum diluted 1:100 in 2% normal goat serum/PBS (Vector Laboratories) for 60 min. Sections were washed and then incubated with components of the avidin–biotin–peroxidase complex (Vectastain ABC, Vector Laboratories) for 60 min. IR was visualized by using the diaminobenzidine method (15).
HPLC Analysis.
Brain tissues were collected from adult and P2 pups for quantitation of norepinephrine (NE), dopamine, and the dopamine metabolite 3,4-dihydroxphenylacetic acid (DOPAC). Adult tissue samples were collected from striata by using the micropunch technique (16). Newborn (P2) forebrains were block-dissected and weighed. Brain specimens were frozen on dry ice and shipped to Bioanalytical Systems (West Lafayette, IN) for HPLC analysis.
Nissl Staining.
Frozen coronal sections (30 μm) of midbrain obtained from adult wt and ak/ak mice were Nissl-stained in a solution containing 0.1% thionin in acetic acid, pH 5.5 (15).
Retrograde Axonal Labeling.
For retrograde labeling of neurons projecting to the striatum, adult wt and ak/ak mice were anesthetized and administered an intracranial injection of 4% Fluoro-Gold (Fluorochrome, Denver, CO)/PBS solution. After a 24-h labeling, brains were dissected and processed for cryostat sectioning. Coronal sections of the midbrain were collected for epifluorescence microscopy.
Results
TH IR in the Adult ak Mouse.
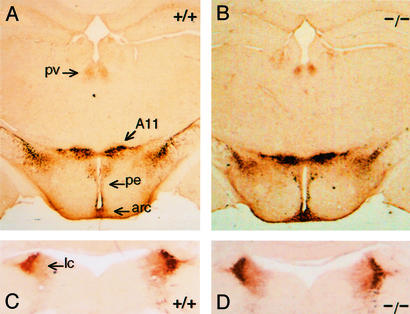
The in vivo role of Pitx3 in the mesDA system was analyzed by using the ak mouse (17). A spontaneous mutation of the Pitx3 locus in the ak mouse resulted in a null allele lacking putative regulator elements and promoter sequences (18, 19). Undetectable levels of Pitx3 mRNA have been reported in the ak mouse, indicating that the sequence deletion resulted in a null mutation (19). Mice that are heterozygous for the ak allele of Pitx3 are phenotypically normal, whereas ak/ak mutants exhibit an obvious ocular defect of microphthalmia secondary to arrested lens development beyond the lens vesicle stage (19). Because published data suggested that Pitx3 may contribute to midbrain DA neuronal function and Pitx3-deficient ak mutants presented with abnormal general motor activity (data not shown), we examined the midbrain TH-IR neuronal population in wt and ak adult mice (>6 weeks of age). TH IR was nearly absent in the SN of mutant mice compared with wt mice (Fig. 1 A–D), yet TH IR of VTA (Fig. 1) and A8 (Fig. 1 E and F) was preserved to essentially wt levels. In addition, subdomains of mutant SN demonstrated a differential loss of TH IR. The dorsal and ventral tiers of SN pars compacta (SNpc) and SN reticulata (SNr) were affected most strongly, whereas SN pars lateralis was less affected (Fig. 1 C and D). We conclude that the loss of TH IR is specific to the SN, because TH IR of other DA cell groups (VTA, A8, paraventricular thalamic nucleus, A11/caudal thalamus, periventricular hypothalamic nucleus, arcuate hypothalamic nucleus; Fig. 2 A and B) and NE cells in locus ceruleus (Fig. 2 C and D) remains intact.
Figure 1.
TH immunohistochemistry of adult wt (+/+) (A, C, and E) and ak/ak (−/−) (B, D, and F) ventral midbrain. Coronal sections through rostral (A and B), middle (C and D), and caudal (E and F) levels were stained. TH IR was strong in VTA, SN pars lateralis (SNpl), and A8 of both wt and mutant sections throughout the midbrain but absent in SNpc and SNr. ml, medial lemniscus.
Figure 2.
TH immunohistochemistry of adult wt (+/+) and ak/ak (−/−) diencephalon (A and B) and brainstem (C and D). Levels of TH IR in DA neurons of the diencephalon (A and B) and noradrenergic neurons in locus ceruleus (lc) (C and D) were similar in sections from wt and mutant mice. pv, paraventricular thalamic nucleus; pe, periventricular hypothalamic nucleus; arc, arcuate hypothalamic nucleus. A11, caudal thalamus.
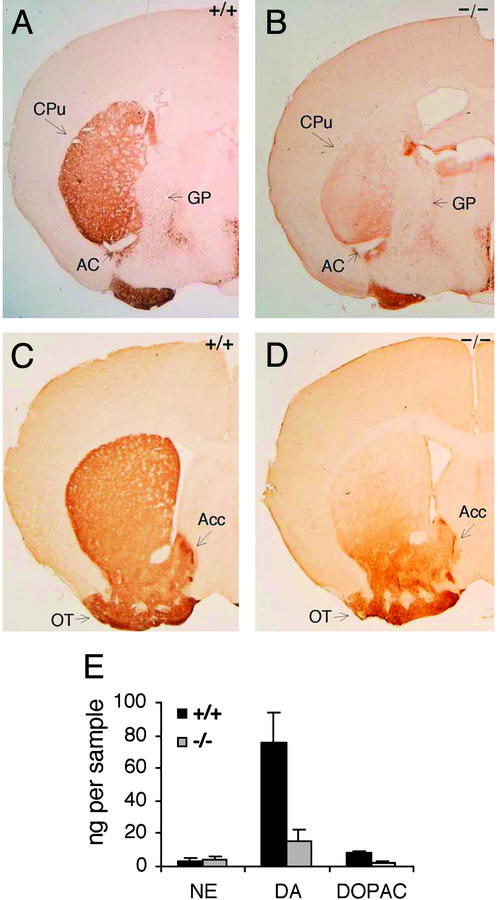
MesDA cells innervate the striatum. Axons from the three subgroups of DA neurons (A8, SN, and VTA) project to different compartments in the striatum (4, 20). The A8 cell group provides DA fibers to dorsal striatum. DA cells in the dorsal and ventral tiers of SNpc project to dorsolateral striatum as well. However, DA projections of VTA are directed to ventromedial striatum, nucleus accumbens, and the olfactory tubercle. To determine whether mesDA projections are intact in the mutants, we characterized striatal DA afferents immunostained for TH in Pitx3-deficient ak mice. A TH-positive plexus of fibers was present in ventromedial caudate putamen (CPu), accumbens, and olfactory tubercle of both mutant and wt sections (Fig. 3 A–D). This fiber staining is consistent with the presence of a largely intact DA-positive cell population in the VTA of mutant mice (Fig. 1). However, significantly reduced TH IR of dorsolateral CPu was observed in ak/ak sections (Fig. 3 A–D), consistent with the decreased TH IR seen in mutant SN (Fig. 1 A–D). Low levels of TH IR seen in dorsal CPu of ak/ak mice presumably reflect the A8 contribution of DA afferent fibers. These data suggest that the lack of Pitx3 does not alter the compartmentalization and circuitry of mesostriatal DA afferents from VTA and A8, but it does result in the loss of nigrostriatal fibers and DA neurons specific to the SN.
Figure 3.
TH immunohistochemistry of adult wt (+/+) (A and C) and ak/ak (−/−) (B and D) striatum. Coronal sections of the entire striatum were analyzed, but sections representative of caudal (A and B) and rostral (C and D) levels are presented. AC, anterior commissure; GP, globus pallidus. Reduced TH staining was most significant in the dorsolateral region of mutant CPu compared with wt mice. TH IR of olfactory tubercle (OT) and accumbens (Acc) was equal among wt and ak sections. (E) NE, DA, and DOPAC levels in micropunches of wt and ak/ak striata were measured by using HPLC. The data are graphed as nanograms of neurotransmitter per micropunch. The tissue volumes in all micropunches were equal. Decreased levels of DA and DOPAC are statistically significant (n = 8, P < 0.001).
HPLC Analysis of DA Levels in Adult Striatum.
To characterize the reduction of striatal DA efferent fibers further, neurotransmitter levels were measured. HPLC was used to measure levels of dopamine and the dopamine metabolite DOPAC in adult striatum. Because dopamine is a precursor for NE and epinephrine production, measured dopamine levels reflect catecholamine neurotransmitter synthesis in both DA and NE neurons. Therefore, NE levels were also measured to distinguish between these two catecholamine systems. Dopamine and DOPAC levels in mutant animals were reduced to 21% and 23% of wt levels, respectively, whereas NE levels in adult wt and ak/ak mice were equal (Fig. 3E). This indicates that the loss of TH IR reflects a specific loss of nigrostriatal fibers.
Nissl Staining and Retrograde Labeling of Adult Ventral Midbrain.
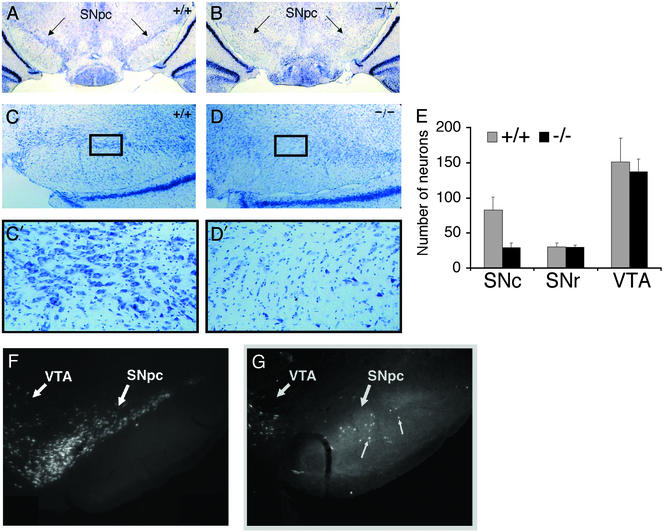
To determine whether decreased TH IR in mutant SN and CPu reflects a decrease in midbrain cell density versus a loss of TH gene expression, we Nissl-stained coronal sections of the midbrain and retrograde-labeled mesDA neurons with Fluoro-Gold (see Materials and Methods). The SN of adult ak/ak mice has significantly less cellularity compared with wt SN as demonstrated in Nissl-stained sections (Fig. 4 A–D). Neuronal cell counts of Nissl-stained midbrain revealed equivalent numbers of neurons in wt and mutant sections of SNr and VTA, whereas 65% less neurons were observed in mutant SNc compared with wt SNc (Fig. 4E). Fluoro-Gold tracer experiments demonstrated that the nigrostriatal fiber bundle emerging from the SN of adult ak mice was essentially unlabeled (Fig. 4 F and G). The absence of Fluoro-Gold labeling most likely reflects the paucity of DA neurons in SNc rather than a defect in intracellular transport. In addition, there is evidence of irregular circuitry between the SN and striatum of ak mice, because cells in the reticulata were retrograde-labeled in the mutant but not in the wt (Fig. 4 F and G). These findings demonstrate that Pitx3 is essential to either the development or maintenance of DA cells specific to the SN.
Figure 4.
Nissl staining of wt (+/+) (A and C) and ak/ak (−/−) (B and D) adult ventral midbrain. Representative coronal sections are presented to document the decreased cell density in mutant SNpc. Photographs at lower (A and B, ×25; C and D ×100) and higher (C′ and D′, boxed fields, ×400) magnification are provided. (E) Neurons in SNc, SNr, and VTA of wt and mutant Nissl-stained sections were counted. Neurons were distinguished from glial cells based on differences in cell size. Cell counts from four fields (×400 magnification) were averaged and graphed. (F and G) Retrograde labeling of ventral midbrain neurons in wt (F) and ak/ak (G) mice with Fluoro-Gold. Note the absence of cell labeling in mutant SN and aberrant labeling of cells in mutant SNr (small arrows). The neuronal population in the VTA of wt and mutant mice was similar with respect to cell density and location.
Characterization of the MesDA System in Newborn ak Mice.
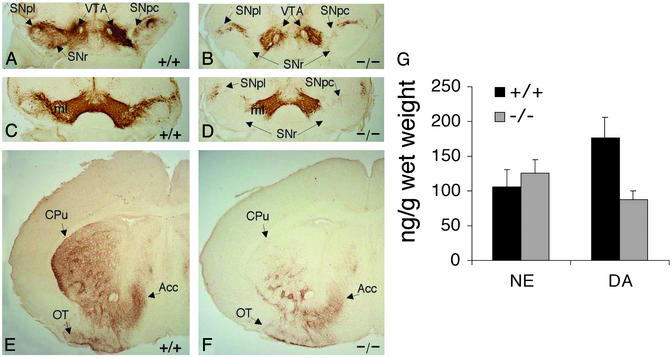
To determine whether the Pitx3-dependent decrease in SN cell density is a developmental or adult degenerative defect, we characterized midbrain and striatal TH IR in newborn wt and ak/ak mice. As observed in the adult, there was significantly reduced TH IR in SNpc and SNr of mutant newborns compared with wt mice (Fig. 5 A–D) as well as in the CPu of mutant mice (Fig. 5 E and F). HLPC was used to measure levels of NE and dopamine present in forebrains of newborn wt and mutant mice. A 50% reduction in dopamine levels was observed in mutant newborn forebrains, whereas equal levels of NE were present in wt and mutant tissues (Fig. 5G). Taken together, these data indicate that the decreased cell density of SN and the absence of nigrostriatal fibers observed in adult ak mice are a developmental and not a postnatal degenerative defect.
Figure 5.
TH immunohistochemistry of newborn (P2) wt (+/+) and ak/ak (−/−) ventral midbrain (A–D) and striata (E and F). Coronal sections through rostral (A and B) and middle (C and D) levels of midbrain were stained. TH IR was strong in the VTA of both wt and mutant mice but absent in mutant SNpc and SNr. ml, medial lemniscus. (E and F) Coronal sections throughout the striata of wt and ak mice were stained, and representative sections are presented. TH IR was reduced significantly in the dorsolateral region of mutant CPu when compared with wt striatum. (G) NE and DA levels in dissected P2 forebrains of wt and ak mice were measured by using HPLC. The data are presented as nanograms of neurotransmitter per gram of dissected tissue. The reduction of DA levels in mutant forebrains is statistically significant (n = 8, P < 0.001).
Discussion
The results here suggest that Pitx3 is a uniquely specific determinant of SN DA neurons. Previous studies describing the birth and differentiation of DA precursors have identified the genes Nurr1, Hnf3β, Lmx1b, and TH, which are expressed in different overlapping areas of the midbrain (8, 12). The loss of these factors disrupts different aspects of development of the mesDA system and other cell types as well. The combination of factors that specify the subgroups of DA neurons that constitute the SN, VTA, and A8 is poorly understood. Our studies indicate that Pitx3 is required for the specification and/or survival of DA precursors unique to the SN and not other DA areas. Cell-lineage studies and identification of additional factors that regulate the differentiation of DA precursors will be required to advance our understanding of how DA neurons specific to VTA, SN, and A8 develop.
The ak/ak mouse is a useful animal model to study the development of SN DA neurons and compensatory neural circuits that develop in the absence of nigrostriatal fibers. We report that mutant animals lack SN DA cells and their respective projections, yet the remainder of the mesDA system seems intact. There is a possibility that the failed differentiation and/or survival of SN DA cells in the mutant mice may result in transdifferentiation of DA precursors and establishment of an aberrant circuit. Results from our retrograde labeling experiments support the speculation that the nigrostriatal circuitry is abnormal in the absence of Pitx3 (Fig. 4 F and G). Preliminary experiments in which Lmx1b protein expression was examined indicate that there is an expansion in the number of midbrain cells that are both Lmx1b-positive and TH-negative in ak pups (data not shown). This suggests that feedback mechanisms regulated by Pitx3 and Lmx1b may be required for the proper development of the midbrain.
The cells affected in the ak/ak mice are the same cells that degenerate first in Parkinson's disease patients, and thus the ak/ak mouse might be proposed to be a valid model for Parkinson's disease. The expected phenotype resulting from adult loss of SN DA cells would be hypoactivity. However, initial behavioral studies indicated that the levels of general motor activity appeared to be higher in ak/ak mice compared with wt mice (data not shown). These experiments have been complicated by additional preliminary findings that mutant mice are not visually responsive to light and that their periods of activity are not light-entrained (data not shown). Perhaps the most significant difference between the cell loss in mutant mice and Parkinson's patients is that in the mice the absence of SN DA cells results from a developmental defect and not from an adult degenerative process.
Cell-replacement treatment of the progressive neurodegenerative disorder Parkinson's disease using stem cell-derived DA neurons has shown great promise (21). However, the controlled differentiation of stem cells into DA neurons that develop and connect as a mature DA cell from the SN is essential to the success and widespread implementation of this therapy (22–24). Understanding how factors specify development of the different classes of midbrain DA neurons would advance this effort. Results presented here show that development of SN-specific DA neurons requires Pitx3, and we speculate that stem cells induced to acquire a Pitx3-regulated gene expression profile may adopt a substantia nigral DA cell fate.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS26836 and NS38370 (to R.E.B.) and EY13424 (to I.N.) and the Michael J. Fox Foundation for Parkinson's Research (to R.M.S.). S.P.G. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- DA
dopaminergic
- VTA
ventral tegmental area
- SN
substantia nigra
- A8
retrorubral field
- mes
mesencephalic
- TH
tyrosine hydroxylase
- ak
aphakia
- wt
wild type
- Pn
postnatal day n
- IR
immunoreactivity
- NE
norepinephrine
- DOPAC
3,4-dihydroxphenylacetic acid
- SNpc
SN pars compacta
- SNr
SN reticulata
- CPu
caudate putamen
Note Added in Proof.
Additional supporting data (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org) are provided to confirm that the SN defect observed in ak newborns is a developmental phenotype. Sections of wt and ak embryonic day 14 midbrains were immunohistochemically stained for TH and Lmx1b. Lmx1b is a known early marker for the developing mesDA system (12). Significantly reduced TH staining of the ventral midbrain was observed in the mutant compared with the wt, and a moderate reduction in Lmx1b staining was observed in the ak midbrain compared with the wt. These findings confirm that the ak midbrain defect reflects an aberrant developmental or degenerative process occurring before embryonic day 14.
References
- 1.Seeman P, Guan H C, Van Tol H H. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- 3.Damier P, Hirsch E C, Agid Y, Graybiel A M. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund A, Lindvall O. In: Classical Transmitters in the CNS: Part I. Bjorklund A, Hokfelt T, editors. Amsterdam: Elsevier Science; 1984. pp. 55–122. [Google Scholar]
- 5.Zetterstrom R H, Solomin L, Jansson L, Hoffer B J, Oson L, Perlmann T. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 6.Wallen A, Zetterstrom R H, Solomin L, Arvidsson M, Olson L, Perlmann T. Exp Cell Res. 1999;253:737–746. doi: 10.1006/excr.1999.4691. [DOI] [PubMed] [Google Scholar]
- 7.Witta J, Baffi J S, Palkovits M, Mezey E, Castillo S O, Nikodem V M. Brain Res Mol Brain Res. 2000;84:67–78. doi: 10.1016/s0169-328x(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 8.Saucedo-Cardenas O, Quintana-Hau J D, Le W-D, Smidt M P, Cox J J, De Mayo F, Burback J P H, Conneely O M. Proc Natl Acad Sci USA. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baffi J S, Palkovits M, Castillo S O, Mezey E, Nikodem V M. Neuroscience. 1999;93:631–642. doi: 10.1016/s0306-4522(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 10.Castillo S O, Baffi J S, Palkovits M, Goldstein D S, Kopin I J, Witta J, Magnuson M A, Nikodem V M. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- 11.Le W, Conneely O M, Zou L, He Y, Saucedo-Cardenas O, Jankovic J, Mosier D R, Appel S H. Exp Neurol. 1999;159:451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- 12.Smidt M P, Asbreuk C H J, Cox J J, Chen H, Johnson R L, Burbach J P H. Nat Neurosci. 2000;3:337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- 13.Gage P J, Suh H, Camper S A. Mamm Genome. 1999;10:197–200. doi: 10.1007/s003359900970. [DOI] [PubMed] [Google Scholar]
- 14.Smidt M P, van Schaick H S A, Lanctôt C, Tremblay J J, Cox J J, van der Kleij A A M, Wolterink G, Drouin J, Burbach J P H. Proc Natl Acad Sci USA. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oo T F, Burke R E. In: Parkinson's Disease: Methods and Protocols. Mouradian M M, editor. Totowa, NJ: Humana; 2001. pp. 101–112. [Google Scholar]
- 16.Palkovits M, Brownstein M, Saavedra J M, Axelrod J. Brain Res. 1974;77:137–149. doi: 10.1016/0006-8993(74)90810-5. [DOI] [PubMed] [Google Scholar]
- 17.Varnum D S, Stevens L C. J Hered. 1968;59:147–150. doi: 10.1093/oxfordjournals.jhered.a107667. [DOI] [PubMed] [Google Scholar]
- 18.Rieger D K, Reichenberger E, McLean W, Sidow A, Olsen B R. Genomics. 2001;72:61–72. doi: 10.1006/geno.2000.6464. [DOI] [PubMed] [Google Scholar]
- 19.Semina E V, Murray J C, Reiter R, Hrstka R F, Graw J. Hum Mol Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- 20.Gerfen C R, Herkenham M, Thibault J. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunnett S B, Bjorklund A, Lindvall O. Nat Rev Neurosci. 2001;2:365–369. doi: 10.1038/35072572. [DOI] [PubMed] [Google Scholar]
- 22.Kim J H, Auerbach J M, Rodríguez-Gómez J A, Velasco I, Gavin D, Lumelsky N, Lee S-H, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S-I, et al. Proc Natl Acad Sci USA. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki H, Mizuseki K, Sasai Y. Methods Mol Biol. 2002;185:217–227. doi: 10.1385/1-59259-241-4:217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.