Abstract
Neural systems for visual processing can focus attention on behaviorally relevant objects, filtering out competing distractors. Neurophysiological studies in animals and brain imaging studies in humans suggest that such filtering depends on top-down inputs to extrastriate visual areas, originating in structures important for attentional control. To test whether the posterior parietal cortex may be a necessary source of signals that filter distractors, we measured the ability of a patient with bilateral parietal lesions to discriminate the features of a target surrounded by distractors of variable contrast. In the presence of distractors, the patient was impaired at discriminating both grating orientation and faces, and the magnitude of the impairment increased with distractor salience. These attentional deficits are remarkably similar to those caused by damage to monkey extrastriate regions V4 and/or TEO, which are thought to be recipients of top-down attentional feedback. In contrast to the effects of V4 and TEO lesions, however, the parietal lesions impaired performance even with widely spaced targets and distractors, a finding consistent with the projections of parietal cortex to visual processing areas covering a wide range of receptive field sizes and eccentricities.
A typical visual scene contains many different objects, not all of which can be fully processed at any given moment. Attentional mechanisms are therefore needed to focus visual processing on the most behaviorally relevant stimuli and to filter out competing distractors. A possible mechanism for this resolution of competition between objects has been described in ventral stream visual areas of monkeys, where neurophysiological studies have found that in the absence of attention, multiple stimuli in the receptive field (RF) of a cell will compete for the response of the cell. However, when attention is directed to a target stimulus in the RF, responses are biased in favor of the target, and the influence of distracting stimuli in the RF is filtered out (1–11). Likewise, brain imaging studies in humans show a similar biasing of competition in favor of attended stimuli (12–20), and human subjects often experience little awareness for distractor stimuli outside the focus of attention (21–23).
Lesion data also support the idea that ventral stream areas are sites where top-down inputs bias the competition in favor of attended targets compared with unattended distractors. Both monkeys with lesions of areas V4 and/or TEO and humans with lesions of area V4 are impaired at visual discrimination tasks when target stimuli are presented in the presence of salient visual distractors (24–26). Consistent with the neurophysiological data, the attentional filtering impairments after these extrastriate lesions are limited to configurations in which both the targets and distractors are located close to one another, within an area equal to the average size of V4 and/or TEO RFs. When normal filtering mechanisms are compromised, the presence of unfiltered distractors presumably degrades the information available about the target stimulus in the RFs of neurons downstream from the lesions, such as area TE.
Other studies in monkeys and humans indicate that the baseline, or resting, activity of cells in ventral visual areas is also increased with focused attention. When instructions or task demands indicate that a target stimulus will later appear at a specific location in the visual field, neurons with RFs at that retinotopic locus increase their activity, even before the appearance of the target stimulus (7, 13, 20, 27–29). These and other data suggest that visual cortex is under the top-down control of an attentional network that increases the sensitivity of the cells for the target stimulus, giving it a competitive advantage compared with distractors. What are the sources of this top-down control?
One major source of top-down control of ventral stream areas is likely to be posterior parietal cortex (27, 28, 30). Anatomical studies have reported multiple reciprocal pathways between parietal cortex and ventral visual processing areas, which could mediate such control (31–34). Furthermore, numerous imaging studies in humans have shown activation of posterior parietal cortex, especially the intraparietal sulcus and superior parietal lobule, in many tasks that require top-down attentional control (13, 27, 28, 35–42). The right intraparietal sulcus and lateral frontal cortex show increased activation when an attended target appears among salient distractors (43, 44). Findings from single-unit neurophysiology also suggest that parietal activity reflects the perceptual salience or behavioral relevance of stimuli. The presence of multiple visual items within an RF yields diminished responses in parietal neurons similar to the competitive interactions documented in extrastriate cells. Moreover, in these studies, competitive interactions were overcome through focused attention (45–47). Neurons in parietal cortex also exhibit increases in baseline activity with directed attention, before stimulus presentation (48, 49).
Given this hypothesized role of parietal cortex in the top-down filtering of distractors, one would expect that damage to parietal cortex would specifically impair this ability. However, attentional filtering has not been a focus of previous neuropsychological investigations of parietal function. Typically, studies of patients with parietal lesions have focused on the movement and disengagement of attention and on visual awareness rather than the ability to ignore irrelevant stimuli per se. Attentional deficits after parietal injury have been characterized in terms of an inability to detect contralesional targets (50–55), a problem in shifting or disengaging attention from ipsilesional to contralesional locations (56–63), or an impairment in visual search, namely, a deficit in the serial deployment of attention across arrays of multiple visual items (64, 65). In such paradigms, however, the location of the target item varies from trial to trial, thus making it difficult to distinguish mechanisms involved in filtering out distracting information from processes associated with the movement of attention through multielement arrays.
Most recently, some researchers have investigated perceptual and response interference from distractors presented in the contralesional visual fields affected by parietal lesions. These studies have most often been motivated by questions of levels of visual awareness: If “neglected” distractors interfere with perceptual processing or motor responses, then patients must process them implicitly, even when explicit recognition is impaired (66–73). The focus of these studies has been to investigate whether equivalent distractor interference effects might be obtained with intact vs. damaged parietal cortex. In contrast, we hypothesize that parietal cortex is necessary for the proper filtering out of irrelevant visual information and that damage to parietal cortex would result in greater interference effects than normally seen in healthy brains.
To test the role of posterior parietal cortex in the top-down biasing of competition between targets and distractors, we studied a patient, R.M., with bilateral parietal lesions, using a behavioral paradigm in which the location of the relevant stimulus was held constant for many trials. This design minimized the necessity to reorient attention from one location to another and, thus, any impairment from the lesion could be more easily attributed to a loss of distractor filtering rather than an impairment in reorienting attention to the target stimulus. Furthermore, to facilitate comparison with the monkey studies, we chose a paradigm similar to that used to test attentional filtering impairments after ventral stream lesions of areas V4 and/or TEO in monkeys, and a lesion of area V4 in a human patient (25, 26). In this paradigm, the discrimination threshold for an attended target is measured as a function of distractor contrast. As distractor contrast is increased, the ability to process the target features should decrease (and thresholds increase) if the top-down bias in favor of the target has been reduced or eliminated, similar to what has been found with ventral stream lesions. However, because parietal cortex has feedback projections to many different ventral stream areas (31, 33, 34) with heterogeneous RF sizes (2–10°) and visual field eccentricities, the bilateral lesion of R.M. might be expected to cause impairments with targets and distractors located anywhere within the visual field.
Methods
Patient.
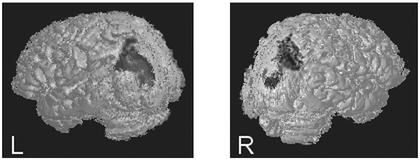
R.M. is a 65-year-old man with bilateral parietal lesions due to two embolic infarcts of the middle cerebral artery, one in June 1991 and one in March 1992. His lesions are focused in Brodmann's areas 7 and 39, and include some of areas 5 and 19 (Fig. 1). The lesions of R.M. do not include any temporal lobe regions such as the superior temporal gyrus. R.M. originally presented with classical symptoms of Balint's syndrome: optic ataxia, simultanagnosia, optic apraxia, and severe spatial dysfunction (see ref. 74). His performance on visual search, visual and auditory orienting tasks, and spatial tasks and his experience of illusory conjunctions have been documented in several publications (69, 75–77). Because his lesion is bilateral, R.M. does not exhibit classic symptoms of visual neglect or extinction, such as systematic orienting toward one visual hemifield. In fact, in keeping with the bilateral damage to parietal cortex, R.M. presents with simultanagnosia. He is frequently aware of only one object in the visual field or one group of clustered objects, thus neglecting all other locations equally. R.M. suffered an additional small stroke in September 1999. Examination by two experienced neurologists suggested the new damage was likely confined to a very small, strategically located lacunae in the internal capsule, which was not visible in computerized tomography scans or T1-weighted MRIs. The basic visual abilities of R.M. were most recently assessed in August 1999, by the School of Optometry, University of California, Berkeley. His acuity was reported to be 20/20 and his visual fields were found to be intact. R.M.'s vision had also been tested at length in June 1994. His color vision, stereopsis, and contrast sensitivity were reported to be normal. However, an earlier Goldmann perimetry test had indicated possible inferior nasal depression, 10° from fixation. It was not possible to monitor the eye movements of R.M. with an eye tracker during testing. However, R.M. has difficulty making saccades and fixates extremely well, as do most patients with Balint's syndrome. From subjective observation by the principal investigator, R.M. appeared to maintain fixation during testing, as instructed.
Figure 1.
Three-dimensional surface reconstructions of the brain of R.M., made from T1-weighted MRIs, show the extent of his bilateral parietal–occipital damage.
R.M. participated in the experiments reported in this article during several testing sessions that occurred March–July 2000. R.M. gave informed consent for behavioral testing and understood that his participation was voluntary and did not affect his medical treatment in any way. R.M. was instructed to notify the experimenter if the testing procedure produced fatigue or if he wished to halt the experiment at any time. All experiments with R.M. and all control subjects were approved by the National Institute of Mental Health Institutional Review Board (IRB). All experiments with R.M. were additionally approved by the University of California, Berkeley, IRB and the Department of Veterans Affairs, Martinez, IRB.
Age-Matched Controls.
Six age-matched subjects (mean age, 60 years; range, 55–64 years; four male/two female) participated as normal volunteers. All were tested on the orientation and face discrimination tasks with disk distractors. Five subjects were also tested on the orientation discrimination task with grating distractors. All control subjects had normal or corrected to normal acuity and reported that they did not have any additional visual or neurological problems. Normal volunteers received a standard clinical screening by an experienced neurologist before behavioral testing. Informed consent was obtained from all normal volunteers. Subjects were reimbursed for their time in accordance with the payment schedules of the National Institutes of Health Clinical Center.
Stimulus Presentation.
Stimuli (1.2°) were displayed (900 ms for R.M.; 300 ms for controls) at an eccentricity of 4° in the left and right upper visual quadrants on a medium gray background (27 cd/m2). The upper visual fields were chosen because some visual field testing had indicated a possible loss of sensitivity in the lower nasal visual field of R.M. Because R.M. may sometimes experience diplopia, all stimuli were presented monocularly to the right eye of R.M. and all control subjects. Subjects were instructed to indicate (verbally by R.M.; button response for controls) the presence or absence of the target by responding as quickly and accurately as possible. The experimenter indicated that accuracy was of greater importance than speed. All subjects were given as much as 25 s to respond but in practice, response times were usually on the order of hundreds of milliseconds for age-matched controls and 1–3 s for R.M. The responses of the subjects initiated advancement to the next trial. When subjects made errors, they received feedback in the form of a brief delay, in which the display screen flashed black before proceeding to the next trial. All subjects understood that errors were an expected part of the staircase procedure.
Orientation Discrimination with Disk and Grating Distractors.
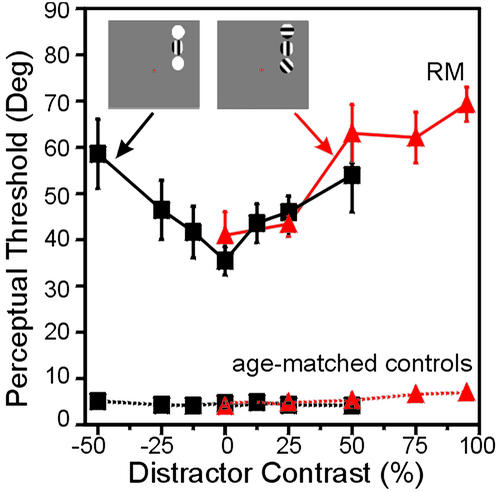
The ability to filter out distracting stimuli was tested by asking R.M. and control subjects to judge the orientation of a sinusoidal target grating (one cycle per degree; phase-randomized; 95% Michelson contrast) when presented eccentrically either alone or with the simultaneous presence of solid disk or grating distractors (Fig. 2). Subjects were instructed to indicate whether the target grating was vertical or nonvertical. The nonvertical orientation was adjusted in a staircase procedure, and perceptual thresholds for determining the just-noticeable difference from vertical were measured. The location of the stimuli (left or right) and the contrast of the distractors (disks: 0%, 12%, 25%, and 50% brighter or darker than background; gratings: 0%, 25%, 50%, 75%, and 95% Michelson contrast) were held constant within a block and randomized across blocks. For all orientation discrimination tests, R.M. performed six staircases (three in the left and three in the right upper visual quadrants). Control subjects performed a total of four staircases (two in the left and two in the right upper quadrants). Student's t tests for each contrast level revealed no differences between left and right hemifields or bright/dark disks; consequently, data were collapsed across hemifields for subsequent analysis.
Figure 2.
(Insets) The stimuli and displays used to test orientation discrimination with luminance disk and grating distractors. The perceptual thresholds of R.M. increased as a function of the distractor contrast: the more salient the distractors, the more difficulty R.M. experienced in determining whether the target grating was vertical or not (black lines are disk distractors; red lines are grating distractors). In contrast to R.M., six age-matched controls exhibited constant perceptual thresholds, regardless of the presence or salience of distractors. In this and all subsequent figures, error bars indicate the SEM. The small error bars for the age-matched controls are barely apparent.
Effect of Spatial Proximity of Distractors.
To examine the effect of distractor proximity to the target, R.M. was tested with disk and grating distractors on two separate occasions. Disk distractors were presented in three different locations (blocked per staircase): vertically aligned with the target, vertically aligned with the fixation point, or at 4° eccentricity in the hemifield opposite the target location. With luminance disk distractors, R.M. completed four staircases for each distractor location. Grating distractors were presented vertically aligned with the target, 2° medial to the target (but in the same hemifield), vertically aligned with the fixation point, or at 2° eccentricity in the hemifield opposite the target location. With grating distractors, R.M. performed only one staircase per distractor location, because he was unable to obtain a threshold even after 200 trials for each of the three conditions in which the grating distractors were not vertically aligned with the target grating.
Morphed Face Discrimination with Disk Distractors.
Stimuli were colored bitmap objects formed by morphing an image of a gorilla face with a photo of a young woman's face. The program used to morph the images completed the morphs in 1,000 steps. We therefore quantified the amount of morphing in terms of the number of processing steps. Thus, an image with a low step number was similar to the original gorilla; an image with a high step number was most similar to the woman. The bitmap objects appeared within a circular aperture whose color and luminance matched the background gray. All subjects viewed samples of the set of objects that could appear in the experiment and were given a chance to study and compare them. During testing, one of the original or morphed images was presented, along with two flanking distractors (luminance disks of varying contrast: 0%, 12.5%, 25%, and 50% brighter or darker than background.). The task for the subject was to indicate whether the object was/was not the original gorilla. The level of morphing was adjusted according to the performance of the subject in a staircase design, and perceptual thresholds for just-noticeable differences were calculated. For each contrast level of distractor, R.M. completed eight staircases (four in each hemifield) and age-matched controls completed four staircases (two in each hemifield).
Psychophysical Methods.
Discrimination thresholds were determined by using a standard staircase procedure (78). Distractor similarity to targets was increased after four consecutive correct answers and decreased after one incorrect answer. This procedure resulted in an overall performance of 84% correct. The staircase was terminated when the subject completed 200 trials or achieved 14 reversal points. For all experimental blocks, the first four reversal points were discarded as practice and the remaining reversal points were averaged to yield a perceptual threshold for that block. Because the staircase procedure demanded that subjects perform near threshold, nonspecific differences in general task difficulty for R.M. vs. neurologically healthy control subjects were minimized.
Statistical Analysis.
Mean thresholds were calculated for each subject and for the group of control subjects as a function of distractor saliency. To assess the statistical significance of distractor-dependent interference effects, we fitted all psychometric functions by using polynomial regression. We report the order and significance of the best fit.
Results
Orientation Discrimination with Disk and Grating Distractors.
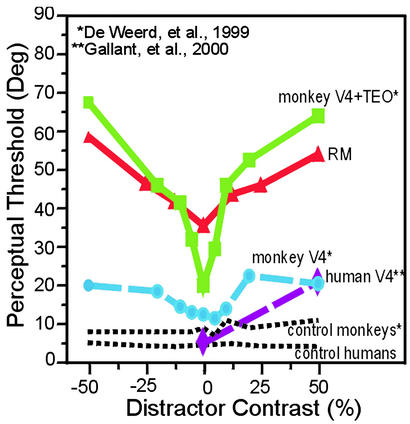
As predicted, the perceptual thresholds of R.M. for the target grating increased as a function of distractor salience (Fig. 2). R.M.'s performance was best with distractors of 0% contrast and steadily worsened as the distractor contrast increased. With disk distractors that were brighter or darker than the background, R.M. exhibited a “V”-shaped psychometric function (Fig. 2, data in black) centered on 0% contrast that was best fit with a second-order polynomial (P = 0.003). This pattern is similar to the increase in perceptual thresholds seen in lesion-affected visual quadrants in monkeys with V4 and/or TEO lesions (25) and a human with a unilateral V4 lesion (26) (Fig. 3). With grating distractors (Fig. 2, data in red), the perceptual thresholds of R.M. were once again elevated in the presence of distractors, and the amount of perceptual interference again increased as a function of distractor saliency (best fit: first-order polynomial, P < 0.0001). That R.M. could still achieve a perceptual threshold with grating distractors of equivalent features and contrast to the target gratings suggests that his elevated thresholds are not due to misorienting to an inappropriate stimulus (i.e., one of the distractors) but reflect instead a decrease in sensitivity due to the competitive interactions of distractors and targets. By comparison, the age-matched controls did not show any increases in threshold in the presence of distractors, even with those of very high contrast (Fig. 2). The mean psychometric functions for the controls were flat and were best fit with zero-order polynomials (disk distractors, P = 0.041; grating distractors, P = 0.003). These findings in neurologically normal subjects are consistent with results from the unaffected visual quadrants of monkeys and a human subject with partial lesions of V4 and/or TEO (25, 26).
Figure 3.
The psychometric function of R.M. for the orientation discrimination task showed a distractor-dependent increase in threshold that was similar to the psychometric functions of a patient with V4 damage and monkeys with V4 and TEO lesions. Extrastriate data are extrapolated from figures in published articles (25, 26).
Effects of Spatial Proximity of Distractors.
In a study of the effect of V4 and TEO lesions in monkeys (25), the animals were impaired in discriminating the orientation of a target grating when distractors were nearby. The impairment therefore seemed to result from the loss of attentional filtering within a spatial range equivalent to the average size of a V4 RF. When the distractors were moved farther away from the target, so that they could not all be contained within the dimensions of a V4 RF, the impairment was eliminated (25). Based on these findings, we have argued that competitive interactions among target and distractors are scaled to the RF sizes of extrastriate visual areas.
Posterior parietal cortex has anatomical projections to many visual cortical regions, including V2, V3, V4, TEO, PO, MT, and areas TEO and TE within inferior temporal cortex (31, 33, 34). This network of connections would presumably allow posterior parietal cortex to provide feedback to neurons whose RFs span many sizes, from the small RFs of V2 neurons to the full-field RFs of TE cells. Consequently, we predicted that the bilateral parietal lesions of R.M. would impair discrimination of the target, regardless of the distance between target and distractors. To test this hypothesis, we presented R.M. with grating targets accompanied by two disk or grating distractors that varied in their spatial proximity to the target.
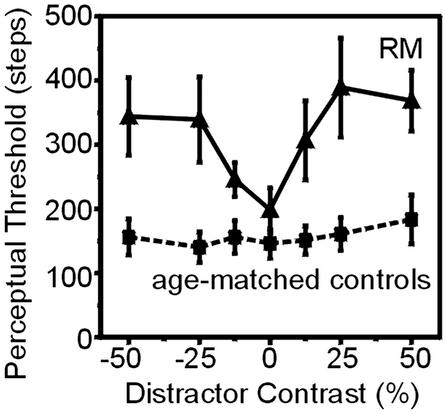
With no distractors present, R.M. achieved a mean orientation threshold of 39° (±10°). We also tested him with flanking luminance disks that appeared in three different configurations: (i) directly above and below the target grating; (ii) above and below fixation; and (iii) in the opposite hemifield. The thresholds of R.M. for these conditions were 54° (±10°), 56° (±8°), and 71° (±5°), respectively. Thus, as predicted, R.M. was impaired by even far-removed distractors. Indeed, the impairment of R.M. was significantly greater with distractors located in the opposite hemifield than in the same hemifield as the target (P = 0.037).
What could account for the fact that the impairment of R.M. was even larger with distractors in the opposite hemifield? R.M. has difficulty perceiving more than one item or more than one group of elements at a time, especially when the elements have similar features. He also has problems disengaging attention from items that have captured his attention. When items are close together, there is only a single group of items to attend, and preattentive center of mass effects might guide orienting to the center target (79). However, when items are more dispersed in the display, R.M. (and neurologically normal subjects) would be unlikely to group items, and center of mass information would not then help him select the correct target. If so, the most challenging situation for R.M. would be a display with items of similar features (e.g., grating target and distractors) that are spatially offset. Because the target and distractors share visual features (i.e., stripes), R.M. would not be able to distinguish whether the item that had captured his attention was the target or not. In fact, as predicted, when grating distractors were presented in locations offset from the target (in the same hemifield but 2° closer to fixation than the target, aligned with the fixation point, or in the opposite hemifield), his performance worsened. For each of these conditions, he completed 200 trials but the staircase did not converge on a threshold.
Face Discrimination with Disk Distractors.
Even when no distractors were present, R.M. was impaired relative to the control group on the orientation discrimination task (for example, see the threshold of R.M. with 0% distractor contrast in Fig. 2). This is consistent with studies reporting that parietal lesions in monkeys impair discrimination of objects that differ only in orientation (80) and also with imaging studies in humans that have found parietal activation during orientation discriminations of gratings and complex visual objects (81). We tried several experimental manipulations to increase the difficulty of the orientation discrimination task for the age-matched controls (e.g., low contrast target, rapid presentations), with little effect on the control subjects' performances. Consequently, we designed a perceptual task that we thought R.M. and control subjects might perform equally well when no distractors were present.
To determine whether distractors would interfere with perceptual processing even for a task that R.M. was quite good at performing, we tested him in a face discrimination task (Fig. 4), which should require processing primarily by ventral stream visual areas. Without distractors, R.M. performed this task very well; his thresholds for single faces were nearly equivalent to those achieved by normal subjects (Fig. 5), suggesting that his ventral visual pathways were intact and that complex object discrimination (of faces) was spared. In contrast, the thresholds of R.M. increased as a function of distractor salience, just as they did for the orientation discrimination task (best fit: fourth-order polynomial, P = 0.038). The age-matched controls showed a negligible increase in threshold when distractors flanked the target object (best fit: zero-order polynomial, P = 0.143).
Figure 4.
Examples of morphed stimuli. Stimuli with a large “step number” are very different from the target; stimuli with smaller “step numbers” are most similar to the target. The morphed stimuli were presented with flanking disk distractors, similar to the displays illustrated in Fig. 2.
Figure 5.
The thresholds of R.M. on the face discrimination task were comparable to those achieved by age-matched controls when the morphed target face appeared alone. However, when visual distractors flanked the target, the thresholds of R.M. increased as a function of distractor salience, whereas the thresholds of age-matched controls remained relatively constant.
Discussion
We have found that a patient with bilateral posterior parietal lesions is consistently and severely impaired at filtering out irrelevant visual information. R.M. demonstrated elevated perceptual thresholds on several tasks that required him to perform visual discriminations in the presence of distractors, and this deficit increased as a function of distractor salience. This was true not only for orientation discrimination of a target but also for face discrimination, a task in which R.M. was completely normal when tested without distractors. Thus, the deficits of R.M. seem to be caused by an inability to suppress the influence of irrelevant objects rather than an impairment in object discrimination per se.
The filtering deficit of R.M. was similar to the elevated thresholds that have been observed in monkeys with extrastriate lesions of V4 and/or TEO (25) and in one human patient with focal V4 damage (26). These previous studies used the same task and stimuli as we used in some of our experiments with R.M., which allowed us to make a direct comparison of the perceptual thresholds after various cortical lesions. That lesions of extrastriate visual areas and posterior parietal cortex produce similar filtering deficits is consistent with the idea that these areas are part of a common attentional network. As predicted, damage to one of the sources of top-down control (parietal cortex), as well as damage to one of the targets of top-down control (extrastriate cortex), can lead to similar behavioral consequences.
It might be considered surprising that R.M. was impaired by solid disk distractors during the orientation discrimination task. It was possible that the target grating would “pop-out” of the visual display because of its unique features (i.e., stripes), and thus it would not require any top-down attentional modulation to process it preferentially. Studies of R.M. have found that he is impaired in visual search tasks in which the targets and distractors are similar to each other, but that he experiences preattentional pop-out if the target and distractors have dissimilar features (69). One possible explanation is that visual search tasks typically require a simple detection of the presence or absence of the target, whereas the tasks used in the current study require fine discriminations of orientation or facial identity that may demand more attentional resources. Consequently, distractors may cause little interference for easy detection tasks but lead to much more interference for difficult discrimination tasks.
Based on findings from imaging and patient studies, posterior parietal cortex is already known to be important for visual orienting, spatially directed attention, and visual awareness (30, 82). Is a deficit in filtering out irrelevant information just another example on a long list of spatial and attention difficulties after parietal injury? There are several aspects of our findings that argue for a reassessment of the role parietal cortex plays in selective attention. The concept of two functionally specialized visual pathways, a ventral stream involved in object representation and a dorsal stream optimized for motion and spatial analysis (83), has been a powerful conceptual framework that has inspired and been confirmed by numerous functional imaging investigations (e.g., see ref. 84). However, in focusing on the differences between the two pathways, less emphasis has been placed on how these pathways may interact. The filtering deficit explored in this report, as well as feature binding problems documented in this same patient (75), suggests that the calculus of spatial attention is not strictly modular. Posterior parietal cortex may interact and modulate the activity of ventral visual neurons in ways that fundamentally affect object representations themselves. For example, when there are multiple items present in visual displays, R.M.'s very percept of the objects is altered: he experiences illusory conjunctions of features and his perceptual thresholds are elevated. Objects that could be clearly distinguished when presented one at a time have representations that are no longer distinct (and sometimes not even veridical) when the items appear spatially proximate and simultaneously. Interestingly, the cortical region that has traditionally been identified as playing an important role in the inhibition of irrelevant information is frontal cortex (e.g., see ref. 85). The present results demonstrate that inhibition may also be the provence of parietal cortex.
Acknowledgments
We thank Robert Knight, Nina Dronkers, and Mark D'Esposito for their assistance in obtaining and evaluating the MRIs of R.M.; Alex List and Ting Wong for their help in arranging testing sessions with R.M.; Alan Zametkin for screening age-matched controls at the National Institutes of Health; and Alan Rorie, Ben Harris, Trina Norden-Krichmar, and Jessica Rickert for technical help and manuscript preparation. This study was supported by the National Institute of Mental Health Intramural Research Program, National Science Foundation Grant SBR9631132 (to L.C.R.), and the Medical Research Council of the Department of Veterans Affairs.
Abbreviation
- RF
receptive field
References
- 1.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 2.Sato T. Exp Brain Res. 1989;77:23–30. doi: 10.1007/BF00250563. [DOI] [PubMed] [Google Scholar]
- 3.Miller E K, Gochin P M, Gross C G. Brain Res. 1993;616:25–29. doi: 10.1016/0006-8993(93)90187-r. [DOI] [PubMed] [Google Scholar]
- 4.Motter B C. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 5.Motter B C. J Neurosci. 1994;14:2178–2189. doi: 10.1523/JNEUROSCI.14-04-02178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolls E T, Tovee M J. Exp Brain Res. 1995;103:409–420. doi: 10.1007/BF00241500. [DOI] [PubMed] [Google Scholar]
- 7.Luck S J, Chelazzi L, Hillyard S A, Desimone R. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 8.Chelazzi L, Duncan J, Miller E K, Desimone R. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds J H, Chelazzi L, Desimone R. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdams C J, Maunsell J H. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelazzi L, Miller E K, Duncan J, Desimone R. Cereb Cortex. 2001;11:761–772. doi: 10.1093/cercor/11.8.761. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta M, Miezin F M, Dobmeyer S, Shulman G L, Petersen S E. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbetta M, Kincade J M, Ollinger J M, McAvoy M P, Shulman G L. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 14.Heinze H J, Mangun G R, Burchert W, Hinrichs H, Scholz M, Münte T F, Gös A, Johannes S, Scherg M, Hundeshagen H, et al. Nature. 1994;392:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 15.Mangun G R, Hopfinger J, Kussmaul C, Fletcher E, Heinze H J. Hum Brain Mapp. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Mangun G R, Buonocore M, Girelli M, Jha A. Hum Brain Mapp. 1998;6:383–389. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woldorff M, Fox P, Matzke M, Lancaster J, Veeraswamy J, Zamarripa F, Seabolt M, Glass T, Gao J, Martin C, Jerabek P. Hum Brain Mapp. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Kastner S, De Weerd P, Desimone R, Ungerleider L G. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 19.Brefczynski J A, DeYoe E A. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 20.Chawla D, Rees G, Friston K J. Nat Neurosci. 1999;7:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- 21.Rensink R A, O'Regan J K, Clark J J. Psychol Sci. 1997;8:368–373. [Google Scholar]
- 22.Mack A, Rock I. Inattentional Blindness. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 23.Di Lollo V, Enns J T, Rensink R A. J Exp Psychol Gen. 2000;129:481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- 24.Schiller P H, Lee K. Science. 1991;251:1251–1253. doi: 10.1126/science.2006413. [DOI] [PubMed] [Google Scholar]
- 25.De Weerd P, Peralta M R, Desimone R, Ungerleider L G. Nat Neurosci. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- 26.Gallant J L, Shoup R E, Mazer J A. Neuron. 2000;27:227–235. doi: 10.1016/s0896-6273(00)00032-5. [DOI] [PubMed] [Google Scholar]
- 27.Kastner S, Pinsk M A, De Weerd P, Desimone R, Ungerleider L G. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 28.Hopfinger J B, Buonocore M H, Mangun G R. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 29.Hopfinger J B, Woldorff M G, Fletcher E M, Mangun G R. Neuropsychology. 2001;39:1277–1291. doi: 10.1016/s0028-3932(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 30.Kastner S, Ungerleider L G. Neuropsychology. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- 31.Cavada C, Goldman-Rakic P. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 32.Cavada C, Goldman-Rakic P. Neuroscience. 1991;42:683–696. doi: 10.1016/0306-4522(91)90037-o. [DOI] [PubMed] [Google Scholar]
- 33.Distler C, Boussaoud D, Desimone R, Ungerleider L G. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 34.Webster M J, Bachevalier J, Ungerleider L G. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta M, Miezin F, Shulman G, Petersen S. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbetta M, Akbudak E, Conturo T E, Snyder A Z, Ollinger J M, Drury H A, Linenweber M R, Petersen S E, Raichle M E, Van Essen D C, Shulman G L. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberghe R, Dupont P, De Bruyn B, Bormans G, Michiels J, Mortelmans L, Orban G A. Brain. 1996;119:1263–1276. doi: 10.1093/brain/119.4.1263. [DOI] [PubMed] [Google Scholar]
- 38.Nobre A C, Sebestyen G N, Gitelman D R, Mesulam M M, Frackowiack R S J, Frith C D. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- 39.Corbetta M. Proc Natl Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coull J T, Nobre A C. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le T H, Pardo J V, Hu X. J Neurophysiol. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- 42.Rosen A C, Rao S M, Caffarra P, Scaglioni A, Bobholz J A, Woodley S J, Hammeke T A, Cunningham J M, Prieto T E, Binder J R. J Cognit Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- 43.Marois R, Chun M M, Gore J C. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 44.Chun M M, Marois R. Curr Opin Neurobiol. 2002;12:184–189. doi: 10.1016/s0959-4388(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb J P, Kusunoki M, Goldberg M E. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- 46.Constantinidis C, Steinmetz M A. Cereb Cortex. 2001;11:581–591. doi: 10.1093/cercor/11.7.581. [DOI] [PubMed] [Google Scholar]
- 47.Gottlieb J. Curr Opin Neurobiol. 2002;12:134–140. doi: 10.1016/s0959-4388(02)00312-4. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg M E, Colby C L, Duhamel J R. Cold Spring Harbor Symp Quant Biol. 1990;55:729–739. doi: 10.1101/sqb.1990.055.01.068. [DOI] [PubMed] [Google Scholar]
- 49.Colby C L, Duhamel J R, Goldberg M E. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 50.Mesulam M M. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 51.Mesulam M M. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 52.Kinsbourne M. In: Neurophysiological and Neuropsychological Aspects of Spatial Neglect. Jeannerod M, editor. Amsterdam: Elsevier; 1987. pp. 69–86. [Google Scholar]
- 53.De Renzi E, Gentilini M, Fagliani P, Barbieri C. Cortex. 1989;25:231–237. doi: 10.1016/s0010-9452(89)80039-5. [DOI] [PubMed] [Google Scholar]
- 54.Watson R T, Valenstein E, Day A, Heilman K M. Neurology. 1986;36:636–640. doi: 10.1212/wnl.36.5.636. [DOI] [PubMed] [Google Scholar]
- 55.Lynch J C, McLaren J W. J Neurophysiol. 1989;61:74–90. doi: 10.1152/jn.1989.61.1.74. [DOI] [PubMed] [Google Scholar]
- 56.Ladavas E, Del Pesce M, Provinciali L. Neuropsychologia. 1989;27:353–366. doi: 10.1016/0028-3932(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 57.Posner M I, Cohen Y, Rafal R D. Philos Trans R Soc London B. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- 58.Posner M I, Walker J A, Friedrich F A, Rafal R D. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Posner M I, Petersen S E. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 60.Verfaellie M, Rapcsak S Z, Heilman K M. Brain Cognit. 1990;12:195–204. doi: 10.1016/0278-2626(90)90015-g. [DOI] [PubMed] [Google Scholar]
- 61.Egly R, Driver J, Rafal R D. J Exp Psychol Gen. 1994;123:161–177. doi: 10.1037//0096-3445.123.2.161. [DOI] [PubMed] [Google Scholar]
- 62.Rafal R, Robertson L. In: The Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1995. pp. 625–648. [Google Scholar]
- 63.Friedrich F J, Egly R, Rafal R, Beck D. Neuropsychology. 1998;12:193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- 64.Eglin M, Robertson L C, Knight R T. Cereb Cortex. 1991;1:262–272. doi: 10.1093/cercor/1.3.262. [DOI] [PubMed] [Google Scholar]
- 65.Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Brain. 2001;124:941–952. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- 66.Fuentes L J, Humphreys G W. Cognit Psychol. 1996;13:111–136. [Google Scholar]
- 67.Karnath H-O, Ferber S, Rorden C, Driver J. Neurocase. 2000;6:295–306. [Google Scholar]
- 68.Filoteo V J, Friedrich F J, Rabbel C, Stricker J L. J Int Neuropsychol Soc. 2002;8:461–473. doi: 10.1017/s135561770281325x. [DOI] [PubMed] [Google Scholar]
- 69.Robertson L, Treisman A, Friedman-Hill S, Grabowecky M. J Cognit Neurosci. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- 70.Rafal R, Gershberg F, Egly R, Ivry R, Kingstone A, Ro T. Neuropsychologia. 1996;34:1197–1202. doi: 10.1016/0028-3932(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 71.Ro T, Cohen A, Ivry R B, Rafal R D. Brain Cognit. 1998;37:461–476. doi: 10.1006/brcg.1998.1008. [DOI] [PubMed] [Google Scholar]
- 72.Danckert J, Maruff P, Kinsella G, de Graaff S, Currie J. NeuroReport. 1999;10:1077–1083. doi: 10.1097/00001756-199904060-00032. [DOI] [PubMed] [Google Scholar]
- 73.Danckert J, Maruff P, Ymer C, Kinsella G, Yucel M, de Graaff S, Currie J. Neuropsychology. 2000;14:16–28. doi: 10.1037//0894-4105.14.1.16. [DOI] [PubMed] [Google Scholar]
- 74.Rafal R. In: Behavioral Neurology and Neuropsychology. Feinberg T E, Farah M J, editors. New York: McGraw–Hill; 1997. pp. 337–356. [Google Scholar]
- 75.Friedman-Hill S R, Robertson L C, Treisman A. Science. 1995;269:853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- 76.Phan M L, Schendel K L, Recanzone G H, Robertson L C. J Cognit Neurosci. 2000;12:583–600. doi: 10.1162/089892900562354. [DOI] [PubMed] [Google Scholar]
- 77.Kim M S, Robertson L C. Cognit Neurosci. 2001;13:1080–1087. doi: 10.1162/089892901753294374. [DOI] [PubMed] [Google Scholar]
- 78.Wetherill G B, Levitt R. Br J Math Stat Psychol. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 79.Grabowecky M, Robertson L C, Treisman A. J Cognit Neurosci. 1993;5:288–302. doi: 10.1162/jocn.1993.5.3.288. [DOI] [PubMed] [Google Scholar]
- 80.Eacott M J, Gaffan D. Behav Brain Res. 1991;46:95–98. doi: 10.1016/s0166-4328(05)80100-7. [DOI] [PubMed] [Google Scholar]
- 81.Faillenot I, Sunaert S, Van Hecke P, Orban G A. Eur J Neurosci. 2001;13:585–596. doi: 10.1046/j.1460-9568.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 82.Robertson L C. Space, Objects, Minds and Brains. New York: Psychology; 2003. [Google Scholar]
- 83.Ungerleider L G, Mishkin M. In: Analysis of Visual Behavior. Ingle D G, Goodale M A, Mansfield R J Q, editors. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 84.Haxby J V, Gobbini M I, Furey M L, Ishai A, Shouten J L, Pietrini P. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 85.Stuss D T, Knight R T. Principles of Frontal Lobe Function. New York: Oxford Univ. Press; 2002. [Google Scholar]