Abstract
Acanthamoeba spp. are free-living amebae that inhabit a variety of air, soil, and water environments. However, these amebae can also act as opportunistic as well as nonopportunistic pathogens. They are the causative agents of granulomatous amebic encephalitis and amebic keratitis and have been associated with cutaneous lesions and sinusitis. Immuno compromised individuals, including AIDS patients, are particularly susceptible to infections with Acanthamoeba. The immune defense mechanisms that operate against Acanthamoeba have not been well characterized, but it has been proposed that both innate and acquired immunity play a role. The ameba's life cycle includes an active feeding trophozoite stage and a dormant cyst stage. Trophozoites feed on bacteria, yeast, and algae. However, both trophozoites and cysts can retain viable bacteria and may serve as reservoirs for bacteria with human pathogenic potential. Diagnosis of infection includes direct microscopy of wet mounts of cerebrospinal fluid or stained smears of cerebrospinal fluid sediment, light or electron microscopy of tissues, in vitro cultivation of Acanthamoeba, and histological assessment of frozen or paraffin-embedded sections of brain or cutaneous lesion biopsy material. Immunocytochemistry, chemifluorescent dye staining, PCR, and analysis of DNA sequence variation also have been employed for laboratory diagnosis. Treatment of Acanthamoeba infections has met with mixed results. However, chlorhexidine gluconate, alone or in combination with propamidene isethionate, is effective in some patients. Furthermore, effective treatment is complicated since patients may present with underlying disease and Acanthamoeba infection may not be recognized. Since an increase in the number of cases of Acanthamoeba infections has occurred worldwide, these protozoa have become increasingly important as agents of human disease.
INTRODUCTION
Free-living amebae belonging to the genus Acanthamoeba are the causative agents of granulomatous amebic encephalitis (GAE), a fatal disease of the central nervous system (CNS), and amebic keratitis (AK), a painful sight-threatening disease of the eyes (95, 210, 286, 325). Acanthamoeba spp. also have been associated with cutaneous lesions and sinusitis in AIDS patients and other immunocompromised individuals (128, 143, 164, 179, 282, 295, 446). The first suggestion that Acanthamoeba could cause disease in humans came in 1958 during polio vaccine safety trials. Plaques appeared in cell cultures used to prepare vaccine and were thought to be virus induced because mice and monkeys died from encephalitis following inoculation of tissue culture fluid. However, these plaques were found later to be caused by amebae (98, 99). Both trophozoites and cysts were detected in cell cultures and were identified as belonging to the genus Acanthamoeba. These observations of experimental animals dying from encephalitis led Culbertson et al. (99) to predict a role for free-living amebae as agents of human disease. Human cases of amebic encephalitis were reported soon thereafter from Australia, Europe, Africa, South America, and the United States (35, 57, 58, 64, 74, 142, 201, 280, 284, 344, 476). However, some of these cases were identified later as primary amebic meningoencephalitis, a rapidly fatal disease of the CNS caused by another free-living ameba, Naegleria fowleri (57, 268, 286). The first cases which clearly established Acanthamoeba as causative agents of disease in humans were reported in the early 1970s. These included reports of amebic encephalitis, amebic keratitis, and skin infections (164, 201, 210, 213, 284, 325, 368, 374, 476). Consequently, since different free-living amebae can infect the CNS, the term “granulomatous amebic encephalitis” (GAE) has been used for CNS infections caused by Acanthamoeba spp. while the term “primary amebic meningoencephalitis” has been reserved for CNS infections caused by Naegleria fowleri (64, 286). Acanthamoeba and Naegleria have been termed amphizoic organisms since they have the ability to exist both as free-living amebae and as parasitic pathogens (341). More recently, two other free-living amebae from distinct genera, Balamuthia mandrillaris and Sappinia diploidea, have been associated with CNS infections in humans (156, 461). B. mandrillaris was reported to cause fatal amebic encephalitis in both healthy and immunosuppressed patients (113, 281, 387). S. diploidea, a soil ameba, was identified in an otherwise healthy individual who experienced nonfatal amebic encephalitis following a sinus infection (156). Thus, it is becoming increasingly apparent that free-living amebae cause human disease. Furthermore, with increasing awareness of the potential of free-living amebae to cause disease, amebae from other genera may be found to be causative agents of human infections.
CLASSIFICATION OF ACANTHAMOEBA
Acanthamoeba was first described by Castellani when he reported the presence of an ameba in Cryptococcus pararoseus cultures (70). The genus Acanthamoeba was established later by Volkonsky in 1931 (463), but the actual classification of organisms within this genus is currently under review (12, 41, 42, 50, 56, 61, 151, 232, 395, 434). Acanthamoeba has been placed in the Family Acanthamoebidae (Fig. 1). A second genus, Balamuthia, previously assigned with amebae of uncertain affinities, has recently been included in this family (91, 378). Studies suggested that the genus Balamuthia be transferred from the family Leptomyxidae to Acanthamoebidae on the basis of molecular analysis of 16S-like rRNA genes (12, 434). Furthermore, Acanthamoeba and Balamuthia both possess a multilayered microtubule-organizing center and both can cause disease in humans (345). Identification of Acanthamoeba at the genus level is relatively easy due to the presence of spiny surface projections, termed acanthopodia, on trophozoites (Fig. 2). However, using morphological criteria, identification of these amebae at the species level has been difficult. Acanthamoeba spp. have been placed into three morphological groups (I, II, and III) based on cyst size and shape (340, 363). Species in group I were designated on the basis of having a large cyst in comparison to that of species in the other groups. Species in group II were characterized as having a wrinkled ectocyst and an endocyst which could be stellate, polygonal, triangular, or oval. Species in group III typically exhibited a thin, smooth ectocyst and a round endocyst. Nevertheless, classification of Acanthamoeba based on morphological characteristics of the cyst wall has proved unreliable because cyst morphology can change depending on culture conditions (15, 105, 390, 435). Immunological, biochemical, and physiological criteria also have been applied to the identification of different species of Acanthamoeba (10, 92, 189, 219, 462, 466, 467). However, many species share antigenic determinants. Therefore, results obtained through immunological approaches such as Western blotting and immunofluorescence have been inconclusive in identifying species. Isoenzyme electrophoresis of different enzyme systems also has been used to compare strains of Acanthamoeba (105, 112). Although this method has the potential to provide insight into relationships among species, results have indicated interstrain variation within species as well as similarities between strains of separate species. Furthermore, studies have shown that enzyme patterns change when isolates are grown under different laboratory conditions (199, 472).
FIG. 1.
Phylogenetic scheme of Acanthamoeba, Balamuthia, and Naegleria. Modified from references 91 and 378.
FIG. 2.
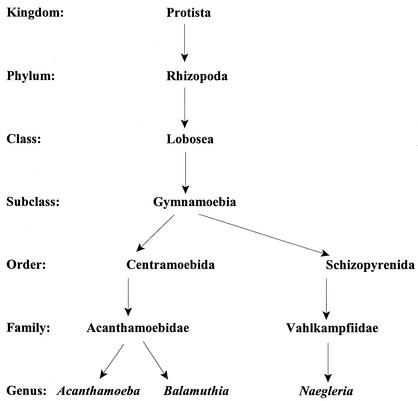
Scanning electron micrograph of an Acanthamoeba trophozoite. Spiny surface structures called acanthopodia (arrows) distinguish Acanthamoeba from other free-living amebae that infect humans, such as B. mandrillaris, N. fowleri, and Sappinia diploidea. Bar, 1 μm.
To address these potential confounds, methods for classification of Acanthamoeba species at the molecular level have been developed (10, 39, 42, 62, 83, 152, 209, 225, 231, 395). A number of laboratories have applied mitochondrial DNA restriction fragment length polymorphism (RFLP) analysis to cluster strains of Acanthamoeba (39, 154, 219, 483). However, Gast et al. (152) reported that although assessment of mitochondrial DNA was useful for typing Acanthamoeba isolates, an inherent drawback to this approach was the relatively large number of amebae required for analysis. Johnson et al. (209) used reverse transcription to determine the partial nucleotide sequences of small-subunit rRNAs of ameba isolates. They reported a high degree of 18S rRNA sequence diversity within the genus Acanthamoeba. However, subsequent DNA sequencing results from a number of laboratories have not confirmed these observations, and so conclusions regarding large sequence differences among Acanthamoeba strains appear unwarranted. As opposed to the RNA sequencing approach reported by Johnson et al. (209), Gast et al. (152) developed a classification scheme based on nuclear rRNA gene sequences (18S rDNA). The complete gene sequence of nuclear small ribosomal subunit RNA (Rns) was determined. Using this approach, Stothard et al. (434) classified 53 isolates of Acanthamoeba species on the basis of 12 rDNA sequence types (Rns genotypes) designated typing units T1 to T12 (Table 1). Additional sequence types may exist (151). Sequences of either nuclear (Rns) or mitochondrial (rns) rRNA genes are suitable for classifying isolates. Current classification schemes integrate the morphological groups which were established by Pussard and Pons (363) with the 12 sequence types (Rns genotypes) (T1 to T12) such that group I includes sequence types T7, T8, and T9, group II includes sequence types T3, T4, and T11, and group III includes sequence types T1, T2, T5, T6, T10, and T12. Studies in which clinical isolates have been identified based on sequence types have shown that the majority of strains causing keratitis belong to sequence type 4 (i.e., T4) (395, 434, 464). Chung et al. (83) used a riboprinting approach for subgenus classification of Acanthamoeba. Genomic DNA was extracted, and small-subunit rDNA was amplified by PCR and digested with restriction enzymes for analysis of RFLP. The resultant dendrogram based on riboprinting coincided with the grouping scheme of Pussard and Pons (363), which was based on morphological criteria, and with that of Stothard et al. (434), who examined 18S rDNA gene sequence variation (Table 1). In summary, comparison of results for classification of Acanthamoeba species obtained through DNA-based approaches with those based on morphological and biochemical criteria has revealed major inconsistencies. A revision of the taxonomy of the genus based on sequence comparisons is under way (42, 61).
TABLE 1.
Acanthamoeba spp. isolated from the environment and from humans
| Speciesa | Type strainb | Sequence typec | Groupd | Isolatione | Reference(s) |
|---|---|---|---|---|---|
| A. astronyxis | 30137 | T7 | I | Water from termite colony | 340, 366 |
| A. castellanii | 30011 | T4 | II | Yeast culture | 70, 119, 463 |
| A. commandoni | 30135 | T9 | I | Garden humus | 90, 362 |
| A. culbertsoni | 30171 | T10 | III | Monkey kidney cell culture | 99, 413 |
| A. divionensisi | 50238 | —f | II | Soil | 363 |
| A. echinulata | 50239 | — | I | Compost | 363 |
| A. griffini | 30731 | T3 | II | Seawater bottom sample | 389 |
| A. hatchetti | 30730 | T11 | II | Harbor sediment | 393 |
| A. healyi | CDC:1283:V013g | T12 | III | Brain tissue | 320 |
| A. jacobsi | 30732 | — | III | Marine sediment | 392 |
| A. lenticulata | 30841 | T5 | III | Swimming pool | 313 |
| A. lugdunensis | 50240 | T4 | II | Pool | 350, 363 |
| A. mauritaniensis | 50253 | T4 | II | Sewer sludge | 363 |
| A. palestinensis | 30870 | T2 | III | Soil | 340, 367 |
| A. pearcei | 50435 | T3 | I | Sewage sediments | 327 |
| A. polyphaga | CCAP1501/3Ah | T4 | II | Pond | 340, 361 |
| A. pustulosaj | 50252 (GE3a) | T2 | III | Pool | 112, 350, 363 |
| A. quina | 50241 | — | II | Swimming pool | 350, 363 |
| A. rhysodes | 30973 | T4 | II | Soil | 418 |
| A. royreba | 30884 | T4 | III | Human choriocarcinoma cells | 478 |
| A. stevensoni | 50438 | T11 | II | Shellfish beds | 391 |
| A. triangularis | 50254 | T4 | II | Human feces | 363 |
| A. tubiashi | 30867 | T8 | I | River water | 259 |
Identification of strains should be based on comparisons with type strains (395).
ATCC designation.
Genotypes Rns T1 to T12 based on Ohio State University Acanthamoeba nuclear small-subunit ribosomal DNA (rDNA). Available at www.biosci-ohiostate/∼tbyers/byers.htm.
Genera are divided into three morphological groups based on cyst size and shape (363).
Original isolation of type strain.
—, sequence type not yet determined (395).
CDC, Centers for Disease Control and Prevention.
CCAP, Culture Collection of Algae and Protozoa.
A. paradivonensis may be the same as A. divionensis.
A. pustulosa (A. palestinensis) 50252 GE3a.
BIOLOGY AND DISTRIBUTION OF ACANTHAMOEBA
Ecology and Distribution
Acanthamoeba spp. are among the most prevalent protozoa found in the environment (301, 340, 369, 370, 376). They are distributed worldwide and have been isolated from soil, dust, air, natural and treated water, seawater, swimming pools, sewage, sediments, air-conditioning units, domestic tap water, drinking water treatment plants, bottled water, dental treatment units, hospitals and dialysis units, eyewash stations, and contact lenses and lens cases and as contaminants in bacterial, yeast, and mammalian cell cultures (23, 68, 70, 111, 202, 228, 301, 305, 343, 372, 412, 437). Acanthamoeba spp. also have been isolated from vegetation, from animals including fish, amphibia, reptiles, and mammals (129, 266, 267, 465), from the nasal mucosa and throats of apparently healthy humans (75, 304, 330), from infected brain and lung tissue, from skin lesions of immunosuppressed patients, and from corneal tissue of patients with AK (111, 245, 286).
Life Cycle
The life cycle of Acanthamoeba consists of two stages: an actively feeding, dividing trophozoite and a dormant cyst (Fig. 3 and 4). The trophozoite varies in size from 25 to 40 μm and feeds on bacteria, algae, and yeast in the environment but also can exist axenically on nutrients in liquid taken up through pinocytosis (44, 47). Uptake of food by trophozoites can occur by pseudopod formation and phagocytosis or by food cup formation (Fig. 5) and ingestion of particulate matter. Food cups formed on the ameba surface are temporary structures used to ingest bacteria, yeast, or cells (351). Locomotion involves the formation of a hyaline pseudopodium and is sluggish in all species of Acanthamoeba (358). One species, A. castellanii, has been used extensively to study the molecular mechanisms of actin polymerization during ameboid locomotion (235, 356).
FIG. 3.
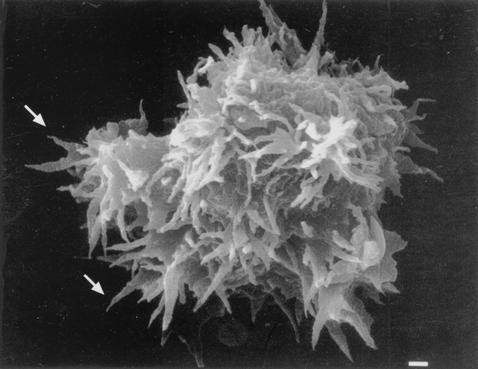
Light micrographs of cultures depicting life cycle stages of Acanthamoeba spp. (A and C) Unstained preparations of cultures of A. astronyxis trophozoites (A) and cysts (C). (B and D) H-&-E-stained preparations of A. castellanii trophozoites (B) and cysts (D). Bars, represent 50 μm (A and C) and 25 μm (B and D).
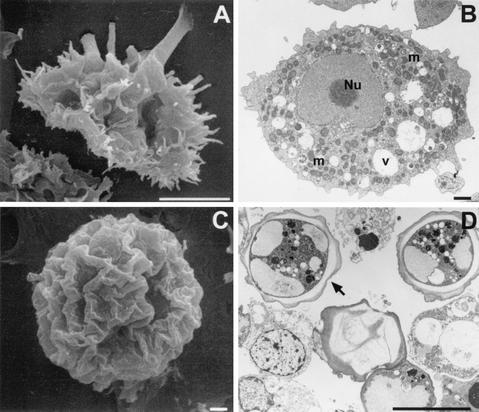
FIG. 4.
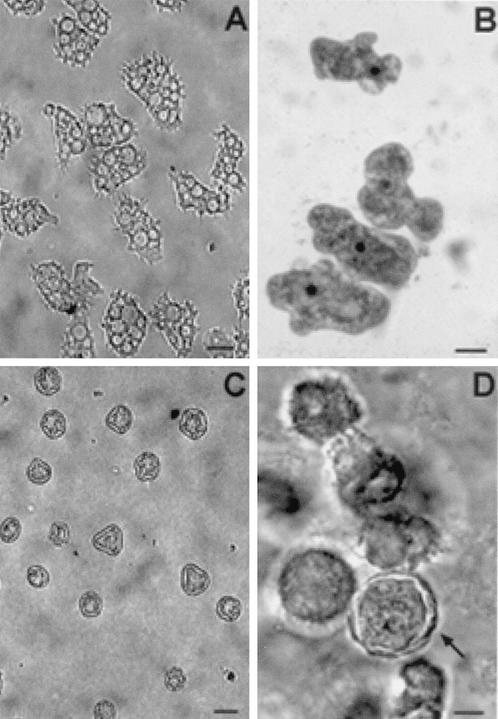
Scanning (A and C) and transmission (B and D) electron micrographs depicting the life cycle stages of Acanthamoeba spp. (A) A. polyphaga trophozoite; (B) trophozoite of A. castellanii showing the prominent central nucleolus (Nu), mitochondria (m), and cytoplasmic food vacuoles (v); (C) wrinkled cyst of A. polyphaga; (D) double-walled cyst of A. castellanii. Bars, 10 μm (A and D) and 1 μm (B and C).
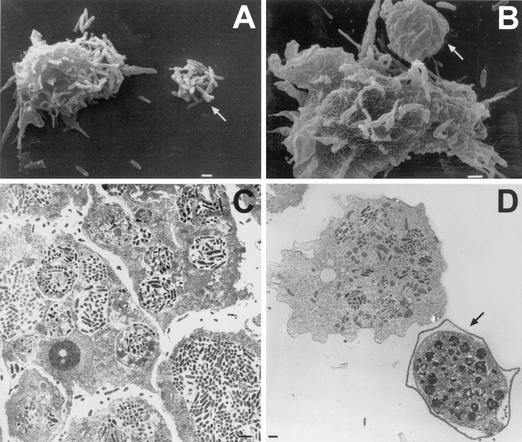
FIG. 5.
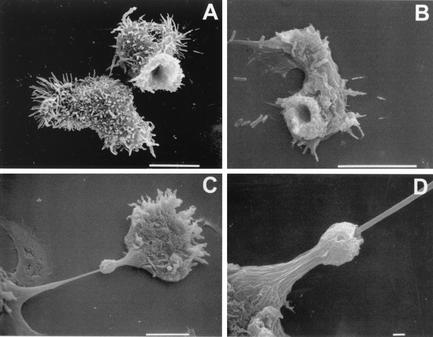
Scanning electron micrographs of trophozoites illustrating the presence of surface structures termed food cups. (A) Food cups present on the surface of a trophozoite of A. culbertsoni are temporary structures that form and reform for the intake of bacteria, yeast, or cellular debris. (B) Food cup present on the surface of A. astronyxis trophozoite used to ingest bacteria. (C) Food cup present on the surface of an A. castellanii trophozoite in the apparent process of ingesting a cultured nerve cell. (D) Higher magnification of the trophozoite in panel C to illustrate the food cup structure in the apparent process of ingestion. Bars, 10 μm (A to C), and 1 μm (D).
Morphology
The cellular organization of Acanthamoeba has been studied using electron microscopy (45, 46, 158, 379). Organelles typically found in higher eucaryotic cells have been identified in Acanthamoeba (Fig. 4). Bowers and Korn (45) indicated the presence of a Golgi complex, smooth and rough endoplasmic reticula, free ribosomes, digestive vacuoles, mitochondria, and microtubules in Acanthamoeba trophozoites. A trilaminar plasma membrane was found to surround the cytoplasmic contents of the trophozoite. In addition, distinguishing features of the trophozoite were the presence of spiny surface projections called acanthopodia (Fig. 2), a prominent contractile vacuole in the cytoplasm that controls the water content of the cell, and a nucleus with a large central nucleolus. Generally, the amebae are uninucleate, although multinucleated cells are common when Acanthamoeba are maintained in suspension culture. Reproduction occurs by binary fission (59, 340).
A double-walled wrinkled cyst (Fig. 4) composed of an ectocyst and an endocyst ranges in size from 13 to 20 μm and varies from species to species (46). Cyst formation occurs under adverse environmental conditions such as food deprivation, desiccation, and changes in temperature and pH (46, 60, 76). Villemez and coworkers (457, 484) have reported that antibody binding to a specific membrane protein also causes A. castellanii to encyst. Cysts are resistant to biocides, chlorination, and antibiotics (109, 217, 262, 448) and survive low temperatures (0 to 2°C) (55). Meisler et al. (300), however, have shown that treatment with Freon or methylene oxide or autoclaving destroys cysts. Excystment occurs when trophozoites emerge from the cyst under suitable environmental conditions. Mazur et al. (297) demonstrated that cysts retained viable amebae for over 24 years after storage in water at 4°C. Of 17 environmental isolates of A. polyphaga or A. castellanii maintained as cysts, 14 gave rise to trophozoites on inoculation on nonnutrient agar (NNA) containing bacteria. After the excystment process, amebae were tested for pathogenicity by intranasal inoculation of BALB/c mice. Fewer deaths were recorded for mice inoculated with amebae which had been encysted for 24 years than for mice inoculated with the same environmental isolates when tested initially (297). Thus, although virulence was shown to decline with the passage of time, the isolates retained pathogenicity for mice.
Because Acanthamoeba trophozoites can be induced to transform into cysts in nonnutrient media and excystation occurs under favorable conditions, the amebae have been used to study differentiation. Acanthamoeba has been used as a model system to study eucaryotic RNA transcription, RNA polymerase functions, and cellular differentiation in trophozoites and cysts (29, 63, 79, 190, 203, 338, 396). Paule and coworkers (346) have purified RNA polymerases from Acanthamoeba and have performed extensive studies on initiation and regulation of RNA transcription in Acanthamoeba (8, 194, 347, 348, 355, 364).
CULTURE METHODS FOR ACANTHAMOEBA
A variety of undefined liquid media, different mammalian cell types, and NNA seeded with bacteria support the growth of Acanthamoeba spp. Acanthamoeba can be grown on NNA (1.5%) containing a lawn of Escherichia coli (458). Acanthamoeba also can be grown axenically in PYG medium consisting of 2% proteose peptone, 0.2% yeast extract, and 0.1 M glucose (459) or in Oxoid medium (Cline medium) containing serum and hemin, which has been used to culture Naegleria spp. (268). Mammalian cells which support the growth of Acanthamoeba include African green monkey kidney (Vero), human embryonic lung (HEL), human embryonic kidney (HEK), HeLa, B103 rat neuroblastoma, and L929 fibroblasts (101, 110, 351). Conditions and medium formulations for the cultivation of pathogenic and opportunistic free-living amebae have been reviewed recently (399).
ACANTHAMOEBA SPP. AS OPPORTUNISTIC PATHOGENS
Granulomatous Amebic Encephalitis
GAE is a disease which is characterized by a chronic protracted slowly progressive CNS infection (Fig. 6) which also may involve the lungs (127, 284). Serological laboratory diagnosis has indicated that several species of Acanthamoeba are associated with GAE. Although the incubation period for Acanthamoeba infections is unknown, several weeks or months may be necessary to establish clinical signs. Table 2 summarizes differences in terms of disease, portal of entry, clinical signs, pathology, and diagnosis for free-living amebae causing human infections. GAE is generally associated with individuals who already have underlying diseases such as malignancies (133), systemic lupus erythematosus (163, 230), diabetes (172), renal failure, cirrhosis, tuberculosis, skin ulcers, human immunodeficiency virus (HIV) infection or Hodgkin's disease (201, 277, 286, 426, 477). Predisposing factors include alcoholism, drug abuse, steroid treatment, cancer chemotherapy, radiotherapy, and organ transplantation (14, 282, 408). Although enhanced susceptibility to infection is associated with immune suppression and debilitating conditions, cases of GAE caused by Acanthamoeba have been found in immunocompetent children and adults (35, 336, 368, 388, 419). The route of infection is thought to be by inhalation of amebae through the nasal passages and lungs or introduction through skin lesions. Access to the CNS may be by hematogenous spread from a primary site in the lungs or skin or directly through the olfactory neuroepithelium (282). Symptoms of CNS infection (Table 3) include headache, confusion, nausea, vomiting, fever, lethargy, stiff neck, focal neurologic deficits, or signs of increased intracranial pressure (280, 286). Pathological findings generally include severe hemorrhagic necrosis, fibrin thrombi, and inflammation. The cerebral hemispheres show moderate to severe edema. Multifocal lesions are present in the midbrain, brain stem, corpus callosum, and cerebellum. A chronic inflammatory exudate is observed over the cortex and is composed mainly of polymorphonuclear leucocytes and mononuclear cells. Severe angiitis with perivascular cuffing by lymphocytes is seen in some cases. In addition, numerous trophozoites can be identified within tissue (Fig. 7). However, other than these clinical and laboratory observations, there is a paucity of information concerning the pathogenesis of infection and the response of the host to infection, particularly as it involves the CNS. For example, in immunocompetent individuals, well developed granulomas form (Fig. 8) around the organisms, while in immunocompromised individuals, granuloma formation is weak or lacking (277) Furthermore, it is unknown whether severe necrosis of the brain is due to direct destruction of tissue by Acanthamoeba trophozoites or by induction of inflammatory cytokines such as interleukin-1 (IL-1) or tumor necrosis factor (TNF-α) or through the interactive action of both pathways (271). In addition, dissemination of amebae to other organs such as the liver, kidneys, trachea, and adrenals can occur in immunocompromised individuals (214, 277, 282, 322). Individuals with GAE also may have lung involvement. Trophozoites and cysts have been found in pulmonary alveoli from infected individuals, and pneumonitis is a characteristic feature (149, 178, 214, 278, 460). Acanthamoeba has also been recovered from ear infections (257) and from necrotic bone tissue of a patient with osteomyelitis of a bone graft of the mandible (43).
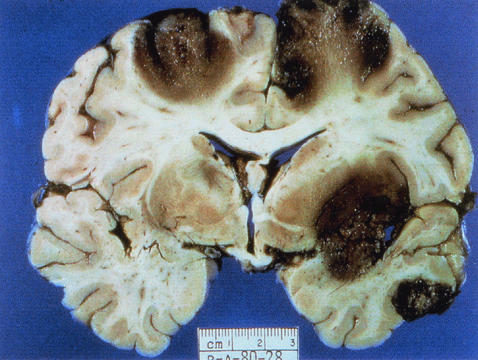
FIG. 6.
Coronal section of the cerebral hemispheres with cortical and subcortical necrosis from a fatal human case of GAE. (Courtesy of A. J. Martinez; reprinted from reference 277 with permission of the publisher.)
TABLE 2.
Differences among the free-living amebae causing disease in humansa
| Characteristic |
Acanthamoeba
|
Balamuthia |
Naegleria
|
||
|---|---|---|---|---|---|
| GAE | AK | Cutaneous lesions, sinusitis | GAE | PAME | |
| Portal of entry | Olfactory epithelium, respiratory tract, skin, sinuses | Corneal abrasion | Skin, sinuses, respiratory tract | Olfactory epithelium, skin, respiratory tract | Olfactory epithelium |
| Incubation Period | Weeks to months | Days | Weeks to months | Weeks to months | Days |
| Clinical signs | Confusion, headache, stiff neck, irritability | Blurred vision, photophobia, inflammation, corneal ring | Skin lesions, nodules, sinus lesions, sinusitis | Slurred speech, muscle weakness, headache, nausea, seizures | Headache, nausea, vomiting, confusion, fever, stiff neck |
| Pathology | Focal necrosis, granulomas | Ulceration of cornea | Granulomatous reaction in skin, inflammation | Multiple necrotic foci, inflammation, cerebral edema | Hemorrhagic necrosis |
| Diagnosis | Brain biopsy, CSF smear/wet prep, culture, IIFb of tissue, PCR | Corneal scrape, corneal biopsy, calcofluor white, culture of material, confocal microscopy | Skin lesion biopsy, culture, IIF of tissue | Brain biopsy, culture on mammalian cells, IIF of tissue | Brain biopsy, CSF wet prep, CSF culture, IIF of tissue, PCR |
TABLE 3.
Signs and symptoms of Acanthamoeba infectionsa
| Symptoms of infection of: | |
|---|---|
| CNS | Eyes (AK) |
| Headache | Eyelid ptosis |
| Mental status abnormalities | Conjunctival hyperemia |
| Seizures | Photophobia |
| Stiff neck | Watering (tearing) |
| Irritability | Blurred vision |
| Nausea and vomiting | Ocular pain |
| Hemiparesis | Corneal ring |
| Cranial nerve palsies | Perineural infiltrates |
| Hallucinations | Opacities |
| Gait ataxis | Loose corneal epithelium |
| Diplopia | Irritation |
| Photophobia | |
| Sleep disturbances | |
| Anorexia | |
| Babinski's sign | |
| Kernig's sign | |
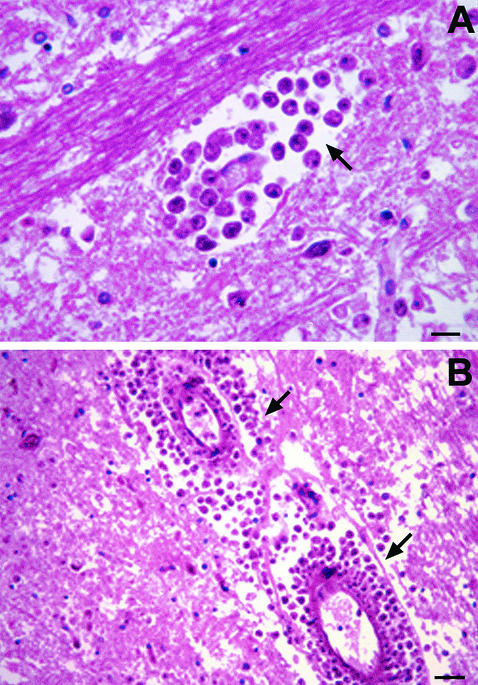
FIG. 7.
H & E stain of brain tissue from a human with GAE. (A). Numerous trophozoites can be identified within the tissue (arrow). (B) Trophozoites identified within vascular walls (arrow). Bars, 150 μm (A) and 300 μm (B). Photographs courtesy of A. J. Martinez.
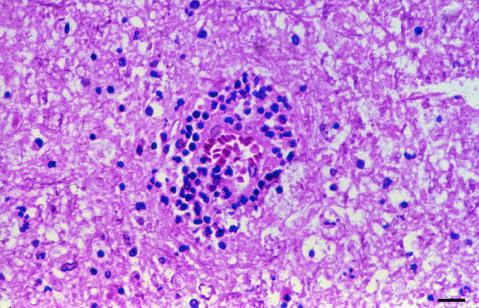
FIG. 8.
H-&-E-stained section of paraffin-embedded brain tissue demonstrating granuloma formation in Acanthamoeba infection. Bar, 200 μm.
Infections in Patients with AIDS
The first reported case of Acanthamoeba infection in a patient with AIDS was in 1986 (157). Since then, an increasing number of cases of disseminated Acanthamoeba infections have been reported in individuals with AIDS (40, 65, 69, 77, 125, 128, 143, 149, 159, 173, 178, 192, 214, 224, 258, 295, 307, 322, 373, 375, 380, 406, 407, 420, 438, 442, 446, 474) (see Table 4). Most of these have been diagnosed postmortem (65, 143, 322, 407, 420, 474). It has been postulated that impairment of host defense mechanisms in immunocompromised individuals results in, or contributes to, infection which can spread from the primary site of infection to other organs and tissues. In immunosuppressed individuals, a well-developed granulomatous reaction may not occur. Such individuals usually exhibit advanced HIV disease with a low CD4 + T cell count (less than 200/mm 3) at the time of infection with Acanthamoeba (173). The clinical course can be fulminant, with rapid progression to death. Most patients die in less than 1 month after onset of neurological symptoms (65, 149, 159, 406, 438, 474). While cerebrospinal fluid (CSF) lymphocytosis is observed in non-AIDS Acanthamoeba-infected individuals, CSF may be devoid of cells in HIV-positive patients (149). Other prevalent manifestations of Acanthamoeba infections in HIV-positive individuals are chronic sinusitis, otitis (115, 224, 373, 442), and cutaneous lesions with Acanthamoeba organisms present in sinus lesions and skin ulcers (40, 69, 128, 157, 224, 442, 446). The nasal passage is thought to be the portal of entry for Acanthamoeba, although skin lesions may serve as the primary site of infection. Indeed, skin lesions are most often the presenting manifestation of Acanthamoeba infection in AIDS patients (322, 438). Also, there have been reports of separate cases of leukocytoclastic vasculitis, amebic osteomyelitis, and endophthalmitis in AIDS patients with Acanthamoeba infections (178, 179, 407). Because other opportunistic infections occur in AIDS patients, those due to Acanthamoeba are often overlooked. In fact, patients with CNS symptoms have been diagnosed empirically with toxoplasmosis, although serological testing for Toxoplasma may be negative (149, 159, 173, 406). Other patients have been misdiagnosed with CNS vasculitis (159), squamous cell carcinoma (373), or bacterial meningitis (173). Acanthamoeba in tissues also has been identified incorrectly as macrophages or fungi (69, 157, 214, 258, 271, 322, 337, 420, 438, 474).
TABLE 4.
Treatment regimens and outcomes of Acanthamoeba infections
| Patient no. | Underlying disease | Amebic disease | Therapya | Outcome | Reference |
|---|---|---|---|---|---|
| 1 | AIDS | Sinusitis | Rifampin/KC | Died/septicemia | 157 |
| 2 | AIDS | Cutaneous | TMP/AmpB/clin/gentamicin | Died/GAE | 474 |
| 3 | AIDS | GAE | Pyrimethamine + flucon | Died/GAE | 149 |
| 4 | AIDS | Otitis | Chloramphenicol/AmpB/ceftizoxime | Died/GAE | 114 |
| 5 | Connective tissue | Cutaneous/vasculitis | Ampicillin/chloramphenicol/AmpB/ adenine arabinoside | Died/vasculitis | 159 |
| 6 | AIDS | GAE | Pyrimethamine/sulfadiazine | Died/GAE | 159 |
| 7 | AIDS | GAE | Pyrimethamine/sulfadiazine | Died GAE | 159 |
| 8 | AIDS | Sinusitis/cutaneous | KC/flucon + sulfadiazine | Died/GAE | 143 |
| 9 | AIDS | Cutaneous | AmpB + broad-spectrum antibiotics | Died/GAE | 438 |
| 10 | AIDS | Cutaneous | Flucon + sulfadiazine | Died/GAE? | 438 |
| 11 | AIDS | Cutaneous/sinusitis | Ketoconazole + IV FC | Resolution of lesions | 179 |
| No CNS involvement | |||||
| 12 | AIDS | Cutaneous lesions | AmpB + FC + flucon | Died GAE? | 420 |
| 13 | AIDS | Cutaneous lesions | KC + FC | Lesions resolved/died inanition | 420 |
| 14 | Renal transplant | Cutaneous lesions, IgA deficiency | Pentamidine + chlorhexidine + 2% KC cream; maintenance on oral IT | Lesions resolved, no CNS involvement | 421 |
| 15 | AIDS | Sinusitis + cutaneous | FC alone | Died | 322 |
| 16 | AIDS | Cutaneous + CNS | KC/pentamidine | No response | 322 |
| Oral FC + IV pentamidine | Skin lesions improved-Died GAE | 322 | |||
| 17 | AIDS | Sinusitis + cutaneous | Pentam, KC, flucon, IT + metron + AmpB | Died | 322 |
| 18 | AIDS | Cutaneous/sinusitis | AmpB + rifamp + FC + IT + pentam | Multidrug toxicity, died | 192 |
| 19 | AIDS | Endophthalmic + cutaneous | 5FC + pentam/propam/clotrim/neomycin eye drops | No response | 178 |
| 20 | AIDS | Cutaneous/sinusitis/otitis | FC | Minimal response | 128 |
| 21 | AIDS | Cutaneous | Oral IT + FC | Died | 77 |
| 22 | AIDS | Cutaneous | IV pentam + oral KC + flucon | Died | 77 |
| 23 | AIDS | Cutaneous | Prednisone + clin + TMP/SMX + flucon | Died GAE | 173 |
| 24 | AIDS | Cutaneous | TMP/SMX + flucon + rifabutin + pyrimethamine + sulfadiazine | Died GAE | 173 |
| 25 | AIDS | Cutaneous | Flucon + pentam + IT | ||
| Amebic osteomyelitis | FC and pentam, extended therapy for over 1 yr | 407 | |||
| 26 | AIDS | Cutaneous | Pentam + IT + chlorhexidine + topical KC | Pentamidine toxicity, Died sepsis | 307 |
| 27 | Lung transplant | Cutaneous | AmpB + IC + pentam + FC + chlorhex/KC cream + azithro + FC + pentam, maintenance FC + clarithromycin | Survived | 337 |
| 28 | AIDS | Cutaneous | 5FC + oral IT | Died, inanition | 69 |
| 29 | AIDS | Sinusitis | IV pentam + oral IT + IV metron | Died/GAE | 224 |
| 30 | AIDS | CNS lesion | Sulfadiazine + pyrimethamine, flucon + sulfadiazine; one localized brain lesion excised | Survived | 406 |
| 31 | HIV positive | Sinusitis | Debridement of sinuses, IT + gentamicin nasal wash | Resolution of lesions | 442 |
| 32 | AIDS | Sinusitis/cutaneous | Debridement of sinuses + pentam + topical chlorhexidine + 2% KC; pentam toxicity, maintenance on FC | Survived | 442 |
| 33 | AIDS | Cutaneous | Pentam + flucon + azithromycin + topical econazole + chlorhexidine | Died | 446 |
| 34 | AIDS | Sinusitis/cutaneous | AmpB + pentam IV + FC + itraconazole + 2% KC cream; pentam toxicity, oral FC, oral IC + KC cream | Skin lesions healed, died of septicemic shock | 258 |
| 35 | CNS | Immunocompetent | TMP/SMZ + KC + rifampin | Survived | 419 |
| 36 | CNS | Immunocompetent | TMP/SMZ + KC + rifampin | Survived | 419 |
| 37 | HIV positive | Sinusitis/cutaneous, lobular panniculitis | Surgical debridement + IT, azithromycin, 5FC, rifampin | Survived | 380 |
| 38 | Lung transplant | Cutaneous | Pentam + IC + KC + chlorhexidine | Died | 449 |
| 39 | AIDS | Rhinosinusitis | Removal of all diseased nasal mucosa, pentam, levofloxacin, FC, AmpB | Survived | 373 |
FC, fluorocytosine; IT, itraconazole; azithro, azithromycin; AmpB, amphotericin B; KC, ketoconazole; TMP/SMZ, trimethoprim-sulfamethoxazole; pentam, pentamidine; flucon, fluconazole; metron, metronidazole; chlorhex, chlorhexidine; IV, intravenous; clin, clindamycin.
Cutaneous Acanthamebiasis
Cutaneous infections caused by Acanthamoeba are most common in patients with AIDS, with or without CNS involvement (69, 125, 179, 322). Cutaneous disease has also been documented for non-HIV-infected patients with amebic encephalitis, for patients undergoing immunosuppressive therapy for organ transplantation (421, 449), or for individuals with immunological diseases (159, 277, 337, 421, 460). The cutaneous form of the disease is characterized by the presence of hard erythematous nodules or skin ulcers (40, 77, 143, 157, 179, 258, 295, 322, 380, 438, 446). Early manifestations of the cutaneous form of acanthamoebiasis include the presence of firm papulonodules that drain purulent material and then develop into nonhealing indurated ulcerations (295, 380). Occurrence of disseminated skin lesions may be the presenting manifestation of Acanthamoeba infection (322, 438). Whether skin lesions represent a primary focus of infection or are the result of hematogenous dissemination from other sites such as the respiratory tract, sinuses, or the CNS is not known (143). The reported mortality rate from cutaneous infection for individuals without CNS involvement is approximately 73%, while that from cutaneous infection accompanied by CNS disease is 100% (446).
Histologic examination of cutaneous lesions generally shows foci of necrosis surrounded by inflammatory cells, vasculitis, trophozoite and cyst forms (143). However, the histologic appearance of skin lesions may mimic that of fungi, viruses, mycobacteria, or inflammation due to a foreign body (77, 159, 419). Organisms in tissue sections have been mistaken for yeast forms of Blastomyces dermatiditis (438), sporangia of Rhinosporidium seeberi, Cryptococcus neoformans, or Prototheca wickerhamii (420). Also, cases of cutaneous acanthamebiasis have been misdiagnosed as bacillary angiomatosis (214), cat scratch fever (420), Penicillium marneffei infection (69), Kaposi's sarcoma (77) or cells with cytomegalovirus inclusions (442). Thus, when a single biopsy specimen does not reveal trophozoites when Acanthamoeba is suspected, the examination should be repeated with a different specimen (307, 337).
Therapeutic options for treatment of cutaneous acanthamebiasis are not clearly established. Patients given combination treatments have shown improvement, but the majority have died (128, 192, 420). Therapy is less successful when CNS involvement occurs. However, successful treatments of cutaneous acanthamebiasis using itraconazole, pentamidine, 5-fluocytosine, and topical chlorhexidine gluconate and ketoconazole cream have been reported (179, 421).
ACANTHAMOEBA SPP. AS NONOPPORTUNISTIC PATHOGENS
Amebic Keratitis
AK, first reported by Nagington et al. (325) in Great Britain and by Jones et al. (210) in the United States, is a painful progressive sight-threatening corneal disease (Fig. 9). Several species of Acanthamoeba, including A. castellanii, A. polyphaga, A. hatchetti, A. culbertsoni, A. rhysodes, A. griffini, A. quina, and A. lugdunensis, have been reported to cause AK (17, 315, 316, 394). Unlike debilitated patients with GAE or cutaneous acanthamebiasis, individuals with AK generally are immunocompetent. Nevertheless, these individuals do not develop protective immunity, and reinfection can occur (334). In the mid-1980s, an epidemic of AK occurred which was attributed to the increased use of contact lenses and poor lens hygiene (403, 404). Acanthamoeba organisms have been cultured from lens cases and saline cleaning solutions. It is now recognized that the wearing of contact lenses is the leading risk factor for AK (116, 196, 425). Conditions which promote disease include the use of home-made saline solutions, poor contact lens hygiene, and corneal abrasions (16, 117, 195, 333, 409). Corneal trauma due to injury by a foreign body and exposure to contaminated water also may be associated with Acanthamoeba infection. Disease symptoms (Table 3) include redness, tearing, photophobia, and lid edema. The histopathologic picture of AK varies (237, 306). Initially, amebae are restricted to the corneal epithelium, but as the disease progresses, they invade the underlying stroma, cause extensive damage, and provoke mild to severe inflammation. The most characteristic clinical feature of AK is the presence of a ring-like stromal infiltrate, thought to be composed of infiltrating inflammatory cells such as neutrophils. Clinically, conjunctival hyperemia, corneal inflammation, episcleritis, and scleritis occur. Trophozoites can infiltrate corneal nerves, causing neuritis and necrosis (150, 288). In rare circumstances, Acanthamoeba can spread from the cornea to the retina, causing chorioretinitis (208, 319). In severe cases which do not respond to medical and surgical therapy, enucleation of the eye may be required (118). An in-depth discussion of the clinical progression of AK has been presented by Illingworth and Cook (195).
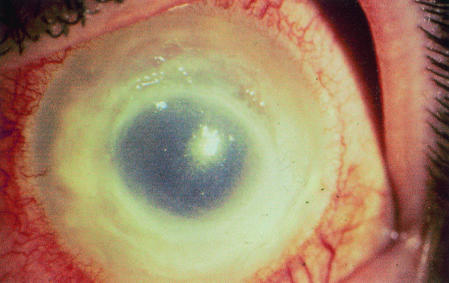
FIG. 9.
Stromal infiltrate in AK. Photograph of a human with AK provided by P. C. Maudgal, Katholieke Universiteit Leuven, Leuven, Belgium.
AK can be difficult to diagnose and treat. It has been diagnosed mistakenly as atypical herpes simplex keratitis or fungal keratitis (294, 394, 440). Diagnosis is complicated by the frequent occurrence of secondary bacterial infections. Antibacterial, antiviral, antifungal, or corticosteroid treatment may complicate the diagnosis because there is initial improvement following application of these therapeutic approaches which is followed by a worsening of the disease. Proper diagnosis is important for early treatment, since amebic cysts which form in tissues are resistant to many drugs.
Since contaminated contact lens cases and cleaning solutions are a source of infection, decontamination of lens cases and storage solutions is essential for preventing Acanthamoeba infection. A 3% solution of hydrogen peroxide has been used for disinfection of contact lenses and lens cases because it has been shown to be active against cysts and trophozoites (191). Hiti et al. (182) evaluated commercially available contact lens storage solutions for amebicidal activity. It was indicated that a two-step hydrogen peroxide system (0.6% H2O2) was an effective disinfectant after an 8-h soaking of contact lenses and lens cases. However, two of the three strains used to test cysticidal activity were not those typically associated with AK. Zanetti et al. (486) demonstrated that a 1:2 dilution of a 3% hydrogen peroxide solution killed cysts of A. castellani after a 9-h exposure. It has been recommended that a two-step hydrogen peroxide system be used at concentrations of 3% rather than 0.6% since bacteria present in lens cases produce catalase, which neutralizes the peroxide (30). Microwave irradiation also has been reported to effectively kill Acanthamoeba spp. after 3 min of treatment (181). Pinna (354) recommended the use of “1-day” disposable contact lenses to reduce the risk of keratitis.
DIAGNOSIS OF ACANTHAMOEBA INFECTIONS
Granulomatous Amebic Encephalitis
For patients who present with CNS symptoms, diagnosis of Acanthamoeba can include direct microscopy of wet mounts of CSF or stained smears of CSF sediment (64, 88, 245, 419). However, while assessment of CSF may be of value in the diagnosis of GAE, lumbar puncture may be contraindicated because of increased intracranial pressure (265, 286). CSF is centrifuged at low speed (250 × g) for 10 min to avoid rupture of trophozoites (265). Trophozoites may be observed in wet preparations of CSF but may be unrecognized because they resemble macrophages. CSF sediment can be smeared on a glass slide, fixed in methanol, and stained with Giemsa-Wright stain. Pleocytosis with abundant lymphocytes and polymorphonuclear leukocytes and low glucose levels with high protein levels are suggestive of GAE. CSF or bronchoalveolar lavage fluid cytospin preparations also have been used to identify amebae in patients with GAE or respiratory illnesses (31, 330). However, certain characteristic features of Acanthamoeba trophozoites such as a prominent nucleolus, contractile vacuole, and cytoplasmic vacuoles may be visualized more readily using trichrome or hematoxylin and eosin (H & E) stains on fixed preparations after cytocentrifugation rather than using air-dried preparations (330).
Computed tomography and magnetic resonance imaging have been performed on some patients (149, 286). These procedures have shown that while enhancing lesions are present in many individuals, nonenhancing lesions are present in others. Computed tomography or magnetic resonance imaging of the brain may reveal multifocal areas of signal intensity or discrete lesions suggestive of abscesses or brain tumors (218, 291, 397, 408). However, although imaging analysis reveals CNS abnormalities, it does not provide a definitive diagnosis of GAE. In fact, amebic meningoencephalitis has been misdiagnosed as neurocysticercosis based on neuroimaging findings (291).
Methods for in vitro cultivation of Acanthamoeba also have been used for laboratory diagnosis. CSF, brain tissue, or material from cutaneous or sinus lesions can be inoculated into ameba growth medium, onto NNA plates containing a layer of Escherichia coli or Enterobacter aerogenes cells, or onto monolayers of cultured mammalian cells (285). Clear plaques are observed after a week of growth where amebae have ingested bacteria or cells. However, isolation of Acanthamoeba from CSF or infected tissues is difficult because many of the amebae may be encysted (222).
In addition, histological diagnosis can be made on the basis of frozen or paraffin-embedded sections of brain or cutaneous lesion biopsy material stained with H & E (Fig. 7) (286, 380, 406, 442). Although Gram, Giemsa, and H & E staining are not differential, macrophages and other immune cells can be distinguished from ameba trophozoites based on nuclear morphology. The nuclear structure of Acanthamoeba is characterized by a pronounced karyosome and surrounding “halo,” which is completely unlike that for any inflammatory cell. However, several staining methods have proved useful for identification of cysts in tissue sections. Periodic acid-Schiff stains the cyst wall red, while Gomori-methenamine silver stains the cyst black. In addition, calcofluor white (Fig. 10) has been used to identify cysts in brain tissue (414). Microscopic findings in infected tissue stained with H & E generally reveal granulomas (Fig. 8) with multinucleated giant cells, but these may be absent in immunosuppressed individuals. H-&-E-stained tissues (Fig. 7) reveal focal necrosis and amebic trophozoites and cysts throughout lesions which are located primarily in perivascular spaces and invading blood vessels (279).
FIG. 10.
Calcofluor white fluorescent staining of a mouse brain section to identify trophozoites and cysts in infected tissues. Calcofluor white has been used for the identification of cysts (arrow) in cases of AK and can be used to identify cysts in brain tissue or cutaneous lesions. Bar, 50 μm.
Immunofluorescent or immunoperoxidase cytochemical staining of cryostat sections or infected tissues embedded in paraffin, as well as transmission electron microscopy of infected tissues, has been employed for identification of Acanthamoeba with greater success (Fig. 11) (265, 428, 461, 476). Use of specific antibodies to different species of Acanthamoeba in conjunction with immunofluorescent staining has allowed the identification of amebae in tissue sections. However, while organisms can be identified as members of the genus Acanthamoeba, discrimination of distinctive species is difficult since many species are antigenically related (285).
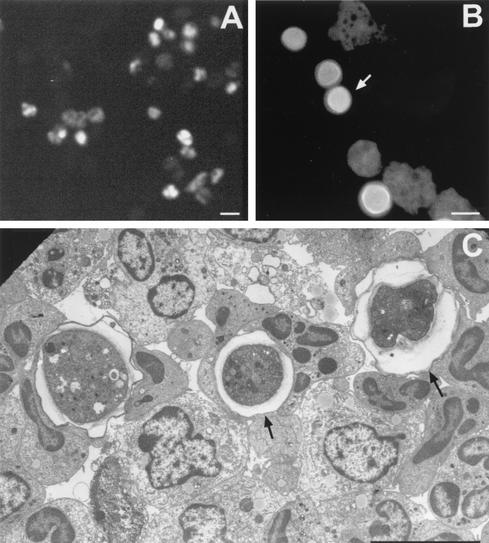
FIG. 11.
Micrographs illustrating diagnostic methods used to identify Acanthamoeba in infected tissues. (A) Immunofluorescence of trophozoites using anti-ameba antibodies; (B) calcofluor white staining of trophozoites and cysts (arrow) of Acanthamoeba. (C) Electron micrograph of material from infected mice. Electron microscopy has been used to identify cysts (arrows) and trophozoites more readily in infected tissues. Bars, 50 μm (A), 25 μm (B) and 10 μm (C).
Amebic Keratitis
Early detection and diagnosis is critical to the outcome of the clinical course of AK infections. A diagnosis of AK should be considered when chronic corneal ulcers are unresponsive to antibiotic therapy. However, ocular infections with Acanthamoeba are difficult to diagnose because they can resemble those due to herpes simplex virus, Pseudomonas aeruginosa, or fungal infection. As a result, there is often a significant delay in formulating an appropriate diagnosis before treatment is started. Corneal or conjunctival swabs are generally not suitable for isolation of Acanthamoeba (482). Corneal scrapes or corneal biopsy specimens are used for culture or for identification of cysts or trophozoites in stained tissue sections (108). For culture, material from a corneal scrape can be placed onto nonnutrient agar containing E. coli or inoculated into liquid medium (174, 220). However, corneal scrapes may contain bacteria or yeast, which can confuse the diagnosis (16, 394). Acanthamoeba has been cultured from contact lenses, lens cases, and lens-cleaning solutions when cultured corneal tissues were negative (15, 196, 314). A positive culture of the lens case or cleaning solution does not confirm the diagnosis but suggests infection with Acanthamoeba (329). In a retrospective study in which records were examined for amebae in clinical specimens, Acanthamoeba were recovered from approximately 73% of clinical specimens inoculated onto commercially available buffered charcoal-yeast extract agar, 71% of specimens inoculated onto NNA with E. coli, and 70% of specimens inoculated onto Trypticase soy agar containing horse or sheep blood (349). Other investigators have reported less satisfactory results using corneal scrapings to culture Acanthamoeba (196, 290, 440). NNA plates seeded with E. coli and inoculated with corneal specimens should be incubated at 28 to 35°C and held for an extended interval (10 days or more to ensure time for excystment) because some species of Acanthamoeba do not grow well at 35°C or above (222). Corneal biopsy has been suggested when repeated cultures of corneal scrapings are negative (16, 17). For cytological diagnosis, various staining methods can be employed. The indirect immunofluorescent-antibody assay (Fig. 11A) has been used to detect amebae in corneal scrapings or in biopsy tissue (131). Calcofluor white, a chemofluorescent dye with an affinity for the polysaccharide polymers of amebic cysts, has been used to identify amebic cysts in corneal tissue (475). Calcofluor white stains amebic cyst walls bright apple green, and this effect can be enhanced by prolonging the staining period (Fig. 11B). Evans blue is used to counterstain the background (475). Trophozoites and cysts in paraffin-embedded tissues can also be rapidly and differentially stained with calcofluor white (273, 414). In addition, acridine orange staining of corneal scrapings or CSF has been recommended as a simple and reliable method for rapid histological diagnosis of AK or GAE (100, 167).
In addition to various staining methods, the usefulness of PCR for detection of Acanthamoeba has been demonstrated, although a number of probes which have been developed are not species specific (189, 216, 223, 231, 256, 289, 462). A procedure based on application of a nonradioactive DNA probe prepared from a variable region of cloned 26S rDNA in concert with PCR has been used for the specific detection of Acanthamoeba. Using this technique, as few as 10 Acanthamoeba cells could be detected (243). In addition, a method which employs a PCR primer pair which produces an amplimer that is specific for the genus Acanthamoeba has been used for detection of Acanthamoeba in environmental samples and corneal scrapings from AK patients (129, 251, 395, 434). More recently, a promising novel means of identification of Acanthamoeba in clinical specimens was reported which consists of fluorescence in situ hybridization using a genus-specific probe or a sequence type 4 (T4)-specific probe to enhance the detection of Acanthamoeba (433). In this procedure, the fluorescein-labeled 22-mer genus-specific probe hybridizes specifically to all Acanthamoeba 18S rDNA sequence types but does not react with Balamuthia mandrillaris or Hartmanella vermiformis. To date, a T4 probe has been used because most species which have been identified as associated with AK belong to sequence type T4. Results can be obtained in 1 to 2 days without the need for culturing the organisms, which could take 1 to 2 weeks or longer. However, when negative results are obtained with corneal scrapings by fluorescence in situ hybridization, it has been recommended that these results be confirmed by culturing the corneal sample (433). Additionally, Lehmann et al. (256) have reported the use of a PCR assay to detect Acanthamoeba DNA in corneal and tear specimens containing as few as one to five amebae. These investigators suggested that a PCR assay of tears not only could serve as a diagnostic tool but also could be used to monitor the response to treatment.
A variety of molecular methods of identification of Acanthamoeba in samples have been employed. These consist of analysis of DNA sequence variation through RFLPs of complete or partial nuclear 18S rRNA gene sequences (83, 225, 231, 234, 437), variation in complete mitochondrial 16S rRNA (83, 231) or the complete mitochondrial genome (62, 154, 219, 234, 483, 483), analysis of DNA sequences of complete or partial DNA fragments coding for 18S rRNA (129, 256, 434, 466), and randomly amplified polymorphic DNA analysis of whole-cell DNA (10). Immunodiagnostic probes for identification of Acanthamoeba also have been employed. A bacteriophage antibody display library has been used to isolate antibody fragments that bind specifically to Acanthamoeba spp. in specimens by immunofluorescence or flow cytometry (215). Finally, several investigators have reported the use of tandem scanning confocal microscopy, a noninvasive technique, for in vivo diagnosis of AK. Corneal examination by scanning confocal microscopy has been associated with an increase in the detection of Acanthamoeba (6, 80, 290, 352). The confocal microscope allows the visualization of high-contrast images of coronal corneal sections containing trophozoites or cysts on a video monitor. Thus, a number of rapid techniques are now available for the detection of Acanthamoeba in tissues. These methods should be considered when formulating a laboratory diagnosis so that treatment can be started as soon as possible.
Cutaneous Acanthamoebiasis
Material from cutaneous lesions can be inoculated into ameba growth medium, onto NNA plates containing Escherichia coli or Enterobacter aerogenes, or onto mammalian cell culture monolayers and assessed for ameba growth as described for the laboratory diagnosis of GAE. Histological diagnosis of cutaneous lesion biopsy material can be performed using H & E, periodic acid-Schiff, or calcofluor white staining procedures. In addition, DNA-based molecular methods such as RFLP and randomly amplified polymorphic DNA analysis, as well as immunocytochemical approaches, can be applied.
TREATMENT OF ACANTHAMOEBA INFECTIONS
Disseminated Acanthamoeba Infections
A number of therapeutic agents and plant extracts have been tested in vitro for amebicidal activity against pathogenic Acanthamoeba spp. (Table 4). However, conflicting results have been reported. Ketoconazole, pentamidine, hydroxystilbamidine, paromomycin, 5-fluorocytosine, polymyxin, sulfadiazine, trimethoprim-sulfamethoxazole, azithromycin, and extracts of medicinal plants have been indicated as being active against Acanthamoeba in vitro, but no direct evidence has been obtained that these agents are efficacious in individuals with GAE (49, 67, 82, 126, 339, 377, 398, 400, 401, 429, 444). Therapeutic agents have also been tested in experimental animals. In mice, sulfadiazine, rifampin and flucytosine are effective against Acanthamoeba if administered before or within 24 h of exposure (107, 383).
In human infections, combination therapies have proven more successful than single-drug therapies because many drugs exhibit amebostatic but not amebicidal activity. No single drug has yet been shown to be effective against both the trophozoite and cyst stages of Acanthamoeba. Furthermore, Acanthamoeba infections are not readily recognized by clinicians or pathologists because many patients present with underlying disease (269). Depending on the immune status of the host, infection with Acanthamoeba spp. can result in dissemination to the skin, lungs, CNS, and other organs. Therefore, increased awareness of the potential for infection with Acanthamoeba is important for early detection and treatment. Early treatment is important because most patients with disease disseminated to the CNS die. In early reports of amebic encephalitis, corticosteroid therapy was instituted because of cerebral edema and inflammation (164, 422). However, it has been suggested that steroids exacerbate Acanthamoeba infection and should not be used (96, 274, 276, 283, 298).
Reports of successful treatment of Acanthamoeba infection have been few (Table 4). Ketoconazole and rifampin added to trimethroprim-sulfamethoxazole therapy was used successfully for treatment of two immunocompetent pediatric patients with CNS infection (419). Slater et al. (421) reported successful treatment of disseminated Acanthamoeba infection in a renal transplant patient who was HIV negative. Therapy consisted of a 4-week course of IV pentamidine isethionate, topical chlorhexidine gluconate, and 2% ketoconazole cream. The success of this therapeutic regimen was attributed to early treatment before onset of CNS infection. Resolution of cutaneous lesions and sinusitis with 5-fluorocytosine treatment in a patient with HIV infection without CNS involvement has also been reported (179). Seijo et al. (406) reported a successful outcome in an HIV-positive individual with CNS involvement. Surgical removal of one localized CNS lesion followed by therapy with fluconzole and sulfadiazine resulted in effective treatment and survival of the patient. Teknos et al. (442) suggested the use of surgical debridement of nasal and paranasal sinuses as soon as possible after identification of organisms in nasal tissue, followed by long-term therapy. These investigators recommended 5-fluorocytosine for CNS infection and for renal transplant patients rather than pentamidine because of the nephrotoxicity of the latter compound and the fact that it does not cross the blood brain barrier. Nephrotoxicity caused by pentamidine isethionate treatment has been reported in other patients with Acanthamoeba infection (307, 449). Rivera and Padhya (373) successfully treated an AIDS patient with rhinosinusitis by surgical removal of all diseased areas of the nasal mucosa followed by prolonged therapy with pentamidine, amphotericin B, flucytosine, rifampin, itraconazole, and chlorhexidine.
Treatment with multidrug regimens for humans with disseminated Acanthamoeba infections have met with mixed results. An HIV-positive patient with recurrent sinusitis and cutaneous lesions was stabilized after 7 weeks of treatment with itraconazole, azithromycin, 5-fluorocytosine and rifampin (380). Multidrug therapy was used successfully in a lung transplant recipient without CNS involvement (337). On the other hand, multidrug treatment regimens have proven less successful for some patients because of the induction of multidrug toxicity. Levine et al. (258) attempted multidrug therapy in an HIV-positive patient with cutaneous acanthamoebiasis. Use of a combination of amphotericin B, rifampin, and 5-fluorocytosine resulted in initial improvement but the patient died of gram-negative bacterial septicemia. Hunt et al. (192) treated lesions with a combination of amphotericin B, rifampin, and 5-fluorocytosine, followed by itraconazole, rifampin, and 5-fluorocytosine after recurrence of disease. However, treatment was not successful and the patient died. While many patients respond to initial treatment, a number of these die of related illnesses (69, 128, 258, 307, 322). Thus, to date, the collective data which have been obtained indicate that effective treatment is predicated on early diagnosis and initiation of treatment before dissemination of amebae to the CNS.
Cutaneous Acanthamoebiasis
In patients who are immunocompromised because of HIV infection or patients undergoing immunosuppressive treatment for organ transplantation, cutaneous Acanthamoeba infections with dissemination to other organs has been reported (337). Patients with cutaneous acanthamoebiasis have been treated with various drugs. Helton et al. (179) reported a successful treatment outcome using 40 mg of 5-fluorocytosine per kg for 2 weeks in an AIDS patient with cutaneous and sinus lesions. Therapy with intravenous pentamidine and itraconazole along with topical ketoconazole and chlorhexidine was reported by Van Hamme et al. (449) to be ineffective in a lung transplant patient, who developed cutaneous lesions and died. Treatment with pentamidine, ketoconazole, fluconazole, itraconazole, metronidazole, and amphotericin B consecutively did not resolve lesions in an HIV-positive patient with sinusitis and cutaneous lesions. Cardiac toxicity due to pentamidine treatment was noted in another patient with cutaneous disease (258).
Amebic Keratitis
Because diagnosis is difficult and treatment is often delayed, infection with Acanthamoeba may result in total loss of sight in the infected eye. If infection is recognized early, wide epithelial debridement may be curative if the epithelium alone is involved (53, 184, 261). Debridement may remove infectious organisms and enhance the delivery of topical medications (17, 198). When Acanthamoeba infection proceeds without early treatment, the organisms invade deeper into the corneal region. Under these circumstances, when therapy is instituted it is often continued for several months to 1 year or longer (17). Furthermore, patients must be monitored for recurrence of disease because cysts are resistant to many drugs. Treatment failures are frequently reported; this may be attributed to poor penetration of the agent, insufficient duration of treatment, or acquired resistence to the drugs. A number of therapeutic agents are not effective at the late stage of infection, especially when the amebae have invaded tissues beneath the cornea (118, 139, 161, 186, 323, 447). Binder (36) suggested cryotherapy for patients showing a poor response to medicinal and surgical treatment.
In vitro susceptibility testing of isolates may prove beneficial for application of early treatment regimens. An in vitro susceptibility test for Acanthamoeba isolated from AK patients has been developed (326). Isolates are grown for 1 week on NNA seeded with E. coli to elicit encystation. The cysts are then scraped from the agar, washed, and incubated in dilutions of anti-ameba drugs for 48 h. Following removal of drugs, the cysts are placed on fresh NNA plates to assess the growth of amebae. The minimal cysticidal concentration is determined and is defined as the lowest concentration of test solution that results in no excystment and growth of trophozoites after 7 days of culture.
Wright et al. (482) reported successful treatment of AK using 0.1% propamidine isethionate (Brolene) topically with 0.15% dibromopropamidine. This treatment regimen was effective only when initiated early in infection (17, 120, 294, 314, 402, 482). In vitro studies have demonstrated that trophozoites of Acanthamoeba isolated from AK patients are susceptible to chlorhexidine and propamidine (402). Topical administration of these two drugs was found to be effective for treating AK, provided that the drugs were given for extended periods. However, propamidine is not recommended for all cases since some patients with AK have developed corneal abnormalities following prolonged treatment (206, 323). A successful outcome has been reported for patients with superficial AK treated with 0.1% hexamidine (51). A series of 12 patients with culture-proven AK were monitored during and after therapy with topical chlorhexidine and propamidine. Chlorhexidine in combination with propamidine provided rapid and successful treatment for corneal Acanthamoeba infections (402). Kosrirukvongs et al. (236) reported that four of five culture-proven AK patients were treated successfully with 0.006% chlorhexidine solution alone.
Imidazoles such as miconazole, itraconazole, and ketoconazole have been used with limited success (32, 108, 186, 198, 261, 315, 482). Topical treatment with miconazole, however, has lead to epithelial toxicity. Ketoconazole is more effective for treatment of AK but must be given systemically. Polyhexamethylene biguanide (PHMB), manufactured as an environmental disinfectant known as Baquacil by Zeneca Pharmaceuticals, was shown to exhibit both amebicidal and cysticidal activity against a number of Acanthamoeba strains (405). Although not licensed for therapeutic use, it has been employed as a successful experimental treatment for AK by Larkin et al. (250). A combination of chlorhexidine digluconate (a bisbiguanide) with PHMB (a polymeric biguanide) or with aromatic diamidines such as hexamidine, pentamidine, and propamidine isethionate or PHMB and hexamidine with debridement has been reported for the treatment of AK (124, 161, 171, 175, 195, 196, 250, 260, 323, 365, 405, 447, 454, 482). PHMB alone does not appear to be associated with toxicity, but other compounds used in combination with PHMB may exert untoward effects (195). Recently, a patient with unconfirmed but suspected AK was reported to have developed progressive ulcerative keratitis related to the use of topical chlorhexidine gluconate for 8 weeks (324). Penetrating keratoplasty was performed because of the possibility of corneal perforation. However, the usefulness of penetrating keratoplasty in the treatment of AK has been debated (17, 89, 180, 261, 394), and it has been used as a last resort for some patients. Penetrating keratoplasty may not be required if AK patients are treated within 6 weeks of presentation (196). Bacon et al. (17) indicated that this procedure is more successful if performed after resolution of inflammation. Cremona et al. (94) reported that deep lamellar keratectomy with a conjunctival flap was effective for controlling infection and relieving pain in two patients with advanced keratitis.
Corticosteroids have been used in conjunction with therapeutic agents for the treatment of AK (16). Although corticosteroids reduce inflammation, recent studies suggest that the use of corticosteroids should be avoided if possible (108). In vitro and in vivo studies using experimental animals suggest that exposure of cysts to corticosteroids such as dexamethasone phosphate increases the pathogenicity of the amebae (298). Keratitis in dexamethasone-treated hamsters was found to be more severe than in untreated animals. Additionally, locally administered corticosteroids were shown to be detrimental in a rabbit model of AK (205).
New therapeutic agents are being sought for the treatment of Acanthamoeba infections. More recently, the emergence of resistance to commonly used antimicrobial agents and biocides in the treatment of Acanthamoeba keratitis and in contact lens disinfection systems has been assessed (323, 448). Resistance to biocides apparently results from the physical barrier of the cyst wall rather than being a consequence of the presence of metabolically dormant cysts (448). In one study, the amebicidal activity of eight different alkylphosphocholines against Acanthamoeba spp. was investigated (182). Treatment with hexadecylphosphocholine resulted in complete lysis of Acanthamoeba in vitro within 1 h of addition of the compound. Although alkylphosphocholines are in the experimental stage of development as amebicidal agents, hexadecylphosphocholine may be useful for treatment of GAE as well as AK since this drug has been shown to cross the blood-brain barrier in experimental animals. Table 5 summarizes treatment regimens and outcomes for AK.
TABLE 5.
Treatment regimens and outcomes for AK
| Patient no. | Therapya | Outcome | Reference |
|---|---|---|---|
| 1 | Propamidine isethionate, Neosporin | Toxicity | 482 |
| 2 | PHMBb | Successful | 250 |
| 3 | 1% clotrimazole eye drops | ||
| Dibromopropamidine isethionate | Recurrence | 118 | |
| Oral fluconazole, clotrimazole, PHMB | Progression to enucleation | ||
| 4 | Propamidine isethionate, neosporin | Treatment failure | 161 |
| Miconazole, PNA, itraconazole | Toxicity | ||
| PHMB | Resolution of disease | ||
| 5 | Miconazole, metronidazole, PNA, neomycin, ketoconazole | Residual infiltrate | 186 |
| 6 | Miconazole, metronidazole, propamidine, ketoconazole | Recurrence | 186 |
| 7 | Hexamidine | Well tolerated cure | 51 |
| 8 | PHMB and propamidine isethionate; steroids only after clinical improvement | Cure, long-term treatment necessary | 196 |
| 9 | Chlorhexidine and propamidine | Effective if used for the long term | 402 |
| 10 | Itraconazole, miconazole | Unresponsive | 323 |
| Penetrating keratoplasty | Successful | ||
| 11 | Propamidine, neosporin, itraconazole, miconazole | Corneal perforation, keratoplasty | 323 |
| 12 | Propamidine, PHMB | Resolution of disease | 323 |
| 13 | PHMB, propamidine, PNA, chlorhexidine, PHMB | Propamidine toxicity | 323 |
| 14 | Chlorhexidine, PHMB, propamidine isethionate | Treatment of choice | 260 |
Therapeutic agents reported for individual cases and treatment outcomes. PNA, prednisolone acetate.
PHMB is not licensed for therapeutic use. It is used as experimental drug.
IMMUNOLOGY AND PATHOLOGY OF ACANTHAMOEBA INFECTIONS
Role of the Immune System in Acanthamoeba Infections
The immune defense mechanisms that operate against Acanthamoeba have not been well characterized. Exposure to Acanthamoeba appears to be common since the presence of antibodies to the ameba have been demonstrated in serum samples (Fig. 12) from most asymptomatic healthy individuals (73, 78, 104, 271, 330, 357). Nevertheless, although the prevalence of Acanthamoeba cysts and trophozoites is high in the environment, the incidence of fatal infection appears low. Indeed, Acanthamoeba have been isolated from the nasopharynx of apparently healthy individuals (19, 73, 75, 304, 330, 370, 371). Whether Acanthamoeba causes transient infections in these individuals and stimulates host defense responses which control infection and result in the elimination of the organism is not known. Chappell et al. (78) suggested that while serious ocular disease and CNS infections are rare, mucosal infections may contribute significantly to large numbers of undiagnosed sinus or pulmonary infections. The presence of a higher frequency of A. polyphaga-specific immunoglobulin M (IgM) and a lower frequency of IgG antibodies in serum from rheumatoid arthritis (RA) patients than from matched controls has been reported (204). The higher titers of A. polyphaga-specific IgM in RA patients than in matched controls were thought to be due to persistent or repeated antigenic stimulation by A. polyphaga. However, it is not known whether an immune reaction to A. polyphaga antigens results in symptoms in RA patients.
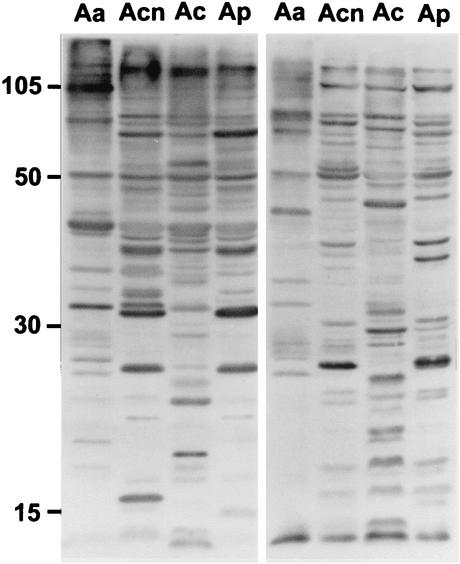
FIG. 12.
Western immunoblot of Acanthamoeba whole-cell lysates reacted with normal human serum, demonstrating immunoreactivity against Acanthamoeba antigens. Serum samples from two asymptomatic individuals served as a source of natural antibodies to four species of Acanthamoeba. Whole-cell lysates of A. astronyxis (lanes Aa), A. castellanii (lanes Acn), A. culbertsoni (lanes Ac), and A. polyphaga (lanes Ap) were prepared and used as the antigen source. Acanthamoeba protein (50 μg) was added to each well of a sodium dodecyl sulfate-12% polyacrylamide gel. Separated proteins were electrophoretically transferred to a nitrocellulose membrane and were incubated with normal human serum as the source of primary antibody and horseradish peroxidase-labeled goat anti-human IgG as the secondary antibody. The blots were then subjected to enhanced chemiluminescence.
Protection from lethal infection may involve both innate and acquired immunity (103, 104). In experimental animal infections, the age of the animal, the mouse strain, the immune status of the host, the infecting dose, temperature tolerance, and the virulence of the ameba strain appear to be important factors in the outcome of a murine infection (270). Complement, an innate resistance factor which provides the first line of defense against invading organisms, is activated by Acanthamoeba infection (138, 445). However, the consequences of such activation in response to infection with Acanthamoeba are not known. Whether complement activation results in increased pathogenesis by generating C3a and C5a components, which act as mediators of inflammation and tissue damage, or aids in eliminating amebae remains to be defined. Activation of complement may result in generation of opsonic factors such as C3b, which plays a role in recognition of amebae by phagocytic cells (138). Alternatively, complement may protect the host by lysing amebae, although in vitro, highly pathogenic species of Acanthamoeba such as A. culbertsoni are more resistant to complement lysis than are nonpathogenic Acanthamoeba spp. (445). It has also been shown that Acanthamoeba interacts with human C1q and that pathogenic strains appear to bind C1q more efficiently than nonpathogenic strains do. Binding of C1q by amebae blocked binding sites for C1, the first component of the classical complement cascade, which serves to inhibit the classical pathway (469). Thus, resistance to complement lysis may constitute a mode of immune evasion which contributes to the establishment of infection and dissemination of Acanthamoeba within the host (445, 469).
The role of antibodies in Acanthamoeba infection also remains unresolved. Antibodies may prevent attachment to host cells, inhibit the motility of amebae, or neutralize ameba cytotoxic factors (104, 136, 272, 431). However, it has been reported that Acanthamoeba can degrade human IgG and IgA antibodies by serine proteases (233). In mice, immunization with A. culbertsoni antigens using intranasal, intraperitoneal, intravenous, or oral routes of administration purportedly provided protection against a lethal challenge (382). However, multiple immunizations were required to impart protection. Of the immunogens tested (e.g., culture fluid, amebic sonicate, freeze-thawed extract, or live amebae), amebic sonicate elicited the best protection against intranasal challenge with A. culbertsoni. Protection was specific in that immunization with other species of Acanthamoeba did not protect mice against challenge with A. culbertsoni (34, 96, 135).
There have been few studies to assess the interaction of Acanthamoeba with specified cells of the immune system. It has been reported that the earliest response of the host to amebae consists of an influx of neutrophils to the site of infection (136). However, human neutrophils fail to kill Acanthamoeba unless the neutrophils are treated with TNF. In vitro, killing of Acanthamoeba by lymphokine-treated neutrophils requires the presence of both antibodies and complement (136). Studies in our laboratory with experimental animals such as mice exposed to Acanthmoeba have shown that neutrophils and macrophages are the major inflammatory cells to migrate into the area of infection. Macrophages may play a more important role than neutrophils in killing Acanthamoeba (Fig. 13 and 14). These cells are capable of injuring amebae and comprise the major cellular component of granulomas frequently encountered in tissues containing Acanthamoeba cysts (Fig. 14) (272). Masihi et al. (287) studied the effect of the mycobacterium-derived immunopotentiating agents muramyl dipeptide and trehalose dimycolate against intranasal Acanthamoeba infections in mice. Treatment of mice with these macrophage-activating agents prior to infection protected 40 and 30% of the animals, respectively, against a lethal infection with A. culbertsoni. In vitro studies with murine macrophages activated in vivo with Bacillus Calmette-Guérin demonstrated that activated macrophages were more efficient in injuring Acanthamoeba than were unstimulated macrophages (272). The activated macrophages also were more efficient in injuring Acanthamoeba than were unstimulated macrophage-like cells maintained as continuous cell lines. TNF-α and IL-1α or IL-1β, cytokine products of activated macrophages, were found not to be amebicidal for Acanthamoeba when used either alone or in combination (272). Hydroxy radicals, hydrogen peroxide, and nitric oxide may be important amebicidal factors since it has been reported that Acanthamoeba strains are sensitive to hydrogen peroxide (134). In addition, Stewart et al. (430) reported that rat macrophages, similar to murine macrophages, undergo chemotaxis to amebae and kill trophozoites in vitro. Thus, although the full range of specific macrophage factors responsible for injuring Acanthamoeba has yet to be defined, it is apparent that macrophages activated with immunomodulators are capable of phagocytizing and destroying amebae (272).
FIG. 13.
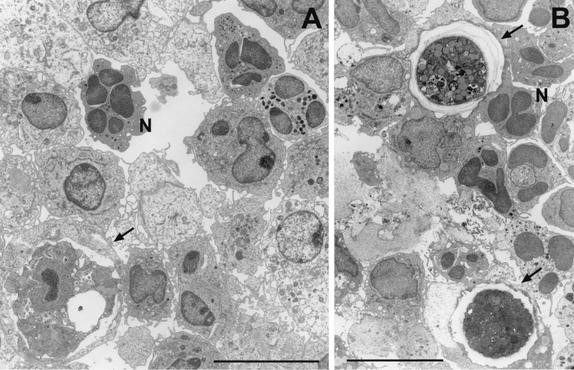
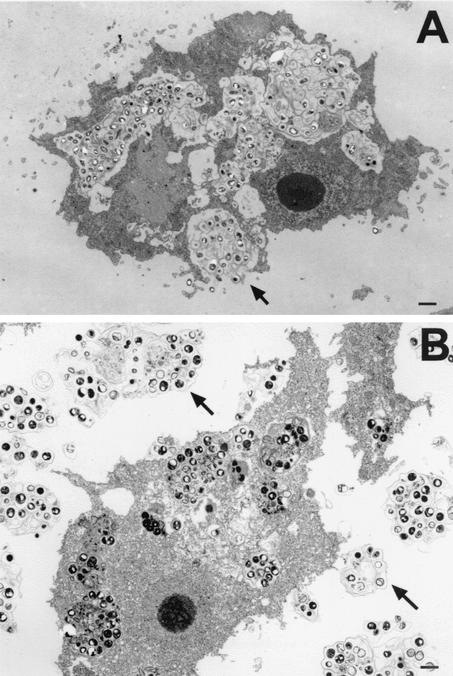
Transmission electron micrographs demonstrating cellular events in an experimental mouse model of infection with Acanthamoeba. (A) Focal area of infection containing amebae after a 24-h exposure to A. castellanii. (B) Focal area of infection containing amebae after a 48-h exposure to A. castellanii. Neutrophils (N) are prominent in focal areas containing amebae (arrow) during the early phase of infection. Bars, 10 μm.
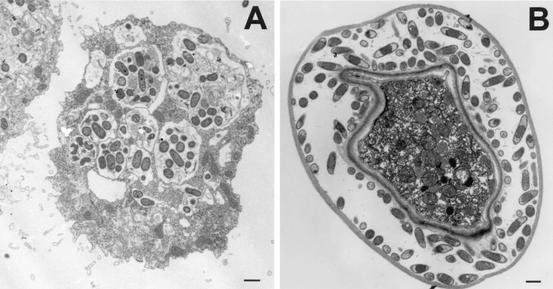
FIG. 14.
Transmission electron micrograph illustrating the accumulation of macrophages in focal areas containing amebae in an experimental mouse model of A. castellanii infection. Macrophages migrate to sites containing amebae during the later phase of infection. (A) Accumulation of macrophages around an A. castellanii cyst (72 h postinfection). (B) Accumulation of macrophages at a site containing amebae (96 h postinfection). Note the presence of an ingested cyst within the macrophage (arrow). Bars, 10 μm (A) and 1 μm (B).
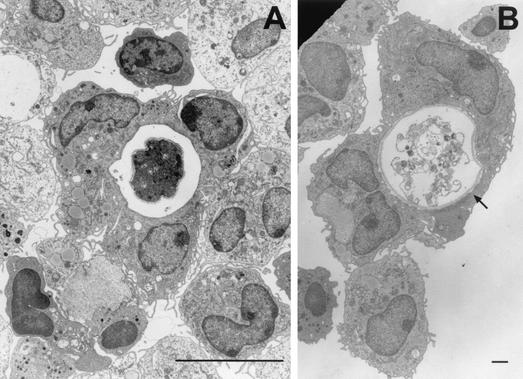
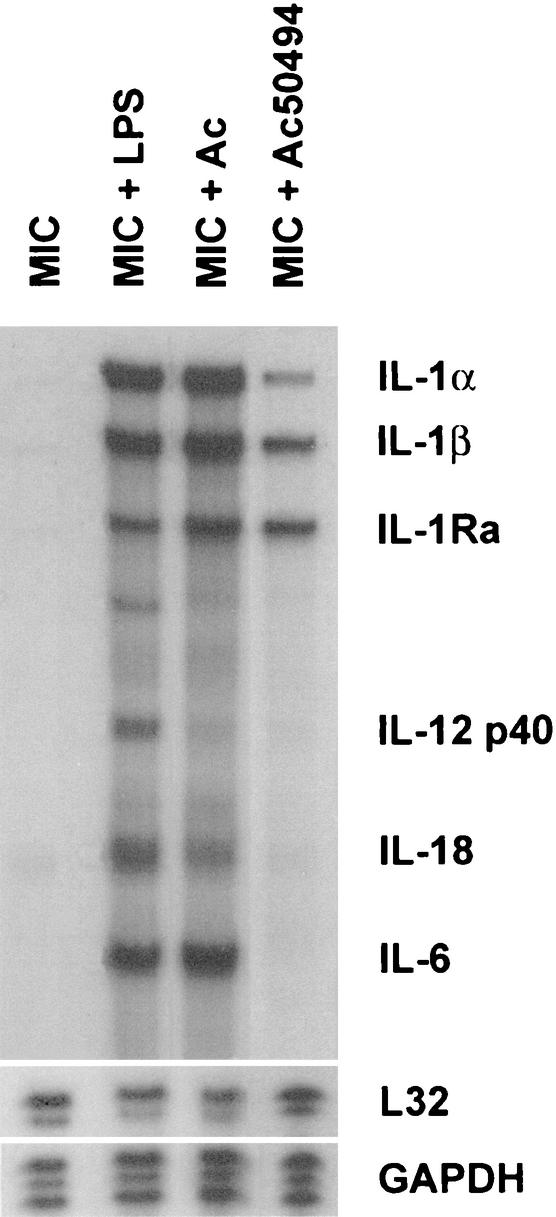
Recent reports indicate that microglial cells, resident macrophages of the brain, also exert amebicidal activity (271). Microglial cells obtained from newborn rat pups and cocultured with A. castellanii were shown to destroy amebae by both phagocytic and lytic processes (Fig. 15). Furthermore, A. castellanii and A. culbertsoni cocultured with microglial cells induced the production of mRNAs for the cytokines IL-1α, IL-1β, and TNF-α (Fig. 16). These observations that microglia undergo inducible expression of proinflammatory cytokine genes suggest a mode by which Acanthamoeba effects neuropathology (271). Studies also have been performed on Acanthamoeba-microglia interactions by using highly pathogenic A. culbertsoni and weakly pathogenic A. royreba amebae. Shin et al. reported that microglial cells cocultured with virulent A. culbertsoni exhibited cytopathic changes consistent with those described for cells undergoing apoptosis while microglial cells cocultured with weakly pathogenic A. royreba did not (411). In view of these observations, it has been postulated that virulent Acanthamoeba strains escape the amebicidal activity of macrophages and macrophage-like cells while, in contrast, weakly pathogenic species are targeted by macrophages and are lysed, ingested, and destroyed (271, 272, 411).
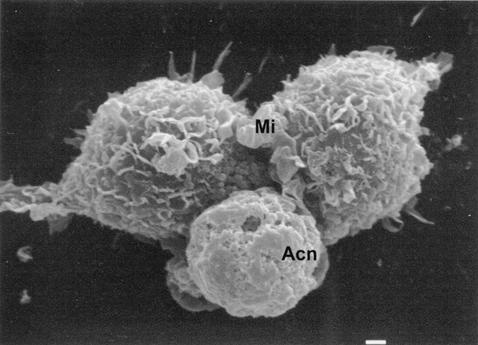
FIG. 15.
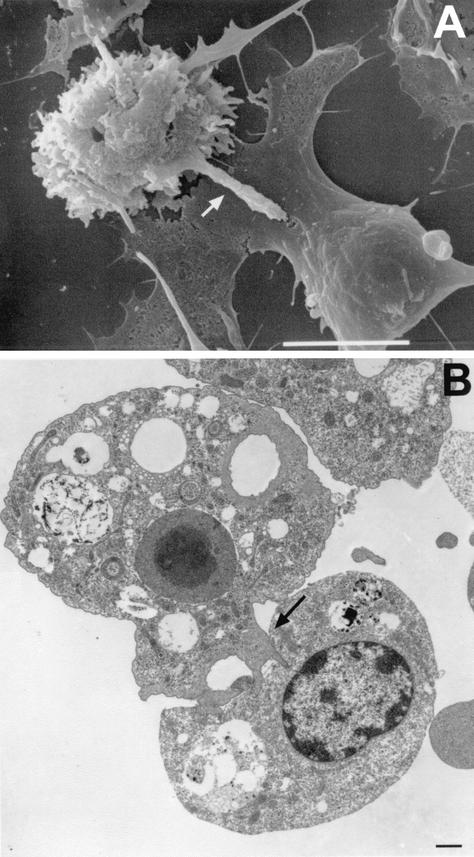
Scanning electron micrograph of brain microglial cells (Mi) cocultured with A. castellanii (Acn). Microglial cells, the resident macrophages in the brain, are capable of injuring A. castellanii by cell contact-dependent lysis. Bar, 1 μm.
FIG. 16.
RNase protection assay illustrating multiple mRNAs elicited by microglia in response to Acanthamoeba. Primary microglial cells obtained from brain cerebral cortices of newborn rat were cocultured with A. castellanii, A. culbertsoni, or bacterial lipopolysaccharide (LPS) for 6 h. The cultures then were harvested with Trizol, and the RNA was subjected to RNase protection assay analysis for assessment of inducible cytokine gene expression. Microglial cells alone (lane MIC), microglia incubated with LPS (lane MIC + LPS), microglia incubated with A. culbertsoni (lane MIC + Ac), and microglia incubated with A. castellanii (lane MIC + Ac50494) are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Few studies have been conducted to investigate the interactions of Acanthamoeba with T lymphocytes. Tanaka et al. (439) examined T-cell responses to Acanthamoeba antigens in healthy human subjects. T-cell clones from these subjects were established and analyzed for biological and functional activities. Those derived from asymptomatic healthy individuals proliferated in response to Acanthamoeba antigens. The T-cell clones were categorized as being Th1 cells involved in delayed-type hypersensitivity reactions in that they produced gamma interferon in response to amebic antigens. Based on their observations, Tanaka et al. (439) suggested that the low incidence of symptomatic disease in humans might be due to acquired protective immunity induced by T-cell responses.
Role of the Immune System in Amebic Keratitis
The cellular immune response to ocular infections with Acanthamoeba in a limited number of human patients and in experimental animals has been studied (237, 288). Macrophages and neutrophils are the major inflammatory cell types found in tissues surrounding amebae or cysts. In one set of studies, corneal buttons from two AK patients with chronic inflammation were examined using electron microscopy and immunohistochemistry to identify cell types involved in the inflammatory response (288). The use of monoclonal antibodies to cell surface markers demonstrated that the corneal stroma contained polymorphonuclear leukocytes and macrophages with few to no stromal lymphocytes. Despite evidence of a vigorous corneal infiltration by neutrophils and a measurable humoral response, infection in these patients proceeded to corneal opacity or perforation. Based on these observations, Mathers et al. (288) suggested that Acanthamoeba masks its antigens from cellular immune responses, thereby suppressing macrophage functions or preventing lymphocyte recruitment.
A variety of animal models including Wistar rats, pigs, mice, and Chinese hamsters have been used for studying immune responses in AK patients after exposure to trophozoites or cysts of human isolates of A. polyphaga or A. castellanii. An intense inflammatory response consisting of neutrophils and macrophages was shown to develop in Wistar rats following inoculation of A. polyphaga directly into the corneal stroma (248, 249). The profile of inflammatory cells changed during progression and clearance of disease. Initially, neutrophils were prominent at sites of infection; this was followed by the accumulation of macrophages, which persisted. A few T cells were present late in infection, but B cells were absent. Protection against ocular infection by A. castellanii has also been investigated by using a pig model (6). Pigs immunized with a crude aqueous extract of Acanthamoeba intramuscularly, subconjunctivally, or by both routes were exposed to Acanthamoeba-laden “soft contact lenses” made of dialysis tubing. Intramuscular injection of ameba extracts failed to protect against AK after Acanthamoeba challenge, even though high IgG titers and lymphocyte blastogenic responses developed. Furthermore, previous ocular infection did not protect from reinfection. In contrast, 50% of pigs immunized with amebic extract subconjunctivally and 100% of pigs immunized with both a subconjunctival and intramuscular injection of amebic extract were protected from infection. However, protection did not correlate with levels of humoral antibody or blastogenic responses by peripheral blood lymphocytes. Van Klink et al. (452) studied the immune response during ocular infection with Acanthamoeba in Chinese hamsters. Corneal infection with A. castellanii failed to stimulate a delayed-type hypersensitivity reaction or serum IgG against Acanthamoeba antigens. Subsequent intramuscular immunization with amebic antigens elicited a vigorous delayed-type hypersensitivity and IgG response, indicating that tolerance to amebic antigens had not occurred. The inability to induce a cell-mediated or humoral response during corneal infection was attributed to the absence of resident antigen-presenting cells in the central cornea (452).
Collectively, animal studies indicate that macrophages play a prominent role in providing protection against Acanthamoeba ocular infections. Depletion of macrophages with the drug dichloromethylene diphosphonate exacerbated severity of infection in the Chinese hamster and prolonged disease, even though AK is a self- limiting disease in this animal (453). Niederkorn et al. (333) suggested that while macrophages limit the severity and chronicity of corneal disease, they do not inhibit corneal infection. On the other hand, the role of neutrophils in AK remains unclear. McCulley et al. (299) reported that neutrophils are abundant in AK and that the most severe stromal necrosis in Acanthamoeba lesions is mediated by proteases released by neutrophils rather than from amebae. However, in the cornea of the Chinese hamster, resolution of disease occurs with production of macrophage inflammatory protein 2 (MIP-2) a chemotactic factor for neutrophils (193). It was demonstrated further that selective inhibition of neutrophil migration in the cornea of Chinese hamsters through exposure to anti-MIP-2, an antibody to neutrophil chemotactic factor, or through elimination of neutrophils by using anti-neutrophil antiserum resulted in prolonged and more severe corneal disease. Furthermore, more rapid resolution of disease occurred when recombinant MIP-2 was injected into the cornea prior to infection.
The role of serum antibodies in AK in humans or experimental animals is not apparent. Walochnik et al. (468) examined sera from 20 individuals, including 2 AK patients, for anti-Acanthamoeba IgM, IgG, and IgA by using Western blotting (468). Differences in the immunological reactivity between a highly pathogenic AK isolate and a nonpathogenic isolate were evaluated. All sera were positive for anti-Acanthamoeba antibodies, but marked differences in IgA reactivity were observed between sera from infected patients and those from uninfected individuals. In IgA immunoblots, IgA immunoreactivity was significantly higher against the nonpathogenic strain than against the pathogenic strain, suggesting that pathogenic and nonpathogenic strains elicit distinct IgA antibody profiles. Furthermore, sera from AK patients showed weak immunoreactivity to the pathogenic strain (468). In Chinese hamsters, intramuscular immunization with Acanthamoeba antigens elicited a vigorous antibody response but corneal infection proceeded (452). On the other hand, oral immunization with Acanthamoeba antigens induced resistance to corneal infection with Acanthamoeba in both a Chinese hamster and pig model of AK (252, 255). Protection correlated with production of IgA antibodies. Furthermore, passive transfer of Acanthamoeba-specific monoclonal IgA antibodies protected against AK in the Chinese hamster (254). Thus, it appears that a mucosal immune response involving IgA is effective in protecting against Acanthamoeba but does not influence the course of infection once it is established (255, 332). Alizadeh et al. (5) have suggested that secretory IgA (sIgA), but not serum immunoglobulin, is important in alleviating AK. In a series of studies, the levels of Acanthamoeba-specific serum IgG and tear IgA in 23 amebic keratitis patients and 25 healthy human subjects were assessed by an enzyme-linked immunosorbent assay. Levels of total serum immunoglobulins were shown not to differ significantly between healthy subjects and patients with AK, but levels of ameba-specific tear IgA were significantly lower in patients with AK. Thus, it has been postulated that low levels of tear IgA may be associated with AK. In summary, the cumulative data suggest that both innate and acquired immune responses come into play in AK. IgA may act to prevent the attachment of amebae to corneal cells, while neutrophils and macrophages may migrate to focal areas of infection and injure or destroy amebae.
Virulence Factors
Pathogenic and nonpathogenic strains of Acanthamoeba have been isolated from the environment, but the pathogenesis of infection and the biochemical determinants of virulence are poorly understood. Temperature tolerance, growth rate, adherence properties, cytolytic products produced by amebae, and immune evasion mechanisms appear to constitute important factors in pathogenicity. The virulence of pathogenic amebae wanes during continuous culture in axenic medium but can be restored by brain passage in mice (197, 296). It has been proposed that virulence may be related to distinct physiological characteristics of a strain and not to a dependence on environmental conditions (464).
Studies to define pathogenic mechanisms have been conducted more often on strains of Acanthamoeba associated with AK rather than on those obtained from the brain. Adherence of trophozoites to cells followed by injury and invasion of tissue are thought to represent important steps in the establishment of infection. Clinical isolates of A. polyphaga, A. castellanii, and A. culbertsoni have been shown to attach to corneal epithelial cells through a process which involves binding to cell surface carbohydrate moieties (318). A. castellanii binds to mannose-containing glycoproteins on the corneal epithelium through a 136-kDa mannose-binding protein on the ameba surface (485). The adherence of Acanthamoeba to corneal epithelial cells can be inhibited by mannose and methylmannose pyranoside but not by other sugars (318). Glycolipids of corneal epithelium reactive with Acanthamoeba may also play a role in the pathogenesis of AK by mediating the adherence of the amebae to the cornea (342). Studies in which monoclonal antibodies were shown to be reactive with different surface membrane epitopes suggest the presence of more than one adhesion molecule on the ameba surface (212). Kennett et al. (212) proposed that an adhesion molecule distinct from the 136-kDa mannose-binding protein, or an adhesion molecule composed of two different subunits, plays a role in attachment of amebae to host cells. Gordon et al. (160), using binding assays, reported that Acanthamoeba binds preferentially to collagen IV, laminin, and fibronectin.
Following adherence to cells, invasion and extensive tissue destruction occur in the host. Human epithelial cells, stromal keratocytes, and stromal cell homogenates have been used in vitro as models of AK (432). Damage to cells and tissue is thought to occur by phagocytic processes and by cytotoxic substances released by amebae. Using cultured human epithelial cells, Stopak et al. (432) proposed that pathology associated with AK elicited by A. polyphaga occurred when organisms became bound to the epithelial surface and fed directly on cells. Such “direct feeding” resulted in disruption of epithelial layers and invasion of amebae into the stromal layers. Studies with corneal epithelial cells indicated that Acanthamoeba can also cause direct target cell cytolysis (18, 247, 432). Electron microscopy studies of human corneal buttons exposed to Acanthamoeba indicated that entry into the cornea apparently involved a sequential process in which trophozoite adherence was followed by penetration. This process appeared to involve both secretion of lytic enzymes and phagocytosis (317). Consistent with these observations, Alfieri et al. (4) indicated that enzymes secreted by Acanthamoeba may play a role in contact-independent injury to cells and facilitate the invasion of host tissue. Hadas and Mazur (165) compared serine and cysteine proteinases of pathogenic and nonpathogenic species of Acanthamoeba. Their studies suggested that cysteine proteinases could serve as markers for pathogenicity since larger amounts were detected in pathogenic strains than in nonpathogenic species of Acanthamoeba. Mitro et al. (311) examined excretory and secretory products of a corneal isolate of A. polyphaga ATCC 30461 for protease activity. Partial characterization of the products demonstrated that A. polyphaga trophozoites secrete multiple proteinases. These included serine proteinases, cysteine proteinases, and metalloproteinases which are able to degrade type I collagen, the main component of the corneal stromal matrix. In addition, Cho et al. (81) purified and characterized a 42-kDa extracellular serine protease which degrades collagen and rabbit corneal extract. The purified proteinase also exerted cytopathic effects (CPE) on corneal epithelial cells and fibroblasts. Secretion of serine proteinases which degrade a broad spectrum of extracellular matrix proteins and serum proteins has been proposed as correlating with invasive properties of Acanthamoeba (451).
Ferrante and Bates (137) and He et al. (177) reported that acanthamoebae elaborate elastase, which degrades connective tissue proteins and causes damage to target cells. High levels of elastase activity were found in A. culbertsoni and were implicated as being linked to tissue destruction (137). Collagenolytic enzymes which degrade collagen in vitro were studied using A. castellanii ATCC 30868, a strain isolated originally from a human cornea (177). Intrastromal injections of conditioned medium obtained from A. castellanii cultures containing collagenase produced corneal lesions clinically similar to those found in biopsy specimens from human patients diagnosed with AK. Van Klink et al. (451) compared the fibrinolytic activity and collagenolytic activity of two strains of A. castellanii, one a soil isolate and the other an eye isolate. Fibrinolytic activity was found in the ocular isolate but not in the soil isolate, whereas collagenolytic activity was found in both the soil and ocular isolates. Thus, production of fibrinolytic enzymes and collagenase, or a combination of the two enzymes, by Acanthamoeba may correlate with the induction of corneal pathology (451). Plasminogen activator, a 40-kDa serine proteinase, also may play an important role in pathogenesis of AK (310). Collectively, these studies suggest that the pathogenic potential of A. castellanii to cause keratitis may be related to its capacity to bind to corneal epithelium, elaborate plasminogen activator, and produce cytopathology on the corneal epithelium (310, 451). Furthermore, proteinases produced by Acanthamoeba not only may play a role in pathogenesis of disease and cytopathogenicity of cultured cells but also may serve as markers for differentiating pathogenic and nonpathogenic amebae.
In addition, phospholipase activity is higher in virulent Acanthamoeba strains and species than in avirulent amebae (102, 130, 309, 455, 456, 459). For example, phospholipases have been identified in the media of virulent A. culbertsoni. Cursons et al. (102) postulated that secreted phospholipases were responsible for extensive demyelination of brain issue. Studies of mice infected with A. culbertsoni demonstrated that phospholipase A and sphingomyelinase were elaborated in brain during infection and caused the degradation of phospholipids with an accumulation of free fatty acids and lipid peroxides (244).
Cytopathogenicity of Acanthamoeba spp.
The first report that Acanthamoeba caused CPE for cells in culture was published in 1957, when these protozoa were found as tissue culture contaminants (202). Since then, Acanthamoeba strains have been assessed for CPE by using various mammalian cell lines. Studies have been conducted on biochemical parameters, adherence properties, pathogenic and cytopathogenic mechanisms, and antiamebic drug efficacy. Both contact-dependent and contact-independent mechanisms of target cell injury have been reported (253, 271, 351). Cao et al. (66) suggested that lectin-mediated adherence to host cells was a prerequisite for ameba-induced cytolysis of host cells. However, it also has been reported that cell-free medium containing soluble factors released from trophozoites can cause injury to host cells (253). Leher et al. (253) indicated that cell-free medium obtained from mannose-treated Acanthamoeba induced the lysis of corneal epithelial cells. In addition, treatment of the medium containing ameba soluble factors with the serine protease inhibitor phenylmethylsulfonyl fluoride was reported to inhibit most but not all of the cytolytic activity. On the other hand, mannose blocks the binding of trophozoites to cells and inhibits the phagocytosis of red blood cells and yeast (9, 54).
Comparative assessments of the ability of human or environmental isolates of Acanthamoeba spp. to destroy mammalian cells have been undertaken (18, 242, 247, 410). In a study of the CPE of an AK isolate of A. polyphaga and an environmental isolate of A. castellanii using confluent monolayers of human and rabbit corneal cells as targets, total destruction of target cell monolayers was observed (247). The CPE was dependent on the incubation time and on the effector/target ratio of amebae to corneal cells. Badenoch et al. (18) assessed the virulence of strains of Acanthamoeba derived from the cornea, the nasal mucosa, or the environment. Isolates were screened for virulence by using a rat model of AK and were assessed for CPE on human keratocytes. An 86% correlation was obtained between virulence of trophozoites in vivo and CPE in vitro. However, not all strains which were virulent in rat corneas in vivo produced CPE on keratocytes in vitro. Eight of 13 corneal isolates, 2 of 9 environmental isolates, and 1 of 6 nasal isolates induced CPE on keratocytes, while 6 of 13 corneal strains, 3 of 9 environmental strains, and 1 of 6 nasal strains induced AK in the rat. Pidherney et al. (353) reported that A. castellanii ATCC 30868 isolated originally from human corneal tissue produced CPE on primary pig epithelium, human corneal endothelium, murine neuroblastoma cells, human ocular melanomas, and murine fibrosarcomas. In another study, the ability of A. castellanii ATCC 30868 to induce CPE on normal rabbit kidney cells was found to be dependent on the ameba-to-target-cell ratio (335); CPE reportedly did not occur when cocultures were maintained at a low ameba-to-target-cell ratio.
An active phagocytic process in which amebostomes (i.e., food cups) were used to ingest Vero cells was described by Diaz et al. (114) for A. lenticulata and an unidentified species of Acanthamoeba. Lagmay et al. (242) reported that live amebae, as well as cell-free supernatants prepared from amebae isolated from AK patients and from an environmental isolate, produced CPE on C6 rat glial cells in a dose- and time-dependent manner. These investigators suggested that cytotoxic factors released from amebae during the logarithmic phase of growth accounted for target cell death. Alizadeh et al. (7) reported that A. castellanii lysed tumor cells by a process involving apoptosis. Further characterization of the CPE revealed that calcium channels and cytoskeletal elements were involved in the cytopathology of ocular melanoma cells by A. castellanii (441). Phagocytosis or trogocytosis of target cells by the ameba was not observed in these studies. In contrast, Pettit et al. (351) demonstrated that trophozoites of four species of Acanthamoeba induced the cytolysis of cultured rat B103 nerve cells in addition to ingesting these cells through food cups (Fig. 5). Transmission and scanning electron microscopy studies revealed that A. culbertsoni, A. castellanii, A. polyphaga, and A. astronyxis apparently caused cytolysis through a contact-dependent process involving the extension of digipodia from the amebal surface to the target cell membrane (Fig. 17). Membrane blebbing and margination of nuclear chromatin were observed on target cells with which digipodia were in contact, consistent with the induction of apoptosis. In addition, studies of the interaction of A. castellanii with human epithelial Wish cells have shown that amebae release ADP into the culture medium, resulting in a biphasic rise in [Ca]i, which culminates in apoptosis (292, 293). We have demonstrated that cell death induced by Acanthamoeba spp. also can occur by necrosis (351). Studies indicated that a subset of target neuroblastoma cells cocultured with Acanthamoeba exhibited cell swelling and dissolution of cytoplasmic and cell surface membranes.
FIG. 17.
Scanning (A) and transmission (B) electron micrographs of Acanthamoeba cocultured with rat B103 neuroblastoma cells. (A) A. castellanii with extended digipodium (arrow) into the cytoplasm of the target B103 cell. (B) A. culbertsoni cocultured with B103 cells, demonstrating fingerlike projections extending into the target cell. Cells targeted by digipodia (arrow) eventually die by apoptosis or necrosis. Bars, 10 μm (A) and 1 μm (B).
Although confluent monolayers of mammalian cells have been used to assess the pathogenicity of isolates, both pathogenic and nonpathogenic strains of Acanthamoeba are capable of producing CPE on cell cultures under appropriate conditions (101, 335). However, the data also suggest that not all human or environmental isolates cause symptomatic disease in experimental animals, particularly after the isolates have been maintained in axenic culture. Thus, studies to assess for pathogenicity of Acanthamoeba isolates should be complemented with those utilizing in vivo models of infection.
MODELS OF ACANTHAMOEBA INFECTIONS
Animal Models of Granulomatous Amebic Encephalitis
Culbertson et al. (99) first established the pathogenicity of Acanthamoeba for experimental animals after intracerebral and intraspinal inoculations of amebae which had been isolated as a tissue culture contaminant. These studies indicated that Acanthamoeba caused extensive choriomeningitis and destructive encephalomyelitis in monkeys and mice. Intravenous and intranasal inoculations of amebae into mice also resulted in fatal infections. However, intraperitoneal and subcutaneous inoculations did not result in the death of the animals (95, 98). Since these initial studies, the mouse has been the animal model of choice to study CNS infections with Acanthamoeba. Several strains of Acanthamoeba isolated from the environment have been tested for pathogenicity by using this model. In these experimental animals, CNS infection can be produced by intranasal instillation of amebae. Amebic rhinitis and pneumonitis also develop. Some of the environmental isolates produce an acute fatal encephalitis, while others produce a chronic, nonfatal disease consisting of a subacute and chronic granulomatous response. Lesions present in the nasal mucosa and brain contain trophozoites and cysts. Mice exhibit symptoms of neurological disorders including circling movement, difficulties with equilibrium, hyperactivity, and convulsive seizures (95, 97, 98).
Culbertson et al. (97) reported that A. castellanii HN-3 isolated from a human nasal swab and inoculated intranasally into mice produced nasal lesions and then demonstrated passage along nerve fibers and invasion of the olfactory bulb. An intense neutrophil response occurred, which was followed by a mononuclear response of the cerebral hemispheres. In addition, granulomas formed in the brain. Cerva (71, 72) reported that mice inoculated with the Neff strain of A. castellanii survived without any sign of illness. However, infection of mice with the A-1 strain of A. culbertsoni following intranasal inoculation resulted in the death of most of the animals. The mice died within 10 to 13 days postinoculation and exhibited acute pneumonia and CNS lesions. The growth phase of the amebae appeared to be an important factor influencing mortality in mice. The highest mortality rate was observed when amebae harvested at the beginning of the logarithmic phase of growth were used for inoculation. Cerva (71) also reported that continuous cultivation of amebae in culture medium resulted in decreased virulence. Virulence could be restored by use of amebae in the early logarithmic phase of growth.
Studies by Martinez et al. (283) of experimentally induced murine infections with Acanthamoeba demonstrated that the earliest signs of infection were respiratory distress, in which difficulty in breathing was noted. Pneumonia was followed by neurological symptoms consisting of head tilt, circling, twirling, and limb paresis. Fatal encephalitis occurred after the appearance of amebic rhinitis and pneumonitis. Trophozoites were observed frequently in the capillaries of the lungs and brains. In addition, in the brain, Acanthamoeba produced a chronic granulomatous encephalitis in which both trophozoites and cysts were found. Based on these observations, Martinez et al. (283) suggested that Acanthamoeba invasion of the CNS in mice was a secondary outcome of infection and represented a metastatic spread from the primary focus of infection in the lungs. Intranasal inoculation of Acanthamoeba also results in CNS infection due to invasion of amebae through the olfactory nerves to the olfactory bulbs (98, 270).
Experimental murine models of immune suppression also have been developed. Mice treated with corticosteroids to suppress host defenses have been reported to have greater mortalities (274). Animals maintained on the corticosteroid methylprednisolone or the antibiotic tetracycline and then inoculated intranasally with A. castellanii showed significantly higher mortalities than did untreated mice. Steroids also impair neutrophil migration into sites of infection as well as phagocytosis of organisms (274). Recently, mouse models of drug abuse also have been used to assess the effects of illicit drugs on Acanthamoeba infection. Marciano-Cabral et al. (270) demonstrated that treatment of mice with delta-9-tetrahydrocannabinol (THC), the major psychoactive and immunosuppressive component of marijuana, exacerbated infection with A. castellanii or A. culbertsoni. Mice treated with THC had higher mortalities from infection than did similarly infected drug-free control animals. Amebae were isolated from the lungs and brains of drug-treated mice at the time of death, suggesting that THC-induced immune suppression contributed to the disseminated disease.
Animal Models of Amebic Keratitis
A number of experimental animals, including both immunocompetent and immunosuppressed hosts, have been exposed to Acanthamoeba by using “contact lenses” made of dialysis tubing or by intrastromal injection of amebae (93, 141, 207). Font et al. (141), using a rabbit model, indicated that necrosis and an inflammatory response were induced after administration of subconjunctival or topical steroids followed by intrastromal inoculations of A. castellanii. Treatment of animals with steroids did not enhance the severity of AK or the rate of isolation of amebae from cultures inoculated with ocular material. Badenoch et al. (20, 21) reported that suppurative AK could be induced in rats only when amebae were injected directly into the corneal stroma or when the amebae were coinjected with bacteria such as Corynebacterium. A model of AK in pigs also has been developed by using “contact lenses” laden with amebae to mimic human exposure. This model is advantageous for studying ocular infections since the anatomic similarities of the pig eye to the human eye allow for clinical correlation (176). However, unlike the human infection with Acanthamoeba, which is prolonged, spontaneous resolution of disease in pigs occurred in 8 to 10 weeks. In addition, Van Klink et al. (450) have developed a Chinese hamster model of AK which has been used extensively because clinically and histopathologically the infection closely resembles the acute stages of infection in humans. Using this model, corneal infections were produced when “contact lenses” containing Acanthamoeba were applied to abraded corneal surfaces and maintained in place for at least 5 to 7 days. Typical features included neutrophil infiltration, epithelial ulceration, edema, corneal opacity, and neovascularization. Corneal disease was not produced when “contact lenses” containing amebae were applied to intact corneal surfaces (450). Although the Chinese hamster model of AK provides a means of studying the immune response to Acanthamoeba infection and testing therapeutic methods to combat infection, the disease in this model is acute and self-limiting. Thus, to date, animal models which elicit the full spectrum of host-pathogen interactions for progressive chronic AK are unavailable. Furthermore, not all animals are susceptible to infection, which complicates the search for an ideal animal model of AK.
ACANTHAMOEBA-BACTERIUM INTERACTIONS
It is becoming increasingly apparent that free-living amebae interact with bacterial species in the environment. The outcome of the interaction of free-living amebae such as Acanthamoeba with different bacteria is complex. Amebae have been reported to exhibit different capabilities for binding and internalizing different species of bacteria (358). Bacterium-amebae interactions may lead to the establishment of an endosymbiotic state or, alternatively, to destruction of either the bacterium or the ameba.
Acanthamoeba spp. have been reported to be predators of bacteria and to control the bacterial populations in soil habitats (376). Soil amebae produce bacteriolytic enzymes which enable the amebae to degrade bacterial cell wall components (122, 381, 473). In aquatic environments, Acanthamoeba organisms can be found predominantly at the water-air interface, feeding on bacteria which support their growth (359). Some genera of bacteria are “edible,” while others are “nonedible” and are not digested, possibly due to the presence of bacterial toxins, toxic pigments, or outer membrane structures (106, 416, 417). Nonpigmented enterobacteriaceae such as E. coli K-12 and Klebsiella aerogenes serve as better food sources for Acanthamoeba than do bacteria such as Bacillus subtilis or Serratia marcescans (471). Strains of A. castellanii isolated from soil and water sites exhibit predatory activity on cyanobacteria in culture (481). The survival and growth of Acanthamoeba in the presence of specific bacteria appears to be influenced by the species and density of bacteria. At low densities, gram-negative bacteria support the growth of Acanthamoeba. However, at high densities of bacteria to amebae (i.e., >10 to 1), bacteria such as Pseudomonas inhibit the growth of Acanthamoeba (470). It is not known whether exotoxins produced by bacteria or changes in pH induced by bacterial growth contribute to the death of the amebae.
A number of Acanthamoeba species appear to harbor endosymbiotic bacteria which divide and survive within their cytoplasm. Proca-Ciobanu et al. (360) used electron microscopy to describe rod-shaped bacterial endosymbionts in an environmental isolate of Acanthamoeba. Hall and Voelz (168) described the growth and reproduction of bacterial endosymbionts in the cytoplasm of Acanthamoeba spp. No evidence of a phagosomal or phagolysosomal membrane surrounding bacterial inclusions was observed. Additionally, Acanthamoeba organisms isolated from potable water treatment plants have been shown to harbor Archaea-like endocytobiotic organisms (183). The occurrence of nonculturable bacterial endosymbionts in Acanthamoeba isolates also has been reported (144, 145). In both clinical and environmental Acanthamoeba isolates, chlamydia-like and rickettsial-related organisms have been identified (11, 38, 144, 145, 153, 162, 185, 304). In one study, at least 26% of isolates obtained from human corneal specimens and 24% of environmental samples contained intracellular endosymbionts. However, attempts to culture the bacteria apart from amebae were unsuccessful (144). Horn et al. (185) studied a novel group of endosymbionts of Acanthamoeba which included environmental and clinical isolates. Endosymbionts belonging to the α subclass of Proteobacteria closely related to kappa particles of Paramecium were identified. Based on 16S rDNA sequence similarities of bacteria, the endosymbionts were assigned tentatively to existing and new genera, Candidatus Caedibacter acanthamoebae, Candidatus Paracaedibacter acanthamoebae, and Candidatus Paracaedibacter symbiosus. Since these bacteria are related to the Rickettsiaceae, it was suggested that the interaction of Acanthamoeba with these bacteria might be of clinical significance in the transfer of rickettsia in human disease (185). Additionally, chlamydia-like endosymbionts of Acanthamoeba have been implicated in respiratory disease (38). In addition to bacterial endosymbionts which stably infect amebae, other bacteria apparently utilize Acanthamoeba as their natural host in the environment, wherein they multiply. The bacteria then lyse the amebae in order to disperse.
The role of Acanthamoeba spp. as reservoirs or vectors for human pathogens has been examined. Rowbotham studied the interaction of Acanthamoeba with Legionella pneumophila, the causative agent of atypical pneumonia in humans (384). Several strains of L. pneumophila, normally intracellular pathogens of alveolar macrophages, were found to infect Acanthamoeba, indicating that free-living amebae could serve as natural hosts of Legionella. Following phagocytosis by Acanthamoeba, Legionella organisms were observed to “escape” into the cytoplasm rather than being sequestered into lysosomal structures and being degraded by hydrolytic enzymes. Following multiplication, the Legionella organisms lysed the amebae and were released into the surrounding environment. Based on these observations, Rowbotham (384) suggested that vesicles filled with Legionella or amebae filled with bacteria, rather than free bacteria, were the source of legionellosis. Consistent with the postulate of an interaction between Acanthamoeba and Legionella is the observation that Legionella and amebae have been isolated from the same aquatic environments (22, 52, 140, 239). Indeed, amebae isolated directly from river water or soil have been shown to contain Legionella spp. (169, 170, 331). Studies have demonstrated that the temperature at which Legionella and the amebae interact is important. Legionella-induced lysis of host Acanthamoeba cells has been observed to occur at an ambient temperature of 37°C. However, at lower temperatures such as 20°C, a converse interaction occurs in that the amebae phagocytize and digest Legionella (13).
Since the initial observations of Rowbotham, the interaction of Legionella with free-living amebae has been widely studied (Fig. 18). Uptake of Legionella can occur through “coiling” phagocytosis. Bozue and Johnson (48) indicated that following uptake, inhibition of lysosomal fusion with phagosomes containing Legionella occurs within A. castellanii. Subsequently, intracellular multiplication of Legionella and killing of host macrophages and amebae have been described (1, 2, 166, 229, 240, 321). However, while Legionella induces apoptotic cell death in macrophages, it reportedly does not do so in A. castellanii. Gao and Kwaik (148), using L. pneumophila and A. polyphaga, confirmed that L. pneumophila does not kill amebae by apoptosis but, rather, does so by necrosis. Necrotic cell death induced by L. pneumophila and subsequent release of these bacteria from the amebae apparently is mediated by the pore-forming activity of the bacteria. Mutants defective in pore-forming activity fail to exit Acanthamoeba.
FIG. 18.
Scanning (A and B) and transmission (C and D) electron micrographs of co-cultures of A. astronyxis and L. pneumophila. (A) Trophozoite of A. astronyxis extending a fingerlike projection toward a “clump” of Legionella (arrow). (B) Trophozoite of A. astronyxis infected with L. pneumophila and in the apparent process of expelling a vesicle filled with bacteria (arrow). (C) A. astronyxis trophozoites harboring numerous L. pneumophila cells in cytoplasmic vesicles following coculture in vitro. (D) Cyst of A. astronyxis containing L. pneumophila organisms (arrow). Bars, 1 μm.
The mechanisms for recognition, entry, and intracellular proliferation of bacteria in amebae and in mammalian cells may be similar (48, 85, 187, 188). Adaptation of such bacteria to both mammalian cells and amebae suggests an interaction which may confer specified functional attributes to the bacteria. Survival and intracellular growth of bacteria in amebae may prime bacteria for intracellular growth in mammalian cells (24). Cirillo et al. (86) suggested that survival of Legionella strains from intracellular digestion in Acanthamoeba preadapted the bacteria for invasion of human and animal host cells. Recent studies have shown that the interaction of bacteria with amebae may result in changes in the morphology and physiology of the bacteria. For example, L. pneumophila cells grown in Acanthamoeba were reported to be smaller than when cultured in vitro, to display different surface properties, and to exhibit enhanced motility (25, 27, 28, 226). In addition, de novo synthesis of select L. pneumophila antigens has been demonstrated in bacteria grown in amebae (436). Also, bacteria grown in amebae are resistant to chemical disinfectants, and vesicles containing Legionella released from amebae are highly resistant to biocides (25, 33). Furthermore, it has been reported that growth in A. castellanii enhances the capacity of Legionella to invade macrophages and increases the intracellular replication of the bacteria (84, 85, 312, 328). Mycobacterium avium grown in Acanthamoeba also demonstrated enhanced entry and intracellular replication in macrophages (87).
The interaction of Legionella with free-living amebae raises intriguing possibilities for a functional relevance regarding human disease. For example, Legionella-like ameba pathogens (LLAP) which may represent a source of respiratory disease in humans have been identified. These pathogens were first described in 1956 by Drozanski (121) and later named Sarcobium lyticum. S. lyticum is an obligate intracellular parasite of small free-living amebae isolated from soil and water and has been shown to enter amebae by phagocytosis, to divide in the cytoplasm, and to result eventually in lysis of the amebae (123). Comparative sequence analysis of the 16S rRNA gene of Sarcobium with that of related bacteria revealed the closest relationship to Legionella species (147, 423). A number of nonculturable, gram-negative bacilli which multiply intracellularly within amebae have since been isolated and named LLAP 1 through 12 (3, 386). Analysis of the rRNA of the LLAPs has shown that these organisms are related closely to the S. lyticum described in 1956 by Drozanski (147). Since the LLAPs do not grow on conventional artificial media, amebae have been used for isolation and propagation of the organisms. One LLAP was isolated originally by amebal enrichment of a sputum specimen from a patient with pneumonia. The patient demonstrated a fourfold rise in antibody titer to the bacteria from “infected” amebae (3, 386). Thus, while the role of LLAPs as human pathogens remains unresolved, serum specimens from patients with pneumonia tested for LLAPs suggest that the organisms play a role in community-acquired pneumonia (275).
Ameba-bacterium interactions may extend to bacteria beyond Legionella and Legionella-like species. Acanthamoeba organisms recovered from environmental or clinical specimens have been shown to harbor bacterial species including Candidatus Parachlamydia acanthamoebae a member of the Chlamydiaceae, Candidatus Odyssella thessalonicensis, Rickettsia spp., Pseudomonas aeruginosa, and Comamonas acidovoran (11, 37, 144, 302, 303, 464). Laboratory studies using ameba-bacterium microcosms have shown that a number of clinically relevant bacteria such as Mycobacterium avium, M. leprae, Burkholderia cepacia, B. pseudomallei, Simkania negevensis, E. coli O157:H7, Helicobacter pylori, Chlamydia pnuemoniae, and at least three species of Listeria survive and multiply within Acanthamoeba (26, 132, 200, 211, 238, 246, 263, 264, 427, 480). Thom et al. (443) showed that Vibrio cholerae strains multiply and survive in Acanthamoeba. La Scola and Raoult (250a) demonstrated that under experimental conditions, Coxiella burnetti is capable of infecting A. castellanii, within which it undergoes differentiation into spore-like forms. Gaze et al. (155) reported that in cocultures of A. polyphaga and Salmonella enterica serovar Typhimurium, invasion of large contractile vacuoles by bacteria occurred. Barker et al. (26) reported that lysed A. polyphaga could serve as a substrate for pathogenic E. coli O157:H7, the causative agent of hemolytic-uremic syndrome. We have determined that Helicobacter pylori multiplies within Acanthamoeba and is released in vesicles (Fig. 19). Additionally, Pseudomonas aeruginosa was observed in vacuoles when cocultured with A. polyphaga under laboratory conditions. Encystment of A. polyphaga occurred after several days of cocultivation, with Pseudomonas remaining within the cyst compartment (Fig. 20). Steenbergen et al. (424) indicated that the encapsulated yeast Cryptococcus neoformans also replicates in Acanthamoeba and is not degraded by these amebae. Thus, a variety of bacteria, as well as one species of yeast, are able to survive and/or proliferate within Acanthamoeba.
FIG. 19.
Electron micrographs of cocultures of A. astronyxis and Helicobacter pylori. (A) Trophozoite replete with bacteria and in the apparent process of releasing (arrow) a “bolus” of bacteria (96 h coincubation in vitro). (B) Trophozoite containing numerous intracellular vesicles replete with bacteria and surrounded by bacterium-filled vesicles which apparently have been released from the ameba (96 h coincubation in vitro). Bars, 1 μm.
FIG. 20.
Transmission electron micrographs of A. astronyxis cocultured with Pseudomonas aeruginosa. (A) Trophozoite filled with bacteria (72 h of coculture). (B) Cyst of A. astronyxis filled with Pseudomonas (6 days of coculture). Bars, 1 μm.
The intracellular growth of bacteria in Acanthamoeba has been associated with enhanced survival of bacteria in the environment, increased resistance of bacteria to biocides, and increased bacterial virulence. Intracellular survival within the amebae has been postulated as a mode by which bacteria survive in substrate-limiting environmental ecosystems (227, 240). Intracellular growth of bacteria in amebae apparently also affects the resistance of bacteria to antibiotics (28). For example, L. pneumophila grown in A. polyphaga and M. avium grown in A. castellanii were reported to be protected from antimicrobials. Survival studies indicated that L. pneumophila isolates grown in Acanthamoeba, and tested after release from these host cells, were approximately 1,000-fold more resistant to rifampin or ciprofloxacin than were L. pneumophila cells grown exclusively in vitro (27). Miltner and Bermudez (308) reported that no significant activity against M. avium was observed with rifabutin, azithromycin, and clarithromycin when these antimicrobials were employed to treat infected A. castellanii. In contrast, these antibiotics exhibited significant anti-M. avium activity when tested against infected U937 macrophage-like cells. These investigators proposed that growth of M. avium in amebae reduced the effectiveness of these antimicrobials. It has also been shown that amebae survive chlorination and apparently protect engulfed bacteria such as S. enterica serovar Typhimurium, Yersinia enterocolitica, Shigella sonnei, and Campylobacter jejuni from free chlorine (227). Such resistance to chlorination could have health implications, especially for treatment regimens applied to “drinking” water. In addition, Acanthamoeba organisms also appear to protect bacteria when the amebae form cysts (385). The double wall of the cyst is resistant to many drugs and chemicals (448). Bacteria which remain in cysts after trophozoites have encysted appear to be protected from chlorine and other disinfectants or biocides (221).
There are accumulating data which suggest a strong potential for Acanthamoeba to serve as bacterial reservoirs for human infection. Larkin and Easty (248) demonstrated experimentally that coinfection of rat corneas with Corynebacterium and Acanthamoeba led to suppurative keratitis whereas infection with either alone did not, suggesting that the presence of bacteria played a role in the establishment of corneal infection. It has also been postulated that a bacterium-related inflammatory response occurs in AK as a result of the release of bacterial endosymbionts from amebae (479). The isolation of Legionella, Pseudomonas spp., and Acanthamoeba from eye wash stations (343) and of Acanthamoeba spp. naturally infected with P. aeruginosa from a contaminated drinking water system in a hospital (302) further indicates a potential for Acanthamoeba to serve as reservoirs for human infection. Acanthamoeba and bacteria were present on shower heads in bathrooms in a hospital setting in Austria (465). Thermotolerant Acanthamoeba organisms were identified which contained an ocular pathogen, Comamonas acidovorans. The presence of a P. aeruginosa biofilm on the surface of contact lenses has been reported to increase the adsorption of amebic trophozoites to the lens, suggesting an increased opportunity for the development of AK (415). It has also been shown that of several environmental isolates of Acanthamoeba, whether obtained through natural or experimental infection, those harboring endosymbiotic bacteria demonstrate CPE on cultured human embryonic fibroblast cells more quickly than do those not containing endosymbionts (146). Induction of CPE was most rapid on monolayers infected with Acanthamoeba which included Chlamydia-like endosymbionts. In addition, specific antibodies to chlamydia-related organisms, as well as DNA sequences, have been detected in patients with respiratory disease (38). In summary, a body of data indicates that Acanthamoeba spp. have the potential to act as cofactors in human infection or as reservoirs for a number of bacterial pathogens.
CONCLUSIONS
Free-living amebae of the genus Acanthamoeba are found worldwide and inhabit a variety of water, air, and soil environments. These amebae are the causative agents of GAE, AK, and cutaneous and sinus lesions. While AK may occur in both immunocompetent and immunodeficient individuals, the preponderance of cases of GAE and cutaneous acanthamebiasis have been found in individuals with compromised immune systems, including those with AIDS. Rapid diagnosis of Acanthmoeba infections is requisite to the application of successful therapy. Diagnosis and treatment of Acanthamoeba infections are difficult due to the rarity of the infections, the lack of familiarity of most clinicians with the disease syndromes, and the limitations of therapeutic options, given the similarity of protozoal physiology to that of other eukaryotic cells, including those of mammals. In vitro culture of amebae and application of specialized staining procedures for tissue sections or cytological preparations are useful in the formulation of a laboratory diagnosis. The use of newer molecular methods to identify Acanthamoeba in tissue could provide a more rapid means to diagnose infection. The mechanisms by which Acanthamoeba cause disease are unresolved, although it is apparent that multiple modes of action including cell contact-dependent and -independent processes may be involved. Similarly, the role of the immune system in resistance to infection remains unresolved. Recent studies suggest that sIgA, complement, neutrophils, and macrophages may play important roles in resistance to infection. The recognition that Acanthamoeba spp. sequester a variety of bacteria with known potential for causing human disease suggests that these amebae serve as reservoirs for bacterial pathogens. Finally, the increase in the reported incidence of Acanthamoeba infections may be the result of greater recognition of the disease potential of these organisms. In addition, a number of factors may account for an increased incidence of infection, such as a large number of HIV-infected individuals and more patients undergoing cancer chemotherapy or immunosuppressive therapy for organ transplantation.
Acknowledgments
This work was supported in part by NIH/NIDA Center grant P50 DA05274 from the National Institutes of Health.
We thank Thomas Byers, Ohio State University and Andrew Rogerson, Nova Southeastern, for numerous discussions concerning classification of Acanthamoeba; Julio Martinez for providing a photograph of the human brain and a tissue sample from a patient who died of GAE; The Journal of Neuropathology and Experimental Neurology and P. C. Maudgal, Katholieke Universiteit Leuven, for providing a photograph of a patient with AK; and S. Gaylen Bradley for discussions.
REFERENCES
- 1.Abu Kwaik, Y., L. Y. Gao, B. J. Stone, and O. S. Harb. 1998. Invasion of mammalian and protozoan cells by Legionella pneumophila. Bull. Inst. Pasteur 96:237-247. [Google Scholar]
- 2.Abu Kwaik, Y., L. Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeleke, A., J. Pruckler, R. Benson, T. Rowbotham, M. Halablab, and B. Fields. 1996. Legionella-like amebal pathogens—phylogenetic status and possible role in respiratory disease. Emerg. Infect. Dis. 2:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfieri, S. C., C. E. Correia, S. A. Motegi, and E. M. Pral. 2000. Proteinase activities in total extracts and in medium conditioned by Acanthamoeba polyphaga trophozoites. J. Parasitol. 86:220-227. [DOI] [PubMed] [Google Scholar]
- 5.Alizadeh, H., S. Apte, M. S. El Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh, H., Y. He, J. P. McCulley, D. Ma, G. L. Stewart, M. Via, E. Haehling, and J. Y. Niederkorn. 1995. Successful immunization against Acanthamoeba keratitis in a pig model. Cornea 14:180-186. [PubMed] [Google Scholar]
- 7.Alizadeh, H., M. S. Pidherney, J. P. McCulley, and J. Y. Niederkorn. 1994. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect. Immun. 62:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Khouri, A. M., and M. R. Paule. 2002. A novel RNA polymerase I transcription initiation factor, TIF-IE, commits rRNA genes by interaction with TIF-IB, not by DNA binding. Mol. Cell. Biol. 22:750-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen, P. G., and E. A. Dawidowicz. 1990. Phagocytosis in Acanthamoeba. I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J. Cell. Physiol. 145:508-513. [DOI] [PubMed] [Google Scholar]
- 10.Alves, J. M., C. X. Gusmao, M. M. Teixeira, D. Freitas, A. S. Foronda, and H. T. Affonso. 2000. Random amplified polymorphic DNA profiles as a tool for the characterization of Brazilian keratitis isolates of the genus Acanthamoeba. Braz. J. Med. Biol. Res. 33:19-26. [DOI] [PubMed] [Google Scholar]
- 11.Amann, R., N. Springer, W. Schonhuber, W. Ludwig, E. N. Schmid, K. D. Muller, and R. Michel. 1997. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl. Environ. Microbiol. 63:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaral Zettler, L. A., T. A. Nerad, C. J. O'Kelly, M. T. Peglar, P. M. Gillevet, J. D. Silberman, and M. L. Sogin. 2000. A molecular reassessment of the Leptomyxid amoebae. Protist 151:275-282. [DOI] [PubMed] [Google Scholar]
- 13.Anand, C. M., A. R. Skinner, A. Malic, and J. B. Kurtz. 1983. Interaction of L. pneumophila and a free living amoeba (Acanthamoeba palestinensis). J. Hyg. 91:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderlini, P., D. Przepiorka, M. Luna, L. Langford, M. Andreeff, D. Claxton, and A. B. Deisseroth. 1994. Acanthamoeba meningoencephalitis after bone marrow transplantation. Bone Marrow Transplant. 14:459-461. [PubMed] [Google Scholar]
- 15.Armstrong, M. 2000. The pathogenesis of human Acanthamoeba infection. Infect. Dis. Rev. 2:65-73. [Google Scholar]
- 16.Auran, J. D., M. B. Starr, and F. A. Jakobiec. 1987. Acanthamoeba keratitis. A review of the literature. Cornea 6:2-26. [PubMed] [Google Scholar]
- 17.Bacon, A. S., D. G. Frazer, J. K. Dart, M. Matheson, L. A. Ficker, and P. Wright. 1993. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye 7:719-725. [DOI] [PubMed] [Google Scholar]
- 18.Badenoch, P. R., M. Adams, and D. J. Coster. 1995. Corneal virulence, cytopathic effect on human keratocytes and genetic characterization of Acanthamoeba. Int. J. Parasitol. 25:229-239. [DOI] [PubMed] [Google Scholar]
- 19.Badenoch, P. R., T. R. Grimmond, J. Cadwgan, S. E. Deayton, and M. S. L. Essery. 1988. Nasal carriage of free-living amoebae. Microb. Ecol. Health Dis. 1:209-211. [Google Scholar]
- 20.Badenoch, P. R., A. M. Johnson, P. E. Christy, and D. J. Coster. 1990. Pathogenicity of Acanthamoeba and a Corynebacterium in the rat cornea. Arch. Ophthalmol. 108:107-112. [DOI] [PubMed] [Google Scholar]
- 21.Badenoch, P. R., A. M. Johnson, P. E. Christy, and D. J. Coster. 1991. A model of Acanthamoeba keratitis in the rat. Rev. Infect. Dis. 13(Suppl. 5):S445.. [DOI] [PubMed] [Google Scholar]
- 22.Barbaree, J. M., B. S. Fields, J. C. Feeley, G. W. Gorman, and W. T. Martin. 1986. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl. Environ. Microbiol. 51:422-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbeau, J., and T. Buhler. 2001. Biofilms augment the number of free-living amoebae in dental unit waterlines. Res. Microbiol. 152:753-760. [DOI] [PubMed] [Google Scholar]
- 24.Barker, J., and M. R. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 25.Barker, J., M. R. Brown, P. J. Collier, I. Farrell, and P. Gilbert. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 58:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker, J., T. J. Humphrey, and M. W. Brown. 1999. Survival of Escherichia coli O157 in a soil protozoan: implications for disease. FEMS Microbiol. Lett. 173:291-295. [DOI] [PubMed] [Google Scholar]
- 27.Barker, J., P. A. Lambert, and M. R. Brown. 1993. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect. Immun. 61:3503-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker, J., H. Scaife, and M. R. Brown. 1995. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 39:2684-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateman, E. 1998. Autoregulation of eukaryotic transcription factors. Prog. Nucleic Acid Res. Mol. Biol. 60:133-168. [DOI] [PubMed] [Google Scholar]
- 30.Beattie, T. K., A. Tomlinson, and D. V. Seal. 2002. Anti-Acanthamoeba efficacy in contact lens disinfecting systems. Br. J. Ophthalmol. 86:1319-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson, R. L., L. Ansbacher, R. E. Hutchison, and W. Rogers. 1985. Cerebrospinal fluid centrifuge analysis in primary amebic meningoencephalitis due to Naegleria fowleri. Arch. Pathol. Lab. Med. 109:668-671. [PubMed] [Google Scholar]
- 32.Berger, S. T., B. J. Mondino, R. H. Hoft, P. B. Donzis, G. N. Holland, M. K. Farley, and J. E. Levenson. 1990. Successful medical management of Acanthamoeba keratitis. Am. J. Ophthalmol. 110:395-403. [DOI] [PubMed] [Google Scholar]
- 33.Berk, S. G., R. S. Ting, G. W. Turner, and R. J. Ashburn. 1998. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 64:279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaduri, C. R., K. Janitschke, and K. N. Masihi. 1987. Immunity to Acanthamoeba culbertsoni: experimental studies with Acanthamoeba and control antigen preparations. Trans. R. Soc. Trop. Med. Hyg. 81:768-770. [DOI] [PubMed] [Google Scholar]
- 35.Bhagwandeen, S. B., R. F. Carter, K. G. Naik, and D. Levitt. 1975. A case of hartmannellid amebic meningoencephalitis in Zambia. Am. J. Clin. Pathol. 63:483-492. [DOI] [PubMed] [Google Scholar]
- 36.Binder, P. S. 1989. Cryotherapy for medically unresponsive acanthamoeba keratitis. Cornea 8:106-114. [PubMed] [Google Scholar]
- 37.Birtles, R. J., T. J. Rowbotham, R. Michel, D. G. Pitcher, B. Lascola, S. Alexiou-Daniel, and D. Raoult. 2000. “Candidatus Odyssella thessalonicensis' gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int. J. Syst. E vol. Microbiol. 50:63-72. [DOI] [PubMed] [Google Scholar]
- 38.Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349:925-926. [DOI] [PubMed] [Google Scholar]
- 39.Bogler, S. A., C. D. Zarley, L. L. Burianek, P. A. Fuerst, and T. J. Byers. 1983. Interstrain mitochondrial DNA polymorphism detected in Acanthamoeba by restriction endonuclease analysis. Mol. Biochem. Parasitol. 8:145-163. [DOI] [PubMed] [Google Scholar]
- 40.Bonilla, H. F., A. Whitehurst, and C. A. Kauffman. 1999. Acanthamoeba sinusitis and disseminated infection in a patient with AIDS. Infect. Med. 16:397-400. [Google Scholar]
- 41.Booton, G. C., D. J. Kelly, Y. W. Chu, D. V. Seal, E. Houang, D. S. Lam, T. J. Byers, and P. A. Fuerst. 2002. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 40:1621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Booton, G. C., D. R. Ledee, M. H. Awwad, S. Sharma, I. Nizsl, M. M. Markus, P. A. Fuerst, and T. J. Byers. 2001. Acanthamoeba mitochondrial 16S rDNA sequences: inferred phylogeny and support of nuclear ribosomal 18S rDNA gene sequence types, p. 227-234. In S. Billot-Bonef, P. A. Cabanes, F. Marciano-Cabral, P. Pernin, and E. Pringuez (ed.), IXth International Meeting on the Biology and Pathogenicity of Free-Living Amoebae Proceedings. John Libbey Eurotext, Paris, France.
- 43.Borochovitz, D., A. J. Martinez, and G. T. Patterson. 1981. Osteomyelitis of a bone graft of the mandible with Acanthamoeba castellanii infection. Hum. Pathol. 12:573-576. [DOI] [PubMed] [Google Scholar]
- 44.Bowers, B. 1977. Comparison of pinocytosis and phagocytosis in Acanthamoeba castellanii. Exp. Cell Res. 110:409-417. [DOI] [PubMed] [Google Scholar]
- 45.Bowers, B., and E. D. Korn. 1968. The fine structure of Acanthamoeba castellanii. I. The trophozoite. J. Cell Biol. 39:95-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowers, B., and E. D. Korn. 1969. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell Biol. 41:786-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers, B., and T. E. Olszewski. 1983. Acanthamoeba discriminates internally between digestible and indigestible particles. J. Cell Biol. 97:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozue, J. A., and W. Johnson. 1996. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradley, S. G. 1996. Inhibitors of the free-living amoebae Naegleria and Acanthamoeba. Recent Res. Dev. Antimicrob. Agents Chemotherapy 1:53-63. [Google Scholar]
- 50.Bradley, S. G., and F. Marciano-Cabral. 1996. Diversity of free-living ”naked' amoeboid organisms. J. Ind. Microbiol. 17:314-321. [Google Scholar]
- 51.Brasseur, G., L. Favennec, D. Perrine, J. P. Chenu, and P. Brasseur. 1994. Successful treatment of Acanthamoeba keratitis by hexamidine. Cornea 13:459-462. [DOI] [PubMed] [Google Scholar]
- 52.Breiman, R. F., W. Cozen, B. S. Fields, T. D. Mastro, S. J. Carr, J. S. Spika, and L. Mascola. 1990. Role of air sampling in investigation of an outbreak of legionnaires' disease associated with exposure to aerosols from an evaporative condenser. J. Infect. Dis. 161:1257-1261. [DOI] [PubMed] [Google Scholar]
- 53.Brooks, J. G., Jr., D. J. Coster, and P. R. Badenoch. 1994. Acanthamoeba keratitis. Resolution after epithelial debridement. Cornea 13:186-189. [PubMed] [Google Scholar]
- 54.Brown, R. C., H. Bass, and J. P. Coombs. 1975. Carbohydrate binding proteins involved in phagocytosis by Acanthamoeba. Nature 254:434-435. [DOI] [PubMed] [Google Scholar]
- 55.Brown, T. J., and R. T. Cursons. 1977. Pathogenic free-living amebae (PFLA) from frozen swimming areas in Oslo, Norway. Scand. J. Infect. Dis. 9:237-240. [DOI] [PubMed] [Google Scholar]
- 56.Burger, G., I. Plante, K. M. Lonergan, and M. W. Gray. 1995. The mitochondrial DNA of the amoeboid protozoon, Acanthamoeba castellanii: complete sequence, gene content and genome organization. J. Mol. Biol. 245:522-537. [DOI] [PubMed] [Google Scholar]
- 57.Butt, C. G. 1966. Primary amebic meningoencephalitis. N. Engl. J. Med. 274:1473-1476. [DOI] [PubMed] [Google Scholar]
- 58.Butt, C. G., C. Baro, and R. W. Knorr. 1968. Naegleria (sp.) identified in amebic encephalitis. Am. J. Clin. Pathol. 50:568-574. [DOI] [PubMed] [Google Scholar]
- 59.Byers, T. J. 1979. Growth, reproduction, and differentiation in Acanthamoeba. Int. Rev. Cytol. 61:283-338. [DOI] [PubMed] [Google Scholar]
- 60.Byers, T. J., R. A. Akins, B. J. Maynard, R. A. Lefken, and S. M. Martin. 1980. Rapid growth of Acanthamoeba in defined media; induction of encystment by glucose-acetate starvation. J. Protozool. 27:216-219. [DOI] [PubMed] [Google Scholar]
- 61.Byers, T. J., G. C. Booton, D. R. Stothard, R. J. Gast, D. R. Ledee, J. M. Schroeder, M. H. Awwad, and P. A. Fuerst. 2001. Studies of the phylogeny, systematics and pathogenicity of Acanthamoeba using ribosomal and transfer RNA gene sequences, p. 211-218. In S. Billot-Bonef, P. A. Cabanes, F. Marciano-Cabral, P. Pernin, and E. Pringuez (ed.), IXth International Meeting on the Biology and Pathogenicity of Free-Living Amoebae Proceedings. John Libbey Eurotext, Paris, France.
- 62.Byers, T. J., E. R. Hugo, and V. J. Stewart. 1990. Genes of Acanthamoeba: DNA, RNA and protein sequences (a review). J. Protozool. 37:17S-25S. [DOI] [PubMed] [Google Scholar]
- 63.Byers, T. J., B. G. Kim, L. E. King, and E. R. Hugo. 1991. Molecular aspects of the cell cycle and encystment of Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S373-S384. [DOI] [PubMed] [Google Scholar]
- 64.Callicott, J. H., Jr. 1968. Amebic meningoencephalitis due to free-living amebas of the Hartmannella (Acanthamoeba)-Naegleria group. Am. J. Clin. Pathol. 49:84-91. [DOI] [PubMed] [Google Scholar]
- 65.Calore, E. E., M. J. Cavaliere, and N. M. Calore. 1997. Cerebral amebiasis in the acquired immunodeficiency syndrome. Acta Neurol. Belg. 97:248-250. [PubMed] [Google Scholar]
- 66.Cao, Z., D. M. Jefferson, and N. Panjwani. 1998. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J. Biol. Chem. 273:15838-15845. [DOI] [PubMed] [Google Scholar]
- 67.Casemore, D. P. 1970. Sensitivity of Hartmannella (Acanthamoeba) to 5-fluorocytosine, hydroxystilbamidine, and other substances. J. Clin. Pathol. 23:649-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casemore, D. P. 1977. Free-living amoebae in home dialysis unit. Lancet ii:1078.. [DOI] [PubMed] [Google Scholar]
- 69.Casper, T., D. Basset, C. Leclercq, J. Fabre, N. Peyron-Raison, and J. Reynes. 1999. Disseminated acanthamoeba infection in a patient with AIDS: response to 5-fluorocytosine therapy. Clin. Infect. Dis. 29:944-945. [DOI] [PubMed] [Google Scholar]
- 70.Castellani, A. 1930. An amoeba found in cultures of a yeast: preliminary note. J. Trop. Med. Hyg. 33:160. [Google Scholar]
- 71.Cerva, L. 1967. Intracerebral inoculation of experimental animals in pathological studies of Hartmanella castellanii. Folia Parasitol. (Prague) 14:171-175. [Google Scholar]
- 72.Cerva, L. 1967. Intranasal, intrapulmonary and intracardial inoculation of experimental animals with Hartmanella castellanii. Folia Parasitol. (Prague) 14:207-215. [Google Scholar]
- 73.Cerva, L. 1989. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J. Hyg. Epidemiol. Microbiol. Immunol. 33:99-103. [PubMed] [Google Scholar]
- 74.Cerva, L. and K. Novak. 1968. Amoebic meningoencephalitis: 16 fatalities. Science 160:92.. [DOI] [PubMed] [Google Scholar]
- 75.Cerva, L., C. Serbus, and V. Skocil. 1973. Isolation of limax amoebae from the nasal mucosa of man. Folia Parasitol. (Prague) 20:97-103. [PubMed] [Google Scholar]
- 76.Chagla, A. H., and A. J. Griffiths. 1974. Growth and encystation of Acanthamoeba castellanii. J. Gen. Microbiol. 85:139-145. [DOI] [PubMed] [Google Scholar]
- 77.Chandrasekar, P. H., P. S. Nandi, M. R. Fairfax, and L. R. Crane. 1997. Cutaneous infections due to Acanthamoeba in patients with acquired immunodeficiency syndrome. Arch. Intern. Med. 157:569-572. [PubMed] [Google Scholar]
- 78.Chappell, C. L., J. A. Wright, M. Coletta, and A. L. Newsome. 2001. Standardized method of measuring acanthamoeba antibodies in sera from healthy human subjects. Clin. Diagn. Lab. Immunol. 8:724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen, L., and E. Bateman. 2000. Linker scanning analysis of TBP promoter binding factor DNA binding, activation, and repression domains. J. Biol. Chem. 275:2771-2776. [DOI] [PubMed] [Google Scholar]
- 80.Chew, S. J., R. W. Beuerman, M. Assouline, H. E. Kaufman, B. A. Barron, and J. M. Hill. 1992. Early diagnosis of infectious keratitis with in vivo real time confocal microscopy. CLAO J. 18:197-201. [PubMed] [Google Scholar]
- 81.Cho, J. H., B. K. Na, T. S. Kim, and C. Y. Song. 2000. Purification and characterization of an extracellular serine proteinase from Acanthamoeba castellanii. IUBMB Life 50:209-214. [DOI] [PubMed] [Google Scholar]
- 82.Chu, D. M., H. Miles, D. Toney, C. Ngyuen, and F. Marciano-Cabral. 1998. Amebicidal activity of plant extracts from Southeast Asia on Acanthamoeba spp. Parasitol. Res. 84:746-752. [DOI] [PubMed] [Google Scholar]
- 83.Chung, D. I., H. S. Yu, M. Y. Hwang, T. H. Kim, T. O. Kim, H. C. Yun, and H. H. Kong. 1998. Subgenus classification of Acanthamoeba by riboprinting. Korean J. Parasitol. 36:69-80. [DOI] [PubMed] [Google Scholar]
- 84.Cianciotto, N. P., and B. S. Fields. 1992. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc. Natl. Acad. Sci. USA 89:5188-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cirillo, J. D., S. Falkow, and L. S. Tompkins. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62:3254-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cleland, P. G., R. V. Lawande, G. Onyemelukwe, and H. C. Whittle. 1982. Chronic amebic meningoencephalitis. Arch. Neurol. 39:56-57. [DOI] [PubMed] [Google Scholar]
- 89.Cohen, E. J., C. J. Parlato, J. J. Arentsen, G. I. Genvert, R. C. Eagle, Jr., M. R. Wieland, and P. R. Laibson. 1987. Medical and surgical treatment of Acanthamoeba keratitis. Am. J. Ophthalmol. 103:615-625. [DOI] [PubMed] [Google Scholar]
- 90.Comandon, J., and P. de Fonbrune. 1935. Observations sur une amibe. Ann. Physiol. 11:1004-1007. [Google Scholar]
- 91.Corliss, J. O. 1998. Classification of protozoa and protists: the current status, p. 409-447. In G. H. Coombs, K. Vickerman, M. A. Sleigh, and A. Warren (ed.), Evolutionary relationships among protozoa. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 92.Costas, M., and A. J. Griffiths. 1985. Enzyme composition and the taxonomy of Acanthamoeba. J. Protozool. 32:604-607. [DOI] [PubMed] [Google Scholar]
- 93.Cote, M. A., J. A. Irvine, N. A. Rao, and M. D. Trousdale. 1991. Evaluation of the rabbit as a model of Acanthamoeba keratitis. Rev. Infect. Dis. 13(Suppl. 5):S443-S444. [DOI] [PubMed] [Google Scholar]
- 94.Cremona, G., M. A. Carrasco, A. Tytiun, and M. J. Cosentino. 2002. Treatment of advanced acanthamoeba keratitis with deep lamellar keratectomy and conjunctival flap. Cornea 21:705-708. [DOI] [PubMed] [Google Scholar]
- 95.Culbertson, C. G. 1961. Pathogenic Acanthamoeba (Hartmannella). Am. J. Clin. Pathol. 35:195-202. [DOI] [PubMed] [Google Scholar]
- 96.Culbertson, C. G. 1971. The pathogenicity of soil amebas. Annu. Rev. Microbiol. 25:231-254. [DOI] [PubMed] [Google Scholar]
- 97.Culbertson, C. G., P. W. Ensminger, and W. M. Overton. 1966. Hartmannella (Acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. Am. J. Clin. Pathol. 46:305-314. [DOI] [PubMed] [Google Scholar]
- 98.Culbertson, C. G., J. W. Smith, I. Cohen, and J. R. Minner. 1959. Experimental infection of mice and monkeys by Acanthamoeba. Am. J. Pathol. 35:185-197. [PMC free article] [PubMed] [Google Scholar]
- 99.Culbertson, C. G., J. W. Smith, and H. Minner. 1958. Acanthamoebae: observations on animal pathogenicity. Science 127:1506.. [DOI] [PubMed] [Google Scholar]
- 100.Cursons, R. 1981. A simple staining method for the detection of amoebae. N. Z. Med. J. 94:471.. [PubMed] [Google Scholar]
- 101.Cursons, R. T., and T. J. Brown. 1978. Use of cell cultures as an indicator of pathogenicity of free-living amoebae. J. Clin. Pathol. 31:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cursons, R. T., T. J. Brown, and E. A. Keys. 1978. Virulence of pathogenic free-living amebae. J. Parasitol. 64:744-745. [PubMed] [Google Scholar]
- 103.Cursons, R. T., T. J. Brown, E. A. Keys, K. M. Moriarty, and D. Till. 1980. Immunity to pathogenic free-living amoebae: role of cell-mediated immunity. Infect. Immun. 29:408-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cursons, R. T., T. J. Brown, E. A. Keys, K. M. Moriarty, and D. Till. 1980. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect. Immun. 29:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daggett, P. M., D. Lipscomb, T. K. Sawyer, and T. A. Nerad. 1985. A molecular approach to the phylogeny of Acanthamoeba. Biosystems 18:399-405. [DOI] [PubMed] [Google Scholar]
- 106.Danso, S. K., and M. Alexander. 1975. Regulation of predation by prey density: the protozoan-Rhizobium relationship. Appl. Microbiol. 29:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Das, S. R., S. Asiri, A. el Soofi, and H. P. Baer. 1991. Protective and curative effects of rifampicin in Acanthamoeba meningitis of the mouse. J. Infect. Dis. 163:916-917. [DOI] [PubMed] [Google Scholar]
- 108.D'Aversa, G., G. A. Stern, and W. T. Driebe, Jr. 1995. Diagnosis and successful medical treatment of Acanthamoeba keratitis. Arch. Ophthalmol. 113:1120-1123. [DOI] [PubMed] [Google Scholar]
- 109.De Jonckheere, J., and H. Van de Voorde. 1976. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl. Environ. Microbiol. 31:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Jonckheere, J. F. 1980. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl. Environ. Microbiol. 39:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Jonckheere, J. F. 1991. Ecology of Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S385-S387. [DOI] [PubMed] [Google Scholar]
- 112.De Jonckheere, J. F. 1983. Isoenzyme and total protein analysis by agarose isoelectric focusing, and taxonomy of the genus Acanthamoeba. J. Protozool. 30:701-706. [Google Scholar]
- 113.Denney, C. F., V. J. Iragui, L. D. Uber-Zak, N. C. Karpinski, E. J. Ziegler, G. S. Visvesvara, and S. L. Reed. 1997. Amebic meningoencephalitis caused by Balamuthia mandrillaris: case report and review. Clin. Infect. Dis. 25:1354-1358. [DOI] [PubMed] [Google Scholar]
- 114.Diaz, J., A. Osuna, M. J. Rosales, J. Cifuentes, and C. Mascaro. 1991. Sucker-like structures in two strains of Acanthamoeba: scanning electron microscopy study. Int. J. Parasitol. 21:365-367. [DOI] [PubMed] [Google Scholar]
- 115.Di Gregorio, C., F. Rivasi, N. Mongiardo, B. De Rienzo, S. Wallace, and G. S. Visvesvara. 1992. Acanthamoeba meningoencephalitis in a patient with acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 116:1363-1365. [PubMed] [Google Scholar]
- 116.Donzis, P. B., B. J. Mondino, B. A. Weissman, and D. A. Bruckner. 1987. Microbial contamination of contact lens care systems. Am. J. Ophthalmol. 104:325-333. [DOI] [PubMed] [Google Scholar]
- 117.Doren, G. S., E. J. Cohen, S. E. Higgins, I. J. Udell, R. C. Eagle, Jr., J. J. Arentsen, and P. R. Laibson. 1991. Management of contact lens associated Acanthamoeba keratitis. CLAO J. 17:120-125. [PubMed] [Google Scholar]
- 118.Dougherty, P. J., P. S. Binder, B. J. Mondino, and B. J. Glasgow. 1994. Acanthamoeba sclerokeratitis. Am. J. Ophthalmol. 117:475-479. [DOI] [PubMed] [Google Scholar]
- 119.Douglas, M. 1930. Notes on the classification of the amoeba found by Castellani in cultures of a yeast-like fungus. J. Trop. Med. Hyg. 33:258. [Google Scholar]
- 120.Draulans, E., and P. C. Maudgal. 1992. Acanthamoeba keratitis. Bull. Soc. Belge Ophtalmol. 243:115-121. [PubMed] [Google Scholar]
- 121.Drozanski, W. 1956. Fatal bacterial infection in soil amoebae. Acta Microbiol. Pol. 5:315-317. [PubMed] [Google Scholar]
- 122.Drozanski, W. 1969. Bacteriolytic enzyme produced by Acanthamoeba castellanii. Acta Microbiol. Pol. Ser. A 1:155-168. [PubMed] [Google Scholar]
- 123.Drozanski, W. 1991. Sarcobium lyticum gen. nov., sp. nov., an obligate intracellular bacterial parasite of small free-living amoebae. Int. J. Syst. Bacteriol. 41:82-87. [Google Scholar]
- 124.Duguid, I. G., J. K. Dart, N. Morlet, B. D. Allan, M. Matheson, L. Ficker, and S. Tuft. 1997. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology 104:1587-1592. [DOI] [PubMed] [Google Scholar]
- 125.Duluol, A. M., M. F. Teilhac, and J. L. Poirot. 1996. Cutaneous lesions due to Acanthamoeba sp. in patients with AIDS. J. Eukaryot. Microbiol. 43:130-131. [DOI] [PubMed] [Google Scholar]
- 126.Duma, R. J., and R. Finley. 1976. In vitro susceptibility of pathogenic Naegleria and Acanthamoeba species to a variety of therapeutic agents. Antimicrob. Agents Chemother. 10:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duma, R. J., W. B. Helwig, and A. J. Martinez. 1978. Meningoencephalitis and brain abscess due to a free-living amoeba. Ann. Intern. Med. 88:468-473. [DOI] [PubMed] [Google Scholar]
- 128.Dunand, V. A., S. M. Hammer, R. Rossi, M. Poulin, M. A. Albrecht, J. P. Doweiko, P. C. DeGirolami, E. Coakley, E. Piessens, and C. A. Wanke. 1997. Parasitic sinusitis and otitis in patients infected with human immunodeficiency virus: report of five cases and review. Clin. Infect. Dis. 25:267-272. [DOI] [PubMed] [Google Scholar]
- 129.Dykova, I., J. Lom, J. M. Schroeder-Diedrich, G. C. Booton, and T. J. Byers. 1999. Acanthamoeba strains isolated from organs of freshwater fishes. J. Parasitol. 85:1106-1113. [PubMed] [Google Scholar]
- 130.Elson, C., R. P. Geyer, and R. S. Chang. 1970. A lecithinase from the amoeba Hartmannella rhysodes. J. Protozool. 17:440-445. [DOI] [PubMed] [Google Scholar]
- 131.Epstein, R. J., L. A. Wilson, G. S. Visvesvara, and E. G. Plourde, Jr. 1986. Rapid diagnosis of Acanthamoeba keratitis from corneal scrapings using indirect fluorescent antibody staining. Arch. Ophthalmol. 104:1318-1321. [DOI] [PubMed] [Google Scholar]
- 132.Essig, A., M. Heinemann, U. Simnacher, and R. Marre. 1997. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl. Environ. Microbiol. 63:1396-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feingold, J. M., J. Abraham, S. Bilgrami, N. Ngo, G. S. Visvesara, R. L. Edwards, and P. J. Tutschka. 1998. Acanthamoeba meningoencephalitis following autologous peripheral stem cell transplantation. Bone Marrow Transplant. 22:297-300. [DOI] [PubMed] [Google Scholar]
- 134.Ferrante, A. 1991. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 13:31-47. [DOI] [PubMed] [Google Scholar]
- 135.Ferrante, A. 1991. Immunity to Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S403-S409. [DOI] [PubMed] [Google Scholar]
- 136.Ferrante, A., and T. J. Abell. 1986. Conditioned medium from stimulated mononuclear leukocytes augments human neutrophil-mediated killing of a virulent Acanthamoeba sp. Infect. Immun. 51:607-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferrante, A., and E. J. Bates. 1988. Elastase in the pathogenic free-living amoebae Naegleria and Acanthamoeba spp. Infect. Immun. 56:3320-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferrante, A., and B. Rowan-Kelly. 1983. Activation of the alternative pathway of complement by Acanthamoeba culbertsoni. Clin. Exp. Immunol. 54:477-485. [PMC free article] [PubMed] [Google Scholar]
- 139.Ficker, L., D. Seal, D. Warhurst, and P. Wright. 1990. Acanthamoeba keratitis—resistance to medical therapy. Eye 4:835-838. [DOI] [PubMed] [Google Scholar]
- 140.Fields, B. S., G. N. Sanden, J. M. Barbaree, W. E. Morrill, R. M. Wadowsky, E. H. White, and J. C. Feeley. 1989. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 18:131-137. [Google Scholar]
- 141.Font, R. L., M. J. Tapert, N. M. Robinson, G. Visvesvara, D. Murphy, and M. S. Osato. 1982. An animal model of Acanthamoeba keratitis. Further studies with emphasis on the early destruction of trophozoites. Investig. Ophthalmol. Visual Sci. 22:S163. [Google Scholar]
- 142.Fowler, M., and R. F. Carter. 1965. Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br. Med. J. 5464:740-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Friedland, L. R., S. A. Raphael, E. S. Deutsch, J. Johal, L. J. Martyn, G. S. Visvesvara, and H. W. Lischner. 1992. Disseminated Acanthamoeba infection in a child with symptomatic human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 11:404-407. [DOI] [PubMed] [Google Scholar]
- 144.Fritsche, T. R., R. K. Gautom, S. Seyedirashti, D. L. Bergeron, and T. D. Lindquist. 1993. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J. Clin. Microbiol. 31:1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fritsche, T. R., M. Horn, S. Seyedirashti, R. K. Gautom, K. H. Schleifer, and M. Wagner. 1999. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl. Environ. Microbiol. 65:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fritsche, T. R., D. Sobek, and R. K. Gautom. 1998. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol. Lett. 166:231-236. [DOI] [PubMed] [Google Scholar]
- 147.Fry, N. K., T. J. Rowbotham, N. A. Saunders, and T. M. Embley. 1991. Direct amplification and sequencing of the 16S ribosomal DNA of an intracellular Legionella species recovered by amoebal enrichment from the sputum of a patient with pneumonia. FEMS Microbiol. Lett. 67:165-168. [DOI] [PubMed] [Google Scholar]
- 148.Gao, L. Y., and Y. A. Kwaik. 2000. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ. Microbiol. 2:79-90. [DOI] [PubMed] [Google Scholar]
- 149.Gardner, H. A., A. J. Martinez, G. S. Visvesvara, and A. Sotrel. 1991. Granulomatous amebic encephalitis in an AIDS patient. Neurology 41:1993-1995. [DOI] [PubMed] [Google Scholar]
- 150.Garner, A. 1993. Pathogenesis of acanthamoebic keratitis: hypothesis based on a histological analysis of 30 cases. Br. J. Ophthalmol. 77:366-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gast, R. J. 2001. Development of an Acanthamoeba-specific reverse dot-blot and the discovery of a new ribotype. J. Eukaryot. Microbiol. 48:609-615. [DOI] [PubMed] [Google Scholar]
- 152.Gast, R. J., D. R. Ledee, P. A. Fuerst, and T. J. Byers. 1996. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J. Eukaryot. Microbiol. 43:498-504. [DOI] [PubMed] [Google Scholar]
- 153.Gautom, R. K., and T. R. Fritsche. 1995. Transmissibility of bacterial endosymbionts between isolates of Acanthamoeba spp. J. Eukaryot. Microbiol. 42:452-456. [DOI] [PubMed] [Google Scholar]
- 154.Gautom, R. K., S. Lory, S. Seyedirashti, D. L. Bergeron, and T. R. Fritsche. 1994. Mitochondrial DNA fingerprinting of Acanthamoeba spp. isolated from clinical and environmental sources. J. Clin. Microbiol. 32:1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gaze, W. H., N. Burroughs, M. P. Gallagher, and E. M. H. Wellington. 2001. Interactions between Salmonella typhimurium and Acanthamoeba polyphaga: a new mode of intracellular growth within contractile vacuoles, p. 155-157. In S. Billot-Bonef, P. A. Cabanes, F. Marciano-Cabral, P. Pernin, and E. Pringuez (ed.), IXth International Meeting on the Biology and Pathogenicity of Free-Living Amoebae Proceedings. John Libbey Eurotext, Paris, France.
- 156.Gelman, B. B., S. J. Rauf, R. Nader, V. Popov, J. Borkowski, G. Chaljub, H. W. Nauta, and G. S. Visvesvara. 2001. Amoebic encephalitis due to Sappinia diploidea. JAMA 285:2450-2451. [DOI] [PubMed] [Google Scholar]
- 157.Gonzalez, M. M., E. Gould, G. Dickinson, A. J. Martinez, G. Visvesvara, T. J. Cleary, and G. T. Hensley. 1986. Acquired immunodeficiency syndrome associated with Acanthamoeba infection and other opportunistic organisms. Arch. Pathol. Lab. Med. 110:749-751. [PubMed] [Google Scholar]
- 158.Gonzalez-Robles, A., A. Flores-Langarica, M. Omana-Molina, and M. Shibayama. 2001. Acanthamoeba castellanii: ultrastructure of trophozoites using fast freeze-fixation followed by freeze-substitution. J. Electron Microsc. (Tokyo) 50:423-427. [DOI] [PubMed] [Google Scholar]
- 159.Gordon, S. M., J. P. Steinberg, M. H. DuPuis, P. E. Kozarsky, J. F. Nickerson, and G. S. Visvesvara. 1992. Culture isolation of Acanthamoeba species and leptomyxid amebas from patients with amebic meningoencephalitis, including two patients with AIDS. Clin. Infect. Dis. 15:1024-1030. [DOI] [PubMed] [Google Scholar]
- 160.Gordon, V. R., E. K. Asem, M. H. Vodkin, and G. L. McLaughlin. 1993. Acanthamoeba binds to extracellular matrix proteins in vitro. Investig. Ophthalmol. Visual Sci. 34:658-662. [PubMed] [Google Scholar]
- 161.Gray, T. B., K. A. Gross, R. T. Cursons, and J. F. Shewan. 1994. Acanthamoeba keratitis: a sobering case and a promising new treatment. Aust. N. Z. J. Ophthalmol. 22:73-76. [DOI] [PubMed] [Google Scholar]
- 162.Grimm, D., W. F. Ludwig, B. C. Brandt, R. Michel, K. H. Schleifer, J. Hacker, and M. Steinert. 2001. Development of 18S rRNA-targeted oligonucleotide probes for specific detection of Hartmannella and Naegleria in Legionella-positive environmental samples. Syst. Appl. Microbiol. 24:76-82. [DOI] [PubMed] [Google Scholar]
- 163.Grunnet, M. L., G. H. Cannon, and J. P. Kushner. 1981. Fulminant amebic meningoencephalitis due to Acanthamoeba. Neurology 31:174-176. [DOI] [PubMed] [Google Scholar]
- 164.Gullett, J., J. Mills, K. Hadley, B. Podemski, L. Pitts, and R. Gelber. 1979. Disseminated granulomatous acanthamoeba infection presenting as an unusual skin lesion. Am. J. Med. 67:891-896. [DOI] [PubMed] [Google Scholar]
- 165.Hadas, E., and T. Mazur. 1993. Proteolytic enzymes of pathogenic and non-pathogenic strains of Acanthamoeba spp. Trop. Med. Parasitol. 44:197-200. [PubMed] [Google Scholar]
- 166.Hagele, S., J. Hacker, and B. C. Brand. 1998. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FEMS Microbiol. Lett. 169:51-58. [DOI] [PubMed] [Google Scholar]
- 167.Hahn, T. W., T. P. O'Brien, W. J. Sah, and J. H. Kim. 1998. Acridine orange staining for rapid diagnosis of Acanthamoeba keratitis. Jpn. J. Ophthalmol. 42:108-114. [DOI] [PubMed] [Google Scholar]
- 168.Hall, J., and H. Voelz. 1985. Bacterial endosymbionts of Acanthamoeba sp. J. Parasitol. 71:89-95. [PubMed] [Google Scholar]
- 169.Harf, C. 1994. Free-living amoeba: interactions with environmental pathogenic bacteria. Endocytobiosis Cell Res. 10:167-183. [Google Scholar]
- 170.Harf, C., and H. Monteil. 1988. Interaction between free-living amoebae and Legionella in the environment. Water Sci. Technol. 20:235-239. [Google Scholar]
- 171.Hargrave, S. L., J. P. McCulley, Z. Husseini, and the Brolene Study Group. 1999. Results of a trial of combined propamidine isethionate and neomycin therapy for Acanthamoeba keratitis. Ophthalmology 106:952-957. [DOI] [PubMed] [Google Scholar]
- 172.Harwood, C. R., G. E. Rich, R. McAleer, and G. Cherian. 1988. Isolation of Acanthamoeba from a cerebral abscess. Med. J. Aust. 148:47-49. [DOI] [PubMed] [Google Scholar]
- 173.Hawley, H. B., J. S. Czachor, V. Malhotra, J. W. Funkhouser, and G. S. Visvesvara. 1997. Acanthamoeba encephalitis in patients with AIDS. AIDS Reader 7:134-137. [Google Scholar]
- 174.Hay, J., F. Kinnear, and C. M. Kirkness. 1995. Acanthamoeba keratitis: laboratory diagnosis, characterisation of protozoa and treatment. Scottish Centre Infect. Environ. Health 29:90-91. [Google Scholar]
- 175.Hay, J., and D. V. Seal. 1996. Acanthamoeba keratitis: risk factors and outcome. Br. J. Ophthalmol. 80:773.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He, Y. G., J. P. McCulley, H. Alizadeh, M. Pidherney, J. Mellon, J. E. Ubelaker, G. L. Stewart, R. E. Silvany, and J. Y. Niederkorn. 1992. A pig model of Acanthamoeba keratitis: transmission via contaminated contact lenses. Investig. Ophthalmol. Visual Sci. 33:126-133. [PubMed] [Google Scholar]
- 177.He, Y. G., J. Y. Niederkorn, J. P. McCulley, G. L. Stewart, D. R. Meyer, R. Silvany, and J. Dougherty. 1990. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Investig. Ophthalmol. Visual Sci. 31:2235-2240. [PubMed] [Google Scholar]
- 178.Heffler, K. F., T. J. Eckhardt, A. C. Reboli, and D. Stieritz. 1996. Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am. J. Ophthalmol. 122:584-586. [DOI] [PubMed] [Google Scholar]
- 179.Helton, J., M. Loveless, and C. R. White, Jr. 1993. Cutaneous acanthamoeba infection associated with leukocytoclastic vasculitis in an AIDS patient. Am. J. Dermatopathol. 15:146-149. [DOI] [PubMed] [Google Scholar]
- 180.Hirst, L. W., W. R. Green, W. Merz, C. Kaufmann, G. S. Visvesvara, A. Jensen, and M. Howard. 1984. Management of Acanthamoeba keratitis. A case report and review of the literature. Ophthalmology 91:1105-1111. [DOI] [PubMed] [Google Scholar]
- 181.Hiti, K., J. Walochnik, C. Faschinger, E. M. Haller-Schober, and H. Aspock. 2001. Microwave treatment of contact lens cases contaminated with acanthamoeba. Cornea 20:467-470. [DOI] [PubMed] [Google Scholar]
- 182.Hiti, K., J. Walochnik, E. M. Haller-Schober, C. Faschinger, and H. Aspock. 2002. Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br. J. Ophthalmol. 86:144-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoffmann, R., R. Michel, K. D. Muller, and E. N. Schmid. 1998. Archaea like endocytobiotic organisms isolated from Acanthamoeba sp (GR II). Endocytobiosis Cell Res. 12:185-188. [Google Scholar]
- 184.Holland, G. N., and P. B. Donzis. 1987. Rapid resolution of early Acanthamoeba keratitis after epithelial debridement. Am. J. Ophthalmol. 104:87-89. [DOI] [PubMed] [Google Scholar]
- 185.Horn, M., T. R. Fritsche, R. K. Gautom, K. H. Schleifer, and M. Wagner. 1999. Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ. Microbiol. 1:357-367. [DOI] [PubMed] [Google Scholar]
- 186.Horne, D. D., M. E. Frizell, L. Ingham, R. G. Jans, S. M. Gubash, C. M. Anand, and M. A. Athar. 1994. Acanthamoeba keratitis: an emerging clinical problem. CMAJ 150:923-925. [PMC free article] [PubMed] [Google Scholar]
- 187.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Horwitz, M. A. 1984. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27-33. [DOI] [PubMed] [Google Scholar]
- 189.Howe, D. K., M. H. Vodkin, R. J. Novak, G. Visvesvara, and G. L. McLaughlin. 1997. Identification of two genetic markers that distinguish pathogenic and nonpathogenic strains of Acanthamoeba spp. Parasitol. Res. 83:345-348. [DOI] [PubMed] [Google Scholar]
- 190.Huang, W., and E. Bateman. 1997. Transcription of the Acanthamoeba TATA-binding protein gene. A single transcription factor acts both as an activator and a repressor. J. Biol. Chem. 272:3852-3859. [DOI] [PubMed] [Google Scholar]
- 191.Hughes, R., and S. Kilvington. 2001. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob. Agents Chemother. 45:2038-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hunt, S. J., S. L. Reed, W. C. Mathews, and B. Torian. 1995. Cutaneous Acanthamoeba infection in the acquired immunodeficiency syndrome: response to multidrug therapy. Cutis 56:285-287. [PubMed] [Google Scholar]
- 193.Hurt, M., S. Apte, H. Leher, K. Howard, J. Niederkorn, and H. Alizadeh. 2001. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect. Immun. 69:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iida, C. T., P. Kownin, and M. R. Paule. 1985. Ribosomal RNA transcription: proteins and DNA sequences involved in preinitiation complex formation. Proc. Natl. Acad. Sci. USA 82:1668-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Illingworth, C. D., and S. D. Cook. 1998. Acanthamoeba keratitis. Surv. Ophthalmol. 42:493-508. [DOI] [PubMed] [Google Scholar]
- 196.Illingworth, C. D., S. D. Cook, C. H. Karabatsas, and D. L. Easty. 1995. Acanthamoeba keratitis: risk factors and outcome. Br. J. Ophthalmol. 79:1078-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Im, K. I., K. M. Park, T. S. Yong, Y. P. Hong, and T. E. Kim. 1999. Upregulated expression of the cDNA fragment possibly related to the virulence of Acanthamoeba culbertsoni. Korean J. Parasitol. 37:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ishibashi, Y., Y. Matsumoto, T. Kabata, R. Watanabe, S. Hommura, K. Yasuraoka, and K. Ishii. 1990. Oral itraconazole and topical miconazole with debridement for Acanthamoeba keratitis. Am. J. Ophthalmol. 109:121-126. [DOI] [PubMed] [Google Scholar]
- 199.Jacobson, L. M., and R. N. Band. 1987. Genetic heterogeneity in a natural population of Acanthamoeba polyphaga from soil, an isoenzyme analysis. J. Protozool. 34:83-86. [Google Scholar]
- 200.Jadin, J. B. 1975. Free-living pathogenic amoebae. Wiad. Parazytol. 21:493-498. (In Polish.) [PubMed] [Google Scholar]
- 201.Jager, B. V., and W. P. Stamm. 1972. Brain abscesses caused by free-living amoeba probably of the genus Hartmannella in a patient with Hodgkin's disease. Lancet ii:1343-1345. [DOI] [PubMed] [Google Scholar]
- 202.Jahnes, B., H. M. Fullmer, and C. P. Li. 1957. Free-living amoebae as contaminants in monkey kidney tissue cultures. Proc. Soc. Exp. Biol. Med. 96:484-488. [DOI] [PubMed] [Google Scholar]
- 203.Jantzen, H., I. Schulze, and M. Stohr. 1988. Relationship between the timing of DNA replication and the developmental competence in Acanthamoeba castellanii. J. Cell Sci. 91:389-399. [DOI] [PubMed] [Google Scholar]
- 204.Jeansson, S., and T. K. Kvien. 2001. Acanthamoeba polyphaga in rheumatoid arthritis: possibility for a chronic infection. Scand. J. Immunol. 53:610-614. [DOI] [PubMed] [Google Scholar]
- 205.John, T., J. Lin, D. Sahm, and J. H. Rockey. 1991. Effects of corticosteroids in experimental Acanthamoeba keratitis. Rev. Infect. Dis. 13(Suppl. 5):S440-S442. [DOI] [PubMed] [Google Scholar]
- 206.Johns, K. J., W. S. Head, and D. M. O'Day. 1988. Corneal toxicity of propamidine. Arch. Ophthalmol. 106:68-69. [DOI] [PubMed] [Google Scholar]
- 207.Johns, K. J., W. S. Head, R. D. Robinson, T. E. Williams, and D. M. O'Day. 1991. Examination of the contact lens with light microscopy: an aid in diagnosis of Acanthamoeba keratitis. Rev. Infect. Dis. 13(Suppl. 5):S425.. [DOI] [PubMed] [Google Scholar]
- 208.Johns, K. J., D. M. O'Day, and S. S. Feman. 1988. Chorioretinitis in the contralateral eye of a patient with Acanthamoeba keratitis. Ophthalmology 95:635-639. [DOI] [PubMed] [Google Scholar]
- 209.Johnson, A. M., R. Fielke, P. E. Christy, B. Robinson, and P. R. Baverstock. 1990. Small subunit ribosomal RNA evolution in the genus Acanthamoeba. J. Gen. Microbiol. 136:1689-1698. [DOI] [PubMed] [Google Scholar]
- 210.Jones, B. R., G. S. Visvesvara, and N. M. Robinson. 1975. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. U. K. 95:221-232. [PubMed] [Google Scholar]
- 211.Kahane, S., B. Dvoskin, M. Mathias, and M. G. Friedman. 2001. Infection of Acanthamoeba polyphaga with Simkania negevensis and S. negevensis. Survival within amoebal cysts. Appl. Environ. Microbiol. 67:4789-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kennett, M. J., R. R. Hook, Jr., C. L. Franklin, and L. K. Riley. 1999. Acanthamoeba castellanii: characterization of an adhesin molecule. Exp. Parasitol. 92:161-169. [DOI] [PubMed] [Google Scholar]
- 213.Kenney, M. 1971. The Micro-Kolmer complement fixation test in routine screening for soil ameba infection. Health Lab. Sci. 8:5-10. [PubMed] [Google Scholar]
- 214.Khalife, G. E., S. W. Pambuccian, G. S. Visvesvara, and B. Horten. 1994. Disseminated Acanthamoeba infection masquerading as bacillary angiomatosis in a patient with AIDS. Int. J. Surg. Pathol. 2:11-16. [Google Scholar]
- 215.Khan, N. A., J. Greenman, K. P. Topping, V. C. Hough, G. S. Temple, and T. A. Paget. 2000. Isolation of Acanthamoeba-specific antibodies from a bacteriophage display library. J. Clin. Microbiol. 38:2374-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khan, N. A., E. L. Jarroll, and T. A. Paget. 2001. Acanthamoeba can be differentiated by the polymerase chain reaction and simple plating assays. Curr. Microbiol. 43:204-208. [DOI] [PubMed] [Google Scholar]
- 217.Khunkitti, W., D. Lloyd, J. R. Furr, and A. D. Russell. 1998. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J. Infect. 36:43-48. [DOI] [PubMed] [Google Scholar]
- 218.Kidney, D. D., and S. H. Kim. 1998. CNS infections with free-living amebas: neuroimaging findings. Am. J. Roentgenol. 171:809-812. [DOI] [PubMed] [Google Scholar]
- 219.Kilvington, S., J. R. Beeching, and D. G. White. 1991. Differentiation of Acanthamoeba strains from infected corneas and the environment by using restriction endonuclease digestion of whole-cell DNA. J. Clin. Microbiol. 29:310-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kilvington, S., D. F. Larkin, D. G. White, and J. R. Beeching. 1990. Laboratory investigation of Acanthamoeba keratitis. J. Clin. Microbiol. 28:2722-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kilvington, S., and J. Price. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519-525. [DOI] [PubMed] [Google Scholar]
- 222.Kilvington, S., and D. G. White. 1994. Acanthamoeba: biology, ecology and human disease. Rev. Med. Micobiol. 5:12-20. [Google Scholar]
- 223.Kim, J. Y., Y. S. Choi, and J. H. Lee. 1997. Keratitis from corneal anesthetic abuse after photorefractive keratectomy. J. Cataract Refract. Surg. 23:447-449. [DOI] [PubMed] [Google Scholar]
- 224.Kim, S. Y., M. J. Syms, M. R. Holtel, and K. K. Nauschuetz. 2000. Acanthamoeba sinusitis with subsequent dissemination in an AIDS patient. Ear Nose Throat J. 79:168, 171-168, 174. [PubMed] [Google Scholar]
- 225.Kim, Y. H., M. S. Ock, H. C. Yun, M. Y. Hwang, H. S. Yu, H. H. Kong, and D. I. Chung. 1996. Close relatedness of Acanthamoeba pustulosa with Acanthamoeba palestinensis based on isoenzyme profiles and rDNA PCR-RFLP patterns. Korean J. Parasitol. 34:259-266. [DOI] [PubMed] [Google Scholar]
- 226.King, C. H., B. S. Fields, E. B. Shotts, Jr., and E. H. White. 1991. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect. Immun. 59:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kingston, D. and D. C. Warhurst. 1969. Isolation of amoebae from the air. J. Med. Microbiol. 2:27-36. [DOI] [PubMed] [Google Scholar]
- 229.Kirby, J. E., J. P. Vogel, H. L. Andrews, and R. R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323-336. [DOI] [PubMed] [Google Scholar]
- 230.Koide, J., E. Okusawa, T. Ito, S. Mori, T. Takeuchi, S. Itoyama, and T. Abe. 1998. Granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with systemic lupus erythematosus. Clin. Rheumatol. 17:329-332. [DOI] [PubMed] [Google Scholar]
- 231.Kong, H. H., and D. I. Chung. 1996. PCR and RFLP variation of conserved region of small subunit ribosomal DNA among Acanthamoeba isolates assigned to either A. castellanii or A. polyphaga. Korean J. Parasitol. 34:127-134. [DOI] [PubMed] [Google Scholar]
- 232.Kong, H. H., and D. I. Chung. 2002. A riboprinting scheme for identification of unknown Acanthamoeba isolates at species level. Korean J. Parasitol. 40:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kong, H. H., T. H. Kim, and D. I. Chung. 2000. Purification and characterization of a secretory serine proteinase of Acanthamoeba healyi isolated from GAE. J. Parasitol. 86:12-17. [DOI] [PubMed] [Google Scholar]
- 234.Kong, H. H., J. H. Park, and D. I. Chung. 1995. Interstrain polymorphisms of isoenzyme profiles and mitochondrial DNA fingerprints among seven strains assigned to Acanthamoeba polyphaga. Korean J. Parasitol. 33:331-340. [DOI] [PubMed] [Google Scholar]
- 235.Kong, H. H., and T. D. Pollard. 2002. Intracellular localization and dynamics of myosin-II and myosin-IC in live Acanthamoeba by transient transfection of EGFP fusion proteins. J. Cell Sci. 115:4993-5002. [DOI] [PubMed] [Google Scholar]
- 236.Kosrirukvongs, P., D. Wanachiwanawin, and G. S. Visvesvara. 1999. Treatment of acanthamoeba keratitis with chlorhexidine. Ophthalmology 106:798-802. [DOI] [PubMed] [Google Scholar]
- 237.Kremer, I., E. J. Cohen, R. C. Eagle, Jr., I. Udell, and P. R. Laibson. 1994. Histopathologic evaluation of stromal inflammation in Acanthamoeba keratitis. CLAO J. 20:45-48. [PubMed] [Google Scholar]
- 238.Krishna Prasad, B. N., and S. K. Gupta. 1978. Preliminary report on the engulfment and retention of Mycobacteria by trophozoites of exenically grown Acanthamoeba castellanii Douglas, 1930. Curr. Sci. 47:245-247. [Google Scholar]
- 239.Kurtz, J. B., C. L. Bartlett, U. A. Newton, R. A. White, and N. L. Jones. 1982. Legionella pneumophila in cooling water systems. Report of a survey of cooling towers in London and a pilot trial of selected biocides. J. Hyg. 88:369-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kwaik, Y. A. 1998. Fatal attraction of mammalian cells to Legionella pneumophila. Mol. Microbiol. 30:689-695. [DOI] [PubMed] [Google Scholar]
- 241.Reference deleted.
- 242.Lagmay, J. P., R. R. Matias, F. F. Natividad, and G. L. Enriquez. 1999. Cytopathogenicity of Acanthamoeba isolates on rat glial C6 cell line. Southeast Asian J. Trop. Med. Public Health 30:670-677. [PubMed] [Google Scholar]
- 243.Lai, S., M. Asgari, and H. R. Henney, Jr. 1994. Non-radioactive DNA probe and polymerase chain reaction procedures for the specific detection of Acanthamoeba. Mol. Cell Probes 8:81-89. [DOI] [PubMed] [Google Scholar]
- 244.Lal, A. A., and N. K. Garg. 1979. Hartmanella culbertsoni: biochemical changes in the brain of the meningoencephalitic mouse. Exp. Parasitol. 48:331-336. [DOI] [PubMed] [Google Scholar]
- 245.Lalitha, M. K., V. Anandi, A. Srivastava, K. Thomas, A. M. Cherian, and S. M. Chandi. 1985. Isolation of Acanthamoeba culbertsoni from a patient with meningitis. J. Clin. Microbiol. 21:666-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Landers, P., K. G. Kerr, T. J. Rowbotham, J. L. Tipper, P. M. Keig, E. Ingham, and M. Denton. 2000. Survival and growth of Burkholderia cepacia within the free-living amoeba Acanthamoeba polyphaga. Eur. J. Clin. Microbiol. Infect. Dis. 19:121-123. [DOI] [PubMed] [Google Scholar]
- 247.Larkin, D. F., M. Berry, and D. L. Easty. 1991. In vitro corneal pathogenicity of Acanthamoeba. Eye 5:560-568. [DOI] [PubMed] [Google Scholar]
- 248.Larkin, D. F., and D. L. Easty. 1990. Experimental Acanthamoeba keratitis. I. Preliminary findings. Br. J. Ophthalmol. 74:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Larkin, D. F., and D. L. Easty. 1991. Experimental Acanthamoeba keratitis. II. Immunohistochemical evaluation. Br. J. Ophthalmol. 75:421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Larkin, D. F., S. Kilvington, and J. K. Dart. 1992. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 99:185-191. [DOI] [PubMed] [Google Scholar]
- 250a.La Scola, B., and D. Raoult. 2001. Survival of Coxiella burnetii within free-living amoeba Acanthamoeba castellanii. Clin. Microbiol. Infect. 7:75-79. [DOI] [PubMed] [Google Scholar]
- 251.Ledee, D. R., J. Hay, T. J. Byers, D. V. Seal, and C. M. Kirkness. 1996. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Investig. Ophthalmol. Visual Sci. 37:544-550. [PubMed] [Google Scholar]
- 252.Leher, H., K. Kinoshita, H. Alizadeh, F. L. Zaragoza, Y. He, and J. Niederkorn. 1998. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Investig. Ophthalmol. Visual Sci. 39:2337-2343. [PubMed] [Google Scholar]
- 253.Leher, H., R. Silvany, H. Alizadeh, J. Huang, and J. Y. Niederkorn. 1998. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect. Immun. 66:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Leher, H., F. Zaragoza, S. Taherzadeh, H. Alizadeh, and J. Y. Niederkorn. 1999. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp. Eye Res. 69:75-84. [DOI] [PubMed] [Google Scholar]
- 255.Leher, H. F., H. Alizadeh, W. M. Taylor, A. S. Shea, R. S. Silvany, F. Van Klink, M. J. Jager, and J. Y. Niederkorn. 1998. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Investig. Ophthalmol. Visual Sci. 39:2666-2673. [PubMed] [Google Scholar]
- 256.Lehmann, O. J., S. M. Green, N. Morlet, S. Kilvington, M. F. Keys, M. M. Matheson, J. K. Dart, J. I. McGill, and P. J. Watt. 1998. Polymerase chain reaction analysis of corneal epithelial and tear samples in the diagnosis of Acanthamoeba keratitis. Investig. Ophthalmol. Visual Sci. 39:1261-1265. [PubMed] [Google Scholar]
- 257.Lengy, J., R. Jakovljevich, and B. Talis. 1971. Recovery of a hartmannelloid ameba from a purulent ear discharge. Harefuah 80:23-24. [PubMed] [Google Scholar]
- 258.Levine, S., A. E. Goldstein, M. Dahdouh, P. Blank, C. Hoffman, and C. A. Gropper. 2001. Cutaneous Acanthamoeba in a patient with AIDS: a case study with a review of new therapy. Cutis 67:377-380. [PubMed] [Google Scholar]
- 259.Lewis, E. J., and T. K. Sawyer. 1979. Acanthamoeba tubiashi n. sp., a new species of fresh water amoebida (Acanthamoebidae). Trans. Am. Microsc. Soc. 98:543-549. [Google Scholar]
- 260.Lindquist, T. D. 1998. Treatment of Acanthamoeba keratitis. Cornea 17:11-16. [DOI] [PubMed] [Google Scholar]
- 261.Lindquist, T. D., N. A. Sher, and D. J. Doughman. 1988. Clinical signs and medical therapy of early Acanthamoeba keratitis. Arch. Ophthalmol. 106:73-77. [DOI] [PubMed] [Google Scholar]
- 262.Lloyd, D., N. A. Turner, W. Khunkitti, A. C. Hann, J. R. Furr, and A. D. Russell. 2001. Encystation in Acanthamoeba castellanii: development of biocide resistance. J. Eukaryot. Microbiol. 48:11-16. [DOI] [PubMed] [Google Scholar]
- 263.Ly, T. M. C., and H. E. Muller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 264.Ly, T. M. C. and H. E. Muller. 1990. Interactions of Listeria monocytogenes, Listeria seeligeri, and Listeria innocula with protozoans. J. Gen. Appl. Microbiol. 36:143-150. [Google Scholar]
- 265.Ma, P., G. S. Visvesvara, A. J. Martinez, F. H. Theodore, P. M. Daggett, and T. K. Sawyer. 1990. Naegleria and Acanthamoeba infections: review. Rev. Infect. Dis. 12:490-513. [DOI] [PubMed] [Google Scholar]
- 266.Madrigal Sesma, M. J. 1988. Isolation of free-living amoebae, potentially pathogenic for humans, from 3 species of saurians from the western Canary Islands. Rev. Sanid. Hig. Publica 62:1405-1409. [PubMed] [Google Scholar]
- 267.Madrigal Sesma, M. J., and L. M. Zapatero Ramos. 1989. Isolation of free-living amoebas from the intestinal contents of reptiles. J. Parasitol. 75:322-324. [PubMed] [Google Scholar]
- 268.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Marciano-Cabral, F., and S. G. Bradley. 2001. Current research on free-living amebae causing granulomatous amebic encephalitis. J. Eukaryot. Microbiol. 48:3S.. [DOI] [PubMed] [Google Scholar]
- 270.Marciano-Cabral, F., T. Ferguson, S. G. Bradley, and G. Cabral. 2001. Delta-9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana, exacerbates brain infection by Acanthamoeba. J. Eukaryot. Microbiol. Suppl:4S-5S. [DOI] [PubMed] [Google Scholar]
- 271.Marciano-Cabral, F., R. Puffenbarger, and G. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 272.Marciano-Cabral, F., and D. M. Toney. 1998. The interaction of Acanthamoeba spp. with activated macrophages and with macrophage cell lines. J. Eukaryot. Microbiol. 45:452-458. [DOI] [PubMed] [Google Scholar]
- 273.Marines, H. M., M. S. Osato, and R. L. Font. 1987. The value of calcofluor white in the diagnosis of mycotic and Acanthamoeba infections of the eye and ocular adnexa. Ophthalmology 94:23-26. [DOI] [PubMed] [Google Scholar]
- 274.Markowitz, S. M., T. Sobieski, A. J. Martinez, and R. J. Duma. 1978. Experimental Acanthamoeba infections in mice pretreated with methylprednisolone or tetracycline. Am. J. Pathol. 92:733-743. [PMC free article] [PubMed] [Google Scholar]
- 275.Marrie, T. J., D. Raoult, B. La Scola, R. J. Birtles, and E. de Carolis. 2001. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 7:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Martinez, A. J. 1980. Is Acanthamoeba encephalitis an opportunistic infection? Neurology 30:567-574. [DOI] [PubMed] [Google Scholar]
- 277.Martinez, A. J. 1982. Acanthamoebiasis and immunosuppression. Case report. J. Neuropathol. Exp. Neurol. 41:548-557. [DOI] [PubMed] [Google Scholar]
- 278.Martinez, A. J. 1987. Clinical manifestations of free-living amebic infections, p. 161-177. In E. G. Rondanelli (ed.), Amphizoic amoebae: human pathology. Piccin Nuova Libraria, Padua, Italy.
- 279.Martinez, A. J. 1991. Infection of the central nervous system due to Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S399-S402. [DOI] [PubMed] [Google Scholar]
- 280.Martinez, A. J., C. A. Garcia, M. Halks-Miller, and R. Arce-Vela. 1980. Granulomatous amebic encephalitis presenting as a cerebral mass lesion. Acta Neuropathol. (Berlin) 51:85-91. [DOI] [PubMed] [Google Scholar]
- 281.Martinez, A. J., A. E. Guerra, J. Garcia-Tamayo, G. Cespedes, J. E. Gonzalez-Alfonzo, and G. S. Visvesvara. 1994. Granulomatous amebic encephalitis: a review and report of a spontaneous case from Venezuela. Acta Neuropathol. (Berlin) 87:430-434. [DOI] [PubMed] [Google Scholar]
- 282.Martinez, A. J., and K. Janitschke. 1985. Acanthamoeba, an opportunistic microorganism: a review. Infection 13:251-256. [DOI] [PubMed] [Google Scholar]
- 283.Martinez, A. J., S. M. Markowitz, and R. J. Duma. 1975. Experimental pneumonitis and encephalitis caused by Acanthamoeba in mice: pathogenesis and ultrastructural features. J. Infect. Dis. 131:692-699. [DOI] [PubMed] [Google Scholar]
- 284.Martinez, A. J., C. Sotelo-Avila, J. Garcia-Tamayo, J. T. Moron, E. Willaert, and W. P. Stamm. 1977. Meningoencephalitis due to Acanthamoeba sp. Pathogenesis and clinico-pathological study. Acta Neuropathol. (Berlin) 37:183-191. [DOI] [PubMed] [Google Scholar]
- 285.Martinez, A. J., and G. S. Visvesvara. 1991. Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, Acanthamoeba, and Leptomyxida. Clin. Lab Med. 11:861-872. [PubMed] [Google Scholar]
- 286.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Masihi, K. N., C. R. Bhaduri, H. Werner, K. Janitschke, and W. Lange. 1986. Effects of muramyl dipeptide and trehalose dimycolate on resistance of mice to Toxoplasma gondii and Acanthamoeba culbertsoni infections. Int. Arch. Allergy Appl. Immunol. 81:112-117. [DOI] [PubMed] [Google Scholar]
- 288.Mathers, W., G. Stevens, Jr., M. Rodrigues, C. C. Chan, J. Gold, G. S. Visvesvara, M. A. Lemp, and L. E. Zimmerman. 1987. Immunopathology and electron microscopy of Acanthamoeba keratitis. Am. J. Ophthalmol. 103:626-635. [DOI] [PubMed] [Google Scholar]
- 289.Mathers, W. D., S. E. Nelson, J. L. Lane, M. E. Wilson, R. C. Allen, and R. Folberg. 2000. Confirmation of confocal microscopy diagnosis of Acanthamoeba keratitis using polymerase chain reaction analysis. Arch. Ophthalmol. 118:178-183. [DOI] [PubMed] [Google Scholar]
- 290.Mathers, W. D., J. E. Sutphin, R. Folberg, P. A. Meier, R. P. Wenzel, and R. G. Elgin. 1996. Outbreak of keratitis presumed to be caused by Acanthamoeba. Am. J. Ophthalmol. 121:129-142. [DOI] [PubMed] [Google Scholar]
- 291.Matson, D. O., E. Rouah, R. T. Lee, D. Armstrong, J. T. Parke, and C. J. Baker. 1988. Acanthameba meningoencephalitis masquerading as neurocysticercosis. Pediatr. Infect. Dis. J. 7:121-124. [DOI] [PubMed] [Google Scholar]
- 292.Mattana, A., F. Bennardini, S. Usai, P. L. Fiori, F. Franconi, and P. Cappuccinelli. 1997. Acanthamoeba castellanii metabolites increase the intracellular calcium level and cause cytotoxicity in wish cells. Microb. Pathog. 23:85-93. [DOI] [PubMed] [Google Scholar]
- 293.Mattana, A., M. G. Tozzi, M. Costa, G. Delogu, P. L. Fiori, and P. Cappuccinelli. 2001. By releasing ADP, Acanthamoeba castellanii causes an increase in the cytosolic free calcium concentration and apoptosis in wish cells. Infect. Immun. 69:4134-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Maudgal, P. C. 1989. Acanthamoeba keratitis: report of three cases. Bull. Soc. Belge Ophtalmol. 231:135-148. [PubMed] [Google Scholar]
- 295.May, L. P., G. S. Sidhu, and M. R. Buchness. 1992. Diagnosis of Acanthamoeba infection by cutaneous manifestations in a man seropositive to HIV. J. Am. Acad. Dermatol. 26:352-355. [DOI] [PubMed] [Google Scholar]
- 296.Mazur, T., and E. Hadas. 1994. The effect of the passages of Acanthamoeba strains through mice tissues on their virulence and its biochemical markers. Parasitol. Res. 80:431-434. [DOI] [PubMed] [Google Scholar]
- 297.Mazur, T., E. Hadas, and I. Iwanicka. 1995. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop. Med. Parasitol. 46:106-108. [PubMed] [Google Scholar]
- 298.McClellan, K., K. Howard, J. Y. Niederkorn, and H. Alizadeh. 2001. Effect of steroids on Acanthamoeba cysts and trophozoites. Investig. Ophthalmol. Visual Sci. 42:2885-2893. [PubMed] [Google Scholar]
- 299.McCulley, J. P., H. Alizadeh, and J. Y. Niederkorn. 1995. Acanthamoeba keratitis. CLAO J. 21:73-76. [PubMed] [Google Scholar]
- 300.Meisler, D. M., I. Rutherford, F. E. Bican, I. H. Ludwig, R. H. Langston, G. S. Hall, E. Rhinehart, and G. S. Visvesvara. 1985. Susceptibility of Acanthamoeba to surgical instrument sterilization techniques. Am. J. Ophthalmol. 99:724-725. [DOI] [PubMed] [Google Scholar]
- 301.Mergeryan, H. 1991. The prevalence of Acanthamoeba in the human environment. Rev. Infect. Dis. 13(Suppl. 5):S390-S391. [DOI] [PubMed] [Google Scholar]
- 302.Michel, R., H. Burghardt, and H. Bergmann. 1995. Acanthamoeba, naturally intracellularly infected with Pseudomonas aeruginosa, after their isolation from a microbiologically contaminated drinking water system in a hospital. Zentbl. Hyg. Umweltmed. 196:532-544. [PubMed] [Google Scholar]
- 303.Michel, R., and B. Hauroder. 1997. Isolation of an Acanthamoeba strain with intracellular Burkholderia pickettii infection. Zentbl. Bakteriol. 285:541-557. [DOI] [PubMed] [Google Scholar]
- 304.Michel, R., B. Hauroder-Philippczyk, K. Muller, and I. Weishaar. 1994. Acanthamoeba from human nasal mucosa infected with an obligate intracellular parasite. Eur. J. Protistol. 30:104-110. [Google Scholar]
- 305.Michel, R., K. D. Muller, and R. Hoffmann. 2001. Enlarged Chlamydia-like organisms as spontaneous infection of Acanthamoeba castellanii. Parasitol. Res. 87:248-251. [DOI] [PubMed] [Google Scholar]
- 306.Mietz, H., and R. L. Font. 1997. Acanthamoeba keratitis with granulomatous reaction involving the stroma and anterior chamber. Arch. Ophthalmol. 115:259-263. [DOI] [PubMed] [Google Scholar]
- 307.Migueles, S., and P. Kumar. 1998. Primary cutaneous acanthamoeba infection in a patient with AIDS. Clin. Infect. Dis. 27:1547-1548. [DOI] [PubMed] [Google Scholar]
- 308.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mishra, S. K., S. C. Maitra, N. K. Garg, and A. M. Kidwai. 1986. Enzyme and chemical composition of plasma membranes of virulent and avirulent strains of Acanthamoeba culbertsoni. Indian J. Biochem. Biophys. 23:5-8. [PubMed] [Google Scholar]
- 310.Mitra, M. M., H. Alizadeh, R. D. Gerard, and J. Y. Niederkorn. 1995. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol. Biochem. Parasitol. 73:157-164. [DOI] [PubMed] [Google Scholar]
- 311.Mitro, K., A. Bhagavathiammai, O. M. Zhou, G. Bobbett, J. H. McKerrow, R. Chokshi, B. Chokshi, and E. R. James. 1994. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp. Parasitol. 78:377-385. [DOI] [PubMed] [Google Scholar]
- 312.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12:693-705. [DOI] [PubMed] [Google Scholar]
- 313.Molet, B., and G. Ermolieff-Braun. 1976. Description d'une amibe d'eau douce: Acanthamoeba lenticulata, sp. nov. (Amoebida). Protistologica 12:571-576. [Google Scholar]
- 314.Moore, M. B., and J. P. McCulley. 1989. Acanthamoeba keratitis associated with contact lenses: six consecutive cases of successful management. Br. J. Ophthalmol. 73:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Moore, M. B., J. P. McCulley, M. Luckenbach, H. Gelender, C. Newton, M. B. McDonald, and G. S. Visvesvara. 1985. Acanthamoeba keratitis associated with soft contact lenses. Am. J. Ophthalmol. 100:396-403. [DOI] [PubMed] [Google Scholar]
- 316.Moore, M. B., J. P. McCulley, C. Newton, L. M. Cobo, G. N. Foulks, D. M. O'Day, K. J. Johns, W. T. Driebe, L. A. Wilson, R. J. Epstein, et al. 1987. Acanthamoeba keratitis. A growing problem in soft and hard contact lens wearers. Ophthalmology 94:1654-1661. [PubMed] [Google Scholar]
- 317.Moore, M. B., J. E. Ubelaker, J. H. Martin, R. Silvany, J. M. Dougherty, D. R. Meyer, and J. P. McCulley. 1991. In vitro penetration of human corneal epithelium by Acanthamoeba castellanii: a scanning and transmission electron microscopy study. Cornea 10:291-298. [DOI] [PubMed] [Google Scholar]
- 318.Morton, L. D., G. L. McLaughlin, and H. E. Whiteley. 1991. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro. Infect. Immun. 59:3819-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Moshari, A., I. W. McLean, M. T. Dodds, R. E. Damiano, and P. L. McEvoy. 2001. Chorioretinitis after keratitis caused by Acanthamoeba: case report and review of the literature. Ophthalmology 108:2232-2236. [DOI] [PubMed] [Google Scholar]
- 320.Moura, H., S. Wallace, and G. S. Visvesvara. 1992. Acanthamoeba healyi n. sp. and the isoenzyme and immunoblot profiles of Acanthamoeba spp., groups 1 and 3. J. Protozool. 39:573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Muller, A., J. Hacker, and B. C. Brand. 1996. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect. Immun. 64:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Murakawa, G. J., T. McCalmont, J. Altman, G. H. Telang, M. D. Hoffman, G. R. Kantor, and T. G. Berger. 1995. Disseminated acanthamebiasis in patients with AIDS. A report of five cases and a review of the literature. Arch. Dermatol. 131:1291-1296. [PubMed] [Google Scholar]
- 323.Murdoch, D., T. B. Gray, R. Cursons, and D. Parr. 1998. Acanthamoeba keratitis in New Zealand, including two cases with in vivo resistance to polyhexamethylene biguanide. Aust. N. Z. J. Ophthalmol. 26:231-236. [DOI] [PubMed] [Google Scholar]
- 324.Murthy, S., N. R. Hawksworth, and I. Cree. 2002. Progressive ulcerative keratitis related to the use of topical chlorhexidine gluconate (0.02%). Cornea 21:237-239. [DOI] [PubMed] [Google Scholar]
- 325.Nagington, J., P. G. Watson, T. J. Playfair, J. McGill, B. R. Jones, and A. D. Steele. 1974. Amoebic infection of the eye. Lancet ii:1537-1540. [DOI] [PubMed] [Google Scholar]
- 326.Narasimhan, S., H. N. Madhavan, and K. LT. 2002. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorhexidine. Cornea 21:203-205. [DOI] [PubMed] [Google Scholar]
- 327.Nerad, T. A., T. K. Sawyer, E. J. Lewis, and S. M. McLaughlin. 1995. Acanthamoeba pearcei n. sp. (Protozoa: Amoebida) from sewage contaminated sediments. J. Eukaryot. Microbiol. 42:702-705. [DOI] [PubMed] [Google Scholar]
- 328.Neumeister, B., G. Reiff, M. Faigle, K. Dietz, H. Northoff, and F. Lang. 2000. Influence of Acanthamoeba castellanii on intracellular growth of different Legionella species in human monocytes. Appl. Environ. Microbiol. 66:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Newman, W., J. Hay, B. Browne, and D. V. Seal. 1997. Acanthamoeba as a “transient' in the corneal scrape of a poorly compliant soft contact lens wearer with peripheral keratitis. Eye 11:937-939. [DOI] [PubMed] [Google Scholar]
- 330.Newsome, A. L., F. T. Curtis, C. G. Culbertson, and S. D. Allen. 1992. Identification of Acanthamoeba in bronchoalveolar lavage specimens. Diagn. Cytopathol. 8:231-234. [DOI] [PubMed] [Google Scholar]
- 331.Newsome, A. L., T. M. Scott, R. F. Benson, and B. S. Fields. 1998. Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl. Environ. Microbiol. 64:1688-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Niederkorn, J. Y. 2002. The role of the innate and adaptive immune responses in Acanthamoeba keratitis. Arch. Immunol. Ther. Exp. (Warsaw) 50:53-59. [PubMed] [Google Scholar]
- 333.Niederkorn, J. Y., H. Alizadeh, H. Leher, and J. P. McCulley. 1999. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1:437-443. [DOI] [PubMed] [Google Scholar]
- 334.Niederkorn, J. Y., H. Alizadeh, H. F. Leher, and J. P. McCulley. 1999. The immunobiology of Acanthamoeba keratitis. Springer Semin. Immunopathol. 21:147-160. [DOI] [PubMed] [Google Scholar]
- 335.Niszl, I. A., R. B. Veale, and M. B. Markus. 1998. Cytopathogenicity of clinical and environmental Acanthamoeba isolates for two mammalian cell lines. J. Parasitol. 84:961-967. [PubMed] [Google Scholar]
- 336.Ofori-Kwakye, S. K., D. G. Sidebottom, J. Herbert, E. G. Fischer, and G. S. Visvesvara. 1986. Granulomatous brain tumor caused by Acanthamoeba. Case report. J. Neurosurg. 64:505-509. [DOI] [PubMed] [Google Scholar]
- 337.Oliva, S., M. Jantz, R. Tiernan, D. L. Cook, and M. A. Judson. 1999. Successful treatment of widely disseminated acanthamoebiasis. South. Med. J. 92:55-57. [DOI] [PubMed] [Google Scholar]
- 338.Orfeo, T., and E. Bateman. 1998. Transcription by RNA polymerase II during Acanthamoeba differentiation. Biochim. Biophys. Acta 1443:297-304. [DOI] [PubMed] [Google Scholar]
- 339.Osato, M. S., N. M. Robinson, K. R. Wilhelmus, and D. B. Jones. 1991. In vitro evaluation of antimicrobial compounds for cysticidal activity against Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S431-S435. [DOI] [PubMed] [Google Scholar]
- 340.Page, F. C. 1967. Re-definition of the genus Acanthamoeba with descriptions of three species. J. Protozool. 14:709-724. [DOI] [PubMed] [Google Scholar]
- 341.Page, F. C. 1974. A further study of taxonomic criteria for Limax amoebae, with descriptions of new species and a key to genera. Arch. Protistenkd. BD 116:S149. [Google Scholar]
- 342.Panjwani, N., Z. Zhao, J. Baum, M. Pereira, and T. Zaidi. 1992. Acanthamoebae bind to glycolipids of rabbit corneal epithelium. Infect. Immun. 60:3460-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Paszko-Kolva, C., H. Yamamoto, M. Shahamat, T. K. Sawyer, G. Morris, and R. R. Colwell. 1991. Isolation of amoebae and Pseudomonas and Legionella spp. from eyewash stations. Appl. Environ. Microbiol. 57:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Patras, D., and J. J. Andujar. 1966. Meningoencephalitis due to Hartmannella (Acanthamoeba). Am. J. Clin. Pathol. 46:226-233. [PubMed] [Google Scholar]
- 345.Patterson, D. J. 1999. The diversity of eukaryotes. Am. Nat. 154:S96-S124. [DOI] [PubMed] [Google Scholar]
- 346.Paule, M. R. 1983. Regulation of ribosomal RNA transcription during differentiation of Acanthamoeba castellanii: a review. J. Protozool. 30:211-214. [DOI] [PubMed] [Google Scholar]
- 347.Paule, M. R., E. Bateman, L. Hoffman, C. Iida, M. Imboden, W. Kubaska, P. Kownin, H. Li, A. Lofquist, P. Risi, et al. 1991. Initiation and regulation mechanisms of ribosomal RNA transcription in the eukaryote Acanthamoeba castellanii. Mol. Cell. Biochem. 104:119-126. [DOI] [PubMed] [Google Scholar]
- 348.Paule, M. R., and R. J. White. 2000. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 28:1283-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Penland, R. L., and K. R. Wilhelmus. 1997. Comparison of axenic and monoxenic media for isolation of Acanthamoeba. J. Clin. Microbiol. 35:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Pernin, P. 1976. Recherche et essai d'identification par microscopie en contraste de phase des amibes libres des piscines lyonnaises. Etude de la pathogenicite des souches de Naegleria sp. et d'Acanthamoeba sp. isolees. Ph.D. thesis. Pharmacie, Lyon, France.
- 351.Pettit, D. A., J. Williamson, G. A. Cabral, and F. Marciano-Cabral. 1996. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study J. Parasitol. 82:769-777. [PubMed] [Google Scholar]
- 352.Pfister, D. R., J. D. Cameron, J. H. Krachmer, and E. J. Holland. 1996. Confocal microscopy findings of Acanthamoeba keratitis. Am. J. Ophthalmol. 121:119-128. [DOI] [PubMed] [Google Scholar]
- 353.Pidherney, M. S., H. Alizadeh, G. L. Stewart, J. P. McCulley, and J. Y. Niederkorn. 1993. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 72:91-98. [DOI] [PubMed] [Google Scholar]
- 354.Pinna, A. 2002. Acanthamoeba and disinfecting contact lens solutions. Br. J. Ophthalmol. 86:1461-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Polakowski, N., and M. R. Paule. 2002. Purification and characterization of transcription factor IIIA from Acanthamoeba castellanii. Nucleic Acids Res. 30:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pollard, T. D., and E. M. Ostap. 1996. The chemical mechanism of myosin-I: implications for actin-based motility and the evolution of the myosin family of motor proteins. Cell Struct. Funct. 21:351-356. [DOI] [PubMed] [Google Scholar]
- 357.Powell, E. L., A. L. Newsome, S. D. Allen, and G. B. Knudson. 1994. Identification of antigens of pathogenic free-living amoebae by protein immunoblotting with rabbit immune and human sera. Clin. Diagn. Lab. Immunol. 1:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Preston, T. M., and C. A. King. 1984. Amoeboid locomotion of Acanthamoeba castellanii with special reference to cell-substratum interactions. J. Gen. Microbiol. 130:2317-2323. [DOI] [PubMed] [Google Scholar]
- 359.Preston, T. M., H. Richards, and R. S. Wotton. 2001. Locomotion and feeding of Acanthamoeba at the water-air interface of ponds. FEMS Microbiol. Lett. 194:143-147. [DOI] [PubMed] [Google Scholar]
- 360.Proca-Ciobanu, M., G. H. Lupascu, A. Petrovici, and M. D. Ionescu. 1975. Electron microscopic study of a pathogenic Acanthamoeba castellani strain: the presence of bacterial endosymbionts. Int. J. Parasitol. 5:49-56. [DOI] [PubMed] [Google Scholar]
- 361.Puschkarew, B. 1913. Uber die Verbreitugen der Susswasserprotozoen durch die Luft. Arch. Protistenkd. BD 23:323-362. [Google Scholar]
- 362.Pussard, M. 1964. Acanthamoeba comandoni n. sp., Comparison avec Ac. terricola. Rev. Ecol. Biol. Sol. 1:587-610. [Google Scholar]
- 363.Pussard, M., and R. Pons. 1977. Morphologies de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica 13:557-610. [Google Scholar]
- 364.Radebaugh, C. A., W. M. Kubaska, L. H. Hoffman, K. Stiffler, and M. R. Paule. 1998. A novel transcription initiation factor (TIF), TIF-IE, is required for homogeneous Acanthamoeba castellanii TIF-IB (SL1) to form a committed complex. J. Biol. Chem. 273:27708-27715. [DOI] [PubMed] [Google Scholar]
- 365.Radford, C. F., D. C. Minassian, and J. K. Dart. 2002. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br. J. Ophthalmol. 86:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ray, D. L., and R. E. Hayes. 1954. Hartmannella astronyxis: A new species of free-living ameba. J. Morphol. 95:159-187. [Google Scholar]
- 367.Reich, K. 1935. Studien uber die bodenprotozoen Palastinas. J. Exp. Zool. 69:497. [Google Scholar]
- 368.Ringsted, J., B. V. Jager, D. Suk, and G. S. Visvesvara. 1976. Probable acanthamoeba meningoencephalitis in a Korean child. Am. J. Clin. Pathol. 66:723-730. [DOI] [PubMed] [Google Scholar]
- 369.Rivera, F., F. Lares, E. Gallegos, E. Ramirez, P. Bonilla, A. Calderon, J. J. Martinez, S. Rodriguez, and J. Alcocer. 1989. Pathogenic amoebae in natural thermal waters of three resorts of Hidalgo, Mexico. Environ. Res. 50:289-295. [DOI] [PubMed] [Google Scholar]
- 370.Rivera, F., F. Lares, E. Ramirez, P. Bonilla, S. Rodriguez, A. Labastida, R. Ortiz, and D. Hernandez. 1991. Pathogenic Acanthamoeba isolated during an atmospheric survey in Mexico City. Rev. Infect. Dis. 13(Suppl. 5):S388-S389. [DOI] [PubMed] [Google Scholar]
- 371.Rivera, F., F. Medina, P. Ramirez, J. Alcocer, G. Vilaclara, and E. Robles. 1984. Pathogenic and free-living protozoa cultured from the nasopharyngeal and oral regions of dental patients. Environ. Res. 33:428-440. [DOI] [PubMed] [Google Scholar]
- 372.Rivera, F., G. Roy-Ocotla, I. Rosas, E. Ramirez, P. Bonilla, and F. Lares. 1987. Amoebae isolated from the atmosphere of Mexico City and environs. Environ. Res. 42:149-154. [DOI] [PubMed] [Google Scholar]
- 373.Rivera, M. A., and T. A. Padhya. 2002. Acanthamoeba: a rare primary cause of rhinosinusitis. Laryngoscope 112:1201-1203. [DOI] [PubMed] [Google Scholar]
- 374.Robert, V. B., and L. B. Rorke. 1973. Primary amebic encephalitis, probably from Acanthamoeba. Ann. Intern. Med. 79:174-179. [DOI] [PubMed] [Google Scholar]
- 375.Robinson, G., S. E. Wilson, and R. A. Williams. 1987. Surgery in patients with acquired immunodeficiency syndrome. Arch. Surg. 122:170-175. [DOI] [PubMed] [Google Scholar]
- 376.Rodriguez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 377.Rodriguez-Zaragoza, S., C. Ordaz, G. Avila, J. L. Munoz, A. Arciniegas, and d. Romo, V. 1999. In vitro evaluation of the amebicidal activity of Buddleia cordata (Loganiaceae, H. B. K.) on several strains of Acanthamoeba. J. Ethnopharmacol. 66:327-334. [DOI] [PubMed] [Google Scholar]
- 378.Rogerson, A., and D. J. Patterson. 2000. The naked ramicristate amoebae (Gymnamoebae), p. 1023-1053. In J. J. Lee, G. F. Leedale, and P. Bradbury (ed.), The illustrated guide to the protozoa. Allen Press, Inc., Lawrence, Kans.
- 379.Rondanelli, E. G., G. Carosi, P. Lanzarini, and G. Filice. 1987. Ultrastructure of Acanthamoeba-Naegleria free-living amoebae, p. 87-125. In E. G. Rondanelli (ed.), Amphizoic amoebae: human pathology. Piccin Nuova Libraria, Padua, Italy.
- 380.Rosenberg, A. S., and M. B. Morgan. 2001. Disseminated acanthamoebiasis presenting as lobular panniculitis with necrotizing vasculitis in a patient with AIDS. J. Cutan. Pathol. 28:307-313. [DOI] [PubMed] [Google Scholar]
- 381.Rosenthal, S., E. J. Reed, and R. A. Weisman. 1969. Effect of lytic enzymes of Acanthamoeba castellanii on bacterial cell walls. J. Bacteriol. 98:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Rowan-Kelly, B., and A. Ferrante. 1984. Immunization with killed Acanthamoeba culbertsoni antigen and amoeba culture supernatant antigen in experimental Acanthamoeba meningoencephalitis. Trans. R. Soc. Trop. Med. Hyg. 78:179-182. [DOI] [PubMed] [Google Scholar]
- 383.Rowan-Kelly, B., A. Ferrante, and Y. H. Thong. 1982. The chemotherapeutic value of sulphadiazine in treatment of Acanthamoeba meningoencephalitis in mice. Trans. R. Soc. Trop. Med. Hyg. 76:636-638. [DOI] [PubMed] [Google Scholar]
- 384.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678-689. [PubMed] [Google Scholar]
- 386.Rowbotham, T. J. 1993. Legionella-like amoebal pathogens, p. 137-140. In J. M. Barbaree, R. F. Breiman, and A. P. Dufour (ed.), Legionella—current status and emerging perspectives. American Society for Microbiology, Washington, D.C.
- 387.Rowen, J. L., C. A. Doerr, H. Vogel, and C. J. Baker. 1995. Balamuthia mandrillaris: a newly recognized agent for amebic meningoencephalitis. Pediatr. Infect. Dis. J. 14:705-710. [PubMed] [Google Scholar]
- 388.Sangruchi, T., A. J. Martinez, and G. S. Visvesvara. 1994. Spontaneous granulomatous amebic encephalitis: report of four cases from Thailand. Southeast Asian J. Trop. Med. Public Health 25:309-313. [PubMed] [Google Scholar]
- 389.Sawyer, T. K., and L. R. Buchanan. 1971. Contamination of tissue sections of the American oyster by cysts of Acanthamoeba sp. J. Invertebr. Pathol. 18:300.. [DOI] [PubMed] [Google Scholar]
- 390.Sawyer, T. K., and J. L. Griffin. 1975. A proposed new family. Acanthamoebidae n. fam. (order Amoebida), for certain cyst-forming filose amoebae. Trans. Am. Microsc. Soc. 94:93-98. [Google Scholar]
- 391.Sawyer, T. K., T. A. Nerad, E. J. Lewis, and S. M. McLaughlin. 1993. Acanthamoeba stevensoni n. sp. (Protozoa: Amoebida) from sewage-contaminated shellfish beds in Raritan Bay, New York. J. Eukaryot. Microbiol. 40:742-746. [DOI] [PubMed] [Google Scholar]
- 392.Sawyer, T. K., T. A. Nerad, and G. S. Visvesara. 1992. Acanthamoeba jacobsi sp. n. (Protozoa: Acanthamoebidae) from sewage contaminated ocean sediments. Helminthol. Soc. Wash. 59:223-226. [Google Scholar]
- 393.Sawyer, T. K., G. S. Visvesvara, and B. A. Harke. 1977. Pathogenic amoebas from brackish and ocean sediments, with a description of Acanthamoeba hatchetti, n. sp. Science 196:1324-1325. [DOI] [PubMed] [Google Scholar]
- 394.Schaumberg, D. A., K. K. Snow, and M. R. Dana. 1998. The epidemic of Acanthamoeba keratitis: where do we stand? Cornea 17:3-10. [DOI] [PubMed] [Google Scholar]
- 395.Schroeder, J. M., G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2001. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 39:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Schulze, I., and H. Jantzen. 1982. Coordinate regulation of synthesis of ribosomal proteins during encystation of Acanthamoeba castellanii. Eur. J. Biochem. 126:285-292. [DOI] [PubMed] [Google Scholar]
- 397.Schumacher, D. J., R. D. Tien, and K. Lane. 1995. Neuroimaging findings in rare amebic infections of the central nervous system. AJNR Am. J. Neuroradiol. 16:930-935. [PMC free article] [PubMed] [Google Scholar]
- 398.Schuster, F. L. 1993. Comparative effects of selected azole compounds on trophic and cystic stages of Acanthamoeba polyphaga. J. Eukaryot. Microbiol. 40:563-569. [DOI] [PubMed] [Google Scholar]
- 399.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Schuster, F. L., T. H. Dunnebacke, and G. S. Visvesvara. 2001. Activity of selected antimicrobials against clinical isolates of pathogenic free-living amebas, p. 45-48. In S. Billot-Bonef, P. A. Cabanes, F. Marciano-Cabral, P. Pernin, and E. Pringuez (ed.), IXth International Meeting on the Biology and Pathogenicity of Free-Living Amoebae Proceedings. John Libbey Eurotext, Paris, France.
- 401.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against clinical isolates of opportunistic amebas. J. Eukaryot. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 402.Seal, D., J. Hay, C. Kirkness, A. Morrell, A. Booth, A. Tullo, A. Ridgway, and M. Armstrong. 1996. Successful medical therapy of Acanthamoeba keratitis with topical chlorhexidine and propamidine. Eye 10:413-421. [DOI] [PubMed] [Google Scholar]
- 403.Seal, D. V., and J. Hay. 1993. The microbiologist as a contact lens wearer. J. Med. Microbiol. 39:1-2. [DOI] [PubMed] [Google Scholar]
- 404.Seal, D. V., and J. Hay. 1994. Acanthamoeba keratitis. Br. Med. J. 309:1019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Seal, D. V., J. Hay, and C. M. Kirkness. 1995. Chlorhexidine or polyhexamethylene biguanide for acanthamoeba keratitis. Lancet 345:136.. [DOI] [PubMed] [Google Scholar]
- 406.Seijo, M. M., G. Gonzalez-Mediero, P. Santiago, D. L. Rodriguez, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Selby, D. M., R. S. Chandra, T. A. Rakusan, B. Loechelt, B. M. Markle, and G. S. Visvesvara. 1998. Amebic osteomyelitis in a child with acquired immunodeficiency syndrome: a case report. Pediatr. Pathol. Lab Med. 18:89-95. [PubMed] [Google Scholar]
- 408.Sell, J. J., F. W. Rupp, and W. W. Orrison, Jr. 1997. Granulomatous amebic encephalitis caused by Acanthamoeba. Neuroradiology 39:434-436. [DOI] [PubMed] [Google Scholar]
- 409.Sharma, S., P. Garg, and G. N. Rao. 2000. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br. J. Ophthalmol. 84:1103-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Shin, H. J., M. S. Cho, S. Y. Jung, H. I. Kim, and K. I. Im. 2000. In vitro cytotoxicity of Acanthamoeba spp. isolated from contact lens containers in Korea by crystal violet staining and LDH release assay. Korean J. Parasitol. 38:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Shin, H. J., M. S. Cho, H. I. Kim, M. Lee, S. Park, S. Sohn, and K. I. Im. 2000. Apoptosis of primary-culture rat microglial cells induced by pathogenic Acanthamoeba spp. Clin. Diagn. Lab. Immunol. 7:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Shin, L. E., and P. B. Hadley. 1936. Note on the spontaneous contamination of a bacterial culture by an organism resembling Hartmannella castellanii. J. Infect. Dis. 58:23-27. [Google Scholar]
- 413.Shukla, O. P., S. M. Kaul, and R. K. Mehlotra. 1989. Development of improved media for axenic cultivation of Acanthamoeba culbertsoni, Singh and Das 1970. Indian J. Exp. Biol. 27:785-791. [PubMed] [Google Scholar]
- 414.Silvany, R. E., M. W. Luckenbach, and M. B. Moore. 1987. The rapid detection of Acanthamoeba in paraffin-embedded sections of corneal tissue with calcofluor white. Arch. Ophthalmol. 105:1366-1367. [DOI] [PubMed] [Google Scholar]
- 415.Simmons, P. A., A. Tomlinson, and D. V. Seal. 1998. The role of Pseudomonas aeruginosa biofilm in the attachment of Acanthamoeba to four types of hydrogel contact lens materials. Optom. Visual Sci. 75:860-866. [DOI] [PubMed] [Google Scholar]
- 416.Singh, B. N. 1942. Selection of bacterial food by soil flagellates and amoebae. Ann. Appl. Biol. 29:18-22. [Google Scholar]
- 417.Singh, B. N. 1946. A method of estimating the numbers of soil protozoa, especially amoebae, based on their differential feeding on bacteria. Ann. Appl. Biol. 33:112-119. [DOI] [PubMed] [Google Scholar]
- 418.Singh, B. N. 1952. Nuclear division in nine species off small free-living amoebae and the bearing of nuclear division on the classification of the order Amoebida. Philos. Trans. R. Soc. Lond. Ser. B 236:405.. [DOI] [PubMed] [Google Scholar]
- 419.Singhal, T., A. Bajpai, V. Kalra, S. K. Kabra, J. C. Samantaray, G. Satpathy, and A. K. Gupta. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 20:623-627. [DOI] [PubMed] [Google Scholar]
- 420.Sison, J. P., C. A. Kemper, M. Loveless, D. McShane, G. S. Visvesvara, and S. C. Deresinski. 1995. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin. Infect. Dis. 20:1207-1216. [DOI] [PubMed] [Google Scholar]
- 421.Slater, C. A., J. Z. Sickel, G. S. Visvesvara, R. C. Pabico, and A. A. Gaspari. 1994. Brief report: successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N. Engl. J. Med. 331:85-87. [DOI] [PubMed] [Google Scholar]
- 422.Sotelo-Avila, C., F. M. Taylor, and C. W. Ewing. 1974. Primary amebic meningoencephalitis in a healthy 7-year-old boy. J. Pediatr. 85:131-136. [DOI] [PubMed] [Google Scholar]
- 423.Springer, N., W. Ludwig, W. Drozanski, R. Amann, and K. H. Schleifer. 1992. The phylogenetic status of Sarcobium lyticum, an obligate intracellular bacterial parasite of small amoebae. FEMS Microbiol. Lett. 75:199-202. [DOI] [PubMed] [Google Scholar]
- 424.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Stehr-Green, J. K., T. M. Bailey, and G. S. Visvesvara. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am. J. Ophthalmol. 107:331-336. [DOI] [PubMed] [Google Scholar]
- 426.Steinberg, J. P., R. L. Galindo, E. S. Kraus, and K. G. Ghanem. 2002. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin. Infect. Dis. 35:E43-E49. [DOI] [PubMed] [Google Scholar]
- 427.Steinert, M., G. Ockert, C. Luck, and J. Hacker. 1998. Regrowth of Legionella pneumophila in a heat-disinfected plumbing system. Zentbl. Bakteriol. 288:331-342. [DOI] [PubMed] [Google Scholar]
- 428.Stevens, A. R., T. Kilpatrick, E. Willaert, and A. Capron. 1977. Serologic analyses of cell-surface antigens of Acanthamoeba spp. with plasma membrane antisera. J. Protozool. 24:316-324. [DOI] [PubMed] [Google Scholar]
- 429.Stevens, A. R., and W. D. O'Dell. 1974. In vitro growth and virulence of Acanthamoeba. J. Parasitol. 60:884-885. [PubMed] [Google Scholar]
- 430.Stewart, G. L., I. Kim, K. Shupe, H. Alizadeh, R. Silvany, J. P. McCulley, and J. Y. Niederkorn. 1992. Chemotactic response of macrophages to Acanthamoeba castellanii antigen and antibody-dependent macrophage-mediated killing of the parasite. J. Parasitol. 78:849-855. [PubMed] [Google Scholar]
- 431.Stewart, G. L., K. Shupe, I. Kim, R. E. Silvany, H. Alizadeh, J. P. McCulley, and J. Y. Niederkorn. 1994. Antibody-dependent neutrophil-mediated killing of Acanthamoeba castellanii. Int. J. Parasitol. 24:739-742. [DOI] [PubMed] [Google Scholar]
- 432.Stopak, S. S., M. I. Roat, R. C. Nauheim, P. W. Turgeon, G. Sossi, R. P. Kowalski, and R. A. Thoft. 1991. Growth of Acanthamoeba on human corneal epithelial cells and keratocytes in vitro. Investig. Ophthalmol. Visual Sci. 32:354-359. [PubMed] [Google Scholar]
- 433.Stothard, D. R., J. Hay, J. M. Schroeder-Diedrich, D. V. Seal, and T. J. Byers. 1999. Fluorescent oligonucleotide probes for clinical and environmental detection of Acanthamoeba and the T4 18S rRNA gene sequence type. J. Clin. Microbiol. 37:2687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledee, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 45:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Stratford, M. P., and A. J. Griffith. 1978. Variations in the properties and morphology of cysts of Acanthamoeba castellanii. J. Gen. Microbiol. 108:33. [Google Scholar]
- 436.Susa, M., J. Hacker, and R. Marre. 1996. De novo synthesis of Legionella pneumophila antigens during intracellular growth in phagocytic cells. Infect. Immun. 64:1679-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Szenasi, Z., T. Endo, K. Yagita, and E. Nagy. 1998. Isolation, identification and increasing importance of ”free-living' amoebae causing human disease. J. Med. Microbiol. 47:5-16. [DOI] [PubMed] [Google Scholar]
- 438.Tan, B., C. M. Weldon-Linne, D. P. Rhone, C. L. Penning, and G. S. Visvesvara. 1993. Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency syndrome. Arch. Pathol. Lab Med. 117:1043-1046. [PubMed] [Google Scholar]
- 439.Tanaka, Y., S. Suguri, M. Harada, T. Hayabara, K. Suzumori, and N. Ohta. 1994. Acanthamoeba-specific human T-cell clones isolated from healthy individuals. Parasitol. Res. 80:549-553. [DOI] [PubMed] [Google Scholar]
- 440.Tay-Kearney, M. L., C. N. McGhee, G. J. Crawford, and K. Trown. 1993. Acanthamoeba keratitis. A masquerade of presentation in six cases. Aust. N. Z. J. Ophthalmol. 21:237-245. [DOI] [PubMed] [Google Scholar]
- 441.Taylor, W. M., M. S. Pidherney, H. Alizadeh, and J. Y. Niederkorn. 1995. In vitro characterization of Acanthamoeba castellanii cytopathic effect. J. Parasitol. 81:603-609. [PubMed] [Google Scholar]
- 442.Teknos, T. N., M. D. Poulin, A. M. Laruentano, and K. K. Li. 2000. Acanthamoeba rhinosinusitis: characterization, diagnosis, and treatment. Am. J. Rhinol. 14:387-391. [DOI] [PubMed] [Google Scholar]
- 443.Thom, S., D. Warhurst, and B. S. Drasar. 1992. Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 36:303-306. [DOI] [PubMed] [Google Scholar]
- 444.Tomlinson, G. 1991. Screening for chemical inhibitors of growth rate, encystment, and excystment in Acanthamoeba castellanii. Rev. Infect. Dis. 13(Suppl. 5):S436-S437. [DOI] [PubMed] [Google Scholar]
- 445.Toney, D. M., and F. Marciano-Cabral. 1998. Resistance of Acanthamoeba species to complement lysis. J. Parasitol. 84:338-344. [PubMed] [Google Scholar]
- 446.Torno, M. S., Jr., R. Babapour, A. Gurevitch, and M. D. Witt. 2000. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 42:351-354. [DOI] [PubMed] [Google Scholar]
- 447.Tseng, S. H., S. C. Lin, and F. K. Chen. 1998. Is polyhexamethylene biguanide alone effective for Acanthamoeba keratitis? Cornea 17:345-346. [DOI] [PubMed] [Google Scholar]
- 448.Turner, N. A., J. Harris, A. D. Russell, and D. Lloyd. 2000. Microbial differentiation and changes in susceptibility to antimicrobial agents. J. Appl. Microbiol. 89:751-759. [DOI] [PubMed] [Google Scholar]
- 449.Van Hamme, C., M. Dumont, M. Delos, and J. M. Lachapelle. 2001. Acanthamibiase cutanee chez un transplante pulmonaire. Ann. Dermatol. Venereol. 128:1237-1240. [PubMed] [Google Scholar]
- 450.Van Klink, F., H. Alizadeh, Y. He, J. A. Mellon, R. E. Silvany, J. P. McCulley, and J. Y. Niederkorn. 1993. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Investig. Ophthalmol. Visual Sci. 34:1937-1944. [PubMed] [Google Scholar]
- 451.Van Klink, F., H. Alizadeh, G. L. Stewart, M. S. Pidherney, R. E. Silvany, Y. He, J. P. McCulley, and J. Y. Niederkorn. 1992. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr. Eye Res. 11:1207-1220. [DOI] [PubMed] [Google Scholar]
- 452.Van Klink, F., H. Leher, M. J. Jager, H. Alizadeh, W. Taylor, and J. Y. Niederkorn. 1997. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocul. Immunol. Inflamm. 5:235-244. [DOI] [PubMed] [Google Scholar]
- 453.Van Klink, F., W. M. Taylor, H. Alizadeh, M. J. Jager, N. van Rooijen, and J. Y. Niederkorn. 1996. The role of macrophages in Acanthamoeba keratitis. Investig. Ophthalmol. Visual Sci. 37:1271-1281. [PubMed] [Google Scholar]
- 454.Varga, J. H., T. C. Wolf, H. G. Jensen, V. C. Parmley, and J. J. Rowsey. 1993. Combined treatment of Acanthamoeba keratitis with propamidine, neomycin, and polyhexamethylene biguanide. Am. J. Ophthalmol. 115:466-470. [DOI] [PubMed] [Google Scholar]
- 455.Victoria, E. J., and E. D. Korn. 1975. Enzymes of phospholipid metabolism in the plasma membrane of Acanthamoeba castellanii. J. Lipid Res. 16:54-60. [PubMed] [Google Scholar]
- 456.Victoria, E. J., and E. D. Korn. 1975. Plasma membrane and soluble lysophospholipases of Acanthamoeba castellanii. Arch. Biochem. Biophys. 171:255-258. [DOI] [PubMed] [Google Scholar]
- 457.Villemez, C. L., P. L. Carlo, and M. A. Russell. 1985. Differentiation in Acanthamoeba castellanii is induced by specific monoclonal antibodies. J. Cell. Biochem. 29:373-379. [DOI] [PubMed] [Google Scholar]
- 458.Visvesvara, G. S. 1987. Laboratory diagnosis, p. 193-212. In E. G. Rondanelli (ed.), Amphizoic amoebae: human pathology. Piccin Nuova Libraria, Padua, Italy.
- 459.Visvesvara, G. S., and W. Balamuth. 1975. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J. Protozool. 22:245-256. [DOI] [PubMed] [Google Scholar]
- 460.Visvesvara, G. S., S. S. Mirra, F. H. Brandt, D. M. Moss, H. M. Mathews, and A. J. Martinez. 1983. Isolation of two strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J. Clin. Microbiol. 18:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Visvesvara, G. S., F. L. Schuster, and A. J. Martinez. 1993. Balamuthia mandrillaris, n. g., n. sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514. [DOI] [PubMed] [Google Scholar]
- 462.Vodkin, M. H., D. K. Howe, G. S. Visvesvara, and G. L. McLaughlin. 1992. Identification of Acanthamoeba at the generic and specific levels using the polymerase chain reaction. J. Protozool. 39:378-385. [DOI] [PubMed] [Google Scholar]
- 463.Volkonsky, M. 1931. Hartmanella castellanii Douglas, et classification des hartmannelles. Arch. Zool. Exp. Gen. 72:317-339. [Google Scholar]
- 464.Walochnik, J., E. Haller-Schober, H. Kolli, O. Picher, A. Obwaller, and H. Aspock. 2000. Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens- wearing keratitis patients in Austria. J. Clin. Microbiol. 38:3932-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Walochnik, J., A. Hassl, K. Simon, G. Benyr, and H. Aspock. 1999. Isolation and identification by partial sequencing of the 18S ribosomal gene of free-living amoebae from necrotic tissue of Basilliscus plumifrons (Sauria: Iguanidae). Parasitol. Res. 85:601-603. [DOI] [PubMed] [Google Scholar]
- 466.Walochnik, J., A. Obwaller, and H. Aspock. 2000. Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:4408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Walochnik, J., A. Obwaller, and H. Aspock. 2001. Immunological inter-strain crossreactivity correlated to 18S rDNA sequence types in Acanthamoeba spp. Int. J. Parasitol. 31:163-167. [DOI] [PubMed] [Google Scholar]
- 468.Walochnik, J., A. Obwaller, E. M. Haller-Schober, and H. Aspock. 2001. Anti-Acanthamoeba IgG, IgM, and IgA immunoreactivities in correlation to strain pathogenicity. Parasitol. Res. 87:651-656. [DOI] [PubMed] [Google Scholar]
- 469.Walochnik, J., A. Obwaller, E. M. Haller-Schober, and H. Aspock. 2001. Differences in immunoreactivities and capacities to bind human complement subcomponent C1q between a pathogenic and a nonpathogenic Acanthamoeba, p. 59-64. In S. Billot-Bonef, P. A. Cabanes, F. Marciano-Cabral, P. Pernin, and E. Pringuez (ed.), IXth International Meeting on the Biology and Pathogenicity of Free-Living Amoebae Proceedings. John Libbey Eurotext, Paris, France.
- 470.Wang, X., and D. G. Ahearn. 1997. Effect of bacteria on survival and growth of Acanthamoeba castellanii. Curr. Microbiol. 34:212-215. [DOI] [PubMed] [Google Scholar]
- 471.Weekers, P. H., P. L. E. Bodelier, J. P. Wijen, and G. D. Vogels. 1993. Effects of grazing by the free-living soil amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl. Environ. Microbiol. 59:2317-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Weekers, P. H., and J. F. De Jonckheere. 1997. Differences in isoenzyme patterns of axenically and monoxenically grown Acanthamoeba and Hartmannella. Antonie Leeuwenhoek 71:231-237. [DOI] [PubMed] [Google Scholar]
- 473.Weekers, P. H., A. M. Engelberts, and G. D. Vogels. 1995. Bacteriolytic activities of the free-living soil amoebae, Acanthamoeba castellanii, Acanthamoeba polyphaga and Hartmannella vermiformis. Antonie Leeuwenhoek 68:237-243. [DOI] [PubMed] [Google Scholar]
- 474.Wiley, C. A., R. E. Safrin, C. E. Davis, P. W. Lampert, A. I. Braude, A. J. Martinez, and G. S. Visvesvara. 1987. Acanthamoeba meningoencephalitis in a patient with AIDS. J. Infect. Dis. 155:130-133. [DOI] [PubMed] [Google Scholar]
- 475.Wilhelmus, K. R., M. S. Osato, R. L. Font, N. M. Robinson, and D. B. Jones. 1986. Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch. Ophthalmol. 104:1309-1312. [DOI] [PubMed] [Google Scholar]
- 476.Willaert, E., and A. R. Stevens. 1976. Indirect immunofluorescent identification of Acanthamoeba causing meningoencephalitis. Pathol. Biol. (Paris) 24:545-547. [PubMed] [Google Scholar]
- 477.Willaert, E., A. R. Stevens, and G. R. Healy. 1978. Retrospective identification of Acanthamoeba culbertsoni in a case of amoebic meningoencephalitis. J. Clin. Pathol. 31:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Willaert, E., A. R. Stevens, and R. L. Tyndall. 1978. Acanthamoeba royreba sp. n. from a human tumor cell culture. J. Protozool. 25:1-14. [DOI] [PubMed] [Google Scholar]
- 479.Winiecka-Krusnell, J., and E. Linder. 2001. Bacterial infections of free-living amoebae. Res. Microbiol. 152:613-619. [DOI] [PubMed] [Google Scholar]
- 480.Winiecka-Krusnell, J., K. Wreiber, A. von Euler, L. Engstrand, and E. Linder. 2002. Free-living amoebae promote growth and survival of Helicobacter pylori. Scand. J. Infect. Dis. 34:253-256. [DOI] [PubMed] [Google Scholar]
- 481.Wright, J. L., K. Redhead, and H. Maudsley. 1981. Acanthamoeba castellanii, a predator of cyanobacteria. J. Gen. Microbiol. 125:293-300. [Google Scholar]
- 482.Wright, P., D. Warhurst, and B. R. Jones. 1985. Acanthamoeba keratitis successfully treated medically. Br. J. Ophthalmol. 69:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Yagita, K., and T. Endo. 1990. Restriction enzyme analysis of mitochondrial DNA of Acanthamoeba strains in Japan. J. Protozool. 37:570-575. [DOI] [PubMed] [Google Scholar]
- 484.Yang, S., and C. Villemez. 1994. Cell surface control of differentiation in Acanthamoeba. J. Cell. Biochem. 56:592-596. [DOI] [PubMed] [Google Scholar]
- 485.Yang, Z., Z. Cao, and N. Panjwani. 1997. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect. Immun. 65:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Zanetti, S., P. L. Fiori, A. Pinna, S. Usai, F. Carta, and G. Fadda. 1995. Susceptibility of Acanthamoeba castellanii to contact lens disinfecting solutions. Antimicrob. Agents Chemother. 39:1596-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]