Abstract
Inhibition of type 4 cAMP-specific phosphodiesterase (PDE4) activity in L6-C5 and L6-E9 abolished myogenic differentiation induced by low-serum medium and IGF-I. L6-C5 cells cultured in low-serum medium displayed a PDE4 activity higher than cells cultured in serum-free medium, a condition not sufficient to induce differentiation. In the presence of serum, PDE4D3, the major isoform natively expressed in L6-C5 cells, translocated to a Triton-insoluble fraction, which increased the PDE specific activity of the fraction, and exhibited a Mr shift typical of phosphorylation of this isoform. Furthermore, serum promoted the localization of PDE4D3 to a vesicular subcellular compartment. In L6-C5 cells, IGF-I is a stronger inducer of myogenic differentiation in the presence than in absence of serum. Its ability to trigger differentiation in the absence of serum was restored by overexpressing wild-type PDE4D3, but not a phosphorylation-insensitive mutant. This finding was confirmed in single cells overexpressing a GFP-PDE4D3 fusion protein by assessing nuclear accumulation of myogenin in both L6-C5 and L6-E9. Overexpression of other PDE isoforms was less efficient, confirming that PDE4D3 is the physiologically relevant phosphodiesterase isoform in the control of myogenesis. These results show that downregulation of cAMP signaling through cAMP-phosphodiesterase stimulation is a prerequisite for induction of myogenesis.
INTRODUCTION
Myogenic differentiation is a complex phenomenon that involves morphological, biochemical, and molecular modifications resulting in the formation of multinucleated postmitotic myotubes expressing an array of muscle-specific proteins such as sarcomeric myosin and creatine kinase (CK). This model of differentiation has been extensively investigated, especially since the discovery of a family of myogenic regulatory factors: Myf5, MyoD, myogenin, and MRF4, belonging to the basic helix-loop-helix (bHLH) transcription factors superfamily (Edmondson and Olson, 1993). Several extracellular factors, such as hormones and growth factors, can positively or negatively modulate myogenic differentiation. Among the few factors that have been shown to promote the myogenic program, one class of peptide growth factors, the insulin-like growth factors (IGFs) I and II, potently stimulate myogenic cells to differentiate in vitro (Florini et al., 1996) and their presence is required in vivo for the development of skeletal muscle (Liu et al., 1993). L6 rat muscle cells are widely used as a model for studying the effects of IGFs on myogenic differentiation because they produce very low amounts of IGF compared with other myogenic cell lines (Florini et al., 1991b). In myogenic cell lines, IGFs can induce either differentiation or proliferation (Florini et al., 1996), suggesting that other factors influence myoblast response. Both responses are elicited through binding to the same type 1 IGF tyrosine protein kinase receptor (Florini et al., 1996). How a single receptor can elicit two opposite responses is not clear. To address this issue, the IGF-I signal transduction pathways in L6 myogenic cells have been extensively dissected. IGF-I activates both the MAP-kinase pathway, which is mainly involved in the mitogenic response (Coolican et al., 1997; Samuel et al., 1999), and PI3-kinase, which notably mediates the differentiative signals (Coolican et al., 1997; Kaliman et al., 1999; Samuel et al., 1999). In particular, IGF-I stimulation of PI3K activity generates phospatidylinositol 3,4,5-trisphosphate and induces the activation of p70S6K and Akt kinases (Kaliman et al., 1999); Akt is involved in the PI3K effect on myogenic differentiation (Jiang et al., 1999) possibly by increasing myogenin expression in L6 cells (Xu and Wu, 2000) .
Because several studies have shown that high intracellular levels of cAMP or high PKA activity can totally suppress the differentiation of myogenic cells (Li et al., 1992; Winter et al., 1993), we have been interested in the involvement of cAMP-phosphodiesterases (PDE) in the myogenic differentiation process. These enzymes are indeed able to determine the local concentrations of cAMP in discrete cell compartments and thus regulate various cell functions (Soderling and Beavo, 2000). We have previously observed that the hormone arg8-vasopressin (AVP) potently induces in vitro differentiation in various myogenic primary cells and cell lines (Nervi et al., 1995; Minotti et al., 1998) and that this effect involves the stimulation of the type 4 cAMP-phosphodiesterase isoform PDE4D3 (Naro et al., 1999), suggesting that a downregulation of the cAMP pathway is required for myogenesis. In this article, we address the more general issue of the role of PDE activity in myogenic differentiation achieved either by reducing serum content in culture medium or by treatment of L6 cells by the physiological effector IGF-I. As mentioned above, IGF-I has various effects on myoblasts, and we hypothesized that the expression of myoblast differentiative response to IGF-I is conditioned by their type 4 PDE activity, which could vary according to culture conditions and the resulting cell status. To investigate the role of PDE4, we used myoblasts of the L6-C5 subclone, which have been thoroughly characterized in our laboratory. These cells are unable to differentiate as far as they are maintained quiescent in the absence of serum and, in these conditions, weakly respond to IGF-I. The ability of IGF-I to induce differentiation is restored by factors present in serum or by the addition of AVP. This cell model enabled us to investigate whether culture conditions allowing IGF-I differentiating effects to develop affect PDE4 activity, and whether in turn overexpression of PDE could restore IGF-I effects. The major findings were verified by using another widely used L6 clone, the L6-E9 line.
Furthermore, we investigated the role and the specificity of the PDE4D3 isoform in the control of myogenin translocation from the cytoplasm to the nucleus, which could represent a novel checkpoint in the differentiation program of L6 cells.
MATERIALS AND METHODS
Cell Culture
Two clones of L6 cells were used: the subclone C5 (L6-C5; Teti et al., 1993; Minotti et al., 1998), and the subclone E9 (L6-E9), widely used as a cell model for studying the factors involved muscle cell differentiation (Nadal-Ginard, 1978). The cells were seeded at the density of 10,000/cm2 in DMEM, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated FBS. Twenty-four hours after plating, cultures were washed with PBS and either shifted to serum-free medium consisting of DMEM supplemented with 1% (wt/vol) fatty acid–free BSA (Roche Molecular Biochemicals, Mannheim, Germany), or to 1% FBS-containing DMEM (differentiative medium), with or without IGF-I addition. IGF-I was purchased from Chemicon (Temecula, CA). Terminal myogenic differentiation was morphologically evaluated after 6 d by assessing the presence of multinucleated myotubes in May-Grunwald-Giemsa–stained cultures. Cell viability was assessed using a colorimetric assay based on the cleavage of a tetrazolium salt by mitochondrial dehydrogenase in viable cells (Cell Proliferation Reagent WST-1, Roche Molecular Biochemicals).
Transfections Experiments
Transient transfections were performed by using Fugene-6 according to manufacturer's instructions (Roche Molecular Biochemicals). Briefly, 1 μg of each PDE construct (Sette and Conti, 1996; Jin et al., 1998; Verde et al., 2001) was mixed with diluted Fugene reagent (97 μl of serum-free medium + 3 μl of Fugene) and incubated for 15 min. The DNA-Fugene mix was then added dropwise to 200,000 cells/ml in suspension. The transfected cells were seeded and incubated for an additional 24 h in 10% FBS-containing DMEM before being treated.
PDE4D Immunoblotting Analysis
The cell soluble and particulate fractions were prepared as previously reported (Jin et al., 1998). Briefly, L6 cells were harvested in hypotonic buffer (20 mM Tris-HCl, pH 8, 1 mM EDTA, 0.2 mM EGTA, 50 mM NaF, 10 mM sodium orthovanadate, and 50 mM benzamidine, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 4 μg/ml aprotinin, and 2 mM phenylmethylsulphonyl fluoride). The homogenate obtained with an all-glass Dounce homogenizer was centrifuged at 20,000 × g for 15 min. The supernatant was collected as the soluble fraction. The pellet was washed twice with the same hypotonic buffer and extracted with Triton X-100 for 30 min at 4°C. After centrifugation at 20,000 × g the supernatant fraction was considered as the Triton X-100–soluble fraction and the pellet as the Triton-insoluble fraction. All the fractions were separated on 10% acrylamide gel, after loading equal amounts of proteins. Proteins were transferred onto a nitrocellulose membrane and blotted as previously reported (Jin et al., 1998). The first antibody incubation was carried out with the PDE4D-specific mAb M3S1 diluted 1:50 and incubated overnight. A second antibody incubation was performed with 1:10,000 dilution of anti-mouse immunoglobulin G antibody conjugated to HRP (Amersham Biosciences Limited, Amersham Place, England).
PDE4D Immunofluorescence
One day after transfection with pCMV5-PDE4D3 expression vector (Sette et al., 1994), L6-C5 cells were shifted to either DMEM + 1% BSA or DMEM + 1% FBS for 24 h. Cells were fixed with 4% PFA/PBS for 20 min at RT and permeabilized with 0.2% Triton X-100/PBS for 10 min. After blocking, overexpressed PDE4D3 was detected using the PDE4D specific mAb M3S1 diluted 1:50 in 5% goat serum/PBS for 1 h, followed by incubation with a 1:500 dilution of AlexaFluor-568 goat anti-mouse IgG (Molecular Probes, Eugene, OR) in 5% goat serum/PBS for 1 h. After extensive washing and mounting in antifade medium the samples were analyzed using a Leica TCS-SP (UV) confocal microscope (Chantilly, VA). In the absence of M3S1 antibody or with untransfected cells no staining could be detected.
Preparation of GFP-PDE4D3 Construct
The green fluorescent protein plasmid pEGFP-N1 was obtained from Clontech (Palo Alto, CA). It was amplified using the maxi-prep kit from Qiagen (Valencia, VA) and DH5α bacterial cells from Life Technologies (Gaithersburg, MD). PDE4D3 was amplified by PCR with Pfu DNA polymerase from Stratagene (La Jolla, CA) and was inserted into the pEGFP-N1 vector with DNA ligase (Roche Diagnostics, Milan, Italy). Stop codon was removed before subcloning to insure continuous translation of the GFP located after the PDE4D3 C terminus. The completed construct was then checked by restriction digests and sequencing.
Myogenin Expression
Cells were fixed in 4% paraformaldehyde in PBS for 30 min at 4°C and permeabilized in 0.2% Triton X-100 in PBS for 30 min. Cells were washed with 1% BSA in PBS and incubated overnight at RT with the undiluted supernatant of F5D hybridoma cells (developed by Dr. W.E. Wright, University of Texas, Dallas, obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA). After washing with 1% BSA in PBS, the cells were incubated for 1 h at RT with fluorescein- or rhodamine-conjugated goat anti-mouse IgG (Cappel Laboratories, West Chester, PA). In some experiments, the cytoskeleton was visualized by using rhodamin-labeled phalloidin, which binds to F-actin, in the conditions indicated by the manufacturer (Molecular Probes, Eugene, OR).
Creatine Kinase Assay
After 6 d of culture, cells were washed with PBS, homogenized in 30 mM Tris, 1 mM EDTA, pH 7.2. The 20,000 × g supernatant was used to measure CK activity as previously described (Nervi et al., 1995).
Myosin Expression and Quantification
mAb MF20, directed against the myosin heavy chain (kindly given by Dr. D. Fischman, Cornell University Medical College, New York, NY; Bader et al., 1982) was used to quantify myosin by indirect ELISA, as described in Naro et al. (1999) or by Western blotting, as in Minotti et al. (1998).
cAMP PDE Assay
At the end of the treatment period, the cells were washed with cold PBS and scraped into 300 μl of homogenization buffer (20 mM Tris/HCl, pH 8.0, 1 mM EDTA, 0.2 mM EGTA, 1.25 mM 2-β-mercaptoethanol, 50 mM benzamidine, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 4 μg/ml aprotinin, and 2 mM phenylmethylsulphonyl fluoride). Cells were homogenized and immediately assayed for PDE activity using 1 μM cAMP as substrate, as previously described (Naro et al., 1999). Alternatively, subcellular fractions prepared as described above were assayed in the same conditions. In some experiments, rolipram (4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone; Calbiochem, La Jolla, CA), a specific inhibitor of the type 4 cAMP-PDEs (Schwabe et al., 1976), was added to the incubation mixture at a final concentration of 10 μM.
RESULTS
PDE4 Activity Is Essential for Low Serum- and IGF-I–induced Myogenesis
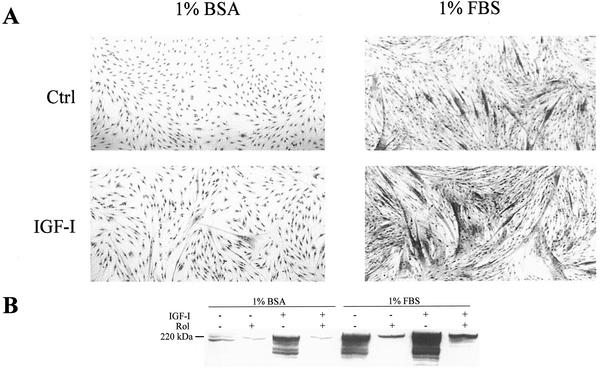
An established way to induce differentiation of myogenic cells is to shift myoblasts from the serum-rich medium (10% FBS) that allows their proliferation, to a low-serum medium (1% FBS). In these conditions, we observed a significant level of differentiation of L6-C5 cells after 6 d of culture, as evaluated by morphological examination (Figure 1A), assessment of the differentiation marker myosin heavy chain by immunoblotting (Figure 1B), and measurement of CK activity (Table 1). Supplementation of 1% FBS-containing medium with 10−9 M IGF-I led to further stimulation of cell fusion with the formation of large multinucleated myotubes (Figure 1A), a strong increase in myosin expression (Figure 1B), and of CK activity (Table 1). By contrast, in the absence of serum (myoblast shifted to 1% BSA-containing medium), the cells were maintained quiescent and no differentiation was observed. In these culture conditions, IGF-I had very little effect (Table 1 and Figure 1, A and B).
Figure 1.
Effect of IGF-I on myogenic differentiation of L6-C5 cells cultured in different conditions. (A) Morphological analysis of L6-C5 cells cultured in 1% BSA- or in 1% FBS-containing medium. Photomicrographs of L6-C5 cells were taken at the 6th day of culture in the absence or presence of 1 nM IGF-I. (B) Myosin heavy-chain immunoblots of extracts of L6-C5 cells cultured for 6 d in different conditions, as indicated on the figure. IGF-I was 1 nM, and rolipram was 10 μM.
Table 1.
CK activity of L6-C5 in various culture conditions
| Culture condition | Control | 10 μM rolipram |
|---|---|---|
| 10% FBS | 3.0 ± 0.25 | 1.2 ± 0.12d |
| 1% FBS | 6.9 ± 2.25a | 2.7 ± 0.36e |
| 1% FBS + IGF-I | 11.1 ± 2.3b | 2.6 ± 0.1d |
| 1% BSA | 0.12 ± 0.032 | 0.1 ± 0.004d |
| 1% BSA + IGF-I | 0.25 ± 0.007c | 0.1 ± 0.002d |
L6-C5 myoblasts were cultured as indicated, in the presence or absence of 1 nM IGFI, and with or without 10 μM rolipram addition. After 6 d of culture, the CK activity in mOD/min/μg protein was measured. Results are expressed as the mean of three independent experiments ± SEM.
10% FBS vs. 1% FBS p ≤ 0.05.
10% FBS vs. 1% FBS+IGF-I p < 0.01.
1% BSA vs. 1% BSA+IGF-I p < 0.001.
Control vs. rolipram p < 0.001.
Control vs. rolipram p < 0.05.
Addition of the selective type 4 PDE inhibitor rolipram to the culture medium efficiently blocked myogenic differentiation (Table 1 and Figure 1B) in all conditions tested, suggesting that PDE4 activity is essential for L6-C5 differentiation regardless of induction conditions. The sensitivity to rolipram is not a distinctive feature of L6-C5 clone, because rolipram could as well inhibit the differentiation of the widely used L6-E9 clone, achieved in various conditions.
L6-C5 Cells Cultured in the Presence of Serum Display a Higher cAMP-PDE Activity than Cells Cultured in Serum-free Medium
As shown above, cells cultured in low serum medium achieved a much higher degree of differentiation compared with cells cultured in the absence of serum. If PDE4 activity is required for differentiation, this difference in behavior could be related to a different level of PDE4 activity in cells undergoing differentiation compared with quiescent cells.
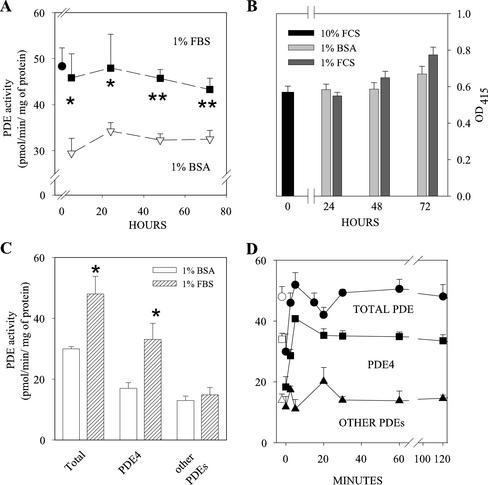
To verify this hypothesis, we compared the PDE activity of cells cultured with 1% serum and 1% BSA at different times. As early as 5 h after medium change, cells cultured with low serum displayed a 50% higher PDE activity, and the difference was still appreciable after 72 h of culture (Figure 2A). The difference in PDE activity could be attributed mainly to PDE4, because no change of rolipram insensitive activity (other PDEs in Figure 2C) was observed, whereas the rolipram-inhibited fraction of activity (representing the PDE4 component) was 95% higher in serum-cultured cells (Figure 2C). The lower PDE4 activity of cells maintained in BSA medium could not be ascribed to cell damage induced by serum deprivation, because the number of living cells was independent of the culture medium used, even after 72 h of culture (Figure 2B). In addition, when cells kept for 24 h in 1% BSA medium were shifted to 1% FBS medium, their PDE4 activity rapidly increased to reach, in 5 min, a level similar to that of cells kept for the same time in 1% FBS medium, demonstrating that alterations in PDE activity induced by medium changes are rapidly reversible (Figure 2D).
Figure 2.
PDE4 activity is higher in L6-C5 cells cultured in 1% FBS than 1% BSA medium. (A) Serum deprivation induces a long-lasting depression of PDE4 activity. L6-C5 cells were cultured for 24 h in 10% FBS medium (●), and shifted to 1% BSA (▿) or 1% FBS medium (▪). Samples were harvested at the indicated times, and total PDE activity was measured. (B) Time course of viability of L6-C5 cells cultured in different conditions. After 24 h in 10% FBS cells were shifted to 1% FBS or to 1% BSA. Viability was assessed by measuring the reduction of WS-1 tetrazolium salt added directly in the culture medium by means of 410-nm absorption. (C) Effects of culture conditions on cAMP-PDE activity of L6-C5 cells. L6-C5 cells were cultured for 24 h in 1% BSA or 1% FBS medium, harvested, and immediately homogenized and assayed for cAMP-PDE activity. Total PDE activity (Total), PDE4 activity (fraction of the activity sensitive to rolipram inhibition), and rolipram-insensitive PDE activity (Other PDEs) were evaluated as described in MATERIALS AND METHODS. Data are the mean ± SEM for n = 9 samples from three different experiments (A and B) and n = 6 samples from three different experiments (C). Statistical significance of the data was evaluated by ANOVA. *Significantly different from PDE activity in 1% BSA medium, p < 0.05; **p < 0.01. (D) Time course of PDE activity changes in L6-C5 cultured in 1% BSA and shifted to 1% FBS medium. L6-C5 cells were cultured for 24 h in 1% BSA medium and, after washing, treated with 1% FBS for the indicated times, harvested, and immediately homogenized and assayed for cAMP-PDE activity. Total PDE activity (Total), PDE4 activity (fraction of the activity sensitive to rolipram inhibition), and rolipram-insensitive PDE activity (Other PDEs) were evaluated as described in MATERIALS AND METHODS. Data are the mean ± SEM for n = 3–6 samples from one experiment representative of three performed. The empty symbols show the PDE activities of cells maintained for 24 h in 1% FBS medium.
It can be concluded that factors present in serum allow the maintenance of an elevated PDE4 activity in these cells, which in turn conceivably allows the myoblasts to differentiate, thus unmasking the positive effects of IGF-I on differentiation. Such effects would not develop in the sole presence of BSA, because, as we previously reported (Naro et al., 1999), IGF-I by itself neither influences PDE activity nor the cAMP pathway.
In the Presence of Serum, a Higher Proportion of Endogenous PDE4D3 Phosphodiesterase Is Found in a Phosphorylated Form Localized to a Triton-insoluble Cell Compartment
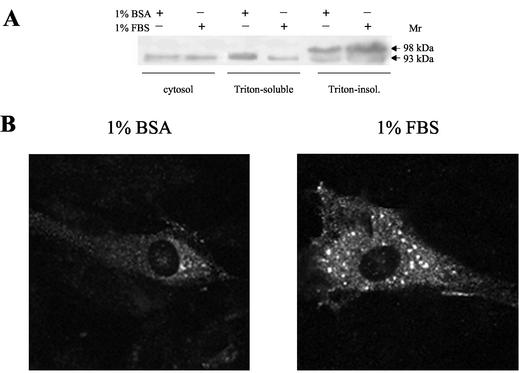
As an approach to elucidating the mechanism underlying the difference in PDE4 activity of cells grown in FBS or BSA medium, extracts of L6-C5 cells cultured in either condition for 24 h were immunoblotted with a PDE4D-specific antibody. Because it has been reported that PDE4D3 is in part bound to the particulate fraction (Jin et al., 1998), we investigated immunoreactive PDE4D3 in both the Triton X-100–extractable and nonextractable particulate cell fractions (Figure 3A). In the 1% Triton X-100–soluble membrane-bound fractions, we observed a single protein band of 93 kDa, typical of nonphosphorylated PDE4D3. The 93 kDa protein amount was markedly lower in cells cultured in the presence of FBS, compared with BSA-cultured cells. By contrast, the Triton X-100–insoluble fractions showed the presence of an additional slower-migrating band at ∼98 kDa, consistent with migration retardation typically associated with PDE4D3 phosphorylation (Oki et al., 2000). The intensity of this band was increased in FBS-cultured cells compared with BSA-cultured ones, suggesting that, in parallel with inducing PDE4D3 phosphorylation, serum induced a translocation of the enzyme from a Triton X-100–soluble to a Triton X-100–insoluble cell compartment.
Figure 3.
Effects of culture conditions on PDE4D3 localization and migration. (A) L6-C5 cells were cultured for 24 h in 1% BSA or 1% FBS. After harvesting the cells, soluble, Triton-X-100–soluble and –insoluble fractions were prepared as described in MATERIALS AND METHODS and submitted to SDS-PAGE. Approximately 50 μg of protein were loaded in each lane. PDE4D3 was detected by using the M3S1 antibody. A representative experiment of the four performed is reported. (B) L6-C5 myoblasts were transiently transfected with pCMV5-PDE4D3 plasmid, cultured, and treated as described in MATERIALS AND METHODS. Immunofluorescence was performed after 24 h of culture in DMEM containing 1% BSA or 1% FBS as indicated, using M3S1 as first antibody.
Serum might thus insure a higher PDE4 activity in L6 cells by promoting phosphorylation of the endogenous PDE4D3 isoform likely on serine 54. Phosphorylation of Ser54 of PDE4D3 is known to stimulate the activity of this enzyme (Sette and Conti, 1996).
PDE activity was then assessed in the above-considered cell fractions. When L6-C5 cells were kept in 1% FBS medium, the specific activity of PDE was significantly higher in the sole Triton-insoluble fraction, compared with the corresponding fraction from BSA-cultured cells (by 2.8-fold, Table 2). Furthermore, a significantly higher percentage of total activity was found in this fraction in FBS-cultured cells (13.6 vs. 3.6% in BSA-cultured cells, Table 2). These data are thus in good agreement with our hypothesis on the influence of culture medium on PDE4D3 activity and localization.
Table 2.
Influence of culture conditions on the distribution of PDE activity between different L6-C5 cell fractions
| Culture condition | Cell fractions | Specific activity (pmol/min/mg protein) | Percent of retrieved activity |
|---|---|---|---|
| 1% BSA | Total homogenate | 31.53 ± 0.24 | |
| Cytosolic fraction | 41.46 ± 3.15 | 80.63 ± 1.98 | |
| Triton-soluble fraction | 28.47 ± 5.67 | 15.60 ± 1.40 | |
| Triton-insoluble fraction | 19.62 ± 2.61 | 4.10 ± 0.84 | |
| 1% FCS | Total homogenate | 49.35 ± 7.17NS | |
| Cytosolic fraction | 54.36 ± 20.07NS | 63.67 ± 9.36NS | |
| Triton-soluble fraction | 37.17 ± 7.38NS | 25.87 ± 8.08NS | |
| Triton-insoluble fraction | 54.81 ± 19.47a | 10.43 ± 2.18b |
L6-C5 cells were cultured for 5 h in 1% BSA- or 1% FBS-medium, homogenized, and fractionated as described in MATERIALS AND METHODS. PDE activity was immediately assayed in the fractions. The results are the means ± SEM of three independent experiments assayed in triplicate.
NS, not statistically different from the corresponding fraction from BSA cells.
Different from Triton-insoluble fraction from BSA cells, p < 0.05 by ANOVA.
Different from Triton-insoluble fraction from BSA cells, p < 0.05 by paired t-test.
To delineate the effects of culture conditions on subcellular localization of PDE4D3, L6-C5 cells overexpressing the enzyme were cultured in 1% BSA or 1% FBS medium, with or without IGF-I, labeled with a PDE4D-specific antibody, and observed by confocal immunofluorescence microscopy (Figure 3B). Interestingly, in addition to a faint diffuse cytosolic background, PDE4D3 staining followed a punctate pattern, suggestive of localization to a vesicular intracellular compartment. Plasma membranes were totally devoid of staining (Figure 3B). Attempts to colocalize the PDE4D3 staining with either mitochondria, Golgi, or nuclei markers conclusively allowed to exclude these structures as the PDE4D3 accumulating compartment. In agreement with experiments showing that culture medium influenced the localization of PDE4D3, compared with serum-free conditions, culture with serum clearly promoted an increase in the accumulation of the protein in the intracellular vesicles, these structures becoming heavily stained (Figure 3B). In contrast, IGF-I treatment had no marked effect on the staining and distribution of PDE4D3, compared with FBS alone.
IGF-I Promotes Differentiation in PDE4D3-transfected L6-C5 Cells in the Absence of Serum
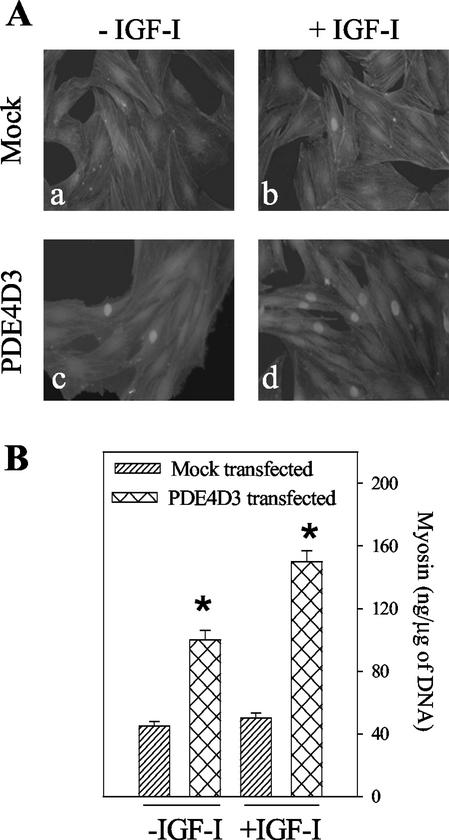
To confirm whether an increase in PDE4 activity can potentiate the effect of IGF-I, the PDE4D3 isoform was overexpressed by transient transfection in L6-C5 myoblasts, and we examined the consequences on myogenic differentiation. Expression of both myogenin (a muscle-specific transcription factor whose nuclear accumulation represents an early marker of myogenic differentiation) and myosin (a marker of terminal myogenic differentiation) was evaluated. As shown in Figure 4A (c vs. a), cells transfected with PDE4D3 and further cultivated for 48 h in the absence of serum showed a significant increase of the number of myogenin positive nuclei compared with mock-transfected cells in basal conditions. This increase was clearly potentiated by IGF-I treatment (during the last 24 h), which resulted in a much higher IGF-I–induced nuclear myogenin accumulation in PDE4D3-transfected cells than in mock-transfected cells (Figure 4A, d vs. b). As a control, the effect of rolipram addition to the culture medium was evaluated: PDE4 inhibition totally prevented IGF-I– and PDE4D3-induced myogenin nuclear accumulation. When transfected L6-C5 cells were further cultured for up to 6 d, a significant increase in myotube formation and expression of myosin (Figure 4B) was evidenced, compared with mock-transfected cells. IGF-I treatment potentiated the PDE4D3-induced expression of the differentiation marker.
Figure 4.
Differentiation of L6-C5 cells transfected with PDE4D3. L6-C5 myoblasts were transiently transfected either with pCMV5 expression vector containing rat PDE4D3 cDNA (Sette et al., 1994) or the empty vector (mock) and plated in 10% FBS-containing medium. After 24 h, the medium was replaced by medium containing 1% BSA with or without the addition of 1 nM IGF-I. (A) Morphological examination and immunofluorescence analysis of myogenin in transfected L6-C5 cells. Mock-transfected cells (a and b) and pCMV5-PDE4D3-transfected cells (c and d) were left untreated (a and c) or were treated with 1 nM IGF-I for 48 h (b and d). Cell morphology was visualized by staining of actin filaments with rhodamin-labeled phalloidin; no signs of toxicity were evidenced either in mock or pCMV5-PDE4D3–transfected cells. Myogenin was detected by using the antimyogenin antibody F5D and fluorescein-conjugated secondary antibody. Fluorescence was examined at 510 nm, optimal for fluorescein detection, which allowed sufficient detection of the intense rhodamin fluorescence. (B) ELISA quantification of myosin expression in transfected L6-C5 cells. Mock-transfected and pCMV5-PDE4D3–transfected cells were left untreated (−IGF-I) or were treated with 1 nM IGF-I (+IGF-I) for 6 d. *Significantly different from myosin content in mock-transfected cells, p < 0.001.
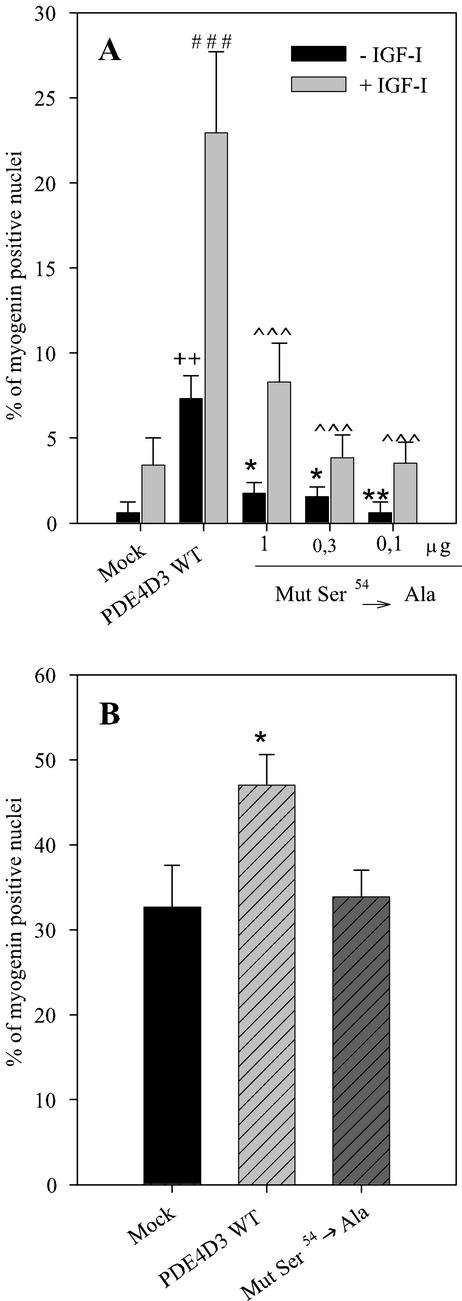
To further verify our hypothesis involving the phosphorylation of PDE4D3 in the control of myogenesis, we compared the efficiencies at stimulating myogenin nuclear import of the transfection of both wild-type PDE4D3 and ser54→ala-PDE4D3 mutant, which lacks the serine residue target of activating phosphorylation (Sette and Conti, 1996). In cells cultured as stated in Figure 4, it clearly appeared that ser54→ala-PDE4D3 induced much less effect on myogenin nuclear accumulation than wild-type enzyme, both in the absence and in the presence of IGF-I (Figure 5A), although the increases in PDE activity induced by transfection of both forms were very similar (see legend of Figure 5). This strongly argues in favor of a role of PDE4D3 phosphorylation in the myogenic response involving this enzyme. When cells were cultured in the presence of 1% FBS after transfection (Figure 5B), serum itself had a marked effect on myogenin nuclear accumulation (as evidenced by comparison of control cells in Figure 5, A and B), which can likely be attributed to its ability to induce phosphorylation and activation of endogenous PDE4D3. Transfection of wild-type PDE4D3 significantly further increased nuclear myogenin. By contrast, overexpression of ser54→ala mutant had no influence on myogenic response to serum, showing that serum effect required PDE4D3 phosphorylation.
Figure 5.
Involvement of PDE4D3 phosphorylation in the myogenic response induced by overexpression of the enzyme. L6-C5 myoblasts were transiently transfected either with 1 μg per dish of pCMV5 containing wild-type PDE4D3 cDNA (Sette et al., 1994) or various amounts of pCMV5 containing mutant ser54ala-PDE4D3 cDNA (Sette and Conti, 1996) or with the empty vector (mock), and plated in 10% FBS-containing medium. (A) After 24 h, the medium was replaced by medium containing 1% BSA with or without the addition of 1 nM IGF-I. Myogenin was detected by immunofluorescence, by using the antimyogenin antibody F5D. The results are expressed as percentages of myogenin-positive nuclei (the total number of nuclei was evaluated by UV microscopy using Hoechst 33342 nuclear dye). Normalization of the efficiency of transfection was obtained by cotransfecting a GFP-carrying plasmid. Results are the mean ± SEM of four independent experiments in which 10 fields were counted. ++p < 0.02 compared with Mock-transfected cells −IGF; *p < 0.05 compared with wt-PDE4D3–transfected cells − IGF; **p < 0.02 compared with wt-PDE4D3–transfected cells −IGF; ###p < 0.0001 compared with Mock-transfected cells +IGF; ∧∧∧p < 0.0001 compared with wt-PDE4D3–transfected cells +IGF. The specific activities of PDE in the homogenates of cells transfected with empty, 1 μg wild-type-PDE4D3-, 1 μg, 0.3 μg, 0.1 μg ser54ala mutant-containing plasmid DNA were, respectively, of 25.2 ± 0.6, 114.8 ± 4.6, 141.4 ± 6.2, 35.4 ± 4.6, 16.6 ± 1.6 pmol·min−1·mg proteins−1. (B) Twenty-four hours after plating in 10% FBS medium, the transfected cells were shifted to 1% FBS medium. Myogenin nuclear accumulation was quantified as above. Results are the mean ±. SEM of three independent experiments in which 10 fields were counted. *p < 0.05 compared with Mock-transfected cells.
L6 Cells Overexpressing a GFP-PDE4D3 Fusion Protein Are Efficiently Driven to Differentiate by IGF-I Treatment
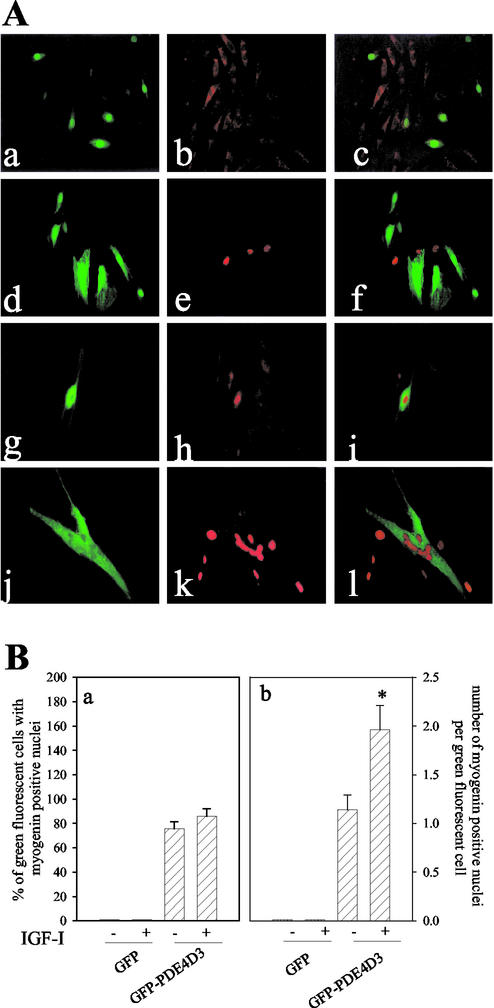
To examine at the level of individual cells if overexpression of PDE4D3 is necessary for enabling the IGF-I differentiating effects, L6-C5 cells were transfected with a GFP-PDE4D3 construct, or with a GFP construct as a control, and kept in absence of serum for 24 h. The chimeric GFP-PDE4D3 protein retains the activity of the native PDE4D3 enzyme because its expression increased by 2.7 ± 0.2-fold the PDE4 activity of transfected cell extracts. As shown in Figure 6A, cells expressing GFP (a) displayed a cytoplasmic, not nuclear, diffuse myogenin immunofluorescence (b and c). In the presence of IGF-I, GFP-transfected cells showed rare and scattered myogenin-positive nuclei without any superposition with GFP expression (d–f). In cells transfected with the GFP-PDE4D3 construct, the fusion protein was efficiently expressed in part of the myoblasts, where intracellular green fluorescence could be visualized (Figure 6A, g). In control preparations (which were not stimulated by IGF-I), myogenin was localized in the cytoplasm of cells expressing no GFP-PDE4D3 (green fluorescence negative), whereas myogenin accumulated in the nuclei exclusively in the GFP-PDE4D3–overexpressing cells (h and i). This result strongly supports the hypothesis that an increase of PDE4 activity is effective by itself to translocate myogenin into the nucleus. Cells overexpressing GFP-PDE4D3 were challenged with IGF-I and appeared to fuse into large myotubes (Figure 6A, j), a differentiation level that was not achieved in the other conditions used. The PDE-overexpressing myotubes contained clusters of myogenin-positive nuclei (Figure 6A, l), reemphasizing the correlation between myogenin nuclear import and terminal myogenic differentiation. Interestingly, in the same cell cultures it could be noticed that IGF-I promoted myogenin accumulation in the nuclei of some cells that did not express GFP-PDE4D3, but these cells appeared to be mononucleated (the nuclei were scattered). Similar results were obtained with L6-E9 cells.
Figure 6.
Myogenin nuclear accumulation in L6-C5 cells overexpressing GFP-PDE4D3. (A) Analysis of GFP expression and myogenin localization by immunofluorescence in transfected L6-C5 cells. L6-C5 myoblasts were transiently transfected with either GFP-PDE4D3 or the GFP vector and plated in 10% FBS-containing medium. After 24 h, the medium was replaced by medium containing 1% BSA with or without the addition of 1 nM IGF-I. GFP-transfected cells (a and d) were left untreated (a) or were treated with 1 nM IGF-I for 24 h (d); myogenin was detected in control cells (b) or IGF-I–treated cells (e) by using the antimyogenin antibody F5D; the GFP and myogenin fluorescences were merged (c and f). GFP-PDE4D3–transfected cells (g and j) were left untreated (g) or were treated with 1 nM IGF-I for 24 h (j); myogenin was detected in control cells (h) or IGF-I–treated cells (k) and the GFP and myogenin fluorescences were merged (i and l). (B) Quantification of myogenin positive nuclei in GFP- and GFP-PDE4D3–expressing L6-C5 cells. The results of the above experiment were quantified by evaluating the percentage of GFP- or GFP-PDE4D3–expressing cells (“green fluorescent cells”) containing myogenin-positive nuclei (a), and the average number of myogenin-positive nuclei per “green fluorescent cell” (b). Results are the mean ± SEM of 10 fields separately examined in three independent experiments. *p < 0.01, GFP-PDE4D3–transfected cells −IGF-I compared with GFP-PDE4D3–transfected cells +IGF-I.
Quantification of the results of the above experiments is shown in Figure 6B. As it appears in panel a, IGF-I did not influence the proportion of GFP or GFP-PDE4D3–expressing cells that contained one or more myogenin-positive nuclei, whereas this proportion was much higher in GFP-PDE4D3–expressing cells compared with GFP-expressing cells. Panel b shows that IGF-I significantly increased the number of myogenin positive nuclei per cell, only in cells overexpressing GFP-PDE4D3. It thus clearly appeared that overexpression of PDE4D3 activity allowed myogenin accumulation in the cell nuclei and that IGF-I treatment further stimulated differentiation in the PDE4D3-overexpressing cells by inducing cell fusion and formation of multinucleated myotubes.
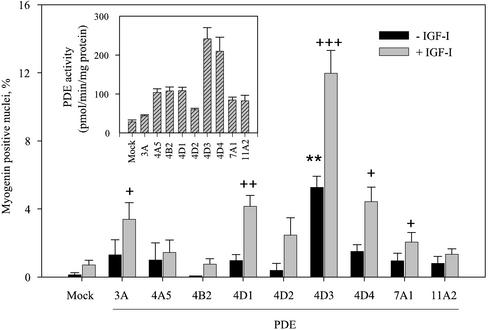
PDE Isoforms Promote Myogenin Nuclear Accumulation with Different Efficiencies
With regard to the multiplicity of isoforms of PDE expressed in mammalian cells the question arose of the specificity of the PDE4D3 isoform as a factor promoting IGF-I effects. To address this point various phosphodiesterase isoforms belonging either to the type 4 PDE family (PDE4D1 and PDE4D2, which are short splicing variants deriving from the expression of gene PDE4D, which also gives rise to the long variants PDE4D3 and PDE4D4; PDE4A5 and PDE4B2 expressed from other type 4 PDE genes) or to other PDE families (PDE3A, PDE7A1, PDE11A2) were ectopically expressed in L6-C5 cells, and nuclear myogenin accumulation in the presence or absence of IGF-I treatment was evaluated. As shown in Figure 7, several PDE isoforms (namely, PDE3A, PDE4D1, PDE4D3, PDE4D4, PDE7A1) were able to promote myogenin nuclear accumulation in the presence of IGF-I, although with markedly different efficiencies. PDE4D3 was the only active isoform in the absence of IGF-I, and the most effective in the presence of this growth factor. PDE4D4, although it increased cAMP-PDE activity of L6-C5 cells similarly to PDE4D3 (Figure 6, inset) had much less effect (approximately threefold) on myogenin nuclear import. Transfection with other isoforms increased cAMP-PDE activity to variable extents (Figure 7, inset), without a correlation between the levels of cAMP-PDE activity and the effects on myogenin labeling: e.g., PDE3A transfection, which induced a very modest increase in cAMP-PDE activity, significantly promoted myogenin accumulation, whereas PDE4A5 or PDE4B2 transfections, which markedly elevated cAMP-PDE activity (by approximately fourfold), had no effect on the proportion of myogenin labeled nuclei.
Figure 7.
Effects of the overexpression of various PDE isoforms on myogenin nuclear accumulation in L6-C5 cells. L6-C5 myoblasts were transiently transfected by different PDE-carrying plasmids or with empty vector (mock) and plated in 10% FBS-containing medium. After 24 h, the medium was replaced by medium containing 1% BSA with or without the addition of 1 nM IGF-I. Myogenin was detected by immunofluorescence, by using the antimyogenin antibody F5D. The results are expressed as percentages of myogenin-positive nuclei (the total number of nuclei was evaluated by UV microscopy using Hoechst 33342 nuclear dye). Normalization of the efficiency of transfection was obtained by cotransfecting a GFP-carrying plasmid. Results are the mean ± SEM of 10 fields counted in a typical experiment. Significantly different from mock-transfected cells cultured without IGF-I: **p < 0.001. Significantly different from mock-transfected cells cultured with IGF-I: +++p < 0.001; ++p < 0.01; +p < 0.05. Overexpression efficiency of the different PDE isoforms was checked by measuring PDE activity in transfected cell homogenates, and normalizing as above. The means ± SEM of 4–5 experiments is shown (inset).
DISCUSSION
In this study, we demonstrate that cAMP-PDE activity is essential for the full expression of the IGF-I capability to induce a differentiated phenotype in L6-C5 cells. L6-C5 cells differentiate when serum concentration in the culture medium is reduced (e.g., to 1% FBS) and respond, in these conditions, to IGF-I treatment by an enhanced differentiation, a property shared with other L6 clones (Florini et al., 1991b). Rolipram, a specific PDE4 inhibitor, inhibited myogenic differentiation of both L6-C5 and L6-E9 clones, either in the absence or presence of IGF-I, showing that PDE4 activity is involved in this process.
L6 cells require the presence of a low serum concentration or other factors in order to start the differentiation program because cells cultured in serum-free medium do not fuse or very poorly fuse into myotubes (Engert et al., 1996; Coolican et al., 1997; Kaliman et al., 1998). Furthermore, in serum-free medium, IGF-I is a weak inducer of L6-C5 cell differentiation. We observed that L6-C5 cells cultured with 1% serum have a higher PDE activity than cells cultured in 1% BSA. The difference in total cAMP-PDE activity was entirely attributable to a difference in type 4, rolipram-inhibitable PDE activity (Figure 2). Because we previously showed that the major form of PDE4 expressed in L6-C5 is PDE4D3, that its expression is not modulated during cell differentiation, and that no other PDE4 isoforms are expressed in the course of myoblast differentiation into myotubes (Naro et al., 1999), it is likely that serum maintains PDE4D3 under an activated form and that this active PDE4D3 is a determining factor allowing the progression of differentiation and the expression of the positive effects of IGF-I on this process.
The mechanism by which serum ensures the maintenance of an elevated PDE4D3 activity seems to involve the phosphorylation of the enzyme, as reflected by migration retardation in immunoblotting experiments (Figure 3A). The time course of the reversion of cAMP-PDE activity loss, observed after serum restitution to serum-deprived cells (Figure 2D), denotes a rapid phenomenon. PDE activity reached a maximum in 5 min, compatible with the kinetics of PDE4D3 phosphorylation and activation, as it has been, for example, described in thyrotropin-treated FRTL5 cells (Oki et al., 2000). Another effect of serum presence or absence in the culture medium was to influence the subcellular localization of PDED3. Immunoblotting experiments, in agreement with PDE activity measurement, showed that in the presence of serum PDE4D3 was accumulated into a different subcellular compartment, characterized by resistance to Triton X-100 extraction. This still unidentified compartment, as evidenced by immunofluorescence labeling of PDE4D, followed a punctate pattern in confocal microscopy. The serum-promoted changes in PDE4D3 localization could take part in the mechanism of enzyme activation or alternatively be a mere consequence of enzyme phosphorylation.
Myoblast requirement for elevated PDE4D3 activity in order to undergo differentiation was further investigated by overexpressing PDE4D3 in transiently transfected L6-C5 cells cultured in serum-free medium. As shown in Figure 4, PDE4D3-transfected cells displayed increased myogenin nuclear localization in the absence of exogenous stimuli. The localization of the muscle-specific transcription factor in the nucleus probably allowed the cells to start the differentiation program, inasmuch as a significant increase of myosin expression was seen in PDE4D3-transfected cells compared with mock-transfected cells, after 6 d of culture in serum free-medium. In agreement with the hypothesis that PDE4D3 exerts a permissive role on the induction of differentiation by IGF-I, we observed that IGF-I was much more efficient in inducing myogenin nuclear accumulation and myosin expression in PDE4D3-transfected L6-C5 cells than in mock-transfected cells. The involvement of phosphorylation-mediated PDE4D3 activation in the induction of the myogenic process was shown by the loss of myogenic effect of ectopic PDE4D3 expression when the site of PKA-dependent phosphorylation on the enzyme was suppressed by mutation (Figure 5). With such a mutant, the positive effect of serum on myogenesis was not enhanced by PDE ovexpression in contrast with what observed when using the wild-type form, which comes in confirmation of the hypothesis that serum exerts its effects through phosphorylation of PDE4D3.
The conclusion that elevated PDE4D3 activity is required for myogenic differentiation of L6-C5 and L6-E9 myoblasts was further supported at the single-cell level by means of expression of a GFP-PDE4D3 fusion protein. Considering that GFP-PDE4D3-transfection increased the PDE4 activity of cell cultures, we can infer that the cells overexpressing the fusion protein have a higher PDE4 activity than the others, and we could more accurately evaluate the effects of elevated PDE activity in this cell subset. It is shown, in Figure 6A, that in mock-transfected cells (transfected with the GFP construct) cultured in the absence of serum, myogenin was exclusively localized in the cytoplasm, and IGF-I treatment induced its translocation to the nucleus in a limited number of cells, without any correlation with the expression of the GFP protein. After transfection with the GFP-PDE4D3 construct, in a large portion of cells overexpressing the fusion protein, myogenin was localized inside the nucleus, whereas myogenin remained in the cytoplasm in all the cells not expressing the fusion protein. This result shows that an increase of PDE4D3 expression and, as a consequence, an increase of PDE4 activity, is sufficient to cause myogenin translocation to the nucleus. This can be viewed as the PDE4D3-operated opening of a cAMP-dependent gate that keeps myogenin trapped in the cytoplasm in the absence of specific differentiative signals. We have previously established that PDE4 inhibition, through elevation of cAMP levels, totally suppresses myogenin nuclear import without affecting the synthesis of this factor (Naro et al., 1999). Phosphorylation of myogenin by PKA has been observed both in cell free systems and in myogenin- and PKA-overexpressing COS cells (Li et al., 1992), but the consequences on myogenin function were not clear. The involvement of cAMP-induced phosphorylation of myogenin, or of an interacting protein, in controlling its translocation process in myogenic cells is a hypothesis that deserves further investigation. We already showed that AVP can increase PDE4D3 activity, induce myogenin localization in the nucleus, and promote terminal differentiation (Naro et al., 1999). The present data confirm the role of PDE4 by showing at the single-cell level that an increase of PDE4 activity is sufficient by itself to determine myogenin nuclear localization, a crucial step in the differentiation program of L6 cells. Even if this is not sufficient to induce full differentiation (as evidenced by the absence of cell fusion) in the rather short period examined, this would enable IGF-I–differentiating effects to develop. These effects could rely on the amplification of myogenin synthesis/translocation, and/or on the triggering of more downstream steps. A relationship between IGF-I stimulatory myogenic effects and myogenin expression has been established (Florini and Ewton, 1990). However, myogenin expression is clearly not sufficient for the enhanced myogenesis caused by IGF-I (Engert et al., 1996). IGF-I effects, in the GFP-PDE4D3–transfected cells, resulted in the achievement of a complete differentiated phenotype after only 24 h from the time of the peptide addition. By contrast, in the cells that did not express GFP-PDE4D3, even though in a few cases myogenin started accumulating in the nucleus under the influence of IGF-I, it appeared that the steps of fusion and myotube formation could not be reached in this span of time, possibly because endogenous cAMP is able to slow down the process of myogenin accumulation.
Interestingly, overexpression of PDE isoforms different from PDE4D3 had markedly lower effects on myoblast differentiation, as assessed by myogenin nuclear accumulation, either in the absence or presence of IGF-I (Figure 7). Although the preferential effect of PDE4D3 could be explained by the higher cAMP-PDE activity that it induces in transfected cells, there was no correlation between the increases in cAMP-PDE activity induced by the various transfected isoforms and their effects on myogenin transport. In particular, PDE4D4 transfection had little effect on differentiation, whereas it efficiently increased cAMP-PDE activity of myoblasts. We can thus speculate that subcellular localization of the PDE4D isoforms is a determining factor in the ability of these PDEs to promote myogenesis. Indeed, PDE4D3 is largely present in the particulate fraction (Jin et al., 1998). Its interaction with the newly described protein myomegalin might anchor it to the Golgi/centrosomal region of nonmuscle cells and sarcomeric structures of skeletal muscle (Verde et al., 2001). Furthermore, it has been recently demonstrated that PDE4D3 is assembled together with protein kinase A through binding to the anchoring protein mAKAP in the perinuclear region of hyperthrophic rat neonatal ventriculocytes (Dodge et al., 2001). In L6-C5 cells, PDE4D3 was clearly distributed in punctate structures, evocative of a vesicular compartment. The localization to discrete subcellular structures might thus confer to the PDE4D3 isoform a preferential ability to control cAMP signaling in a compartment involved in myogenin translocation. Instead, PDE4D1 and PDE4D2 are predominantly found in the cytosolic fraction (Bolger et al., 1997; Iona et al., 1998; Jin et al., 1998). Furthermore, PDE4D4 is present in a compartment different from PDE4D3, because it is not extracted in conditions that allow PDE4D3 solubilization, and seems to be associated with cytoskeletal structures (Jin et al., 1998). Another type 4 PDE isoform such as PDE4A5 had no significant influence on myogenin transport (Figure 7). This result underlines the variety in functional roles of the multiple type 4 PDE isoforms. Although they share important sequence homologies, they strongly diverge in their N-terminal extremities, which seems to confer to each isoform specific intracellular targeting and function. As an example, PDE4A5, unlike PDE4D3, interacts with certain SH3 domain-containing proteins, and this might be involved in the recently reported PDE4A5 redistribution accompanying apoptosis and possibly in the protective effects of this isoform against cell death (Huston et al., 2000).
On the whole, these studies suggest that the cAMP-phosphodiesterase PDE4D3 plays a critical role in myogenic differentiation of L6 cells by controlling myogenin transcriptional activity through its subcellular localization. In these cells, myogenin is the main factor responsible for differentiation and maintenance of the terminally differentiated myotube phenotype, whereas other MRFs seem to play minor roles (Florini et al., 1991a). In adult skeletal muscle as well, myogenin is the only MRF expressed in slow fibers (Hughes et al., 1993; Voytik et al., 1993), and PDE4D3 similarly might be essential to ensure its effects on the maintenance of a terminally differentiated state. In agreement with this hypothesis, PDE4D3 has been shown to be expressed in adult rat skeletal muscle (our unpublished results).
Furthermore, the fact that PDE4D3 activity allows the expression of IGF-I myogenic effects in L6 cells might have a physiological significance. Because IGF-I has a major function in preserving muscle mass and performance in the adult, as demonstrated by localized expression of IGF-I transgene in mouse (Coleman et al., 1995; Musaro et al., 2001), PDE4D3 might be involved in the myocyte hypertrophic response to IGF-I. Interestingly, it has been noticed that PDE4D null mice have a decreased body weight and, in particular, a lowered muscle mass, in parallel with reduced circulating IGF-I levels (Jin et al., 1999). These observations highlight the integration between PDE4D activity and the IGF-I pathway during muscle development.
ACKNOWLEDGMENTS
We thank Prof. Joseph Beavo for kindly sharing the PDE7A1 and PDE11A2 plasmids, Dr. Antonio Musarò for the gift of L6-E9 clone, and Dr. Claudio Sette for helpful discussions. The exchanges between the collaborating institutions were supported by a Consiglio Nazionale delle Ricerche–Institut National de la Santé et de la Recherche Médicale joint program grant (to G.N. and S.A.). The research was supported by grants from MIUR (Ministero Istruzione Università Ricerca) to S.A. and M.M., and from P.F. 83/2001 (Ministero della Salute) to M.M.
Abbreviations used:
- AVP
Arg8-vasopressin
- CK
creatine kinase
- FBS
fetal bovine serum
- IGF-I
insulin like growth factor 1
- PDE
cAMP-phosphodiesterase
- PKA
protein kinase A
- GFP
green fluorescent protein
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0156. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0156.
REFERENCES
- Bader D, Masaki T, Fishman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger GB, Erdogan S, Jones RE, Loughney K, Scotland G, Hoffmann R, Wilkinson I, Farrell C, Houslay MD. Characterization of five different proteins produced by alternatively spliced mRNAs from the human cAMP-specific phosphodiesterase PDE4D gene. Biochem J. 1997;328:539–548. doi: 10.1042/bj3280539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson DG, Olson EN. Helix-loop-helix proteins as regulators of muscle-specific transcription. J Biol Chem. 1993;268:755–758. [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ. Highly specific inhibition of IGF-1 stimulated differentiation by an antisense oligodeoxyribonucleotide to myogenin mRNA. No effects on other actions of IGF-1. J Biol Chem. 1990;265:13435–13437. [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Roof SL. Insulin-like growth factor-1 stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol Endocrinol. 1991a;5:718–724. doi: 10.1210/mend-5-5-718. [DOI] [PubMed] [Google Scholar]
- Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem. 1991b;266:15917–15923. [PubMed] [Google Scholar]
- Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Huston E, Beard M, McCallum F, Pyne NJ, Vandenabeele P, Scotland G, Houslay MD. The cAMP-specific phosphodiesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3. Consequences for altered intracellular distribution. J Biol Chem. 2000;275:28063–28074. doi: 10.1074/jbc.M906144199. [DOI] [PubMed] [Google Scholar]
- Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M. Characterization of the rolipram-sensitive, cAMP specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Aoki M, Zheng JZ, Li J, Vogt PK. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SLC, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. J Biol Chem. 1998;273:19672–19678. doi: 10.1074/jbc.273.31.19672. [DOI] [PubMed] [Google Scholar]
- Jin SL, Richard FJ, Kuo WP, D'Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96:11998–12003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Shepherd PR, Beeton CA, Testar X, Palacin M, Zorzano A. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- Li L, Heller-Harrison R, Czech M, Olson EN. Cyclic AMP-dependent protein kinase activity inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol. 1992;12:4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Minotti S, Scicchitano BM, Nervi C, Scarpa S, Lucarelli M, Molinaro M, Adamo S. Vasopressin and insulin-like growth factors synergistically induce myogenesis in serum-free medium. Cell Growth Diff. 1998;9:155–163. [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978;15:855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Naro F, et al. Involvement of type 4 cAMP-phosphodiesterase in the myogenic differentiation of L6 cells. Mol Biol Cell. 1999;10:4355–4367. doi: 10.1091/mbc.10.12.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi C, Benedetti L, Minasi A, Molinaro M, Adamo S. Arginine-vasopressin induces differentiation of skeletal myogenic cells and upregulation of myogenin and myf-5. Cell Growth Diff. 1995;6:81–89. [PubMed] [Google Scholar]
- Oki N, Takahashi SI, Hidaka H, Conti M. Short term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]
- Samuel DS, Ewton DZ, Coolican SA, Petley TD, McWade FJ, Florini JR. Raf-1 activation stimulates proliferation and inhibits IGF-stimulated differentiation in L6A1 myoblasts. Horm Metab Res. 1999;31:55–64. doi: 10.1055/s-2007-978699. [DOI] [PubMed] [Google Scholar]
- Schwabe U, Miyake M, Ohga Y, Daly JW. 4-(-3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3′ 5′-mono-phosphate-phosphodiesterases in homogenates and tissue slices from rat brain. Mol Pharmacol. 1976;12:900–910. [PubMed] [Google Scholar]
- Sette C, Vicini E, Conti M. The ratPDE3/IVd phosphodiesterase gene codes for multiple proteins differentially activated by cAMP-protein kinase. J Biol Chem. 1994;269:18271–18274. [PubMed] [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Teti A, Naro F, Molinaro M, Adamo S. Transduction of the arginine-vasopressin signal in skeletal myogenic cells. Am J Physiol. 1993;265(C34):C113–C121. doi: 10.1152/ajpcell.1993.265.1.C113. [DOI] [PubMed] [Google Scholar]
- Verde I, Pahlke G, Salanova M, Zhang G, Wang S, Coletti D, Onuffer J, Jin SL, Conti M. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- Voytik SL, Przyborski M, Badylak SF, Konieczny SF. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev Dyn. 1993;198:214–224. doi: 10.1002/aja.1001980307. [DOI] [PubMed] [Google Scholar]
- Winter B, Braun T, Arnold HH. cAMP-dependent protein kinase represses myogenic differentiation and the activity of the muscle-specific helix-loop-helix transcription factors Myf-5 and MyoD. J Biol Chem. 1993;268:9869–9878. [PubMed] [Google Scholar]
- Xu Q, Wu Z. The IGF-PI3K-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in Rhabdomyosarcoma derived RD cells. J Biol Chem. 2000;275:36750–36757. doi: 10.1074/jbc.M005030200. [DOI] [PubMed] [Google Scholar]