Abstract
We have determined that the previously identified dual-specificity protein kinase TTK is the human orthologue of the yeast MPS1 kinase. Yeast MPS1 (monopolar spindle) is required for spindle pole duplication and the spindle checkpoint. Consistent with the recently identified vertebrate MPS1 homologues, we found that hMPS1 is localized to centrosomes and kinetochores. In addition, hMPS1 is part of a growing list of kinetochore proteins that are localized to nuclear pores. hMPS1 is required by cells to arrest in mitosis in response to spindle defects and kinetochore defects resulting from the loss of the kinesin-like protein, CENP-E. The pattern of kinetochore localization of hMPS1 in CENP-E defective cells suggests that their interaction with the kinetochore is sensitive to microtubule occupancy rather than kinetochore tension. hMPS1 is required for MAD1, MAD2 but not hBUB1, hBUBR1 and hROD to bind to kinetochores. We localized the kinetochore targeting domain in hMPS1 and found that it can abrogate the mitotic checkpoint in a dominant negative manner. Last, hMPS1 was found to associate with the anaphase promoting complex, thus raising the possibility that its checkpoint functions extend beyond the kinetochore.
INTRODUCTION
The mitotic checkpoint is a fail-safe mechanism that ensures accurate chromosome segregation by preventing cells from prematurely exiting mitosis in the presence of unaligned chromosomes (Nicklas, 1997; Rieder and Salmon, 1998; Amon, 1999). This checkpoint system is highly sensitive, because even a single unaligned chromosome is sufficient to block cells from entering anaphase (Rieder et al., 1994; Li and Nicklas, 1997). The mitotic checkpoint has been shown to monitor both microtubule attachment and tension generated across sister kinetochores by poleward forces (Rieder et al., 1994; Li and Nicklas, 1997; Waters et al., 1998). Failure of the mitotic checkpoint causes cells to exit mitosis in the presence of unaligned chromosomes and is a major mechanism responsible for aneuploidy (Jallepalli and Lengauer, 2001). Seven mitotic checkpoint genes, BUB1, BUB2, BUB3, MAD1, MAD2, MAD3, and MPS1, were originally identified via genetic screens in Saccharomyces cerevisiae (Hoyt et al., 1991; Li and Murray, 1991; Weiss and Winey, 1996). These genes act along two separate mitotic checkpoint pathways (Clarke and Gimenez-Abian, 2000; Daum et al., 2000; Gardner and Burke, 2000). MPS1, BUB1, BUB3, MAD1, MAD2, and MAD3 monitor kinetochore microtubule attachments and prevent premature chromosome segregation by inhibiting degradation of securin/Pds1 and mitotic cyclins (Wassmann and Benezra, 2001; Peters, 2002). BUB2 acts along a different pathway that monitors spindle integrity and orientation and prevents premature cytokinesis by inhibiting the degradation of the mitotic cyclin Clb2 (Alexandru et al., 1999; Fesquet et al., 1999; Fraschini et al., 1999; Li, 1999; Bardin et al., 2000; Bloecher et al., 2000; Pereira et al., 2000).
Many of the mitotic checkpoint genes in yeast are evolutionarily conserved, because orthologues of MAD1, MAD2, MAD3, BUB1, and BUB3 have been identified in worms, flies, and mammals (Chen et al., 1996; Li and Benezra, 1996; Taylor and McKeon, 1997; Basu et al., 1998; Cahill et al., 1998; Chan et al., 1998; Gorbsky et al., 1998; Jablonski et al., 1998; Jin et al., 1998; Taylor et al., 1998; Basu et al., 1999; Kitagawa and Hieter, 2001). Importantly, many of these mitotic checkpoint proteins bind preferentially to unattached kinetochores, where they are postulated to function in generating the “wait anaphase signal” (Hoffman et al., 2001, and references therein). How this is done remains uncertain but appears to be dependent on kinetochore microtubule occupancy and tension. Indeed, the ability of hBUBR1 to interact with the kinesin-related protein CENP-E suggests that it may monitor the kinetochore microtubule interactions mediated by CENP-E (Chan et al., 1999). Likewise, studies of MAD2 have revealed that its interactions at kinetochores are sensitive to microtubule occupancy rather than tension (Waters et al., 1998). Despite these observations, the mechanism by which unattached kinetochores generate the “wait anaphase signal” remains unresolved (Shah and Cleveland, 2000). One explanation has come from the finding that MAD2 exhibits a rapid rate of turnover at unattached kinetochores (Howell et al., 2000). This behavior is proposed to reflect the release of the inhibitor that diffuses through the cell to inhibit the anaphase promoting complex (APC). However, the biochemical nature of the “wait anaphase signal” is likely to be complex, and its genesis is probably dependent on the interactions among many different checkpoint proteins at the kinetochores.
Recently, the vertebrate orthologues of the yeast MPS1 kinase were identified and found to localize to kinetochores (Poch et al., 1994; Abrieu et al., 2001; Fisk and Winey, 2001; Stucke et al., 2002). MPS1 encodes a tyrosine and serine/threonine dual-specificity kinase (Poch et al., 1994; Lauze et al., 1995) that was originally identified in a genetic screen for mutants defective in spindle pole duplication (Winey et al., 1991). Subsequently, it was discovered to be an essential component of the mitotic checkpoint (Hardwick et al., 1996; Weiss and Winey, 1996). Consistent with yeast MPS1, mouse MPS1 is localized at centrosomes throughout the cell cycle and is essential for accurate centrosome duplication (Fisk and Winey, 2001; Castillo et al., 2002). However, a recent study indicated that human MPS1 was not localized at centrosomes in human U2OS cells (Stucke et al., 2002). Despite the discrepancy in the centrosome localization of MPS1 in mouse and human cells, it is clear that MPS1 is present at kinetochores during mitosis, where it may participate in the checkpoint. This possibility was verified by studies of the Xenopus MPS1, which was shown to be a critical component of the spindle checkpoint in egg extracts (Abrieu et al., 2001). Furthermore, xMPS1 was found to be critical for the localization of CENP-E and the checkpoint proteins MAD1 and MAD2 to kinetochores (Abrieu et al., 2001). Likewise, disruption of hMPS1 prevented cells from arresting in mitosis in the presence of spindle damage (Stucke et al., 2002).
In this study, we show that the previously identified human TTK kinase (Mills et al., 1992; Hogg et al., 1994; Schmandt et al., 1994) is the human MPS1 kinase. We found that hMPS1 is hyperphosphorylated in mitosis and is dephosphorylated when cells exit mitosis. We show that hMPS1 localizes to kinetochores during early stages of mitosis. Consistent with mouse MPS1 (Fisk and Winey, 2001), hMPS1 is localized to the centrosome throughout the cell cycle. In addition, we show that hMPS1, as was shown for human MAD1 and MAD2 (Campbell et al., 2001), also localizes to the nucleoplasmic side of the nuclear pore complex (NPC). Consistent with other studies, we show that hMPS1 is an essential component of the mitotic checkpoint in HeLa cells. Furthermore, hMPS1 is part of the checkpoint pathway that is required to arrest cells defective for CENP-E functions in mitosis. In addition to its localization at kinetochores, we found hMPS1 to associate with the APC. This suggests that hMPS1 may have multiple roles in the mitotic checkpoint.
MATERIALS AND METHODS
Cloning of hMPS1
The cDNA of hMPS1 was identified in GenBank as human TTK and isolated through PCR amplification (TJY357, CAGGATCCATAATGAACAAAGTGAGAGAC; TJY358, CTGGATCCTATCTGACATTACGAATAACTG) from HeLa Marathon ready cDNA (Clontech, Palo Alto, CA). The 5′ end of hMPS1 was isolated by 5′ RACE and found to have an additional 16 amino acids at its N-terminus. The full-length hMPS1 cDNA was isolated by PCR (TJY782, GGATTCGAAATGGAATCCGAGGATTTAAGTGGC; TJY358). The green fluorescent protein (GFP) expression constructs GFP-A1–301, GFP-B267–482, GFP-C400–841, GFP-D1–239, GFP-E55–310, GFP-F267–638, and GFP-G493–857 were made by inserting PCR-amplified fragments into pECEGFP vector (a kind gift from Drs. H. Fisk and M. Winey, University of Colorado, Boulder). The constructs were introduced into HeLa cells with Fugene 6 (Roche Products, Indianapolis, IN) or linear PEI (Durocher et al., 2002) as transfection reagents.
Preparation of Anti-hMPS1 Antibody
Human MPS1 cDNA encoding amino acids 400–507 was subcloned into pGEX-KT, and GST-hMPS1400–507 was expressed in Escherichia coli. Purified GST-hMPS1400–507 recombinant protein was used to immunize a rabbit. For affinity purification, immune serum from hMPS1-injected rabbit was first incubated with Affi-gel 10 (Bio-Rad, Hercules, CA) that was coupled with a bacterial lysate that contained glutathione S-transferase (GST) to remove antibodies against GST and other bacterial proteins. The preadsorbed serum was then incubated overnight at 4°C with Affi-gel 10 that was coupled with GST–hMPS1400–507. The columns were washed extensively with TBS-500 (10 mM Tris-HCl, pH 7.4, and 500 mM NaCl). Antibodies were eluted with 0.5% acetic acid and 150 mM NaCl and immediately neutralized with 1 M Tris, pH 9.0. Fractions were monitored by light absorbance at 280 nm, and the peak fractions were pooled, desalted, and concentrated into 0.5×PBS/50% glycerol. Antibodies to be used for microinjections were concentrated to ∼5 mg/ml in Ca2+- and Mg2+-free PBS (Life Technologies, Grand Island, NY), passed through a 0.22-μm filter, divided into aliquots, and frozen at −80°C.
Cell Culture and Microinjections
HeLa cells were grown in DMEM supplemented with 10% FBS in the presence of antibiotics in a humidified incubator at 37°C. Cells were synchronized at the G1/S boundary by a double thymidine block. For microinjections, HeLa cells were blocked at the G1/S boundary, and antibodies were injected into the nuclei of cells with an Eppendorf semiautomated microinjector and Femtotip needles (Brinkmann Instruments Inc., Westbury, NY). Injections were performed on a Nikon TE300 inverted microscope. After microinjection, cells were washed and released into HEPES-buffered DMEM plus 10% FBS, returned to the incubator, and then fixed at a later time. Typically, mock-injected or nonimmune-IgG–injected cells enter mitosis 10–12 h after release from the G1/S block. In cases in which injected cells were tested for their response to spindle damage, nocodazole (60 ng/ml) was added ∼8 h after release from the G1/S boundary. In some cases, injections were performed on cells arrested in mitosis. Injected cells were identified by staining with the appropriate secondary antibodies.
RNA Interference
RNA interference was carried out as described previously (Elbashir et al., 2001). The small interfering RNA (siRNA) corresponding to nucleotides 1077–1097 from the start codon of hMPS1 (mRNA sequence: AACGGAAUCAAGUCUUCUAGC) was synthesized by Dharmacon Inc. (Lafayette, CO) siRNA was delivered into cells either by Oligofectamine (Invitrogen, Carlsbad, CA) or with Transit TKO (Mirus, Madison, WI) at 100 nM and 20 nM final concentrations, respectively. The plates were replaced with fresh medium 16 h later, and coverslips were usually harvested 36–48 h after transfection.
Immunofluorescence
Cells used for immunofluorescence staining or for microinjections were plated onto No. 1.5 glass coverslips and used 1–2 d later. For staining, cells were fixed for 7 min in freshly prepared 3.5% paraformaldehyde/PBS, pH 7.0, extracted in KB medium (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% BSA) plus 0.2% Triton X-100 for 5 min at room temperature and rinsed in KB. For optimal staining of hMPS1 at kinetochores, cells were simultaneously fixed and extracted with 3.5% paraformaldehyde/PBS, pH 7.0, plus 1% Triton X-100 for 10 min at room temperature. The coverslips were then rinsed in KB before use. Primary and secondary antibodies were diluted in KB and added to coverslips for 30–60 min at 37°C in a humidified chamber. Human anti-centromere autoimmune antibodies (ACAs) were gifts from K. F. Sullivan (Scripps Institute). Rat antibodies against CENP-E, hBUBR1, hBUB1, hROD, and hMAD1 were used at a final concentration of 0.5–1 μg/ml (Chan et al., 1998; Campbell et al., 2001). Monoclonal antibody mAb414 (Covance, Richmond, VA), mouse anti-cyclin B1 (PharMingen, San Diego, CA), mouse anti-α-tubulin (Sigma Chemical Co., St. Louis, MO), and mouse anti-γ-tubulin (Sigma Chemical Co.) antibodies were used at 1:1000 dilution. Secondary antibodies conjugated to Alexafluor 488, Alexafluor 594 (Molecular Probes, Eugene, OR), Texas Red, and Cy5 (Jackson ImmunoResearch, West Grove, PA) were all used at 2 μg/ml.
Gel Filtration, Immunoprecipitation, and Immunoblots
Asynchronous HeLa cells or mitotic cells shaken off the plates after 16 h of nocodazole treatment were lysed in ice-cold lysis buffer (Gately et al., 1998) (PBS with 0.5% NP40 and protease and phosphatase inhibitors). Lysates were centrifuged at 16,000 × g for 10 min, and the supernatants were either incubated with antibodies for immunoprecipitation or used directly for Western blot analysis. For gel filtration, ∼1 mg of clarified lysates was filtered through 0.45-μm membrane before being loaded onto a Superose 6 FPLC column (Amersham, Piscataway, NJ). For Western blots, rabbit anti-CDC27 (gift from Dr. V. Sudakin, Fox Chase Cancer Center, Philadelphia, PA), anti-CDC16 (gift from Dr. P. Hieter, University of British Columbia, Vancouver, Canada), and anti-APC7 (gift from Dr. J.M. Peters, IMP, Vienna, Austria) antibodies were used at 1:1000 dilution. Primary antibodies were detected with alkaline phosphatase–conjugated secondary antibodies (Sigma) that were diluted to 1:30,000 and then processed for chemiluminescence detection (CDPStar, Applied Biosystems, Foster City, CA). For immunoprecipitation, ∼250 μg of cell lysate was mixed with 1.5 μg rabbit nonimmune IgG or anti-hMPS1 antibody and rocked at 4°C for 2 h before addition of 15 μl of protein A–agarose. The protein A–agarose beads were presoaked in 1 mg/ml BSA to block nonspecific binding sites. After 30 min of rocking, the beads were washed four times with 0.4 ml lysis buffer, and 10 μl SDS gel sample buffer was added to boil the samples before loading onto SDS-PAGE gels.
RESULTS
Human MPS1 Is Localized to Centrosomes, Nuclear Pores, and Kinetochores
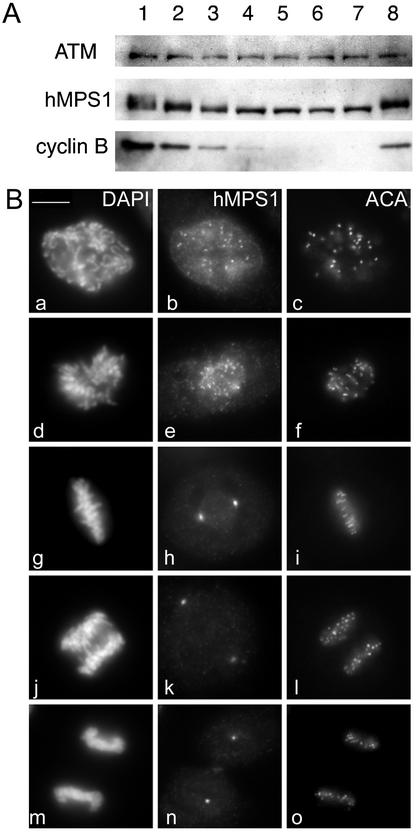
The human TTK kinase was originally identified in a screen for novel tyrosine kinases by using a phosphotyrosine antibody to screen a T-cell cDNA expression library (Mills et al., 1992). Using a similar strategy, the mouse homologue, esk, was also cloned from an embryonal carcinoma cell line (Douville et al., 1992). It was determined subsequently that esk was the mouse orthologue of yeast MPS1 (Fisk and Winey, 2001), and thus, it was likely that TTK is the human orthologue of MPS1. A recent report claimed that human MPS1 differed from mouse MPS1 in that the human protein was not found at centrosomes (Stucke et al., 2002). To resolve this discrepancy, we raised antibodies against amino acids 400–507 of hMPS1. Western blots of HeLa lysates with the affinity-purified antibody revealed a single band of ∼100 kDa that closely matched the calculated molecular weight of hMPS1 (Supplementary Figure 1A, lane 1). As shown by Stucke et al. (2002), we found that hMPS1 is hyperphosphorylated in mitosis (Supplementary Figure 1A, lane 2). We examined the phosphorylation status of hMPS1 at various times after release from a mitotic block. hMPS1 was rapidly dephosphorylated within 30 min after release from the mitotic block (Figure 1A). Dephosphorylation of hMPS1 coincided with entry into anaphase, as determined by microscopy and also by the decrease in the steady-state levels of cyclin B1. We found that hMPS1 remained hyperphosphorylated in the cells that were arrested in metaphase for up to 3 h with the proteosome inhibitor ALLN (Calbiochem, La Jolla, CA). Thus, dephosphorylation of hMPS1 is likely to be coordinated with entry into anaphase. There may also be a modest change at the protein level of hMPS1 when cells exit mitosis, which is consistent with the report by Stucke et al. (2002).
Figure 1.
Mitotic phosphorylation and subcellular distribution of hMPS1. (A) hMPS1 is dephosphorylated when cells exit mitosis. HeLa cells that were blocked in mitosis with nocodazole were collected and released into drug-free medium, and samples were taken every 30 min up to 3 h (lanes 1–7; lane 1 is time 0). One sample was released into medium containing the proteosome inhibitor ALLN and harvested 3 h later (lane 8). Equal amounts of protein obtained from the different lysates were probed for hMPS1, cyclin B, and ATM. ATM was used as a loading control because its level has been shown to not fluctuate during the cell cycle (Gately et al., 1998). (B) Affinity-purified rabbit anti-hMPS1 antibodies were used to stain HeLa cells at various stages of mitosis: prophase (a–c), prometaphase (d–f), metaphase (g–i), anaphase (j–l), and telophase (m–o). DAPI and ACA (anticentromere autoimmune serum) were used to stain chromosomes and kinetochores, respectively. Note the bright foci of centrosome staining at spindle poles. (C) hMPS1 is localized to centrosomes and nuclear pores during interphase. Interphase HeLa cells were costained with rabbit anti-hMPS1 and mouse anti-γ tubulin (a–c) to verify hMPS1 at centrosomes. The presence of hMPS1 at nuclear pores was revealed by costaining with mAb414 (d and e). A merged image shows coincident localization of hMPS1 with mAb414 (f). Cells permeabilized with digitonin were stained with hMPS1 and mAb414 antibodies (g–i). Rabbit anti-hMPS1 was visualized with Alexafluor 488 anti-rabbit secondary antibodies. Mouse anti-γ-tubulin and mAb414 were visualized with Texas Red anti-mouse secondary antibodies. DNA was stained with DAPI. Bar, 10 μm.
We examined the distribution of hMPS1 in mitotic HeLa cells by immunofluorescence microscopy and found that it was first detected at the kinetochores soon after nuclear envelope breakdown and persisted through prometaphase (Figure 1B, a–f). hMPS1 staining at kinetochores was no longer detectable from metaphase through telophase (Figure 1B, g–o). Consistent with mouse MPS1, we found that hMPS1 was concentrated at the spindle poles at all stages of mitosis (Figure 1B, h, k, and n). In interphase cells, we confirmed that hMPS1 was localized to centrosomes on the basis of colocalization with γ-tubulin (Figure 1C, a–c). The centrosome and kinetochore staining patterns were confirmed independently by a transfected GFP-hMPS1 that localized to both centrosomes and kinetochores (see Figure 4C).
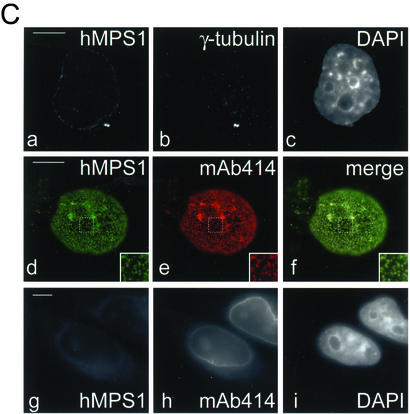
Figure 4.
Depletion of hMPS1 by siRNA prevented binding of MAD1 but not BUB1, BUBR1, and hROD to kinetochores. HeLa cells were transfected with hMPS1 siRNA and then processed for immunofluorescence 38 h later. Rat anti-MAD1, BUBR1, BUB1, and hROD antibodies were used with Alexafluor 488–conjugated secondary antibodies. Endogenous hMPS1 was stained with rabbit anti-hMPS1 antibody and Alexafluor 594–conjugated secondary antibody. ACA- and Cy5-labeled anti-human secondary antibody was used to visualize kinetochores, and DAPI was stained for chromosomes. Arrows indicate pairs of centromeres/kinetochores for comparison. Bar, 10 μm.
In addition to centrosome staining, the hMPS1 antibody also stained the nuclear rim, which was reminiscent of nuclear pores. To confirm this, HeLa cells were costained with hMPS1 antibodies and mAb414, a mAb that recognizes several nuclear pore proteins that are localized at the cytoplasmic and nucleoplasmic side of the NPC (Davis and Blobel, 1986). Comparison of individual optical slices from a z-series showed clearly that hMPS1 colocalized with mAb414 (Figure 1C, d–f). To test whether hMPS1 is concentrated at the cytoplasmic or nucleoplasmic side of the NPC, cells were permeabilized with digitonin and then stained with hMPS1 antibodies. Because digitonin selectively permeabilizes the cell membrane but not the nuclear membrane, proteins on the nucleoplasmic side would not be accessible to antibodies. hMPS1 antibodies failed to stain the nuclear envelope of the digitonin-permeabilized cells, indicating that it is located on the nucleoplasmic face (Figure 1C, g–i). mAb414, which recognizes a number of nuclear pore proteins located on both the nucleoplasmic and the cytoplasmic faces, stains the nuclear pores in the digitonin-permeabilized cells (Figure 1C, e).
hMPS1 Is Essential for the Mitotic Checkpoint
We next examined whether hMPS1 is required for HeLa cells to arrest in mitosis after the spindle was disrupted by the microtubule-depolymerizing drug nocodazole. To avoid disrupting its role in centrosome duplication, we performed experiments on cells after they had reached the G1/S boundary, when their centrosomes have already duplicated (Fisk and Winey, 2001; and our unpublished results). Affinity-purified hMPS1 antibodies were injected into the nucleus of cells shortly after they were released from the G1/S boundary. Using this approach, we did not detect defects in centrosome duplication or assembly of a bipolar spindle (see below). Injected cells were challenged with nocodazole and examined at 16 h after G1/S release. Consistent with previous reports (Stucke et al., 2002), we found that a high proportion of cells (52%) injected with hMPS1 antibody exited mitosis, as determined by loss of cyclin B1 and formation of polyploid nuclei (compared with 9% of cells injected with nonimmune IgG; see Supplementary Figure 2, A and B). To test the importance of hMPS1 in maintaining the mitotic checkpoint, hMPS1 antibodies were injected into cells that were arrested in mitosis after treatment with nocodazole. A total of 57 mitotic cells were injected in a period of 10 min and fixed 42 min later (42 min from the start of microinjection). Approximately half of the injected mitotic cells (28 of 57) escaped the nocodazole block, degraded cyclin B, and reformed aberrant multilobed nuclei. As a control, all 35 cells injected with nonimmune IgG remained arrested in mitosis after 1 h.
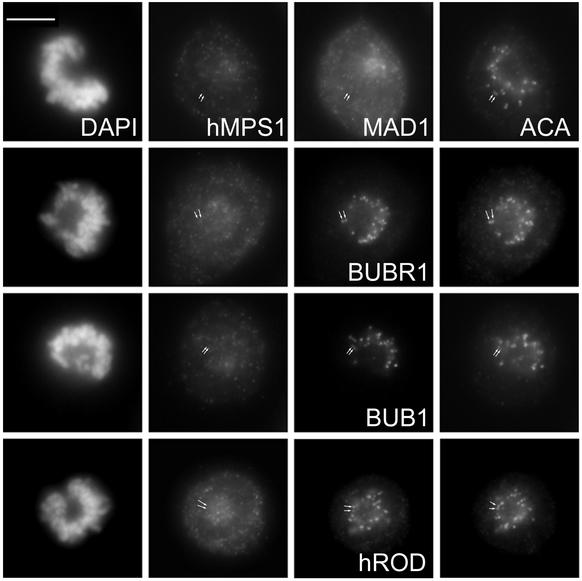
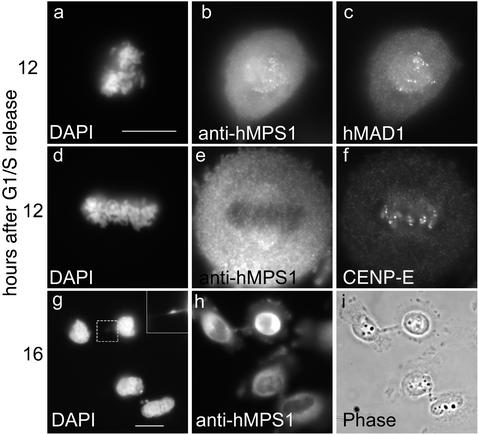
If hMPS1 is a critical component of the mitotic checkpoint, its function during a normal mitosis should be to prevent cells from exiting mitosis with unaligned chromosomes. Thus, inhibiting hMPS1 function might cause cells to enter anaphase prematurely, before chromosome alignment was completed. HeLa cells were injected with hMPS1 antibodies shortly after being released from the G1/S arrest. Cells were fixed and analyzed at various times. Examination of cells that had entered mitosis showed that the injected antibody was concentrated at kinetochores of the unaligned chromosomes. These antibodies did not interfere with the assembly of MAD1 at kinetochores (Figure 2, a–c). In some rare cases in which chromosomes had aligned at the spindle equator, the injected antibody was not detected at kinetochores, presumably because they were released along with hMPS1. Despite the lack of detectable hMPS1 at kinetochores, CENP-E was still detected there (Figure 2, d–f). At 16 h after release from the G1/S boundary, many of the newly divided cells had chromatin bridges between them. This suggested that the cells exited mitosis before all of their chromosomes were properly aligned (Figure 2, g–i). Thus, hMPS1 is important for normal mitotic progression.
Figure 2.
hMPS1 is critical for normal mitotic progression. HeLa cells synchronized at the G1/S boundary were injected with hMPS1 antibodies, fixed 12 and 16 h later, and stained with Cy5 anti-rabbit antibodies to identify the injected cells. Samples at the 12-h time point were also stained with rat anti-MAD1 (a–c) and rat anti-CENP-E (d–f) and counterstained with Alexafluor 488 anti-rat secondary antibodies. At the 16-h time point (g–i), injected cells had divided, as seen by phase-contrast microscopy. DNA was stained with DAPI. The inset shows chromatin bridges or “cut” phenotype in an anaphase cell that had been injected with hMPS1 antibodies. Bar, 10 μm.
hMPS1 Is Necessary for CENP-E–Dependent Mitotic Arrest
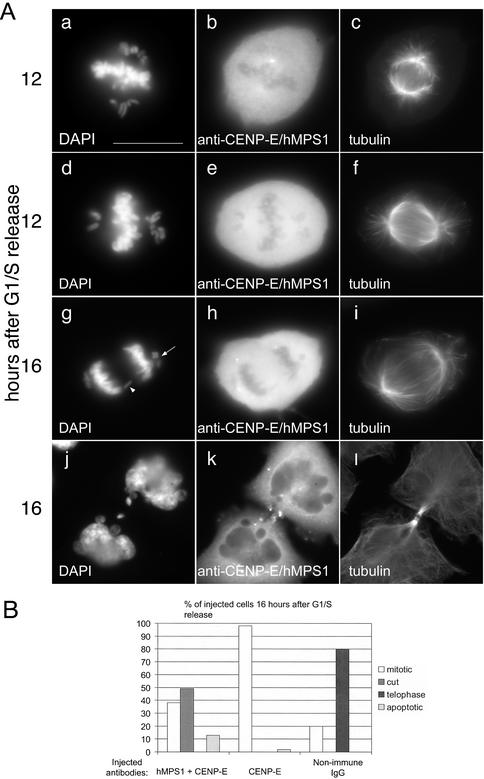
In human cells, the mitotic checkpoint monitors CENP-E functions at kinetochores, because disruption of CENP-E arrests cells in mitosis for prolonged periods (Schaar et al., 1997; Chan et al., 1998; Yao et al., 2000; McEwen et al., 2001). We previously reported that this arrest depends on hBUBR1 kinase (Chan et al., 1999). However, the preferential localization of hMPS1 at unattached kinetochores in CENP-E–depleted cells suggested that it might be required for this arrest (Supplementary Figure 3). HeLa cells synchronized at the G1/S boundary were coinjected with CENP-E antibodies and nonimmune IgG or with CENP-E and hMPS1 antibodies. The injected cells were fixed and processed for immunofluorescence at 12 and 16 h after G1/S release (Figure 3A). Nearly all the cells (>90%) injected with CENP-E and nonimmune antibodies accumulated in mitosis with mono-oriented chromosomes at the 16-h time point that is typical of cells whose kinetochores were depleted of CENP-E (Figure 3B). By comparison, 80% of cells injected with nonimmune IgG alone had exited mitosis and divided normally (Figure 3B). Cells coinjected with CENP-E and nonimmune IgG were arrested in mitosis with mono-oriented chromosomes, which is typical of a CENP-E defect (Schaar et al., 1997; Wood et al., 1997; Chan et al., 1998; Yao et al., 2000; McEwen et al., 2001). When cells coinjected with CENP-E and hMPS1 antibodies were examined at the 12-h time point, they were also found to contain mono-oriented chromosomes that were indicative of a CENP-E defect (Figure 3A, a–f). The presence of a bipolar spindle suggests that centrosome function was not disrupted by the injected antibodies (Figure 3A, c and f). Despite the presence of unaligned chromosomes, these cells failed to arrest in mitosis. At the 16-h time point, ∼50% of the cells coinjected with CENP-E and hMPS1 antibodies were in anaphase or had completed cell division (Figure 3B). Examination of anaphase cells revealed the presence of lagging chromosomes (Figure 3A, g, arrowhead). In addition, the chromosomes that failed to establish bipolar attachment remain stranded at one of the poles when cells entered anaphase (Figure 3A, g, arrow). These defects are probably responsible for the presence of micronuclei and chromatin bridges in the newly divided cells (Figure 3A, j). These data show that hMPS1 is essential for the mitotic arrest that results from the loss of CENP-E from kinetochores.
Figure 3.
Mitotic arrest induced by loss of CENP-E depends on hMPS1. Synchronized HeLa cells were coinjected with CENP-E and hMPS1 antibodies, CENP-E and nonimmune IgG, or nonimmune IgG alone and sampled 12 and 16 h after release from the G1/S boundary. (A) Cells coinjected with CENP-E and hMPS1 antibodies were stained for the injected antibodies and α-tubulin to visualize the spindle and DAPI to visualize chromosomes. At the 12-h time point, the presence of mono-oriented chromosomes indicated a CENP-E defect. At the 16-h time point, the doubly injected cells entered anaphase with chromatin bridges (“cut” phenotype, g) or divided to form multilobed nuclei or micronuclei (j). Bar, 10 μm. (B) Cells injected with different antibodies were fixed at the 16-h time point, visualized by phase-contrast microscopy, and stained with DAPI to determine their fates. Cells in mitosis, those that prematurely exited mitosis with chromatin bridges (“cut” phenotype), those that exited mitosis normally, and apoptotic cells were quantified and compared. An average of 200 cells were counted for each experiment.
We next tested whether hMPS1 is also important for maintaining the arrest that resulted from the disruption of CENP-E functions. Cells were injected with CENP-E antibodies to induce a mitotic arrest and then reinjected with nonimmune IgG, anti-hMPS1, or anti-MAD2 antibodies, and the fates of the cells were examined 1 and 2 h later. All cells (20 of 20) injected with nonimmune IgG remained arrested in mitosis with unaligned chromosomes 2 h after injection. One hour after injection of hMPS1 antibodies, 15 of 16 cells remained in mitosis, whereas one cell exited mitosis. By comparison, 12 of 16 cells injected with MAD2 antibodies had exited mitosis 1 h after injection. Two hours after injection of hMPS1 antibodies, 21 of 30 cells exited mitosis, whereas 9 of 30 cells were still in mitosis. All cells (23 of 23) injected with MAD2 antibodies had exited mitosis by 2 h. We believe that the quantitative difference between cells injected with hMPS1 and MAD2 antibodies is likely to reflect differences in how efficiently the antibodies inhibited their targets. Regardless, these results show that hMPS1 is required for maintaining the mitotic checkpoint.
hMPS1 Is Required for MAD1 but Not hBUB1 or hBUBR1 to Bind Kinetochores
Injection of hMPS1 antibodies did not interfere with the localization of MAD1, even though a recent report showed that cells depleted of hMPS1 failed to assemble MAD1 and MAD2 onto kinetochores (Martin-Lluesma et al., 2002). The difference is probably because the injected antibodies did not deplete hMPS1 from kinetochores. To examine the relationship between hMPS1 and other checkpoint proteins, we used siRNA to block hMPS1 expression. We screened prometaphase cells for hMPS1 staining, because this stage of mitosis is when hMPS1 can be easily detected at kinetochores. We found many prometaphase cells that showed at least a 10-fold reduction of hMPS1 staining (Figure 4; quantified by fluorescence microscopy), even though control cells exhibited normal levels of staining (our unpublished results). As reported previously, kinetochores lacking hMPS1 lacked MAD1. However, the loss of hMPS1 from kinetochores did not affect the ability of other checkpoint proteins such as hBUB1, hBUBR1, and hROD to bind to kinetochores (Figure 4). Thus, hMPS1 is required only for the assembly of a subset of checkpoint proteins, including MAD1 and MAD2 to kinetochores.
The Kinetochore Binding Domain of hMPS1 Kinase Disrupts the Mitotic Checkpoint
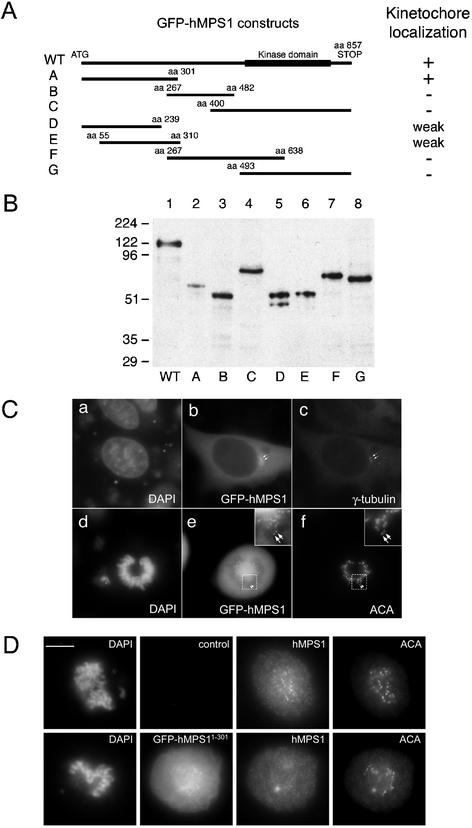
To understand the molecular basis of the interaction between hMPS1 and kinetochores, we sought to identify its kinetochore-targeting domain. Different segments of hMPS1 were fused to GFP, and the localization of the fusion proteins in transfected HeLa cells was monitored by fluorescence microscopy. Seven hMPS1 fragments that overlapped each other and spanned the entire coding region of hMPS1 were fused at their amino termini to GFP (Figure 5A). Western blot of transfected lysates showed that all the constructs, including full-length GFP-hMPS1, expressed proteins of the predicted size (Figure 5B). To verify that the GFP tag would not interfere with localization of hMPS1, the full-length GFP-hMPS1 was tested first. GFP-MPS1 was found to localize to the centrosomes during interphase (Figure 5C, a–c, arrows). In prometaphase cells, GFP-MPS1 was also detected at kinetochores (Figure 5C, d–f, insets and arrows). Rim staining around nuclei was also observed in interphase cells transfected with GFP-hMPS1, suggesting the nuclear envelope localization of the GFP fusion protein, but because of the background of GFP expression, we cannot be certain whether GFP-hMPS1 was targeted to nuclear pores (our unpublished results).
Figure 5.
Mapping the kinetochore targeting domain of hMPS1. (A) Schematic depiction of hMPS1 fragments that were used to map the kinetochore binding domain. WT, wild-type. (B) Lysates prepared from HeLa cells transfected with various GFP-hMPS1 constructs were probed with anti-GFP antibodies. (C) Cells transfected with full-length hMPS1 fused to GFP were stained with γ-tubulin (c) or ACA (f). (D) GFP-hMPS11–301 depletes endogenous hMPS1 from kinetochores. An untransfected control cell (top) and a transfected cell (bottom) on the same coverslip were stained with hMPS1 antibody and visualized with Alexafluor 594 conjugated anti-rabbit secondary antibody. DAPI and ACA followed by Cy5 conjugated anti-human secondary antibody were used to stain chromosomes and kinetochores. Bar, 10 μm.
We then attempted to localize the domain in hMPS1 that specified kinetochore binding. Among the six fragments of hMPS1 that were examined, only the fragment that encompassed amino acids 1–301 was clearly localized to kinetochores (Figure 5D). Fragments D and E, which encompassed amino acids 1–239 and 55–310, respectively, were found to localize to kinetochores infrequently. Furthermore, their staining intensity at kinetochores was very weak compared with hMPS11–301 or full-length hMPS1 (our unpublished results). Thus, the kinetochore-binding domain of hMPS1 resides within the amino-terminal 301 amino acids. Immunoprecipitation experiments did not reveal an interaction between hMPS11–301 and endogenous hMPS1 (our unpublished results). Thus, the localization of hMPS11–301 at kinetochores was not because of its interaction with endogenous hMPS1. This was further confirmed by the observation that kinetochores containing the GFP-hMPS11–301 lacked detectable endogenous hMPS1 (Figure 5D, bottom).
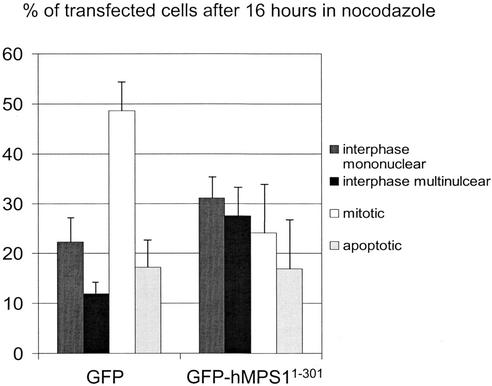
We next tested whether GFP-hMPS11–301 might interfere with endogenous hMPS1 functions and disrupt the checkpoint. As shown in Figure 6, when transfected cells were examined after 16 h of nocodazole treatment, there was a twofold reduction in the number of mitotic cells that expressed GFP-hMPS11–301 compared with cells transfected with just GFP. Correspondingly, there was a twofold increase in the number of cells with multiple nuclei or multilobed nuclei, which were indicative of checkpoint override. We believe that cell-to-cell variation in expression levels of the transfected protein can explain why a larger proportion of cells that expressed GFP-hMPS11–301 did not overcome the checkpoint.
Figure 6.
GFP-hMPS11–301 disrupts mitotic checkpoint. HeLa cells were transfected with pECEGFP or pECEGFP-hMPS11–301 at the time of release from double thymidine block, and nocodazole was added 8 h later. After 16 h in nocodazole, transfected cells were examined live by phase-contrast and fluorescence microscopy to determine mitotic index. The average of three experiments is presented here.
hMPS1 Associates with APC in Both Interphase and Mitosis
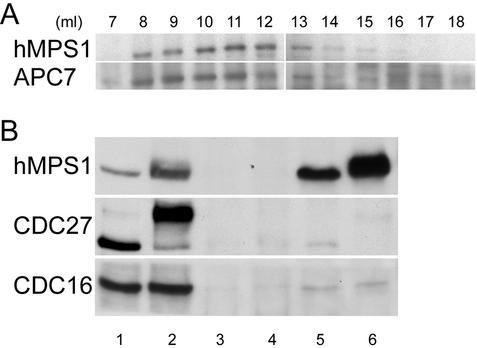
The mitotic checkpoint pathway is initiated at unattached kinetochores, and its target is the APC. Checkpoint proteins such as MAD2 and hBUBR1 not only act at kinetochores but also directly inhibit the APC (Sudakin et al., 2001; Tang et al., 2001; Fang, 2002). We therefore examined whether hMPS1 might have roles beyond the kinetochores. The elution profile of hMPS1 from a Superose 6 gel filtration column suggested that it existed in a large complex that overlapped with the peak fractions of the APC (Figure 7A). Immunoprecipitation of hMPS1 from lysates prepared from mitotically arrested cells brought down the APC, as determined by the presence of CDC27 and CDC16 (Figure 7B, lane 6). Surprisingly, the APC also coimmunoprecipitated with hMPS1 from interphase cell lysates (Figure 7B, lane 5). Although it was only a very small fraction of APC that coimmunoprecipitated with hMPS1, the specificity of these interactions was demonstrated by the fact that a similar amount of nonimmune IgG did not bring down CDC27 and CDC16 from both interphase and mitotic cell lysates (Figure 7B, lanes 3 and 4). The fraction of the APC subunits associated with hMPS1 did not seem to differ dramatically between mitosis and interphase (compare lanes 5 and 6 with lanes 1 and 2).
Figure 7.
hMPS1 associates with APC in interphase and mitosis. (A) Elution profiles of hMPS1 and APC7 from a Superose 6 column. Asynchronous HeLa cell lysate, ∼1 mg, was loaded onto the column, and 1-ml fractions were collected. The fractions across the elution profile were probed with hMPS1 and APC7 antibodies. (B) hMPS1 coimmunoprecipitated with the APC subunits CDC27 and CDC16 in interphase and mitotic cell lysates. Asynchronous (lanes 1, 3, and 5) or mitotic (lanes 2, 4, and 6) lysates, ∼250 μg, were immunoprecipitated with 1.5 μg nonimmune IgG (lanes 3–4) or anti-hMPS1 antibody (lanes 5–6). Then ∼16 μg of the lysates (1/15 of the amount used for immunoprecipitation) were run in lanes 1 and 2 as controls.
DISCUSSION
Multiple Subcellular Localizations of hMPS1
We have identified and characterized the human MPS1 kinase and found that it is localized to centrosomes and kinetochores. These findings are consistent with the results reported for mouse MPS1 (Fisk and Winey, 2001). However, our findings differ from a recent report that indicated that hMPS1 does not localize to the centrosomes (Stucke et al., 2002). We believe that this discrepancy is probably a result of differences in specificity of the antibodies that were used in the respective studies. Stucke et al. used a mAb raised against hMPS1 in their studies. Thus, it is possible that the epitope recognized by their mAb was not accessible at centrosomes. We are certain that our antibodies identified hMPS1 at centrosomes, because we independently confirmed the immunofluorescence data by showing that a GFP–hMPS1 fusion protein was localized to centrosomes in transfected interphase and mitotic cells.
Our studies also revealed the novel finding that hMPS1 is localized to nuclear pores. The localization of a kinetochore protein to nuclear pores is not unique to hMPS1, because we have shown that the MAD1 and MAD2 checkpoint proteins are also localized there (Campbell et al., 2001). The significance of this remains to be determined but may reflect a previously unrecognized connection between nuclear pores and kinetochores. In this regard, the nuclear pore proteins Nup133, Nup107 (Belgareh et al., 2001), Rae1 (Wang et al., 2001), RanGAP1, and RanBP2 (Joseph et al., 2002) have been found to localize to kinetochores during mitosis. Thus, it is possible that these two groups of proteins depend on each other for their localization at kinetochores.
We found that hMPS1 exhibits a dynamic pattern of interaction with kinetochores during mitosis. hMPS1 is not detected at kinetochores during interphase and is first detected there after nuclear envelope breakdown. At later stages of prometaphase, when chromosomes are congressing toward the spindle equator, the level of hMPS1 at kinetochores was reduced significantly. By metaphase, hMPS1 was no longer detected at kinetochores. This pattern is in general agreement with that reported for mouse, human, and Xenopus MPS1.
We have localized the kinetochore targeting domain of hMPS1 to lie within the amino-terminal 301 amino acids. Because constructs lacking this region failed to localize to kinetochores, the amino-terminal 301 amino acids must be necessary and sufficient for kinetochore localization. The primary sequence of the amino-terminal 301 residues does not reveal any apparent motifs that might provide clues as to the biochemical interactions mediated between hMPS1 and kinetochores.
Human MPS1 Is Essential for the Mitotic Checkpoint
We demonstrated that hMPS1 is an essential component of the mitotic checkpoint. Cells defective for hMPS1 function failed to arrest in mitosis in the presence of spindle defects and kinetochore defects resulting from the loss of CENP-E. In addition, hMPS1 is critical for normal mitotic progression, because cells defective for hMPS1 functions exited mitosis with lagging chromosomes. This phenotype is indicative of a checkpoint defect that caused cells to exit mitosis prematurely, before all their chromosomes are aligned properly. This phenotype is very similar to that observed when the checkpoint functions of MAD1, MAD2, hBUB1, hBUBR1, hZW10, and hROD were disrupted. Our results are consistent with those reported for xMPS1 and for hMPS1 (Abrieu et al., 2001; Stucke et al., 2002).
The localization of hMPS1 to kinetochores, like many other checkpoint proteins (Hoffman et al., 2001), is sensitive to microtubule interactions. hMPS1 appears to bind preferentially to unattached kinetochores, because it was not detected at kinetochores that were aligned. On the basis of the localization pattern of hMPS1 in cells that lack CENP-E, it appears that hMPS1 maybe sensitive to microtubule occupancy rather than kinetochore tension. We previously showed that chromosomes are able to establish bipolar attachments despite the loss of CENP-E from kinetochores. Although kinetochores lacking CENP-E are able to establish nearly the same number of microtubule attachments as normal kinetochores, they lack tension (McEwen et al., 2001). Because hMPS1 was not detected at the bipolar attached kinetochores that lacked tension, its association with the kinetochore must be sensitive to microtubule occupancy.
Recent studies of HeLa cells showed that the localization of hMPS1 is specified by HEC1, a protein that shares some similarities with the NDC80p kinetochore protein in yeast (Martin-Lluesma et al., 2002). Furthermore, they showed that hMPS1 was required for the localization of MAD1 and MAD2 to kinetochores. We confirmed this finding and extended it by showing that hMPS1 was not required by hBUB1, hBUBR1, and hROD to bind to kinetochores. This observation is significant in light of the report that kinetochores depleted of hMPS1, MAD1, and MAD2 by blocking HEC1 expression were arrested in mitosis in the presence of unaligned chromosomes. The ability of HEC1-depleted cells to arrest in mitosis (Martin-Lluesma et al., 2002) would seem to be at odds with the finding that direct inhibition of hMPS1 by antibody injection or siRNA abrogated the mitotic checkpoint (our results and Stucke et al., 2002). This discrepancy can be resolved if we assume that hMPS1 plays roles both at kinetochores and downstream of kinetochores. HEC1 depletion may affect hMPS1 only at kinetochores but not its downstream functions. Conversely, direct inhibition of hMPS1 by antibody injection, siRNA, and overexpression of a dominant negative hMPS1 mutant would disrupt all the activities of hMPS1 and thus cause a defective mitotic checkpoint. The proposition that checkpoint proteins act at different steps along the mitotic checkpoint pathway is not novel; hBUBR1 and MAD2 are thought to act downstream of the kinetochores by inhibiting the APC (Chen et al., 1998; Sudakin et al., 2001; Tang et al., 2001; Fang, 2002). Our finding that hMPS1 can associate with the APC suggests that it may also act at this step of the checkpoint pathway. Unlike hBUBR1 and MAD2, which bind the APC only in mitosis, hMPS1 was found to associate with the APC during interphase and mitosis. Earlier characterization of TTK showed that its kinase activity is low in interphase but peaks during mitosis (Hogg et al., 1994; Stucke et al., 2002). Thus, it is possible that hMPS1 phosphorylates the APC during mitosis and that these modifications may be part of the mechanism by which the checkpoint inhibits the APC.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge expert technical support by B. Conner. S.T.L. was supported by the Greenwald Fellowship. This work was supported by National Institutes of Health grant GM-44762, core grant CA-06927, and an appropriation from the Commonwealth of Pennsylvania.
Footnotes
Online version of this article contains supplemental figures. The online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–05–0074. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–05–0074.
REFERENCES
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Alexandru G, Zachariae W, Schleiffer A, Nasmyth K. Sister chromatid separation and chromosome re-duplication are regulated by different mechanisms in response to spindle damage. EMBO J. 1999;18:2707–2721. doi: 10.1093/emboj/18.10.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9:69–75. doi: 10.1016/s0959-437x(99)80010-0. [DOI] [PubMed] [Google Scholar]
- Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. doi: 10.1016/s0092-8674(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Basu J, Bousbaa H, Logarinho E, Li Z, Williams BC, Lopes C, Sunkel CE, Goldberg ML. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J Cell Biol. 1999;146:13–28. doi: 10.1083/jcb.146.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Logarinho E, Herrmann S, Bousbaa H, Li Z, Chan GK, Yen TJ, Sunkel CE, Goldberg ML. Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma. 1998;107:376–385. doi: 10.1007/s004120050321. [DOI] [PubMed] [Google Scholar]
- Belgareh N, Rabut G, Bai SW, van Overbeek M, Beaudouin J, Daigle N, Zatsepina OV, Pasteau F, Labas V, Fromont-Racine M, Ellenberg J, Doye V. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol. 2001;154:1147–1160. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloecher A, Venturi GM, Tatchell K. Anaphase spindle position is monitored by the BUB2 checkpoint. Nat Cell Biol. 2000;2:556–558. doi: 10.1038/35019601. [DOI] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Wilson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Campbell MS, Chan GK, Yen TJ. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J Cell Sci. 2001;114:953–963. doi: 10.1242/jcs.114.5.953. [DOI] [PubMed] [Google Scholar]
- Castillo AR, Meehl JB, Morgan G, Schutz-Geschwender A, Winey M. The yeast protein kinase Mps1p is required for assembly of the integral spindle pole body component Spc42p. J Cell Biol. 2002;156:453–465. doi: 10.1083/jcb.200111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol. 1999;146:941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Schaar BT, Yen TJ. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J Cell Biol. 1998;143:49–63. doi: 10.1083/jcb.143.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R-H, Shevchenko A, Mann M, Murray AW. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol. 1998;143:283–295. doi: 10.1083/jcb.143.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Gimenez-Abian JF. Checkpoints controlling mitosis. Bioessays. 2000;22:351–363. doi: 10.1002/(SICI)1521-1878(200004)22:4<351::AID-BIES5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Daum JR, Gomez-Ospina N, Winey M, Burke DJ. The spindle checkpoint of Saccharomyces cerevisiae responds to separable microtubule-dependent events. Curr Biol. 2000;10:1375–1378. doi: 10.1016/s0960-9822(00)00780-6. [DOI] [PubMed] [Google Scholar]
- Davis LI, Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Douville EM, Afar DE, Howell BW, Letwin K, Tannock L, Ben-David Y, Pawson T, Bell JC. Multiple cDNAs encoding the esk kinase predict transmembrane and intracellular enzyme isoforms. Mol Cell Biol. 1992;12:2681–2689. doi: 10.1128/mcb.12.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol Biol Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesquet D, Fitzpatrick PJ, Johnson AL, Kramer KM, Toyn JH, Johnston LH. A Bub2p-dependent spindle checkpoint pathway regulates the Dbf2p kinase in budding yeast. EMBO J. 1999;18:2424–2434. doi: 10.1093/emboj/18.9.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk HA, Winey M. The mouse Mps1p-like kinase regulates centrosome duplication. Cell. 2001;106:95–104. doi: 10.1016/s0092-8674(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Fraschini R, Formenti E, Lucchini G, Piatti S. Budding yeast Bub2 is localized at spindle pole bodies and activates the mitotic checkpoint via a different pathway from Mad2. J Cell Biol. 1999;145:979–991. doi: 10.1083/jcb.145.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RD, Burke DJ. The spindle checkpoint: two transitions, two pathways. Trends Cell Biol. 2000;10:154–158. doi: 10.1016/s0962-8924(00)01727-x. [DOI] [PubMed] [Google Scholar]
- Gately DP, Hittle JC, Chan GK, Yen TJ. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol Biol Cell. 1998;9:2361–2374. doi: 10.1091/mbc.9.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Chen RH, Murray AW. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg D, Guidos C, Bailey D, Amendola A, Groves T, Davidson J, Schmandt R, Mills G. Cell cycle dependent regulation of the protein kinase TTK. Oncogene. 1994;9:89–96. [PubMed] [Google Scholar]
- Howell BJ, Hoffman DB, Fang G, Murray AW, Salmon ED. Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J Cell Biol. 2000;150:1233–1250. doi: 10.1083/jcb.150.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Jablonski SA, Chan GK, Cooke CA, Earnshaw WC, Yen TJ. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma. 1998;107:386–396. doi: 10.1007/s004120050322. [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- Jin DY, Spencer F, Jeang KT. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J Cell Biol. 2002;156:595–602. doi: 10.1083/jcb.200110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Hieter P. Evolutionary conservation between budding yeast and human kinetochores. Nat Rev Mol Cell Biol. 2001;2:678–687. doi: 10.1038/35089568. [DOI] [PubMed] [Google Scholar]
- Lauze E, Stoelcker B, Luca FC, Weiss E, Schutz AR, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 1995;14:1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. Bifurcation of the mitotic checkpoint pathway in budding yeast. Proc Natl Acad Sci USA. 1999;96:4989–4994. doi: 10.1073/pnas.96.9.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Tension-sensitive kinetochore phosphorylation and the chromosome distribution checkpoint in praying mantid spermatocytes. J Cell Sci. 1997;110(Pt 5):537–545. doi: 10.1242/jcs.110.5.537. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma S, Stucke VM, Nigg EA. Role of hec1 in spindle checkpoint signaling and kinetochore recruitment of mad1/mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell. 2001;12:2776–2789. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Schmandt R, McGill M, Amendola A, Hill M, Jacobs K, May C, Rodricks AM, Campbell S, Hogg D. Expression of TTK, a novel human protein kinase, is associated with cell proliferation. J Biol Chem. 1992;267:16000–16006. [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Pereira G, Hofken T, Grindlay J, Manson C, Schiebel E. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10. [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Poch O, Schwob E, de Fraipont F, Camasses A, Bordonne R, Martin RP. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Mol Gen Genet. 1994;243:641–653. doi: 10.1007/BF00279573. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandt R, Hill M, Amendola A, Mills GB, Hogg D. IL-2-induced expression of TTK, a serine, threonine, tyrosine kinase, correlates with cell cycle progression. J Immunol. 1994;152:96–105. [PubMed] [Google Scholar]
- Shah JV, Cleveland DW. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell. 2000;103:997–1000. doi: 10.1016/s0092-8674(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Stucke VM, Sillje HH, Arnaud L, Nigg EA. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 2002;21:1723–1732. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Ha E, McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J Cell Biol. 1998;142:1–11. doi: 10.1083/jcb.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, McKeon F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Babu JR, Harden JM, Jablonski SA, Gazi MH, Lingle WL, de Groen PC, Yen TJ, van Deursen JM. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J Biol Chem. 2001;276:26559–26567. doi: 10.1074/jbc.M101083200. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Waters JC, Chen RH, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Goetsch L, Baum P, Byers B. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol. 1991;114:745–754. doi: 10.1083/jcb.114.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol. 2000;2:484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.