Abstract
The (11;19)(q23;p13.1) translocation in acute leukemia results in the formation of a chimeric MLL-ELL fusion protein. ELL is an RNA Polymerase II (Pol II) transcriptional elongation factor that interacts with the recently identified EAF1 protein. Here, we show that ELL and EAF1 are components of Cajal bodies (CBs). Although ELL and EAF1 colocalize with p80 coilin, the signature protein of CBs, ELL and EAF1 do not exhibit a direct physical interaction with p80 coilin. Treatment of cells with actinomycin D, DRB, or α-amanitin, specific inhibitors of Pol II, disperses ELL and EAF1 from CBs, indicating that localization of ELL and EAF1 in CBs is dependent on active transcription by Pol II. The concentration of ELL and EAF1 in CBs links the transcriptional elongation activity of ELL to the RNA processing functions previously identified in CBs. Strikingly, CBs are disrupted in MLL-ELL leukemia. EAF1 and p80 coilin are delocalized from CBs in murine MLL-ELL leukemia cells and in HeLa cells transiently transfected with MLL-ELL. Nuclear and cytoplasmic fractionation revealed diminished expression of p80 coilin and EAF1 are not present in the nuclei of MLL-ELL leukemia cells. These studies are the first demonstration of a direct role of CB components in leukemogenesis.
INTRODUCTION
The ELL gene was first identified as a fusion partner gene of MLL in the (11;19)(q23;p13.1) translocation, a recurring chromosomal aberration in acute myeloid leukemia (Thirman et al., 1994). Subsequent studies revealed that ELL functions as an RNA polymerase II transcriptional elongation factor (Shilatifard et al., 1996). In addition to ELL, several different factors with elongation activity have been identified including TFIIS, p-TEFb, TFIIF, Elongin, ELL2, and FACT. The function of one group of these factors, which includes ELL, TFIIF, and Elongin, is to facilitate the processivity of transcription by suppressing transient pausing by Pol II (Conaway et al., 2000).
Using ELL as the bait in a yeast two-hybrid screen, we recently isolated a novel protein that we named EAF1 for ELL-Associated Factor 1 (Simone et al., 2001). We found that endogenous ELL and EAF1 coimmunoprecipitated as a complex in multiple cell types. We identified an EAF1 interaction domain that mapped to amino acids 508–621 of ELL. EAF1 contains a region that is rich in serine, aspartic acid, and glutamic acid residues that exhibits limited homology with the transactivation domains of AF4, LAF4, and AF5q31 proteins that fuse to MLL in 11q23 chromosome translocations. We identified a similar transactivation domain within this region of EAF1. By confocal microscopy, ELL and EAF1 colocalized in a distinct stippled pattern within nuclei. In dividing cells, ELL and EAF1 were distributed diffusely, consistent with the dissolution of the nuclear membrane and the lack of transcription by Pol II during mitosis.
Recently, we showed that retroviral infection of murine hematopoietic progenitor cells with MLL-ELL followed by transplantation into lethally irradiated littermates resulted in the development of acute myeloid leukemia (Lavau et al., 2000). Similarly, murine hematopoietic progenitor cells transduced with MLL-ELL became immortalized in vitro. In structure function studies undertaken to assess the critical contributions of ELL to the MLL-ELL fusion, we found that the elongation domain of ELL was dispensable but that its EAF1 interaction domain was necessary and sufficient for the immortalization of hematopoietic progenitor cells and for the development of acute leukemia (Luo et al., 2001). To address whether the EAF1 interaction domain was the critical functional contribution of ELL to the MLL-ELL fusion protein, we examined the transforming properties of a heterologous MLL-EAF1 fusion. Although EAF1 has not been identified as a partner protein in 11q23 translocations, MLL-EAF1 demonstrated the capacity to immortalize primary hematopoietic cells in vitro, and mice transplanted with cells transduced with MLL-EAF1 developed acute leukemia similar to that induced by MLL-ELL. At this time, human cell lines derived from MLL-ELL leukemia cells have not been generated. To examine the effect of MLL-ELL on EAF1 nuclear foci, we previously examined cell lines derived from MLL-ELL leukemic mice and found that EAF1 foci were not detectable. These data suggested that the nuclear compartment that normally contains ELL and EAF1 might play a critical role in the pathogenesis of MLL-ELL leukemia.
We have now determined that ELL and EAF1 are components of Cajal bodies. This nuclear organelle, previously referred to as the coiled body, contains numerous factors involved in RNA processing (Gall, 2000). The compartmentalization of proteins within nuclear structures is critical to the regulation of gene expression (Carmo-Fonseca, 2002). CBs have also been detected adjacent to specific genetic loci including snRNA genes and histone gene clusters (Frey and Matera, 1995; Smith et al., 1995). The localization of ELL and EAF1 within CBs suggests that the elongation activity of ELL may be targeted to sites of RNA processing either within or adjacent to CBs. In MLL-ELL murine leukemia cells, CBs are disrupted, resulting in the delocalization of EAF1 and p80 coilin from nuclei. The disruption of nuclear architecture is a feature of several other disease states including viral infection, spinal muscular atrophy, and acute promyelocytic leukemia (Lamond and Earnshaw, 1998). The studies described herein are the first to implicate CB components in leukemogenesis.
MATERIALS AND METHODS
Immunofluorescence
HeLa cells were grown for 24 h on glass coverslips coated with 0.2% gelatin, washed with PBS, and then fixed in one of two conditions: 3.7% formaldehyde in PBS at room temperature followed by permeabilization with 0.2% TX-100 or fixed at −20°C in methanol with or without further permeabilization in −20°C acetone. Incubation with the primary and secondary antibodies, quenching, and staining with 4′,6-diamidino-2-phenylindole (DAPI) were performed as previously described (Simone et al., 2001). For the cytospins of the murine cell lines, 0.3 ml of cells at a concentration of 1 × 106/ml were adhered to a poly-l-lysine–coated glass coverslip mounted in a cytocentrifuge (Wescor, Logan, UT). Following centrifugation, mouse cells were processed like HeLa cells. Fluorescence images were obtained with a Zeiss Axiophot microscope and confocal images were obtained with a Zeiss/NORAN system.
Antibodies
For immunofluorescence with human cells, the affinity-purified polyclonal anti-ELL antisera was used at a 1:250 dilution and the anti-EAF1 mAb at a 1:50 dilution. Additional antibodies used and the dilutions are as follows: rabbit 288 anti-p80 coilin (Dr. Chan) at a dilution of 1:250, biotinylated mouse anti-FLAG (Sigma, St. Louis, MO) at a 1:350 dilution, mouse anti-cyclin E (Calbiochem, La Jolla, CA) at 1:100, mouse antifibrillarin (Cytoskeleton, Denver, CO) at 1:1000, mouse anti-human nucleoli antibody (Chemicon, Temulcala, CA) at 1:2000, human auto-antibody against p80 coilin (Dr. Bloch) at 1:200, mouse anti-Gemin2 (Dr. Dreyfuss) at 1:500, rabbit anti-Nopp140(RF12) at 1:250 (Dr. Meier), guinea pig anti–NOH61–4.2 (Dr. Schmidt-Zachmann) at 1:100 (−20°C methanol fixation, acetone permeabilization), rabbit anti-TFIIF(RAP74) (Santa Cruz, Santa Cruz, CA) at 1:50, goat anti-SMN (Santa Cruz) at 1:10 (−20°C methanol fixation), goat anti-Elongin A(R 19) (Santa Cruz) at 1:10, mouse anti-Sm(Y12) (Neo Markers, Fremont, CA) at 1:500. For immunofluorescence with mouse cells, affinity-purified polyclonal anti-ELL antiserum was used at a 1:25 dilution, the anti-EAF1 mAb at a 1:10 dilution and the human auto-antibody against p80 coilin (Dr. Bloc, West Grove, PA) at 1:125 dilution. Secondary antibodies (Jackson ImmunoResearch) were diluted 1:1 with glycerol, stored at −20°C, and then used at a further dilution of 1:100. For the immunoprecipitations, the cell extracts were incubated with the EAF1 monoclonal or the isotype control monoclonal at 1:10, and with the anti-FLAG monoclonal (Sigma) at 1:500. For the Western blots, the membranes were incubated with anti–FLAG-M2 (Sigma) at 1:1000, polyclonal anti-ELL antisera at 1:1000, anti-EAF1 at 1:10, anti–p80-coilin at 1:500, and anti-HDAC1 (Santa Cruz) at 1:1000.
Cell Culture, Transient Transfection, and Immunoprecipitation
The open reading frames of ELL, EAF1, and p80-coilin were cloned in the pFLAG-CMV2 expression vector and transiently transfected in the human 293 cell line using Effectene (Qiagen, Valencia, CA). Cell pellets were resuspended in 1 ml TEN (40 mM Tris, 1 mM EDTA, 150 mM NaCl) buffer, centrifuged for 5 min at 1200 × g at 4°C, lysed with 0.5 ml NETN (100 mM NaCl, 20 mM Tris, pH 8.0, 1 mM EDTA, and 0.2% NP-40) containing a cocktail of protease inhibitors (Sigma), incubated on ice for 10 min, and centrifuged at 2500 × g for 30 min at 4°C. To precipitate the complexes, supernatants were precleared with 30 μL protein A/G agarose beads (Santa Cruz) for 30 min and then incubated for 1 h with the FLAG-M2 antibody at 1:500. Thirty microliters of a 50% slurry of protein A/G agarose beads was then added, incubated overnight at 4°C, washed five times at 4°C with lysis buffer, boiled in Laemmli sample buffer, and fractionated by SDS-PAGE. For the incubations with inhibitors of Pol II, cells were incubated with either actinomycin D (Sigma) at 5 μg/ml for 3 h, 5,6-dichlorobenzimidazole riboside (DRB; Sigma) at 50 μM for 16 h, or α-amanitin (Sigma) at 20 μg/ml for 5 h.
Western Blot Analysis
Extracts were prepared from cultured cells, electrophoresed in SDS-PAGE gels, and blotted onto PVDF membranes (Millipore, Bedford, MA) using a transfer buffer with 25 mM Tris, 192 mM glycine, 0.1% SDS, and 20% methanol (pH 8.3). The membranes were blocked in 5% nonfat dry milk in TBS with 0.05% Tween 20 (TBST) and incubated with the indicated primary antibody. The membranes were washed in TBST and then incubated with HRP-conjugated second antibodies (Santa Cruz). After five washes with TBST, the protein bands were detected with an enhanced chemiluminescence protocol (Amersham, Piscataway, NJ).
Preparation of Nuclear and Cytoplasmic Extracts
Cell lines were grown as a suspension in RPMI supplemented with 10% FBS, 4 mM l-glutamine, and penicillin/streptomycin. Cells were harvested by centrifugation at 600 × g for 3 min. The resulting pellet was resuspended and washed in HBSS with another centrifugation. The cell pellet was resuspended in 10 pellet volumes of RSB buffer containing 10 mM NaCl, 1.5 mM MgCl2, and 10 mM Tris-HCl, pH 7.4 for 10 min on ice. The swollen cells were disrupted with a tight-fitting glass dounce homogenizer using 36 strokes with a fast upward movement of the pestle. Complete cellular disruption was monitored by phase contrast microscopy. Nuclei were pelleted for 3 min at 1000 × g in a microfuge at 4°C. The cytoplasmic supernatant fraction was spun once more to ensure complete removal of nuclei. The nuclear pellet was washed two more times with RSB buffer with centrifugation as above. The nuclei were extracted, with rotation, for 30 min at 4°C after resuspension of the pellet in one-half pellet volume of low salt buffer (20 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2, 20 mM KCl, and 0.2 mM EDTA), followed by one-half pellet volume of high salt buffer (substituting 1.2 M for 20 mM KCl). All solutions contained a eukaryotic cell protease inhibitor cocktail at a 20× dilution (Sigma), 1 mM EDTA, and Antipain at a 100× dilution (Sigma). The nuclear extract was pelleted for 30 min in a microfuge at 14,500 rpm at 4°C. The resulting supernatant was dialyzed against 20 mM HEPES, pH 7.9, 20% glycerol, 100 mM KCl, and 0.2 mM EDTA and labeled as the nuclear extract. Using the Bradford method, protein concentration was determined for both the fractions, which were then boiled in Laemmli sample buffer before SDS-PAGE.
RESULTS
ELL and EAF1 Are Components of Cajal Bodies
We previously observed that ELL and EAF1 colocalized in a nuclear stippled pattern in multiple cell types. In dividing cells, ELL and EAF1 antibodies detected a diffuse punctate pattern, consistent with the dissolution of the nuclear membrane during mitosis. To determine the nature of the ELL/EAF1 nuclear foci, we previously examined antibodies to a number of candidate proteins present in nuclear structures including SC-35 and PML, with no evidence for colocalization with ELL or EAF1. To investigate possible associations of ELL and EAF1 within the nucleus, we incubated multiple cell types with antibodies to p80 coilin, ELL, and EAF1. In HeLa cells incubated simultaneously with a human polyclonal antiserum reactive to p80 coilin, the ELL antiserum, and the EAF1 monoclonal, the merged images demonstrated colocalization of these three proteins, confirming the presence of ELL and EAF1 within CBs (Figure 1A). The specificity of these antibodies is shown in Figure 2. To confirm these findings, we used a rabbit polyclonal antiserum to p80 coilin and a mouse mAb to EAF1 and observed colocalization by confocal microscopy (Figure 1B).
Figure 1.
(A) ELL and EAF1 colocalize with p80 coilin in CBs. HeLa cells were incubated with antibodies to ELL, EAF1, and p80 coilin. In the top left, immunofluorescence with a mouse mAb to EAF1 detected with donkey anti-mouse antibodies conjugated to RRX; in the bottom left, immunofluorescence with a rabbit polyclonal antiserum to ELL detected with donkey anti-rabbit antibodies conjugated to Cy5; and in the top right immunofluorescence with a human anticoilin serum detected with donkey anti-human antibodies conjugated to FITC. A merged image in the bottom right demonstrates colocalization of the three proteins as indicated by white foci formed by the merging of red, green, and blue signals. (B) ELL and EAF1 colocalize with multiple CB components. In the left column, confocal microscopy images of HeLa cells that were incubated with rabbit polyclonal antibodies to CB components and detected with goat anti-rabbit antibodies conjugated to FITC. In the middle column, confocal images of cells incubated with mouse monoclonal antibodies detected with goat anti-mouse antibodies conjugated to Cy5. Merged images are in the right column. The top panel shows colocalization of EAF1 with p80 coilin detected with the rabbit 288 antiserum. In the second panel, the images demonstrate colocalization of ELL with the Sm antigen. Sm also exhibits a diffuse nucleoplasmic distribution. In the third panel, ELL colocalizes with fibrillarin. Fibrillarin is found primarily in nucleoli and also in CBs. In the fourth panel, colocalization of ELL with cyclin E is observed. In the bottom panel, merged images demonstrate colocalization of NOPP140 with EAF1. Similar to fibrillarin, NOPP140 is localized in nucleoli and in CBs. (C) ELL and EAF1 are not components of Gems. (A) In the top panel, confocal microscopy images of HeLa cells incubated with a rabbit polyclonal antiserum to SMN and a mouse monoclonal to EAF1. In the second panel, confocal images of HeLa cells incubated with a polyclonal antiserum to ELL and a mouse monoclonal to Gemin2. In the merged images, SMN and Gemin2 are adjacent but not colocal with ELL and EAF1. (D) TFIIF and Elongin A do not colocalize with ELL and EAFI. In the top panel, confocal microscopy images of HeLa cells incubated with rabbit polyclonal antibodies to TFIIF and the mouse mAb to EAF1. In the bottom panel, confocal images of a goat polyclonal antibody to Elongin A and the rabbit polyclonal antiserum to ELL. TFIIF and Elongin A both exhibit a diffuse nucleoplasmic distribution. In merged images, colocalization with ELL or EAF1 is not detected.
Figure 2.
Specificity of antibodies to ELL, EAF1, and p80 coilin. Human 293 cells were transfected with FLAG-tagged ELL, EAF1, and p80 coilin. In the left panel, cell lysates were probed with the FLAG antibody demonstrating expression of each of the constructs. In the second panel, the cell lysates were immunoblotted with the ELL polyclonal antiserum, the EAF1 mAb in the third panel, the rabbit 288 polyclonal antiserum to p80 coilin in the fourth panel, and the human antiserum to p80 coilin in the fifth panel. Each of the antibodies exhibited specificity with no cross-reactivity to other CB components.
ELL and EAF1 Colocalize with Sm, Fibrillarin, NOPP140, and Cyclin E
The detection of proteins that colocalize with p80 coilin has facilitated the identification of other CB components. To examine the relationship of ELL and EAF1 to other CB proteins, we incubated HeLa cells with antibodies to other CB components including Sm, fibrillarin, NOPP140, and cyclin E (Figure 1B). In addition to their presence in CBs, these proteins have additional sites of subcellular localization. Fibrillarin and NOPP140 are found primarily in nucleoli. The Sm antigen is the core protein of small ribonucleoproteins, which are present in the nucleoplasm and concentrated in CBs. The cyclin E-CDK2 complex is present in CBs and also in the nucleoplasm (Liu et al., 2000). We observed that ELL colocalized with Sm, fibrillarin, NOPP140, and cylin E within CBs. Although p80 coilin has both cytoplasmic and nucleoplasmic components, it is primarily concentrated within CBs. In addition to nuclear foci, ELL and EAF1 also exhibit faint cytoplasmic and nucleoplasmic distributions within cells. However, ELL and EAF1 are also predominantly localized to CBs. The striking similarity in the subcellular localization of ELL, EAF1, and p80 coilin suggests that antibodies to ELL and EAF1 could also be used to define the presence of CBs.
ELL and EAF1 Are Not Present within Gems
Gems are structures adjacent to CBs that contain the SMN and Gemin2 proteins (Liu and Dreyfuss, 1996). Gems are not found in all cell types, and the presence of gems varies in different strains of the HeLa cell line (Matera and Frey, 1998). SMN and Gemin2 have also been identified in CBs (Carvalho et al., 1999). In the HeLa strain that we examined, SMN and Gemin2 were distinct from ELL and EAF1, indicating that ELL and EAF1 are restricted to CBs and not to gems (Figure 1C).
Nuclear Distribution of Elongation Factors
To examine the relationship of other general elongation factors to CBs, we incubated HeLa cells with antibodies to Elongin A and to the RAP74 subunit of TFIIF. Both of these factors exhibited a diffuse nucleoplasmic distribution (Figure 1D). We did not observe a more focal expression pattern for Elongin A and TFIIF either in CBs or elsewhere in the nucleus. In light of the diffuse nucleoplasmic distribution of these factors, the presence of Elongin A and TFIIF in CBs cannot be excluded. In contrast, ELL and EAF1 are targeted primarily to CBs, with a minimal distribution in the remainder of the nucleoplasm. The predominant localization of ELL and EAF1 in CBs suggests that specific targets for the transcriptional elongation activity of ELL might exist either within or adjacent to CBs.
The Presence of ELL and EAF1 in CBs Correlates with RNA Synthesis
To determine the relationship of mRNA synthesis to the localization of ELL and EAF1 in CBs, we incubated HeLa, U-2OS, and WI-38 cells with specific inhibitors of Pol II–mediated transcription. Actinomycin D inhibits Pol I at low concentrations (0.04 μg/ml) and Pol II at higher concentrations (5 μg/ml). DRB inhibits RNA synthesis by Pol II by causing premature termination. In addition, α-amanitin inhibits Pol II at low levels and Pol III at high levels. In cells treated with actinomycin D at 5 μg/ml for 3 h, ELL and EAF1 exhibited a diffuse nucleoplasmic distribution with no apparent localization within CBs (Figure 3A). In contrast, p80 coilin localized to discrete ringed foci at the periphery of nucleoli. A similar pattern of p80 coilin localization has previously been observed in cells treated with actinomycin D (Raska et al., 1990). Treatment with actinomycin D and DRB also changes the morphology of nucleoli due to segregation and disintegration (Raska et al., 1990). In cells treated with DRB at 50 μM for 16 h, p80 coilin also demonstrated a ringed pattern on the periphery of nucleoli (Figure 3B). In contrast to the pattern observed with actinomycin D treatment, ELL exhibited a similar pattern to p80 coilin in DRB treated cells, with a ringed localization at the periphery of nucleoli. However, EAF1 exhibited a diffuse nuclear distribution, with no localization apparent near nucleoli or within CBs. In cells treated with α-amanitin at 20 μg/ml, ELL and EAF1 also exhibited a diffuse nucleoplasmic distribution, whereas p80 coilin localized within ringed foci near nucleoli (Figure 3C). This cap-like appearance of p80 coilin has previously been observed in cells treated with α-amanitin (Carmo-Fonseca et al., 1992; Frey et al., 1999).
Figure 3.
The localization of ELL and EAF1 in CBs is dependent on active transcription by Pol II. (A) HeLa, U-2 OS, and WI-38 cells were treated with actinomycin D, 5 μg/ml, for 3 h. In confocal microscopy images, ELL and EAF1 exhibited a diffuse nucleoplasmic pattern, with a loss of the typical nuclear distribution in CBs. In the actinomycin D–treated cells, p80 coilin exhibited a ring-like pattern distributed around the nucleoli. (B) HeLa, U-2 OS, and WI-38 cells were treated with 50 μM DRB for 16 h. In confocal images, EAF1 exhibited a diffuse nucleoplasmic distribution. In contrast, ELL and p80 coilin exhibited a spherical staining around nucleoli. The nucleoli are smaller and more numerous than in untreated cells. (C) HeLa, U-2 OS, and WI-38 cells were treated with 20 μg/ml α-amanitin for 5 h. ELL and EAF1 exhibited a diffuse nucleoplasmic distribution and p80 coilin localized in ringed foci near nucleoli.
ELL and EAF1 Do Not Exhibit a Direct Physical Interaction with p80 Coilin
ELL and EAF1 interact to form a heterodimer that is stable in conditions of high salt and nonionic detergent concentrations (Simone et al., 2001). Interactions of p80 coilin with SMN and other CB components have previously been identified (Hebert et al., 2001). In light of the similarity in the subcellular localization patterns of ELL, EAF1, and p80 coilin, we examined whether ELL or EAF1 might physically interact with p80 coilin. We transiently transfected the human 293 embryonic kidney cell line with epitope-tagged version of ELL, EAF1, and p80 coilin (Figure 4). In cells transfected with FLAG-tagged ELL, cell lysates were immunoprecipitated with the FLAG antibody and immunoblotted with antibodies to EAF1. A 43-kDa band corresponding to endogenous EAF1 was observed in cells transfected with FLAG-ELL. However, in cells transfected with FLAG-coilin, endogenous EAF1 did not coimmunoprecipitate with p80 coilin. Similarly, in cells transfected with FLAG-EAF1, the FLAG antibody immunoprecipitated a 75-kDa band corresponding to endogenous ELL. However, in cells transfected with FLAG-coilin, endogenous ELL did not coimmunoprecipitate with p80 coilin. The lack of a direct physical interaction between these proteins suggests that distinct signals target ELL/EAF1 and p80 coilin to CBs.
Figure 4.
ELL and EAF1 do not exhibit a direct physical interaction with p80 coilin. Human 293 cells were transfected with FLAG-tagged ELL, EAF1, and p80 coilin. Expression of these constructs with the FLAG antibody is shown in the left panel. The cell lysates were immunoprecipitated with the FLAG antibody and immunoblotted with either the EAF1 or ELL antibodies. In the middle panel, FLAG-ELL immunoprecipitated with endogenous EAF1, but FLAG-coilin did not immunoprecipitate with EAF1. In the right panel, FLAG-EAF1 immunoprecipitated with endogenous ELL, but FLAG-coilin did not immunoprecipitate with ELL.
EAF1 and p80 Coilin Are Delocalized from CBs in MLL-ELL Leukemia
To determine the impact of MLL-ELL on CBs, we examined leukemia cell lines derived from mice transplanted with hematopoietic cells transduced with MLL-ELL and MLL-EAF1 retroviruses (Luo et al., 2001). As a control for the normal distribution of ELL, EAF1, and p80 coilin in normal hematopoietic cells, we examined the BaF3 progenitor cell line. To determine whether CBs are affected in leukemias that contain MLL fusions to partner proteins other than ELL, we examined a leukemia cell line derived from mice transplanted with hematopoietic cells containing an MLL-ENL retrovirus (Lavau et al., 1997). Strikingly, p80 coilin foci were absent from the nuclei of MLL-ELL and MLL-EAF1 leukemia cells (Figure 5). In merged images with DAPI staining of nuclei, the expression of p80 coilin appeared primarily cytoplasmic. However, we cannot exclude the possibility that the cytoplasmic signal represents autofluorescence due to low signal intensity. In the MLL-EAF1 cells, the EAF1 antibody, which recognizes both the MLL-EAF1 and wild-type EAF1 proteins, detected a faint, nuclear stippled pattern, possibly representing the MLL-EAF1 fusion protein. In the MLL-ELL cells, EAF1 was absent from nuclei and exhibited only minimal expression in the cytoplasm. In both the BaF3 and MLL-ENL cell lines, ELL, EAF1, and p80 coilin exhibited colocalization. Thus, CBs do not appear to be altered by expression of MLL-ENL.
Figure 5.
EAF1 and p80 coilin are delocalized from CBs in MLL-ELL and MLL-EAF1 leukemias. Fluorescence microscopy images of murine cell lines incubated with antibodies to ELL, EAF1, and p80 coilin. As controls, the murine hematopoietic progenitor cell line BaF3 and a leukemia cell line from MLL-ENL mice were examined. In the control cell lines, ELL, EAF1, and p80 coilin exhibited colocalization. In MLL-ELL and MLL-EAF1 cells, p80 coilin foci were absent from nuclei. In MLL-ELL cells, EAF1 foci were also absent from nuclei. In MLL-EAF1 cells, the EAF1 antibody detected a faint, nuclear stippled pattern, possibly representing the MLL-EAF1 fusion protein. The merged images with DAPI staining revealed that the majority of p80 coilin staining was in the cytoplasm of MLL-ELL and MLL-EAF1 cells.
To confirm the direct relationship of expression of MLL-ELL to the delocalization of p80 coilin, we transiently transfected HeLa cells with FLAG-tagged MLL-ELL, FLAG-ELL, and the FLAG-tagged amino-terminus of MLL (Figure 6, A–C). Transfection of FLAG-MLL did not affect the localization of p80 coilin. In FLAG-ELL transfected cells, the overexpressed ELL exhibited a nuclear stippled pattern as well as a diffuse nucleoplasmic distribution with exclusion of nucleoli. The nuclear foci of transfected FLAG-ELL colocalized with endogenous p80 coilin. In addition to CBs, endogenous p80 coilin also localized within nucleoli. The redistribution of p80 coilin to nucleoli increased during the course of the transfection of FLAG-ELL. We observed nucleolar expression of p80 coilin in ∼24% of transfected cells after 16 h, 57% of cells after 24 h, and 71% of cells after 40 h of transfection. In addition, the cells that demonstrated the highest expression of FLAG-ELL, as observed by confocal microscopy, exhibited a greater level of expression of p80 coilin in nucleoli, suggesting that redistribution of p80 coilin appears to occur because of overexpression of ELL. In previous reports of transfections of epitope-tagged p80 coilin, overexpression of p80 coilin also resulted in its localization within nucleoli, suggesting that the nuclear compartmentalization of diverse CB components may be linked (Hebert and Matera, 2000). In MLL-ELL–transfected cells, p80 coilin exhibited a faint dispersed pattern that lacked the typical stippled appearance of CBs observed in nontransfected cells. Similar to the MLL-ELL murine leukemia cells, p80 coilin failed to localize to CBs in MLL-ELL–transfected cells.
Figure 6.
Transient transfection of MLL-ELL delocalizes p80 coilin from CBs. HeLa cells were transiently transfected with FLAG-MLL (A), FLAG-ELL (B), and FLAG-MLL-ELL (C and E) followed by incubation with a biotinylated FLAG antibody detected by streptavidin-Cy5 and the rabbit p80 coilin antibody detected with goat anti-rabbit conjugated to FITC. Transfection with FLAG-MLL did not affect the localization of p80 coilin in CBs (A). In B, two examples (I, II) of cells transfected with FLAG-ELL are shown. The overexpressed ELL exhibited nuclear foci along with a diffuse nucleoplasmic distribution with exclusion of nucleoli. In the FLAG-ELL–transfected cells, endogenous p80 coilin colocalized with transfected ELL nuclear foci. In addition, endogenous p80 coilin also localized in nucleoli. In C, an untransfected cell (bottom right) exhibited a typical CB pattern with the p80 coilin antibody. In contrast, in the MLL-ELL–transfected cell (top left), CBs were not detected with the p80 coilin antibody. (D–F) Extranucleolar foci that contain Nopp140 and fibrillarin are present in cells that express MLL-ELL. (D) To confirm the specificity of the antinucleolar antibody, untransfected HeLa cells were incubated with an antibody to Nopp140 detected with FITC-conjugated second antibody and the antinucleolar antibody detected with Cy-5–conjugated antibody, which showed exclusive localization of the antinucleolar staining in nucleoli and Nopp140 staining in nucleoli and also in an extranucleolar focus (arrow). In E, a HeLa cell transfected with FLAG-MLL-ELL is shown as detected by a biotinylated anti-FLAG antibody and Cy-5 streptavidin. The antinucleolar antibody was detected with RRX-conjugated second antibody, and the Nopp140 antibody was detected with second antibody conjugated to FITC. An extranucleolar focus (arrow) was observed with the NOPP140 antibody. (F) Left: Murine erythroleukemic cell line (MEL) stained with an antinucleolar antibody (NOH61) detected with FITC-conjugated second antibody and fibrillarin antibody detected with Cy-5–conjugated second antibody. Because the antinuclelolar antibody used in D and E recognizes human but not mouse nucleoli, we used a guinea pig polyclonal antiserum to NOH61, which recognizes mouse nucleoli but not CBs. This antiserum stained nucleoli and exhibited background staining of the cytoplasm. (F) Right: MLL-ELL murine leukemia cells incubated with the antibody to NOH61 detected with FITC-conjugated second antibody and fibrillarin antibody detected with Cy-5–conjugated second antibody. Note the presence of extranucleolar foci (arrows) in both murine cell lines examined.
Extranucleolar Foci That Contain NOPP140 and Fibrillarin Are Present in Cells that Express MLL-ELL
In cells derived from p80 coilin knockout mice, extranucleolar foci were observed that were described as “residual” CBs (Tucker et al., 2001). In cells that express MLL-ELL, we have observed that p80 coilin is delocalized from CBs. To determine whether extranucleolar foci might be present in cells that express MLL-ELL, we used antibodies to NOPP140 and fibrillarin, which are components of both nucleoli and CBs. In HeLa cells transiently transfected with FLAG-MLL-ELL, distinct extranucleolar foci were observed with antibodies to NOPP140 (Figure 6E). To confirm that these foci were in fact outside of nucleoli, the cells were also labeled with an antinucleolar antibody that does not stain CBs (Figure 6D). In untransfected HeLa cells, these foci correspond to CBs (Figure 1B), whereas in HeLa cells transfected with MLL-ELL, these foci do not contain p80 coilin (Figure 6C). Similarly, in murine MLL-ELL leukemia cells and in a control murine leukemia cell line, distinct extranucleolar foci were detected using antibodies to fibrillarin (Figure 6F). As the antinucleolar antibody used with the HeLa cells is specific for human but not mouse nucleoli, we confirmed the presence of extranucleolar foci using a guinea pig polyclonal antiserum to NOH61, which recognizes mouse nucleoli but not CBs (Zirwes et al., 2000). In cells that express MLL-ELL, additional components of the extranucleolar foci other than fibrillarin and NOPP140, remain to be determined.
EAF1 and p80 Coilin Protein Levels Are Decreased in Nuclei of MLL-ELL Leukemia
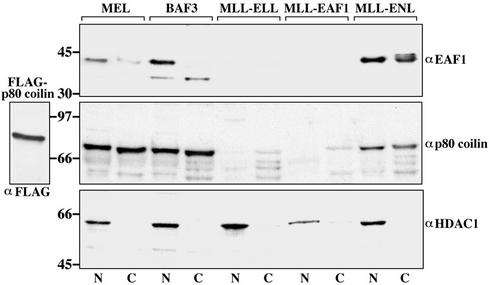
Examination of MLL-ELL and MLL-EAF1 leukemia cells by immunofluorescence suggested that expression of EAF1 and p80 coilin was diminished in cell nuclei. To determine the levels of expression of these proteins, nuclear and cytoplasmic fractions were prepared from these cell lines and compared with control murine hematopoietic cell lines including MEL, BaF3, and MLL-ENL leukemia cells (Figure 7). To confirm the integrity of the proteins in the nuclear extracts, the Western blots were probed with an antibody to HDAC1. In the MEL, BaF3, and MLL-ENL cells, EAF1 bands were detected primarily in the nuclear fractions, with fainter bands present in the cytoplasm. However, the wild-type EAF1 protein was not observed in either the nuclear or cytoplasmic fractions from MLL-ELL or MLL-EAF1 cells. The Western blot was stripped and reprobed with an antibody to p80 coilin. Expression of p80 coilin protein was observed in both nuclear and cytoplasmic fractions of MEL, BaF3, and MLL-ENL cells. In contrast, p80 coilin protein was not detected in the nuclear extracts of MLL-ELL and MLL-EAF1 cells. In line with the cytoplasmic localization observed by immunofluorescence, p80 coilin protein expression was observed in the cytoplasmic fractions of MLL-ELL and MLL-EAF1 cells. However, we cannot exclude that leakage of p80 coilin from the nucleus to the cytoplasm might have occurred during fractionation. Thus, MLL-ELL and MLL-EAF1 delocalize both p80 coilin and wild-type EAF1 from the nuclei of leukemia cells.
Figure 7.
Diminished expression of EAF1 and p80 coilin in nuclei of MLL-ELL and MLL-EAF1 leukemia cells. Nuclear and cytoplasmic extracts were prepared from murine cell lines and immunoblotted with antibodies to EAF1, p80 coilin, and HDAC1. To compare expression with other murine hematopoietic cells, MEL and BaF3 cells were used as controls. The murine MLL-ENL cell line was used a control for MLL fusions with other partner proteins. The levels of p80 coilin were equivalent in the nuclear and cytoplasmic fractions of the control cell lines. The HDAC1 antibody was used as a control for the integrity of the proteins in the nuclear extracts. In MLL-ELL and MLL-EAF1 cells, wild-type EAF1 protein levels were significantly reduced compared with controls, with no expression detected in nuclear extracts. Expression of p80 coilin was also not detected in the MLL-ELL and MLL-EAF1 nuclear extracts. However, expression of p80 coilin was observed in cytoplasmic extracts from these cell lines.
EAF1 and p80 Coilin Are Specific Targets in MLL-ELL Leukemia
To determine whether EAF1 and p80 coilin might be targets of other MLL fusion proteins that result from 11q23 translocations, nuclear and cytoplasmic extracts from multiple leukemia cell lines were examined. A series of non-11q23 cell lines including U937, MP-1, Jurkat, and K562 cells were also examined. The 11q23 cell lines included THP-1 that contains a (9;11)(p22;q23) translocation and expresses MLL-AF9, and MV4;11 that contains a (4;11)(q21;q23) translocation and expresses MLL-AF4. In both the 11q23 and non-11q23 leukemia cell lines, expression of EAF1 and p80 coilin was not decreased in nuclear extracts (Figure 8). Similarly, expression of EAF1 and p80 coilin was not diminished in nuclear extracts from a murine MLL-ENL leukemia cell line (Figure 7). The MLL-ENL fusion results from the (11;19)(q23;p13.3) translocation. Although we cannot exclude that EAF1 and p80 coilin might be disrupted in one of the other more than 30 alternative MLL translocations that have been observed, no changes in the localization were observed in cells expressing three of the most common types of 11q23 translocations, namely the t(4;11)(q21;q23), t(9;11)(p22;q23), and the t(11;19)(q23;p13.3).
Figure 8.
ELL and EAF1 are specific targets in MLL-ELL leukemia. Nuclear and cytoplasmic extracts were prepared from a series of cell lines and immunoblotted with antibodies to EAF1, p80 coilin, and HDAC1. MV4;11 expresses MLL-AF4, and THP-1 expresses MLL-AF9. The K562, Jurkat, MP-1, and U-937 hematopoietic cell lines do not contain MLL fusion proteins and were used as controls. In contrast to MLL-ELL cells, the levels of EAF1 and p80 coilin were not diminished in the nuclei of MV4;11 and THP-1 cells or in the control cell lines.
DISCUSSION
Using silver staining of neurons, Santiago Ramon y Cajal identified a small spherical structure adjacent to nucleoli, which he described as an accessory body (Cajal, 1903). In later studies, numerous investigators identified similar organelles in a wide variety of cell types in plant and animal nuclei, which were referred to as coiled bodies (Monneron and Bernhard, 1969). Because this designation is not an accurate reflection of the ultrastructure of this organelle, Gall has proposed that it be named the Cajal body (Gall et al., 1999). The identification of human sera containing autoantibodies that recognized Cajal bodies (CBs) led to the expression cloning of an 80-kDa protein named p80 coilin (Andrade et al., 1991; Raska et al., 1991). Antibodies generated to recombinant p80 coilin have been used to define CBs by immunofluorescence and immunoelectron microscopy. Subsequently, multiple CB components have been delineated by their colocalization with p80 coilin. CBs are enriched in factors involved in RNA processing, and functional analyses of these components have suggested a critical role for CBs in the preassembly of transcription complexes (Gall, 2000).
The predominant localization of ELL and EAF1 in CBs suggests that specific targets for the transcriptional elongation activity of ELL might exist either within or adjacent to CBs. The pattern of distribution of ELL, EAF1, and p80 coilin is strikingly similar, suggesting that ELL and EAF1 might also serve as useful markers for the detection of CBs. Although these proteins colocalize, ELL and EAF1 do not exhibit a direct physical interaction with p80 coilin. In contrast, p80 coilin interacts with several other CB components including NOPP140 and SMN (Isaac et al., 1998; Hebert et al., 2001). The identification of an interaction between SMN and fibrillarin suggests that multiple networks of interactions exist among other CB components (Jones et al., 2001; Pellizzoni et al., 2001). The lack of a direct physical interaction between ELL/EAF1 and p80 coilin suggests that other signals or protein–protein interactions target ELL and EAF1 to CBs.
The Sm antigen was first identified within CBs using electron microscopy (Eliceiri and Ryerse, 1984; Fakan et al., 1984). Subsequently, small nuclear RNAs (snRNAs) and small nuclear ribonucleoproteins (snRNPs) were also found in CBs (Raska et al., 1991). In addition to their frequent close proximity to nucleoli, an overlap with CB and nucleolar components exists, including fibrillarin, NOPP140, and small nucleolar RNAs (Raska et al., 1990; Isaac et al., 1998). Cell cycle regulatory proteins have also been found in CBs. Four components of the general transcription factor TFIIH have been identified within CBs, including CDK7, cyclin H, MAT1, and p62 (Jordan et al., 1997). The CDK2-cyclin E complex has also been identified within CBs (Liu et al., 2000). CBs exhibit specific patterns of compartmentalization within the nucleus. This was first observed in newt lampbrush chromosomes, with the identification of CBs adjacent to histone genes (Gall et al., 1981). In mammalian cells, CBs also associate preferentially with histone gene clusters (Frey and Matera, 1995). In addition, CBs not only are enriched in snRNAs, they also associate with the genes that encode multiple snRNAs. CBs associate with the loci for U1, U2, U3, U4, U11, and U12, which are all transcribed by Pol II (Frey and Matera, 1995; Smith et al., 1995; Jacobs et al., 1999). In contrast, CBs do not associate with U6 snRNA genes, which are transcribed by Pol III (Jacobs et al., 1999). The association of snRNA genes with CBs appears to be mediated by nascent snRNA transcripts, suggesting the possibility of a regulatory feedback mechanism (Frey and Matera, 1999; Frey and Matera, 2001).
A structure adjacent to CBs has been designated the “gem” for Gemini of coiled bodies (Liu and Dreyfuss, 1996). Gems and CBs overlap in multiple cell types (Matera and Frey, 1998). However, in fetal tissues, gems are distinct from CBs, with colocalization increasing with developmental age (Young et al., 2000). Subtle differences in the localization of gems and CBs have been observed in different strains of the same cell line. In the HeLa PV line, gems are predominantly distinct from CBs, whereas gems and CBs are colocalized in other HeLa strains (Matera and Frey, 1998). In HeLa cells that contain both gems and CBs, we observed that ELL and EAF1 localized exclusively to CBs. In cultured cells grown at 32°, gems separate completely from CBs (Liu and Dreyfuss, 1996). The SMN protein is a component of both gems and CBs and in addition, exhibits a diffuse cytoplasmic distribution (Liu and Dreyfuss, 1996). The telomeric copy of the SMN gene is deleted or mutated in spinal muscular atrophy, an autosomal recessive disorder that results in loss of spinal motor neurons (Lefebvre et al., 1995). SMN interacts with Gemin2 as part of a complex with spliceosomal snRNP core proteins (Fischer et al., 1997). In a mouse model of spinal muscular atrophy, deletion of exon 7 of the SMN gene results in a failure of proper nuclear targeting of SMN-Gemin2 and an altered distribution of p80 coilin (Frugier et al., 2000). Similarly, the failure of EAF1 and p80 coilin to localize in CBs in MLL-ELL leukemia suggest that altered nuclear compartmentalization of factors involved in RNA processing may be a critical feature of multiple disease states.
The localization of ELL and EAF1 within CBs suggests a link between Pol II–mediated transcriptional elongation and the RNA processing activities previously identified within CBs. Recent studies have established that transcription by Pol II is coupled to multiple aspects of pre-mRNA processing, including capping, splicing, and polyadenylation (Bentley, 1999). Several factors have been identified that regulate the elongation phase of mRNA synthesis, including Elongin, TFIIF, and ELL, which facilitate the processivity of transcription by suppressing transient pausing of Pol II (Conaway et al., 2000). Although ELL, Elongin, and TFIIF demonstrate similar activities in vitro, we have found that these elongation factors exhibit diverse patterns of distribution within the nucleus. Elongin A and the RAP74 subunit of TFIIF exhibited a diffuse nucleoplasmic pattern, whereas ELL localized preferentially in CBs. The RAP74 subunit of TFIIF has previously been found either within or adjacent to CBs in mammalian cells (Grande et al., 1997; Gall, 2000). However, in light of the diffuse nuclear distribution observed for TFIIF, it does not appear that CBs are the predominant site of its localization. Previous studies have shown that localization of snRNPs and p80 coilin in CBs is dependent on transcription, supporting a dynamic nature for the association of these factors with CBs (Raska et al., 1990; Carmo-Fonseca et al., 1992). In our studies, treatment of cells with actinomycin D, DRB, and α-amanitin resulted in the failure of ELL and EAF1 to localize in CBs, indicating that localization in CBs depends on active transcriptional elongation by Pol II. The targeting of ELL and EAF1 to CBs supports the model of the CB as a site of preassembly of transcription complexes referred to as “transcriptosomes” that function in both transcription and RNA processing (Gall et al., 1999).
The involvement of CBs in MLL-ELL leukemia is the first evidence that implicates this organelle in leukemia. In view of the relationship of CBs to fundamental aspects of RNA processing within the cell, it might appear that disruption of CBs would be cell lethal. However, several lines of evidence indicate that alterations in CB components do not necessarily affect cellular viability. In Xenopus, nuclei that contain typical CBs can be assembled from egg extract in vitro. However, depletion of p80 coilin from Xenopus egg extract by immunoprecipitation had no obvious effect on the morphology of the nuclei, and residual CBs could be detected using antibodies to other CB components (Bauer and Gall, 1997). In HeLa cells, microinjection of antibodies to p80 coilin induced the disappearance of CBs (Almeida et al., 1998). However, splicing of pre mRNA transcripts was maintained, and no other nuclear abnormalities were detected. Using a gene knockout strategy, mice have been generated with a targeted disruption of exons 2 through 7 of p80 coilin (Tucker et al., 2001). Homozygous p80 coilin null mice appeared normal, but reduced viability was observed when the knockout mice were crossed to inbred strains. Cells from the knockout mice exhibited extranucleolar foci that contained NOPP140 and fibrillarin but failed to recruit snRNPs and the SMN complex to these “residual” CBs. In cells that express MLL-ELL, we have observed that p80 coilin is delocalized from CBs. However, the MLL-ELL cells also exhibited extranucleolar foci that contain NOPP140 and fibrillarin. In the p80 coilin knockout mice, transient expression of p80 coilin restored the formation of CBs and rescued the recruitment of snRNPs and the SMN complex, suggesting that p80 coilin is essential for either the generation or maintenance of CBs. Although p80 coilin is not essential for viability, the effect of its depletion from CBs has not previously been examined for a potential role in tumorigenesis.
In acute promyelocytic leukemia (APL), a chromosome translocation also results in the altered compartmentalization of a subnuclear organelle (Dyck et al., 1994; Weis et al., 1994). The t(15;17)(q22;q21) translocation in APL leads to the formation of a PML-RARα fusion protein. PML is a component of nuclear bodies that are also referred to as PODs for PML oncogenic domains. A number of proteins colocalize with PML in these nuclear bodies, including Sp100, SUMO-1, Daxx, BLM, and eIF-4 (Zhong et al., 2000). In APL, PML-RARα functions as a dominant negative that delocalizes wild-type PML and other nuclear body proteins. In patients with APL, treatment with all-trans retinoic acid results in the reconstitution of nuclear bodies. In addition to its effects on PML dependent pathways, PML-RARα also acts to repress RARα dependent pathways that are critical for myeloid differentiation (Melnick and Licht, 1999). Thus in APL, the PML-RARα fusion protein functions as a dominant negative for both PML-dependent and RARα-dependent pathways (Pandolfi, 2001). Similarly, MLL-ELL may exhibit dominant effects on both MLL- and ELL-specific pathways.
In addition to ELL, MLL fuses to >30 other proteins in acute leukemia (Thirman et al., 1993). The large number and diverse nature of the motifs in MLL partner proteins have led to the hypothesis that MLL fusion proteins act to disrupt pathways regulated normally by MLL. However, our data suggest that the pathways regulated by the MLL partner proteins may also be disrupted as a result of 11q23 translocations. In MLL-ELL leukemias, EAF1, the heterodimeric partner of ELL, exhibits diminished expression and delocalization from CBs. In addition, p80 coilin, which does not bind directly to either ELL or to EAF1, exhibits a similar pattern of delocalization and decreased expression. Thus, the MLL-ELL fusion protein appears to function as a dominant negative for ELL/EAF1 pathways (Figure 9). Future studies will be necessary to determine whether other MLL fusions function as dominant negatives for MLL partner protein pathways.
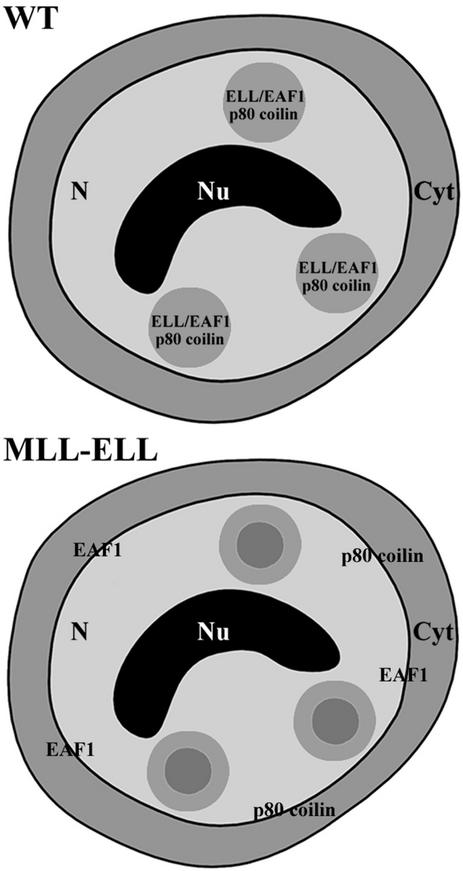
Figure 9.
Potential consequences of MLL-ELL expression. In wild-type cells, ELL, EAF1, and p80 coilin localize to CBs. In MLL-ELL cells, EAF1 and p80 coilin are delocalized from CBs. N, nucleus; Cyt, cytoplasm; Nu, nucleolus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. D. Bloch, Dr. E. Chan, Dr. G. Dreyfuss, Dr. C. Lavau, Dr. U. Meier, and Dr. M. Schmidt-Zachmann for generously providing reagents used in this study. We thank the Al Robin Laser Scanning Confocal Microscopy Core of the University of Chicago Digestive Disease Center. This work was supported by grant CA78431 from the National Cancer Institute and by the family of Robert A. Chapski. M.J.T. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
DOI: 10.1091/mbc.E02–07–0394.
REFERENCES
- Andrade LEC, Chan EKL, Raska I, Peebles CL, Roos G, Tan EM. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80–coilin. J Exp Med. 1991;173:1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F, Saffrich R, Ansorge W, Carmo-Fonseca M. Microinjection of anti-coilin antibodies affects the structure of coiled bodies. J Cell Biol. 1998;142:899–912. doi: 10.1083/jcb.142.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DW, Gall JG. Coiled bodies without coilin. Mol Biol Cell. 1997;8:73–82. doi: 10.1091/mbc.8.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. Coupling RNA polymerase II transcription with pre-mRNA processing. Curr Opin Cell Biol. 1999;11:347–351. doi: 10.1016/S0955-0674(99)80048-9. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Un sencillo metodo de coloracion seletiva del reticulo protoplasmatico y sus efectos en los diversos organos nerviosos de vertebrados e invertebrados. Trab Lab Invest Biol (Madrid) 1903;2:129–221. [Google Scholar]
- Carvalho T, Almeida F, Calapez A, Lafarga M, Berciano MT, Carmo-Fonseca M. The spinal muscular atrophy disease gene product, SMN: a link between snRNP biogenesis and the Cajal (coiled) body. J Cell Biol. 1999;147:715–727. doi: 10.1083/jcb.147.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M, Pepperkok R, Carvalho MT, Lamond AI. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992;117:1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M. The contribution of nuclear compartmentalization to gene regulation. Cell. 2002;108:513–521. doi: 10.1016/s0092-8674(02)00650-5. [DOI] [PubMed] [Google Scholar]
- Conaway JW, Shilatifard A, Dvir A, Conaway RC. Control of elongation by RNA polymerase II. Trends Biochem Sci. 2000;25:375–380. doi: 10.1016/s0968-0004(00)01615-7. [DOI] [PubMed] [Google Scholar]
- Dyck JA, Maul GG, Miller WH, Chen JD, Kakizuka A, Evans RM. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Eliceiri GL, Ryerse JS. Detection of intranuclear clusters of Sm antigens with monoclonal anti-Sm antibodies by immunoelectron microscopy. J Cell Physiol. 1984;121:449–451. doi: 10.1002/jcp.1041210226. [DOI] [PubMed] [Google Scholar]
- Fakan S, Leser G, Martin TE. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984;98:358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90:1023–1029. doi: 10.1016/s0092-8674(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Frey MR, Matera AG. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc Natl Acad Sci USA. 1995;92:5915–5919. doi: 10.1073/pnas.92.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MR, Bailey AD, Weiner AM, Matera AG. Association of snRNA genes with coiled bodies is mediated by nascent snRNA transcripts. Curr Biol. 1999;9:126–135. doi: 10.1016/s0960-9822(99)80066-9. [DOI] [PubMed] [Google Scholar]
- Frey MR, Matera AG. RNA-mediated interaction of Cajal bodies, and U2 snRNA genes. J Cell Biol. 2001;154:499–509. doi: 10.1083/jcb.200105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier T, Tiziano FD, Cifuentes-Diaz C, Miniou P, Roblot N, Dierich A, Le Meur M, Melki J. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum Mol Genet. 2000;9:849–858. doi: 10.1093/hmg/9.5.849. [DOI] [PubMed] [Google Scholar]
- Gall JG, Stephenson EC, Erba HP, Diaz MO, Barsacchi-Pilone G. Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma. 1981;84:159–171. doi: 10.1007/BF00399128. [DOI] [PubMed] [Google Scholar]
- Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell. 1999;10:4385–4402. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG. Cajal bodies. the first 100 years. Annu Rev Cell Dev Biol. 2000;16:273–300. doi: 10.1146/annurev.cellbio.16.1.273. [DOI] [PubMed] [Google Scholar]
- Grande MA, van der Kraan I, de Jong L, van Driel R. Nuclear distribution of transcription factors in relation to sites of transcription and RNA polymerase II. J Cell Sci. 1997;110:1781–1791. doi: 10.1242/jcs.110.15.1781. [DOI] [PubMed] [Google Scholar]
- Hebert MD, Matera AG. Self-association of coilin reveals a common theme in nuclear body localization. Mol Biol Cell. 2000;11:4159–4171. doi: 10.1091/mbc.11.12.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MD, Szymczyk PW, Shpargel KB, Matera AG. Coilin forms the bridge between Cajal bodies, and SMN, the spinal muscular atrophy protein. Genes Dev. 2001;15:2720–2729. doi: 10.1101/gad.908401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac C, Yang Y, Meier UT. Nopp140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol. 1998;142:319–329. doi: 10.1083/jcb.142.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EY, Frey MR, Wu W, Ingledue TC, Gebuhr TC, Gao L, Marzluff WF, Matera AG. Coiled bodies preferentially associate with U4, U11 and U12 small nuclear RNA genes in interphase HeLa cells but not with U6 and U7 genes. Mol Biol Cell. 1999;10:1653–1663. doi: 10.1091/mbc.10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Cunha C, Carmo-Fonseca M. The cdk-cyclin H-MAT1 complex associated with TFIIH is localized in coiled bodies. Mol Biol Cell. 1997;8:1207–1217. doi: 10.1091/mbc.8.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KW, Gorzynski K, Hales CM, Fischer U, Badbanchi F, Terns RM, Terns MP. Direct interaction of the spinal muscular atrophy disease protein SMN with the small nucleolar RNA-associated protein fibrillarin. J Biol Chem. 2001;276:38645–38651. doi: 10.1074/jbc.M106161200. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Earnshaw WC. Structure and function in the nucleus. Science. 1998;280:547–553. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavau C, Luo RT, Du C, Thirman MJ. Retrovirus mediated gene transfer of MLL-ELL transforms primary myeloid progenitors, and causes acute myeloid leukemias in mice. Proc Natl Acad Sci USA. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- Liu JL, Hebert MD, Ye Y, Templeton DJ, Kung HJ, Matera AG. Cell cycle-dependent localization of the CDK2–cyclin E complex in Cajal (coiled) bodies. J Cell Sci. 2000;113:1543–1552. doi: 10.1242/jcs.113.9.1543. [DOI] [PubMed] [Google Scholar]
- Liu Q, Dreyfuss G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996;15:3555–3565. [PMC free article] [PubMed] [Google Scholar]
- Luo RT, Lavau C, Du C, Simone F, Polak PE, Kawamata S, Thirman MJ. The elongation domain of ELL is dispensable but its EAF1 interaction domain is essential for MLL-ELL induced leukemogenesis. Mol Cell Biol. 2001;21:5678–5687. doi: 10.1128/MCB.21.16.5678-5687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera AG, Frey MR. Coiled bodies and gems: Janus or Gemini? Am J Hum Genet. 1998;63:317–321. doi: 10.1086/301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- Monneron A, Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969;27:266–288. doi: 10.1016/s0022-5320(69)80017-1. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP. Oncogenes and tumor suppressors in the molecular pathogenesis of acute promyelocytic leukemia. Hum Mol Genet. 2001;7:769–775. doi: 10.1093/hmg/10.7.769. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Baccon J, Charroux B, Dreyfuss G. The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin, and GAR1. Curr Biol. 2001;11:1079–1088. doi: 10.1016/s0960-9822(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Raska I, Ochs RL, Andrade LE, Chan EK, Burlingame R, Peebles C, Gruol D, Tan EM. Association between the nucleolus and the coiled body. J Struct Biol. 1990;104:120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Raska I, Andrade LEC, Ochs RL, Chan EKL, Chang CM, Roos G, Tan EM. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991;195:27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Shilatifard A, Lane WS, Jackson KW, Conaway RC, Conaway JW. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- Simone F, Polak PE, Luo RT, Kaberlein JJ, Levitan DA, Thirman MJ. EAF1, a novel ELL associated factor that is delocalized by expression of the MLL-ELL fusion protein. Blood. 2001;98:201–209. doi: 10.1182/blood.v98.1.201. [DOI] [PubMed] [Google Scholar]
- Smith KP, Carter KC, Johnson CV, Lawrence JB. U2 and U1 snRNA gene loci associate with coiled bodies. J Cell Biochem. 1995;59:473–485. doi: 10.1002/jcb.240590408. [DOI] [PubMed] [Google Scholar]
- Thirman MJ, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993;329:909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- Thirman MJ, Levitan DA, Kobayashi H, Simon MC, Rowley JD. Cloning of ELL, a gene that fuses to MLL in a t(11;19)(q23;p13.1) in acute myeloid leukemia. Proc Natl Acad Sci USA. 1994;91:12110–12114. doi: 10.1073/pnas.91.25.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KE, et al. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs, and SMN, the spinal muscular atrophy gene product. J Cell Biol. 2001;154:293–307. doi: 10.1083/jcb.200104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Young PJ, Le TT, Thi Man N, Burghes AH, Morris GE. The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues, and cultured cells. Exp Cell Res. 2000;256:365–374. doi: 10.1006/excr.2000.4858. [DOI] [PubMed] [Google Scholar]
- Zhong S, Salomoni P, Pandolfi PP. The transcriptional role of PML, and the nuclear body. Nat Cell Biol. 2000;5:85–90. doi: 10.1038/35010583. [DOI] [PubMed] [Google Scholar]
- Zirwes RF, Eilbracht J, Kneissel S, Schmidt-Zachmann MS. A novel helicase-type protein in the nucleolus: protein NOH61. Mol Biol Cell. 2000;11:1153–1167. doi: 10.1091/mbc.11.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.