Abstract
The expression of the intermediate filament (IF) protein nestin is closely associated with rapidly proliferating progenitor cells during neurogenesis and myogenesis, but little is known about its function. In this study, we examine the effects of nestin expression on the assembly state of vimentin IFs in nestin-free cells. Nestin is introduced by transient transfection and is positively correlated with the disassembly of vimentin IFs into nonfilamentous aggregates or particles in mitotic but not interphase cells. This nestin-mediated disassembly of IFs is dependent on the phosphorylation of vimentin by the maturation/M-phase–promoting factor at ser-55 in the amino-terminal head domain. In addition, the disassembly of vimentin IFs during mitosis appears to be a unique feature of nestin-expressing cell types. Furthermore, when the expression of nestin is downregulated by the nestin-specific small interfering RNA in nestin-expressing cells, vimentin IFs remain assembled throughout all stages of mitosis. Previous studies suggest that nonfilamentous vimentin particles are IF precursors and can be transported rapidly between different cytoplasmic compartments along microtubule tracks. On the basis of these observations, we speculate that nestin may play a role in the trafficking and distribution of IF proteins and potentially other cellular factors to daughter cells during progenitor cell division.
INTRODUCTION
Cell division marks a period in the cell cycle during which both the cytoplasmic and nuclear compartments are disassembled, reorganized, and partitioned into daughter cells. In vertebrate cells, this process is orchestrated by the three major cytoskeletal systems: intermediate filaments (IFs), microtubules, and microfilaments. Although the changes in organizational states of microtubules and microfilaments are highly conserved during both the assembly of the mitotic spindle and the formation of the contractile ring in cytokinesis, the structural changes in cytoplasmic IF networks appear to be cell- and IF-type specific (Chou et al., 1996). For example, in mitotic BHK-21 cells, the interphase IF network, which is composed primarily of vimentin and desmin, is completely disassembled from 10-nm IFs into nonfilamentous particles in late prophase (Rosevear et al., 1990). In PtK-2 epithelial cells, both vimentin and keratin IF networks remain intact during mitosis (Aubin et al., 1980). In HeLa cells, which also possess keratin and vimentin networks, the keratin network is disassembled into spheroid bodies, whereas vimentin remains filamentous (Franke et al., 1982; Jones et al., 1985). The various organizational fates of cytoplasmic IFs in different cell types undergoing mitosis suggest that the biochemical factors regulating their restructuring during mitosis are not identical.
Although the factors involved in regulating the structural changes of IFs in vivo are not completely understood, protein phosphorylation is known to play an essential role in determining the assembly states (Inagaki et al., 1997). In dividing BHK-21 cells, it has been shown that the disassembly of vimentin IFs is correlated with an elevated phosphorylation of vimentin mediated by two mitotic protein kinases, maturation/M-phase promoting factor (MPF; p34cdc2/cyclin B) and p37 kinase (Chou et al., 1990; Tsujimura et al., 1994; Chou et al., 1996). Whereas MPF phosphorylates vimentin at ser-55 and plays an essential role in the disassembly of vimentin IFs, p37 kinase phosphorylates vimentin at thr-457 and ser-458 and has no apparent impact on the disassembly of vimentin IFs in mitotic BHK-21 cells (Chou et al., 1996). The phosphorylation of vimentin by MPF is a universal feature of mitotic cells expressing vimentin. Yet the breakdown of vimentin networks during mitosis has been reported only in MDBK (Franke et al., 1982), BHK-21 (Rosevear et al., 1990), and ST15A (Sahlgren et al., 2001) cells. Therefore, additional factors, unique to these three cell lines, are required for the disassembly of IFs.
Clues regarding the identity of these cell type–specific factors come from our recent studies of the high-molecular-weight proteins present in purified IF preparations of BHK-21 cells (Steinert et al., 1999). One of these has been identified as nestin, a protein known to be expressed in neuroepithelial cells and developing muscle cells (Lendahl et al., 1990; Sejersen and Lendahl, 1993; Kachinsky et al., 1995; Vaittinen et al., 1999). Nestin cannot form filaments on its own, but it can readily form copolymer IFs when combined with type III IF proteins such as vimentin both in vitro and in vivo (Marvin et al., 1998; Eliasson et al., 1999; Steinert et al., 1999). The inability of nestin to form IFs is most likely because of its very short N-terminus, a domain known to be essential for IF assembly (Fuchs and Weber, 1994; Herrmann and Aebi, 2000). This possibility is supported by in vitro studies of nestin-vimentin coassembly, which demonstrate that nestin inhibits filament formation in a concentration-dependent manner (Steinert et al., 1999). These observations have led us to investigate the possible role of nestin in regulating the structural dynamics of vimentin IFs in vivo.
MATERIALS AND METHODS
Cell Culture and Transfection
Hamster BHK-21 cells were cultured as described previously (Prahlad et al., 1998). Mouse 3T3 cells were grown in DMEM supplemented with 10% calf serum. Chinese hamster ovary (CHO) and bovine MDBK cells were grown in Ham's F12 medium with 10% fetal calf serum. African green monkey CV-1 cells were grown in MEM containing 10% fetal calf serum. Rat embryonic fibroblasts (REFs) were obtained and cultured as described (Goldman, 1998). Human MCF-7 and rat C6–2 glioma cells were cultured in DMEM with 10% fetal calf serum. Penicillin and streptomycin (100 U/ml) were added to all culture media. Rat C6 glioma cells express various levels of vimentin as determined by immunofluorescence, but the expression of vimentin and nestin is always coincidental. The C6–2 glioma cells used in this study were derived from a subclone that expresses both vimentin and nestin.
Transient transfection was performed by electroporation as described previously (Prahlad et al., 1998) in OPTI-MEM (R) medium (Life Technologies/Invitrogen, San Diego, CA) at 0.27 V/960 μF (Gene Pulser II, Bio-Rad Laboratories, Hercules, CA). Transfected cells were allowed to grow for 48–72 h before fixation and staining. Under our experimental conditions, transfection rates of ∼30–50% and ∼10–25%, respectively, were obtained in single and double transfection assays. In the case of doubly transfected cells, ∼3–5% were mitotic. The numbers in Figure 3M were pooled from three to five experiments.
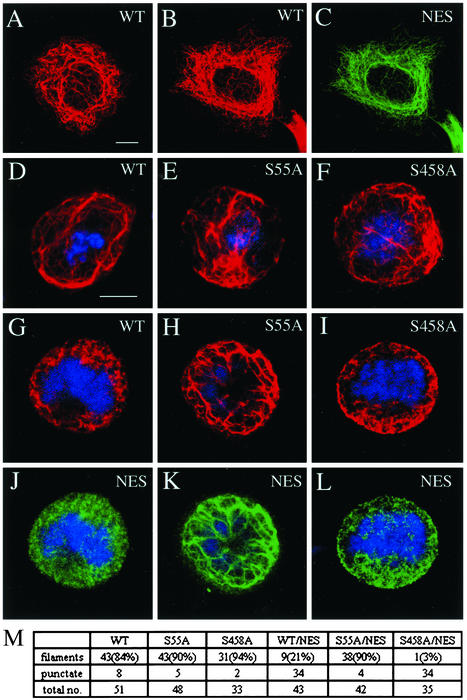
Figure 3.
Phosphorylation of vimentin at ser-55 is required for the nestin-mediated disassembly of IFs during mitosis. Wild-type vimentin (WT) or either the ser-55:ala (S55A) or thr-457:ala/ser-458:ala (S458A) mutant vimentins were transfected individually or together with nestin in MCF-7 cells. Subsequently, the assembly state of vimentin was examined by indirect immunofluorescence with a rabbit antibody directed against vimentin (red) and a monoclonal antibody directed against rat nestin (NES, green). In interphase cells, typical vimentin IF networks assembled from WT vimentin alone (A) or from WT vimentin and nestin as demonstrated by double-label immunofluorescence (B and C). During mitosis, all three forms of vimentin, when expressed individually, retained their filamentous networks (D–F). However, when vimentin was coexpressed with nestin, IFs formed with WT vimentin/nestin (G and J) or with thr-457:ala/ser-458:ala-vimentin/nestin (I and L) were disassembled into punctate and diffuse structures, whereas IFs assembled with ser-55:ala-vimentin/nestin remained intact (H and K). Images G and J, H and K, and I and L are pairs of the same cells using double immunofluorescence. Mitotic cells were identified by the presence of condensed chromosomes (blue). (M) Summary table of the quantitative results of singly and doubly transfected mitotic MCF-7 cells. Bar in interphase (A–C) and mitotic (D–L) cells, 10 μm.
Indirect Immunofluorescence
Cells were fixed and processed for indirect immunofluorescence as previously described (Helfand et al., 2002). Images were acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Inc., Oberkochen, Germany). Fluorescein, rhodamine, and toto-3 images were visualized using excitation at 488, 543, 633 nm and emission at 505–530, 585–615, and > 670 nm, respectively. Mitotic cells were identified by both phase contrast and the fluorescence patterns of condensed chromosomes stained with toto-3 iodide (Molecular Probes, Eugene, OR). To visualize vimentin, two polyclonal antibodies (Prahlad et al., 1998; Helfand et al., 2002) and one monoclonal antibody V9 (Sigma Chemical Co., St. Louis, MO) were used. For following the fate of nestin and immunoblotting, a polyclonal antibody (No. 268) raised against purified hamster nestin (Steinert et al., 1999) was used. This antibody was affinity-purified using Affi-gel-10 beads (Bio-Rad) coupled with two glutathione S-transferase–nestin-tail fusion proteins described below. Two monoclonal antibodies, 411C for hamster nestin (Yang et al., 1992) and 401 for rat nestin (BD PharMingen, San Diego, CA), were also used.
Cloning and Expression of Nestin
The complete rat nestin cDNA was amplified by RT-PCR with total C6 glioma cell RNA as template using the Thermoscript RT-PCR and Elongase systems (Invitrogen). Sense and antisense primers were made corresponding to the starting and the ending sequences of the open reading frame of the published rat nestin cDNA (accession No. m34384). Primers were designed to create two different sets of unique restriction sites at the 5′ and the 3′ ends of the amplified cDNA to facilitate the subsequent cloning into either mammalian or bacterial expression vectors. For expression in bacteria, the nestin cDNA was cloned into the pET-24 vector (Novagen, Madison, WI) between the NdeI and EcoRI sites. For expression in cultured mammalian cells, the nestin cDNA was cloned into the pCMV-myc vector (Clontech Laboratories, Cambridge, UK) between the SalI and NotI sites. The nestin cDNA (accession No. AF538924) amplified from rat C6 glioma cells is longer than the published sequence and is predicted to encode a protein of 1893 amino acids, which is 88 amino acids longer than those predicted from the published rat sequence (Lendahl et al., 1990). This additional sequence is located in the 11-amino-acid repeat region of the C-terminal domain. The size of the C6 nestin cDNA does not appear to be the result of mispriming during RT-PCR amplification or rearrangements during cloning. PCR reactions using genomic C6 cell DNA as template and two different pairs of primers corresponding to the flanking sequences of the repeat region produced the expected size of the repeat region (i.e., 49 × 33 base pairs; data not shown).
The C-terminal 1350 amino acids of hamster nestin (accession No. af110498) were cloned as two separate nestin tail fragments (NT334–949 and NT950–1683) into pGEX vector (Pharmacia/Amersham Biosciences, Arlington Heights, IL) between the EcoRI and SalI sites and expressed as glutathione fusion proteins. These fusion proteins were expressed in BL-21 bacterial cells (Stratagene, La Jolla, CA) and subsequently purified using a glutathione–Sepharose 4B column according to the manufacturer's protocol (Pharmacia). These purified fusion proteins were used for antibody purification mentioned above.
Small Interfering RNA Studies
A 21 nucleotide sequence (AAG AUG UCC CUU AGU CUG GAG) which is conserved in rat and hamster nestin (residues 299–319, accession No. af110498) was selected for synthesizing the small interfering RNA (siRNA) duplex with two 2′-deoxythymidine overhangs at the 3′ ends. This nestin siRNA duplex as well as the negative control luciferase–GL2 siRNA duplex (Harborth et al., 2001) were purchased from Dharmacon (Lafayette, CO). Transient transfection of siRNA-nestin into BHK-21 and C6–2 cells was performed exactly as described previously (Harborth et al., 2001), and the effects of siRNAs were examined 60 h after transfection.
Immunoblotting and Two-Dimensional Gel Electrophoresis
IF-enriched cytoskeletal fractions were prepared according to established procedures (Prahlad et al., 1998; Steinert et al., 1999). Then 2.5 μg of each sample was used for immunoblotting analyses. The same amount of the BL-21 cell lysates expressing the full-length rat nestin was used as a positive control. Because of the strong reactivity of the antibody toward the hamster nestin, the amount of the BHK-21 IF sample was reduced to 0.5 μg. For two-dimensional gel electrophoresis studies, 5 μg of each of the IF-enriched samples was used, and 0.5 μg of bacterially expressed tailless vimentin (Correia et al., 1999) (a gift of Brian Helfand) was included in samples and served as a reference mobility marker for unphosphorylated vimentin and its acidic variants. Isoelectric focusing and SDS-PAGE were performed using a published protocol (O'Farrell, 1975). Mitotic cells were enriched by treating untransfected or transfected CHO cells with 2 μM of nocodazole for 4 h and then collected by mechanical shake-off as described previously (Chou et al., 1989).
RESULTS
Nestin Expression Affects the Assembly State of Vimentin in Mitotic Cells
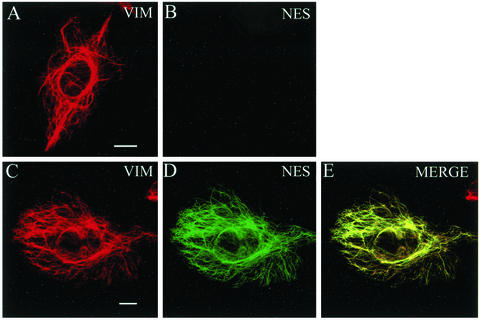
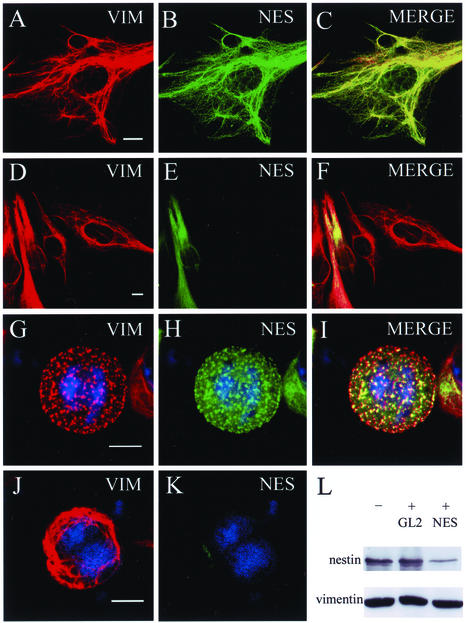
To begin to determine whether nestin plays a role in the organization of IF networks, CHO cells that express vimentin but not nestin (see Figure 1, A and B, and 4A) were used to study the effects of nestin expression. CHO cells were transiently transfected with an expression vector carrying the rat nestin cDNA. As is shown in Figure 1, C–E, ectopically expressed nestin colocalized with vimentin in a filamentous pattern, suggesting that it was incorporated into the vimentin IF network in interphase CHO cells. The expression of nestin did not appear to alter the overall distribution or the assembly state of endogenous IFs. In contrast, nestin expression caused significant changes in the organization of the vimentin IF network in mitotic cells. In untransfected mitotic cells, vimentin remained filamentous, and it formed a cage-like structure surrounding the mitotic apparatus during all stages of mitosis (Aubin et al., 1980; Zieve et al., 1980). Polymerized IFs persisted through mid to late cytokinesis (Figure 2D). In contrast, vimentin IFs in mitotic cells expressing nestin were disassembled, as indicated by the transformation from a filamentous into a punctate and diffuse pattern of fluorescence (Figure 2, E and F). In addition, the majority of the vimentin and nestin patterns in these cells appeared to be coincident (Figure 2G). This disassembled vimentin pattern persisted throughout all mitotic stages until the completion of cytokinesis, when cells began to reassemble their typical interphase IF networks (data not shown; see Figure 1, A and C, for examples of interphase pattern).
Figure 1.
Nestin expression in interphase CHO cells. In untransfected interphase cells, vimentin (VIM, red) is seen in a typical filamentous pattern (A). These cells express no detectable nestin (NES, green) after staining with nestin antibody (B). When nestin is expressed in CHO cells, it is incorporated into the endogenous vimentin IF network with no apparent impact on its assembly state or organization (C–E). Bar, 10 μm.
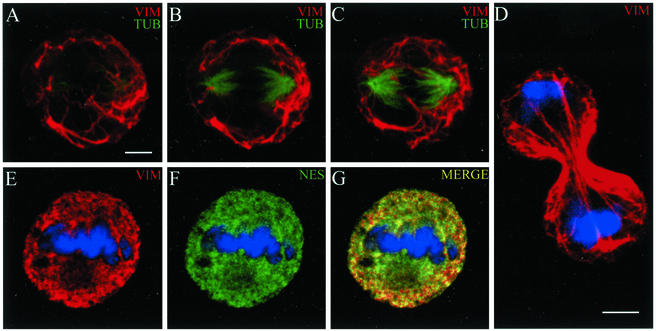
Figure 2.
Nestin expression in mitotic CHO cells. During mitosis, vimentin IFs remain intact and form a filamentous network surrounding the mitotic spindle. The relationship between vimentin IFs (red) and spindle tubulin microtubules (green) in an untransfected mitotic cell is shown in three consecutive optical sections (A–C). The vimentin IFs persist into mid to late cytokinesis (D). In mitotic cells expressing nestin, the endogenous vimentin IFs are disassembled and vimentin (red) and nestin (green) are extensively colocalized in punctate and diffuse structures (E–G). Mitotic cells were identified by the presence of condensed chromosomes (blue). Bar, 5 μm.
Nestin-Mediated Disassembly of Vimentin IFs Depends on the MPF-specific Phosphorylation of the N-Terminal Domain of Vimentin at ser-55
Because an elevated phosphorylation level is the major biochemical modification of vimentin that has been characterized during the interphase–mitosis transition, studies were performed to determine whether vimentin phosphorylation is required for the nestin-mediated disassembly of IFs during mitosis. To approach this, we performed transient transfection assays in MCF-7 cells, a cell line that expresses keratin IFs but is devoid of vimentin. This cell type allowed us to assemble a vimentin network solely from wild-type (WT) or either one of the two mitotic phosphorylation mutant vimentins (Chou et al., 1996). When these proteins were expressed individually, each formed filamentous IF networks in interphase MCF-7 cells that were indistinguishable from each other (for example, see Figure 3A, and data not shown). During mitosis, in each case, dense arrays of vimentin IFs surrounded the mitotic apparatus (see Figure 3, D–F). The fraction of cells that gave filamentous patterns was ∼84% (n = 51) for WT-vimentin, ∼90% (n = 48) for ser-55:ala-vimentin, and ∼94% (n = 33) for thr-457:ala/ser-458:ala vimentin. These observations are consistent with the idea that elevated phosphorylation alone is not sufficient to cause the disassembly of vimentin IFs during mitosis.
When nestin was coexpressed with either the WT or one of the two vimentin mutants in MCF-7 cells, nestin and vimentin colocalized in indistinguishable filamentous networks in interphase cells (for example, see Figure 3, B and C). In mitotic cells, however, the structural changes associated with the nestin/WT-vimentin and the nestin/thr-457:ala/ser-458:ala-vimentin networks were significantly different from those associated with the nestin/ser-55:ala-vimentin networks (Figure 3, G–L). The filamentous networks of the nestin/WT-vimentin (Figure 3, G and J) were disassembled in the majority (∼79%, n = 43) of doubly transfected cells. A similar result was observed in mitotic cells expressing both nestin and thr-457:ala/ser-458:ala-vimentin (Figure 3, I and L; ∼97%, n = 35). In contrast, IF networks formed from the nestin and ser-55:ala-vimentin remained largely intact (Figure 3, H and K; ∼90%, n = 42). Taken together, these observations suggest that the disassembly of vimentin IFs during mitosis requires both the presence of nestin and phosphorylation of vimentin at the MPF-specific site, ser-55.
IF Breakdown During Mitosis Is a General Feature of Cells Expressing Nestin
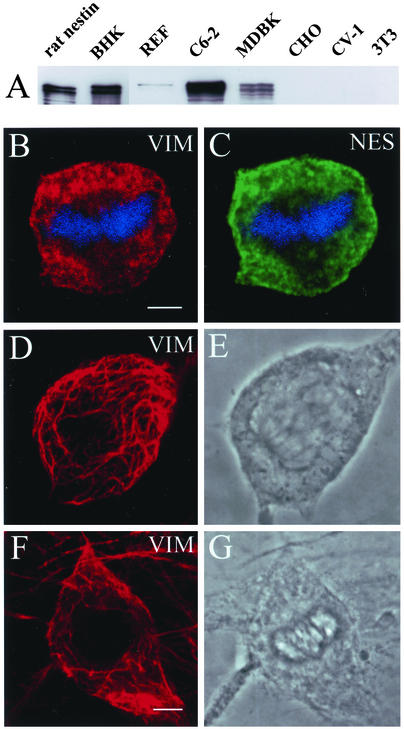
The conversion of vimentin IFs into nonfilamentous structures during mitosis has been reported in BHK-21, ST15A, and MDBK cells. Furthermore, it has been demonstrated that nestin is expressed in BHK-21 (Steinert et al., 1999) and ST15A (Sahlgren et al., 2001) cells. This led us to examine whether MDBK cells also express nestin and whether there is a correlation between the expression of nestin and the disassembly of mitotic vimentin networks in other cultured cell types. We first screened, by immunoblotting with an affinity-purified polyclonal nestin antibody (No. 268), a number of commonly used cell lines. Bacterially expressed full-length rat nestin was used as a positive control. Among the seven cell lines screened, the strongest nestin reaction was detected in IF-enriched preparations obtained from BHK-21 cells (Figure 4A) (note: the amount of the BHK IF protein sample loaded for this blotting assay was only 20% of that used for other samples). C6–2 glioma cell IF preparations gave the second strongest reaction, whereas a relatively weaker signal was obtained in IF samples prepared from MDBK cells. A very faint band in the REF sample could also be detected after longer exposure. In all positive immunoblots, the antibodies recognized primarily a doublet in the 240- to 280-kDa range, which is consistent with the sizes of nestin predicted from published cDNA sequences (Dahlstrand et al., 1992; Steinert et al., 1999; Yang et al., 2001) (also see Materials and Methods).
Figure 4.
Nestin expression in cultured cell types. (A) Immunoblotting analysis of IF-enriched cytoskeletal samples from seven cultured cell lines with an affinity-purified polyclonal nestin antibody (see Materials and Methods). In cell types such as C6–2, vimentin (VIM, red) and nestin (NES, green) are present in a nonfilamentous punctate pattern during mitosis (B and C). The mitotic state (metaphase) of this cell is indicated by the presence of condensed chromosomes (blue). In cell types such as 3T3 (D and E) and CV-1 (F and G) that do not express nestin, the vimentin IFs remain filamentous, as shown in an anaphase 3T3 cell (D and E) and a metaphase/early anaphase CV-1 cell (F and G). Bar for B–E, F, and G, 5 μm.
The cell lines expressing nestin were also examined by immunofluorescence. Double labeling of C6–2 glioma and BHK-21 cells showed that nestin expression could be detected in the majority (>95%) of the cells and that there was extensive coincidence of the vimentin and nestin staining patterns during interphase. During mitosis, the majority of these filamentous networks were disassembled into nonfilamentous structures that in C6–2 cells appeared as punctate structures surrounded by more diffuse staining (Figure 4, B and C). This latter morphological feature is very similar to the one seen in ST15A cells, which express a similar level of nestin (Sahlgren et al., 2001). In mitotic BHK-21 cells, the disassembled vimentin displayed a distinct punctate pattern (Figure 6, G–I). When the fate of IFs in mitotic REF, 3T3, CV-1, and CHO cells was examined, the majority of them appeared to be filamentous (see Figure 2, A–C, and Figure 4, D–G, for examples of CHO, 3T3, and CV-1 cells).
Figure 6.
Nestin-specific siRNA prevents the disassembly of vimentin IFs in mitotic BHK-21 cells. Interphase cells display filamentous vimentin (VIM, red) and nestin (NES, green) networks that are very similar to each other (A–C, double-label and merged images). After cells were treated with nestin siRNA, nestin fluorescence was greatly reduced or nondetectable in many cells. Reduction of nestin expression had no detectable effect on the expression or the organization of endogenous vimentin (D–F, double-label and merged images). Untreated mitotic cells had both vimentin and nestin in a punctate pattern (G–I). In mitotic cells, in which the nestin signal was greatly reduced by nestin siRNA, the vimentin remained in a filamentous pattern (J and K). The mitotic state of the cells was identified by condensed chromosomes in blue. (L) Immunoblotting analysis of vimentin and nestin in cell lysates derived from cells untreated (−) or treated (+) with luciferase GL2 siRNA (GL2) or nestin siRNA (NES). Bar, 10 μm.
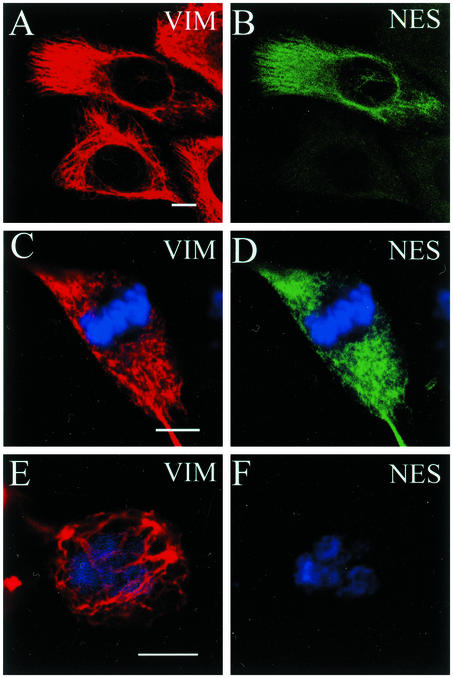
When MDBK cells were examined by immunofluorescence, the nestin signals detected in these cell lines were different from those of BHK-21 and C6–2 cells. Fewer cells (∼63%, n = 553) were fluorescent (Figure 5, A and B), and the intensity of the fluorescence was variable among the nestin-positive cells. The organizational fate of vimentin IF networks in mitotic MDBK cells also appeared to be heterogeneous. In all of the mitotic cells that lacked nestin and in almost half of the mitotic cells that appeared to express low levels of nestin, the vimentin networks remained filamentous (Figure 5, E and F). However, in ∼30% (n = 58) of the mitotic cells, vimentin IF networks appeared to be fragmented, with some punctate vimentin structures (Figure 5, C and D). In general, this latter phenotype was correlated with cells expressing higher levels of nestin, as indicated by the intensity of fluorescence. Together, these results suggest that the various filamentous states of vimentin seen during mitosis can be correlated qualitatively with different expression levels of nestin in different cells. These results provide further support for the idea that nestin expression is causally linked to the mechanism regulating the extent of vimentin IF disassembly during mitosis.
Figure 5.
Nestin expression and the assembly state of vimentin in MDBK cells. (A and B) All cells display a filamentous vimentin pattern (VIM, red), but fewer are positive for nestin (NES, green), as determined by double indirect immunofluorescence. During mitosis, cells exhibiting brighter nestin fluorescence contain a rather punctate vimentin and nestin pattern (C and D), whereas cells expressing no or low levels of nestin have a filamentous vimentin network (E and F). Mitotic chromosomes are stained with toto-3 (blue). Bar, 10 μm.
Downregulation of Nestin Expression Blocks Vimentin IF Network Breakdown during Mitosis
It was recently demonstrated that double-stranded and sequence-specific siRNAs could effectively downregulate the expression of specific genes in cultured cells (Elbashir et al., 2001; Harborth et al., 2001). We therefore designed one 21-nucleotide-long RNA duplex corresponding to sequences shared by both rat and hamster, and the siRNA was then introduced into BHK-21 or C6–2 cells to study the effects on the assembly state of vimentin IFs. As shown in Figure 6, D–F, this treatment resulted in a significant loss of nestin signal from > 50% of the BHK-21 cells. The specificity of these reagents was shown by immunoblotting analysis of siRNA-treated cells. Although the expression level of nestin was significantly reduced in cells treated with nestin siRNA, its expression was not affected in the luciferase GL2 siRNA-treated cells (Figure 6L). Furthermore, the expression levels of vimentin under these conditions appeared to be the same. By immunofluorescence, the partial or complete loss of nestin did not appear to change the organization or the assembly state of vimentin IFs during interphase (compare Figure 6, A–C and D–F). In contrast, during mitosis, the reduction of nestin by siRNA was accompanied by a concurrent change in vimentin IF organization from a punctate to a filamentous pattern (compare Figure 6, G–I and J and K). Similar results were obtained in siRNA-treated C6–2 glioma cells (data not shown).
Ectopic Expression of Nestin Has No Effect on the Basal Phosphorylation Level of Vimentin
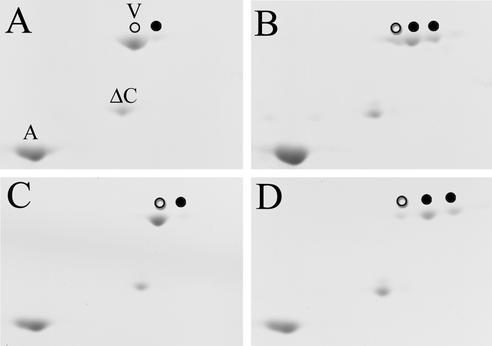
Because the incorporation of nestin into vimentin IFs could potentially change the structural properties of IFs and thereby modulate vimentin phosphorylation, we performed two-dimensional gel electrophoresis analyses to determine whether the basal level of vimentin phosphorylation was affected by the expression of nestin. This approach has been used previously in evaluating the phosphorylation state of vimentin (Evans and Fink, 1982; Celis et al., 1983). As shown in Figure 7A, vimentin derived from untransfected interphase cells migrated as two distinct spots, a major/unphosphorylated vimentin (V, open circle) and a minor acidic/phosphorylated variant (closed circle). Conversely, vimentin prepared from untransfected mitotic cells was resolved into three distinct spots, a minor/unphosphorylated vimentin (Figure 7B, open circle) and two more prominent acidic/phosphorylated variants (Figure 7B, closed circles). When samples from nestin-transfected cells were compared with those derived from untransfected cells, the relative abundance of the unphosphorylated vimentin and its acidic variants was not obviously altered (compare Figure 7, A and B, with C and D). We also performed similar two-dimensional gel analyses with samples prepared from untreated and nestin siRNA–treated BHK-21 cells. No detectable difference in phosphorylation levels was observed between these two sets of samples (data not shown).
Figure 7.
The expression of nestin does not affect the phosphorylation state of endogenous vimentin. IF-enriched preparations from untransfected (A and B) and nestin-transfected (38% transfection rate; C and D) CHO cells were analyzed by two-dimensional gel electrophoresis, and the phosphorylation states of vimentin were determined by the relative abundance of unphosphorylated and phosphorylated (more acidic) electrophoretic variants. In interphase samples (A and C), vimentin is largely unphosphorylated (marked as V, open circle), and only a small fraction of it exits as a more acidic variant (closed circle). However, when cells enter mitosis, the phosphorylation state of vimentin changes significantly (B and D), with the majority of the vimentin present as two more acidic variants (closed circles), and only a small fraction of it remains unphosphorylated (open circle). The positions of the endogenous actin (A), which is frequently associated with crude IF preparations, and the exogenously added ΔC-vimentin (ΔC) were used as mobility reference points.
These results are consistent with our previous observation that vimentin is only slightly phosphorylated at ∼0.3 mol Pi/mol protein during interphase, and the level of phosphorylation is elevated approximately sixfold to ∼1.9 mol Pi/mol protein when cells enter mitosis (Chou et al., 1989). The single acidic vimentin variant associated with the interphase sample is probably a result of the partial phosphorylation of one of many previously identified interphase-specific sites (Inagaki et al., 1997). The two acidic vimentin variants seen in mitotic samples can be accounted for by the two major phosphorylation sites at ser-55 and ser-458 (Chou et al., 1996). Together, our results suggest that the presence or absence of nestin does not significantly alter the phosphorylation state of vimentin. However, the transition from interphase to mitosis appears to be the major contributing factor in elevating the phosphorylation state of vimentin.
DISCUSSION
Although it has been known for many years that there are significant differences in the assembly states of vimentin IFs during mitosis in different cell types, the mechanisms responsible for these variations are not understood. The phosphorylation of vimentin at ser-55 by the ubiquitous mitotic kinase MPF has been shown to be essential for the disassembly of vimentin IFs during mitosis (Chou et al., 1996). Furthermore, this site has been conserved during vertebrate evolution (Herrmann et al., 1996b). However, the persistence of vimentin IFs in many other cell types (Aubin et al., 1980; Jones et al., 1985) during mitosis suggests that the phosphorylation of vimentin by MPF alone is insufficient to disassemble IF networks. The results of this study identify nestin as a modulator of IF structure during mitosis. Furthermore, the results show that nestin works in concert with MPF to induce the disassembly of vimentin IFs during mitosis.
Several lines of evidence support the synergistic roles of nestin and the specific phosphorylation of vimentin by MPF to achieve an extensive disassembly of IFs during mitosis. For example, the ectopic expression of nestin in vimentin+/nestin− cell types, such as CHO cells, promotes the disassembly of vimentin IFs during mitosis, whereas there are no apparent effects on the organization of interphase IF networks. In addition, this nestin-mediated disassembly of IF networks is dependent on vimentin phosphorylation at ser-55, the MPF-specific site. Furthermore, the nestin-facilitated disassembly of IFs in mitosis is blocked by the siRNA-mediated downregulation of nestin expression. Finally, we show that the nestin-mediated disassembly of vimentin IFs during mitosis is a general feature of cell types expressing sufficient amounts of nestin.
The molecular basis of how nestin and vimentin phosphorylation by MPF work synergistically to disassemble the copolymers of vimentin and nestin is unknown. Previous studies indicate that nestin and vimentin can form heterodimers and heterotetramers in vitro. It has also been demonstrated in vitro that nestin-vimentin heterodimers and heterotetramers are less stable than similar oligomers formed by vimentin alone when subjected to increasing concentrations of urea (Steinert et al., 1999). This latter observation may be partly because nestin has only a short amino-terminal head domain. In the case of vimentin homopolymer IFs, it is known that the head domain plays an important role in dimer-dimer interaction (Herrmann et al., 1996a). Therefore, the presence of heterodimers containing only one full-length head (i.e., that of vimentin) may lead to the formation of less stable though still long IFs. As a consequence, it is conceivable that the phosphorylation of the vimentin head domain in a vimentin/nestin heteropolymer would generate a dimer or tetramer unable to sustain interactions essential for maintaining the structural integrity of IFs, thereby leading to the fragmentation of filaments and ultimately disassembly.
Vimentin is phosphorylated throughout the cell cycle, but the phosphorylation level is significantly higher during mitosis. The apparently normal IF networks seen during interphase in nestin-expressing cells suggest that a higher level of phosphorylation is required for nestin to exert its effect on vimentin structure, and this level of phosphorylation may be achieved only when cells enter mitosis (Chou et al., 1989). Consistent with this idea is the observation that interphase vimentin IFs in BHK-21 cells are rapidly disassembled when cells are exposed to low doses of the phosphatase inhibitor calyculin A, which elevates the phosphorylation level of vimentin (Eriksson et al., 1992). Furthermore, phosphorylation of nestin by MPF during mitosis has also been correlated with the reorganization of vimentin IFs in ST15A cells (Sahlgren et al., 2001). Although it has not been explored in this study, the phosphorylation of nestin may also play a role in the extent of disassembly of vimentin IFs during mitosis. Finally, the persistence of vimentin IFs during mitosis in some of the nestin+ MDBK cells described in this study suggests that a critical ratio of nestin/vimentin is required for nestin to exert the above-described effects on vimentin IF assembly states. This latter suggestion is supported by the dose-dependent effects of nestin on the disassembly of vimentin filaments in vitro (Steinert et al., 1999).
Like those of vimentin, the phosphorylation levels of the keratins are elevated in mitosis, and the organizational fates of keratin IF networks vary from one cell type to another (Horwitz et al., 1981; Franke et al., 1982; Lane et al., 1982; Celis et al., 1983; Ku and Omary, 1994). It is therefore likely that variations in the disassembly of keratin IFs observed in different types of epithelial cells are also regulated by keratin phosphorylation, along with the expression of unique keratin-associated proteins (Fuchs and Karakesisoglou, 2001; Leung et al., 2002). Because nestin does not appear to coassemble with keratin IFs in epithelial cell types such as those used in this study (MCF-7, MDBK), proteins other than nestin are most likely responsive for their disassembly. A number of other IF proteins, such as synemin (Bellin et al., 1999), paranemin (Hemken et al., 1997), and syncoilin (Newey et al., 2001), exhibit some properties similar to those of nestin, because they cannot assemble into IFs on their own. Each of these proteins requires a type III IF protein for its assembly into IFs (Schweitzer et al., 2001). Therefore, they could potentially function like nestin, regulating the dynamic properties or assembly states of IF networks in different cells and tissues as well as in different stages of development.
The disassembly of vimentin IFs during mitosis is obviously not required for mitosis per se, because many cell types do not express nestin. Furthermore, phosphorylation at ser-55 is not essential for the distribution of vimentin IFs to daughter cells (Yasui et al., 2001). In mitotic cells in which there is no obvious disassembly of vimentin IFs, the partitioning of IFs into daughter cells is facilitated by a highly localized phosphorylation and disassembly of IFs restricted to the cleavage furrow in late cytokinesis. This involves phosphorylation of vimentin at multiple specific sites by C-kinase, rho-kinase, and an unidentified protein kinase. Mutation of these sites produces an abnormally long IF-enriched bridge between daughter cells (Goto et al., 2000; Yasui et al., 2001).
The potential benefit of the mitotic disassembly of vimentin IFs for cells expressing nestin remains an open question. However, nonfilamentous vimentin in the form of particles has also been observed in spreading interphase cells. These particles move at high speeds along microtubules because of their association with the molecular motors such as kinesin and dynein (Prahlad et al., 1998; Helfand et al., 2002). Motile and nonfilamentous keratin structures have also been reported in mitotic epithelial cells (Windoffer and Leube, 1999). One of the suggested functions for the fast-moving nonfilamentous IF structures is to provide a rapid transit system to move IF precursors between various cytoplasmic compartments. In light of the unusually long C-terminus (>1200 amino acids) of nestin, which is likely to interact with other cellular factors, nonfilamentous IF particles could potentially carry other “cargoes” in their journey. Therefore, the expression of nestin may be associated with an increase in cytoplasmic trafficking required for progenitor cells undergoing rapid rounds of division, interspersed with active interphase migration. These activities are hallmarks of the nestin-expressing cells found in early developing nerve and muscle systems (Lendahl et al., 1990; Sejersen and Lendahl, 1993; Kachinsky et al., 1995; Vaittinen et al., 1999) and in cells responding to the injury and regeneration of adult tissues (Frisen et al., 1995; Vaittinen et al., 1999).
From the developmental perspective, the nestin-expressing progenitor or stem cells of the early developing neural tube are organized in a polarized manner between the inner ventricular edge and the outer pial surface. As the cell number increases, proliferation is confined primarily to the ventricular zone, whereas postmitotic differentiating neurons migrate toward the pial surface (Frederiksen and McKay, 1988; Rakic, 1988). The polarized distribution of the dividing and differentiating cells within the neuroepithelium may be caused by the uneven partitioning of key cellular components during cell divisions of the progenitor cells. In this regard, the nestin-mediated disassembly of IFs and the motility of vimentin particles during mitosis could also take part in the asymmetric allocation of cytoskeletal and other cellular factors to daughter cells.
ACKNOWLEDGMENTS
This study was supported by a MERIT grant from the National Institute of General Medical Sciences.
Abbreviations used:
- IF
intermediate filament
- MPF
maturation/M-phase promoting factor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–08–0545. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–08–0545.
REFERENCES
- Aubin JE, Osborn M, Franke WW, Weber K. Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp Cell Res. 1980;129:149–165. doi: 10.1016/0014-4827(80)90340-7. [DOI] [PubMed] [Google Scholar]
- Bellin RM, Sernett SW, Becker B, Ip W, Huiatt TW, Robson RM. Molecular characteristics and interactions of the intermediate filament protein synemin: interactions with alpha-actinin may anchor synemin-containing heterofilaments. J Biol Chem. 1999;274:29493–29499. doi: 10.1074/jbc.274.41.29493. [DOI] [PubMed] [Google Scholar]
- Celis JE, Larsen PM, Fey SJ, Celis A. Phosphorylation of keratin and vimentin polypeptides in normal and transformed mitotic human epithelial amnion cells: behavior of keratin and vimentin filaments during mitosis. J Cell Biol. 1983;97:1429–1434. doi: 10.1083/jcb.97.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou YH, Opal P, Quinlan RA, Goldman RD. The relative roles of specific N- and C-terminal phosphorylation sites in the disassembly of intermediate filament in mitotic BHK-21 cells. J Cell Sci. 1996;109:817–826. doi: 10.1242/jcs.109.4.817. [DOI] [PubMed] [Google Scholar]
- Chou YH, Rosevear E, Goldman RD. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci USA. 1989;86:1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. Integrating the actin and vimentin cytoskeletons: adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J Cell Biol. 1999;146:831–842. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Brautigan DL, Vallee R, Olmsted J, Fujiki H, Goldman RD. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci USA. 1992;89:11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Fink LM. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982;29:43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Grund C, Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982;30:103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes Dev. 2001;15:1–14. doi: 10.1101/gad.861501. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Goldman A. Isolation and culture of fibroblasts. In: Goldman RD, Spector DL, Leinwand LA, editors. Cells: A Laboratory Manual. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1998. pp. 4.1–4.7. [Google Scholar]
- Goto H, Kosako H, Inagaki M. Regulation of intermediate filament organization during cytokinesis: possible roles of Rho-associated kinase. Microsc Res Tech. 2000;49:173–182. doi: 10.1002/(SICI)1097-0029(20000415)49:2<173::AID-JEMT10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Mikami A, Vallee RB, Goldman RD. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol. 2002;157:795–806. doi: 10.1083/jcb.200202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemken PM, Bellin RM, Sernett SW, Becker B, Huiatt TW, Robson RM. Molecular characteristics of the novel intermediate filament protein paranemin: sequence reveals EAP-300 and IFAPa-400 are highly homologous to paranemin. J Biol Chem. 1997;272:32489–32499. doi: 10.1074/jbc.272.51.32489. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Haner M, Brettel M, Muller SA, Goldie KN, Fedtke B, Lustig A, Franke WW, Aebi U. Structure and assembly properties of the intermediate filament protein vimentin: the role of its head, rod and tail domains. J Mol Biol. 1996a;264:933–953. doi: 10.1006/jmbi.1996.0688. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Munick MD, Brettel M, Fouquet B, Markl J. Vimentin in a cold-water fish, the rainbow trout: highly conserved primary structure but unique assembly properties. J Cell Sci. 1996b;109:569–578. doi: 10.1242/jcs.109.3.569. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Kupfer H, Eshhar Z, Geiger B. Reorganization of arrays of prekeratin filaments during mitosis: immunofluorescence microscopy with multiclonal and monoclonal prekeratin antibodies. Exp Cell Res. 1981;134:281–290. doi: 10.1016/0014-4827(81)90427-4. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Inagaki N, Takahashi T, Takai Y. Phosphorylation-dependent control of structures of intermediate filaments: a novel approach using site- and phosphorylation state- specific antibodies. J Biochem (Tokyo) 1997;121:407–414. doi: 10.1093/oxfordjournals.jbchem.a021603. [DOI] [PubMed] [Google Scholar]
- Jones JC, Goldman AE, Yang HY, Goldman RD. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J Cell Biol. 1985;100:93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem. 1995;43:843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- Ku NO, Omary MB. Identification of the major physiologic phosphorylation site of human keratin 18: potential kinases and a role in filament reorganization. J Cell Biol. 1994;127:161–171. doi: 10.1083/jcb.127.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane EB, Goodman SL, Trejdosiewicz LK. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1:1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leung CL, Green KJ, Liem RK. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 2002;12:37–45. doi: 10.1016/s0962-8924(01)02180-8. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111:1951–1961. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem. 2001;276:6645–6655. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Yoon M, Moir RD, Vale RD, Goldman RD. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J Cell Biol. 1998;143:159–170. doi: 10.1083/jcb.143.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rosevear ER, McReynolds M, Goldman RD. Dynamic properties of intermediate filaments: disassembly and reassembly during mitosis in baby hamster kidney cells. Cell Motil Cytoskeleton. 1990;17:150–166. doi: 10.1002/cm.970170303. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456–16463. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- Schweitzer SC, Klymkowsky MW, Bellin RM, Robson RM, Capetanaki Y, Evans RM. Paranemin and the organization of desmin filament networks. J Cell Sci. 2001;114:1079–1089. doi: 10.1242/jcs.114.6.1079. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein: limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881–9890. doi: 10.1074/jbc.274.14.9881. [DOI] [PubMed] [Google Scholar]
- Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem. 1994;269:31097–31106. [PubMed] [Google Scholar]
- Vaittinen S, Lukka R, Sahlgren C, Rantanen J, Hurme T, Lendahl U, Eriksson JE, Kalimo H. Specific and innervation-regulated expression of the intermediate filament protein nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am J Pathol. 1999;154:591–600. doi: 10.1016/S0002-9440(10)65304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R, Leube RE. Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. J Cell Sci. 1999;112:4521–4534. doi: 10.1242/jcs.112.24.4521. [DOI] [PubMed] [Google Scholar]
- Yang HY, Lieska N, Goldman AE, Goldman RD. Colchicine-sensitive and colchicine-insensitive intermediate filament systems distinguished by a new intermediate filament-associated protein, IFAP-70/280 kD. Cell Motil Cytoskeleton. 1992;22:185–199. doi: 10.1002/cm.970220306. [DOI] [PubMed] [Google Scholar]
- Yang J, Cheng L, Yan Y, Bian W, Tomooka Y, Shiurba R, Jing N. Mouse nestin cDNA cloning and protein expression in the cytoskeleton of transfected cells. Biochim Biophys Acta. 2001;1520:251–254. doi: 10.1016/s0167-4781(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Goto H, Matsui S, Manser E, Lim L, Nagata K, Inagaki M. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene. 2001;20:2868–2876. doi: 10.1038/sj.onc.1204407. [DOI] [PubMed] [Google Scholar]
- Zieve GW, Heidemann SR, McIntosh JR. Isolation and partial characterization of a cage of filaments that surrounds the mammalian mitotic spindle. J Cell Biol. 1980;87:160–169. doi: 10.1083/jcb.87.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]