Abstract
Odor stimulation of olfactory sensory neurons (OSNs) leads to both the activation and subsequent desensitization of a heteromultimeric cyclic-nucleotide-gated (CNG) channel present in these cells. The native olfactory CNG channel consists of three distinct subunits: CNGA2, CNGA4, and CNGB1b. Mice in which the CNGA4 gene has been deleted display defective Ca2+/calmodulin-dependent inhibition of the CNG channel, resulting in a striking reduction in adaptation of the odor-induced electrophysiological response in the OSNs. These mutants therefore afford an excellent opportunity to assess the importance of Ca2+-mediated CNG channel desensitization for odor discrimination and adaptation in behaving animals. By using an operant conditioning paradigm, we show that CNGA4-null mice are profoundly impaired in the detection and discrimination of olfactory stimuli in the presence of an adapting background odor. The extent of this impairment depends on both the concentration and the molecular identity of the adapting stimulus. Thus, Ca2+-dependent desensitization of the odor response in the OSNs mediated by the CNGA4 subunit is essential for normal odor sensation and adaptation of freely behaving mice, preventing saturation of the olfactory signal transduction machinery and extending the range of odor detection and discrimination.
Olfactory sensory neurons (OSNs) respond to odor stimulation with a receptor-mediated increase in intracellular cAMP, which directly activates a cyclic-nucleotide-gated (CNG) channel in the plasma membrane (1–3). Calcium ions, entering the OSN cilia through the open CNG channel (4, 5), mediate adaptation of the sensory response by providing a negative feedback signal that modulates the olfactory signal transduction machinery (4–8). A major mechanism for this rapid adaptation to odors is the Ca2+/calmodulin-mediated reduction in cAMP sensitivity of the CNG channel (9–14). Although it is widely thought that adaptation of the odor-induced sensory current may be critical as a mechanism for odor adaptation in freely behaving animals (8–14), no direct linkage between cellular adaptation of OSNs and sensory adaptation of the olfactory system in vivo has been established.
Recent progress concerning the molecular mechanism underlying odor-response desensitization in OSNs provides an experimental strategy to overcome this problem. Six distinct CNG channel genes have been identified in mammals; these are grouped, according to sequence similarity, into two subfamilies, CNGA and CNGB (15, 16). OSNs express three of these genes, CNGA2 (17, 18), CNGA4 (19, 20), and CNGB1b (21, 22). Native olfactory CNG channels comprise all three subunits, forming CNGA2/A4/B1b heteromultimers (12, 13, 22). Previously, we used gene targeting in embryonic stem cells to disrupt the CNGA4 gene in mice (12). CNGA4−/− mice reveal a striking reduction in the rate of adaptation of the electrophysiological response to odors in OSNs because of defective Ca2+/calmodulin-dependent CNG channel modulation (12). Furthermore, olfactory CNG channels in these mice exhibit a decreased affinity for cAMP, with a dose–response relation shifted by ≈10-fold to higher concentrations, demonstrating that CNGA4 contributes to the high cAMP sensitivity of the native olfactory channel (12). Therefore, the CNGA4-null mice are ideal for examining the relationship between Ca2+-dependent CNG channel inhibition and odor adaptation.
In the present study, we have conducted behavioral testing by using the CNGA4−/− mice. The CNGA4−/− mice display a significantly elevated odor-detection threshold, consistent with the altered affinity for cyclic nucleotides observed in heterologous expression systems. In addition, these mice exhibit profound and unanticipated deficits in the ability to detect and discriminate olfactory stimuli in the presence of adapting background odors. These results demonstrate that CNGA4 is essential for extending both the sensitivity and range of odor detection and provide strong support for the hypothesis that Ca2+/calmodulin-mediated CNG channel desensitization by the CNGA4 subunit in OSNs is a functionally important mechanism for sensory adaptation of the olfactory system in vivo.
Materials and Methods
Animals.
CNGA4+/− breeding pairs of mixed 129Sv × C57BL/6J background were used to produce CNGA4−/− experimental animals and heterozygous (+/−) or WT (+/+) littermate controls. Generation of these mice has been described (12). CNGA4-null mice are fertile and no obvious differences exist in size, weight, and overall activity compared with WT or heterozygous animals. In addition, we previously established that WT and CNGA4+/− mice are phenotypically indistinguishable for odor or cyclic nucleotide responses in OSNs (12). For behavioral testing, 25 adult male mice (8 WT, 4 CNGA4+/−, and 13 CNGA4−/− mice) were housed individually in plastic cages and kept on a 12:12 light/dark cycle. Food was available ad libitum. Mice were water-deprived for 24 h before the first day of testing. For the remainder of the behavioral experiments, these mice were maintained on a 1.5 ml/day water-deprivation schedule. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Olfactometer.
Mice were trained and tested in a fully automated liquid dilution olfactometer (Knosys Instruments, Bethesda; ref. 23). In brief, the apparatus consisted of a 20 × 15 × 13-cm Plexiglas box (operant chamber). A glass tube affixed to the outside of one wall provided a port for odor delivery, exhaust, and water reward. Odors were delivered through the bottom of the glass tube and exhausted through the top with the aid of an exhaust fan. Water reinforcement was delivered through a stainless steel tube on the far side of the glass tube. A ventilation fan affixed to the wall opposite the glass tube served to blow room air continuously into the operant chamber, which provided a steady stream of fresh air for the animal and prevented test odors from leaking out of the delivery tube and into the operant chamber. For the odor-adaptation assays, the apparatus was slightly modified in that a delivery box containing the adapting stimulus was placed into the fresh air stream. In this way, a steady stream of background odor could be delivered to the operant chamber. All experiments were performed at room temperature.
All training and testing procedures were controlled, monitored, and recorded by using a personal computer with software written in QBASIC.
Training for the Operant Task.
Mice were trained using a go/no-go discrete trials operant conditioning procedure (23). An animal was trained to insert its nose into the odor-delivery port, stay there until the odor was delivered, and then begin licking at the water-reinforcement tube. The animal was rewarded with 5 μl of water for licking after the test odor (S+) was delivered. No water was dispensed after the control odor (S−) was delivered. During these experiments, an animal was given no more than one testing session a day. Each session was divided into blocks, a block consisting of 10 trials with the S+ odor and 10 trials with the S− odor. Licking for the S+ odor (hit) or not responding to the S− odor (correct rejection) were scored as correct choices. Not responding during an S+ trial (miss) or responding during an S− trial (false alarm) were scored as errors. At the end of each block the number of correct choices out of 20 possible was recorded as a percentage of correct trials, yielding the performance accuracy.
Odor Stimuli.
Odors [cineole (eucalyptol), 1-heptanol, and 1-octanol] were purchased from Sigma and diluted in mineral oil (Sigma). Mineral oil without added odors served as the control (S−) stimulus in several experiments. Serial dilutions were made from stock solutions. Odors in the saturation flasks were replaced every 2 days. Odor concentration in this study refers to the concentration in the liquid phase of the flask. The exact odor concentration at the odor-delivery port is not known, but previous estimates with the same behavioral paradigms indicate that WT mice have absolute odor-detection thresholds at concentrations near or below 10−10 M (23), one to two logarithmic units below the values obtained when liquid-phase concentrations are used (see Fig. 2).
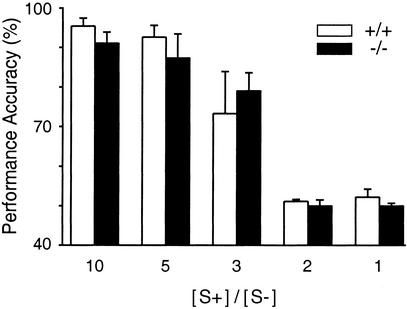
Figure 2.
Mean performance accuracies of WT (open bars, n = 3) and CNGA4−/− (filled bars, n = 4) mice as a function of the ratio between the concentration of S+ and S−, [S+]/[S−]. S+ remained at 10−3 M 1-octanol during each consecutive session, whereas the concentration of S− was 10−4 M, 2 × 10−4 M, 3.3 × 10−4 M, 5 × 10−4 M, or 10−3 M 1-octanol. No significant differences occurred between the two genotypes [two-way repeated measures ANOVA: F(1, 19) = 0.36; P > 0.05]. Both groups were capable of discriminating 10- or 5-fold differences in 1-octanol concentration with high accuracy but failed to discriminate a 2-fold difference.
Odor Quality and Intensity Discrimination.
WT and CNGA4−/− mice that were trained to detect 1-octanol were used in these experiments. Mice were run in a single, 10-block (200 trials) session to examine whether they could distinguish between 10−3 M 1-octanol (S+) and 10−3 M 1-heptanol (S−). In a separate 10-block session these same mice were required to discriminate between 10−3 M 1-octanol (S+) and 10−4 M 1-octanol (S−). In each of the tasks the mean percentage of correct trials for the last three blocks (60 trials) was scored. Data are expressed as mean ± SEM.
Least Discriminable Intensity Difference.
Three WT and four CNGA4−/− were tested in their ability to discriminate small intensity differences of the same odor. All mice were trained and tested by using 10−3 M 1-octanol as S+ stimulus. After initial training, mice were given four testing sessions on separate days consisting of 10 blocks of 20 trials each. During each consecutive session, the S+ stimulus remained 10−3 M 1-octanol, whereas the S− stimulus was 10−4 M, 2 × 10−4 M, 3.3 × 10−4 M, 5 × 10−4 M, or 10−3 M 1-octanol. The mean percentage of correct trials for the last three blocks (60 trials) was recorded for each animal.
Threshold Sensitivity.
Two cohorts of mice were first trained to discriminate between a high concentration (10−2 M) of test odor (S+) and mineral oil (S−). Five CNGA4−/− mice and four CNGA4+/− mice were trained and tested by using cineole as S+ stimulus. Four CNGA4−/− mice and five WT mice were trained and tested with 1-octanol as S+ stimulus. After initial training, mice were given testing sessions consisting of 10 blocks of 20 trials each. Each session was performed on a different day at a progressively lower S+ concentration (with the exception of 3 × 10−7 M and 3 × 10−8 M cineole, which were the final two sessions given for that odor). The mean percentage of correct trials for the last three blocks (60 trials) was recorded for each animal.
Odor-Adaptation Assay.
To examine the effects of odor adaptation on odor-detection behavior, we compared the performance accuracy during an odor quality discrimination task in the absence or presence of a steady background odor. WT or CNGA4−/− mice were initially tasked to discriminate between 10−3 M 1-octanol (S+) and mineral oil (S−). After the mice scored ≥90% correct for two consecutive blocks, the session was suspended and a steady stream of background odor (1-octanol, 3 × 10−5 M or 10−4 M) was added to the operant chamber. Two minutes were given for the background odor to equilibrate before the session was resumed, on the basis of control experiments in which we added TiCl4 (Sigma) to the background stream. This substance generates a white “smoke” that mimics the behavior of odor plumes and thus provides a means to visualize the spatial boundaries of odor stimuli (24). The session then continued for an additional eight blocks (160 trials). In some experiments, we also analyzed recovery from adaptation by measuring performance accuracy after turning off the background odor stream. Identical procedures were used for the cross-adaptation task with the exception of using a background odor of either 1-heptanol or cineole (10−2 M).
Results
Odor Quality and Intensity Discrimination.
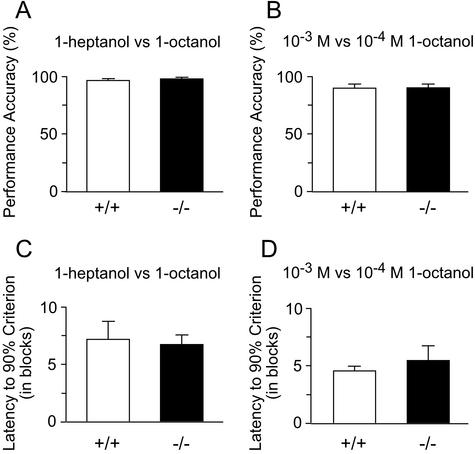
To investigate the behavioral consequences of genetic deletion of the CNGA4 channel subunit for odor detection, we assayed odor quality and intensity discrimination in WT and CNGA4−/− mice. In the first experiment, mice were required to distinguish between two structurally related aliphatic alcohols, 1-heptanol and 1-octanol (each at 10−3 M). WT and CNGA4−/− mice displayed a comparable performance accuracy of ≥90% (Fig. 1A). Likewise, when both groups were required to distinguish between two concentrations of the same odor (1-octanol, 10−3 M and 10−4 M), they were both able to do this with ≥90% accuracy (Fig. 1B). Furthermore, no significant differences occurred between WT and CNGA4−/− mice in the latency it took to learn these tasks; the mean number of blocks needed before mice had a performance accuracy of ≥90% was closely similar between the two genotypes for both quality (Fig. 1C) and intensity discrimination tasks (Fig. 1D), and no significant difference occurred in the average trial duration [odor quality discrimination: 19 ± 1 s for WT mice and 16 ± 2 s for CNGA4−/− mice (t test, P > 0.1); odor intensity discrimination: 21 ± 3 s for WT mice and 23 ± 5 s for CNGA4−/− mice (t test, P > 0.1)].
Figure 1.
(A) Mean performance accuracies of WT (open bars, n = 5) and CNGA4−/− (filled bars, n = 4) mice are indistinguishable when animals were required to discriminate between 10−3 M 1-heptanol and 10−3 M 1-octanol (t = 0.07, P > 0.1, two-tailed t test). (B) Odor intensity discrimination tasks with 1-octanol at 10−3 and 10−4 M also revealed no genotypic differences in performance accuracy (t = 0.08, P > 0.1, two-tailed t test). WT, open bars (n = 5); CNGA4−/−, filled bars (n = 4). (C and D) All subjects attained at least 90% performance accuracy. The latency to achieve this criterion did not differ significantly between genotypes for either task (10−3 M 1-heptanol vs. 10−3 M 1-octanol, t = 0.2, P > 0.1; 10−3 M 1-octanol vs. 10−4 M 1-octanol, t = 0.7, P > 0.1).
We next examined whether WT and CNGA4−/− mice differ in their ability to discriminate small intensity differences of the same odor. This comparison was done by analyzing the performance accuracy as a function of the ratio between the concentration of S+ and S−, [S+]/[S−]. In these experiments the concentration of S+ remained at 10−3 M 1-octanol during each consecutive session, whereas the concentration of S− was 10−4 M, 2 × 10−4 M, 3.3 × 10−4 M, 5 × 10−4 M, or 10−3 M 1-octanol. We found no significant difference between the two genotypes; both groups were capable of discriminating 10- or 5-fold differences in 1-octanol concentration with high accuracy, whereas performance accuracy dropped to chance levels (≈50%) with a 2-fold difference in stimulus concentration in both groups (Fig. 2). A 3-fold difference in 1-octanol concentration was close to the limit for discriminating small intensity differences of the same odor (the least discriminable intensity difference) in both groups. Thus taken together, the results of Figs. 1 and 2 show that CNGA4−/− mice do not display obvious dysfunctions in performing odor quality and intensity discrimination tasks or in the number of sessions required to learn these discrimination problems.
Elevated Odor-Detection Threshold in CNGA4−/− Mice.
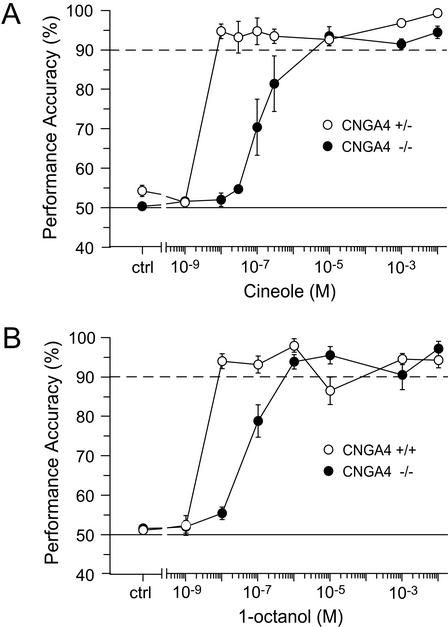
The presence of CNGA4 in the native olfactory CNG channel contributes to the high cAMP sensitivity of the signal transduction cascade in OSNs (12). To define the significance of this cellular feature for stimulus sensitivity of the olfactory system in vivo, we examined whether CNGA4−/− mice exhibit altered odor-detection thresholds as compared with heterozygous or WT controls. Analysis of odor concentration–performance curves shows that both CNGA4−/− and control mice had a performance accuracy of ≥90% for high concentrations of the test odor when either cineole or 1-octanol was used (Fig. 3). CNGA4−/− mice showed a decrease in performance at 3 × 10−7 M cineole and their accuracy dropped to chance levels at ≈3 × 10−8 M cineole (Fig. 3A). By contrast, heterozygous controls had a performance accuracy of >90% at 1 × 10−8 M cineole but dropped to a 50% accuracy at 10−9 M cineole (Fig. 3A). When 1-octanol was used as the rewarded (S+) odor, CNGA4−/− mice exhibited a similarly reduced performance accuracy at low stimulus concentrations (Fig. 3B).
Figure 3.
Elevated odor-detection threshold in CNGA4−/− mice. (A) Comparison of the performance accuracy of CNGA4+/− (○, n = 5) and CNGA4−/− (●, n = 4) mice as a function of stimulus concentration. Mice were required to discriminate between cineole and mineral oil. (B) Comparison of the performance accuracy of WT (○) and CNGA4−/− (●) mice as a function of stimulus concentration. Mice were tasked to discriminate between 1-octanol and mineral oil. Note that in both experiments detection thresholds are shifted to higher concentrations for CNGA4−/− mice. As a control (ctrl) to rule out that the animals used nonchemosensory (i.e., auditory or visual) cues for discrimination between S+ and S− trials, mice were required to discriminate between two samples of mineral oil without adding any odors to the solution. All mice performed at chance level under these conditions.
To quantify these differences between CNGA4−/− and control mice, we estimated detection thresholds for individual mice. Detection threshold was defined as 65% correct detection (25) as determined from the analysis of individual odor concentration–performance curves (individual data not shown). In CNGA4−/− mice, mean detection thresholds were shifted to higher concentrations by 43-fold for cineole (t test; t = 3.4, P < 0.02) and 15-fold for 1-octanol (t test; t = 7.5, P < 0.001), when compared with control mice. Thus, CNGA4-null mice reveal a significantly elevated odor-detection threshold, indicating that CNGA4 plays an essential role in enhancing the overall sensitivity of the main olfactory system.
CNGA4 Is Essential for Odor Discrimination in the Presence of an Adapting Stimulus.
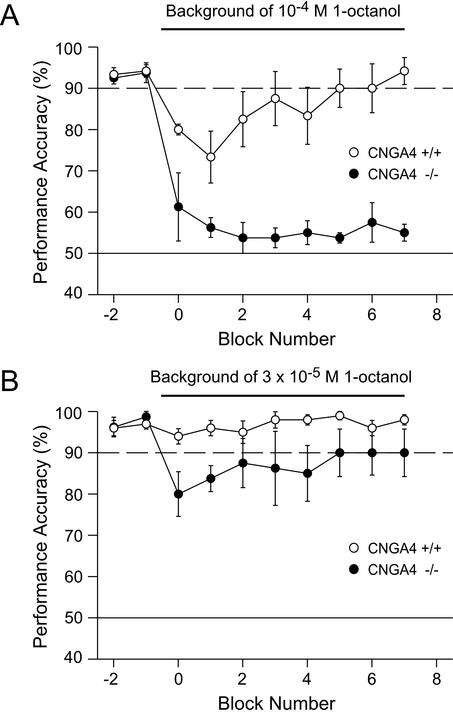
OSNs from CNGA4-null mice exhibit a strikingly reduced rate of odor-induced desensitization; the presence of CNGA4 in the native olfactory channel accelerates the Ca2+-mediated negative feedback in olfactory signaling and allows rapid adaptation of the odor response (12). To investigate the relevance of this mechanism for odor adaptation in vivo, we compared the performance accuracy of WT and CNGA4−/− mice during an odor quality discrimination task in the absence or presence of a steady background odor stream (Fig. 4A). Initially, in the absence of an adapting stimulus, both groups of mice detected the S+ stimulus (10−3 M 1-octanol) at ≥90% accuracy, as seen in the first two blocks of the experiment. We then added the same odor (1-octanol, 10−4 M) to the background stream. WT mice performed with high accuracy under these conditions. Although performance accuracy initially dropped after the addition of the background odor, it never reached chance level and recovered to ≥90% accuracy while the background odor was still present (Fig. 4A). By contrast, CNGA4−/− mice were unable to execute this discrimination problem. Their performance accuracy quickly dropped to chance levels and stayed at this level for the duration of the test (Fig. 4A). Thus, CNGA4−/− mice could no longer detect the stimulus under these conditions. This remarkable deficit was fully reversible; after removal of the background odor, CNGA4−/− mice recovered and attained a ≥90% accuracy generally within one to three blocks (data not shown).
Figure 4.
In vivo odor adaptation is impaired in CNGA4−/− mice. Shown is a comparison of the performance accuracy of WT (n = 5, ○) and CNGA4−/− (n = 4, ●) mice during a discrimination task with 1-octanol (10−3 M) as S+ stimulus in the absence or presence of a steady stream of background odor (A, 10−4 M 1-octanol; B, 3 × 10−5 M 1-octanol).
The effects of the background odor on discrimination performance were strongly dose-dependent (Fig. 4B). When the concentration of the background odor was decreased (1-octanol, 3 × 10−5 M), the ability of CNGA4−/− mice to detect the S+ stimulus was not completely disrupted. Although CNGA4−/− mice showed an initial drop in performance accuracy, they recovered to ≈90% while the background odor was still present. In contrast, WT mice showed no drop in performance after the addition of the background odor, displaying a performance accuracy well above 90%. Together, these results demonstrate that the ability of CNGA4−/− mice to both detect and discriminate odors is significantly impaired in the presence of an adapting background odor. Therefore, we conclude that the function of the CNGA4 subunit, through its ability to mediate rapid Ca2+-dependent modulation of the olfactory CNG channel, is essential for odor adaptation in behaving mice.
Impaired Cross-Adaptation.
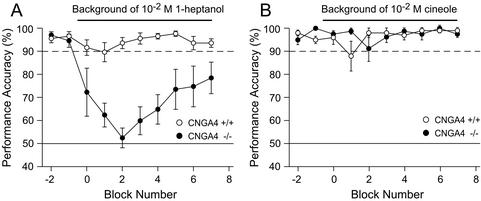
We next examined whether WT and CNGA4−/− mice differ in performance when tested in a cross-adaptation paradigm (Fig. 5). By using 1-octanol as S+ stimulus, we chose either 1-heptanol, as a structurally related, and cineole, as a structurally unrelated background stimulus. When WT mice were tested, 1-heptanol did not elicit significant cross-adaptation, even when used at a concentration of 10−2 M (Fig. 5A). In contrast, the performance accuracy for 1-octanol of CNGA4−/− mice in the presence of 1-heptanol dropped to chance level before recovering gradually (Fig. 5A). We observed no significant differences in performance between WT and CNGA4−/− mice with a structurally unrelated odorant, cineole (10−2 M), as the cross-adapting stimulus (Fig. 5B). A likely explanation for the lack of effect of cineole in this cross-adaptation paradigm is that little overlap exists between the populations of OSNs activated by cineole and those activated by the S+ stimulus (1-octanol). These data reveal that CNGA4−/− mice are significantly impaired in their ability to discriminate an odor stimulus in the presence of an adapting odor and that the extent of this impairment depends on both the concentration and the molecular identity of the adapting stimulus.
Figure 5.
CNGA4−/− mice exhibit impaired cross-adaptation to 1-octanol with 1-heptanol, but not cineole, as the adapting stimulus. Shown is a comparison of the performance accuracy of WT (n = 5, ○) and CNGA4−/− (n = 4, ●) mice during a discrimination task with 1-octanol (10−3 M) as S+ stimulus in the absence or presence of a cross-adapting background odor (A, 10−2 M 1-heptanol; B, 10−2 M cineole).
Discussion
Our experiments with behaving mice trained to discriminate between distinct odor stimuli demonstrate that CNGA4−/− mice are strongly impaired in performing a simple odor-discrimination task in the presence of an adapting background odor. In contrast, the mutant mice perform well in the absence of any adapting background stimuli, even when required to discriminate between only a 5-fold difference in concentration of the same odor or between two aliphatic alcohols that differ only minimally in carbon chain length. We thus conclude that CNGA4 has a prominent role in olfactory adaptation but that this channel subunit is not essential for odor detection per se. Hence, our data provide strong support for the hypothesis that the modulation of cAMP sensitivity of the olfactory CNG channel by Ca2+/calmodulin is a functionally important mechanism for rapid odor adaptation in vivo (8–14), linking odor-response desensitization by CNGA4 in the OSNs with sensory adaptation in the olfactory system of behaving animals. We would expect that the human ortholog of CNGA4, located on chromosome 11, plays a similar role in odor adaptation in humans.
Under natural conditions, OSNs must constantly adapt to the changing odor environment and intensity. This ability to adapt to stimuli, by preventing saturation of the transduction machinery, should allow OSNs to function over a broader stimulus range and provide for finer discrimination of concentration changes in the presence of adapting stimuli. Similarly, detecting changes in concentration of structurally related stimuli is enhanced by adaptation. The results reported here demonstrate that CNGA4−/− mice are indeed impaired in the discrimination among odor stimuli in the presence of an adapting stimulus, whereas WT mice perform well under the same conditions (Fig. 4A). This finding suggests that the ability of OSNs to adapt to sensory stimulation extends the working range of odor detection to higher concentrations (26), thus optimizing discrimination capacity. What is the molecular basis for the failure of CNGA4−/− mice to detect an odor stimulus in the presence of the same or related background stimuli? A likely explanation for this unexpected result is that CNGA4−/− OSNs, through the ability of the CNGA4 subunit to mediate rapid Ca2+-dependent modulation of the olfactory CNG channel (12, 13), are less well inhibited by Ca2+/calmodulin. A sustained background stimulus should therefore produce a much stronger and more persistent depolarization in these cells compared with WT OSNs. At a certain concentration of the background odor, this stimulus should lead to complete inactivation of voltage-gated Na+ channels, causing depolarization block thereby preventing the cells from eliciting action potentials to test odors (26, 27). Because of the threshold of the voltage dependence of Na+ channel inactivation, the extent of this impairment should depend strongly on relatively small changes in concentration of the background odor, consistent with the results shown in Fig. 4. The CNGA4−/− mice should be useful in future work aimed at providing a quantitative model for the regulation of electrical excitability in mammalian OSNs.
In addition to the deficits observed in olfactory adaptation, CNGA4−/− mice displayed as much as 40-fold elevated odor-detection thresholds. These behavioral results fit well with previous data demonstrating that CNGA4 contributes to the cAMP sensitivity of native olfactory CNG channel excised from dendritic knobs of OSNs (12). Thus, the presence of CNGA4 in the native olfactory channel plays an essential role in enhancing the overall sensitivity of the main olfactory system, indicating that specific molecular and physiological features of the olfactory signal transduction cascade in the OSNs are translated into odor perception in a surprisingly precise manner. It will be particularly interesting to determine whether genetic variations in the human CNGA4 gene are associated with elevated odor-detection thresholds.
Despite the remarkable specificity of the odor-detection impairments in CNGA4−/− mice and the close similarity of the behavioral defects with chemosensory defects observed in OSNs (12), it is possible that CNGA4 has other yet undetected roles in the nervous system. CNGA4 mRNA expression has been reported in sensory neurons of the vomeronasal organ (28), but the functional relevance of this result is not yet clear, especially because cyclic nucleotides fail to activate a conductance in vomeronasal neurons (29). Given the role of the vomeronasal organ in modulation of sexual and social behaviors (30–32), interpretation of the performance in the behavioral tests used here should not be complicated by a potential function for CNGA4 in the vomeronasal organ. CNGA4 mRNA expression has also been described in the hippocampus and other brain regions (33), but the need for extensive PCR amplification and long development times for in situ hybridization suggests that the level of channel mRNA is very low (16, 33). Our behavioral tests demonstrate that CNGA4−/− mice do not display obvious dysfunctions in the number of sessions required to learn odor-discrimination tasks. Most strikingly, our result that the extent of impairment in a cross-adaptation paradigm depends on the molecular structure of the adapting stimulus argues strongly that the specific behavioral phenotypes identified here are caused by chemosensory defects in the peripheral OSNs rather than central problems with odor processing such as habituation (34).
In summary, we have demonstrated that CNGA4-null mice are a useful model for investigating the relationship between Ca2+-dependent CNG channel inhibition, odor-response desensitization in OSNs, and the role of adaptation for odor detection and discrimination in vivo. Several other molecular mechanisms, including phosphorylation of olfactory receptors (35, 36) and adenylyl cyclase (37–39), have been proposed to play a role in odor adaptation. By using combined molecular, cellular, and behavioral analyses of gene-targeted mice, we can now begin to evaluate the relative contribution of each of these mechanisms to odor perception.
Acknowledgments
We thank Trese Leinders-Zufall for much discussion and advice, Frank Margolis and Faith Scipio for help with genotyping, and Renee Cockerham for assistance in setting up the olfactometer. This work was supported by the Howard Hughes Medical Institute (R.R.R.) and National Institutes of Health/National Institute on Deafness and Other Communication Disorders Grants to F.Z. (DC05249 and DC00347), S.D.M. (DC04779), and R.R.R. (DC04190). K.R.K. is the recipient of National Institutes of Health/National Institute of Neurological Disorders and Stroke Postdoctoral Training Grant NS07375.
Abbreviations
- OSN
olfactory sensory neuron
- CNG
cyclic-nucleotide-gated
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lancet D. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Gold G H. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 3.Firestein S, Darrow B, Shepherd G M. Neuron. 1991;6:825–835. doi: 10.1016/0896-6273(91)90178-3. [DOI] [PubMed] [Google Scholar]
- 4.Menini A. Curr Opin Neurobiol. 1999;9:419–426. doi: 10.1016/S0959-4388(99)80063-4. [DOI] [PubMed] [Google Scholar]
- 5.Leinders-Zufall T, Greer C A, Shepherd G M, Zufall F. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurahashi T, Shibuya T. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- 7.Zufall F, Shepherd G M, Firestein S. Proc R Soc London Ser B. 1991;246:225–230. doi: 10.1098/rspb.1991.0148. [DOI] [PubMed] [Google Scholar]
- 8.Kurahashi T, Menini A. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen T-Y, Yau K-W. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Chen T-Y, Ahamed B, Li J, Yau K-W. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 11.Varnum M D, Zagotta W N. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 12.Munger S, Lane A P, Zhong H, Leinders-Zufall T, Yau K-W, Zufall F, Reed R R. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley J, Reuter D, Frings S. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 14.Zufall F, Leinders-Zufall T. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]
- 15.Bradley J, Frings S, Yau K-W, Reed R R. Science. 2001;294:2095. doi: 10.1126/science.294.5549.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaupp U B, Seifert R. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 17.Dhallan R S, Yau K-W, Schrader K A, Reed R R. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig J, Margalit T, Eismann E, Lancet D, Kaupp U B. FEBS Lett. 1990;270:24–29. doi: 10.1016/0014-5793(90)81226-e. [DOI] [PubMed] [Google Scholar]
- 19.Liman E R, Buck L B. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 20.Bradley J, Li J, Davidson N, Lester H A, Zinn K. Proc Natl Acad Sci USA. 1994;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sautter A, Zong X, Hofman F, Biel M. Proc Natl Acad Sci USA. 1998;95:4696–4701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bönigk W, Bradley J, Müller F, Sesti F, Boekhoff I, Ronnett G V, Kaupp U B, Frings S. J Neurosci. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodyak N, Slotnick B. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- 24.Baker T C, Linn C E., Jr . In: Wind Tunnels in Pheromone Research. Hummel H A, Miller T A, editors. Heidelberg: Springer; 1984. pp. 75–110. [Google Scholar]
- 25.Youngentob S L, Margolis F L. NeuroReport. 1999;10:15–19. doi: 10.1097/00001756-199901180-00003. [DOI] [PubMed] [Google Scholar]
- 26.Reisert J, Matthews H R. J Physiol (London) 1999;519.3:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trotier D. Semin Cell Biol. 1994;5:47–54. doi: 10.1006/scel.1994.1007. [DOI] [PubMed] [Google Scholar]
- 28.Berghard A, Buck L B, Liman E R. Proc Natl Acad Sci USA. 1996;93:2365–2367. doi: 10.1073/pnas.93.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liman E R, Corey D P. J Neurosci. 1996;16:4625–4637. doi: 10.1523/JNEUROSCI.16-15-04625.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leypold B G, Yu C R, Leinders-Zufall T, Kim M M, Zufall F, Axel R. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowers L, Holy T E, Meister M, Dulac C, Koentges G. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 32.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki C J, Ogawa S, Zufall F, Mombaerts P. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 33.Bradley J, Zhang Y, Bakin R, Lester H A, Ronnett G V, Zinn K. J Neurosci. 1997;17:1993–2005. doi: 10.1523/JNEUROSCI.17-06-01993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson D A. J Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- 35.Dawson T M, Arriza J L, Jaworsky D E, Borisy F F, Attramadal H, Lefkowitz R J, Ronnett G V. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- 36.Peppel K, Boekhoff I, McDonald P, Breer H, Caron M G, Lefkowitz R J. J Biol Chem. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- 37.Wei J, Zhao A Z, Chan G C K, Baker L P, Impey S, Beavo J A, Storm D R. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- 38.Leinders-Zufall T, Ma M, Zufall F. J Neurosci. 1999;19:RC19. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi H, Kurahashi T. J Physiol (London) 2002;541.3:825–833. doi: 10.1113/jphysiol.2002.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]