Abstract
Ion transport in various tissues can be regulated by the cortical actin cytoskeleton. Specifically, involvement of actin dynamics in the regulation of nonvoltage-gated sodium channels has been shown. Herein, inside-out patch clamp experiments were performed to study the effect of the heterodimeric actin capping protein CapZ on sodium channel regulation in leukemia K562 cells. The channels were activated by cytochalasin-induced disruption of actin filaments and inactivated by G-actin under ionic conditions promoting rapid actin polymerization. CapZ had no direct effect on channel activity. However, being added together with G-actin, CapZ prevented actin-induced channel inactivation, and this effect occurred at CapZ/actin molar ratios from 1:5 to 1:100. When actin was allowed to polymerize at the plasma membrane to induce partial channel inactivation, subsequent addition of CapZ restored the channel activity. These results can be explained by CapZ-induced inhibition of further assembly of actin filaments at the plasma membrane due to the modification of actin dynamics by CapZ. No effect on the channel activity was observed in response to F-actin, confirming that the mechanism of channel inactivation does not involve interaction of the channel with preformed filaments. Our data show that actin-capping protein can participate in the cytoskeleton-associated regulation of sodium transport in nonexcitable cells.
INTRODUCTION
Ion transport mechanisms in various tissues are regulated by the cortical actin cytoskeleton. It has been shown that cytoskeletal elements are responsible for localization and clustering of channel molecules in the plasma membrane (Levina et al., 1994; Smith et al., 1995). Several lines of evidence indicate that ion channels can interact with microfilaments via actin-binding proteins. Ankyrin and spectrin were found to be linked to voltage-gated sodium channels from brain (Srinivasan et al., 1988). In epithelia, ankyrin and fodrin are associated with amiloride-sensitive sodium channels from apical microvilli (Smith et al., 1991) suggesting that actin-binding proteins link sodium channels to the cortical actin filament network, and that this association may sequester channels at apical microvilli and maintain their polarized distribution in renal epithelial cells.
Functional characteristics of single channels can be affected by cytoskeleton rearrangements (Janmey, 1998). Disruption of the actin network by cytochalasin has been shown to activate amiloride-sensitive sodium channels in A6 cells (Cantiello et al., 1991), amiloride-insensitive sodium channels in leukemia cells (Negulyaev et al., 1996a), volume-regulated Cl− channels in epithelial cells of the renal cortical collecting duct (Schwiebert et al., 1994) and also to enhance ATP-sensitive K+ channel activity in guinea pig cardiomyocytes (Terzic and Kurachi, 1996). On the other hand, inhibition of K+ channels by cytochalasin has been observed in the rat cortical collecting duct (Wang et al., 1994). In neuronal tissues, cytochalasin affects membrane excitability through the regulation of Ca2+ and N-methyl-d-aspartic acid channels (Johnson and Byerly, 1993; Rosenmund and Westbrook, 1993). In B lymphocytes, cytochalasin modulates the response of volume-regulated chloride channels to the hyposmotic gradient (Levitan et al., 1995). Moreover, ion channel activity can be affected by both DNase I and gelsolin, which destabilize actin filaments (Terzic and Kurachi, 1996; Maximov et al., 1997; Lader et al., 1999). Accordingly, phalloidin, which stabilizes actin filaments, may reverse the effect of actin cytoskeleton disrupters (Terzic and Kurachi, 1996; Lader et al., 1999). Short actin filaments were reported to activate epithelial sodium channels reconstituted in a lipid bilayer (Berdiev et al., 1996). Together, these data clearly show the involvement of the actin cytoskeleton in channel regulation. However, the mechanisms underlying regulation of ion channel activity by the cytoskeleton are yet poorly understood.
We have previously shown that nonvoltage-gated sodium channels in leukemia cells are activated by both cytochalasin D and gelsolin, and inactivated by polymerizing actin (Negulyaev et al., 2000). These results suggest that actin dynamics may be of primary importance in the regulation of channel activity. Recent studies have identified capping proteins as indispensable components of actin dynamics in living cells (Borisy and Svitkina, 2000). These proteins are known to bind to the ends of actin filaments thus preventing further filament elongation (Schafer and Cooper, 1995; Cooper and Schafer, 2000). In this work, we demonstrated the effect of the actin capping protein CapZ on actin-induced regulation of sodium channel activity in leukemia cells.
MATERIALS AND METHODS
Cells
Human myeloid leukemia K562 cells (Cell Culture Collection, Institute of Cytology, Russia) were maintained in glass flasks in RPMI 1640 medium containing 10% fetal bovine serum and antibiotics (100 μg/ml streptomycin and 100 U/ml penicillin) at 37°C. Cells were plated on coverslips 1–3 d before the experiment.
Electrophysiology
Single channel currents were recorded using standard inside-out configuration of the patch-clamp technique (Hamill et al., 1981). The recordings were performed on the stage of an inverted microscope with Nomarsky optics (256×). Pieces of coverslips with adhered cells were transferred into a recording chamber (volume of 0.2 ml). At the beginning of the experiment, the chamber was filled with normal sodium external solution in which giga-seal was formed. Pipettes were pulled from soft glass capillaries to a resistance of 10 to 15 MΩ when filled with external solution. Membrane currents were recorded using a homemade head stage, based on Burr-Brown operational amplifier OPA-128 with 20-GΩ feedback resistor. Computer-controlled set of Bessel filters LM-202 and amplifiers LM-201S (L-Card, Moscow, Russia) were used for signal conditioning. Data were filtered at 200 Hz and sampled at a rate of 1 kHz by 12-bit ADC for analysis and display. Experiments were performed at room temperature (21–23°C). Channel open probability (Po) was determined using the following equation: Po = I/iN, where I is the mean current determined from the amplitude histograms, i is the unitary current amplitude, and N is the number of functional channels in the patch. Averaged data are given as the mean ± SEM (number of experiments).
Solutions
Recording pipettes were filled with normal external solution containing 145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES/Tris-OH (pH 7.3). The bath cytosol-like solution for inside-out measurements contained 140 mM potassium aspartate, 5 mM NaCl, 1 mM MgCl2 (if not otherwise stated), 20 mM HEPES/KOH (pH 7.3), 2 mM EGTA, and an appropriate amount (0.176 mM) of CaCl2 to establish free ionized calcium concentration at the level of 0.01 μM (pCa of 8). Cytochalasin B (CB) was prepared as a stock solution in dimethyl sulfoxide and then diluted in the cytosol-like solution. If not otherwise stated, the procedure of changing solution in the chamber was carried out as follows: the previous solution was removed and completely replaced by the next one. Those cases when the agent was injected in the solution to obtain the mixture are specially indicated. HEPES, EGTA, and cytochalasin B were from Sigma Chemical (St. Louis, MO).
Proteins
G-actin isolated from rabbit skeletal muscle (Spudich and Watt, 1971) was stored in a low ionic strength solution (2 mM Tris-HCl, pH 7.5, 0.1 mM CaCl2, 0.2 mM ATP, 0.02% NaN3) and used within a week. An aliquot of the G-actin stock solution was added to the bath to a final concentration of 0.3 mg/ml. CapZ was isolated from chicken breast muscle as described previously (Remmert et al., 2000) and stored as a pelleted ammonium sulfate precipitate at −70°C. Before use, the precipitate was dissolved and dialyzed intensively against a buffer, containing 0.2 mM EGTA, 0.2 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 10 mM Tris-HCl, pH 7.0. Homogeneity of the protein samples and intrinsic properties of CapZ were tested as in Remmert et al. (2000).
Actin Polymerization
Actin polymerization was registered as an increase in viscosity of the bath cytosol-like solution (140 mM KAsp, 5 mM NaCl, 1 mM MgCl2, 20 mM HEPES/KOH, pH 7.3, 2 mM EGTA, pCa of 8) containing actin (0.3 mg/ml) and CapZ at various CapZ/actin molar ratios. Specific viscosity of the solutions was measured in an Ostwald-type capillary viscometer with a sample volume of 0.7 ml and an outflow time for water of 70 s at 22°C.
RESULTS
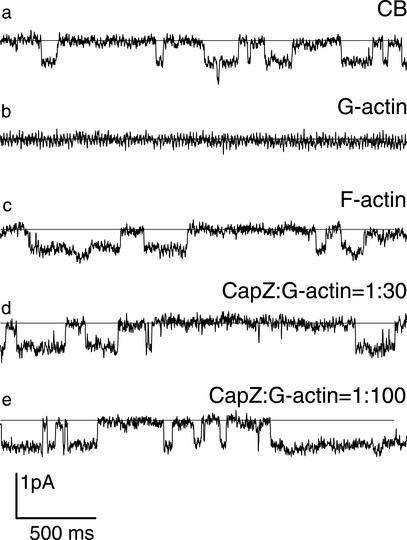
In previous experiments, nonvoltage-gated sodium channels were activated by cytochalasin D (Negulyaev et al., 2000). In the present work, we induced the activity of nonvoltage-gated sodium channels (12 pS, PNa/PK of ∼3) in inside-out patches by addition of cytochalasin B (10 μg/ml) added to the bath cytosol-like solution (Figure 1a). Consistent with our previous data (Negulyaev et al., 2000), these channels were inactivated by addition of G-actin (0.3 mg/ml) under ionic conditions promoting rapid actin polymerization (Figure 1b). On activation by CB, the channel open probability (Po) was 0.57 ± 0.10 (n = 15), G-actin addition decreased Po to zero level (n = 7). However, no inhibition of the cytochalasin-induced channels was observed in response to F-actin polymerized before its addition to the bath solution (Figure 1c); Po was 0.62 ± 0.13 (n = 3).
Figure 1.
Cytochalasin-induced activity of sodium channels is abolished by G-actin but not by F-actin or actin/CapZ complexes. Inside-out current records show sodium channel activation by 10 μg/ml CB (a), inactivation of CB-activated channels upon application of 0.3 mg/ml G-actin (b), and the lack of inactivation of CB-activated channels by 0.3 mg/ml F-actin (c), or by 0.3 mg/ml G-actin in the presence of CapZ at CapZ/actin molar ratios of 1:30 (d) and 1:100 (e). Holding potential was −20 mV, filter 200 Hz.
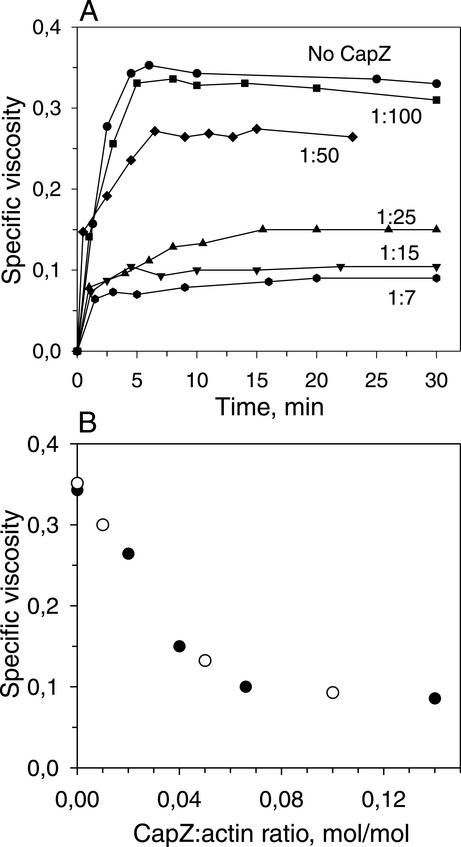
In the next set of experiments, G-actin was added to the bath solution together with CapZ, which caps the fast-polymerizing ends of actin filaments and promotes actin nucleation (Caldwell et al., 1989; Remmert et al., 2000). In a series of viscosity measurements (Figure 2), CapZ decreased the viscosity of actin solutions in a concentration-dependent manner. Because CapZ binds only to the barbed ends of actin filaments, the reduction in viscosity reflects a decrease in average filament length. When actin was polymerized in the presence of CapZ at low CapZ/actin molar ratios (1:50–1:100), the viscosity was reduced by ∼20% compared with the control, indicating that under these conditions a large proportion of long filaments is still formed (Figure 2). At high CapZ/actin ratios (1:5–1:15) the viscosity was reduced by 70–80%, implying that short actin filaments became predominant in the solution. In inside-out experiments, when the channel activity had been induced by CB, we applied CapZ and G-actin simultaneously at different CapZ/actin molar ratios from 1:100 to 1:5 (Figure 1, d and e). Our hypothesis was that the closing effect of actin on channel activity might depend on filament length, and hence differ at different CapZ/actin ratios. We found, however, that in the presence of CapZ, G-actin failed to inhibit channel activity at all CapZ/actin ratios used (7 of 7 experiments) (Figure 1). No decrease of channel activity was observed even at low CapZ/actin ratios (Po of 0.60 ± 0.12, n = 3). When the actin/CapZ mixture was replaced by fresh cytosol-like solution, an injected aliquot of G-actin reduced the channel activity to the background level within 1.5 min after actin addition. These data show that the presence of CapZ prevented the actin-induced channel inactivation.
Figure 2.
CapZ decreases viscosity of F-actin solutions in a concentration-dependent manner. (A) Increase in viscosity of CapZ/actin solutions during polymerization reflects a corresponding increase in the average length of actin filaments. Aliquots of the G-actin stock solution were mixed with CapZ at the CapZ/actin molar ratios indicated, and actin polymerization was initiated by dilution of the mixture with cytosol-like solution to a final actin concentration of 0.3 mg/ml. (B) Viscosity of the steady state CapZ/F–actin solutions indicates that the increase in the CapZ/actin ratio leads to the shift of the average filament length to the shorter values.
In the control experiments, when G-actin was applied in the presence of human serum albumin at albumin/actin molar ratios from 1:50 to 1:10, the channel inactivation was identical to that induced by G-actin alone (n = 3). This confirms that the effect of CapZ was specific.
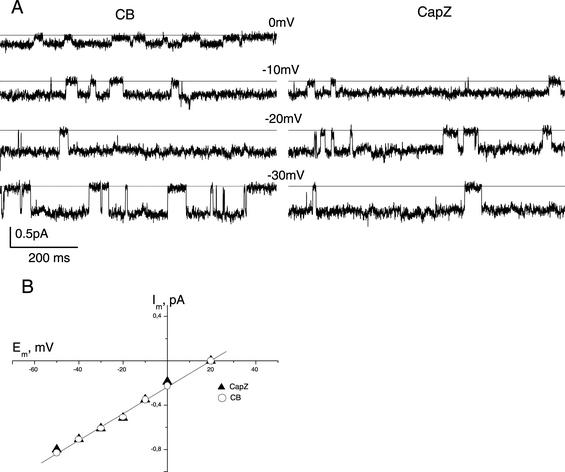
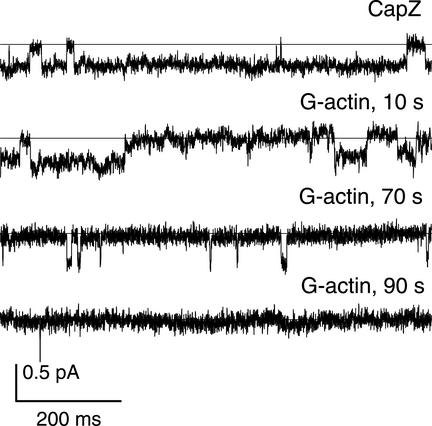
The inability of G-actin to inhibit channel activity in the presence of CapZ might be due to a specific binding of CapZ to the membrane site, which would alter the channel properties and prevent actin polymerization at the cytoplasmic surface of the cell membrane. To test these possibilities, CapZ and G-actin were applied separately to cytochalasin-activated channels (Figures 3 and 4). CapZ was added to the patch at a final concentration of 0.03 mg/ml (corresponding to a 1:5 CapZ/actin molar ratio in the CapZ/actin complexes). Figure 3 shows (4 of 4 experiments) that CapZ alone had no effect on single channel characteristics: the mean conductance (11.35 ± 1.00 pS), Na+/K+ permeability (PNa/PK of ∼3), and Po estimated after addition of CapZ (0.62 ± 0.11, n = 4) (as well as after simultaneous application of CapZ and actin; Figure 1) were not different from those measured after the CB treatment (Figure 3, A and B). After 2–3 min of current recording in the presence of CapZ, we replaced the solution in the chamber with a fresh one containing 0.3 mg/ml G-actin. This resulted in the channel inactivation; in 90 s Po was equal to zero (Figure 4), indicating that CapZ could be washed out from the membrane fragment. These results clearly demonstrate that CapZ does not affect the channel properties directly and is not tightly associated with plasma membrane. Hence, the effect of CapZ on regulation of the channel activity by actin seems to be due to the ability of CapZ to modulate actin dynamics rather than channel properties.
Figure 3.
Sodium channel properties are not affected by application of CapZ. (A) Inside-out current records of CB-induced sodium currents before (left) and after (right) addition of CapZ at different membrane potentials demonstrate that channel activity is not affected by CapZ application. Filter, 100 Hz. (B) Current-voltage relationships of sodium channels measured in the same patch after CB treatment and after CapZ (0.03 mg/ml) application. Mean unitary conductance was 12 pS; the reversal potential obtained by extrapolation was 20 mV. Im, membrane current; Em, membrane potential.
Figure 4.
CapZ can be washed out from the membrane fragment. CB-induced channel activity in the presence of CapZ (0.03 mg/ml) (top trace). The following traces demonstrate development of channel inactivation after CapZ was replaced with fresh portion of cytosol-like solution containing G-actin (0.3 mg/ml). Time intervals after G-actin application are indicated. Currents were recorded at −10 mV; filter, 100 Hz.
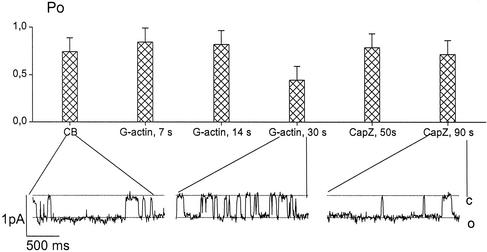
To further analyze the effect of CapZ on the filament-channel coupling, G-actin was applied to the membrane fragment before CapZ (Figures 5 and 6). Two different protocols were applied. First, CapZ was added at an early step of actin polymerization (Figure 5). Activation of the channels with CB was followed by the addition of G-actin in the standard Mg2+-containing cytosol-like solution. After 30 s, an aliquot of the CapZ stock solution was injected into the chamber, so that a 1:50 M ratio of CapZ to actin in the chamber was established. Figure 5 displays the mean Po and typical records of the channel activity under these conditions (4 of 4 experiments). The mean probability of the open state of the channels, Po, was 0.74 ± 0.14 after CB treatment, decreased to nearly half of this value during 30 s after the G-actin application (0.44 ± 0.03) and reincreased to 0.71 ± 0.16 within 1 min after the CapZ application. In additional experiments, when actin polymerization was slowed down by excluding Mg2+ from the bath solution, consistent results were obtained (our unpublished data). These data imply that at an early stage of actin polymerization, the actin-induced channel inactivation could be reversed by CapZ.
Figure 5.
Sodium channel inactivation evoked by actin polymerization is reversed by CapZ at an early stage of actin polymerization. The mean Po values (top) represent sodium channel activity in a CB-treated patch during incubation with G-actin followed by injection of CapZ (final CapZ/actin molar ratio was 1:50). CapZ was injected to the solution 30 s after actin application. Time intervals after G-actin and CapZ application are indicated. Three fragments of current records (bottom) at holding membrane potential of −20 mV demonstrate channel activity after application of CB (in 60 s), of G-actin (in 30 s), and of CapZ (in 90 s); c and o indicate closed and open states, respectively. Filter, 100 Hz.
Figure 6.
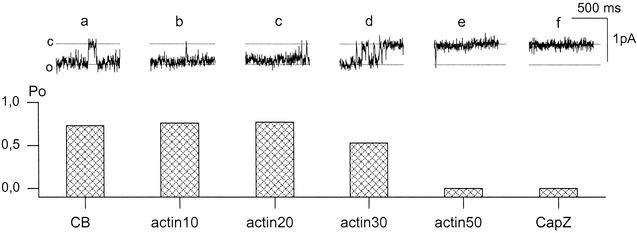
Sodium channel inactivation evoked by actin polymerization is not reversed at the stage of long filaments. Current records (top) and corresponding Po values (bottom) show sodium channel activity induced by CB (a), inhibited by G-actin polymerization (b–e); and not affected by subsequent injection of CapZ to the solution (CapZ:G-actin = 1:50). The Po values are 0.76 (a), 0.76 (b), 0.77 (c), 0.53 (d), and 0 (e and f). Time intervals after G-actin and CapZ application are indicated. CapZ was injected to the solution 60 s after actin application. Holding potential, −10 mV; filter, 200 Hz; c and o indicate closed and open states, respectively.
However, a reversion of the channel inactivation by CapZ was observed only if the channels were partially inactivated by actin. This is demonstrated in the second series of experiments (Figure 6). Inside-out patches were incubated with actin for 60 s or more, until complete channel inactivation had occurred. Subsequent injection of CapZ (to establish CapZ/actin ratio as 1:50) did not restore channel activity; Po remained close to zero (Figure 6).
Together, our experiments suggest that CapZ added at an early stage of actin assembly at the plasma membrane inhibit further filament growth and also seems to promote disassembly of short filaments. These effects of CapZ prevent inactivation of the channels by polymerizing actin and could maintain the channel in an open state.
DISCUSSION
Nonvoltage-gated sodium channels of leukemia cells are activated by cytochalasin-induced or gelsolin-induced actin disassembly, and inactivated by actin assembly on the cytoplasmic surface of the cell membrane (Negulyaev et al.,1996a, 2000; Maximov et al., 1997). The results presented in this work show that the actin-induced inactivation (closure) of these channels is prevented by the actin-capping protein CapZ. This effect was observed when CapZ was injected into the bath solution either simultaneously with G-actin or shortly after actin assembly had started. On the other hand, prepolymerized F-actin failed to inhibit cytochalasin-induced channel activity, confirming our previous data (Negulyaev et al., 2000) that the mechanism of channel inactivation does not involve the interaction of the channel with preformed filaments. Patch-clamp measurements imply that exogenous CapZ is neither tightly bound to the plasma membrane nor alters the channel characteristics. Together, these results allow us to conclude that the effect of CapZ on the channel regulation is due to the CapZ-mediated modification of actin assembly.
While being added simultaneously with G-actin, CapZ prevents actin-induced channel inactivation even at low CapZ/actin molar ratios. Viscosity measurements indicate that CapZ-capped actin filaments formed at low CapZ/actin ratios are not much shorter than the filaments with free barbed ends. Such filaments would inactivate the channels if they were formed at the plasma membrane (Negulyaev et al., 2000). The observed lack of channel inactivation in the presence of Capz suggests, therefore, that the simultaneous addition of CapZ and G-actin leads to the rapid formation of filaments in the bath solution rather than on the plasma membrane.
While being added to the experimental chamber during the process of channel inactivation when actin filaments are growing on the cytoplasmic membrane side, CapZ may bind to the barbed end of assembling actin filaments, thus preventing further association of actin monomers to this end (Isenberg et al., 1980; Schafer et al., 1996; Kuhlman and Fowler, 1997). This would restrict filament elongation to the pointed end only and thereby slow down further assembly. In addition, CapZ will promote the nucleated polymerization of residual actin monomers in solution (Remmert et al., 2000) and thereby decrease the pool of actin monomers recruitable for elongation of membrane-associated filaments. Capping of the barbed filament ends also increases the critical concentration for actin polymerization, which would inhibit further filament elongation (Pollard et al., 2000). In this way, CapZ would both prevent channel inactivation and restore partially declined channel activity.
Capping proteins with properties similar to those of muscle CapZ have been detected in many cells, including platelets (Barkalow et al., 1996), neutrophils (Maun et al., 1996; Huang et al., 1999), erythrocytes (Kuhlman et al., 1997), and various cultivated cells (Schafer et al., 1998). In platelets, the ratio of capping protein to actin was estimated as 1:90 (Barkalow et al., 1996). Capping protein is visualized at the cell periphery (Schafer et al., 1998) and is assumed to play a role in dynamics of cortical actin structures (Cooper and Schafer, 2000). Our results show that the cortical cytoskeleton regulates the channel activity and capping protein can be a component of this regulation.
The effects of CapZ on sodium channel functioning resemble those described previously for gelsolin (Maximov et al., 1997; Negulyaev et al., 2000). At micromolar level of intracellular calcium, gelsolin severs actin filaments and caps the barbed filament ends. Thus, in living cells, gelsolin can induce the calcium-dependent activation of sodium channels, whereas capping protein could maintain an activated state in a calcium-independent manner. In the regulation of channel activity both capping protein and gelsolin may cooperate with cytoskeleton linkers: ankyrin, spectrin (fodrin), band 4.1, and ezrin/radixin/moezin, proteins that are believed to anchor actin filaments to ion transport proteins (Denker and Barber, 2002; Luciani et al., 2002). On the other hand, the linker proteins may play a capping role. The barbed end capping function is reported for radixin (Tsukita et al., 1989), whereas binding of ezrin at the sides of actin filament (Yao et al., 1996) might inhibit disassembly of actin monomers from the filament pointed ends as has been shown for tropomyosin (Broschat, 1990).
A large number of studies with localization and drug-based disruption methods have suggested a close relationship between structural organization of actin cytoskeleton and alterations in cell volume (Henson, 1999) mediated by activation of ion channels (Schwiebert et al., 1994; Levitan et al., 1995; Lang et al., 1998). In some cells, regulatory cell volume increase is accompanied by activation of sodium channels (Lang et al., 1998). For example, in U937 macrophages, sodium-selective channels were activated in response to increasing extracellular osmolarity (cell shrinkage) and up-regulated by corticosteroids (Gamper et al., 2000). Sodium channels in K562 cells were also shown to be up-regulated by corticosteroids (Negulyaev et al., 1996b). It is possible, therefore, that sodium channels in K562 cells can also contribute to the regulation of cell volume. However, these channels are activated by cortical actin depolymerization (Negulyaev et al., 2000), and actin filaments have been found to depolymerize during swelling (not shrinkage) of variety of cells (Lang et al., 1998). Therefore, we assume, that in K562 cells sodium channels are involved in specific modulations of intracellular Na+ concentration that is significant for regulation of different cell signaling systems, including cytoplasmic enzymes, ion-transporting membrane proteins (Basudev et al., 1995; Ruiz-Opazo et al., 1997), and control of intracellular Ca2+ level (Lipp and Niggli, 1994; Fukushi et al., 1997). Thus, activity of nonvoltage-gated sodium channels coupled with actin cytoskeleton can be important both in maintaining water-saline balance and in local signal transduction processes.
In conclusion, our experiments demonstrate that the mechanism of sodium channel inactivation involves assembly of actin filaments at the cytoplasmic surface of the membrane rather than interaction of the channel with preformed filaments. Moreover, we show, for the first time, that capping of actin filaments can be involved in the regulation of sodium channels. The capping protein-induced modification of the cortical actin filaments may be important to maintain a required level of sodium influx to the cytoplasm of nonexcitable cells.
ACKNOWLEDGMENTS
We are grateful to L. Glushankova for cell cultivation. The work was supported by the Russian Basic Research Foundation, grants 01-04-48591, 00-15-97822, 02-04-48251, and 02-04-48253 and by the Deutsche Forschungsgemeinschaft (SFB 549).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–09–0622. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–09–0622.
REFERENCES
- Barkalow K, Witke W, Kwiatkowski DJ, Hartwig JH. Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J Cell Biol. 1996;134:389–399. doi: 10.1083/jcb.134.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basudev H, Romano-Silva MA, Brammar MJ, Campbell LC. Effects of sodium on PKC translocation; relationship to neurotransmitter release. Neuroreport. 1995;6:809–812. doi: 10.1097/00001756-199503270-00026. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Prat AG, Cantiello HF, Ausiello DA, Fuller CM, Jovov B, Benos DJ, Ismailov II. Regulation of epithelial sodium channels by short actin filaments. J Biol Chem. 1996;271:17704–17710. doi: 10.1074/jbc.271.30.17704. [DOI] [PubMed] [Google Scholar]
- Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Cur Opin Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Broschat KO. Tropomyosin prevents depolymerization of actin filaments from the pointed end. J Biol Chem. 1990;265:21323–21329. [PubMed] [Google Scholar]
- Caldwell JE, Heiss SG, Mermall V, Cooper JA. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry. 1989;28:8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- Cantiello HF, Stow JL, Prat AG, Ausiello DA. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991;261:C882–C888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Schafer DA. Control of actin assembly and disassembly at filament ends. Cur Opin Cell Biol. 2000;12:97–103. doi: 10.1016/s0955-0674(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Ion transport proteins anchor and regulate the cytoskeleton. Curr Opin Cell Biol. 2002;14:214–220. doi: 10.1016/s0955-0674(02)00304-6. [DOI] [PubMed] [Google Scholar]
- Fukushi Y, Ozawa T, Kanno T, Wakui M. Na+-dependent release of intracellular Ca2+ induced by purinoceptors in parotid acinar cells of the rat. Eur J Pharmacol. 1997;336:89–97. doi: 10.1016/s0014-2999(97)01228-4. [DOI] [PubMed] [Google Scholar]
- Gamper N, Huber SM, Badawi K, Lang F. Cell volume-sensitive sodium channels upregulated by glucocorticoids in U937 macrophages. Pfluegers Arch. 2000;441:281–286. doi: 10.1007/s004240000410. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henson JH. Relationships between the actin cytoskeleton and cell volume regulation. Microsc Res Tech. 1999;47:155–162. doi: 10.1002/(SICI)1097-0029(19991015)47:2<155::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Huang M, Yang C, Schafer DA, Cooper JA, Higgs HN, Zigmond SH. Cdc42-induced actin filaments are protected from capping protein. Curr Biol. 1999;9:979–982. doi: 10.1016/s0960-9822(99)80428-x. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Aebi U, Pollard TD. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980;288:455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+ Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Kuhlman PA, Fowler VM. Purification and characterization of an α1β2 isoform of CapZ from human erythrocytes: cytosolic location and inability to bind to Mg2+ ghosts suggest that erythrocyte actin filaments are capped by adducin. Biochemistry. 1997;36:13461–13472. doi: 10.1021/bi970601b. [DOI] [PubMed] [Google Scholar]
- Lader AS, Kwiatkowski DJ, Cantiello HF. Role of gelsolin in the actin filament regulation of cardiac L-type calcium channels. Am J Physiol. 1999;277:C1277–C1283. doi: 10.1152/ajpcell.1999.277.6.C1277. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Levina NN, Lew RR, Heath IB. Cytoskeletal regulation of ion channel distribution in the tip-growing organism Saprolegnia ferax. J Cell Sci. 1994;107:127–134. doi: 10.1242/jcs.107.1.127. [DOI] [PubMed] [Google Scholar]
- Levitan I, Almonte C, Mollard P, Garber SS. Modulation of a volume-regulated chloride current by F-actin. J Membr Biol. 1995;147:283–294. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994;474:439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani F, Molinari A, Lozupone F, Calcabrini A, Lugini L, Stringaro A, Puddu P, Arancia G, Cianfriglia M, Fais S. P-glycoprotein-actin association through ERM family proteins: a role in P-glycoprotein function in human cells of lymphoid origin. Blood. 2002;99:641–648. doi: 10.1182/blood.v99.2.641. [DOI] [PubMed] [Google Scholar]
- Maun NA, Speicher DW, DiNubile MJ, Southwick FS. Purification and properties of a Ca2+-independent barbed-end actin filament capping protein, CapZ, from human polymorphonuclear leukocytes. Biochemistry. 1996;35:3518–3524. doi: 10.1021/bi952470p. [DOI] [PubMed] [Google Scholar]
- Maximov AV, Vedernikova EA, Hinssen H, Khaitlina SY, Negulyaev YA. Ca-dependent regulation of Na+-selective channels via actin cytoskeleton modification in leukemia cells. FEBS Lett. 1997;412:94–96. doi: 10.1016/s0014-5793(97)00754-0. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Khaitlina SY, Hinssen H, Shumilina EV, Vedernikova EA. Sodium channel activity in leukemia cells is directly controlled by actin polymerization. J Biol Chem. 2000;275:40933–40937. doi: 10.1074/jbc.M008219200. [DOI] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Maximov AV. Disruption of actin filaments increases the activity of sodium-conducting channels in human myeloid leukemia cells. Mol Biol Cell. 1996a;7:1857–1864. doi: 10.1091/mbc.7.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Maximov AV. Aldosterone increases the activity level of Na+-conducting channels in chronic myeloid leukemia K562 cells. Dokl Akad Nauk. 1996b;349:701–703. (in Russian). [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–76. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Remmert K, Vullhorst D, Hinssen H. In vitro refolding of heterodimeric CapZ expressed in E. coli as inclusion body protein. Protein Expr Purif. 2000;18:11–19. doi: 10.1006/prep.1999.1132. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduced NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Ruiz-Opazo N, Cloix JF, Melis MG, Xiang XH, Herrera VL. Characterization of a sodium-response transcriptional mechanism. Hypertension. 1997;30:191–198. doi: 10.1161/01.hyp.30.2.191. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Cooper JA. Control of actin assembly at filament ends. Annu Rev Cell Dev Biol. 1995;11:497–518. doi: 10.1146/annurev.cb.11.110195.002433. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol. 1996;135:169–179. doi: 10.1083/jcb.135.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Welch MD, Machesky LM, Bridgman PC, Meyer SM, Cooper JA. Visualization and molecular analysis of actin assembly in living cells. J Cell Biol. 1998;143:1919–1930. doi: 10.1083/jcb.143.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Mills JW, Stanton BA. Actin-based cytoskeleton regulates a chloride channel and cell volume in renal cortical collecting duct cell line. J Biol Chem. 1994;269:7081–7089. [PubMed] [Google Scholar]
- Smith PR, Saccomani G, Joe E-h, Angelides KJ, Benos DJ. Amiloride-sensitive sodium channel is linked to cytoskeleton in renal epithelial cells. Proc Natl Acad Sci USA. 1991;88:6971–6975. doi: 10.1073/pnas.88.16.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PR, Stoner LC, Viggiano SC, Angelides KJ, Benos DJ. Effects of vasopressin and aldosterone on the lateral mobility of epithelial Na+ channels in A6 renal epithelial cells. J Membr Biol. 1995;147:195–205. doi: 10.1007/BF00233547. [DOI] [PubMed] [Google Scholar]
- Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. 1. Biochemical studies of the interactions of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Srinivasan Y, Elmer L, Davis J, Benett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Terzic A, Kurachi Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492, 2:395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Hieda Y, Tsukita S. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: purification and characterization. J Cell Biol. 1989;108:2369–2382. doi: 10.1083/jcb.108.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Cassola A, Giebisch G. Involvement of actin cytoskeleton in modulation of apical K channel activity in rat collecting duct. Am J Physiol. 1994;266:F592–F598. doi: 10.1152/ajprenal.1994.267.4.F592. [DOI] [PubMed] [Google Scholar]
- Yao X, Cheng L, Forte JG. Biochemical characterization of ezrin-actin interaction. J Biol Chem. 1996;271:7224–7229. doi: 10.1074/jbc.271.12.7224. [DOI] [PubMed] [Google Scholar]