Abstract
l-Glutamic acid decarboxylase (GAD) exists as both membrane-associated and soluble forms in the mammalian brain. Here, we propose that there is a functional and structural coupling between the synthesis of γ-aminobutyric acid (GABA) by membrane-associated GAD and its packaging into synaptic vesicles (SVs) by vesicular GABA transporter (VGAT). This notion is supported by the following observations. First, newly synthesized [3H]GABA from [3H]l-glutamate by membrane-associated GAD is taken up preferentially over preexisting GABA by using immunoaffinity-purified GABAergic SVs. Second, the activity of SV-associated GAD and VGAT seems to be coupled because inhibition of GAD also decreases VGAT activity. Third, VGAT and SV-associated Ca2+/calmodulin-dependent kinase II have been found to form a protein complex with GAD. A model is also proposed to link the neuronal stimulation to enhanced synthesis and packaging of GABA into SVs.
The rate-limiting enzyme L-glutamic acid decarboxylase (GAD, EC 4.1.1.15) is involved in the synthesis of γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter in the mammalian brain. There are two well-characterized GAD isoforms in the human brain, namely GAD65 and GAD67 (referring to GAD with a molecular mass of 65 kDa and 67 kDa, respectively) (1). Both GAD65 and GAD67 are present as homodimers or heterodimers in soluble GAD (SGAD) and membrane-associated GAD (MGAD) pools (2–4). The ratio of GAD65 to GAD67 is higher in synaptic vesicle (SV) fractions than in the cytosol (5). Some studies suggest that GAD65 binds to the membranes (6, 7) and that GAD67 subsequently interacts with MGAD65 (2, 6). However, the nature of anchorage of GAD to membranes and its physiological significance is still not well understood. GAD is not considered to be an integral membrane protein because it lacks a stretch of hydrophobic amino acids long enough to span the membrane. Subpopulations of GAD65 and GAD67 remain firmly anchored to membranes despite various ionic extraction methods (2, 4, 8). The interaction of GAD with membranes was reported to be through ionic (9–11), hydrophobic (12, 13), protein phosphorylation (14), or protein–protein interaction (15). Previously, we reported that MGAD is activated by phosphorylation that requires an electrochemical gradient across the SV membrane (7). A model for the anchoring mechanism of GAD to SV and its role as a link between GABA synthesis and storage in nerve terminals was also proposed (15). The evidence presented here will demonstrate that GABA synthesized by SV-associated GAD is preferentially transported into the SV by vesicular GABA transporters (VGATs). We have also demonstrated that VGAT, a 10-transmembrane helix protein (16), forms a protein complex with GAD on the SV and could be involved in the anchorage of MGAD to the SV. The formation of this GAD protein complex ensures an efficient coupling between GABA synthesis and packaging into the SV.
Materials and Methods
Preparation of SVs.
SVs were purified from whole rat brain (Sprague–Dawley) at 4°C as described (www.els.net). Briefly, synaptosomes were prepared by homogenizing fresh rat brains in 0.3 M sucrose buffer (5–10 ml/g brain), centrifuging the homogenate at 1,000 × g for 10 min to remove nuclei and debris (P1). The resultant supernatant liquid (S1) was centrifuged at 15,000 × g for 15 min, and the pellet thus obtained was referred to as P2 pellet. Synaptosomes in the pellet (P2) were lysed by rapid resuspension in 10 vol of water, then 1 mM EGTA and 130 mM potassium chloride were added after 1 min. The suspension was centrifuged at 15,000 × g for 20 min. The resulting supernatant solution (S2) containing SVs was further centrifuged at 200,000 × g for 2 h. The pellet (P3 or SV) was suspended in 10 vol of standard GAD buffer containing 50 mM potassium phosphate, 1 mM 2-aminoethylisothiouronium bromide (AET), and 0.2 mM pyridoxal 5′-phosphate (Sigma) at pH 7.2 and referred to as crude SV.
Immunoisolation of GABAergic-Specific SVs.
Specific anti-GAD65 IgG-coupled oxirane acrylic beads (Sigma) were used to purify GABAergic-specific SVs from the crude SV preparation as described above. The coupling of IgG to the beads was performed according to the manufacturer's instructions with some modifications. An aliquot of anti-GAD65 IgG purified from anti-GAD65 sera by a protein G Sepharose column was added to the bead to give a ratio of 4 ml of IgG/g of bead. The mixture was incubated at 20–25°C for 24 h, followed by washing with 0.1 M potassium phosphate buffer, pH 7.5. The unreacted oxirane groups on the beads were blocked by reacting overnight with 1 M mercaptoglycerol, pH 7.5. The beads were then extensively washed with standard GAD buffer. Anti-GAD65-coupled beads were incubated with crude SV preparation for 12–16 h at 4°C, followed by extensive wash with standard GAD buffer. The resulting bead complex was analyzed by GAD activity assays, immunostaining with anti-VGAT, and electron microscopic examination.
Enzyme Assay.
GAD activity was measured by using a radiometric method as described (17).
Immunoblotting.
Immunoblotting tests were conducted as described (15). The protein samples were first separated by SDS/PAGE using BisTris-gel (Invitrogen) and then blotted on a nitrocellulose membrane. The blot was detected by primary antibodies and horseradish peroxidase-conjugated secondary antibodies by using ECL detection reagents (Amersham Pharmacia).
Electron Microscopy.
The examination of SV–bead complex under the electron microscope was conducted as described (18) with modifications. Briefly, SV⋅antibody-coupled beads or beads alone were pelleted by centrifugation and resuspended in 2% agarose. After polymerization, thin agarose blocks were cut out, fixed, and dehydrated. Ultra-thin sections were then cut and stained with uranyl acetate and lead citrate for electron microscopic examination.
Vesicular Uptake Assay with GABA Newly Synthesized from Glutamate.
Uptake assays were performed as described (19, 20) with some modifications. A SV mixture containing a final concentration of SV beads (2 mg of protein per ml), pyruvate kinase (60 μg/ml) in buffer consisting of 9.5 mM KH2PO4, 40.5 mM K2HPO4, 8 mM KCl, 86.6 mM potassium gluconate, pH 7.4 (GPBS), was first incubated at 32°C for 2 min, followed by the addition of the same volume of ATP mixture giving a final concentration of 2 mM ATP, 4.4 mM MgSO4, 12 mM phospho(enol) pyruvate, 50 μM GABA, 2 mM Glu, and 0.1 μCi/μl [3H]Glu. The mixture was further incubated at 32°C, and an aliquot of 30 μl of the reaction mixture was removed and vacuum-filtered through nitrocellulose membrane at 1-, 5-, 10-, 15-, and 20-min intervals. The membranes were washed twice with 5 ml of ice-cold GPBS buffer, and the radioactivity remaining on the membrane was measured in a scintillation counter. For experiments involving GAD inhibitor, hydrazine, or aminooxyacetate, the inhibitor was first preincubated with SV mixture for 10 min before the addition of ATP mixture. For uptake assays involving both [3H]Glu and [14C]GABA (DuPont), the conditions were the same as described above except that both [3H]Glu and [14C]GABA were included in the reaction mixture.
Separation of Glutamate and GABA by an Anion Exchange Column.
To identify the content taken up into SV as GABA, a separation pattern of standard Glu and GABA on an anion exchange column (Bio-Rad AG1X8 200–400 mesh chloride form) was first established as described (21). Briefly, after the application of samples, the samples were allowed to remain in the column for 3 min. The column was first washed with H2O until no radioactivity was detectable, followed by elution with 10 mM HCl. Fractions were collected at 1.5 ml per fraction, and the radioactivity was counted in a scintillation counter. In case dual isotopes were used, two separate channels were used for counting 14C and 3H radioactivity.
Immunopurification of GAD Protein Complex.
All experiments were conducted at 4°C. Purified anti-GAD65 IgG was coupled to HiTrap N-hydroxysuccinimide-activated Sepharose according to the manufacturer's instructions (Amersham Pharmacia). Anti-GAD65 IgG immunoaffinity columns were then used for purification of the GAD-associated proteins from Triton X-100 solubilized crude SV preparation. In a typical experiment, a SV membrane preparation was solubilized with 0.5% Triton X-100 in standard GAD buffer at 4°C overnight. The mixture was cleared by centrifugation at 200,000 × g for 1 h. The supernatant solution was applied to a Bio-Beads SM-2 adsorbent column (Bio-Rad) to remove detergent. After removal of detergent, the sample was recirculated into a 1-ml anti-GAD65 immunoaffinity column at 4°C for 8 h. The column was then washed extensively with standard GAD buffer, followed by 200 mM potassium phosphate GAD buffer. The column was further eluted with 0.2 M acetic acid, pH 2.5. Fractions were collected and dried with a Speed-vac concentrator (Savant). The samples were resuspended in a small volume of 5 mM potassium phosphate buffer, pH 7.2, and dialyzed extensively in the same potassium phosphate buffer. The protein concentration of dialyzed samples was estimated at OD280 nm based on an extinction coefficient of BSA, 6.8 for 1% solution. The samples were analyzed by immunoblotting with anti-VGAT (Chemicon), anti-SV protein 2 (SV2) (a gift from Erik Floor, University of Kansas, Lawrence), preadsorbed anti-GAD65, or anti-calmodulin-dependent kinase II (CaMKII, Chemicon).
Identification of Heat Shock Cognate 70 (HSC70) and Cysteine-String Protein (CSP) on Immunoisolated GABAergic SVs.
GABAergic SVs purified by anti-GAD65 immunobead were suspended in citric acid (0.1 M) to dissociate SVs from the bead complex. The eluate containing GABAergic SVs was collected and neutralized with NaOH, followed by extensive dialysis against 1 mM potassium phosphate buffer (pH = 7.2). The dialysate was concentrated with a Speed-vac concentrator. The protein concentration was determined by using Bio-Rad protein assay reagents as described in the manufacturer's instructions. The sample was then analyzed by immunoblotting test using anti-HSC70 (Upstate Biotechnology, Lake Placid, NY) and anti-CSP antibodies (Chemicon), respectively, as described (15).
Isolation of GAD Protein Complex Using GST-Human GAD65 (HGAD65) Fusion Protein and Glutathione (GSH) Affinity Column.
Briefly, partially purified GST-HGAD65 fusion protein obtained as described (22) was incubated with GSH affinity resin (Sigma) at 4°C overnight. The incubation mixture was then packed, and the column was equilibrated extensively with standard GAD buffer. Solubilized SV solution was loaded to the GSH affinity column at a flow rate of 0.25–0.45 ml/min. After application of the sample, the column was washed with standard GAD buffer extensively, followed by an additional wash with 200 mM potassium phosphate containing 1 mM 2-aminoethylisothiouronium bromide (AET) and 0.2 mM pyridoxal 5′-phosphate (PLP), pH 7.2. The column was then eluted with elution buffer containing 25 mM Tris⋅HCl, 1 mM AET, 0.2 mM PLP, and 0.2% SDS, pH 7.4. The eluates were then analyzed by immunoblotting test using anti-VGAT.
Results
Isolation of GABAergic SVs by Anti-GAD65-Conjugated Beads.
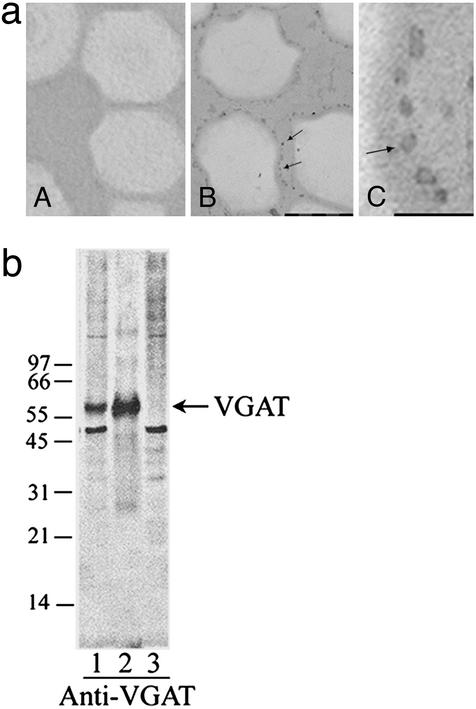
SVs isolated by anti-GAD65-conjugated beads appear as round dark dots associated with oxirane bead as shown in Fig. 1a, lane B. At higher magnification, several hollow vesicles with typical morphological characteristics of SV are clearly visible (Fig. 1a, lane C). No such dark dot is visible in control oxirane bead alone (Fig. 1a, lane A). GAD activity assays show that >90% of the activity in the crude SV preparation is recovered in SV⋅anti-GAD65-conjugated bead complex, suggesting that GABAergic SVs are specifically retained by anti-GAD65-conjugated beads. Immunoblotting tests also show that SVs retained by anti-GAD65-conjugated beads are much more strongly stained by antibodies against VGAT (Fig. 1b, lane 2) than the original preparation (Fig. 1b, lane 1). No VGAT staining is detected with SVs not retained by anti-GAD65-conjugated beads (Fig. 1b, lane 3). These results suggest that the immunoisolated SVs are highly enriched in GABAergic SVs.
Figure 1.
Isolation of specific GABAergic SVs by anti-GAD65-conjugated immunobeads. (a) Electron microscopic examination of SV preparations. (A) Control oxirane beads alone. (B and C) Anti-GAD65-coupled immunobeads coated with SVs. The dark dots associated with oxirane beads are SVs coupled to beads as indicated by the arrowheads. The bar in B indicates 1 μm, and the bar in C indicates 200 nm. (b) Immunoblotting test of various SV preparations with anti-VGAT. An amount of 20 μg of protein per lane was used. Lane 1, original SVs without treatment as control; lane 2, GABAergic SVs purified by anti-GAD65-conjugated immunobeads; lane 3, non-GABAergic SVs that were not retained by anti-GAD65 beads. Arrow indicates the position of VGAT.
Vesicular Uptake of GABA Newly Synthesized from Glutamate by SV-Associated GAD.
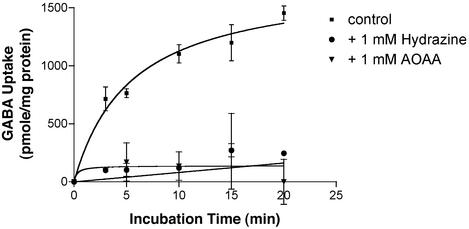
The uptake of newly synthesized GABA by VGAT was found to be increased as a function of time and reached saturation at 10 min (Fig. 2). The specific activity of GABA uptake by VGAT is determined from the slope of the curve as 240 pmol/mg of protein per min (Fig. 2, ▪). VGAT activity is greatly decreased by hydrazine or aminooxyacetate, a GAD inhibitor. At 1 mM, both hydrazine and aminooxyacetate reduce uptake of newly synthesized GABA to near baseline (Fig. 2, ● and ▾, respectively). This result suggests that the VGAT activity is linked to SV-associated GAD. The 3H-labeled content transported into the SV is identified as GABA because it has the same elution position as standard GABA as shown in Fig. 3. These results suggest that the content taken up by GABAergic SVs is not the substrate, [3H]Glu, but [3H]GABA converted from [3H]Glu by SV-associated GAD (Fig. 3b).
Figure 2.
Effect of GAD inhibitors on vesicular uptake of newly synthesized GABA. Uptake of GABA into SV was conducted as described in Materials and Methods under the following conditions: GABAergic SV alone (■), in the presence of the GAD inhibitor, 1 mM hydrazine (●) or 1 mM aminooxyacetate (▾). The uptake of GABA in the presence of the V-type ATPase inhibitor, bafilomycin A1 or in the absence of ATP was taken as nonspecific uptake, which was <5% of the total uptake. [3H]Glu was used as a substrate for the production of [3H]GABA. The specific uptake was obtained by subtracting the nonspecific uptake from the total uptake and was expressed as pmol per mg of protein. Data are presented as mean ± SD (n = 3).
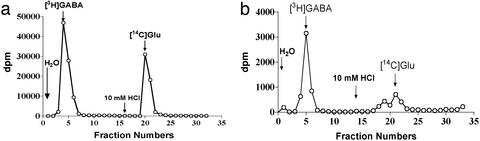
Figure 3.
Separation of GABA and glutamate on an anion exchange column. Separation of standard GABA (100,000 dpm) and Glu (60,000 dpm) as well as the extract of SV on an anion exchange column was conducted as described in Materials and Methods. (a) The elution pattern of standard GABA (first peak) and Glu (second peak) on an anion exchange column. (b) The elution pattern of the content extracted from SV (equivalent to 0.2 mg of protein, first peak) and standard [14C]Glu (second peak) on the same anion exchange column as in a. The first peak is identified as GABA because it has the same elution position as standard GABA in a.
A Comparison of Vesicular Uptake of Newly Synthesized GABA and the Preexisting GABA in Cytosolic Pools.
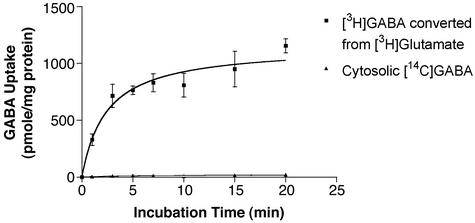
To illustrate that the newly synthesized GABA is preferentially taken up by GABA SVs, dual radioactive-labeled [3H]Glu and [14C]GABA or [14C]Glu and [3H]GABA, respectively, were used to measure the uptake rate, and 10 μCi (2 mM) of [3H]Glu and 2 μCi (50 μM) of [14C]GABA were included in a 150-μl uptake reaction mixture. Based on GAD activity assays under the same conditions, only ≈2.7% of Glu could be converted to GABA. This would give a concentration of newly synthesized [3H]GABA in the uptake mixture around 54 μM (0.27 μCi), which is comparable to the concentration used for the preexisting [14C]GABA, 50 μM. When [3H]Glu was used as the substrate for the production of newly synthesized GABA and [14C]GABA was used as the preexisting GABA, it was found that the newly synthesized [3H]GABA, but not the preexisting [14C]GABA, was taken up by VGAT (Fig. 4).
Figure 4.
A comparison of vesicular uptake of newly synthesized GABA and the preexisting GABA. [3H]Glu was used to generate newly synthesized [3H]GABA, whereas [14C]GABA was included as preexisting GABA in uptake experiments. GABA converted from Glu by SV-associated GAD was actively taken up (■), whereas the uptake of GABA preexisting in the solution was almost zero (▴). Data are presented as mean ± SD (n = 3).
Similar results were obtained when [14C]Glu was used as the substrate for the production of [14C]GABA, and [3H]GABA was used as preexisting cytosolic GABA (data not shown). The identity of the content taken up into SV was determined as GABA based on the similar elution pattern as shown in Fig. 3. These results again indicated that VGAT preferentially transports newly synthesized GABA.
Demonstration of a Protein Complex of GAD, VGAT, and CaMKII on GABA-Specific SV.
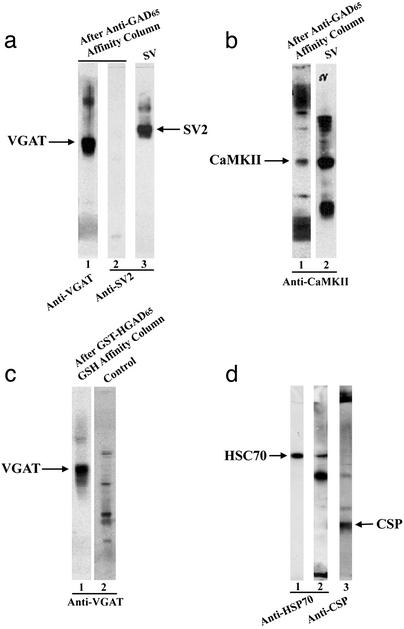
Proteins associated with GAD were isolated from crude SV preparations by using an anti-GAD65 affinity column. The results from immunoblotting assays show that GAD preparations purified by anti-GAD65 immunoaffinity column contain VGAT (Fig. 5a, lane 1) and CaMKII (Fig. 5b, lane 1) in addition to some other unidentified proteins. These unknown proteins may represent the degradative or aggregated forms of VGAT or CaMKII. These results suggest that VGAT and CaMKII form a protein complex with GAD and hence are copurified by an anti-GAD65 immunoaffinity column. When the same blot was reprobed with antibodies specific to SV2, a marker protein for SV, no SV2 was detected (Fig. 5a, lane 2), indicating the absence of the whole SV in anti-GAD65 affinity-purified GAD complex.
Figure 5.
Identification of VGAT, CaMKII, HSC70, and CSP as components of a GAD protein complex. (a) The GAD-associated protein complex in the solubilized SV preparation was purified by an anti-GAD65 affinity column as described in Materials and Methods. The anti-GAD65 immonoaffinity-purified protein complex (4.5 μg of protein per lane) was separated on SDS/PAGE, blotted on a nitrocellulose sheet, and probed with anti-VGAT (lane 1). The arrow indicates the position of VGAT. The same nitrocellulose membrane was stripped and reprobed with anti-SV2 antibodies (lane 2) showing no detectable SV2 in anti-GAD purified preparations. Lane 3 is SV alone as a control. The arrow indicates the position of SV2 protein, which is a marker for SV. (b) Lane 1 is the same as the lane 1 in Fig. 5a, except it was probed with anti-CaMKII showing staining at the position of CaMKII protein as indicated by the arrow. Lane 2 is SV alone as a control. (c) Immunoblotting analysis of the GAD protein complex formed between the solubilized SV solution and the recombinant GST-HGAD65 fusion protein probed with anti-VGAT. The arrow indicates the position of VGAT (lane 1). GST-GAD65 fusion protein alone showed no staining under the same conditions (lane 2). (d) Immunoblotting analysis of GABAergic SV (4.5 μg of protein per lane) isolated by anti-GAD65-conjugated beads with anti-HSC70 (lane 2). The arrow indicates the position of HSC70. Lane 1 is pure HSC70 alone as a marker. The same blot was stripped and restained with anti-CSP (lane 3). The arrow indicates the position of CSP.
Reconstitution of the VGAT–GAD65 Complex Using a GST-HGAD65 Fusion Protein.
To further demonstrate the protein complex between VGAT and GAD65, solubilized SVs were loaded onto a GSH affinity column containing GST-HGAD65 fusion protein. The column was washed extensively with high salt buffer to reduce nonspecific protein–protein interaction after application of detergent-solubilized SV fractions. As shown in Fig. 5c, VGAT was detected with anti-VGAT in the Tris-SDS eluted fraction (Fig. 5c, lane 1), whereas no VGAT was detected in the control (Fig. 5c, lane 2). These results suggest that VGAT forms a protein complex with GST-HGAD65 and hence is specifically retained by GSH affinity resin.
Demonstration of a Protein Complex of GAD, HSC70, and CSP on GABAergic SVs.
GABAergic SVs coupled to anti-GAD65 immunobeads were dissociated from the beads by citric acid. The dissociated GABAergic SVs were analyzed by immunoblotting using anti-HSC70. As shown in Fig. 5d, lane 2, a protein corresponding to the position of HSC70 (Fig. 5d, lane 1) is stained with anti-HSC70, suggesting that HSC70 is present in GABAergic SV. When the same blot was stripped and restained with anti-CSP antibodies, a protein corresponding to CSP is stained (Fig. 5d, lane 3). These results suggest that both HSC70 and CSP form a protein complex with GAD65 in GABAergic SV, confirming our previous findings in synaptosomal preparation (15).
Discussion
At least two isoforms of GAD (GAD65 and GAD67) have been identified (1). These two isoforms, through formation of a homodimer or heterodimer, are found in the membrane-associated as well as the soluble extracts and are responsible for the formation of the major inhibitory neurotransmitter GABA (2). Previously, we have shown that SGAD, presumably GAD67, is activated by protein dephosphorylation through the Ca2+-dependent phosphatase, calcineurin, and is inhibited by phosphorylation through protein kinase A (23). Conversely, MGAD, presumably GAD65, is activated by protein phosphorylation, which is catalyzed by an unidentified membrane-associated kinase (7). Site-directed mutagenesis studies have also shown that several serine residues at the N terminus of MGAD are involved in protein phosphorylation (14). Furthermore, our results indicate that the ATP activation of MGAD activity depends on the integrity of the electrochemical gradient of the SVs (7). This ATP-mediated activation is abolished under conditions that disrupt the vesicular proton gradient, such as in the presence of V-type ATPase inhibitor, protonophore, or ionophore uncouplers (7). It has been well established that GABA uptake into SVs by VGAT depends on the integrity of electrochemical proton gradients (e.g., ΔpH and Δψ) of SVs (24). Newly synthesized neurotransmitters were found to be preferentially released upon stimulation (25, 26). Furthermore, Angel et al. (26) reported that in a reconstituted system GABA taken up into SVs is derived from newly synthesized GABA. The same authors further postulated that the same molecule, namely GAD, is responsible for both GABA synthesis and transport into SVs. Although this is an intriguing hypothesis, separate clonings of VGAT and GAD65/GAD67 suggest that these are distinct proteins encoded by different genes (1, 16). VGAT is an integral membrane protein with 10 transmembrane domains (16), whereas GAD65 and GAD67 are soluble proteins lacking any transmembrane regions (1). Hence, it is clear that two separate protein entities are responsible for synthesis and packaging of GABA into SVs. Recently, we have shown that GAD65 may become anchored to SVs through protein complex formation first with HSC70, followed by interaction with CSP, an intrinsic SV protein (15). In this article, we have shown the presence of HSC70 and CSP in purified GABAergic SV (Fig. 5d). In addition, we provide evidence to show that the protein complex includes two additional SV proteins, namely, VGAT and CaMKII (Fig. 5 a–c). Furthermore, we have used purified GABAergic-specific SVs to demonstrate that [3H]GABA, newly synthesized from [3H]Glu by SV-associated GAD, is taken up preferentially into SV over the preexisting cytosolic GABA (Fig. 4a). The material accumulated inside SVs has also been positively identified as GABA (Fig. 4b). Based on these observations, we proposed that there is a structural and functional coupling between the synthesis and packaging of GABA into SV. This hypothesis is further supported by the following lines of evidence.
First, the newly synthesized [3H]GABA from [3H]Glu by SV-associated GAD is taken up efficiently by VGAT. The VGAT activity is markedly inhibited when the GAD inhibitor, hydrazine or aminooxyacetate, is included in the reaction system, suggesting a functional coupling between MGAD and VGAT. Second, although GAD is a soluble protein, it can anchor to SV through its interaction with HSC70 and several members of SV proteins including CSP, CaMKII, and VGAT (Fig. 5 and ref. 15). This protein machinery provides a structural basis for an efficient coupling between GABA synthesis by GAD and GABA packaging into SV by VGAT. Third, a good correlation between the expression of VGAT and GAD65 in various brain regions as well as at various developmental stages (data not shown) is compatible with the above hypothesis. Fourth, in addition to the GABA system, we have found that a similar mechanism may also be involved in the dopaminergic systems.** Recently, we reported that the membrane-associated tyrosine hydroxylase, the rate-limiting enzyme involved in dopaminergic synthesis, is activated by ATP through protein phosphorylation and inactivated by protein dephosphorylation. Furthermore, the activation of tyrosine hydroxylase mediated by ATP is abolished under conditions disrupting the proton gradient on the SV. Because the uptake of dopaminergic into SVs also depends on the proton gradient of the SV, these observations also support a coupling mechanism between dopaminergic synthesis and packaging into SV.
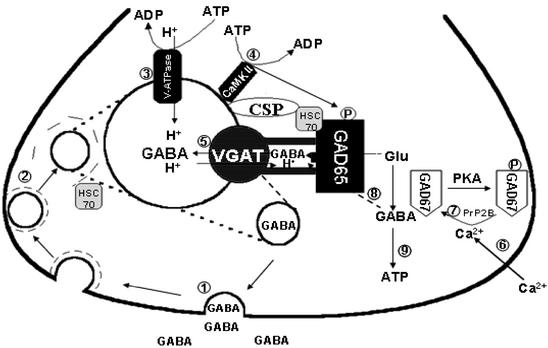
In conclusion, we propose that GAD65 is anchored to SVs by forming a protein complex first with HSC70, followed by the association with proteins on SVs, e.g., CSP, VGAT, and CaMKII. This protein complex functions as a machine to ensure that GABA biosynthesis and packaging into the SV is efficiently coupled. A model that depicts a functional and structural coupling of GABA synthesis, regulation, and packaging into SVs is proposed (Fig. 6). The physiological events leading from neuronal stimulation to activation of SV-associated GAD and subsequent packaging of GABA into the SV can be described as follows. GABA is released by exocytosis after the arrival of an action potential (stage 1). The SV is recycled by means of clathrin-coated pits (stage 2). The clathrin coat is then dissociated from the vesicles through interaction with HSC70 (27). Vesicles are then returned to the resting state of SVs, where the proton gradient is restored by V-ATPase (stage 3). GAD65 is activated through protein phosphorylation by a proton gradient-dependent protein kinase (stage 4). One of the candidates of protein kinase is CaMKII. GABA newly synthesized by GAD65 is then transported into SVs by VGAT (stage 5). These refilled GABA-containing SVs are ready to be released upon arrival of a new action potential (stage 6). Previously, we reported that SGAD is activated by calcineurin-mediated dephosphorylation and inhibited by protein kinase A-mediated protein phosphorylation (22, 23). Here, we propose that when GABA neurons are stimulated, the influx of Ca2+ into the terminal results in dephosphorylation and activation of SGAD/GAD67 (stage 7). GABA synthesized by SGAD/GAD67 in the cytosol may also be transported into SVs, although it represents a minor pathway (stage 8). Cytosolic GABA may also be metabolized to generate ATP through the GABA shunt pathway, which may be used to maintain electrochemical proton gradient for GABA transport (stage 9). The model proposed here could explain the efficiency of vesicular transport of GABA newly synthesized by SV-associated GAD65, but not GABA in the cytosolic pool. One can imagine that the active site of VGAT and the catalytic site of GAD65 are tightly coupled in a key and lock manner. This tight structural coupling would allow an efficient transfer of GABA from its site of synthesis to the site of transport. It also prevents the access of GABA in the cytosol to reach the active site of VGAT, resulting in the minimum rate of transport of cytosolic GABA. However, in the absence of GAD65, the active site of VGAT will become available to cytosolic GABA, and vesicular transport of GABA can be restored to a certain extent. This proposed mechanism can explain the observation that GAD65 knockout mice can survive and grow relatively normal, except with signs of deficiencies in GABA transmission such as increase in seizure susceptibility (28), absence of long-term depression (29), increase in anxiety-like behavior (30, 31), and lack of cortical plasticity (32). However, GAD67 knockout is lethal. GAD67-deficient mice did not survive after birth because of cleft palate (28, 33). These observations are compatible with the notion that GAD65 is primarily responsible for synthesis of GABA to be used as a neurotransmitter, whereas GAD67 is responsible for GABA to be used for other functions such as serving as a signaling molecule in development, a source of energy, and a source of GABA released via nonvesicular mechanism. Further studies on the 3D structure of GAD and its protein complex are needed to elucidate the molecular mechanism of coupling between GABA synthesis and its packaging into SV.
Figure 6.
A model depicting a structural and functional coupling between GABA synthesis and vesicular GABA transport into SV. GAD65 is anchored to SVs first through forming a protein complex with the chaperone protein, HSC70, followed by association of HSC70⋅GAD65 complex to CSP, VGAT, and CaMKII on SVs. The sequence of events leading from neuronal stimulation to activation of GAD65 and packaging of GABA into SVs is discussed in the text.
Acknowledgments
The expert assistance on electron microscopy from Dr. Bruce Cutler is much appreciated. This work was supported in part by National Institutes of Health Grant NS37851 (to J.-Y.W.), National Science Foundation Grant IBN-9723079 (to J.-Y.W.), the Research Development Fund, the J. R. and Inez Jay Fund, the University of Kansas (to J.-Y.W.), and the Schmidt Family Foundation at Florida Atlantic University.
Abbreviations
- GAD
l-glutamic acid decarboxylase
- GABA
γ-aminobutyric acid
- MGAD
membrane-associated GAD
- SGAD
soluble GAD
- HGAD
human GAD
- VGAT
vesicular GABA transporter
- CaMKII
Ca2+/calmodulin-dependent kinase II
- SV
synaptic vesicle
- HGAD65
human GAD65
- HSC70
heat shock cognate 70
- CSP
cysteine-string protein
- GSH
glutathione
Footnotes
Chen, R., Di, S., Wei, J.-N. & Wu, J. Y. (2001) Soc. Neurosci. Abstr. 27, 124.8.
References
- 1.Erlander M G, Tobin A J. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- 2.Dirkx R, Jr, Thomas A, Li L S, Lernmark Å, Sherwin R S, De Camilli P, Solimena M. J Biol Chem. 1995;270:2241–2246. doi: 10.1074/jbc.270.5.2241. [DOI] [PubMed] [Google Scholar]
- 3.Sheikh S N, Martin D L. J Neurochem. 1996;66:2082–2090. doi: 10.1046/j.1471-4159.1996.66052082.x. [DOI] [PubMed] [Google Scholar]
- 4.Kanaani J, Lissin D, Kash S F, Baekkekov S. J Biol Chem. 1999;274:37200–37209. doi: 10.1074/jbc.274.52.37200. [DOI] [PubMed] [Google Scholar]
- 5.Solimena M, Aggujaro D, Muntzel C, Dirkx R, Jr, Butler M, De Camilli P, Hayday A. Proc Natl Acad Sci USA. 1993;90:3073–3077. doi: 10.1073/pnas.90.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman D L, Houser C R, Tobin A J. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu C C, Thomas C, Chen W Q, Davis K M, Foos T, Chen J L, Wu E, Schloss J V, Wu J Y. J Biol Chem. 1999;274:24366–24371. doi: 10.1074/jbc.274.34.24366. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Veit B, Baekkeskov S. J Cell Biol. 1994;124:927–934. doi: 10.1083/jcb.124.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonnum F. Biochem J. 1968;106:401–412. doi: 10.1042/bj1060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin D L, Martin S B. J Neurochem. 1982;39:1001–1008. doi: 10.1111/j.1471-4159.1982.tb11489.x. [DOI] [PubMed] [Google Scholar]
- 11.Westhead E W. Ann NY Acad Sci. 1987;493:92–100. doi: 10.1111/j.1749-6632.1987.tb27186.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y C, Gottlieb D I. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christgau S, Aanstoot H J, Schierbeck H, Begley K, Tullin S, Hejnaes K, Baekkeskov S. J Cell Biol. 1992;118:309–320. doi: 10.1083/jcb.118.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namchuk M, Lindsay L, Turk C W, Kanaani J, Baekkeskov S. J Biol Chem. 1997;272:1548–1557. doi: 10.1074/jbc.272.3.1548. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C C, Davis K M, Jin H, Foos T, Floor E, Chen W Q, Tyburski J B, Yang C Y, Schloss J V, Wu J Y. J Biol Chem. 2000;275:20822–20828. doi: 10.1074/jbc.M001403200. [DOI] [PubMed] [Google Scholar]
- 16.McIntire S L, Reimer R, Schuske K, Edwards R H, Jorgensen E M. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 17.Wu J Y, Denner L A, Wei S C, Lin C T, Song G X, Xu Y F, Liu J W, Lin H S. Brain Res. 1986;373:1–14. doi: 10.1016/0006-8993(86)90309-4. [DOI] [PubMed] [Google Scholar]
- 18.Takamori S, Riedel D, Jahn R. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Floor E. Neurosci Lett. 1994;180:175–178. doi: 10.1016/0304-3940(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 20.Floor E, Phillip S L, Wang Y L, Meng L H, Chen W Q. J Neurochem. 1995;64:689–699. doi: 10.1046/j.1471-4159.1995.64020689.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu J Y, Moss L G, Chen M S. Neurochem Res. 1979;4:201–212. doi: 10.1007/BF00964144. [DOI] [PubMed] [Google Scholar]
- 22.Davis K M, Foos T, Bates C S, Tucker E, Hsu C C, Chen W, Jin H, Tyburski J B, Schloss J V, Tobin A J, Wu J Y. Biochem Biophys Res Commun. 2000;267:777–782. doi: 10.1006/bbrc.1999.2038. [DOI] [PubMed] [Google Scholar]
- 23.Bao J, Cheung W Y, Wu J Y. J Biol Chem. 1995;270:6464–6467. doi: 10.1074/jbc.270.12.6464. [DOI] [PubMed] [Google Scholar]
- 24.Hell J W, Maycox P R, Jahn R. J Biol Chem. 1990;265:2111–2117. [PubMed] [Google Scholar]
- 25.Reubi J C. Neuroscience. 1980;5:2145–2150. doi: 10.1016/0306-4522(80)90130-x. [DOI] [PubMed] [Google Scholar]
- 26.Angel I, Fleissner A, Seifert R. Neurochem Int. 1983;5:697–712. doi: 10.1016/0197-0186(83)90095-5. [DOI] [PubMed] [Google Scholar]
- 27.Stahl B, Tobaben S, Sudhof T C. Eur J Cell Biol. 1999;78:375–381. doi: 10.1016/S0171-9335(99)80079-X. [DOI] [PubMed] [Google Scholar]
- 28.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R G, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S Y, Morales B, Lee H K, Kirkwood A. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stork O, Ji F Y, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- 31.Kash S F, Tecott L H, Hodge C, Baekkeskov S. Proc Natl Acad Sci USA. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mataga N, Nagai N, Hensch T K. Proc Natl Acad Sci USA. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condie B G, Bain G, Gottlieb D I, Capecchi M R. Proc Natl Acad Sci USA. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]