Abstract
Acute otitis media is usually considered a simple bacterial infection that is treated with antibiotics. However, ample evidence derived from studies ranging from animal experiments to extensive clinical trials supports a crucial role for respiratory viruses in the etiology and pathogenesis of acute otitis media. Viral infection of the upper respiratory mucosa initiates the whole cascade of events that finally leads to the development of acute otitis media as a complication. The pathogenesis of acute otitis media involves a complex interplay between viruses, bacteria, and the host’s inflammatory response. In a substantial number of children, viruses can be found in the middle-ear fluid either alone or together with bacteria, and recent studies indicate that at least some viruses actively invade the middle ear. Viruses appear to enhance the inflammatory process in the middle ear, and they may significantly impair the resolution of otitis media. Prevention of the predisposing viral infection by vaccination against the major viruses would probably be the most effective way to prevent acute otitis media. Alternatively, early treatment of the viral infection with specific antiviral agents would also be effective in reducing the occurrence of acute otitis media.
INTRODUCTION
Otitis media is a major worldwide health care problem of childhood. In addition to the distress that it brings on the patient and the family, otitis media also causes an enormous economic burden to the society in terms of physician visits, medications, surgical procedures, and absences from work, school, or day care (40, 79, 97). Acute otitis media (AOM) is the most frequent reason for outpatient antibiotic therapy in children in the United States and most other developed countries (54, 90). According to a recent estimate, AOM accounts for 33% of all visits to physicians and approximately 40% of all antibiotic use in children younger than 5 years (135). The peak incidence of AOM occurs in children between 6 and 24 months of age (4, 107, 122). By the age of 2 years, 70% of children have experienced at least one episode of AOM, and approximately 20% of children have suffered four or more attacks of AOM (4, 122).
AOM is generally considered a bacterial infection that is treated with antibiotics. However, bacterial pathogens cannot be isolated from the middle-ear fluid (MEF) in approximately 30% of AOM cases (19, 31). Although the causes of such sterile cultures might include technical inadequacies resulting in loss of bacterial growth or the presence of fastidious bacterial organisms, the substantial proportion of AOM cases without a proven bacterial etiology has initiated an intensive search for the role of viruses in the etiopathogenesis of this condition. During the past 2 decades, research in this area has produced strong evidence for the crucial role of viruses in the development of AOM.
EPIDEMIOLOGICAL STUDIES
Association of Respiratory Viruses with AOM
The occurrence of AOM shows an extensive seasonal variation. In temperate regions, the incidence rates are highest in the wintertime and lowest during the summer months, which parallels the incidence of upper respiratory infections (107, 108). The occurrence of AOM peaks in the youngest children, who suffer an average of six to eight respiratory infections per year, and it decreases rapidly after the age of 2 to 3 years, at which time also respiratory infections become less prevalent (107, 122). During the first years of life, boys appear to have more respiratory infections than girls, and the incidence of AOM is also higher in boys (3, 91).
Everyday clinical experience clearly indicates that AOM is closely associated with upper respiratory tract infections. In a study of 363 children with newly diagnosed AOM, symptoms of upper respiratory infection were present in 94% of the patients at the time of diagnosis (5). Several signs and symptoms that are traditionally associated with AOM, e.g., fever, purulent rhinitis, cough, poor appetite, vomiting, diarrhea, and tiredness, have been shown to be unspecific to AOM but to be caused by the concurrent viral infection (67, 82, 96, 131).
Extensive clinical studies have documented the tight association between AOM and laboratory-documented viral respiratory infections. In a 14-year follow-up study among children attending day care, viral infections, especially those caused by respiratory syncytial virus (RSV), influenza virus, and adenovirus, conferred a greater risk of AOM than did colonization of the nasopharynx with Streptococcus pneumoniae or Haemophilus influenzae (72). Another study carried out in a pediatric department included 4,524 cases of AOM in children seen during a 6-year period (108). There was a significant correlation between AOM and laboratory-documented epidemics of respiratory viruses, especially RSV epidemics.
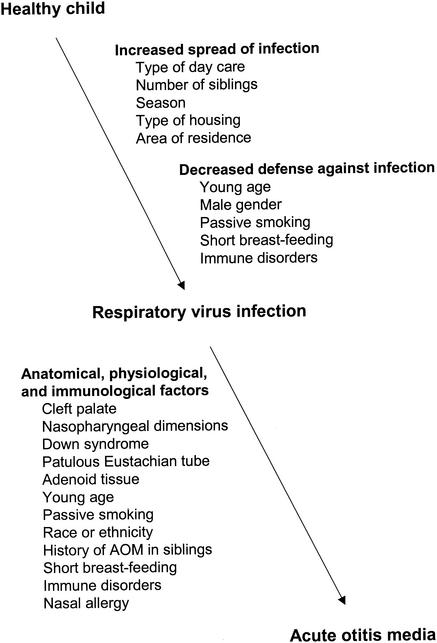
Several factors such as young age, day care attendance, male gender, number of siblings, passive smoking, and short duration of breast-feeding have been identified as important risk factors for AOM (3, 47, 101, 122, 130). However, it is evident from several studies that, in fact, all of the above risk factors for AOM predispose individuals to upper respiratory infection which, in turn, can be considered the most important factor predisposing to AOM (Fig. 1). In most cases, AOM can be clearly regarded as a complication of a preceding or concomitant upper respiratory infection. This concept was corroborated by a recent study of 596 infants monitored from birth to 6 months of age (43). Respiratory tract infection was found as the most important predictor for AOM early in life, unquestionably outweighing all other risk factors, including day care attendance.
FIG. 1.
Proposed sites of influence of some risk factors for AOM.
The rate of development of AOM during upper respiratory infection is largely dependent on the age of the child and potentially also on the type of virus causing the infection. The comparative ability of different viruses to predispose an individual to AOM has not been convincingly determined because of important confounding factors, especially age, in clinical studies. The frequency of AOM in children with respiratory infections has usually ranged between 10 and 50%, with the highest rates associated with RSV infection (72, 108, 129). As a rough estimate across all viral infections, AOM can be considered to occur in approximately 20% of children with upper respiratory infection (71, 121).
Temporal Development of AOM during Viral Respiratory Infection
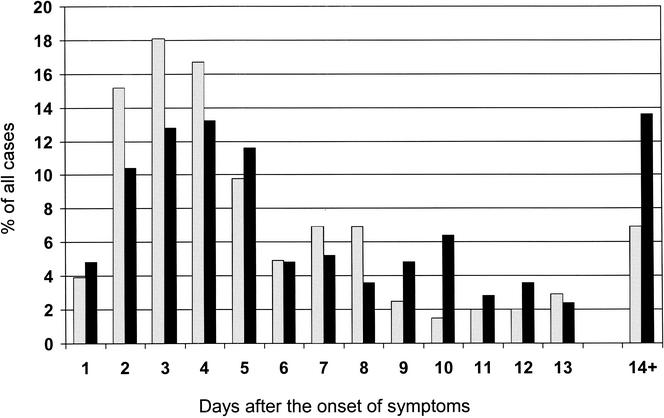
Although in many children AOM is diagnosed concurrently with upper respiratory infection, the development of AOM only occurs after a certain time interval after the onset of the viral infection. Knowledge about this time interval is important, because it represents the window of opportunity for intervention to prevent the development of AOM in children in whom the symptoms of the viral infection are already present. Two studies including a total of more than 1,200 children have specifically attempted to determine the temporal development of AOM during the course of the upper respiratory infection (Fig. 2). In the first of these, 204 episodes of AOM in children less than 4 years old attending day care were analyzed (66). The highest incidence of AOM was observed on day 3 after the onset of upper respiratory infection, and the median day for diagnosing AOM was day 4. Of all AOM episodes, 54% were diagnosed during the first 4 days and 75% were diagnosed during the first week after the onset of respiratory symptoms. No significant differences were observed in the time to development of AOM between subgroups of children grouped according to age, gender, history of otitis media, smoking in the household, previous adenoidectomy, or presence of tympanostomy tubes.
FIG. 2.
Temporal development of AOM after the onset of upper respiratory symptoms. Data derived from reference 66 (grey bars) and reference 81 (black bars).
The second study included 250 episodes of AOM in children with a mean age of 3 years (81). The incidence of AOM peaked on day 4, and 63% of cases were diagnosed during the first week after the onset of symptoms. Age, gender, or history of AOM recurrences did not affect the temporal development of AOM. In children with two separate episodes of AOM during the study, the time to development of AOM during the first occurrence did not correlate with the time lag during the second episode. These findings suggest that the type of virus may have a greater impact on the temporal development of AOM than any host-related individual factor.
Presence of Viruses in Nasopharyngeal Specimens from Children with AOM
Considering the clear connection between AOM and laboratory-documented viral upper respiratory infections, it could be expected that viruses had been found in the nasopharynges of virtually all children with AOM. However, the rates of detection of respiratory viruses have conventionally ranged between 30 and 50% (5, 6, 7, 35, 37, 80, 113). The most frequently found viruses have been RSV; influenza A and B viruses; parainfluenza type 1, 2, and 3 viruses; and adenovirus. The relatively low rates of viral detection in nasopharyngeal specimens from children with AOM have raised doubts about the extent of viral involvement in the development of this disease. With the increasing availability of PCR-based assays, however, it has become obvious that the low rates have been caused by underdetection of existing viruses in studies where viral detection has been based only on viral culture and/or antigen detection methods. PCR has proved especially valuable in diagnosing rhinovirus infections, for which other methods have been suboptimal (8, 74, 87). Rhinoviruses are estimated to account for 30 to 40% of all respiratory illnesses also in children (91), and a recent follow-up study demonstrated that by the age of 2 years more than 90% of children have experienced a laboratory-confirmed rhinovirus infection (17). Because of the existence of more than 100 different serotypes of rhinoviruses, antigen detection techniques cannot be routinely used to detect these viruses. In a study utilizing PCR for the detection of rhinovirus, RSV, and coronavirus only, at least one of these viruses was identified in nasopharyngeal aspirates from 62% of children with AOM (106). Another recent study searched for viruses using antigen detection for RSV; influenza A and B viruses; parainfluenza type 1, 2, and 3 viruses; and adenovirus—together with PCR for rhinovirus, enterovirus, and coronavirus. Viral infection could be documented in the nasopharyngeal specimens of 90% of the children with AOM (T. Heikkinen, A. Ruohola, M. Waris, and O. Ruuskanen, Abstr. 4th Extraordinary Int. Symp. Recent Adv. Otitis Media, abstr. 133, 2001).
ROLE OF VIRUSES IN THE PATHOGENESIS OF AOM
Eustachian Tube Dysfunction
Viral infection of the upper respiratory tract results in congestion of the nasal and nasopharyngeal mucosa. Congestion in and around the nasopharyngeal orifice of the Eustachian tube leads to dysfunction of the tube, which is considered the most important factor in the development of AOM (18). Eustachian tube dysfunction results in (i) impairment of pressure equilibration between the nasopharynx and the middle ear cavity; (ii) decreased drainage into the nasopharynx of secretions produced in the middle ear; and (iii) loss of protection of the middle ear from nasopharyngeal secretions. Even at the normal stage, the muscular opening function of the Eustachian tube is poorer in children than in adults; this function improves with increasing age (27, 28).
Animal studies.
Experimental studies in animals have provided important information on the role of viruses in the pathogenesis of AOM. Compared with studies in humans, the clear advantage of animal experiments is the possibility to have a better control over various confounding factors. Most experimental studies of viral involvement in otitis media have been performed with chinchillas. Chinchillas are well suited for otitis media research because their middle ears are easily accessible; middle ear infections can be produced by intranasal inoculation of microbes; the disease remains localized to the middle ear; and, unlike many other animal species, chinchillas are not susceptible to naturally occurring middle ear infections (57). In one of the early studies, chinchillas were inoculated intranasally with influenza A virus, S. pneumoniae, or both of these pathogens (58). Otitis media developed in 4% of the animals inoculated with influenza virus alone and in 21% of those inoculated with pneumococci alone, whereas it developed in 67% of chinchillas inoculated with both influenza virus and S. pneumoniae. The synergistic effect between viruses and bacteria has also been demonstrated in infant chinchillas inoculated with adenovirus and nontypeable H. influenzae (120). All animals inoculated with both of these pathogens developed more-severe otitis media than those receiving either pathogen alone. The most severe and prolonged episodes of otitis media were observed in chinchillas that were initially inoculated with adenovirus, followed by inoculation of H. influenzae 7 days after the virus. It seems, however, that the synergistic effect of viruses and bacteria is not a constant feature across all types of microbes, because a subsequent experiment in chinchillas using Moraxella catarrhalis instead of H. influenzae did not demonstrate a similar synergy (9).
Intranasal inoculation of chinchillas with influenza A virus was shown to result in negative middle-ear pressure that was not observed in animals inoculated with S. pneumoniae only (2). The extent of epithelial damage in the Eustachian tube caused by influenza A virus infection was later demonstrated in a histopathologic study in which the development of negative middle-ear pressure was associated with marked ciliary damage, disappearance of ciliated epithelial cells, and increased accumulation of mucus and cellular debris in the tubal lumen (59). The substantial damage to ciliated epithelial cells in the Eustachian tube was also shown after direct inoculation of influenza A virus into the middle ear of chinchillas, in which the restoration of the mucosal architecture was not observed until 4 weeks after viral inoculation (38). Impairment of the Eustachian tube function following experimental infections with influenza A virus or adenovirus has also been demonstrated in many other animal species, including guinea pigs and ferrets (10, 22, 99).
Human studies.
Clinical studies of both children and adults have provided strong evidence for a causal role of respiratory viruses in the disruption of normal Eustachian tube function. Several studies have documented the impairment of Eustachian tube function in children with upper respiratory infections (20, 26). In a prospective tympanometric study of preschool children, significant negative middle-ear pressures were measured in 13% of asymptomatic children, but the rate of abnormal tympanograms increased to 75% during upper respiratory infections (112). More than 80% of the negative middle-ear pressures were already detectable by the second day of illness, and 97% were detectable after 4 days of illness. These findings are well in accordance with the clinical studies demonstrating that the peak incidence of AOM occurs 3 to 4 days after the onset of respiratory symptoms (66, 81). A recent study among children 2 to 12 years old corroborated the earlier findings of the effect of viral infection on Eustachian tube function (138). In children with normal tympanograms before the onset of illness, abnormal negative middle-ear pressures occurred at least once during the 2 weeks after the onset of symptoms in 66% of cases. Approximately 90% of the tympanometric abnormalities were observed during the first week of illness. In another tympanometric follow-up study of children through an entire winter season, high negative middle-ear pressures during upper respiratory infections preceded the development of middle-ear effusion (92).
The development of AOM as a complication of upper respiratory infection is uncommon in adults. Nonetheless, experimental viral challenge studies carried out among adult volunteers have provided much detailed information about the effect of viral infection on Eustachian tube function. Substantial deterioration of normal Eustachian tube function has developed in 50 to 80% of adults following intranasal challenge with rhinoviruses, and significant negative middle-ear pressures have been observed in 30 to 50% of the subjects (24, 46, 89). Even higher rates of abnormalities have been demonstrated in adults infected with influenza A virus. By the fourth of fifth day after intranasal challenge with influenza A virus, Eustachian tube dysfunction has occurred in more than 80% of volunteers, and significant middle-ear underpressures occurred in 60 to 80% of the volunteers (23, 44). In one of the influenza challenge studies, one volunteer developed AOM as a complication, and PCR analysis of the MEF revealed both influenza A virus and S. pneumoniae (23). A recent experimental study of adults challenged with RSV showed that by day 6 after viral inoculation, more than half of the subjects had abnormal negative middle-ear pressures (25). The frequent development of substantial negative middle-ear pressures has also been observed during naturally occurring rhinovirus infections in adults (49).
The detailed mechanisms by which viral infection causes Eustachian tube dysfunction are still incompletely understood. However, extensive research during the recent years into the role of inflammatory mediators in the pathogenesis of viral upper respiratory infections has disclosed some potential mechanisms. Numerous studies, both in vivo and in vitro, have demonstrated that respiratory viruses are fully capable of inducing the release of several inflammatory mediators in the nasopharynx. Increased concentrations of mediators such as kinins, histamine, leukotrienes, interleukins (interleukin-1 [IL-1], IL-6, and IL-8), tumor necrosis factor, and RANTES have been found in nasal secretions of patients with respiratory infections (94, 98, 111, 133, 136), and the concentrations in nasal secretions of many of these substances are correlated with the severity of the symptoms (53, 128, 141). Experimental studies in adult volunteers have, in turn, documented that after intranasal challenge, several of these mediators induce Eustachian tube dysfunction (45). The inflammatory mechanisms triggered by viral infection are, however, extremely complex and interrelated, affecting each other in a time-dependent manner (12, 104). It is likely that despite rapidly accumulating data on the host inflammatory response during a viral infection, several important factors contributing to the inflammatory process in the nasopharyngeal mucosa still remain to be determined.
Alteration of Host's Immune Defense
Some viruses, particularly influenza A virus, are known to induce polymorphonuclear leukocyte dysfunction, which could be thought to increase the risk of secondary bacterial infections (1). Several studies have shown that influenza A viruses decrease the oxidative, chemotactic, secretory, and bactericidal activities of neutrophils (reviewed in reference 1). In experimental studies with chinchillas, this neutrophil dysfunction was observed 4 to 8 days after influenza virus inoculation, but it did not occur after inoculation of S. pneumoniae alone (2). The highest incidence of pneumococcal otitis media occurred when the bacteria were inoculated 4 days after the virus, i.e., just before the emergence of virus-induced neutrophil dysfunction. Furthermore, no difference was observed in the rate of otitis media between ears with and without a tympanostomy tube, suggesting that in this animal model neutrophil dysfunction contributed more to the pathogenesis of otitis media than did negative middle-ear pressure. Suppression of polymorphonuclear leukocyte function has also been shown in humans infected with influenza viruses (83, 115). Scarce clinical data are available on other effects of respiratory viral infections on the host's immune defense, but some studies have documented several changes in systemic immune and inflammatory parameters during infections with rhinovirus, RSV, or influenza virus (56, 73, 109, 118). For example, experimental rhinovirus infection of adult volunteers resulted in increased mitogen-stimulated production of IL-2 and gamma interferon by peripheral blood mononuclear cells, as well as increased natural killer cell-mediated cytotoxicity of the mononuclear cells on day 5 after rhinovirus challenge (73).
Bacterial Colonization and Adherence
In addition to eliciting various local and systemic immune and inflammatory responses in the host, viral infection of the upper respiratory tract appears to have a substantial impact on the nasopharyngeal bacterial flora. Pneumococcal colonization of the nasopharynx has been reported to be increased in adult patients infected with influenza A virus (134). Influenza A virus-induced promotion of nasopharyngeal colonization by S. pneumoniae has also been observed in chinchillas (127). However, chinchillas infected with adenovirus did not show enhanced colonization by pneumococci (127). In cotton rats, colonization with H. influenzae increased within 4 days of RSV infection (102). Additional, albeit indirect, evidence for the effect of viruses on the bacterial colonization of the nasopharynx was derived from a study of children with AOM, in whom the occurrence and the quantity of pathogenic bacteria in the nasopharynx were significantly increased during AOM episodes (50).
Viruses have also been reported to increase the adherence of bacteria to epithelial cells. In laboratory experiments, influenza A virus infection significantly increased the binding of S. pneumoniae to HEp-2 cells compared with uninfected cells (48). Certain types of adenoviruses enhance the binding of pneumococci to human A549 cells (63). Further, RSV infection has been shown to increase the attachment of nontypeable H. influenzae to human respiratory epithelial cells (75). Increased adherence of S. pneumoniae and H. influenzae to pharyngeal cells has also been demonstrated in vivo in adult volunteers infected with influenza A virus (51). However, despite a considerable amount of new information on the interaction between viruses and bacteria on the cellular level, the detailed mechanisms by which bacteria colonizing the nasopharyngeal mucosa turn into disease-causing and even invasive organisms are still unknown. Based on available data, it seems likely that by various mechanisms the viral infection activates the epithelial cells, which could then result in increased adherence of bacteria to the cells (42). Recent discoveries on the molecular mechanisms have, however, clearly demonstrated that also other factors such as phase variation in the colonial opacity of pneumococci play an important role in epithelial adhesion and invasion (42, 126).
VIRUSES IN THE MIDDLE EAR
Presence of Viruses in MEF during AOM
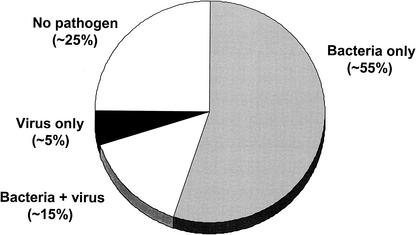
The detection of viruses directly in the MEF of children with AOM is a central piece of evidence for the role of viruses in the pathogenesis of this disease. The first attempts to search for viruses in the MEF were carried out already in the 1950s and 1960s, when detection of viruses was limited to viral culture only (Table 1). Although viruses could be isolated from the MEF of several patients during influenza or RSV outbreaks (15, 16, 140), the general rate of viral detection remained lower than 5%, and in many studies viruses could not be found at all (61, 62, 64, 84, 125). Improvements in viral culture techniques and the development of various antigen detection methods during the subsequent decades substantially increased the rates of viral detection in the MEF. Since the 1980s, viruses have been detected in the MEF of children with AOM in approximately 20% of cases using viral culture and/or antigen detection techniques (6, 7, 33, 34, 35, 37, 80, 113, 114) (Fig. 3). In about one-third of virus-positive cases, the virus has been the sole pathogen found in the MEF. The most common viruses detected in the MEF in these studies were RSV, influenza viruses, adenovirus, and parainfluenza viruses, i.e., the same viruses that had been identified in the nasopharyngeal specimens from children with AOM. However, several other viruses, such as cytomegalovirus and herpes simplex virus, have also been isolated in the MEF specimens from children with AOM (36).
TABLE 1.
Detection of viruses in MEF by different viral diagnostic methods
| Method | Reference | Yr | No. of children or MEF specimens | No. (%) virus positive |
|---|---|---|---|---|
| Culture alone | 140 | 1955 | 10 | 4 (40) |
| 61 | 1964 | 326 | 0 (0) | |
| 16 | 1966 | 27 | 9 (33) | |
| 84 | 1966 | 16 | 0 (0) | |
| 125 | 1967 | 90 | 2 (2) | |
| 15 | 1967 | 33 | 7 (21) | |
| 64 | 1968 | 74 | 1 (1) | |
| 62 | 1968 | 11 | 6 (55) | |
| Total | 587 | 29 (5) | ||
| Culture and/or antigen detection | 80 | 1982 | 53 | 13 (25) |
| 114 | 1983 | 84 | 7 (8) | |
| 113 | 1985 | 137 | 24 (18) | |
| 37 | 1986 | 84 | 17 (20) | |
| 7 | 1988 | 143 | 16 (11) | |
| 6 | 1990 | 88 | 17 (19) | |
| 34 | 1990 | 58 | 11 (19) | |
| 35 | 1992 | 271 | 66 (24) | |
| 33 | 1996 | 106 | 19 (18) | |
| Total | 1,024 | 190 (19) |
FIG. 3.
Presence of bacteria and viruses in MEF specimens of children with AOM. Bacteria were detected by culture; viruses were detected by viral culture and/or antigen detection. Data derived from references 29, 34, 37, and 69.
As with nasopharyngeal specimens, the advent of PCR technique has also dramatically increased the detection rates of viruses in MEF specimens. In one of the first PCR-based studies, the use of PCR for the detection of RSV yielded positive results in 53% of children with AOM, and the rate exceeded 80% in those children in whom RSV could be cultured in the nasopharyngeal secretions at the same time (100). In another study, the use of PCR for detection of rhinovirus, RSV, and coronavirus revealed at least one of these viruses in the MEF specimens from 48% of children with AOM (106). The power of PCR in comparison with conventional methods was well demonstrated in a recent study of 65 MEF specimens from children with AOM (30). The investigators used multiplex PCR for the detection of RSV; influenza A and B viruses; and parainfluenza type 1, 2, and 3 viruses. Viruses had been previously found by culture in 29% of the MEF specimens, but the use of PCR increased the rate to 72%. Two-thirds of specimens that were negative by virus culture turned out to be positive by PCR. Such high rates of viral detection by PCR inevitably raise the question of the real clinical significance of these viruses in the middle ear. Theoretically, the extreme sensitivity of the PCR technique could result in positive viral findings in the MEF even in cases where minute amounts of viral genomic materials have entered the middle ear along with nasal secretions.
Recent data from a study of the relative prevalences of viruses in the MEF of 456 children with AOM have provided additional evidence for the active role of at least some viruses in the pathogenesis of AOM (69). In children with AOM during laboratory-confirmed RSV infection, the same virus could be found in the MEF in 74% of the cases, a rate which was significantly higher than the corresponding ones for parainfluenza viruses (52%) or influenza viruses (42%). The relative prevalences of all these viruses, in turn, were higher than those for enteroviruses (11%) or adenoviruses (4%). These differences imply that while it is possible that some viruses get to the middle ear passively along with nasal secretions, some other viruses seem to actively invade the middle ear cavity. If the presence of all viruses in the MEF were the result of passive influx only, they should be detected in the MEF at roughly similar rates.
In the above study (69), the relative prevalence of rhinoviruses in the MEF could not be analyzed because PCR techniques were not used and the rates of rhinovirus detection by culture were too low for meaningful analysis. However, the impact of rhinoviruses may be equal to that of RSV, because a PCR-based study of children with AOM detected rhinoviruses in the MEF of 69% of children infected with this virus (106).
In approximately two-thirds of cases where virus has been detected in the MEF by culture or antigen detection, bacteria have also been identified, which indicates a mixed viral-bacterial infection. Overall, combined viral-bacterial infections have accounted for approximately 15% of all AOM cases. It is very likely, however, that this proportion does not represent the true occurrence of mixed infections, because it is obvious that underdetection of viruses is still very common. Apart from well-known problems in culturing some respiratory viruses, antigen detection and PCR also have their disadvantages in this context. The use of antigen detection or PCR requires specific antibodies or primers for each virus being searched for. Even with the availability of an extensive array of assays for different viral types, the relatively small volume of MEF would be a limiting factor for the detection of viruses. On the other hand, it has also been reported that the routine bacteriologic techniques are not sensitive enough for complete detection of bacteria in the MEF specimens (132).
Although clear synergism between certain viruses and bacteria has been shown in the development of AOM (58, 120), scarce data are available on potential relationships between types of viruses and bacteria in the MEF. One study of children with AOM has demonstrated a close connection between influenza virus and S. pneumoniae (69). In the MEF specimens that contained both bacteria and virus, S. pneumoniae was cultured significantly more often in MEF specimens containing influenza viruses than in those containing RSV or parainfluenza viruses.
Effect of Viruses on the Outcome of AOM
One of the major issues suggesting an active role for viruses in otitis media is that despite the use of wide-spectrum antibiotics that are effective against the causative bacteria, the clinical response to antibiotic treatment is often poor. This usually results in the change of the antibiotic and even the use of several courses of different antibiotics with the assumption that the poor response was due to bacterial resistance to the antibiotic used. Studies of children with persistent symptoms of AOM have shown, however, that resistant bacteria only account for approximately 20% of these cases (105, 123). It is logical to think that if the middle ear mucosa is infected with both bacteria and viruses simultaneously, eradication of the bacteria with antibiotics may not be sufficient to stop the inflammatory cascades in the middle ear, and the continuing viral infection may lead to more prolonged disease.
The results of clinical studies of viral AOM during the past decade suggest that viruses play an important role in the resolution of otitis media. In a study of 58 children with AOM, MEF samples were obtained by tympanocentesis both before the start of the antibiotic treatment and 2 to 4 days into the treatment (34). The bacteria found in the MEF were susceptible to the antibiotic used. At the time of the second tympanocentesis, bacteriologic failure was observed in 33% of the children who had both bacteria and virus in the initial MEF specimen. In children with only bacteria in the initial MEF specimen, the rate of bacteriologic failure was 3%; the difference was statistically significant. Similar results were obtained in a subsequent study of 271 children, in which otitis media persisted in a higher proportion of children with evidence of mixed viral-bacterial infection than in those with only bacteria in the MEF (35). Further, another study of 88 children with AOM involved 22 children who had persistent symptoms of AOM despite 2 days of antibiotic therapy (6). Bacteria were isolated in the MEF of only four of the children with poor response to antibiotic treatment, and only one of these isolates was resistant to the drug used. By using viral culture and antigen detection, viruses were found in the MEF of 32% of the children with clinical failure, compared with 15% of children in a comparison group with newly diagnosed untreated AOM. The follow-up of the children demonstrated that in children with prolonged symptoms, viruses were found in the MEF in 44% of cases, whereas the corresponding rate was 11% in those with good response to the treatment.
Based on the recent findings about the varying activity of different viruses in invading the middle ear cavity (69), it could be hypothesized that the impact of different viruses on the clinical outcome of otitis media could also vary. Whether this is true is largely unknown, but one study has suggested that rhinoviruses may be more commonly associated with bacteriologic failure in the middle ear than are other respiratory viruses (119).
Mechanisms of Virus-Bacterium Interaction
Despite increasing evidence for interaction between viruses and bacteria, the detailed mechanisms by which the presence of viruses in the middle ear might interfere with the resolution of otitis media are still unclear. Some hypothetical virus-induced mechanisms that have been investigated include (i) production or inhibition of inflammatory mediators in the middle ear, (ii) delayed clearance of bacteria from the middle ear, and (iii) decreased penetration of antibiotics into the middle ear.
Inflammatory mediators.
Clinical signs and symptoms of AOM are most likely caused by the host's inflammatory response to infection in the middle ear (110). Both bacteria and viruses are known to induce the production of cytokines and other inflammatory mediators at the sites of infection (94, 98, 133, 136, 141). Numerous inflammatory mediators, e.g., interleukins, tumor necrosis factor, histamine, leukotrienes, and prostaglandins, have been found in the MEF of children with AOM (11, 14, 21, 77, 86, 139). Recent studies suggest that virus-induced increased production of various inflammatory mediators in the middle ear is the most plausible explanation for the adverse effect of viruses on the resolution of otitis media.
Histamine is a potent mediator of inflammation that causes vasodilatation and increased vascular permeability (52). In a study of 248 children with AOM, the concentrations of histamine in the MEF were higher in children with evidence of concurrent viral infection than in those without, both in bacterium-positive and bacterium-negative cases (32). Bacteria and viruses together appeared to have an additive effect on histamine content in the MEF, and antibiotic treatment for 3 to 5 days did not reduce the histamine concentrations.
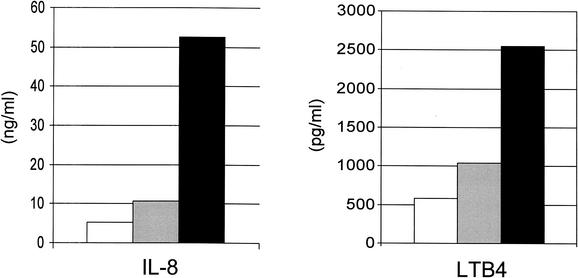
IL-8 is a potent neutrophil-chemotactic cytokine, and leukotriene B4 (LTB4) is an inflammatory product of neutrophils related to the degree of inflammation (78, 93). The concentrations of IL-8 and LTB4 were determined in MEF specimens from 106 children with AOM (33). The highest concentrations of both mediators were measured in MEF specimens that contained both bacteria and virus (Fig. 4). Five of the children had bilateral AOM with identical presence or absence of bacteria in the MEF. The concentrations of IL-8 and LTB4 were consistently higher in the ear that contained the virus than in the opposite ear without virus. High concentrations of LTB4 at the initiation of antibiotic treatment were associated with an increased rate of treatment failure. Antibiotic treatment had no effect on the concentrations of these mediators.
FIG. 4.
Concentrations of IL-8 and LTB4 in MEF specimens of children with AOM according to the presence of viruses or bacteria in MEF. Open bars, viruses only; grey bars, bacteria only; black bars, both viruses and bacteria in the MEF. Data derived from reference 33.
In a study quantifying the concentrations of macrophage inflammatory protein-1α and monocyte chemotactic protein 1, which are both considered histamine-releasing chemokines, higher levels of both mediators were found in MEF specimens with both bacteria and viruses than in specimens with bacteria alone (103). A similar trend has been observed with tryptase concentrations in the MEF, but because of small sample size the differences were not statistically significant (55).
Delayed clearance of bacteria.
Studies with both humans and experimental animals have provided convincing evidence for virus-induced dysfunction of the Eustachian tube (38, 59, 112, 138). The impaired drainage to the nasopharynx of middle ear secretions produced during AOM could be thought to mechanically delay the clearance of bacteria from the middle ear cavity. Another potential mechanism for delayed bacterial clearance is the dysfunction of polymorphonuclear leukocytes that has been documented to occur during influenza A virus infections in both chinchillas and humans (1, 83, 115). This neutrophil dysfunction is associated with decreased chemiluminescence, chemotaxis, and bactericidal activity, which could affect the killing of bacteria in the middle ear.
Decreased penetration of antibiotics.
It has been hypothesized that viral infection of the middle ear mucosa causes local inflammation that interferes with the penetration of antibiotics into the middle ear, resulting in low concentrations of antibiotics in the MEF. In an experimental animal model, reduced penetration of antibiotics into the middle ear has been shown in animals with pure viral and mixed viral-bacterial infections, compared with animals with bacterial infections alone (76). In humans, the pharmacokinetics of amoxicillin was studied in 30 children with AOM (29). The mean concentrations of amoxicillin were highest in children with bacterial infection, lower in children with combined viral and bacterial infection, and lowest in those with viral infection only. However, the small sample size in the study prevented any firm conclusions, and further studies to confirm the findings are needed.
PREVENTION OF AOM
Based on ample evidence that viral infection plays a decisive role in the initiation of events that finally lead to development of AOM, prevention or effective attenuation of the viral infection could be expected to reduce the incidence of AOM as a complication of the viral illness.
Viral Vaccines
Perhaps the most convincing evidence for the role of respiratory viruses in the development of AOM has been derived from direct interventional studies investigating the efficacy of viral vaccines in the prevention of AOM. In the first of these trials, including 374 children attending day care, half of the children received the trivalent inactivated influenza vaccine before the expected influenza epidemic (70). During the influenza outbreak, the children were examined during each episode of respiratory infection and nasopharyngeal specimens were obtained for viral detection. In children who received the vaccine, the incidence of AOM associated with influenza A virus was decreased by 83%, which resulted in a 36% reduction in the overall incidence of AOM regardless of the viral etiology. The efficacy of the vaccination approach was later confirmed in another study of 186 children in day care (39). Children who had received the influenza vaccine had 32% fewer episodes of AOM during the influenza outbreak. The efficacy of the newly developed live, attenuated intranasal influenza vaccine was recently studied among 1,602 children between 15 and 71 months old (13). The efficacy of the vaccine against influenza was 93%, and the incidence of febrile AOM was reduced by 30% in the children who received the vaccine. Another study using the intranasal virosomal influenza vaccine demonstrated an overall 44% efficacy of vaccination in preventing AOM during a 6-month follow-up period (88). A recent study assessing the efficacy of inactivated influenza vaccine failed to show a reduction in the proportion of children with at least one episode of AOM during an entire respiratory season (A. Hoberman, D. P. Greenberg, J. L. Paradise, H. E. Rockette, J. Lave, D. Kearney, D. K. Colborn, M. Kurs-Lasky, M. A. Haralam, C. Byers, L. Zoffel, I. Fabian, B. Bernard, and J. Kerr, Abstr. Pediatr. Acad. Soc. Annu. Meet., abstr. 906, 2002). The design of this study and the subject population differed from those of the previous studies: the efficacy of vaccination in the prevention of influenza-associated AOM was not reported; the subjects were younger than those in other studies (75% of them were younger than 18 months); and they were not all day care attendees, who generally are at higher risk of exposure to infection. More importantly, the influenza activity was low during the study period, and influenza vaccine was not proven effective during the second season of the study.
Unfortunately, influenza vaccine is currently the only vaccine available for the control of respiratory viruses. It is very likely that also other vaccines against major respiratory viruses, especially RSV, would be effective in preventing the development of AOM as a complication. Several types of vaccines against RSV and parainfluenza viruses are currently being developed and some of them are already in early clinical trials (41, 85, 117).
Viral Immune Globulins
Epidemiologic studies have suggested that high levels of transplacentally acquired neutralizing antibodies protect infants from severe RSV infections (60), and passive administration of antibodies has been proposed as one way to prevent AOM. The incidence of AOM was retrospectively analyzed in children (median age, 6 months) who had participated in a clinical trial of RSV-enriched immune globulin (116). The occurrence of AOM was significantly lower in the high-dose group compared with the controls, but the potential confounding effects of tympanostomy tubes, antibiotic prophylaxis, and the presence of antibacterial antibodies in the immune globulin preparation were not ruled out. In addition, it is obvious that this approach that requires monthly intravenous injections is not a feasible way of AOM prevention in everyday clinical practice.
Palivizumab, a recombinant humanized monoclonal antibody directed against the F glycoprotein of RSV, has been shown to reduce RSV-related hospitalizations in premature children (74a). However, palivizumab does not prevent RSV infection and it has not been shown to reduce the incidence of AOM. In a proof-of-concept study, intranasally administered polyclonal immune globulin containing high titers of RSV immunoglobulin A and immunoglobulin G antibodies reduced episodes of rhinitis in children, but the small sample size prevented any conclusions from being made about prevention of AOM as a complication (68).
Antiviral Agents
In principle, antiviral agents could be used in both prevention and treatment of the viral infection. Sufficient attenuation of the viral illness with antiviral drugs could be expected to suppress the inflammatory response, which, in turn, could lead to decreased incidence of complications such as AOM. At present, specific antiviral treatments for respiratory viruses are commercially available only for influenza viruses (65, 95). One study has evaluated the efficacy of oseltamivir, a new influenza neuraminidase inhibitor, in the prevention of AOM in children (137). The study included 695 children 1 to 12 years old who had acute respiratory symptoms of less than 48 h of duration during an influenza epidemic. Influenza virus infection could be confirmed in 65% of the children. In influenza-infected children, oseltamivir treatment provided a 44% reduction in the development of AOM compared with that observed for children receiving placebo. The results of this study indicate that early treatment of viral infection with a specific antiviral drug can prevent the development of AOM, and similar efficacy might be seen also with antiviral drugs against other common viruses that predispose individuals to AOM. However, even with the availability of effective antivirals against all major viruses, two practical problems remain. First, the clinical manifestations of different viral infections are usually indistinguishable, especially in children, and the optimal use of virus-specific antiviral agents would require positive identification of the specific virus causing the infection. Second, the initiation of antiviral treatment should occur as soon as possible after the onset of respiratory symptoms and at the latest within 48 h. The need to see a doctor already in the early course of illness might cause problems in many health care systems.
CONCLUSIONS
There is growing evidence that the etiopathogenesis of AOM is far more complicated than is currently understood, consisting of a network of factors affecting each other. It is also probable that many essential factors involved in the disease process still remain to be identified. Further research into the host inflammatory response and mechanisms of viral-bacterial interaction is needed to elucidate pathways that could serve as targets for intervention to improve the treatment of AOM or to prevent the disease. This need is underscored by the increasing antimicrobial resistance of bacteria that may increase the pressure against routine use of antibiotics in all cases of AOM. More information is also required about the effect of specific respiratory viruses on the resolution of otitis media and the potential role of antiviral agents and adjunctive treatments in improving the outcome. The availability of vaccines against the major respiratory viruses predisposing to AOM would likely be the most effective way to prevent AOM.
REFERENCES
- 1.Abramson, J. S., and J. G. Wheeler. 1994. Virus-induced neutrophil dysfunction: role in the pathogenesis of bacterial infections. Pediatr. Infect. Dis. J. 13:643-652. [PubMed] [Google Scholar]
- 2.Abramson, J. S., G. S. Giebink, and P. G. Quie. 1982. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect. Immun. 36:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alho, O. P., M. Koivu, M. Sorri, and P. Rantakallio. 1990. Risk factors for recurrent acute otitis media and respiratory infection in infancy. Int. J. Pediatr. Otorhinolaryngol. 19:151-161. [DOI] [PubMed] [Google Scholar]
- 4.Alho, O. P., M. Koivu, M. Sorri, and P. Rantakallio. 1991. The occurrence of acute otitis media in infants: a life-table analysis. Int. J. Pediatr. Otorhinolaryngol. 21:7-14. [DOI] [PubMed] [Google Scholar]
- 5.Arola, M., O. Ruuskanen, T. Ziegler, J. Mertsola, K. Näntö-Salonen, A. Putto-Laurila, M. K. Viljanen, and P. Halonen. 1990. Clinical role of respiratory virus infection in acute otitis media. Pediatrics 86:848-855. [PubMed] [Google Scholar]
- 6.Arola, M., T. Ziegler, and O. Ruuskanen. 1990. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J. Pediatr. 116:697-701. [DOI] [PubMed] [Google Scholar]
- 7.Arola, M., T. Ziegler, O. Ruuskanen, J. Mertsola, K. Näntö-Salonen, and P. Halonen. 1988. Rhinovirus in acute otitis media. J. Pediatr. 113:693-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arruda, E., A. Pitkäranta, T. J. Witek, Jr., C. A. Doyle, and F. G. Hayden. 1997. Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 35:2864-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakaletz, L. O., D. M. Murwin, and J. M. Billy. 1995. Adenovirus serotype 1 does not act synergistically with Moraxella (Branhamella) catarrhalis to induce otitis media in the chinchilla. Infect. Immun. 63:4188-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakaletz, L. O., R. L. Daniels, and D. J. Lim. 1993. Modeling adenovirus type 1-induced otitis media in the chinchilla: effect on ciliary activity and fluid transport function of Eustachian tube mucosal epithelium. J. Infect. Dis. 168:865-872. [DOI] [PubMed] [Google Scholar]
- 11.Barzilai, A., B. Dekel, R. Dagan, J. H. Passwell, and E. Leibovitz. 1999. Cytokine analysis of middle ear effusions during acute otitis media: significant reduction in tumor necrosis factor alpha concentrations correlates with bacterial eradication. Pediatr. Infect. Dis. J. 18:301-303. [DOI] [PubMed] [Google Scholar]
- 12.Becker, S., H. S. Koren, and D. C. Henke. 1993. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1, and interleukin-6. Am. J. Respir. Cell Mol. Biol. 8:20-27. [DOI] [PubMed] [Google Scholar]
- 13.Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza vaccine in children. N. Engl. J. Med. 338:1405-1412. [DOI] [PubMed] [Google Scholar]
- 14.Berger, G., M. Hawke, D. W. Proops, N. S. Ranadive, and D. Wong. 1984. Histamine levels in middle ear effusions. Acta Otolaryngol. 98:385-390. [DOI] [PubMed] [Google Scholar]
- 15.Berglund, B., A. Salmivalli, and J. A. Grönroos. 1967. The role of respiratory syncytial virus in otitis media in children. Acta Otolaryngol. 63:445-454. [DOI] [PubMed] [Google Scholar]
- 16.Berglund, B., A. Salmivalli, and P. Toivanen. 1966. Isolation of respiratory syncytial virus from middle ear exudates of infants. Acta Otolaryngol. 61:475-487. [DOI] [PubMed] [Google Scholar]
- 17.Blomqvist, S., M. Roivainen, T. Puhakka, M. Kleemola, and T. Hovi. 2002. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J. Med. Virol. 66:263-268. [DOI] [PubMed] [Google Scholar]
- 18.Bluestone, C. D. 1996. Pathogenesis of otitis media: role of Eustachian tube. Pediatr. Infect. Dis. J. 15:281-291. [DOI] [PubMed] [Google Scholar]
- 19.Bluestone, C. D., and J. O. Klein. 2001. Microbiology, p. 79-101. In C. D. Bluestone and J. O. Klein (ed.), Otitis media in infants and children, 3rd ed. W. B. Saunders, Philadelphia, Pa.
- 20.Bluestone, C. D., E. I. Cantekin, and Q. C. Beery. 1977. Effect of inflammation on the ventilatory function of the Eustachian tube. Laryngoscope 87:493-507. [PubMed] [Google Scholar]
- 21.Brodsky, L., H. Faden, J. Bernstein, J. Stanievich, G. DeCastro, B. Volovitz, and P. L. Ogra. 1991. Arachidonic acid metabolites in middle ear effusions of children. Ann. Otol. Rhinol. Laryngol. 100:589-592. [DOI] [PubMed] [Google Scholar]
- 22.Buchman, C. A., J. D. Swarts, J. T. Seroky, N. Panagiotou, F. Hayden, and W. J. Doyle. 1995. Otologic and systemic manifestations of experimental influenza A virus infection in the ferret. Otolaryngol. Head Neck Surg. 112:572-578. [DOI] [PubMed] [Google Scholar]
- 23.Buchman, C. A., W. J. Doyle, D. P. Skoner, J. C. Post, C. M. Alper, J. T. Seroky, K. Anderson, R. A. Preston, F. G. Hayden, P. Fireman, and G. D. Ehrlich. 1995. Influenza A virus-induced acute otitis media. J. Infect. Dis 172:1348-1351. [DOI] [PubMed] [Google Scholar]
- 24.Buchman, C. A., W. J. Doyle, D. Skoner, P. Fireman, and J. M. Gwaltney. 1994. Otologic manifestations of experimental rhinovirus infection. Laryngoscope 104:1295-1299. [DOI] [PubMed] [Google Scholar]
- 25.Buchman, C. A., W. J. Doyle, O. Pilcher, D. A. Gentile, and D. P. Skoner. 2002. Nasal and otologic effects of experimental respiratory syncytial virus infection in adults. Am. J. Otolaryngol. 23:70-75. [DOI] [PubMed] [Google Scholar]
- 26.Bylander, A. 1984. Upper respiratory tract infection and Eustachian tube function in children. Acta Otolaryngol. 97:343-349. [DOI] [PubMed] [Google Scholar]
- 27.Bylander, A., A. Ivarsson, and Ö. Tjernström. 1981. Eustachian tube function in normal children and adults. Acta Otolaryngol. 92:481-491. [DOI] [PubMed] [Google Scholar]
- 28.Bylander, A., and Ö. Tjernström. 1983. Changes in eustachian tube function with age in children with normal ears. Acta Otolaryngol. 96:467-477. [DOI] [PubMed] [Google Scholar]
- 29.Canafax, D. M., Z. Yuan, T. Chonmaitree, K. Deka, H. Q. Russlie, and G. S. Giebink. 1998. Amoxicillin middle ear fluid penetration and pharmacokinetics in children with acute otitis media. Pediatr. Infect. Dis. J. 17:149-156. [DOI] [PubMed] [Google Scholar]
- 30.Chonmaitree, T., and K. Henrickson. 2000. Detection of respiratory viruses in the middle ear fluids of children with acute otitis media by multiplex reverse transcription-polymerase chain reaction assay. Pediatr. Infect. Dis. J. 19:258-260. [DOI] [PubMed] [Google Scholar]
- 31.Chonmaitree, T., and V. M. Howie. 1987. Bacteriology of otitis media, p. 231-247. In J. M. Bernstein and P. L. Ogra (ed.), Immunology of the ear. Raven Press, New York, N.Y.
- 32.Chonmaitree, T., J. A. Patel, M. A. Lett-Brown, T. Uchida, R. Garofalo, M. J. Owen, and V. M. Howie. 1994. Virus and bacteria enhance histamine production in middle ear fluids of children with acute otitis media. J. Infect. Dis. 169:1265-1270. [DOI] [PubMed] [Google Scholar]
- 33.Chonmaitree, T., J. A. Patel, R. Garofalo, T. Uchida, T. Sim, M. J. Owen, and V. M. Howie. 1996. Role of leukotriene B4 and interleukin-8 in acute bacterial and viral otitis media. Ann. Otol. Rhinol. Laryngol. 105:968-974. [DOI] [PubMed] [Google Scholar]
- 34.Chonmaitree, T., M. J. Owen, and V. M. Howie. 1990. Respiratory viruses interfere with bacteriologic response to antibiotic in children with acute otitis media. J. Infect. Dis. 162:546-549. [DOI] [PubMed] [Google Scholar]
- 35.Chonmaitree, T., M. J. Owen, J. A. Patel, D. Hedgpeth, D. Horlick, and V. M. Howie. 1992. Effect of viral respiratory tract infection on outcome of acute otitis media. J. Pediatr. 120:856-862. [DOI] [PubMed] [Google Scholar]
- 36.Chonmaitree, T., M. J. Owen, J. A. Patel, D. Hedgpeth, D. Horlick, and V. M. Howie. 1992. Presence of cytomegalovirus and herpes simplex virus in middle ear fluids from children with acute otitis media. Clin. Infect. Dis. 15:650-653. [DOI] [PubMed] [Google Scholar]
- 37.Chonmaitree, T., V. M. Howie, and A. L. Truant. 1986. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics 77:698-702. [PubMed] [Google Scholar]
- 38.Chung, M. H., S. R. Griffith, K. H. Park, D. J. Lim, and T. F. DeMaria. 1993. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol. 113:81-87. [DOI] [PubMed] [Google Scholar]
- 39.Clements, D. A., L. Langdon, C. Bland, and E. Walter. 1995. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch. Pediatr. Adolesc. Med. 149:1113-1117. [DOI] [PubMed] [Google Scholar]
- 40.Coyte, P. C., C. V. Asche, and L. M. Elden. 1999. The economic cost of otitis media in Canada. Int. J. Pediatr. Otorhinolaryngol. 49:27-36. [DOI] [PubMed] [Google Scholar]
- 41.Crowe, J. E., Jr. 2001. Respiratory syncytial virus vaccine development. Vaccine 20(Suppl. 1):S32-S37. [DOI] [PubMed] [Google Scholar]
- 42.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daly, K. A., J. E. Brown, B. R. Lindgren, M. H. Meland, C. T. Le, and G. S. Giebink. 1999. Epidemiology of otitis media onset by six months of age. Pediatrics 103:1158-1166. [DOI] [PubMed] [Google Scholar]
- 44.Doyle, W. J., D. P. Skoner, F. Hayden, C. A. Buchman, J. T. Seroky, and P. Fireman. 1994. Nasal and otologic effects of experimental influenza A virus infection. Ann. Otol. Rhinol. Laryngol. 103:59-69. [DOI] [PubMed] [Google Scholar]
- 45.Doyle, W. J., S. Boehm, and D. P. Skoner. 1990. Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J. Allergy Clin. Immunol. 86:924-935. [DOI] [PubMed] [Google Scholar]
- 46.Doyle, W. J., T. P. McBride, D. P. Skoner, B. R. Maddern, J. M. Gwaltney, Jr., and M. Uhrin. 1988. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear, and Eustachian tube to provocative rhinovirus challenge. Pediatr. Infect. Dis. J. 7:229-238. [DOI] [PubMed] [Google Scholar]
- 47.Duncan, B., J. Ey, C. J. Holberg, A. L. Wright, F. D. Martinez, and L. M. Taussig. 1993. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 91:867-872. [PubMed] [Google Scholar]
- 48.El Ahmer, O. R., M. W. Raza, M. M. Ogilvie, D. M. Weir, and C. C. Blackwell. 1999. Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol. Med. Microbiol. 23:331-341. [DOI] [PubMed] [Google Scholar]
- 49.Elkhatieb, A., G. Hipskind, D. Woerner, and F. G. Hayden. 1993. Middle ear abnormalities during natural rhinovirus colds in adults. J. Infect. Dis. 168:618-621. [DOI] [PubMed] [Google Scholar]
- 50.Faden, H., J. Stanievich, L. Brodsky, J. Bernstein, P. L. Ogra. 1990. Changes in nasopharyngeal bacterial flora during otitis media of childhood. Pediatr. Infect. Dis. J. 9:623-626. [PubMed] [Google Scholar]
- 51.Fainstein, V., D. M. Musher, and T. R. Cate. 1980. Bacterial adherence to pharyngeal cells during viral infection. J. Infect. Dis 141:172-176. [DOI] [PubMed] [Google Scholar]
- 52.Frady, R. P., W. A. Parker, and R. T. Jackson. 1977. Studies in permeability of the middle ear mucosa. The feasibility of blocking inflammatory mediators. Arch. Otolaryngol. 103:47-51. [DOI] [PubMed] [Google Scholar]
- 53.Fritz, R. S., F. G. Hayden, D. P. Calfee, L. M. Cass, A. W. Peng, W. G. Alvord, W. Strober, and S. E. Straus. 1999. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J. Infect. Dis. 180:586-593. [DOI] [PubMed] [Google Scholar]
- 54.Froom, J., L. Culpepper, M. Jacobs, R. A. DeMelker, L. A. Green, L. van Buchem, P. Grob, and T. Heeren. 1997. Antimicrobials for acute otitis media? A review from the International Primary Care Network. Br. Med. J. 315:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garofalo, R., I. Enander, M. Nilssons, V. M. Howie, M. J. Owen, and T. Chonmaitree. 1996. Mast cell degranulation in the middle ear of children with acute otitis media, p. 191-193. In D. J. Lim, C. D. Bluestone, M. Casselbrant, J. O. Klein, and P. L. Ogra (ed.), Recent advances in otitis media. Decker, Hamilton, Ontario, Canada.
- 56.Gentile, D. A., W. J. Doyle, P. Fireman, and D. P. Skoner. 2001. Effect of experimental influenza A infection on systemic immune and inflammatory parameters in allergic and nonallergic adult subjects. Ann. Allergy Asthma Immunol. 87:496-500. [DOI] [PubMed] [Google Scholar]
- 57.Giebink, G. S. 1999. Otitis media: the chinchilla model. Microb. Drug Resist. 5:57-72. [DOI] [PubMed] [Google Scholar]
- 58.Giebink, G. S., I. K. Berzins, S. C. Marker, and G. Schiffman. 1980. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect. Immun. 30:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giebink, G. S., M. L. Ripley, and P. F. Wright. 1987. Eustachian tube histopathology during experimental influenza A virus infection in the chinchilla. Ann. Otol. Rhinol. Laryngol. 96:199-206. [DOI] [PubMed] [Google Scholar]
- 60.Glezen, W. P., A. Paredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98:708-715. [DOI] [PubMed] [Google Scholar]
- 61.Grönroos, J. A., A. E. Kortekangas, L. Ojala, and M. Vuori. 1964. The aetiology of acute middle ear infection. Acta Otolaryngol. 58:149-158. [DOI] [PubMed] [Google Scholar]
- 62.Grönroos, J. A., L. Vihma, A. Salmivalli, and B. Berglund. 1968. Coexisting viral (respiratory syncytial) and bacterial (pneumococcus) otitis media in children. Acta Otolaryngol. 65:505-517. [DOI] [PubMed] [Google Scholar]
- 63.Håkansson, A., A. Kidd, G. Wadell, H. Sabharwal, and C. Svanborg. 1994. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect. Immun. 62:2707-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halsted, C., M. L. Lepow, N. Balassanian, J. Emmerich, and E. Wolinsky. 1968. Otitis media: clinical observation, microbiology, and evaluation of therapy. Am. J. Dis. Child. 115:542-551. [DOI] [PubMed] [Google Scholar]
- 65.Hayden, F. G., A. D. Osterhaus, J. J. Treanor, D. M. Fleming, F. Y. Aoki, K. G. Nicholson, A. M. Bohnen, H. M. Hirst, O. Keene, K. Wightman, et al. 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N. Engl. J. Med. 337:874-880. [DOI] [PubMed] [Google Scholar]
- 66.Heikkinen, T., and O. Ruuskanen. 1994. Temporal development of acute otitis media during upper respiratory tract infection. Pediatr. Infect. Dis. J. 13:659-661. [DOI] [PubMed] [Google Scholar]
- 67.Heikkinen, T., and O. Ruuskanen. 1995. Signs and symptoms predicting acute otitis media. Arch. Pediatr. Adolesc. Med. 149:26-29. [DOI] [PubMed] [Google Scholar]
- 68.Heikkinen, T., A. Ruohola, O. Ruuskanen, M. Waris, M. Uhari, and L. Hammarström. 1998. Intranasally administered immunoglobulin for the prevention of rhinitis in children. Pediatr. Infect. Dis. J. 17:367-372. [DOI] [PubMed] [Google Scholar]
- 69.Heikkinen, T., M. Thint, and T. Chonmaitree. 1999. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N. Engl. J. Med. 340:260-264. [DOI] [PubMed] [Google Scholar]
- 70.Heikkinen, T., O. Ruuskanen, M. Waris, T. Ziegler, M. Arola, and P. Halonen. 1991. Influenza vaccination in the prevention of acute otitis media in children. Am. J. Dis. Child. 145:445-448. [DOI] [PubMed] [Google Scholar]
- 71.Heikkinen, T., O. Ruuskanen, T. Ziegler, M. Waris, and H. Puhakka. 1995. Short-term use of amoxicillin-clavulanate during upper respiratory tract infection for prevention of acute otitis media. J. Pediatr. 126:313-316. [DOI] [PubMed] [Google Scholar]
- 72.Henderson, F. W., A. M. Collier, M. A. Sanyal, J. M. Watkins, D. L. Fairclough, W. A. Clyde, Jr., and F. W. Denny. 1982. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N. Engl. J. Med. 306:1377-1383. [DOI] [PubMed] [Google Scholar]
- 73.Hsia, J., A. L. Goldstein, G. L. Simon, M. Sztein, and F. G. Hayden. 1990. Peripheral blood mononuclear cell interleukin-2 and interferon-gamma production, cytotoxicity, and antigen-stimulated blastogenesis during experimental rhinovirus infection. J. Infect. Dis. 162:591-597. [DOI] [PubMed] [Google Scholar]
- 74.Hyypiä, T., T. Puhakka, O. Ruuskanen, M. Mäkelä, A. Arola, and P. Arstila. 1998. Molecular diagnosis of human rhinovirus infections: comparison with virus isolation. J. Clin. Microbiol. 36:2081-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74a.The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 75.Jiang, Z., N. Nagata, E. Molina, L. O. Bakaletz, H. Hawkins, and J. A. Patel. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect. Immun. 67:187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jossart, G. H., D. M. Canafax, G. R. Erdmann, M. J. Lovdahl, H. Q. Russlie, S. K. Juhn, and G. S. Giebink. 1994. Effect of Streptococcus pneumoniae and influenza A virus on middle ear antimicrobial pharmacokinetics in experimental otitis media. Pharm. Res. 11:860-864. [DOI] [PubMed] [Google Scholar]
- 77.Juhn, S. K., C. T. Tolan, W. J. Garvis, D. S. Cross, and G. S. Giebink. 1992. The levels of IL-1 beta in human middle ear effusions. Acta Otolaryngol. 493:37-42. [PubMed] [Google Scholar]
- 78.Jung, T. T. 1988. Prostaglandins, leukotrienes, and other arachidonic acid metabolites in the pathogenesis of otitis media. Laryngoscope 98:980-993. [DOI] [PubMed] [Google Scholar]
- 79.Kaplan, B., T. L. Wandstrat, and J. R. Cunningham. 1997. Overall cost in the treatment of otitis media. Pediatr. Infect. Dis. J. 16(Suppl. 2):S9-S11. [DOI] [PubMed] [Google Scholar]
- 80.Klein, B. S., F. R. Dollete, and R. H. Yolken. 1982. The role of respiratory syncytial virus and other viral pathogens in acute otitis media. J. Pediatr. 101:16-20. [DOI] [PubMed] [Google Scholar]
- 81.Koivunen, P., T. Kontiokari, M. Niemelä, T. Pokka, and M. Uhari. 1999. Time to development of acute otitis media during an upper respiratory tract infection in children. Pediatr. Infect. Dis. J. 18:303-305. [DOI] [PubMed] [Google Scholar]
- 82.Kontiokari, T., P. Koivunen, M. Niemelä, T. Pokka, and M. Uhari. 1998. Symptoms of acute otitis media. Pediatr. Infect. Dis. J. 17:676-679. [DOI] [PubMed] [Google Scholar]
- 83.Larson, H. E., and R. Blades. 1976. Impairment of human polymorphonuclear leucocyte function by influenza virus. Lancet i:283. [DOI] [PubMed]
- 84.Laxdal, O. E., R. M. Blake, T. Cartmill, and H. E. Robertson. 1966. Etiology of acute otitis media in infants and children. Can. Med. Assoc. J. 94:159-163. [PMC free article] [PubMed] [Google Scholar]
- 85.Lee, M. S., D. P. Greenberg, S. H. Yeh, R. Yogev, K. S. Reisinger, J. I. Ward, M. M. Blatter, I. Cho, S. J. Holmes, J. M. Cordova, M. J. August, W. Chen, H. B. Mehta, K. L. Coelingh, and P. M. Mendelman. 2001. Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. J. Infect. Dis. 184:909-913. [DOI] [PubMed] [Google Scholar]
- 86.Leibovitz, E., R. Dagan, J. H. Laver, L. Piglansky, S. Raiz, M. R. Abboud, D. M. Fliss, A. Leiberman, and A. Barzilai. 2000. Interleukin 8 in middle ear fluid during acute otitis media: correlation with aetiology and bacterial eradication. Arch. Dis. Child. 82:165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mäkelä, M. J., T. Puhakka, O. Ruuskanen, M. Leinonen, P. Saikku, M. Kimpimäki, S. Blomqvist, T. Hyypiä, and P. Arstila. 1998. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marchisio, P., R. Cavagna, B. Maspes, S. Gironi, S. Esposito, L. Lambertini, A. Massimini, C. Herzog, and N. Principi. 2002. Efficacy of intranasal virosomal influenza vaccine in the prevention of recurrent acute otitis media in children. Clin. Infect. Dis. 35:168-174. [DOI] [PubMed] [Google Scholar]
- 89.McBride, T. P., W. J. Doyle, F. G. Hayden, and J. M. Gwaltney, Jr. 1989. Alterations of the Eustachian tube, middle ear, and nose in rhinovirus infection. Arch. Otolaryngol. Head Neck Surg. 115:1054-1059. [DOI] [PubMed] [Google Scholar]
- 90.McCaig, L. F., and J. M. Hughes. 1995. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 273:214-219. [PubMed] [Google Scholar]
- 91.Monto, A. S., and B. M. Ullman. 1974. Acute respiratory illness in an American community: the Tecumseh study. JAMA 227:164-169. [PubMed] [Google Scholar]
- 92.Moody, S. A., C. M. Alper, and W. J. Doyle. 1998. Daily tympanometry in children during the cold season: association of otitis media with upper respiratory tract infections. Int. J. Pediatr. Otorhinolaryngol. 45:143-150. [DOI] [PubMed] [Google Scholar]
- 93.Mukaida, N., A. Harada, K. Yasumoto, and K. Matsushima. 1992. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF). Microbiol. Immunol. 36:773-789. [DOI] [PubMed] [Google Scholar]
- 94.Naclerio, R. M., D. Proud, L. M. Lichtenstein, A. Kagey-Sobotka, J. O. Hendley, J. Sorrentino, and J. M. Gwaltney. 1988. Kinins are generated during experimental rhinovirus colds. J. Infect. Dis. 157:133-142. [DOI] [PubMed] [Google Scholar]
- 95.Nicholson, K. G., F. Y. Aoki, A. D. M. E. Osterhaus, S. Trottier, O. Carewicz, C. H. Mercier, A. Rode, N. Kinnersley, and P. Ward, on behalf of the Neuraminidase Inhibitor Flu Treatment Investigator Group. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomized controlled trial. Lancet 355:1845-1850. [DOI] [PubMed] [Google Scholar]
- 96.Niemelä, M., M. Uhari, K. Jounio-Ervasti, J. Luotonen, O. P. Alho, and E. Vierimaa. 1994. Lack of specific symptomatology in children with acute otitis media. Pediatr. Infect. Dis. J. 13:765-768. [DOI] [PubMed] [Google Scholar]
- 97.Niemelä, M., M. Uhari, M. Möttönen, and T. Pokka. 1999. Costs arising from otitis media. Acta Paediatr. 88:553-556. [DOI] [PubMed] [Google Scholar]
- 98.Noah, T. L., F. W Henderson, I. A. Wortman, R. B. Devlin, J. Handy, H. S. Koren, and S. Becker. 1995. Nasal cytokine production in viral acute upper respiratory infection of childhood. J. Infect. Dis. 171:584-592. [DOI] [PubMed] [Google Scholar]
- 99.Ohashi, Y., Y. Nakai, Y. Esaki, Y. Ohno, Y. Sugiura, and H. Okamoto. 1991. Influenza A virus-induced otitis media and mucociliary dysfunction in the guinea pig. Acta Otolaryngol. 486(Suppl.):135-148. [DOI] [PubMed] [Google Scholar]
- 100.Okamoto, Y., K. Kudo, K. Ishikawa, E. Ito, K. Togawa, I. Saito, I. Moro, J. A. Pate, and P. L. Ogra. 1993. Presence of respiratory syncytial virus genomic sequences in middle ear fluid and its relationship to expression of cytokines and cell adhesion molecules. J. Infect. Dis. 168:1277-1281. [DOI] [PubMed] [Google Scholar]
- 101.Owen, M. J., C. D. Baldwin, P. R. Swank, A. K. Pannu, D. L. Johnson, and V. M. Howie. 1993. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J. Pediatr. 123:702-711. [DOI] [PubMed] [Google Scholar]
- 102.Patel, J., H. Faden, S. Sharma, and P. L. Ogra. 1992. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int. J. Pediatr. Otorhinolaryngol. 23:15-23. [DOI] [PubMed] [Google Scholar]
- 103.Patel, J. A., T. Sim, M. J. Owen, V. M. Howie, and T. Chonmaitree. 1996. Influence of viral infection on middle ear chemokine response in acute otitis media, p. 178-179. In D. J. Lim, C. D. Bluestone, M. Casselbrant, J. O. Klein, and P. L. Ogra (ed.), Recent advances in otitis media. Decker, Hamilton, Ontario, Canada.
- 104.Patel, J. A., M. Kunimoto, T. C. Sim, R. Garofalo, T. Eliott, S. Baron, O. Ruuskanen, T. Chonmaitree, P. L. Ogra, and F. Schmalstieg. 1995. Interleukin-1α mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13:602-609. [DOI] [PubMed] [Google Scholar]
- 105.Pichichero, M. E., and C. L. Pichichero. 1995. Persistent acute otitis media. I. Causative pathogens. Pediatr. Infect. Dis. J. 14:178-183. [DOI] [PubMed] [Google Scholar]
- 106.Pitkäranta, A., A. Virolainen, J. Jero, E. Arruda, and F. G. Hayden. 1998. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics 102:291-295. [DOI] [PubMed] [Google Scholar]
- 107.Pukander, J., P. Karma, and M. Sipilä. 1982. Occurrence and recurrence of acute otitis media among children. Acta Otolaryngol. 94:479-486. [DOI] [PubMed] [Google Scholar]
- 108.Ruuskanen, O., M. Arola, A. Putto-Laurila, J. Mertsola, O. Meurman, M. K. Viljanen, and P. Halonen. 1989. Acute otitis media and respiratory virus infections. Pediatr. Infect. Dis. J. 8:94-99. [PubMed] [Google Scholar]
- 109.Ruuskanen, O., and P. L. Ogra. 1993. Respiratory syncytial virus. Curr. Probl. Pediatr. 23:50-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saez-Llorenz, X. 1994. Pathogenesis of acute otitis media. Pediatr. Infect. Dis. J. 13:1035-1038. [DOI] [PubMed] [Google Scholar]
- 111.Saito, T., R. W. Deskin, A. Casola, H. Häeberle, B. Olszewska, P. B. Ernst, R. Alam, P. L. Ogra, and R. Garofalo. 1997. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J. Infect. Dis. 175:497-504. [DOI] [PubMed] [Google Scholar]
- 112.Sanyal, M. A., F. W. Henderson, E. C. Stempel, A. M. Collier, and F. W. Denny. 1980. Effect of upper respiratory tract infection on Eustachian tube ventilatory function in the preschool child. J. Pediatr. 97:11-15. [DOI] [PubMed] [Google Scholar]
- 113.Sarkkinen, H., O. Ruuskanen, O. Meurman, H. Puhakka, E. Virolainen, and J. Eskola. 1985. Identification of respiratory virus antigens in middle ear fluids of children with acute otitis media. J. Infect. Dis. 151:444-448. [DOI] [PubMed] [Google Scholar]
- 114.Sarkkinen, H. K., O. Meurman, T. T. Salmi, H. Puhakka, and E. Virolainen. 1983. Demonstration of viral antigens in middle ear secretions of children with acute otitis media. Acta Paediatr. Scand. 72:137-138. [DOI] [PubMed] [Google Scholar]
- 115.Sawyer, W. D. 1969. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J. Infect. Dis. 119:541-556. [DOI] [PubMed] [Google Scholar]
- 116.Simoes, E. A. F., J. R. Groothuis, D. A. Tristram, K. Allessi, M. V. Lehr, G. R. Siber, and R. C. Welliver. 1996. Respiratory syncytial virus-enriched globulin for the prevention of acute otitis media in high risk children. J. Pediatr. 129:214-219. [DOI] [PubMed] [Google Scholar]
- 117.Skiadopoulos, M. H., J. M. Tatem, S. R. Surman, Y. Mitcho, S. L. Wu, W. R. Elkins, and B. R. Murphy. 2002. The recombinant chimeric human parainfluenza virus type 1 vaccine candidate, rHPIV3-1cp45, is attenuated, immunogenic, and protective in African green monkeys. Vaccine 20:1846-1852. [DOI] [PubMed] [Google Scholar]
- 118.Skoner, D. P., T. L. Whiteside, J. W. Wilson, W. J. Doyle, R. B. Herberman, and P. Fireman. 1993. Effect of rhinovirus 39 infection on cellular immune parameters in allergic and nonallergic subjects. J. Allergy Clin. Immunol. 92:732-743. [DOI] [PubMed] [Google Scholar]
- 119.Sung, B. S., T. Chonmaitree, L. D. Broemeling, M. J. Owen, J. A. Patel, D. C. Hedgpeth, and V. M. Howie. 1993. Association of rhinovirus infection with poor bacteriologic outcome of bacterial-viral otitis media. Clin. Infect. Dis. 17:38-42. [DOI] [PubMed] [Google Scholar]
- 120.Suzuki, K., and L. O. Bakaletz. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect. Immun. 62:1710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tapiainen, T., L. Luotonen, T. Kontiokari, M. Renko, and M. Uhari. 2002. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics 109:e19. [DOI] [PubMed]
- 122.Teele, D. W., J. O. Klein, B. Rosner, and the Greater Boston Otitis Media Study Group. 1989. Epidemiology of otitis media during the first seven years of life in children in Greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83-94. [DOI] [PubMed] [Google Scholar]
- 123.Teele, D. W., S. I. Pelton, and J. O. Klein. 1981. Bacteriology of acute otitis media unresponsive to initial antimicrobial therapy. J. Pediatr. 98:537-539. [DOI] [PubMed] [Google Scholar]
- 124.Reference deleted.
- 125.Tilles, J. G., J. O. Klein, R. L. Jao, J. E. Haslam, Jr., M. Feingold, S. S. Gellis, and M. Finland. 1967. Acute otitis media in children. Serologic studies and attempts to isolate viruses and mycoplasmas from aspirated middle-ear fluids. N. Engl. J. Med. 277:613-618. [DOI] [PubMed] [Google Scholar]
- 126.Tong, H. H., J. N Weiser, M. A. James, and T. F. DeMaria. 2001. Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect. Immun. 69:602-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. F. DeMaria. 2000. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann. Otol. Rhinol. Laryngol. 109:1021-1027. [DOI] [PubMed] [Google Scholar]
- 128.Turner, R. B., K. W. Weingand, C. H. Yeh, and D. W. Leedy. 1998. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin. Infect. Dis. 26:840-846. [DOI] [PubMed] [Google Scholar]
- 129.Uhari, M., J. Hietala, and H. Tuokko. 1995. Risk of acute otitis media in relation to the viral etiology of infections in children. Clin. Infect. Dis. 20:521-524. [DOI] [PubMed] [Google Scholar]
- 130.Uhari, M., K. Mäntysaari, and M. Niemelä. 1996. A meta-analytic review of the risk factors for acute otitis media. Clin. Infect. Dis. 22:1079-1083. [DOI] [PubMed] [Google Scholar]
- 131.Uhari, M., M. Niemelä, and J. Hietala. 1995. Prediction of acute otitis media with symptoms and signs. Acta Paediatr. 84:90-92. [DOI] [PubMed] [Google Scholar]
- 132.Virolainen, A., P. Salo, J. Jero, P. Karma, J. Eskola, and M. Leinonen. 1994. Comparison of PCR assay with bacterial culture for detecting Streptococcus pneumoniae in middle ear fluid of children with acute otitis media. J. Clin. Microbiol. 32:2667-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Volovitz, B., H. Faden, and P. L. Ogra. 1988. Release of leukotriene C4 in respiratory tract during acute viral infection. J. Pediatr. 112:218-222. [DOI] [PubMed] [Google Scholar]
- 134.Wadowsky, R. M., S. M. Mietzner, D. P. Skoner, W. J. Doyle, and P. Fireman. 1995. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect. Immun. 63:1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang, E. E., T. R. Einarson, J. D. Kellner, and J. M. Conly. 1999. Antibiotic prescribing for Canadian preschool children: evidence of overprescribing for viral respiratory infections. Clin. Infect. Dis. 29:155-160. [DOI] [PubMed] [Google Scholar]
- 136.Welliver, R. C., D. T. Wong, M. Sun, E. Middleton, Jr., R. S. Vaughan, and P. L. Ogra. 1981. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N. Engl. J. Med. 305:841-846. [DOI] [PubMed] [Google Scholar]
- 137.Whitley, R. J., F. G. Hayden, K. S. Reisinger, N. Young, R. Dutkowski, D. Ipe, R. G. Mills, and P. Ward. 2001. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 20:127-133. [DOI] [PubMed] [Google Scholar]
- 138.Winther, B., F. G. Hayden, E. Arruda, R. Dutkowski, P. Ward, and J. O. Hendley. 2002. Viral respiratory infection in schoolchildren: effects on middle ear pressure. Pediatrics 109:826-832. [DOI] [PubMed] [Google Scholar]
- 139.Yellon, R. F., W. J. Doyle, T. L. Whiteside, W. F. Diven, A. R. March, and P. Fireman. 1995. Cytokines, immunoglobulins, and bacterial pathogens in middle ear effusions. Arch. Otolaryngol. Head Neck Surg. 121:865-869. [DOI] [PubMed] [Google Scholar]
- 140.Yoshie, C. 1955. On the isolation of influenza virus from mid-ear discharge of influenza otitis media. Jpn. J. Med. Sci. Biol. 8:373-377. [DOI] [PubMed] [Google Scholar]
- 141.Zhu, Z., W. Tang, A. Ray, Y. Wu, O. Einarsson, M. L. Landry, J. Gwaltney, Jr., and J. A. Elias. 1996. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. J. Clin. Investig. 97:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]