Abstract
E-Cadherin is a Ca2+-dependent cell-cell adhesion molecule at adherens junctions (AJs) of epithelial cells. A fragment of N-cadherin lacking its extracellular region serves as a dominant negative mutant (DN) and inhibits cell-cell adhesion activity of E-cadherin, but its mode of action remains to be elucidated. Nectin is a Ca2+-independent immunoglobulin-like cell-cell adhesion molecule at AJs and is associated with E-cadherin through their respective peripheral membrane proteins, afadin and catenins, which connect nectin and cadherin to the actin cytoskeleton, respectively. We showed here that overexpression of nectin capable of binding afadin, but not a mutant incapable of binding afadin, reduced the inhibitory effect of N-cadherin DN on the cell-cell adhesion activity of E-cadherin in keratinocytes. Overexpressed nectin recruited N-cadherin DN to the nectin-based cell-cell adhesion sites in an afadin-dependent manner. Moreover, overexpression of nectin enhanced the E-cadherin–based cell-cell adhesion activity. These results suggest that N-cadherin DN competitively inhibits the association of the endogenous nectin-afadin system with the endogenous E-cadherin-catenin system and thereby reduces the cell-cell adhesion activity of E-cadherin. Thus, nectin plays a role in the formation of E-cadherin–based AJs in keratinocytes.
INTRODUCTION
Cells in multicellular organisms recognize their neighboring cells, adhere to them, and form cell-cell junctions, which play essential roles in various cellular functions, including morphogenesis, migration, proliferation, and differentiation (Takeichi, 1991; Gumbiner, 1996; Vlemincks and Kemler, 1999; Takeichi et al., 2000; Tepass et al., 2000; Yagi and Takeichi, 2000). In polarized epithelial cells, cell-cell adhesion is mediated through a junctional complex comprised of tight junctions (TJs), adherens junctions (AJs), and desmosomes (Farquhar and Palade, 1963). These junctional structures are typically aligned from the apical to basal sides, although desmosomes are independently distributed in other areas. At AJs, E-cadherin functions as a Ca2+-dependent cell-cell adhesion molecule (Takeichi, 1991; Gumbiner, 1996; Vlemincks and Kemler, 1999). E-Cadherin is a member of the cadherin superfamily consisting of over 80 members, each of which is expressed in a wide variety of cells not only in epithelial cells but also in nonepithelial cells (Takeichi et al., 2000; Tepass et al., 2000; Yagi and Takeichi, 2000). E-Cadherin consists of an extracellular region with five tandemly repeated domains, EC1–EC5, a single transmembrane region, and a cytoplasmic region (Takeichi, 1991; Gumbiner, 1996; Vlemincks and Kemler, 1999). The cytoplasmic region of E-cadherin is linked to the actin cytoskeleton through many peripheral membrane proteins (Aberle et al., 1996; Gumbiner, 2000; Nagafuchi, 2001). E-Cadherin directly binds β- or γ-catenin that directly binds α-catenin. α-Catenin binds α-actinin and vinculin. Of these proteins, α-catenin, α-actinin, and vinculin are actin filament (F-actin)-binding proteins. The association of E-cadherin with the actin cytoskeleton strengthens its cell-cell adhesion activity (Aberle et al., 1996; Gumbiner, 2000; Nagafuchi, 2001). The formation and disruption of AJs are dynamically regulated by many extracellular and intracellular signals: the formation of AJs is enhanced by Rac and Cdc42 small G proteins (Gumbiner, 2000), whereas AJs are disrupted by many extracellular signals, such as scatter factor/HGF and phorbol esters, and activated oncogenes, such as Ras and Src (Barth et al., 1997; Gumbiner, 2000). However, the mechanisms of such dynamic organization are largely unknown.
Important roles of E-cadherin in the dynamic organization of AJs have been substantiated by several lines of evidence, one of which has been obtained by the use of a dominant negative mutant (DN) of E- or N-cadherin (Kintner, 1992; Fujimori and Takeichi, 1993; Zhu and Watt, 1996; Nieman et al., 1999; Troxell et al., 1999). A fragment of E- or N-cadherin in which the extracellular region is largely deleted but the cytoplasmic region remains intact is widely used as a DN. Expression of N- or E-cadherin DN disrupts the E-cadherin–based AJs in a variety of cultured cells, such as keratinocytes and MDCK cells (Fujimori and Takeichi, 1993; Nieman et al., 1999; Troxell et al., 1999). When N-cadherin DN is expressed in Xenopus embryos, the E-cadherin–dependent organization of ectoderm is impaired (Kintner, 1992). These effects of DNs are not specific for the cadherin isotype. These results suggest the key role of E-cadherin in the formation of AJs, but the mode of action of N- or E-cadherin DN has not been fully understood. One explanation is that N- or E-cadherin DN competitively binds the α- and β-catenin complex and prevents it from associating with endogenous E-cadherin, eventually inhibiting the cell-cell adhesion activity of E-cadherin (Kintner, 1992). Another explanation is that expression of N- or E-cadherin DN reduces the amount of endogenous E-cadherin (Zhu and Watt, 1996; Nieman et al., 1999; Troxell et al., 1999).
Nectin and afadin constitute an emerging cell-cell adhesion system at AJs (Mandai et al., 1997; Takahashi et al., 1999). Nectin is a Ca2+-independent immunoglobulin-like cell-cell adhesion molecule (Aoki et al., 1997; Lopez et al., 1998; Takahashi et al., 1999; Miyahara et al., 2000; Satoh-Horikawa et al., 2000; Reymond et al., 2001), whereas afadin is an F-actin-binding protein that connects nectin to the actin cytoskeleton (Mandai et al., 1997; Takahashi et al., 1999). Nectin comprises a family of four members, nectin-1, -2, -3, and -4, each of which has two or three splicing variants (Morrison and Racaniello, 1992; Aoki et al., 1994; Eberléet al., 1995; Lopez et al., 1995; Cocchi et al., 1998; Satoh-Horikawa et al., 2000; Reymond et al., 2001). Nectin-1 was originally identified as one of the poliovirus receptor–related proteins (PRR1; Lopez et al., 1995). Nectin-2 was originally identified as the murine homolog of human poliovirus receptor protein (Morrison and Racaniello, 1992), but turned out to be another poliovirus receptor-related protein (PRR2; Eberléet al., 1995; Lopez et al., 1995). Neither PRR-1 nor -2 has thus far been shown to serve as a poliovirus receptor. Nectin-1 and -2 have recently been shown to serve as the α-herpes virus entry and cell-cell spread mediators (Cocchi et al., 1998, 2000; Geraghty et al., 1998; Warner et al., 1998; Sakisaka et al., 2001). It remains unknown whether nectin-3 and -4 serve as receptors for viruses. Each member of the nectin family first forms homo-cis-dimers and then homo-trans-dimers, causing cell-cell adhesion (Lopez et al., 1998; Miyahara et al., 2000; Satoh-Horikawa et al., 2000; Sakisaka et al., 2001; Momose et al., 2002). Nectin-3 furthermore forms hetero-trans-dimers with either nectin-1 or -2, and these hetero-trans-dimers are stronger than homo-trans-dimers (Satoh-Horikawa et al., 2000). Nectin-4 also forms hetero-trans-dimers with nectin-1 (Reymond et al., 2001). Most of the nectin family members have a conserved four-residue motif (Glu/Ala-X-Tyr-Val), which interacts with the PDZ domain of afadin (Takahashi et al., 1999; Satoh-Horikawa et al., 2000; Reymond et al., 2001). Afadin has at least two splicing variants, l- and s-afadins (Mandai et al., 1997). l-Afadin, the larger splicing variant, is an F-actin–binding protein with one PDZ domain and three proline-rich domains, and connects nectin to the actin cytoskeleton (Mandai et al., 1997; Takahashi et al., 1999). s-Afadin, the smaller splicing variant, has one PDZ domain but lacks the F-actin–binding domain and the third proline-rich domain (Mandai et al., 1997). Human s-afadin is identical to the product of the AF-6 gene that has been identified as an ALL-1 fusion partner involved in acute myeloid leukemias (Prasad et al., 1993). In this study, afadin simply refers to l-afadin. The nectin-afadin system is ubiquitously expressed not only in epithelial cells but also in nonepithelial cells, such as fibroblasts and neurons (Morrison and Racaniello, 1992; Eberléet al., 1995; Lopez et al., 1995; Mandai et al., 1997; Nishioka et al., 2000; Satoh-Horikawa et al., 2000; Mizoguchi et al., 2002). Accumulating evidence suggests that nectin and E-cadherin are associated through afadin and the α- and β-catenin complex and that the nectin-afadin system is involved in the formation of AJs cooperatively with the E-cadherin-catenin system (Ikeda et al., 1999; Takahashi et al., 1999; Tachibana et al., 2000), but the precise role and the mode of action of the nectin-afadin system in the organization of AJs has not yet been fully understood.
The evidence that nectin and E-cadherin are associated through afadin and the α- and β-catenin complex has raised the possibility that N- or E-cadherin DN competitively inhibits the association of the endogenous nectin-afadin system with the endogenous E-cadherin-catenin system and thereby reduces the cell-cell adhesion activity of E-cadherin. We examined here this possibility and found that this is indeed the case and that nectin is functionally associated with E-cadherin and plays a critical role in the formation of AJs cooperatively with E-cadherin.
MATERIALS AND METHODS
Cell Culture and Establishment of Transfectants
A mouse keratinocyte cell line PAM212 (Yuspa et al., 1980) and its transfectant PAMcNΔ2A (Fujimori and Takeichi, 1993) were kindly supplied by Dr. M. Takeichi (RIKEN Center for Developmental Biology, Kobe, Japan) and maintained in the DH10 medium (1:1 mixture of Dulbecco's modified Eagle's minimal essential medium and Ham's F12 supplemented with 10% fetal calf serum) as described (Fujimori and Takeichi, 1993). PAMcNΔ2A cells were generated by the introduction of the N-cadherin DN (cN390Δ) cDNA attached to the metallothionein promoter into PAM212 cells. cN390Δ was a mutant of chicken N-cadherin in which its extracellular region was largely deleted (amino acid [aa] 1–913 carrying an internal deletion of aa 272–662). To induce the expression of cN390Δ, PAMcNΔ2A cells were cultured in DH10 containing 125 μM ZnCl2 for more than 24 h as previously described (Fujimori and Takeichi, 1993). PAMcNΔ2A cell lines stably expressing full-length mouse nectin-3α (nectin-3-full, aa 1–549; nectin-3-full-PAMcNΔ2A cells) and its C-terminal four aa-deleted mutant (nectin-3-ΔC, aa 1–545; nectin-3-ΔC-PAMcNΔ2A cells) were prepared as described (Satoh-Horikawa et al., 2000). In brief, PAMcNΔ2A cells were transfected with pCAGIPuro-nectin-3-full or -nectin-3-ΔC using LipofectAMINE 2000 reagent (Life Technologies, Carlsbad, CA) and cultured for 24 h. The cells were then replated and selected by culturing in the presence of 5 μg/ml puromycin. L cells stably expressing nectin-1 (nectin-1-L cells) and nectin-3 (nectin-3-L cells) were prepared as described (Takahashi et al., 1999; Satoh-Horikawa et al., 2000).
Antibodies
A mouse anti-afadin mAb, a rabbit anti–nectin-1 polyclonal antibody (pAb) that recognized the cytoplasmic region of nectin-1α, a rat anti–nectin-2 mAb that recognized both the extracellular regions of nectin-2α and -2δ, and a rabbit anti–nectin-3 pAb that recognized the cytoplasmic region of nectin-3α were prepared as described (Takahashi et al., 1999; Sakisaka et al., 1999; Satoh-Horikawa et al., 2000). A rat anti–E-cadherin mAb (ECCD-2) was kindly supplied by Dr. M. Takeichi (RIKEN Center for Developmental Biology, Kobe, Japan). A rat anti–N-cadherin mAb NCD-2 (Takara, Otsu, Japan), a rabbit antipan-cadherin pAb (Sigma Chemical Co., St. Louis, MO), a mouse anti–β-catenin mAb (Transduction Laboratories, Lexington, KY), a rabbit anti-α-catenin pAb, and a goat anti-human IgG (Fc specific) antibody (Sigma Chemical Co.) were purchased from commercial sources.
Cell Dissociation Assay
The cell dissociation assay was done as described (Nagafuchi et al., 1994). In brief, cells (8 × 105) were cultured in a 35-mm dish at 37°C for 36 h in the presence or absence of ZnCl2. The confluent cells were washed with HEPES-buffered saline (HBS, pH 7.4) and treated with 0.01% trypsin supplemented with 1 mM CaCl2 in HBS (TC treatment) or 0.01% trypsin supplemented with 1 mM EDTA in HBS (TE treatment) at 37°C for 2 h, followed by dissociation by ten-time pipetting. The extent of dissociation of cells was represented by the index NTC/NTE, where NTC and NTE were the total particle number after the TC and TE treatments, respectively.
Preparation of Nef-1–coated Beads and Cell-Bead Adhesion Assay
The extracellular fragment of nectin-1 fused to the human IgG Fc (Nef-1) was prepared as described (Fukuhara et al., 2002; Honda et al., 2003). Latex-sulfate beads (5.2-μm diameter; Interfacial Dynamics Corporation, Portland, OR) coated with Nef-1 were prepared as described (Fukuhara et al., 2002; Honda et al., 2003). In brief, 3 × 108 beads were washed and suspended in 0.2 ml of 0.1 M borate buffer (pH 8.0). The suspended beads were incubated with 100 μg of the goat anti-human IgG (Fc specific) antibody for 18 h at room temperature and suspended in 1 ml of phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). An aliquot (100 μl) of the beads was then incubated with 15 μg of Nef-1 at room temperature for 3 h, washed three times with PBS containing 0.5% BSA, and suspended in 100 μl of the same buffer.
For the cell-bead adhesion assay, 1.25 × 105 cells were cultured in a 35-mm culture dish for 48 h in the DH10 medium containing ZnCl2, and then 3.75 × 105 Nef-1–coated beads were added to the culture medium, followed by incubation for 6 h. The cultured cells were washed with the DH10 medium and fixed with 3% formaldehyde in PBS for 10 min. The fixed cells were treated with 0.5% Triton X-100 in PBS for 5 min and incubated with PBS containing 5% BSA for 1 h. The sample was then double-stained with NCD-2 and the anti–nectin-3 pAb, followed by immunofluorescence microscopy.
Subcellular Fractionation by Sucrose Density Gradient Ultracentrifugation
The subcellular fractionation was done as described by Yamamoto et al. (2002). In brief, after incubation for 36 h in the presence or absence of ZnCl2, confluent cultured cells were washed with PBS and harvested. The cells were then sonicated in buffer A (10 mM HEPES-NaOH at pH 7.5, 100 mM KCl, 1 mM MgCl2, and 25 mM NaHCO3), followed by centrifugation at 1000 × g at 4°C for 5 min. The postnuclear supernatant (290 μg of protein) was overlaid on 4.2 ml of continuous sucrose density gradient (10–40%) over 0.3 ml of 50% sucrose and centrifuged at 200,000 × g at 4°C for 60 min. After the ultracentrifugation, 15 fractions of 0.3 ml each were collected. An aliquot of each fraction (20 μl) was subjected to SDS-PAGE, followed by Western blotting.
Other Procedures
Immunofluorescence microscopy of cultured cells was performed as described (Mandai et al., 1997; Takahashi et al., 1999). Protein concentrations were determined with BSA as a reference protein (Bradford, 1976). SDS-PAGE was done as described (Laemmli, 1970).
RESULTS
Reduction by Overexpression of Nectin-3 of the N-Cadherin DN-induced Dispersion of Cell Colonies
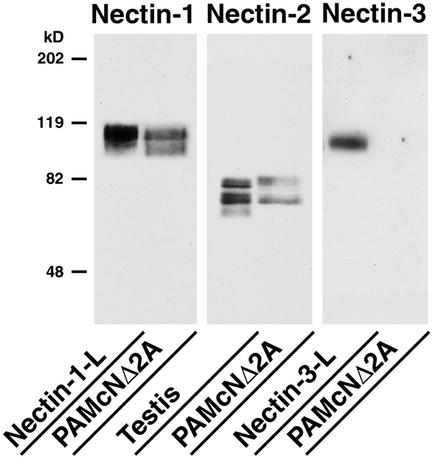
To examine whether overexpression of nectin affects the dominant negative effect of N-cadherin DN on the E-cadherin–based cell-cell adhesion in PAM212 keratinocytes, the cDNA of full-length nectin-3 (nectin-3-full) or the C-terminal four aa-deleted mutant (nectin-3-ΔC) incapable of binding afadin was introduced into PAMcNΔ2A cells. Each type of the transfectants was established: nectin-3-full-PAMcNΔ2A cells stably expressing nectin-3-full; and nectin-3-ΔC-PAMcNΔ2A cells stably expressing nectin-3-ΔC. PAMcNΔ2A cells are PAM212 keratinocytes transfected with the N-cadherin DN cDNA (Fujimori and Takeichi, 1993). The cDNA is attached to the metallothionein promoter that is activated by Zn2+. N-Cadherin DN is a fragment of chick N-cadherin, termed cN390Δ, in which a large portion of the extracellular domain is deleted but the transmembrane and cytoplasmic regions remain intact, and functions as a DN for the cell-cell adhesion activity of E-cadherin (Fujimori and Takeichi, 1993). PAMcNΔ2A cells express endogenous E-cadherin and P-cadherin, of which the former is more abundant (Fujimori and Takeichi, 1993), and furthermore expressed endogenous nectin-1 and -2, but not nectin-3, as estimated by Western blotting (Figure 1). Nectin-1 and -2 showed two bands. The upper and lower bands of nectin-2 were identified to be its splicing variants, nectin-2 δ and –2 α, respectively, by their pAbs (unpublished data). On the other hand, the relationship of the two bands of nectin-1 remains unknown, but this may be due to different levels of the posttranslational modifications such as glycosylation.
Figure 1.
Expression levels of endogenous nectin-1, -2, and -3 in PAMcNΔ2A cells. The lysate of PAMcNΔ2A cells (20 μg of protein) and the control samples were subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting using the anti–nectin-1, -2 or -3 antibody. The lysates of nectin-1-L cells (10 μg of protein), mouse testis (5 μg of protein), and nectin-3-L cells (10 μg of protein) were used as the control samples. The results shown are representative of three independent experiments.
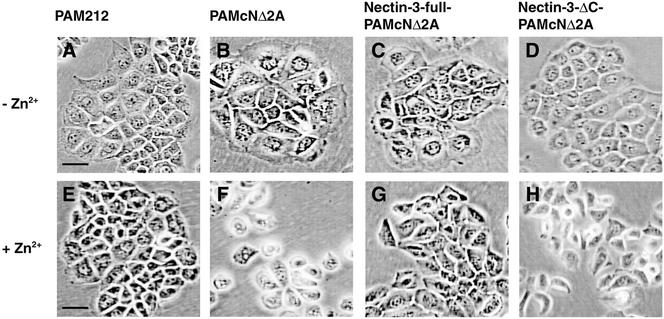
PAM212 cells formed colonies in which cells apparently adhere to each other irrespective of the presence or absence of Zn2+ in the medium (Figure 2, A and E). When PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC- PAMcNΔ2A cells were cultured in the absence of Zn2+not to express N-cadherin DN, each cell line formed colonies and showed apparently similar epithelial morphologies to those of PAM212 cells (Figure 2, B–D, and see also Figure 2A). However, when PAMcNΔ2A cells were cultured in the presence of Zn2+to express N-cadherin DN, the cells in the colonies were morphologically changed and dispersed especially at the peripheral regions where the single cells were observed (Figure 2F), consistent with the earlier observations (Fujimori and Takeichi, 1993). When nectin-3-full-PAMcNΔ2A cells were cultured in the presence of Zn2+, the dominant negative effect of N-cadherin DN was markedly reduced (Figure 2G, and see also Figure 2E). The cells formed colonies and showed roughly similar morphologies to those of PAM212. However, when nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence of Zn2+, the dominant negative effect was not reduced and the cells showed similar phenotypes to those of PAMcNΔ2A cells cultured in the presence of Zn2+ (Figure 2H, and see also Figure 2F). These results indicate that overexpression of full-length nectin-3 has a potency to reduce the dominant negative effect of N-cadherin DN and that this activity of nectin-3 requires its binding to afadin. We have previously shown that nectin-based cell-cell adhesion activity is not affected by the binding to afadin (Miyahara et al., 2000). It is therefore likely that the activity of nectin-3 to reduce the dominant negative effect of N-cadherin DN is not simply due to the nectin-3–based cell-cell adhesion activity.
Figure 2.
Reduction by overexpression of nectin-3 of the N-cadherin DN-induced dispersion of cell colonies. PAM212, PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence or absence of 125 μM Zn2+. (A and E) PAM212 cells; (B and F) PAMcNΔ2A cells; (C and G) nectin-3-full-PAMcNΔ2A cells; (D and H) nectin-3-ΔC-PAMcNΔ2A cells; (A–D) in the absence of Zn2+ and (E–H) in the presence of 125 μM Zn2+. Bars, 50 μm. The results shown are representative of three independent experiments.
Reduction by Overexpression of Nectin-3 of the Dominant Negative Effect of N-Cadherin DN on the Concentration of E-Cadherin at Cell-Cell Adhesion Sites
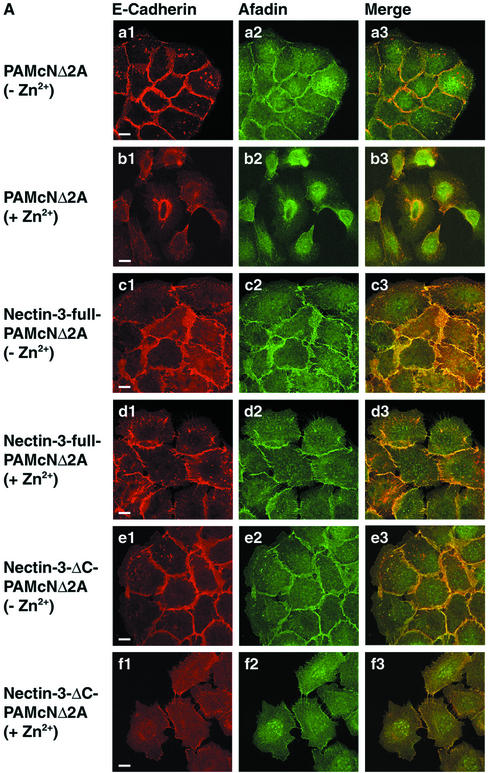
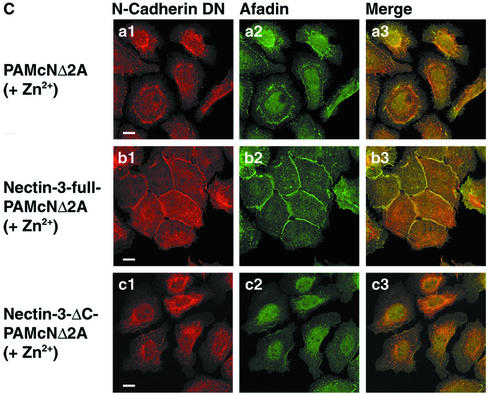
We then examined whether exogenously expressed nectin-3 has a potency to reduce the dominant negative effect of N-cadherin DN on the concentration of E-cadherin at cell-cell adhesion sites. In PAMcNΔ2A cells cultured in the absence of Zn2+, the E-cadherin staining was highly concentrated at the cell-cell adhesion sites and hardly concentrated at the free edges of colonies (Figure 3, Aa1 and Aa3). The afadin staining colocalized with the E-cadherin staining at the cell-cell adhesion sites (Figure 3, Aa2 and Aa3). Nectin-1 and -2 were faintly stained at the same sites as those of E-cadherin and afadin (unpublished data), but the afadin staining was much clearer than the nectin-1 and -2 stainings. Afadin was therefore monitored in the following experiments. In both nectin-3-full-PAMcNΔ2A and nectin-3-ΔC-PAMcNΔ2A cells cultured in the absence of Zn2+, both the E-cadherin and afadin stainings were highly concentrated at the cell-cell adhesion sites (Figure 3, Ac1-Ac3 and Ae1-Ae3). The staining patterns of these proteins were apparently similar to those in PAMcNΔ2A cells cultured in the absence of Zn2+(see Figure 3, Aa1-Aa3). When PAMcNΔ2A cells were cultured in the presence of Zn2+, the cells at the periphery of colonies were dispersed and the E-cadherin staining mostly disappeared (Figure 3, Ab1 and Ab3), consistent with the earlier observations (Fujimori and Takeichi, 1993). It may be noted, however, that the E-cadherin staining appeared to be filopodium-like between two dispersed neighboring cells. This staining was not observed at the free edges of colonies. The afadin staining colocalized with the E-cadherin staining (Figure 3, Aa2, Aa3, Ab2, and Ab3). E-Cadherin and afadin are likely to localize on these thin protrusions through which two neighboring cells form cell-cell adhesion. When nectin-3-full-PAMcNΔ2A cells were cultured in the presence of Zn2+, the cells at the periphery of colonies were not markedly dispersed and both the E-cadherin and afadin stainings remained highly concentrated at the cell-cell adhesion sites (Figure 3, Ad1-Ad3). However, both the stainings were not so linear as those observed in PAMcNΔ2A cells that were cultured in the absence of Zn2+ (see Figure 3, Aa1-Aa3). E-Cadherin and afadin showed interdigitated staining patterns between two neighboring cells. When nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence of Zn2+, the cells at the periphery of colonies were dispersed and the E-cadherin and afadin stainings mostly disappeared (Figure 3, Af1-Af3). The filopodium-like E-cadherin and afadin stainings were observed between two dispersed neighboring cells. The staining patterns of E-cadherin and afadin were similar to those in PAMcNΔ2A cells cultured in the presence of Zn2+ (see Figure 3, Ab1-Ab3). These results indicate that overexpression of full-length nectin-3 has a potency to reduce the dominant negative effect of N-cadherin DN on the concentration of E-cadherin at the cell-cell adhesion sites and that this activity of nectin-3 requires its binding to afadin.
Figure 3.
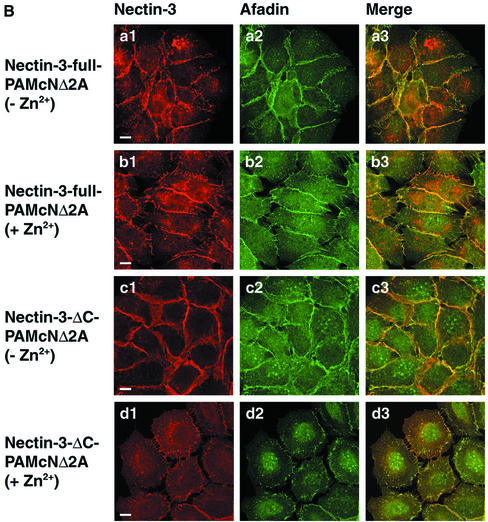
Reduction by overexpression of nectin-3 of the dominant negative effect of N-cadherin DN on the concentration of E-cadherin at cell-cell adhesion sites. PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence or absence of 125 μM Zn2+, followed by double staining with various combinations of the anti–E-cadherin, anti–N-cadherin, anti–nectin-3, and anti-afadin antibodies. There was nuclear staining with the anti-afadin antibody, but its significance is not known. (A) Double staining of E-cadherin and afadin. (a1-b3) PAMcNΔ2A cells; (c1-d3) nectin-3-full-PAMcNΔ2A cells; (e1-f3) nectin-3-ΔC-PAMcNΔ2A cells; (a1-a3, c1-c3, e1-e3) in the absence of Zn2+, (b1-b3, d1-d3, f1-f3) in the presence of 125 μM Zn2+, and (a1, b1, c1, d1, e1, f1) E-cadherin, (a2, b2, c2, d2, e2, f2) afadin, and (a3, b3, c3, d3, e3, f3) merge. (B) Double staining of nectin-3 and afadin. (a1-b3) Nectin-3-full-PAMcNΔ2A cells; (c1-d3) Nectin-3-ΔC-PAMcNΔ2A cells; (a1-a3, c1-c3) in the absence of Zn2+ and (b1-b3, d1-d3) in the presence of 125 μM Zn2+; (a1, b1, c1, d1) nectin-3; (a2, b2, c2, d2) afadin; and (a3, b3, c3, d3) merge. (C) Double staining of N-cadherin DN and afadin. (a1-a3) PAMcNΔ2A cells in the presence of 125 μM Zn2+; (b1-b3) nectin-3-full-PAMcNΔ2A cells in the presence of 125 μM Zn2+; (c1-c3) nectin-3-full-PAMcNΔ2A cells in the presence of 125 μM Zn2+; (a1, b1, c1) N-cadherin DN; (a2, b2, c2) afadin; and (a3, b3, c3) merge. Bars, 10 μm. The results shown are representative of three independent experiments.
The nectin-3 staining was highly concentrated at the cell-cell adhesion sites and not concentrated at the free edges of colonies in nectin-3-full-PAMcNΔ2A cells cultured in the absence of Zn2+ (Figure 3, Ba1-Ba3). This staining pattern was similar to those in nectin-3-full-PAMcNΔ2A cells cultured in the presence of Zn2+ and nectin-3-ΔC-PAMcNΔ2A cells cultured in the absence of Zn2+ (Figure 3, Bb1-Bb3 and Bc1-Bc3). In nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence of Zn2+, the nectin-3-ΔC staining mostly disappeared, but the filopodium-like nectin-3-ΔC staining was observed between two dispersed neighboring cells, and this staining pattern was apparently similar to that of afadin (Figure 3, Bd1-Bd3). The staining patterns of N-cadherin DN in PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence of Zn2+were apparently similar to those of afadin in the respective cell lines cultured in the presence of Zn2+ (Figure 3, Ca1-Ca3, Cb1-Cb3, and Cc1-Cc3).
Recruitment of N-Cadherin DN by Nectin-3 to the Nectin-3–based Cell-Bead Contact Sites
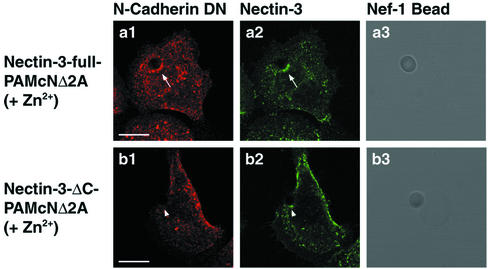
The above results suggest that exogenously expressed nectin-3 has a potency to recruit exogenously expressed N-cadherin DN to nectin-3–based cell-cell adhesion sites in an afadin-dependent manner. To confirm this possibility, we took advantage of the cell-bead adhesion assay using microbeads coated with the extracellular fragment of nectin-1 fused to the IgG Fc (Nef-1), which we have recently established (Fukuhara et al., 2002; Honda et al., 2003). Nef-1–coated beads were put on nectin-3-full-PAMcNΔ2A cells cultured in the presence of Zn2+ and further cultured for 6 h. Both the cellular full-length nectin-3 and N-cadherin DN stainings were concentrated at the cell-bead contact sites and colocalized (Figure 4, a1-a3). In contrast, Nef-1–coated beads were put on nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence of Zn2+ and further cultured for 6 h. The cellular nectin-3-ΔC staining was concentrated at the cell-bead contact sites, but the N-cadherin DN staining was not concentrated there (Figure 4, b1-b3). These results indicate that exogenously expressed nectin-3 has a potency to recruit exogenously expressed N-cadherin DN to the nectin-based cell-cell adhesion sites in an afadin-dependent manner. Taken together, the above results suggest that exogenously expressed nectin-3 associates with exogenous N-cadherin DN through afadin and the α- and β-catenin complex and thereby reduces the dominant negative effect of N-cadherin DN on the E-cadherin–based cell-cell adhesion.
Figure 4.
Recruitment of N-cadherin DN by nectin-3 to the nectin-3–based cell-bead contact sites. Nectin-3-full-PAMcNΔ2A and nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence of 125 μM Zn2+, followed by incubation with Nef-1–coated beads. The cells were double stained with the anti–N-cadherin and anti–necitn-3 antibodies. (a1-a3) Nectin-3-full-PAMcNΔ2A cells; (b1-b3) nectin-3-ΔC-PAMcNΔ2A; (a1, b1) N-cadherin DN; (a2, b2) nectin-3; and (a3, b3) differential interference contrast image. Arrows, cell-bead contact site (nectin-3-full-PAMcNΔ2A cells); arrowheads, cell-bead contact site (nectin-3-ΔC-PAMcNΔ2A cells). Bars, 10 μm. The results shown are representative of three independent experiments.
Reduction by Overexpression of Nectin-3 of the Dominant Negative Effect of N-Cadherin DN on the E-Cadherin–based Cell-Cell Adhesion Activity
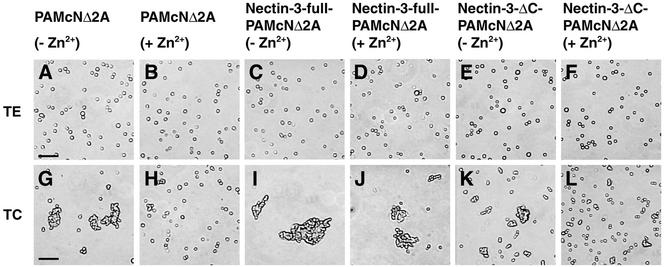
We next examined by the use of the cell dissociation assay (Nagafuchi et al., 1994) whether overexpression of nectin-3 has a potency to reduce the dominant negative effect of N-cadherin DN on the E-cadherin–based cell-cell adhesion activity. Confluent cultured PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence or absence of Zn2+ were treated with 0.01% trypsin containing 1 mM EDTA (TE treatment) or 0.01% trypsin containing 1 mM Ca2+ (TC treatment) and then dissociated by ten-time pipetting. In all the three cell lines, the cells were dissociated into single cells in the presence of EDTA (Figure 5, A–F). We have previously shown that nectin-3–based cell-cell adhesion activity is Ca2+ independent (Satoh-Horikawa et al., 2000). Therefore, these results indicate that the nectin-3- and nectin-3-ΔC–based cell-cell adhesion activities in nectin-3-full-PAMcNΔ2A and nectin-3-ΔC-PAMcNΔ2A cells, respectively, are undetectable as estimated by the cell dissociation assay irrespective of the presence or absence of Ca2+, and that the cell-cell adhesion activities detected in all the three cell lines in the presence of Ca2+ are based on Ca2+-dependent cadherin, mainly E-cadherin.
Figure 5.
Reduction by overexpression of nectin-3 of the dominant negative effect of N-cadherin DN on the E-cadherin–based cell-cell adhesion activity. PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence or absence of 125 μM Zn2+ and treated with 0.01% trypsin containing 1 mM EDTA (TE treatment) or 0.01% trypsin containing 1 mM CaCl2 (TC treatment), followed by dissociation through ten-time pipetting. (A, B, G, and H) PAMcNΔ2A cells; (C, D, I, and J) nectin-3-full-PAMcNΔ2A cells; (E, F, K, and L) nectin-3-ΔC-PAMcNΔ2A cells; (A, G, C, I, E, and K) in the absence of Zn2+; and (B, H, D, J, F, and L) in the presence of 125 μM Zn2+; (A–F) with TE treatment; and (G–L) with TC treatment. Bars, 100 μm. The results shown are representative of three independent experiments.
PAMcNΔ2A cells cultured in the absence of Zn2+ formed aggregates in the presence of Ca2+ (NTC/NTE = 0.15; Figure 5G). PAMcNΔ2A cultured in the presence of Zn2+ did not form aggregates even in the presence of Ca2+, although a few aggregates with two or three cells were observed to a small extent (NTC/NTE = 0.35; Figure 5H). These results are consistent with the earlier observations (Fujimori and Takeichi, 1993). Nectin-3-full-PAMcNΔ2A cells cultured in the absence of Zn2+ formed aggregates in the presence of Ca2+(NTC/NTE = 0.09; Figure 5I). The aggregates were significantly bigger than those of PAMcNΔ2A cells (see also Figure 5G). Nectin-3-full-PAMcNΔ2A cells cultured in the presence of Zn2+ similarly formed aggregates in the presence of Ca2+ (NTC/NTE = 0.16; Figure 5J). The aggregates were slightly smaller than those of nectin-3-full-PAMcNΔ2A cells cultured in the absence of Zn2+ (see also Figure 5I), but similar to those of PAMcNΔ2A cells cultured in the absence of Zn2+ (see also Figure 5G). Nectin-3-ΔC-PAMcNΔ2A cells cultured in the absence of Zn2+ formed aggregates in the presence of Ca2+ (NTC/NTE = 0.25; Figure 5K). The aggregates were smaller than those of nectin-3-full-PAMcNΔ2A cells cultured in the absence of Zn2+(see also Figure 5I), but almost similar to those of PAMcNΔ2A cells cultured in the absence of Zn2+ (see also Figure 5G). Nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence of Zn2+ did not form aggregates in the presence of Ca2+, although a few aggregates with two or three cells were observed to a small extent (NTC/NTE = 0.43; Figure 5L). Taken together, these results indicate that overexpressed nectin-3 has a potency to reduce the dominant negative effect of N-cadherin DN on the E-cadherin–based cell-cell adhesion activity, and furthermore that overexpression of nectin-3 increases the cell-cell adhesion activity of E-cadherin.
No Change of Amount of E-Cadherin at the Plasma Membrane by Expression of N-Cadherin DN
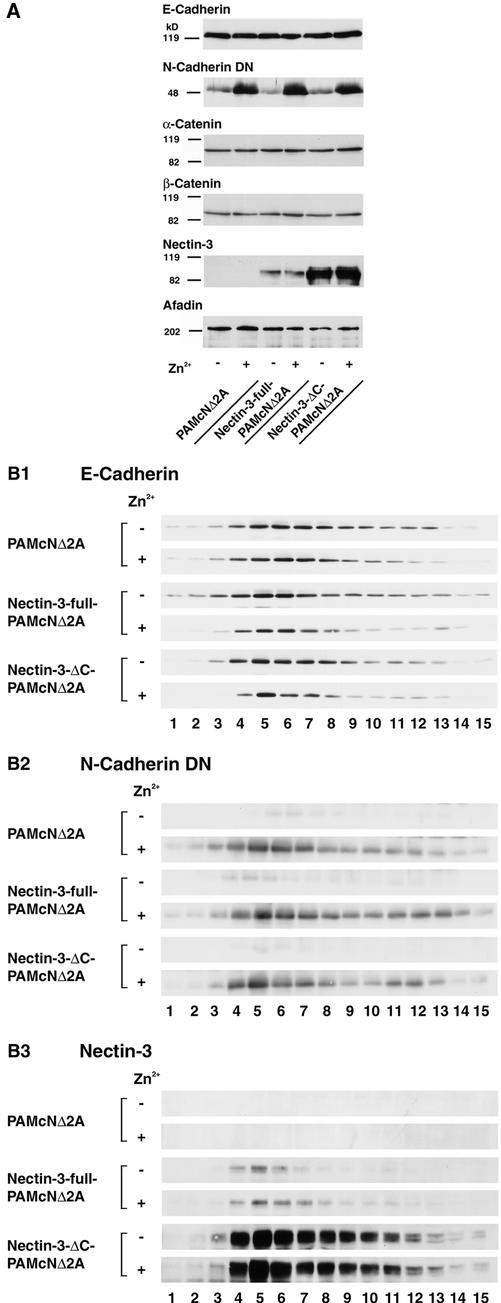
It has previously shown that the dominant negative effect of N-cadherin DN may be due to decrease in the expression level of endogenous E-cadherin (Nieman et al., 1999; Troxell et al., 1999), although it has been shown that the expression level of endogenous E-cadherin is not significantly changed by expression of N-cadherin DN in PAM212 (Fujimori and Takeichi, 1993). In the last set of experiments, we examined the expression levels of the cadherin-catenin system and the nectin-afadin system of PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence or absence of Zn2+. The total cell lysate of each cell line cultured in the presence or absence of Zn2+ was subjected to Western blotting using the anti–E-cadherin, pan-cadherin, α-catenin, β-catenin, nectin-3, and afadin antibodies. The expression levels of E-cadherin were not significantly different among the three cell lines cultured in the presence or absence of Zn2+ (Figure 6A), consistent with the previous observations (Fujimori and Takeichi, 1993). In terms of α-catenin, β-catenin, and afadin, the expression levels of each protein were not significantly different among the three cell lines cultured in the presence or absence of Zn2+. N-Cadherin DN was expressed equally in each of the three cell lines cultured in the presence of Zn2+. The cells cultured in the absence of Zn2+ expressed N-cadherin DN to a small extent. This small expression might be due to the presence of a small amount of Zn2+ in the culture medium. The expression levels of nectin-3-full and -3-ΔC were not different in the respective cell lines cultured in the absence and presence of Zn2+, but the expression levels of nectin-3-ΔC in nectin-3-ΔC-PAMcNΔ2A were much higher than those of nectin-3-full in nectin-3-full-PAMcNΔ2A cells.
Figure 6.
No change of amount of E-cadherin at the plasma membrane by expression of N-cadherin DN. PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells were cultured in the presence or absence of 125 μM Zn2+. (A) Expression levels of the component of the nectin-afadin and cadherin-catenin systems. The homogenate of each cell line (10 μg of protein each) was subjected to SDS-PAGE (10% polyacrylamide gel), followed by Western blotting using the anti–E-cadherin, anti-pan-cadherin, anti–α-catenin, anti–β-catenin, anti–nectin-3, and anti-afadin antibodies. (B) Subcellular distribution of the component of the nectin-afadin and cadherin-catenin systems. The postnuclear supernatant of each cell line (290 μg of protein each) was subjected to sucrose density gradient ultracentrifugation and 15 fractions were collected from the bottom (fraction 1) to the top (fraction 15). An aliquot of each fraction (20 μl) was subjected to SDS-PAGE (8% polyacrylamide gel), followed by Western blotting using the anti–E-cadherin, anti-pan-cadherin, and anti–nectin-3 antibodies. (B1) E-Cadherin; (B2) N-cadherin DN; and (B3) nectin-3. The results shown are representative of three independent experiments.
We furthermore examined whether the amount of E-cadherin at the plasma membrane is changed by the expression of N-cadherin DN. PAMcNΔ2A, nectin-3-full-PAMcNΔ2A, and nectin-3-ΔC-PAMcNΔ2A cells cultured in the presence or absence of Zn2+ were homogenized and centrifuged to collect each postnuclear supernatant. Each supernatant was then subjected to 10–40% linear sucrose gradient ultracentrifugation and 15 fractions were collected after the centrifugation. In all the three cell lines cultured in the presence or absence of Zn2+, E-cadherin was concentrated in fractions 4–7 (Figure 6B1). α- and β-Catenins also appeared in fractions 4–7 (unpublished data). These fractions appeared to contain the plasma membrane components. EEA1, a marker for early endosome, appeared in fractions 11–13, indicating that these fractions contained the early endosome (unpublished data). The amounts of E-cadherin in the plasma membrane fractions from the three cell lines were not significantly different irrespective of the culture in the presence or absence of Zn2+, although a slightly wider distribution profile was seen in the sample from nectin-3-full-PAMcNΔ2A cells. N-Cadherin DN appeared in the same fractions and their amounts were not significantly different among the three cell lines cultured in the presence of Zn2+ (Figure 6B2). Nectin-3-full and -3-ΔC were similarly concentrated in fractions 4–7 from nectin-3-full-PAMcNΔ2A and nectin-3-ΔC-PAMcNΔ2A cells, respectively, cultured in the presence or absence of Zn2+ (Figure 6B3). Nectin-3 was not detected in any fraction from PAMcNΔ2A cells. The amounts of nectin-3-full and -3-ΔC were not different in the respective cell lines cultured in the absence and presence of Zn2+, but the amounts of nectin-3-ΔC in the plasma membrane fraction of nectin-3-ΔC-PAMcNΔ2A were much bigger than those of nectin-3-full in the same fractions of nectin-3-full-PAMcNΔ2A cells. These results indicate that an approximately equal amount of E-cadherin is expressed at the plasma membrane among the three cell lines, and its expression levels are hardly affected by expression of N-cadherin DN.
DISCUSSION
We have first confirmed here the previous observation that N-cadherin DN inhibits the cell-cell adhesion activity of E-cadherin in keratinocytes, PAM212 cells (Fujimori and Takeichi, 1993). When N-cadherin DN is expressed, the E-cadherin–based cell-cell adhesion is mostly, but not completely, disrupted and the cell-cell adhesion activity is much inhibited as estimated by the cell dissociation assay. We have then shown here that overexpression of full-length nectin-3 capable of binding afadin reduces the inhibitory effect of N-cadherin DN. The C-terminal four aa-deleted mutant of nectin-3 incapable of binding afadin does not show this activity, suggesting that the binding of afadin to nectin-3 is required for this activity of nectin-3. We have moreover shown here that full-length nectin-3, but not the truncated mutant, recruits N-cadherin DN to the sites where nectin-3 is laterally clustered by Nef-1 (bead-cell contact sites). We have previously shown using L cells stably expressing the full-length or various mutants of nectin-1 or -2 and E-cadherin that nectin recruits, through afadin, α-catenin alone or the α- and β-catenin complex, to the nectin-based cell-cell adhesion sites even in the absence of E-cadherin (Tachibana et al., 2000). Moreover, nectin recruits the E-cadherin complex through afadin and the α- and β-catenin complex to the nectin-based cell-cell adhesion sites without the formation of the trans-dimer of E-cadherin (Tachibana et al., 2000). We have recently shown that the microbeads coated with the extracellular fragment of nectin-3 fused to the IgG Fc (Nef-3) recruit first the nectin-afadin complex and then the E-cadherin complex to the bead-cell contact sites (Honda et al., 2003). The present results are consistent with these earlier observations and indicate that nectin-3 recruits N-cadherin DN to the nectin-3–based cell-cell adhesion sites presumably through afadin and the α- and β-catenin complex. It is conceivable, therefore, that endogenous nectin-1 or -2 is associated with endogenous E-cadherin through endogenous afadin and the α- and β-catenin complex when N-cadherin DN is not exogenously expressed in PAM212 cells. When N-cadherin DN is exogenously expressed, endogenous nectin is furthermore associated with N-cadherin DN, eventually reducing the amount of endogenous nectin that is associated with endogenous E-cadherin. Overexpressed exogenous nectin is associated with both endogenous E-cadherin and exogenous N-cadherin DN. The proper cell-cell adhesion activity of E-cadherin is observed only when E-cadherin is associated with either endogenous or exogenous nectin. Taken together, the most likely mechanism of the inhibitory effect of N-cadherin DN on the cell-cell adhesion activity of E-cadherin is that N-cadherin DN associates with endogenous nectin in a manner competitive with endogenous E-cadherin and prevents E-cadherin from associating with nectin, eventually inhibiting its cell-cell adhesion activity. A possibility is unlikely that exogenous expression of N-cadherin DN prevents the α- and β-catenin complex from associating with E-cadherin. If this is the case, overexpression of nectin-3 associates with the α- and β-catenin complex and thereby prevents the complex from associating with E-cadherin, inhibiting its cell-cell adhesion activity. It has furthermore been shown that endogenous E-cadherin associates with the α- and β-catenin complex even when N-cadherin DN is exogenously expressed (Fujimori and Takeichi, 1993). Another possibility, that expression of N-cadherin DN reduces the amount of endogenous E-cadherin on the plasma membrane, is also neglected in PAM212 cells, consistent with the earlier observation (Fujimori and Takeichi, 1993). Thus, our present results have clarified the mechanism of the inhibitory effect of N-cadherin DN on the cell-cell adhesion activity of E-cadherin and furthermore have provided another line of evidence that nectin is functionally associated with E-cadherin to organize AJs. On the other hand, it remains to be clarified why nectin does not completely inhibit the effect of N-cadherin DN. An unidentified molecule(s) in addition to nectin may associate with E-cadherin and be required to fully organize AJs. N-Cadherin DN may associate with this molecule(s) as well as nectin in a competitive manner with E-cadherin.
We have furthermore shown here by use of the cell dissociation assay that PAM212 cells, which overexpress nectin but do not express N-cadherin DN, are more hardly dissociated than the cells that do not express nectin-3 or N-cadherin DN, suggesting that the association of E-cadherin with nectin enhances its cell-cell adhesion activity. This result appears to be consistent with the mode of inhibitory effect of N-cadherin DN on the cell-cell adhesion activity of E-cadherin. N-Cadherin DN is likely to associate with endogenous nectin and to thereby prevent it from associating with E-cadherin, inhibiting its cell-cell adhesion activity. This suggests that nectin is necessary for E-cadherin to fully exert its cell-cell adhesion activity. Taken together, it is likely that the physical association of nectin and E-cadherin is involved in not only the physical organization of AJs but also the functional organization of AJs to exert sufficient cell-cell adhesion activity of E-cadherin. The detailed mechanism of the stimulatory effect of nectin on the cell-cell adhesion activity of E-cadherin is not known, but N-cadherin DN may be useful for future studies on not only the role of E-cadherin but also that of nectin.
We have previously shown that when full-length nectin-1 is expressed in L cells stably expressing E-cadherin (EL cells), it is recruited to the E-cadherin staining sites where endogenous nectin-2 and afadin colocalize, but that when the C-terminal four aa-deleted mutant of nectin-1 (nectin-1-ΔC) is expressed in EL cells, it is not recruited to the E-cadherin staining sites where endogenous nectin-2 and afadin colocalize (Takahashi et al., 1999). On the other hand, we have shown here that nectin-3-ΔC as well as full-length nectin-3 apparently colocalizes with endogenous afadin and E-cadherin in nectin-3-ΔC-PAMcNΔ2A cells. The present result is apparently inconsistent with the previous observation. The exact reason for this inconsistency is currently unknown, but may be due to the following reason: each nectin family member forms homo-trans-dimers but nectin-3 furthermore forms hetero-trans-dimers with nectin-1 or -2, but nectin-1 and -2 do not form hetero-trans-dimers (Satoh-Horikawa et al., 2000). The affinity of nectin-3 and nectin-1 is the strongest among all the combinations of nectin-1, -2, and -3 for the formation of the trans-dimers (Fabre et al., 2002; Honda et al., 2003). The C-terminal four-residue of nectin is not essential for the formation of the trans-dimers (Miyahara et al., 2000). In nectin-3-ΔC-PAMcNΔ2A cells, nectin-3-ΔC might form trans-dimers with endogenous nectin-1 or -2 that binds afadin and thereby might be recruited to the afadin staining sites. In L cells, nectin-1 and -2, but not nectin-3, are expressed (unpublished data). Because nectin-1-ΔC expressed in EL cells does not form trans-dimers with endogenous nectin-2 and form them with endogenous nectin-1 with a weak affinity, nectin-1-ΔC may not be recruited to the afadin staining sites. Further studies are necessary for a better understanding of the role and mode of action of the nectin-afadin system in the organization of AJs.
ACKNOWLEDGMENTS
We thank Dr. Takeichi (RIKEN Center for Developmental Biology, Kobe, Japan) for providing us with PAM212 and PAMcNΔ2A cells and the anti–E-cadherin mAb. This work was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (2001, 2002).
Abbreviations used:
- aa
amino acid(s)
- AJs
adherens junctions
- F-actin
actin filaments
- N-cadherin DN
a dominant negative mutant of N-cadherin
- Nef-1
the extracellular fragment of nectin-1 fused to the human IgG Fc
- pAb
polyclonal antibody
- TJs
tight junctions
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–10–0632. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–10–0632.
REFERENCES
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- Barth AI, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Dubreuil P, Lopez M, Campadelli-Fiume G. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB) J Virol. 2000;74:3909–3917. doi: 10.1128/jvi.74.8.3909-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlé F, Dubreuil P, Mattei MG, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, Lopez M. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C"-D beta-strands of the nectin1 V domain. J Biol Chem. 2002;277:27006–27013. doi: 10.1074/jbc.M203228200. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M. Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell. 1993;4:37–47. doi: 10.1091/mbc.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara A, et al. Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene. 2002;21:7642–7655. doi: 10.1038/sj.onc.1205875. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, et al. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells. 2003;8:51–63. doi: 10.1046/j.1365-2443.2003.00616.x. [DOI] [PubMed] [Google Scholar]
- Ikeda W, et al. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez M, Eberlé F, Mattei MG, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- Mandai K, et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose Y, Honda T, Inagaki M, Shimizu K, Irie K, Nakanishi H, Takai Y. Role of the second immunoglobulin-like loop of nectin in cell-cell adhesion. Biochem Biophys Res Commun. 2002;294:45–49. doi: 10.1016/S0006-291X(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Morrison ME, Racaniello VR. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-a-catenin fusion molecules. J Cell Biol. 1994;127:235–247. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13:600–603. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Kim J-B, Johnson KR, Wheelock MJ. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112:1621–1632. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- Nishioka H, Mizoguchi A, Nakanishi H, Mandai K, Takahashi K, Kimura K, Satoh-Moriya A, Takai Y. Localization of l-afadin at puncta adhaerentia-like junctions between the mossy fiber terminals and the dendritic trunks of pyramidal cells in the adult mouse hippocampus. J Comp Neurol. 2000;424:297–306. doi: 10.1002/1096-9861(20000821)424:2<297::aid-cne8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Prasad R, et al. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4: A new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–43215. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Nakanishi H, Takahashi K, Mandai K, Miyahara M, Satoh A, Takaishi K, Takai Y. Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junctions. Oncogene. 1999;18:1609–1617. doi: 10.1038/sj.onc.1202451. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, et al. Requirement of interaction of nectin-1alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J Virol. 2001;75:4734–4743. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1176. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a P.D.Z. domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptor as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Nakagawa S, Aono S, Usui T, Uemura T. Patterning of cell assemblies regulated by adhesion receptors of the cadherin superfamily. Pilos Trans R Soc Lond Biol Sci. 2000;355:885–890. doi: 10.1098/rstb.2000.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- Troxell ML, Chen Y-T, Cobb N, Nelson WJ, Marrs JA. Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am J Physiol. 1999;276:C404–418. doi: 10.1152/ajpcell.1999.276.2.C404. [DOI] [PubMed] [Google Scholar]
- Vlemincks K, Kemler R. Cadherin and tissue formation: integrating adhesion and signaling. Bioessay. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitebeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. J Virol. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- Yamamoto Y, Mandai K, Okabe N, Hoshino T, Nakanishi H, Takai Y. Localization of mLin-7 to nectin-based cell-cell junctions. Oncogene. 2002;21:2545–2554. doi: 10.1038/sj.onc.1205335. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Hawley-Nelson P, Koehler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J Cell Sci. 1996;109:3013–3023. doi: 10.1242/jcs.109.13.3013. [DOI] [PubMed] [Google Scholar]