Abstract
Several microtubule-binding proteins including EB1, dynactin, APC, and CLIP-170 localize to the plus-ends of growing microtubules. Although these proteins can bind to microtubules independently, evidence for interactions among them has led to the hypothesis of a plus-end complex. Here we clarify the interaction between EB1 and dynactin and show that EB1 binds directly to the N-terminus of the p150Glued subunit. One function of a plus-end complex may be to regulate microtubule dynamics. Overexpression of either EB1 or p150Glued in cultured cells bundles microtubules, suggesting that each may enhance microtubule stability. The morphology of these bundles, however, differs dramatically, indicating that EB1 and dynactin may act in different ways. Disruption of the dynactin complex augments the bundling effect of EB1, suggesting that dynactin may regulate the effect of EB1 on microtubules. In vitro assays were performed to elucidate the effects of EB1 and p150Glued on microtubule polymerization, and they show that p150Glued has a potent microtubule nucleation effect, whereas EB1 has a potent elongation effect. Overall microtubule dynamics may result from a balance between the individual effects of plus-end proteins. Differences in the expression and regulation of plus-end proteins in different cell types may underlie previously noted differences in microtubule dynamics.
INTRODUCTION
The microtubule network in the cell is highly dynamic. Microtubules grow and retract, continually probing the cellular environment. Several proteins including CLIP-170, APC, EB1, and dynactin have been localized to the plus-ends of growing microtubules (reviewed in Tirnauer and Bierer, 2000). Evidence for interactions among these plus-end proteins (Su et al., 1995; Berrueta et al., 1999; Valetti et al., 1999; Vaughan et al., 1999) has led to the hypothesis of a microtubule plus-end complex (Schroer, 2001). It is not clear, however, if such a complex exists in vivo and if it does, whether all plus-end proteins are obligate members of the complex. All plus-end proteins thus far identified have microtubule-binding domains and may, therefore, be independently targeted to microtubules. But interactions among plus-end proteins may also allow one protein to recruit others to the complex. Specific binding interactions among plus-end proteins and between plus-end proteins and microtubules remain unclear, as does the temporal sequence with which plus-end proteins are recruited to microtubules.
The specific functions of individual plus-end proteins as well as that of a plus-end complex also remain unclear. Plus-end proteins are perfectly positioned to regulate microtubule growth and dynamics as well as to act as sensors to mediate interactions between probing microtubules and potential targets. EB1 has been suggested to play a role in microtubule dynamics (Tirnauer et al., 1999; Rogers et al., 2002), but the precise nature of that role remains unclear. In addition, it has been suggested that EB1 may only act in concert with APC (Nakamura et al., 2001).
One potential target for probing microtubule plus-ends is the cell cortex, in particular specific cortical domains such as migrating edges or areas of contact with the other cells or the matrix. For example, interactions between microtubule plus-ends and the cell cortex are thought to be important for the proper orientation and function of the mitotic spindle (Schuyler and Pellman, 2001). During mitosis in budding yeast, Bim1, the yeast EB1 homologue is localized to the tips of astral microtubules. When growing microtubules reach the bud cortex, Bim1 interacts with the cortical protein Kar9. This interaction tethers the microtubules and is critical for correct spindle positioning and the movement of the nucleus to the bud neck (Adames and Cooper, 2000; Korinek et al., 2000; Lee et al., 2000; Miller et al., 2000). A second step involving the microtubule motor dynein and its accessory complex dynactin is thought to be involved in the subsequent movement of the nucleus into the bud neck (Carminati and Stearns, 1997; Adames and Cooper, 2000).
Although these two steps are genetically separable in yeast, there is evidence suggesting that EB1 may interact more directly with components of the dynein and/or dynactin complexes in higher eukaryotes (Berrueta et al., 1999), but the precise nature and function of this interaction was unclear. Here we have investigated the interaction between EB1 and dynein and dynactin and shown that EB1 binds directly to the p150Glued subunit of dynactin. EB1 and p150Glued each seem to play a role in microtubule dynamics and/or stability in vitro and in vivo, but these roles are distinct. EB1 appears to have a potent microtubule elongation effect, whereas p150Glued appears to have a potent microtubule nucleation effect. These data suggest that the balance between EB1 and p150Glued may regulate the state of microtubule dynamics within the cell, and regulatory differences between cell types may account for differences seen in microtubule organization and dynamics.
MATERIALS AND METHODS
Generation of EB1 and Dynactin cDNA Constructs
The EST for human EB1 (AAI285786) was obtained from ATCC and subcloned into pGEX(6p-2) (Amersham Pharmacia Biotech, Piscataway, NJ), pEGFP-N1 (Clontech, Palo Alto, CA), pDsRed2-N1 (Clontech), and pET-15b (Novagen, Madison, WI). The p150Glued and p135 human brain cDNA library clones were subcloned into pcDNA3 (Invitrogen, Carlsbad, CA; Tokito et al., 1996) and p150Glued deletion constructs pMT7-1 (aa 237-1255) and pMT1-1 (aa 345-1255) were subcloned into pBluescript SK− (Stratagene, La Jolla, CA). To make a GST-p150 fusion protein, p150 MTB-DICB (aa 8–566) from rat full-length cDNA clone (Holzbaur et al., 1991) was subcloned into pGEX-6p-1 (Pharmacia). pET21a-p150[1–330]wt was a gift from Kevin Vaughan (Vaughan et al., 2002). Other dynactin constructs were made as previously described: Arp1 (Holleran et al., 1996), p50/dynamitin (LaMonte et al., 2002), p62 (Karki et al., 2000), and p22 (Karki et al., 1998).
Antibodies
Affinity-purified rabbit polyclonal antibodies to dynactin subunits p150Glued (Tokito et al., 1996), Arp1 (Holleran et al., 1996), p62 (Karki et al., 2000), p50/dynamitin (Tokito et al., 1996), and p22 (Karki et al., 1998) were produced as previously described. An additional polyclonal antibody to p150Glued was generously provided by Kevin Vaughan (University of Notre Dame). Monoclonal antibodies to EB1 (E4620 from Transduction Laboratories), tubulin (YL1/2 from Serotec and DM1A from Sigma), p150Glued (P41920 from Transduction Laboratories, Lexington, KY), dynamitin (D74620 from Transduction Laboratories), and dynein IC (MAB1618 from Chemicon, Temecula, CA) were obtained commercially. Peroxidase-conjugated secondary antibodies were obtained from Jackson Immunochemicals (West Grove, PA) and Alexa 350–, 488–, and 594–conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR).
Dynein Purification from Rat Brain
Frozen rat brains (Pel Freez, Rogers, AZ) were dounce homogenized at a 1:1 wt/vol ratio in ice-cold PHEM with 10 mM NaCl (50 mM Na-PIPES, 50 mM Na-HEPES, 1 mM EDTA, 2 mM MgCl2, pH 6.9) containing leupeptin (10 μg/ml), pepstatin (1 μg/ml), n-tosyl-l-arginine methylester (10 μg/ml), and 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol, and the resulting homogenate was clarified by centrifugation at 39,000 × g for 20 min. The supernatant was further clarified by high-speed centrifugation (135,000 × g, 1 h) to obtain cytosol. Cytoplasmic dynein and dynactin complexes were obtained by microtubule affinity followed by ATP release essentially as described by (Paschal et al., 1991). ATP extracts containing dynein and dynactin were then dialyzed at 4°C into the appropriate buffer for use in affinity experiments.
Purification of Recombinant Proteins
GST, GST-EB1, GST-p150, and pET-EB1 His-tagged recombinant proteins were expressed in Escherichia coli strain BL21, induced with 0.4 mM isopropyl-β-d-thiogalactoside (IPTG). Cells were harvested by centrifugation and pellets were resuspended in one tenth volume phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 10 mM KH2PO4, pH 7.3) with 1 mg/ml lysozyme, lysed by freeze/thaw at −80°C, incubated with 1 mg/ml DNase and RNase, and sonicated 3 × 15 s if necessary, and lysates were clarified by centrifugation at 39,000 × g. GST-tagged protein-soluble supernatants were filtered with a 25-mm Aerodisc syringe filter of 0.45 μm (Gelman Laboratories, East Hills, NY), loaded onto glutathione Sepharose 4B beads (Amersham Pharmacia Biotech), and washed extensively with phosphate-buffered saline. Proteins were eluted with modified glutathione elution buffer (20 mM reduced glutathione, 50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, pH 8.0) and dialyzed into the appropriate buffer. When necessary the GST group was removed from recombinant EB1 and p150Glued proteins using on-column cleavage by PreScission Protease (Amersham Pharmacia Biotech) as described in the product literature. pET21a-p150[1–330]wt was expressed in Rosetta Blue (DE3) cells (Novagen) and induced with 1 mM IPTG. Cells were harvested by centrifugation and lysed using a French Pressure Cell Press. His-tagged recombinant proteins were purified on a Ni2+ affinity column. Purified proteins were eluted with imidazole and transfered to the appropriate buffer by dialysis or a PD-10 Desalting column (Amersham Pharmacia Biotech).
Affinity Chromatography
ATP extracts containing dynein and dynactin or purified recombinant protein, with 0–0.5% Triton X-100, were loaded onto the appropriate affinity matrix and incubated for 10–15 min at 25°C. The columns were washed extensively with phosphate-buffered saline, and specifically retained proteins were eluted with modified glutathione elution buffer. Fractions were collected and resolved by SDS-PAGE, transfered to Immobilon-P (Millipore, Bedford, MA), blocked using 5% nonfat milk in Tris-buffered saline, pH 8.0, 0.05% IGEPAL, and 0.05% sodium azide, and probed with the appropriate antibody detected by Renaissance Western blot chemiluminescence reagent (NEN, Boston, MA; Karki and Holzbaur, 1995).
In Vitro Transcription/Translation
The Promega TNT T7 Quick and T3 coupled transcription/translation systems (Promega, Madison, WI) were used to express dynactin constructs. TNT T7 Master Mix, [35S]methionine, and 1 μg of cDNA were incubated at 30°C for 1–1.5 h. In vitro synthesized proteins were loaded onto GST or GST-EB1 column or batch-bound beads and incubated for 10–15 min at 25°C. Columns were washed extensively with phosphate-buffered saline containing 0–0.5% Triton X-100 and eluted with glutathione elution buffer or denaturing buffer (2% SDS and 5% β-mercaptoethanol). Fractions were analyzed by SDS-PAGE and autoradiography (Holleran et al., 2001).
Cell Culture, Immunocytochemistry, and Transient Transfection
PtK2 epithelial cells or Rat2 fibroblasts were seeded onto 18 × 18-mm square or 40-mm round glass coverslips. Cells for immunocytochemistry were grown to ∼75% confluency, rapidly fixed in −20°C 100% methanol with 1 mM EGTA for 10 min, and then processed for immunocytochemistry as previously described (Karki et al., 1998). Cells for transient transfection experiments were grown to ∼35% confluency and transfected with the appropriate cDNA using the lipid-mediated transfection reagent Fugene (Roche, Indianapolis, IN). Forty-eight hours after transfection, cells were fixed and prepared for immunocytochemistry as described above. Fluorescent images were acquired with an Orca ER CCD camera (Hamamatsu, Bridgewater, NJ) controlled by OpenLab image acquisition software (Improvision, Lexington, MA).
To investigate the effects of nocodazole and cold on EB1-induced microtubule bundles, cells were either treated with nocodazole (5 μg/ml) for 30–60 min at 37°C or placed at 4°C for 60 min. Cells were then immediately fixed in cold methanol and processed for immunocytochemistry as above. To assess the effect of disruption of the dynactin complex on EB1-induced bundles, cells were transfected with both EB1 and the p50 subunit of dynactin. Fifty cells from each of three different experiments (150 cells total) were scored for the expression level of EB1 (high or low), p50 (high or low) and presence or absence of microtubule bundles.
Tubulin Purification
Tubulin was isolated from porcine brain by three cycles of assembly/disassembly, phosphocellulose ion exchange purification, and glutamate cycling and then aliquoted and stored at −80°C (Vasquez et al., 1997). No microtubule-associated proteins were detectable by Coomassie staining of heavily loaded SDS-PAGE gels. Additional bovine tubulin, porcine tubulin, rhodamine- and fluorescein-labeled tubulin were purchased from Cytoskeleton (Denver, CO).
MT Assembly Assay and MT Pelleting
Purified tubulin and recombinant proteins were thawed and clarified by centrifugation before use. Assembly assays were carried out in PHEM with 50 mM NaCl containing 1 mM MgGTP, 16 μM tubulin (1:40–1:150 rhodamine-labeled to unlabeled tubulin), 2.4 μM p150[1–330]wt and/or 2.4 μM pET-EB1 in the presence or absence of 1–100 μg of microtubule seeds in an 80-μl reaction volume. After incubation, reactions were centrifuged at 39,000 × g and gel samples were made of the supernatant and pellet fractions. Samples were analyzed by SDS-PAGE and either scanned with a Molecular Dynamics phosphorimager (Sunnyvale, CA) or analyzed with Coomassie blue staining. Alternately, after assembly microtubules were fixed in 1% glutaraldehyde, pelleted onto poly-l-lysine–coated coverslips (Evans et al., 1985), and processed for immunocytochemistry as previously described (Karki et al., 1998).
Microtubule seeds were prepared by polymerization of tubulin with 20 μM paclitaxel (Cytoskeleton), 1 mM MgGTP, and incubated at 37°C for 10 min. Microtubules were pelleted at 39,000 × g, resuspended to 10 μg/μl in PHEM with 50 mM NaCl, and then sheared with a 26G needle. Microtubule assembly was performed in a 125-μl reaction volume in a water-jacketed cuvette at 37°C measuring absorbance at 350 nm using a UV-160 spectrophotometer (Shimadzu, Columbia, MD).
RESULTS
EB1 Binds Directly to the p150Glued Subunit of Dynactin
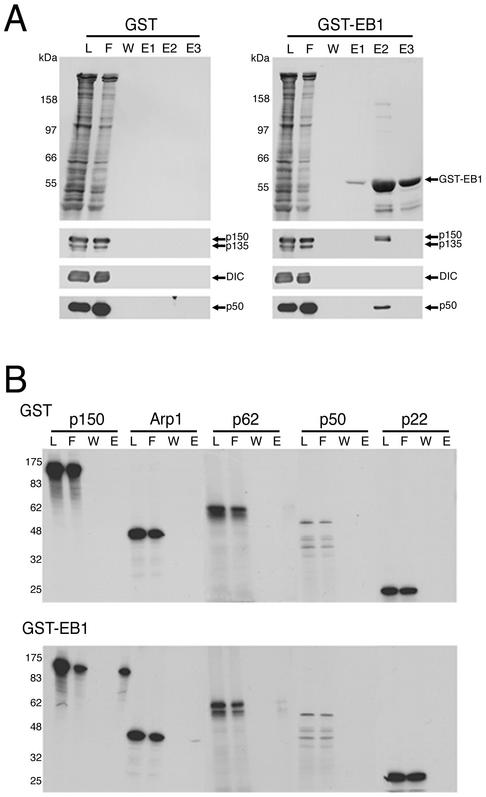
Several proteins, including EB1, p150Glued, and APC have been localized to the plus-ends of growing microtubules, and evidence for interactions among these plus-end proteins has led to the hypothesis of a microtubule plus-end complex (Schroer, 2001). EB1 has been reported to interact with components of the dynein and/or dynactin complexes, but the precise nature of this interaction was unclear (Berrueta et al., 1999). To explore this interaction further, we used affinity chromatography. A GST-EB1 fusion protein was bound to glutathione Sepharose beads and an extract of ATP-releasable microtubule-binding proteins from rat brain cytosol was applied to the column. This ATP extract is highly enriched in both dynein and dynactin (Paschal et al., 1991). Fractions were then analyzed by gel electrophoresis and Western blotting with antibodies to subunits of dynein and dynactin. Dynein was not retained on the GST-EB1 column, but subunits of dynactin were retained, suggesting that EB1 interacts specifically with dynactin, but not dynein (Figure 1A). An alternatively spliced isoform of the p150Glued subunit of dynactin that does not contain the amino terminal microtubule-binding domain (p135) is also expressed in brain (Tokito et al., 1996). Strikingly, p135 was not retained on the GST-EB1 column, suggesting that only a subset of dynactin may interact with EB1 (Figure 1A).
Figure 1.
EB1 interacts with the p150Glued subunit of dynactin. (A) Affinity chromatography of ATP extract from rat brain microtubules over GST-EB1 and GST control columns resulted in the binding of dynactin but not dynein to GST-EB1. The top panel was stained for total protein, and the bottom panels show Western blots. Dynactin subunits p150Glued and p50 as well as Arp1 and p62 (unpublished data) were retained by GST-EB1 but not by GST alone. The p135 isoform was not retained by GST-EB1, suggesting p150Glued binds EB1 through its amino-terminal domain. Dynein IC (DIC) was not retained on the GST-EB1 column. (B) Affinity chromatography of in vitro–translated dynactin subunits over GST-EB1 and GST control columns showed that the dynactin subunit p150Glued bound to GST-EB1 but not to GST alone. Dynactin subunits Arp1, p62, p50, and p22 did not bind significantly to either GST or GST-EB1.
To determine the specific dynactin subunit that interacts with EB1, we generated [35S]methionine-labeled p150Glued, Arp1, p62, dynamitin (p50), and p22 subunits using a reticulocyte lysate in vitro transcription/translation system (Promega). Only p150Glued bound to GST-EB1 (Figure 1B), suggesting that EB1 interacts with the dynactin complex through p150Glued.
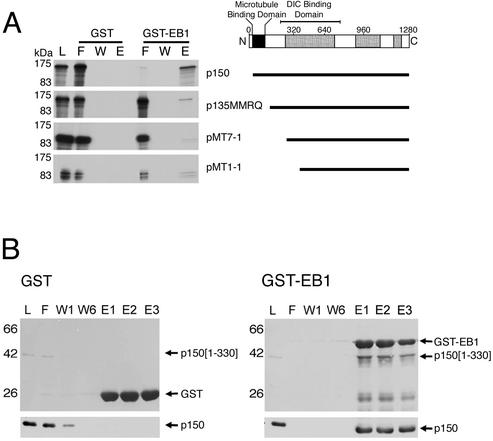
Because EB1 interacts with p150Glued, but not p135, we hypothesized that the site of interaction may be at the N-terminus of p150Glued. The microtubule-binding site of p150Glued is also near the N-terminus and the proximity of these two sites of interaction raises the possibility of cooperative binding interactions between p150Glued, EB1, and microtubules. To identify the EB1 binding domain, we generated a series of N-terminal deletion constructs of p150Glued, synthesized [35S]methionine-labeled peptides, and applied the peptides to the GST-EB1 column. Only the full-length p150Glued construct showed appreciable binding to the column in comparison to the GST control column. Constructs missing the N-terminal domain showed little interaction with EB1, confirming that the site of interaction is near the N-terminus (Figure 2A).
Figure 2.
EB1 binds directly to the N-terminal domain of p150Glued. (A) In vitro–translated full-length p150Glued was retained on a GST-EB1 column, but amino-terminal p150Glued deletion constructs p135MMRQ, pMT7-1, and pMT1-1 did not bind significantly to GST-EB1. (B) A recombinant amino-terminal fragment of p150Glued (residues 1–330) bound to a GST-EB1 column, but not significantly to a GST control column. The top panel was stained for total protein, and the bottom panel was blotted with an antip150Glued antibody. This result shows EB1 interacts directly with the amino-terminal domain of p150Glued, which also includes the microtubule-binding motif.
The interaction between EB1 and p150Glued observed in these assays may not, however, be direct, as other proteins in cytosol or the reticulocyte lysate could mediate the interaction. To determine if the two proteins can interact directly, we isolated and purified a recombinant fragment of p150Glued consisting of the first 330 amino acids and applied it to a GST-EB1 column. Recombinant p150Glued[1–330] was specifically retained on the GST-EB1 column, indicating that the two proteins can interact directly (Figure 2B). The converse experiment was also performed and showed that recombinant EB1 bound to a GST-p150Glued column (unpublished data).
EB1 and p150Glued Exhibit Different Patterns of Microtubule Localization
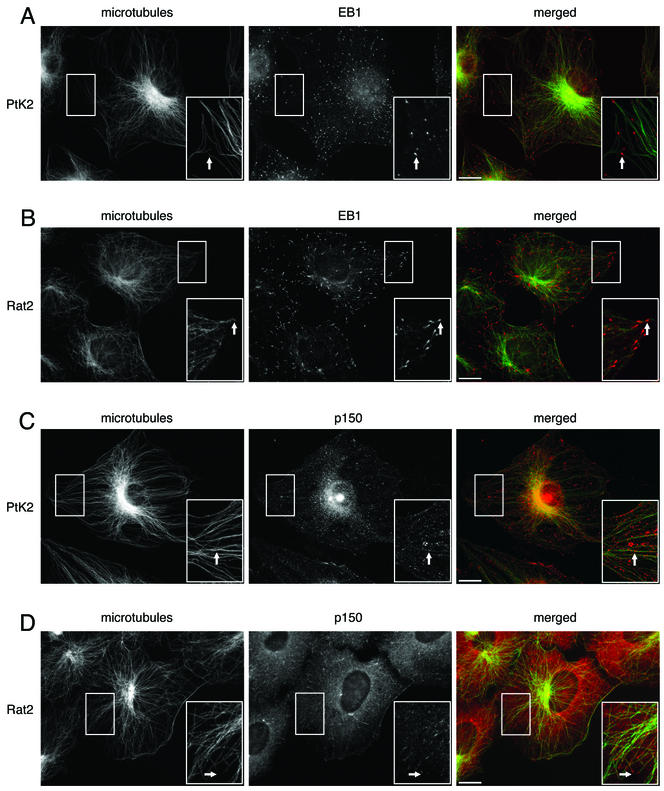
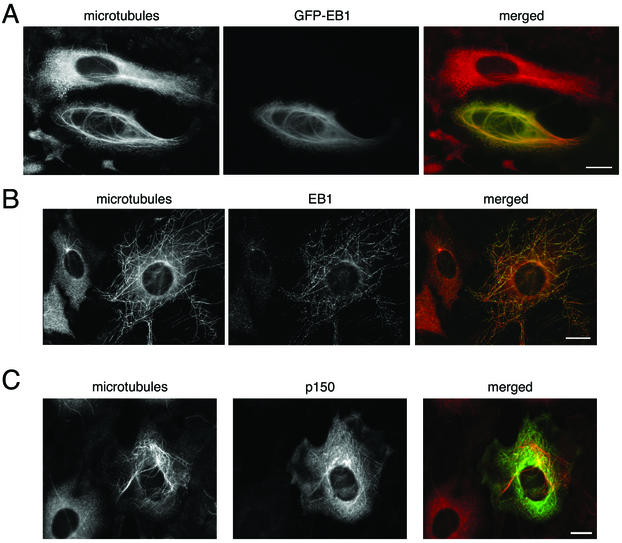
Immunocytochemistry shows that EB1 and p150Glued exhibit different patterns of microtubule localization and, further, that these patterns may vary with cell type. In cultured PtK2 epithelial cells, EB1 is localized to a discrete point at the most distal tip of the plus-end of the microtubule (Figure 3A). In cultured Rat2 fibroblasts, however, EB1 exhibits a more comet-tail expression pattern with a bright spot at the tip and tapering label that extends 1–2 μm toward the minus-end of the microtubule (Figure 3B). This comet-tail pattern of EB1 labeling has also been seen in another fibroblast-like cell line (COS-7; Morrison et al., 1998) and in a fibrosarcoma cell line (HT 1080; Juwana et al., 1999).
Figure 3.
EB1 and p150Glued both localize to microtubules in epithelial cells and fibroblasts, but the pattern of localization differs. For each panel, the individual labels are shown in black and white, whereas the overlays are shown in color. The area in the box is shown magnified in the inset. Scale, 10 μm. (A) Cultured PtK2 epithelial cells labeled with antibodies to EB1 and tubulin. Arrows show EB1 (red) localized to discrete points at the plus ends of microtubules (green). (B) Cultured Rat2 fibroblasts labeled with antibodies to EB1 and tubulin. Arrows show EB1 (red) localized to comet-tails at the plus-ends of microtubules (green). (C) PtK2 cells labeled with antibodies to p150Glued and tubulin. Arrows show microtubule (green) decoration by p150Glued (red), but decoration was not limited to microtubule tips. (D) Rat2 cells labeled with antibodies to p150Glued and tubulin. Arrows show p150Glued labeling (red) was localized to comet tails at the plus-ends of microtubules (green).
p150Glued is more broadly distributed in both PtK2 and Rat2 cells (Figure 3, C and D). Consistent with the essential role of dynactin in dynein-mediated vesicular transport (Waterman-Storer et al., 1997), p150Glued shows a punctate “vesicular” distribution in the cytoplasm of both cell types. A population of the protein is also localized to microtubules in both cell types, but the pattern of this localization differs strikingly between the two. In Rat2 cells, p150Glued is prominently localized to microtubule plus-ends with a comet-tail like distribution (Figure 3D), similar to that previously reported in COS-7 fibroblasts (Vaughan et al., 1999). In PtK2 epithelial cells, however, p150Glued decorates microtubules, but is not limited to the plus-ends. Rather, labeling is distributed along the shaft of the microtubule (Figure 3C).
Overexpression of both EB1 and p150Glued Induces Microtubule Bundling
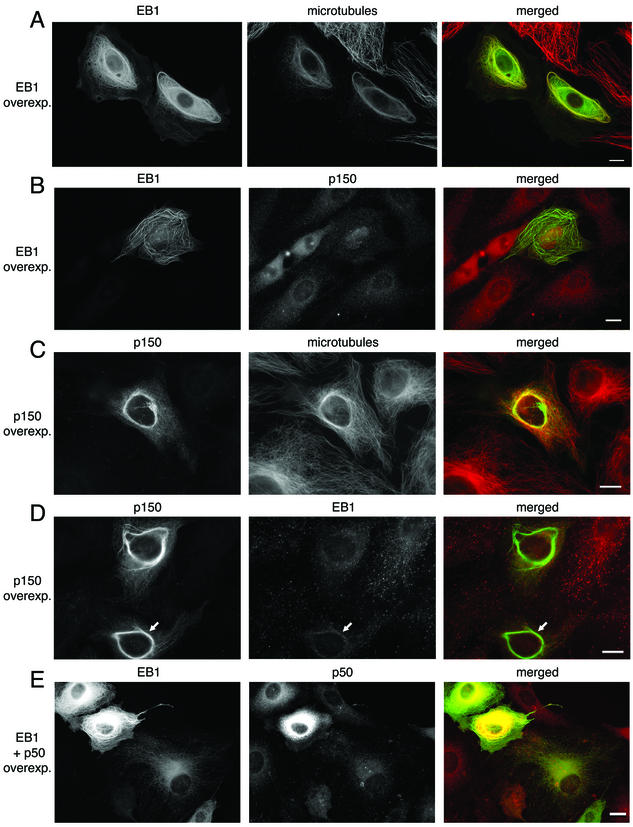
Although the localization of endogenous EB1 is restricted to the plus-ends of microtubules, overexpression of recombinant EB1 suggests that the distribution of the protein depends on the level of expression. We and others have observed that at low expression levels, exogenous EB1 mimics the distribution of the endogenous protein and is concentrated at microtubule tips, but at higher levels of expression, exogenous EB1 can decorate the entire microtubule array (unpublished data; Mimori-Kiyosue et al., 2000; Bu and Su, 2001). At very high levels of EB1 expression, the microtubules become bundled and EB1 is intensely localized to the bundles (Figure 4A). Often these EB1-microtubule bundles are very long and loop throughout the cell. Some EB1-microtubule bundles grow very large and encircle the cell. Similar results were obtained with transient transfection of cDNAs for a GFP-EB1 fusion protein (Figure 4A), a DsRed2-EB1 fusion protein (unpublished data), and EB1 without a fluorescent tag (unpublished data).
Figure 4.
Overexpression of both EB1 and p150Glued can bundle microtubules. (A) PtK2 cells transiently transfected with a cDNA for GFP-EB1 (green) and labeled with an antibody to tubulin (red) show extensive bundling of microtubules. (B) PtK2 cells expressing GFP-EB1 and labeled with an antibody to p150Glued (red) show that p150Glued is not recruited to EB1-microtubule bundles. (C) PtK2 cells transiently transfected with a cDNA for p150Glued and labeled with antibodies to p150Glued (green) and tubulin (red) show short circumnuclear microtubule bundles. (D) PtK2 cells expressing p150Glued and labeled with antibodies to p150Glued (green) and EB1 (red) show that EB1 is weakly recruited to p150Glued-microtubule bundles (arrows). (E) Overexpression of p50 (dynamitin) disrupts the dynactin complex. In cells overexpressing high levels of both GFP-EB1 (green) and p50 (red), an increased bundling effect was evident when compared with cells overexpressing GFP-EB1 alone. Scale, 10 μm.
It has previously been reported that microtubule bundles induced by the overexpression of EB1 are very stable (Bu and Su, 2001). In these experiments, we saw that EB1-microtubule bundles persisted after a 60-min incubation with the microtubule-depolymerizing drug nocodazole (Figure 5A) or a 60-min incubation at 4°C (unpublished data), suggesting that these bundles are both drug- and cold-stable.
Figure 5.
EB1 and p150Glued are associated with stable microtubules. (A) PtK2 cells expressing GFP-EB1 were treated with the microtubule-depolymerizing drug nocodazole (5 μg/ml) for 30 min and then fixed and labeled with an antibody to tubulin (red). The EB1-microtubule bundles resist depolymerization. (B) A small population of stable microtubules persisted after nocodazole treatment in (untransfected) PtK2 cells. These stable microtubules were highly decorated with endogenous EB1. Cells were treated with nocodazole (5 μg/ml) for 30 min and then fixed and labeled with antibodies to tubulin (red) and EB1 (green). (C) PtK2 cells expressing p150Glued were treated with the microtubule depolymerizing drug nocodazole as above, fixed, and labeled with antibodies to p150Glued (green) and tubulin (red). p150Glued-microtubules resisted depolymerization. Scale, 10 μm.
Overexpression of p150Glued has also been shown to bundle microtubules (Waterman-Storer et al., 1995). However, the microtubules bundled by p150Glued look very different from those bundled by the overexpression of EB1. p150Glued-microtubule bundles are generally short extensions from the centrosome. In some cells, thick bundles encircle the nucleus, but rarely do these bundles extend toward the periphery of the cell (Figure 4C). p150Glued-microtubule bundles are also very stable and resist nocodazole treatment (Figure 5C and see Waterman-Storer et al., 1995).
A small population of highly stable microtubules in PtK2 cells resists both cold and drug treatment. In most cells, this population consists of a few microtubules emanating from the centrosome, but some cells have a population of stable microtubules that appears to be noncentrosomal. These noncentrosomal stable microtubules, shown here in nocodazole-treated cells, are highly decorated with EB1, whereas the centrosomal stable microtubules are not (Figure 5B). p150Glued was not observed to be associated with either population of stable microtubules in this cell type (unpublished data).
EB1 Does Not Recruit Dynactin to Microtubules, But p150Glued May Recruit EB1 to Microtubules
Because we had observed a direct interaction between EB1 and p150Glued in vitro, we sought to determine if p150Glued or other dynactin components were recruited to the microtubule bundles induced by EB1 overexpression and if EB1 was recruited to bundles induced by p150Glued overexpression. Immunocytochemistry with several different antibodies to p150Glued (monoclonal and polyclonals; one polyclonal is shown in Figure 4B) and antibodies to the p62, p22, dynamitin (p50), and Arp1 (unpublished data) subunits of dynactin showed that little or no dynactin was present on EB1-microtubule bundles, suggesting that EB1 does not recruit dynactin to microtubules.
Immunocytochemistry with an antibody to EB1 shows some recruitment of EB1 to p150Glued-microtubule bundles, but these results were variable. Some bundles showed prominent EB1 recruitment (unpublished data), but most showed a weak recruitment (Figure 4D). These data suggest that although p150Glued may recruit EB1 to microtubules, this process is likely to be tightly regulated within the cellular environment.
Dynactin May Regulate the Function of EB1
Overexpression of the dynamitin subunit of dynactin has been shown to disrupt the dynactin complex and interfere with its functions (Echeverri et al., 1996). To determine if the disruption of dynactin affects the function of EB1, we transiently transfected cells with cDNAs for both dynamitin and EB1. We qualitatively scored the level of expression (low or high) of each protein (EB1 and dynamitin) in 150 cells (50 cells from each of three separate experiments) and correlated these data with the presence or absence of microtubule bundles. Thirty-three percent of cells with high levels of exogenous EB1 but low levels of dynamitin had bundles (7/21), but 68% of cells with high levels of both EB1 and dynamitin had bundles (21/31; Figure 4E). Dynamitin alone does not induce microtubule bundling in these cells (unpublished data), suggesting that the disruption of the dynactin complex enhances the bundling effect of EB1.
p150Glued and EB1 Have Different Effects on Microtubule Polymerization in Vitro
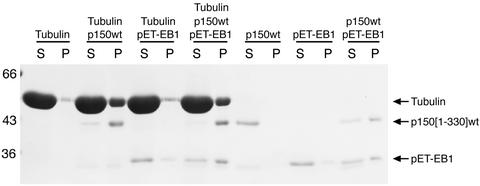
Because both EB1 and p150Glued appear to affect microtubule polymerization and/or stability within the cell, we tested the effects of these two proteins in in vitro microtubule polymerization and sedimentation assays. First, we polymerized tubulin either alone, in the presence of recombinant EB1, in the presence of recombinant p150Glued or in the presence of both EB1 and p150Glued. Under these conditions (no seeds or other nucleation factors), tubulin alone polymerizes very inefficiently. A small amount of polymerized microtubules was seen in the pellet after centrifugation, but most of the tubulin remained in the supernatant (Figure 6). The addition of EB1 did not significantly increase the extent of microtubule polymerization, but the addition of p150Glued dramatically enhanced polymerization. The addition of the two proteins in combination, however, had no effect beyond that of p150Glued alone (Figure 6).
Figure 6.
p150Glued enhances microtubule polymerization, but EB1 appears to have little effect. Microtubules were polymerized in the presence of tubulin alone, tubulin with recombinant p150Glued, tubulin with recombinant EB1, or tubulin with both EB1 and p150Glued and then centrifuged at 39,000 × g. Supernatant and pellet fractions were analyzed by SDS-PAGE. The addition of p150Glued increased the amount of tubulin in the pellet, but EB1 had little effect. The addition of EB1 and p150Glued together had no apparent effect beyond that of p150Glued alone. We note that the combination of EB1 and p150Glued in the absence of tubulin results in the appearance of both proteins in the pellet. This could result from the formation of aggregates of EB1 and p150Glued or a change in the solubility of the proteins in solution.
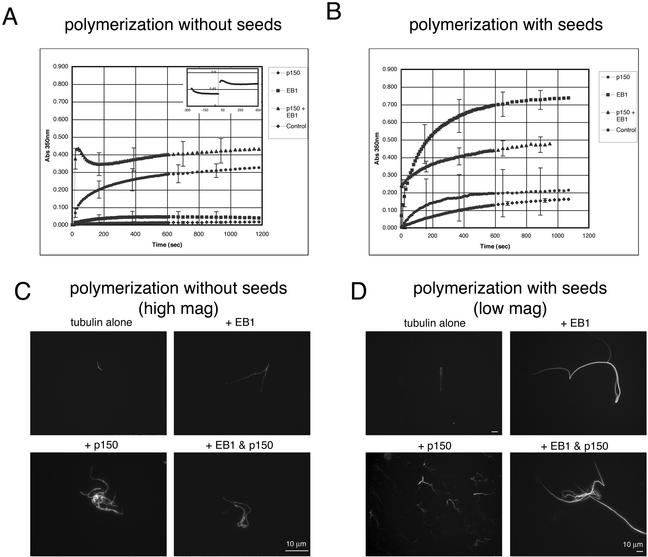
Tubulin polymerizes more efficiently in the presence of nucleating microtubule seeds. To examine the effect of EB1 and p150Glued on microtubule polymerization in the absence and in the presence of seeds, we used a spectrophotometric light scattering assay. In the absence of microtubule seeds, the addition of either recombinant EB1 or p150Glued alone was very similar to that seen with the microtubule pelleting assay. The addition of EB1 increased light scattering only slightly over that seen with tubulin alone, whereas the addition of p150Glued caused a significant increase in light scattering (Figure 7A). In this assay, the addition of p150Glued and EB1 in combination appeared to show an increased effect beyond that of p150Glued alone. It is possible that this assay is more sensitive than the pelleting assay and can therefore identify smaller differences in the extent of polymerization. However, light scattering may also be enhanced by microtubule bundling. Because both EB1 and p150Glued appear to bundle microtubules in vivo, we pelleted the microtubules onto coverslips after polymerization to examine their morphology (Figure 7C). Relatively short microtubules were pelleted after the polymerization of tubulin alone. Some bundling was seen, but it was not extensive. Microtubules pelleted after the addition of EB1 were slightly longer than those seen with tubulin alone, but the difference was not large. The microtubules pelleted after the addition of p150Glued, however, were dense tangles that appeared to consist of many short, splayed bundles of microtubules. The microtubules pelleted after the addition of EB1 and p150Glued in combination were also densely tangled, but the tangles often contained long microtubule bundles, which could account for the increase seen in light scattering.
Figure 7.
p150Glued has a potent microtubule nucleation effect and EB1 has a potent microtubule elongation effect. (A) The effect of p150Glued, EB1, both proteins, and tubulin only (control) on microtubule polymerization over time was examined using a light-scattering assay. When no microtubule seeds are present, EB1 has little effect over that of tubulin alone, but p150Glued has a potent effect on the extent of polymerization. The combination of EB1 and p150Glued had an even greater effect. However, the combination of EB1 and p150Glued appeared to cause a confounding light-scattering aggregation that settled out of solution over time, similar to that seen in the pelleting experiment. The inset graph shows this effect before and after the addition of tubulin. (B) In the presence of microtubule seeds, p150Glued had little effect on microtubule polymerization over that of the addition of tubulin alone, but the addition of EB1 had a potent effect. (C) Fluorescently labeled microtubules were pelleted onto coverslips after polymerization in the above conditions. Without seeds, very few microtubules were polymerized with tubulin alone. Long slender bundles were polymerized in the presence of EB1, and dense tangled bundles were polymerized in the presence of p150Glued. Microtubules polymerized in the presence of both EB1 and p150Glued had characteristics of both. (D) Microtubules polymerized much more efficiently in the presence of seeds, necessitating a larger microscopic field of view. Relatively short, simple microtubules were polymerized in the presence of tubulin alone, short, highly branched microtubule bundles were polymerized in the presence of p150Glued, and extremely long microtubule bundles were polymerized in the presence of EB1. Again, microtubules polymerized in the presence of both EB1 and p150Glued had characteristics of both. Scale, 10 μm.
In the presence of microtubule seeds, the results were strikingly different (Figure 7B). The addition of p150Glued slightly enhanced light scattering above that of tubulin alone, but the addition of EB1 caused a dramatic increase in light scattering. Interestingly, the effect of the two proteins in combination was less than that of EB1 alone and was approximately the same as that seen without seeds. Microtubules pelleted after the polymerization of tubulin with seeds were longer than those polymerized without seeds, but did not appear to be more bundled (Figure 7D). The microtubules polymerized with EB1, on the other hand, were extremely long and thickly bundled, but the bundles did not show any of the splaying or tangling seen with p150Glued. Microtubules polymerized with seeds in the presence of p150Glued were similar to those polymerized without seeds, but the tangles were smaller and less complex. In addition, there appeared to be more bundles of very short microtubules. Microtubules polymerized with seeds in the presence of both EB1 and p150Glued had characteristics of both. Many of the microtubule bundles were extremely long, but they often contained tangles of shorter microtubules as well (Figure 7D). Although the light scattering induced by the addition of EB1 to seeded assembly reactions increased by ∼ 400% over that seen with tubulin alone, sedimentation assays indicate that the actual increase in polymer is only ∼50% (unpublished data). The high degree of microtubule bundling observed by microscopy could account for the larger increase seen with light scattering.
DISCUSSION
A class of microtubule-binding proteins has been identified that preferentially localize to the plus-ends of growing microtubules. Included among this class are CLIP-170, APC, EB1, and dynactin. Each of these proteins has a microtubule-binding domain and may be independently targeted to microtubules. However, interactions between individual plus-end proteins have also been identified: APC interacts with EB1 (Su et al., 1995), EB1 interacts with dynein and/or dynactin (Berrueta et al., 1999), and CLIP-170 may interact with dynactin (Valetti et al., 1999; Vaughan et al., 1999). These data have led to the hypothesis of a microtubule plus-end complex (Schroer, 2001), but the details of interactions among plus-end proteins and between plus-end proteins and microtubules have remained unclear. Here we have clarified the interaction between EB1 and the dynein and dynactin complexes. We have shown that EB1 interacts with the dynactin complex by binding directly to the p150Glued subunit. EB1 binds to p150Glued near the N-terminus of the protein, in close proximity to the CAP-Gly microtubule-binding site of p150Glued, suggesting that EB1 and p150Glued may form a ternary complex with microtubules. A recent crystal structure of the CAP-Gly motif has identified a groove in the protein structure that may mediate microtubule binding (Li et al., 2002). It will be interesting to determine the structural relationship of the EB1 binding domain to this motif. While the present article was in review, another study was published that also noted a direct interaction between EB1 and p150Glued (Askham et al., 2002). Askham et al. demonstrated that the microtubule binding domain on EB1 is at the N terminus of the protein, whereas the dynactin binding domain is at the C terminus. However, both domains are necessary to increase microtubule stability and bundling. Together these data suggest a multipartite complex that might allow for tight regulation within the cellular environment.
If a plus-end complex does exist, it may assemble in the cytoplasm and copolymerize with microtubules or it could assemble in situ on the microtubule. Here we have shown that purified recombinant EB1 and p150Glued interact, suggesting that the proteins can associate independently of microtubules. It has also previously been shown that EB1 coimmunoprecipitates with dynein and dynactin from nocodazole-treated cells (Berrueta et al., 1999). This is not a universal feature of interactions among plus-end proteins, however, because it has been shown that EB1 does not interact with APC in the absence of intact microtubules (Mimori-Kiyosue et al., 2000). These data suggest that the formation of a microtubule plus-end complex may involve several distinct steps, some that occur in the cytoplasm and some that occur on the microtubule.
Interactions between plus-end proteins may allow one protein to recruit others to microtubules or a plus-end complex. For example, overexpression of CLIP-170 has been shown to recruit p150Glued to microtubules, but the overexpression of p150Glued does not recruit CLIP-170 to microtubules (Valetti et al., 1999). It has also been shown that the overexpression of APC recruits EB1 to microtubules (Mimori-Kiyosue et al., 2000). This finding is complicated, however, by the observation that EB1 is localized to microtubules in cells lacking functional APC (Berrueta et al., 1998). The temporal sequence with which proteins are recruited and the hierarchies of binding are largely unknown. Here we have shown that the overexpression of p150Glued may recruit EB1 to microtubules, but the overexpression of EB1 cannot recruit p150Glued to microtubules. Interactions between plus-end proteins, however, are likely to be highly regulated, and overexpression of a protein may circumvent this regulation. Potential regulatory factors include the state of the microtubule (GTP cap, posttranslational modifications) and posttranslational modifications of the plus-end proteins. EB1 has few potential phosphorylation sites, but APC and p150Glued are likely to be regulated by phosphorylation (Ashkam et al., 2000; Vaughan et al., 2000). The details of this regulation, however, remain an open question.
The function of a plus-end complex or plus-end proteins may be twofold: they may play a role in regulating microtubule stability and/or dynamics and they mediate interactions between microtubules and cellular targets (for example, see Kaverina et al., 1998; Ligon et al., 2001). Here we have shown that both EB1 and p150Glued appear to affect microtubules growth and/or stability, but in very different ways. Increased levels of EB1 lead to the formation of cold- and drug-stable microtubule bundles. (This was also shown by Bu and Su [2001].) Likewise, increased levels of p150Glued lead to the formation of stable microtubule bundles (shown here and in Waterman-Storer et al., 1995). However, the microtubule bundles formed by the overexpression of EB1 and p150Glued appear very different. Those induced by EB1 overexpression are very long and wrap around the cell, whereas those induced by p150Glued overexpression are short and more circumnuclear. The overexpression of another plus-end protein, CLIP-170, also causes the formation of microtubule bundles (Pierre et al., 1994). It is interesting to note that while CLIP-170 has a microtubule binding domain similar to that of p150Glued, the bundles induced by its overexpression appear much more like those induced by EB1.
In addition, we have shown that high levels of endogenous EB1 are associated with a population of stable microtubules in epithelial cells, but we did not see p150Glued associated with stable microtubules in this cell type. It has previously been reported that high levels of endogenous p150Glued are associated with stable microtubules in fibroblasts (Vaughan et al., 1999). In both of these experiments, however, it is not clear if increased levels of EB1 or p150Glued caused the microtubules to be stable or if the proteins merely opportunistically bound to the microtubules remaining after drug treatment.
Both EB1 and p150Glued have an effect on microtubule polymerization in vitro, but their effects are distinct. In the absence of microtubule seeds, the addition of EB1 does not increase microtubule polymerization much beyond that of tubulin alone, consistent with previous data (Nakamura et al., 2001). p150Glued, on the other hand, decreases the critical concentration for microtubule polymerization, thus increasing the extent of polymerization. In the presence of seeds, however, the results were very different. p150Glued has little effect beyond that of tubulin alone, whereas the addition of EB1 results in an increase in light scattering in a spectrophotometric assay. Examination of the polymerized microtubules by microscopy suggests that p150Glued may play a role in nucleating new microtubules. In the absence of seeds, this role is critical. But when seeds are present, this nucleating effect is not necessary. It is not clear if p150Glued can bind to tubulin subunits and nucleate microtubules de novo or if it binds to and potentially stabilizes very short microtubules, thus enhancing their nucleating effect. Examination of microtubules polymerized in the presence of EB1, on the other hand, suggests that EB1 appears to have more potent elongation and bundling effects. In the absence of seeds, EB1 is ineffectual, but when seeds are present, the addition of EB1 results in extremely long microtubule bundles. It has been shown that EB1 can bind to tubulin in the absence of microtubules (Juwana et al., 1999). Perhaps EB1 binds to free tubulin and this complex is more likely to add to polymer than tubulin alone.
These in vitro results also clarify the results observed in cells overexpressing EB1 and p150Glued. The overexpression of EB1 causes very long microtubule bundles due to its elongation and bundling effects and the overexpression of p150Glued causes many short microtubules due to its nucleation effect. Together, these data suggests that the overall dynamics of the microtubule array in the cell may depend upon the balance of activities of various plus-end proteins, including EB1 and p150Glued. We have shown that disruption of the dynactin complex enhances the effect of EB1 on microtubule bundling and stability, suggesting that the activities of these two plus-end proteins are linked, but other plus-end proteins may be involved as well.
Finally, differences in the expression and regulation of plus-end proteins may result in cell type differences in microtubule dynamics. Although EB1 and p150Glued are both expressed in fibroblasts and epithelial cells and localize to microtubules in each cell type, the patterns of their localizations differ in the two cell types. It has long been known that the microtubule arrays of epithelial cells and fibroblasts differ in their organization and dynamics (Bershadsky et al., 1979; Spiegelman et al., 1979; Shelden and Wadsworth, 1993). In several cultured epithelial cell lines, microtubules have been shown to array from a single centrosome, whereas in cultured fibroblasts, multiple microtubule-nucleating sites are often seen in a cell, resulting in a large population of noncentrosomal microtubules (Bershadsky et al., 1979; Spiegelman et al., 1979). Differences in microtubule dynamics are more complicated. Microtubules in some epithelial cell types have been shown to be very dynamic on a short time scale, but stable on a longer time scale, i.e., undergoing brief periods of growth and shortening, with little overall change. In contrast, microtubules in fibroblasts are more stable on a short time scale and more dynamic on a long time scale, i.e., they undergo longer periods of growth and shortening, resulting in large net changes (Shelden and Wadsworth, 1993). EB1 and p150Glued as well as other plus-end proteins are perfectly positioned to regulate these differences. The localization of the two proteins differs between the two cell types, they have differential effects on microtubule dynamics, and these proteins interact with one another. The function of differential microtubule dynamics in different cell types, however, remains an unanswered question.
ACKNOWLEDGMENTS
We thank Lynne Cassimeris and Jennifer Tirnauer for insightful comments and discussions, Kevin Vaughan for his kind generosity with reagents, and Krithika Balasubramanian for initiating this project in our laboratory. This work was supported by National Institutes of Health grant GM48661 and a postdoctoral fellowship to L.A.L.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0155. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0155.
REFERENCES
- Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol. 2000;149:863–874. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkam J, Moncur P, Markham A, Morrison E. Regulation and function of the interaction between the APC tumor suppressor and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- Askham JM, Vaughan KT, Goodson HV, Morrison EE. Evidence that an interaction between EB1 and p150Glued is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell. 2002;10:3627–3645. doi: 10.1091/mbc.E02-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Kraeft S-K, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, Pellman D, Bierer BE. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:435–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Tint IS, Gelfand VI, Rosenblat VA, Vasiliev JM. Morphology of the microtubule system in mouse kidney epithelial cells. Ontogenez. 1979;10:231–235. [PubMed] [Google Scholar]
- Bu W, Su LK. Regulation of microtubule assembly by human EB1 family proteins. Oncogene. 2001;20:3185–3192. doi: 10.1038/sj.onc.1204429. [DOI] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L, Mitchison T, Kirschner M. Influence of the centrosome on the structure of nucleated microtubules. J Cell Biol. 1985;100:1185–1191. doi: 10.1083/jcb.100.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Tokito MK, Karki S, Holzbaur EL. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur EL, Hammarback JA, Paschal BM, Kravit NG, Pfister KK, Vallee RB. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Juwana J-P, et al. EB/RP gene family encodes tubulin binding proteins. Int J Cancer. 1999;81:275–284. doi: 10.1002/(sici)1097-0215(19990412)81:2<275::aid-ijc18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S, LaMonte B, Holzbaur EL. Characterization of the p22 subunit of dynactin reveals the localization of cytoplasmic dynein and dynactin to the midbody of dividing cells. J Cell Biol. 1998;142:1023–1034. doi: 10.1083/jcb.142.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Tokito MK, Holzbaur EL. A dynactin subunit with a highly conserved cysteine-rich motif interacts directly with Arp1. J Biol Chem. 2000;275:4834–4839. doi: 10.1074/jbc.275.7.4834. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek WS, Copeland MJ, Chaudhuri A, Chant J. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 2000;287:2257–2259. doi: 10.1126/science.287.5461.2257. [DOI] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur ELF. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Lee L, Tirnauer JS, Li J, Schuyler SC, Liu JY, Pellman D. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 2000;287:2260–2262. doi: 10.1126/science.287.5461.2260. [DOI] [PubMed] [Google Scholar]
- Li, S. et al. Crystal structure of the cytoskeleton-associated protein (CAP-Gly) domain. J. Biol. Chem. 277, 48597–48601. [DOI] [PubMed]
- Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- McNally FJ. Cytoskeleton. CLASPing the end to the edge. Curr Biol. 2001;11:R477–R480. doi: 10.1016/s0960-9822(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Miller RK, Cheng SC, Rose MD. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol Biol Cell. 2000;11:2949–2959. doi: 10.1091/mbc.11.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–868. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumor suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–3477. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001;11:1062–1067. doi: 10.1016/s0960-9822(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. Purification of brain cytoplasmic dynein and characterization of its in vitro properties. Methods Enzymol. 1991;196:181–191. doi: 10.1016/0076-6879(91)96018-m. [DOI] [PubMed] [Google Scholar]
- Pierre P, Pepperkok R, Kreis TE. Molecular characterization of two functional domains of CLIP-170 in vivo. J Cell Sci. 1994;107:1909–1920. doi: 10.1242/jcs.107.7.1909. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Rogers GC, Sharp DJ, Vale RD. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J Cell Biol. 2002;158:873–874. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA. Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr Opin Cell Biol. 2001;13:92–96. doi: 10.1016/s0955-0674(00)00179-4. [DOI] [PubMed] [Google Scholar]
- Schuyler SC, Pellman D. Search, capture and signal games microtubules and centrosomes play. J Cell Sci. 2001;114:247–255. doi: 10.1242/jcs.114.2.247. [DOI] [PubMed] [Google Scholar]
- Su L-K, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- Shelden E, Wadsworth P. Observations and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993;120:935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Lopata MA, Kirschner MW. Multiple sites for the initiation of microtubule assembly in mammalian cells. Cell. 1979;16:239–252. doi: 10.1016/0092-8674(79)90002-3. [DOI] [PubMed] [Google Scholar]
- Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer JS, O'Toole E, Berrueta L, Bierer BE, Pellman D. Yeast Bim1p promotes the G1-specific dynamics of microtubules. J Cell Biol. 1999;145:993–1007. doi: 10.1083/jcb.145.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokito MK, Howland DS, Lee VM, Holzbaur EL. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol Biol Cell. 1996;7:1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KT, Tynan SH, Faulkner NE, Echeverri CJ, Vallee RB. Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J Cell Sci. 1999;112:1437–1447. doi: 10.1242/jcs.112.10.1437. [DOI] [PubMed] [Google Scholar]
- Vaughan PS, Miura PE, Henderson M, Vaughan KT. Regulation of dynactin targeting to microtubule plus-ends by PKA. Mol Biol Cell. 2000;11,,Supplement:365a. [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki SB, Kuznetsov SA, Tabb JS, Weiss DG, Langford GM, Holzbaur EL. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc Natl Acad Sci USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]