Abstract
Macula densa cells are unique renal biosensor cells that detect changes in luminal NaCl concentration ([NaCl]L) and transmit signals to the mesangial cell/afferent arteriolar complex. They are the critical link between renal salt and water excretion and glomerular hemodynamics, thus playing a key role in regulation of body fluid volume. Since identification of these cells in the early 1900s, the nature of the signaling process from macula densa cells to the glomerular contractile elements has remained unknown. In patch–clamp studies of macula densa cells, we identified an [NaCl]L-sensitive ATP-permeable large-conductance (380 pS) anion channel. Also, we directly demonstrated the release of ATP (up to 10 μM) at the basolateral membrane of macula densa cells, in a manner dependent on [NaCl]L, by using an ATP bioassay technique. Furthermore, we found that glomerular mesangial cells respond with elevations in cytosolic Ca2+ concentration to extracellular application of ATP (EC50 0.8 μM). Importantly, we also found increases in cytosolic Ca2+ concentration with elevations in [NaCl]L, when fura-2-loaded mesangial cells were placed close to the basolateral membrane of macula densa cells. Thus, cell-to-cell communication between macula densa cells and mesangial cells, which express P2Y2 receptors, involves the release of ATP from macula densa cells via maxi anion channels at the basolateral membrane. This mechanism may represent a new paradigm in cell-to-cell signal transduction mediated by ATP.
Macula densa cells are located within the thick ascending limb (TAL) and have their basolateral membrane in contact with glomerular mesangial cells, which, in turn, are contiguous with smooth muscle cells and renin-containing granular cells of the afferent arteriole (Fig. 1A Left). Their function is to sense changes in the luminal NaCl concentration ([NaCl]L) and to transmit signals that cause alterations both in vascular tone of the afferent arteriole, termed tubuloglomerular feedback (TGF), and in renin secretion from granular cells of the juxtaglomerular apparatus (1–3). Numerous investigations have sought to understand how macula densa cells sense changes in [NaCl]L and transmit information to the underlying mesangial cells. Morphological evidence for a lack of gap junctions between macula densa cells and mesangial cells argues against direct cell to cell coupling (4), and therefore it seems that the macula densa cells most likely release a humoral factor at the basolateral membrane. The list of potential mediators has included angiotensin II, thromboxane, prostaglandins, adenosine, and most recently ATP (1–3). It has been recently reported by Yang et al. (5) that a low chloride environment stimulates prostaglandin E2 release from a mouse immortalized macula densa cell line. Presumably, this release of prostaglandins may be related to the role of macula densa cells in controlling renin release.
Figure 1.
Macula densa-glomerular preparation and NaCl-activated ion channel in the macula densa plasma membrane. (A Left) Schematic diagram of the macula densa–juxtaglomerular apparatus, which illustrates that the macula densa lies within the TAL and is adjacent to the mesangial/arteriolar complex. (Right) Photomicrograph of macula densa–glomerular preparation. The glomerulus was fixed to the bottom of the chamber with a holding pipette (HP). The TAL was removed by dissection, thereby allowing free access of patch pipette (PP) to the macula densa cells. (B) Single-channel recordings from a cell-attached patch on a macula densa cell. An active channel in control Ringer's bath ([NaCl] = 135 mM; Left) was inactivated after removal of NaCl from the bath (Center). Return of NaCl to the bathing solution reactivated the channel (Right). Arrowheads, the closed level of channel current. Arrows, onset of step pulses. The voltage applied to the cell (−Vp) is indicated beside each current trace. Data represent nine similar experiments.
As originally proposed by Burnstock (6), it is now well established that ATP can serve as an extracellular signaling molecule. This role of ATP, and previous work suggesting an involvement of ATP in the tubuloglomerular mechanism (3, 7), prompted us to examine whether ATP was involved in macula densa signaling. To directly establish a role of ATP in TGF signaling, it is first necessary to determine whether there is a [NaCl]L-sensitive pathway for the release of ATP at the basolateral membrane of macula densa cells. Although ATP permeability via cystic fibrosis transmembrane conductance regulator (CFTR) is somewhat controversial or questionable, there has recently been an upsurge of interest in ATP conductance through anion channels in other epithelial cells (8–17). Thus, studies were performed to determine whether such a pathway might exist in macula densa cells and whether the pathway might be sensitive to [NaCl]L.
Materials and Methods
Tissue and Cell Preparations.
By using manual dissection of kidneys obtained from rabbits, glomeruli were isolated that contained the macula densa plaque but with the removal of the surrounding TAL. This maneuver provided direct access for patch-clamping the plasma (apical and lateral) membranes of macula densa cells. For experiments shown in Fig. 3 C and D, the TAL remained intact and the basolateral membrane of the macula densa plaque was exposed by partially removing the adjacent glomerulus. Individual preparations were transferred to a Lucite chamber mounted on a Nikon TMD or Leitz Fluovert inverted microscope, and the glomerulus was mechanically fixed to the bottom of the chamber with a holding pipette.
Figure 3.
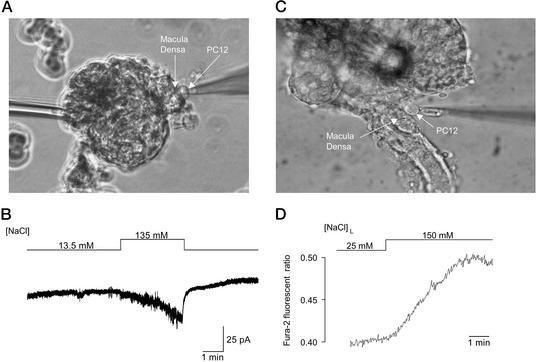
NaCl-dependent ATP release from macula densa cells detected by bioassay techniques. (A) The patch pipette with a PC12 cell in whole-cell configuration is positioned on the macula densa plaque. (B) In response to an increase in bath [NaCl], there was activation of a P2X channel. When the PC12 cell was not in contact with the macula densa, no channel activity was present when the bath [NaCl] was increased (data not shown, n = 7). (C) In an isolated perfused TAL in which the glomerulus was partially removed, thereby exposing the basolateral membrane of macula densa plaque, a fura-2-loaded PC12 cell was positioned and held with a holding pipette at the basolateral membrane of the macula densa. (D) In response to an increase in [NaCl]L from 25 to 150 mM, there was an increase in the fura-2 ratio, indicating the activation of P2X receptors and increases in the cytosolic calcium concentration. Data represent 10 similar experiments.
PC12 cells were cultured as reported (18). For experiments in Fig. 3 C and D, fura-2-loaded PC12 cells were prepared by incubation in culture media containing 5 μM fura-2-AM and 1 mg/ml Pluronic F-127 for 1 h at 37°C to facilitate dye loading.
A glomerular mesangial cell line established from an SV40 transgenic mouse was obtained from the American Type Culture Collection and cultured in a 3:1 medium of DMEM and Ham's medium F12 supplemented with 5% FBS. Fura-2-loaded mesangial cells were prepared, and measurements were performed similar to those described for PC12 cells.
Patch–Clamp Single-Channel Recordings.
Cell-attached and inside-out patches were obtained from macula densa cells. Step pulses were applied from a pipette potential (Vp) of 0–50 mV in 10-mV increments. Currents were recorded at room temperature (23–26°C) with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) or an EPC-7 or EPC-9 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany), filtered at 1 kHz, digitized at 5 kHz, and processed through pclamp or puls+pulsfit software.
Ringer's solution, which was used for the bathing solution consisted of (in mM) 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 d-glucose, and 5 Hepes/NaOH (pH 7.4). In Fig. 1, zero NaCl solution was obtained by substituting mannitol for 135 mM NaCl and contained 11 mM Cl−. In Fig. 2, the low Cl− solutions had a [Cl−] of 24.5 mM where NaCl was replaced with mannitol, Na-gluconate, or Na-asparate. In the low-Na+ solution, N-methyl-d-glucamine (NMDG) was used to substitute for Na+. ATP solution was composed of 100 mM Na2ATP (pH 7.4 adjusted with NaOH), kept on ice until use, and used after washout. Pipette solution was always in mM [140 KCl, 2 CaCl2, 1 MgCl2, 5 d-glucose, and 5 Hepes/NaOH (pH 7.4)].
Figure 2.
Anion selectivity and ATP permeability of macula densa maxi channel. (A) Single-channel recording from inside-out patches. Shown are current traces in control Ringer's ([NaCl] = 135 mM) solution (Left), low Cl− Ringer's solution ([Cl−] = 24.5 mM) (Center), and 100 mM ATP solution (Right). The pipette solution always contained 146 mM Cl−. (Right) Note the presence of channel activity at negative voltages that represents inward current generated by ATP4− efflux. Arrowheads represent the closed level of channel current, and vertical arrows indicate the step pulse onset. The membrane potential (−Vp) is indicated beside each current trace. (B) Single-channel I-V curves obtained from inside-out patches from macula densa cells. (Left) I-V curves in Ringer's solution containing [NaCl] = 135 mM (n = 12), low Cl− (substituted with gluconate; n = 8), or N-methyl-d-glucamine (NMDG; Na+-free; n = 3). (Right) I-V curves in which the bath contained either control Ringer's solution ([NaCl] = 135 mM) or 100 mM ATP solution (n = 7). (C) Expanded trace of single-channel current, presented in A (Right Lower), recorded at −50 mV in 100 mM ATP solution.
Biosensor ATP Assays.
The local concentration of released ATP at the single macula densa surface was monitored at 37°C by two biosensor techniques with PC12 cells that express P2X receptor channels. In experiments in Fig. 3 A and B, whole-cell currents were recorded at a holding potential of −50 mV from a PC12 cell that was attached to the macula densa surface as described (18). The current responses were observed after increasing the bath [NaCl] by substituting NaCl for mannitol. The pipette (intracellular) solution contained (in mM) 150 CsCl, 1 MgCl2, 10 EGTA, and 10 Hepes/CsOH (pH 7.4). In experiments in Fig. 3 C and D, fura-2 fluorescence was measured in a PC12 cell that was placed at the macula densa basolateral membrane while the TAL was cannulated and perfused with a modified Ringer's solution. This solution was composed of (in mM) 148 or 25 NaCl, 0 or 124 N-methyl-d-glucamine-cyclamate, 5 KCl, 1 MgSO4, 1.6 Na2HPO4, 0.4 NaH2PO4, 1.5 CaCl2, and 5 d-glucose (pH 7.4). A pipette was used to gently hold a PC12 cell and to position it either at the basolateral membrane of the macula densa plaque or at the basolateral membrane of an adjacent TAL cell. Fura-2 ratio, which reflects [Ca2+]i, was measured at an emission wavelength of 510 nm in response to excitation wavelengths of 340 and 380 nm, alternated at a rate of 25 Hz by a computer-controlled chopper assembly. Autofluorescence-corrected ratios (340 nm/380 nm) were calculated at a rate of 5 points per sec by using PTI (Photon Technologies, Princeton) software.
Results
At the apical membrane of macula densa cells, the major NaCl entry pathway is through a furosemide-sensitive Na+-2Cl−-K+ cotransporter (19–21). Also, previous microelectrode studies measuring membrane potentials suggested that Cl− channels are the major charge carrier across the basolateral membrane (17, 18). To identify the presence and determine the characteristics of the anion pathway, patch-clamp studies were performed on the “lateral” membrane of macula densa cells, as shown in Fig. 1A Right. A large-conductance channel of ≈380 pS (382 ± 11 pS, n = 11) was identified in cell-attached patches in the presence of 135 mM NaCl in the bathing solution (Fig. 1B Left). Interestingly, when extracellular NaCl was removed from the bathing solution, the channel became quiescent (Center). Channel activity was regained within some 10 sec after the readdition of NaCl to the bathing solution (Right).
After excision, channel activity was maintained, and the single-channel conductance in control Ringer's solution was 380 ± 9 pS (n = 14). Fig. 2 illustrates channel recordings (A) and I-V relationships (B) in inside-out patches. There was a linear I-V relationship with a reversal potential close to zero (2.6 ± 0.6 mV, n = 12) (Fig. 2B). This channel exhibited voltage-dependent inactivation at large potentials [>±50 mV under symmetrical ionic conditions (Fig. 2A Left)]. Evidence for a Cl−-permeable pathway was provided by the finding that the I-V relationship was unaltered when N-methyl-d-glucamine was substituted for Na+ (Fig. 2B, triangles). Also, inward currents were reduced and the reversal potential was shifted to the left when bath [Cl−] was lowered by substituting with gluconate (Fig. 2 A Center and B Left, filled circles). The maxi Cl− channel was also permeable to large monovalent anions. In ion substitution experiments, the permeability ratio of this channel for gluconate relative to Cl− (Pgluconate/PCl) was 0.29 ± 0.02 (n = 8) whereas in other experiments with aspartate, Paspartate/PCl was estimated to be 0.23 ± 0.03 (n = 6, data not shown).
After changing the intracellular (bath) solution from control Ringer's to 100 mM ATP solution, the single-channel current profile was altered, as shown in the right side of Fig. 2 A and B, in a fully reversible manner. Significant suppression of outward Cl− conductance may suggest a blocking effect of ATP. At negative potentials there was evidence for single-channel activity with inward current jumps clearly detected at −50 mV (Fig. 2C). Because we have shown anion selectivity of this maxi channel and because the only anions present at the intracellular surface of the patch (bathing solution) were anionic forms of ATP (under our conditions, ATP4− and H-ATP3− account for >87% and 12% of the ATP present, respectively), then movement of ATP must be responsible for the inward currents. In seven experiments, the permeability ratio was estimated to be PATP/PCl = 0.14 ± 0.02 by assuming a valence of −4 for ATP. For comparison, a study demonstrating ATP flux through a cystic fibrosis transmembrane conductance regulator-related channel reported current jumps of 0.1 pA at −60 mV and a PATP/PCl value of 0.33 under very comparable experimental conditions (13). In addition, a recent study from our laboratory has demonstrated ATP permeability of a maxi anion channel in C127 cells (16, 17). Thus, ATP permeability may be a generalized property of these large anion channels.
Macula densa maxi anion channels were completely inhibited by 50 μM Gd3+ (n = 6, data not shown), which is known to block ATP release from other cell types (14, 15, 22) and ATP-permeable maxi anion channels in C127 cells (16). The Gd3+ effect was rapidly reversible after washout. In the presence of a stilbene-derivative Cl− channel blocker, SITS (100 μM, n = 4, data not shown), the anion channel current was not suppressed but became noisy, suggesting a flickery block. The effect of 4-acetamido-4′-isothiocyanostilbene (SITS) was also fully reversible. However, channel activity was not sensitive to a carboxylate analog Cl− channel blocker, diphenylamine-2-carboxylate (DPC; 2 mM), or 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB; 100 μM; n = 3; data not shown). Also, this maxi anion channel, in the inside-out patch, did not exhibit an apparent sensitivity to cytosolic Ca2+, because removal of bath Ca2+ and addition of 1 mM EGTA did not alter channel activity (n = 5, data not shown).
These results provide evidence for a maxi anion channel that serves as an [NaCl]L-sensitive ATP-permeable pathway in macula densa cells. What remains to be demonstrated, however, is that these cells do in fact release ATP, in an [NaCl]L-dependent manner, without mechanical perturbation by giga-sealed patch pipettes. To address this issue, we used a biosensor technique (18) for cellular ATP release by using a rat pheochromocytoma clonal cell line (PC12) that endogenously expressed a purinergic P2X receptor. P2X receptors are ligand-gated Ca2+-permeable cation channels that are activated by ATP. Therefore, ATP release can be detected from a given cell by measuring either whole-cell currents by using a patch-clamp technique or the cytosolic Ca2+ concentration ([Ca2+]i) by using the Ca2+-sensitive dye fura-2 in a PC12 cell when it is placed next to the host cell. The advantage of this bioassay is that it can be used to detect, in real time, quantitative release of ATP from single cells. As shown in Fig. 3A, a PC12 cell was placed on the macula densa plaque via a patch pipette, and whole-cell currents were measured. In response to an increase in bath [NaCl] there was the appearance of inward currents (Fig. 3B), which represent the P2X receptor channel activity. This channel activity indicates that macula densa cells release ATP in response to an increase in bath [NaCl]. Changes in bath [NaCl] when the PC12 cell was not in contact with the macula densa failed to produce any detectable channel activity in PC12 cells. From the mean peak current density (−8.25 ± 1.25 pA/pF, n = 38), the ATP concentration in the vicinity of the macula densa surface was estimated to increase up to 14.3 μM (95% confidence interval 6.5–26.9 μM) based on a calibration curve of ATP-induced PC12 responses (18). A disadvantage of the preparation shown in Fig. 3A is that removal of the TAL exposes both the apical and lateral membranes of the macula densa plaque to the bathing solution so that it is difficult to ascertain the polarity of ATP release. For ATP to be involved in TGF signaling it was necessary to obtain unequivocal evidence that ATP is released at the basolateral membrane of these cells. Results shown in Fig. 3 C and D were obtained from a preparation in which the basolateral membrane of the macula densa plaque was exposed and the intact TAL was perfused with Ringer's solutions of different [NaCl]L (23, 24). An increase in [NaCl]L was found to significantly increase PC12 [Ca2+]i (Fig. 3D), supporting our suggestion that macula densa cells release ATP at the basolateral membrane. Based on a dose–response curve of PC12 [Ca2+]i vs. exogenously added ATP, we estimate that the ATP concentration at the basolateral surface of the macula densa in the presence of increased [NaCl]L approached 10 μM. In contrast, no release of ATP was found when the PC12 cell was placed on the basolateral membrane of the adjacent TAL cells and [NaCl]L was increased (n = 12, data not shown). This finding eliminates the possibility that ATP from the TAL contributed to the increase in PC12 [Ca2+]i when this cell was placed at the basolateral membrane of the macula densa. Finally, adding the P2 receptor blocker suramin (100 μM, n = 5, data not shown) to the bath reversibly inhibited [NaCl]L-dependent increases in PC12 [Ca2+]i. Thus, we have concluded that a significant amount of ATP is released from the basolateral membrane of macula densa cells in response to increases in [NaCl]L, thereby raising the local interstitial ATP concentration up to a level that is sufficient to stimulate P2 purinergic receptors.
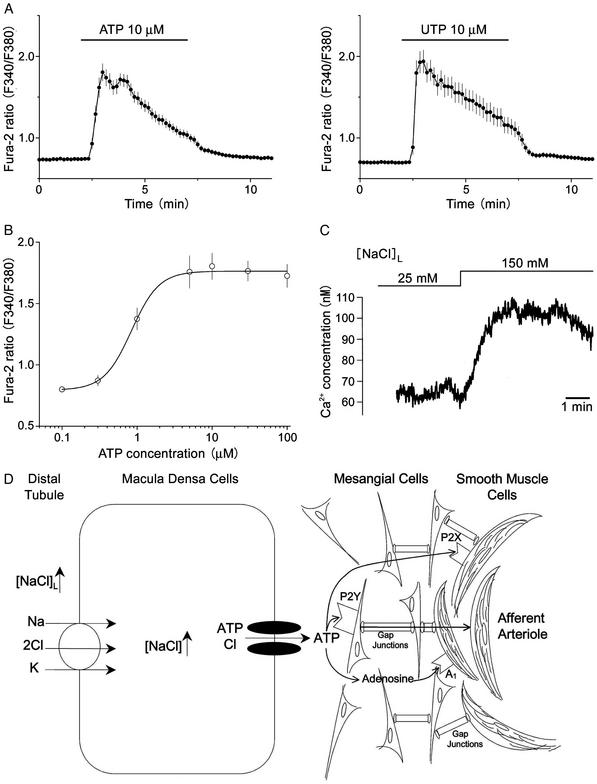
To establish a role of released ATP in macula densa cell-to-mesangial cell signal transduction, it is next necessary to determine whether mesangial cells respond to ATP released at the basolateral membrane of macula densa cells. By using mouse-immortalized mesangial cells loaded with fura-2, application of 10 μM ATP (Fig. 4A Left) resulted in substantial increases in intracellular Ca2+ concentration. This ATP-induced Ca2+ response was abolished by suramin, an antagonist of the P2-purinergic receptor (100 μM, n = 26, data not shown). Essentially the same Ca2+ response was induced by 10 μM UTP (Fig. 4A Right), whereas 10 μM to 1 mM adenosine, 10 μM AMP, and 10 μM ADP failed to induce Ca2+ responses (data not shown, n = 8–13). Thus, it seems that ATP-induced Ca2+ responses were induced by activation of P2Y2-purinergic receptors. This finding is in good agreement with previous studies (25–27). The concentration-response curve (Fig. 4B) shows that ATP can induce Ca2+ responses at concentrations as low as 0.3 μM with a half-maximal concentration of 0.84 ± 0.13 μM. These results support the hypothesis that mesangial cells can respond to an estimated concentration of ATP released from macula densa cells. To support this point further, a mesangial cell was placed in close proximity to the basolateral membrane of the macula densa plaque under the same experimental conditions as those shown in Fig. 3C. As shown in Fig. 4C, an increase in [NaCl]L elicited a fast and rapid increase in cytosolic Ca2+ concentration (n = 5). This increase in mesangial cell Ca2+ concentration could also be completely inhibited by suramin (100 μM, n = 4, data not shown). Thus, these studies demonstrate that the cell-to-cell communication process that occurs between these two different types of cells involves the regulated release of ATP via a macula densa maxi anion channel and the subsequent stimulation of mesangial cells through P2Y2-purinergic receptors (as illustrated in Fig. 4D).
Figure 4.
The effects of ATP on cultured mesangial cells. (A) Effects of ATP and UTP on the cytosolic free Ca2+ concentration in single mesangial cells. Each symbol represents the mean values of 47 (for ATP) or 33 (for UTP) experiments, and each bar represents SEM. (B) The concentration-response curve for ATP-induced Ca2+ elevations in mesangial cells. Each symbol represents the mean value of 26–47 experiments, and each bar represents SEM. A curve represents sigmoidal fit with an EC50 value given in the text and with a Hill coefficient of 2.4. The ratio values of basal level and of peak response to 10 μM ATP correspond to the free Ca2+ concentrations of 80.2 ± 4.2 nM (n = 47) and 336.5 ± 25.5 nM (n = 47), respectively. (C) Effect of an increase in [NaCl]L from 25 to 150 mM on the cytosolic free Ca2+ concentration in a single mesangial cell that had been positioned and held with a holding pipette at the basolateral membrane of the macula densa, as shown for PC12 cells in Fig. 3C. The mean Ca2+ concentration increased from 64.7 ± 6.2 to 90.7 ± 9.7 nM (n = 5) in response to an increase in [NaCl]L from 25 to 150 mM. (D) Scheme depicting [NaCl]L-sensitive ATP release from macula densa cells via maxi anion channels at the basolateral membrane, and possible roles of released ATP in mediating signal transduction from macula densa cells to mesangial cells via P2Y receptors and to afferent arteriolar smooth muscle cells via P2X and/or A1 receptors. Signal transduction from mesangial cells to afferent arteriolar smooth muscle cells may take place via gap junctions.
We propose that the entry of NaCl into macula densa cells induces, by an unidentified mechanism, the activation of a maxi anion channel that results in the movement of not only Cl− but also anionic ATP across the basolateral membrane, thereby transmitting signals to adjacent mesangial cells (Fig. 4D). Because the afferent arteriole expresses P2X and A1 receptors (28–30), it is also possible that ATP released from macula densa cells may directly, or indirectly after being metabolized to adenosine, trigger TGF signaling at the afferent arteriolar smooth muscle cells (Fig. 4D). Several recent studies have shown a lack of TGF responses during adenosine receptor blockade or in mice deficient in the adenosine A1 receptor (31–34). These results suggest that adenosine may either play a direct role or at least a permissive but essential role in TGF signaling. However, mesangial cells do not respond to adenosine, at least with increases in intracellular Ca2+. Recent work by Ren et al. (35) showed the necessity of an intact mesangial cell syncytium for TGF signaling and older work by Iijima et al. (36) demonstrated that Ca2+ signal propagation can occur through mesangial cells. Thus, the simplest idea would be that the release of ATP across the basolateral membrane of macula densa cells would activate purinergic receptors on mesangial cells. This receptor activation would then cause the propagation of a Ca2+ signal through the mesangial cells field and on to the smooth muscle cells of the afferent arteriole. Consistent with the idea of ATP-TGF signaling, Navar and colleagues (3, 37, 38) observed that ATP administration caused renal afferent arteriolar vasoconstriction whereas blockade or desensitization of purinergic receptors inhibited autoregulation and TGF responses. Also, Nishiyama et al. (7) have recently observed that the renal interstitial fluid ATP concentration varied directly with blood pressure-induced changes in renal vascular resistance.
In conclusion, this study represents a significant step forward in our understanding of the nature of the TGF signaling pathway by providing direct evidence that ATP is released from macula densa cells via a maxi anion channel in response to increased luminal NaCl concentration and thereby transmits signals to mesangial cells by activating their purinergic receptors. This work may provide a new paradigm that ATP-permeable maxi anion channels are involved in the cell-to-cell signal transduction mediated by ATP.
Acknowledgments
We thank M. Ohara, A. Hattori, B. Wallendorff, and T. Ishibashi for technical assistance, and Drs. E. Schwiebert and S. Morishima for helpful discussions. This work was supported by a Grant-in-Aid for International Scientific Joint Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to Y.O.), and by funds from the Kidney Foundation of Canada (to J.-Y.L.), the National Institutes of Health [National Institute of Diabetes and Digestive and Kidney Diseases Grant 32032 (to P.D.B.)], and the American National Kidney Foundation/American Society of Nephrology (to J.P.-P.).
Abbreviations
- TAL
thick ascending limb
- TGF
tubuloglomerular feedback
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bell P D, Lapointe J-Y. Clin Exp Pharmacol Physiol. 1997;25:541–547. doi: 10.1111/j.1440-1681.1997.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 2.Schnermann J. Am J Physiol. 1998;274:R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 3.Navar L G, Inscho E W, Majid S A, Imig J D, Harrison-Bernard L M, Mitchell K D. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 4.Barajas L. Fed Proc. 1981;40:78–86. [PubMed] [Google Scholar]
- 5.Yang T, Park J M, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs J P, Schnermann J. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama A, Majid D S A, Taher K A, Miyatake A, Navar L G. Circ Res. 2000;86:656–662. doi: 10.1161/01.res.86.6.656. [DOI] [PubMed] [Google Scholar]
- 8.Prat A G, Reisin I L, Ausiello D A, Cantiello H E. Am J Physiol. 1996;270:C538–C545. doi: 10.1152/ajpcell.1996.270.2.C538. [DOI] [PubMed] [Google Scholar]
- 9.Grygorczyk R, Tabcharani J A, Hanrahan J W. J Membr Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- 10.Reddy M M, Quinton P M, Haws C, Wine J J, Grygorczyk R, Tabcharani J A, Hanrahan J W, Gunderson K L, Kopito R R. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Ramjeesingh M, Bear C E. J Biol Chem. 1996;271:11623–11626. doi: 10.1074/jbc.271.20.11623. [DOI] [PubMed] [Google Scholar]
- 12.Devidas S, Guggino W B. Curr Opin Cell Biol. 1997;9:547–552. doi: 10.1016/s0955-0674(97)80032-4. [DOI] [PubMed] [Google Scholar]
- 13.Sugita M, Yue Y, Foskett J K. EMBO J. 1998;17:898–908. doi: 10.1093/emboj/17.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y. J Gen Physiol. 1999;114:525–533. doi: 10.1085/jgp.114.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazama A, Fan H-T, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. J Physiol. 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabirov R Z, Dutta A K, Okada Y. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta A K, Okada Y, Sabirov R Z. J Physiol. 2002;542:803–816. doi: 10.1113/jphysiol.2002.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazama A, Hayashi S, Okada Y. Pflügers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 19.Lapointe J-Y, Bell P D, Cardinal J. Am J Physiol. 1990;258:F1466–F1469. doi: 10.1152/ajprenal.1990.258.5.F1466. [DOI] [PubMed] [Google Scholar]
- 20.Schlatter E, Salomonson M, Persson A E, Greger G. Pflügers Arch. 1990;414:286–290. doi: 10.1007/BF00584628. [DOI] [PubMed] [Google Scholar]
- 21.Lapointe J-Y, Bell P D, Hurst A M, Cardinal J. Am J Physiol. 1991;260:F856–F860. doi: 10.1152/ajprenal.1991.260.6.F856. [DOI] [PubMed] [Google Scholar]
- 22.Taylor A L, Kudlow B A, Marrs K L, Gruenert D C, Guggino W B, Schwiebert E M. Am J Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- 23.Peti-Peterdi J, Bell P D. Am J Physiol. 1999;277:F574–F580. doi: 10.1152/ajprenal.1999.276.4.F574. [DOI] [PubMed] [Google Scholar]
- 24.Peti-Peterdi J, Chambrey R, Bebok Z, Biemesderfer D, St. John P L, Abrahamson D R, Warnock D G, Bell P D. Am J Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- 25.Pavenstadt H, Gloy J, Leipziger J, Klar B, Pfeilschifter J, Schollmeyer P, Greger R. Br J Pharmacol. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohaupt M G, Fischer T, Schwöbel J, Sterzel P R, Schulze-Lohoff E. Am J Physiol. 1998;275:F103–F110. doi: 10.1152/ajprenal.1998.275.1.F103. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez A M, Lou X, Erik A, Persson G, Ring A. Eur J Pharmacol. 1999;369:107–112. doi: 10.1016/s0014-2999(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 28.Schnermann J, Weihprecht H, Briggs J P. Am J Physiol. 1990;258:F553–F561. doi: 10.1152/ajprenal.1990.258.3.F553. [DOI] [PubMed] [Google Scholar]
- 29.Weihprecht H, Lorenz J N, Briggs J P, Schnermann J. Am J Physiol. 1992;263:F1026–F1033. doi: 10.1152/ajprenal.1992.263.6.F1026. [DOI] [PubMed] [Google Scholar]
- 30.Chan C M, Unwin R J, Bardini M, Oglesby I B, Ford A P, Townsend-Nicholson A, Burnstock G. Am J Physiol. 1998;274:F799–F804. doi: 10.1152/ajprenal.1998.274.4.F799. [DOI] [PubMed] [Google Scholar]
- 31.Sun D, Samuelson L C, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Proc Natl Acad Sci USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson A E. Am J Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 33.Ren Y, Arima S, Carretero O A, Ito S. Kidney Int. 2002;61:169–176. doi: 10.1046/j.1523-1755.2002.00093.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomson S, Bao D, Deng A, Vallon V. J Clin Invest. 2000;106:289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Y, Carretero O A, Garvin J L. Kidney Int. 2002;62:525–531. doi: 10.1046/j.1523-1755.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 36.Iijima K, Moore L C, Goligorsky M S. Am J Physiol. 1991;260:F848–F855. doi: 10.1152/ajprenal.1991.260.6.F848. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell K D, Navar L G. Am J Physiol. 1993;264:F458–F466. doi: 10.1152/ajprenal.1993.264.3.F458. [DOI] [PubMed] [Google Scholar]
- 38.Majid D S, Inscho E W, Navar L G. Am Soc Nephrol. 1999;10:492–498. doi: 10.1681/ASN.V103492. [DOI] [PubMed] [Google Scholar]