Abstract
Matrix metalloprotease type 9 (MMP-9) has been functionally implicated in VEGF activation, the induction and maintenance of chronic angiogenesis, and early stage tumor growth in a number of mouse models of cancer. In this article, we have identified two inflammatory cell types that are major sources of MMP-9 in the angiogenic stages of pancreatic islet carcinogenesis that unfold in RIP1-Tag2 transgenic mice. MMP-9-expressing neutrophils were predominantly found inside angiogenic islet dysplasias and tumors, whereas MMP-9-expressing macrophages were localized along the periphery of such lesions. Transient depletion of neutrophils significantly suppressed VEGF:VEGF-receptor association, a signature of MMP-9 activity, and markedly reduced the frequency of initial angiogenic switching in dysplasias. Thus infiltrating neutrophils can play a crucial role in activating angiogenesis in a previously quiescent tissue vasculature during the early stages of carcinogenesis.
Keywords: VEGF, macrophage, granulocyte colony-stimulating factor (G-CSF), angiogenesis, matrix metalloprotease type 9
Tumors and their neoplastic progenitors are composed not only of transformed “cancer cells” but also of other cell types constituting the stroma. These stromal cells include cancer-associated fibroblasts, endothelial cells, pericytes, and a variable representation of leukocytes, including macrophages, neutrophils, mast cells, and B or T lymphocytes (1, 2). Increasing evidence indicates that leukocytic infiltration can either antagonize tumor formation and growth (immune surveillance) (2, 3) or, alternatively, promote tumor phenotypes, such as angiogenesis, growth, and invasion (immune enhancement) (1, 4, 5). Historically, tumor-infiltrating leukocytes have been considered to be manifestations of an intrinsic defense mechanism against developing tumors (2). However, most solid tumors are largely recognized as self and do not evoke effective immune responses capable of killing or antagonizing tumor formation, growth, and progression (3, 6). In contrast, accumulating clinical data for solid tumors show a correlation between high-density leukocytic infiltration into tumors and poor outcome of patients (1). In regard to the innate immune system, despite its capability to stimulate acquired immune responses, the weight of the evidence indicates that infiltration by innate immune cell types is tumor-promoting in many organs and tumorigenesis pathways (1, 7, 8). In addition to protumorigenic activities of tumor associated macrophages (9–11), neutrophils also have been shown to enhance the in vitro invasive and in vivo metastatic potential of syngeneic tumor cells by facilitating invasion into basement membrane (7).
Genetically engineered mouse models of cancer are proving instructive about the immunobiology of tumors, revealing both enhancing and antagonizing roles played by the adaptive and innate immune system (4, 5, 12–14). RIP1-Tag2 transgenic mice constitute a well characterized prototypical model for multistep carcinogenesis, involving the pancreatic islets, which has provided insights into immune interactions during tumorigenesis. In this model, the simian virus 40 (SV40) large T antigen oncogenes are expressed in all islets under control of an insulin gene promoter, resulting in the appearance of hyperplastic/dysplastic islets ≈5 weeks of age (15). By 9 weeks, ≈25% of these islets have switched on angiogenesis, with histological features of high-grade dysplasias (38); a subset of the angiogenic islets in turn progress to solid tumors thereafter. These mice are immunologically self-tolerant of the SV40 large T antigen oncoprotein (6), and the adaptive immune system has no apparent modulatory role in tumorigenesis, as evidenced by breeding that rendered the mice Rag1-null, which had no impact on tumor phenotype (16). By contrast, there have been implications that the innate immune system plays a role in this tumorigenesis pathway. We previously reported that a protease, matrix metalloprotease type 9 (MMP-9), functionally contributes to both angiogenesis and tumor progression in RIP1-Tag2 mice (12). The data indicated that MMP-9 liberates VEGF, an angiogenic factor that is sequestered in normal and hyperplastic pancreatic islets, enabling it to elicit angiogenesis by ligand-dependent activation of its receptor on endothelial cells. In the case of MMP-9 deficiency, the number of angiogenic islets, the number of tumors, and collective tumor burden were all lower than in age-matched wild-type RIP1-Tag2 littermates (12). Moreover, therapeutic application of several MMP inhibitors markedly inhibited initial angiogenic switching and the growth of nascent tumors in the RIP1-Tag2 mice (12, 17). Inflammatory cells have been suspected as the source of MMP-9 in this model (12), a possibility buoyed by data showing that such cells are the critical source of MMP-9 in other mouse models, e.g., of squamous carcinogenesis of the skin and cervix (14, 18, 19).
We sought to identify the cell types expressing MMP-9 in the RIP1-Tag2 model and report herein that neutrophils and macrophages associated with the angiogenic dysplasias and tumors are the evident sources of MMP-9. Interestingly, MMP-9-expressing neutrophils reside within the neoplastic lesions, whereas MMP-9-producing macrophages accumulate as clusters of cells along the lesional margins. We assessed the functional importance of the MMP-9-expressing neutrophils by depleting them with anti-Gr-1 antibody, thereby revealing their involvement in the initial angiogenic switch during islet tumorigenesis.
Results
Identification and Localization of MMP-9-Expressing Cells.
Previous immunohistological examination suggested that infiltrating immune cells might be the source of MMP-9 in angiogenic islets and tumors of RIP1-Tag2 mice (12). To assess this possibility, we performed semiquantitative RT-PCR analysis of the constituent cell types isolated from tumors by flow cytometry, which revealed that the MMP-9 gene was expressed predominantly by Gr-1+/Mac-1+ innate immune cells (Fig. 5, which is published as supporting information on the PNAS web site). Motivated by this result, we next performed double-label immunohistochemical staining of MMP-9 and various leukocyte markers in normal and neoplastic pancreatic tissue.
Tumor-associated macrophages have been reported to express MMP-9 in a number of mouse models of cancer, including squamous carcinomas of the skin (18) and cervix (19) and carcinomas of the breast (20, 21). When stained with anti-CD68 (Fig. 1A) or anti-F4/80 (data not shown) antibodies, macrophages were detected throughout the pancreas of RIP1-Tag2 mice, including normal exocrine tissue as well as within and peripheral to neoplastic islet tissue. Interestingly, the macrophages located inside angiogenic islets and tumors were MMP-9-negative (MMP-9−), whereas the majority of the CD68+ cells residing along the periphery of islets were MMP-9-positive (MMP-9+) (Fig. 1A).
Fig. 1.
Immunolocalization of MMP-9+ cells (red) in RIP1-Tag2 tumors identifies two subsets of cells that either reside along the periphery of the tumors or infiltrate the tumors. White dashed lines indicate borders of tumors. (A) Costaining with CD68 (green) identifies infiltrating MMP-9− macrophages and peripheral MMP-9+ macrophages. (B) Costaining for 7/4 antigen (green) identifies bright MMP-9+ cells as infiltrating neutrophils. (C) Flow cytometry for intracellular levels of MMP-9 in the 7/4+, Gr-1+, and CD68+ cell populations infiltrating RT2 tumors. 7/4+ and Gr-1+ cells represent ≈0.4% of the total tumor cells and express high levels of MMP-9, whereas 2% of the cells are CD68+, and exhibit either no or lower level expression of MMP-9.
As reported in ref. 12, the intratumoral MMP-9+ cells were relatively rare. Using a monoclonal antibody called “7/4” (22), which recognizes a marker that is expressed on neutrophils and a subset of monocytes, we found that all of the MMP-9+ cells inside the lesions were positive for 7/4 antigen (Fig. 1B). These cells were negative for both CD68 and F4/80 and showed polymorphic nuclei (data not shown), strongly suggesting that they are neutrophils. Interestingly, the expression of MMP-9 was highest in the neutrophil population as compared to macrophages, as evidenced both by immunofluorescent intensity in the same tissue sections and by flow cytometry (Fig. 1 A–C). In sum, we detected two distinct MMP-9+ innate immune cell types in association with angiogenic islets and tumors: neutrophils were found inside the lesions, whereas macrophages were present in clusters at the lesional periphery.
Neutrophil Ablation Suppresses the Angiogenic Switch.
Because the predominant MMP-9+ cells found infiltrating inside angiogenic islets and tumors were Gr-1+ neutrophils, we sought to investigate their role in catalyzing the angiogenic switch in premalignant lesions, namely hyperplasias and early dysplasias with a still quiescent vasculature, by using anti-Gr-1 antibodies to selectively ablate these cells. We used an experimental regimen involving daily inoculation of the anti-Gr-1 monoclonal antibody, which had been shown previously to ablate the neutrophil population circulating in the bloodstream (23, 24). The regimen began at 7 weeks, at a time when hyperplastic islets are undergoing angiogenic switching, coincident with neutrophil infiltration.
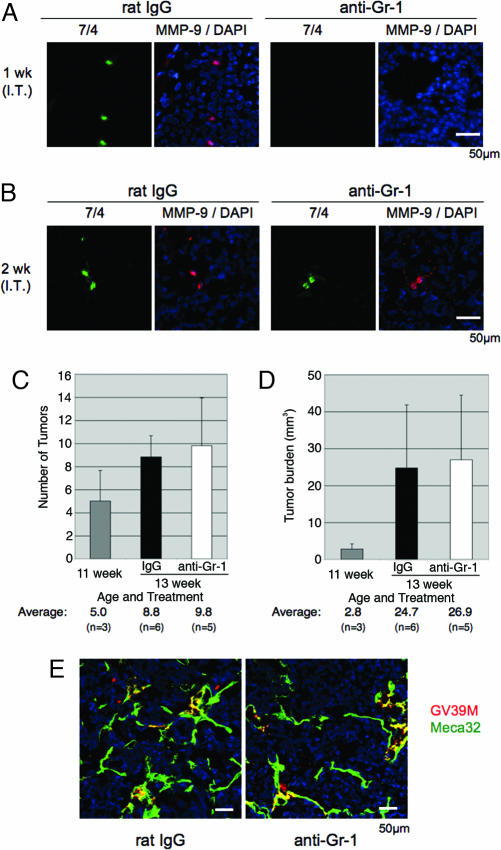
After 1 week of injection of anti-Gr-1 antibodies into RIP1-Tag2 mice, most neutrophils had disappeared from the pancreatic islets compared to rat IgG injection (Fig. 2A). As shown in Fig. 2B, the neutrophil population rebounded by 2 weeks of the regimen in the neoplastic islets. Interestingly, none of the rebound neutrophils infiltrating the premalignant lesions expressed significant immunoreactivity against MMP-9 at the 2-week time point.
Fig. 2.
Neutrophil ablation suppresses the angiogenic switch in a prevention trial. (A) Immunostaining for 7/4 (green) and MMP-9 (red) reveals the depletion of MMP-9+ neutrophils after a 1-week regimen of anti-Gr-1 antibodies. (B) Immunostaining for 7/4 and MMP-9 reveals the return of infiltrating neutrophils that are MMP-9− after a second week of anti-Gr-1 treatment. (C) Neutrophil ablation reduces the number of angiogenic islet dysplasias as compared to untreated and control IgG-treated RIP1-Tag2 mice. The columns indicate mean values, and the bars are SD. (D) Neutrophil ablation decreases bioavailable VEGF in angiogenic islet dysplasias in a prevention trial. Immunostaining for VEGF:VEGF-R2 complex with GV39M (red) and for endothelial cells with Meca-32 (green) in control and anti-Gr-1-injected animals shows a decrease in bioactive VEGF in angiogenic dysplasias consequent to depletion of infiltrating MMP-9+ neutrophils.
The relative synchronicity of neoplastic progression has enabled the design of therapeutic trial regimens that target different stages of tumorigenesis in this model (17). Of these, the “prevention trial” targets the angiogenic switching in hyperplastic/dysplastic islets and assesses the angiogenic switching frequency at a defined endpoint 2–4.5 weeks later in response to candidate inhibitors; depending on the endpoint, 20–50 of the islets have switched on angiogenesis in unmanipulated RIP1-Tag2 mice (17). In this study, we ran a short 2-week prevention trial, from 7 to 9 weeks of age, in part to avoid neutralizing humoral responses against the injected rat IgG and also in recognition of the rebound effect to anti-Gr-1 treatment described above. At the endpoint, the control mice that had been injected with rat IgG showed an average of 30.3 angiogenic islets, a number similar to that observed in 9-week-old untreated RIP1-Tag2 mice (37.7 angiogenic islets) (Fig. 2C). In marked contrast, the anti-Gr-1 antibody regimen decreased the number of angiogenic islets to 12.9 (57% reduction). The reduced frequency of angiogenic switching is remarkably similar to the ≈50% reductions seen in RIP1-Tag2 mice that were homozygous-null for MMP-9 or treated with an MMP-9 inhibitor (12).
Neutrophil Ablation Inhibits the Association of VEGF and VEGF Receptor (VEGF-R) in Neoplastic Lesions.
It has been demonstrated that one of the roles of MMP-9 in regulating initial angiogenic switching and the persistence of angiogenesis involves promoting the bioavailability of VEGF, thereby increasing VEGF association with its receptor VEGFR2, both in the RIP1-Tag2 model (12) and in a model of cervical carcinogenesis (19). The VEGF-A:VEGF-R2 association can be visualized by immunostaining with the GV39M monoclonal antibody, which is indicative of bioavailable VEGF and indirectly of MMP-9 activity (12, 19). We assessed the abundance of VEGF:VEGFR complexes by GV39M staining, together with costaining with a pan-endothelial cell marker, Meca-32, to reveal the endothelial cells. After 2 weeks of control antibody injection, ≈10% of Meca-32+ endothelial cells expressed GV39M in angiogenic islets of control mice (Fig. 2D Left). By contrast, the frequency of GV39M+ endothelial cells decreased to 1.4% in response to the anti-Gr-1 inoculation (7-fold reduction, Fig. 2D Right).
To compare vascularity in angiogenic islets between the rat IgG and anti-Gr-1 antibody-injected animals, CD31 staining was performed. The number of CD31+ cells in islets was similar in the two groups (Fig. 6, which is published as supporting information on the PNAS web site). This result is consistent with previous analysis of MMP-9-null RIP1-Tag2 mice (12), which revealed reduced angiogenic switching frequency, but a similar neovascular morphology in the angiogenic islets that did switch, suggestive of an alternative MMP-9-independent angiogenesis pathway, likely regulated by cysteine cathepsin proteases (25) or heparanase (26).
We further evaluated possible effects of anti-Gr-1 antibodies on the oncogene-expressing cells in those fewer angiogenic islets that did arise angiogenic islets. The analyses did not reveal any difference in proliferation (BrdU+ cells) or apoptosis (TUNEL+ cells) between the two treatment arms (Fig. 7, which is published as supporting information on the PNAS web site). This result is also consistent with that seen in MMP-9-null mice, wherein the angiogenic dysplasias that did arise were fewer in number but similar in neoplastic characteristics.
Transient Ablation and Rapid Rebound of Tumor-Infiltrating Neutrophils.
We also sought to assess the contribution of neutrophils in the tumor stages. Anti-Gr-1 antibodies were administered to tumor-bearing RIP1-Tag2 mice in a 2-week “intervention trial” (from 11 to 13 weeks of age), aimed at a point when small solid tumors are beginning a rapid growth phase (17). The ablation of neutrophils infiltrating tumors was also achieved after 1 week of daily inoculation and rebounded by the end of the trial (Fig. 3A and B). Notably, unlike the rebounding MMP-9− neutrophils infiltrating the angiogenic islets after the prevention trial, the rebounding neutrophils infiltrating tumors after the 2-week-long intervention trial were all MMP-9+ (Fig. 3B).
Fig. 3.
Transient neutrophil ablation does not affect tumor progression in an intervention trial. (A) Immunostaining for 7/4 (green) and MMP-9 (red) reveal the depletion of MMP-9+ neutrophils after 1 week of injection of anti-Gr-1 antibodies. (B) Immunostaining for 7/4 and MMP-9 document the rebound of infiltrating MMP-9+ neutrophils. (C and D) Neutrophil ablation does not lead to the reduction in the number of tumors (C) or in tumor burden (D). The columns indicate mean values, and the bars are SD. (E) Transient neutrophil ablation does not affect bioavailable VEGF in tumors in an intervention trial. Immunostaining for VEGF:VEGF-R2 complex with GV39M (red) and endothelial cells with Meca-32 (green) in control (A) and anti-Gr-1-injected (B) animals shows no difference in bioactive VEGF inside tumors after the depletion of infiltrating MMP-9+ neutrophils.
To address the possible effects of transitory neutrophil ablation on the tumor stage, we assessed tumor burden and performed histological analysis for bioavailable VEGF. The tumor number and tumor burden at the end of trial were virtually identical in the two treatment groups (8.8 tumors and 24.7 mm3 at average in control vs. 9.8 tumors and 26.9 mm3 in anti-Gr-1-injected mice; Fig. 3 C and D). In contrast to the prevention trial, there was no difference observed in the VEGFA:VEGF-R2 association by staining with GV39M, comparing control rat IgG with anti-Gr-1 antibody-injected RIP1-Tag2 mice (Fig. 3E). Both treatment arms showed similar vascular density and similar numbers of proliferating (BrdU+) and apoptotic (TUNEL+) cells in the tumors (data not shown). Thus transient ablation of neutrophils in preexisting solid tumors had no discernable effect on their angiogenic phenotype or other parameters of the transformed state.
Granulocyte Colony-Stimulating Factor (G-CSF) Mobilization of Neutrophils Does Not Increase the Frequency of Infiltrated Islets or of Consequent Angiogenic Switching.
Because ablation of the infiltrating neutrophils suppressed the angiogenic switch, we sought to ask a complimentary question of whether increasing the abundance of activated neutrophils would increase the frequency of angiogenic switching in the preangiogenic hyperplastic/dysplastic islet stage. G-CSF has been shown to enhance the release of neutrophils into the circulation and to stimulate angiogenesis in some animal models (27, 28). RIP-Tag2 mice received daily treatment with recombinant human G-CSF for 2 weeks starting at 7 weeks, when hyperplastic/dysplastic islets are undergoing angiogenic switching. We observed a 4.5-fold increase in circulating neutrophils after 2 weeks of GSF treatment and a 3-fold increase in the number neutrophils present in those islets that were infiltrated (Fig. 4A and B). There was not, however, an increase in the number of islets infiltrated by neutrophils (Fig. 4C), nor was there an apparent difference in the number of angiogenic islet lesions at the end of the experiment (41 angiogenic islets in the PBS control as compared to 35.2 in the G-CSF treated mice) (Fig. 4D). Thus, the GCF-enhanced neutrophil infiltration correlated with angiogenic lesions (as revealed by GV39M immunostaining) and was undetectable/rare in the nonangiogenic islets (Fig. 8A, which is published as supporting information on the PNAS web site). Although the additional neutrophils elicited by G-CSF in angiogenic islets were MMP-9+, they did not further increase the bioavailable VEGF as measured by GV39M immunostaining (see Fig. 4E and Fig. 8B). Thus, in this model, G-CSF does not increase the number of neutrophil-infiltrated islets. Rather, it elevates the abundance of infiltrating neutrophils in those hyperplastic/dysplastic islets that are already attracting the inflammatory cells; we infer that other immunoregulatory signals are rendering a subset of the hyperplastic/dysplastic islets receptive/attractive to neutrophil infiltration and consequent angiogenic switching.
Fig. 4.
Increasing peripheral blood neutrophils by G-CSF treatment does not affect the angiogenic switch. (A) G-CSF increases peripheral blood counts of circulating neutrophils. (B) G-CSF increases neutrophil abundance in islets that are infiltrated. (C) G-CSF does not increase the number of neutrophil infiltrated Rip1-Tag2 islets. (D) The G-CSF mediated increase in neutrophil abundance does not affect the number of angiogenic islet dysplasias, as compared to control PBS-treated RIP1-Tag2 mice. (E) G-CSF treatment does not increase bioavailable VEGF. Immunostaining for VEGF:VEGF-R2 complex with GV39M (red) for endothelial cells with Meca-32 (green) and for 7/4 (blue) in angiogenic islet dysplasias from control and G-CSF-injected mice. The data shown in E are representative of six fields analyzed in two sections each from three G-CSF and three sham-treated RIP-Tag2 mice. The columns show mean values, and the bars are SD.
Discussion
In this study, we have identified the cellular sources of MMP-9, a proangiogenic protease functionally documented as important for angiogenic switching and for initial tumor growth in the RIP1-Tag2 mouse model of pancreatic islet carcinogenesis. MMP-9 is expressed in neutrophils infiltrating the angiogenic islet dysplasias and tumors, whereas MMP-9+ macrophages reside along the periphery of the angiogenic lesions. Because of the localization of the neutrophils within the angiogenic lesions and their relatively high expression of MMP-9, we focused our subsequent analysis on this cell type.
Neutrophils are among the first cells to arrive at sites of infection, where they can release chemokines and proteases that can in turn recruit both nonspecific and specific immune effector cells (1). Neutrophils can also release toxic granules against neighboring cells, suggesting their potential anti-tumor activity (29). However, it has been shown in several tumor transplant models that tumor-associated neutrophils can stimulate tumor angiogenesis via the production of proangiogenic factors, including VEGF, IL-8 (30), and certain proteases such as MMPs (18, 31, 32) and elastases (33, 34), and that they can facilitate metastasis (24). Our study has now demonstrated that neutrophils can switch on angiogenesis in a mouse model of spontaneous tumor development.
As was inferred from our initial analysis of (then unidentified) MMP-9+ cells inside angiogenic lesions (12), the neutrophils infiltrating angiogenic lesions are relatively rare; we estimate that neutrophils variably constitute 0.1–0.4% of the total cells in both angiogenic islets and solid tumors, as compared to macrophages, which represent ≈2–4% of cells (Fig. 1C and data not shown). Nevertheless, ablation of neutrophils by a 2-week-long inoculation of anti-Gr-1 reduced the frequency of angiogenic switching and inhibited the VEGF:VEGF-R association on the islet vasculature. These data are consistent with the proposition that the scarce neutrophils are the key initial source of MMP-9 for catalyzing the angiogenic switch through the activation/release of latent VEGF and consequent proangiogenic signaling. Because the phenotypic effect on angiogenic switching of neutrophil ablation is similar to that of ablating MMP-9 function, we infer that macrophages and other inflammatory cells have little impact on MMP-9-dependent VEGF mobilization and induction of angiogenesis in previously quiescent tissue vasculature. It will be of interest to assess the possible involvement of similarly subtle infiltrations of MMP-9+ neutrophils in angiogenic switching in other organs with incipient malignancy, as well as in other diseases with an angiogenic component.
Longer term functional studies on the continuing role of neutrophils were limited by the rebound of circulating and infiltrating neutrophils in the face of continued anti-Gr-1 antibody injections and likely by the expected induction of a humoral response to the depleting antibody after 2–3 weeks. Interestingly, the rebounding neutrophils were qualitatively different in the prevention trial targeting initial angiogenic switching in dysplastic islets vs. the intervention trial targeting small solid tumors with an already angiogenic vasculature. The infiltrating rebound neutrophils found in premalignant islets in the prevention trial did not express MMP-9 (at least initially) and may represent immature neutrophils (or a distinctive neutrophil subtype), whereas the rebounding neutrophils found in solid tumors in the intervention trial expressed MMP-9, much as neutrophils in the unperturbed stages.
As a complement to ablating the neutrophils, we sought to increase the infiltration of neutrophils via systemic treatment with G-CSF, which is known to increase the abundance of circulating peripheral blood neutrophils. Although G-CSF elevated the neutrophil count in peripheral blood, as expected, it did not increase the frequency of infiltrated islets or the frequency of angiogenic switching among the ≈400 oncogene-expressing islet nodules, nor did it affect the characteristics of the angiogenic islets that appeared. Rather, G-CSF increased the numbers of neutrophils per infiltrated islet. Thus the neutrophil infiltration similarly correlated with the angiogenic lesions (detected with GV39M staining of VEGF-VEGFR complexes) both in untreated and G-CSF-treated RIP-Tag2 mice, and in both cases neutrophils were undetectable/rare within the nonangiogenic lesions. These data suggest that an undefined signal in the preangiogenic islets triggers the recruitment of the MMP-9+ neutrophils and that the infiltration of these neutrophils then catalyzes the angiogenic switch. Intriguingly, the G-CSF mediated increase in the number of neutrophils in those islets that became infiltrated did not qualitatively affect their angiogenic phenotype, suggesting that remarkably few neutrophils are sufficient to facilitate the angiogenic switch. Parenthetically, the inability of G-CSF to enhance angiogenic switching relieves a potential concern, namely that the use of G-CSF to ameliorate neutropenia associated with chemotherapy might be inadvertently proangiogenic and thus tumor-promoting; the data presented here suggest that G-CSF may not have such undesirable effects.
An outstanding question involves the role and relative importance of infiltrating neutrophils in the solid tumor stage. The 2-week-long ablation of neutrophils had no impact on angiogenesis or any other parameter of the tumor phenotype. There are several explanations for this lack of effect, with the most straightforward being that the rapid rebound of MMP-9+ neutrophils was too fast for their brief absence to have an impact. Alternatively, the MMP-9-expressing macrophages at the periphery of the tumors (where angiogenesis and tumor growth are occurring) may take over the job of supplying MMP-9 to sustain continuing neovascularization concomitant with tumor growth. We had sought to ablate the macrophages with clodronate liposome, a reagent that can in some circumstances deplete tissue-associated macrophages (35, 36). Although administration of the reagent successfully depleted macrophages from the liver and spleen in RIP1-Tag2 mice, the pancreatic macrophages proved resistant to this treatment (H.N. and D.H., unpublished observations). Thus we presently do not have the capability to effectively ablate either macrophages or neutrophils for sustained periods in the solid tumors, and as such the definitive resolution of their respective roles in angiogenesis and tumor growth awaits new enabling genetic technologies or pharmacological inhibitors for their inactivation. Meanwhile, our data have revealed a role for infiltrating neutrophils in the initial angiogenic switch that occurs in previously nonangiogenic lesions (38), an essential step in this prototypical model of multistep carcinogenesis (37), one that is evidently important in other mouse models (14, 18, 19) and inferentially in human cancers (39). The result presents the postulate that subtle infiltration by neutrophils may play a similar role in the progression of incipient neoplasias toward expansive angiogenic tumors in many forms of animal and human cancer.
Methods
Mice.
The local committee for animal care at the University of California, San Francisco, approved all animal studies described. From 12 weeks of age, all RIP1-Tag2 mice received 50% sugar food (Harlan Teklad, Madison, WI) to relieve hypoglycemia induced by the insulin-secreting tumors.
Antibodies for in Vivo Injection.
The anti-Gr-1 antibody (RB6–8C5 hybridoma originally produced by R. L. Coffman, DNAX Research Institute, Palo Alto, CA) was produced in serum-free media (Hybridoma-SFM; Invitrogen, Carlsbad, CA), supplemented with MEM nonessential amino acid solution (Sigma-Aldrich, St. Louis, MO), 0.4% glucose, and 2 mM l-glutamine, by using the CELLine system (BD Biosciences, San Jose, CA). The antibody was purified by hydrophobic interaction chromatography using MEP HyperCel (Ciphergen Biosystems, Fremont, CA). Control rat IgG was purchased from Jackson Immunoresearch (West Grove, PA).
Experimental Trials.
Mice received i.p. injections of 200 μg of control rat IgG or anti-Gr-1 antibodies daily for 2 weeks. For the G-CSF trial, mice received i.p. injections of PBS or 100 μg/kg recombinant human G-CSF, Neupogen (Amgen, Thousand Oaks, CA) daily for 2 weeks. Mice were continuously monitored, but no side effects such as weight loss, infection, or hypoglycemic shock due to huge tumor development were observed (data not shown).
The prevention trial was started when the mice reached an age of 7 weeks. After 2 weeks of treatment, mice were anesthetized, and their hearts were perfused with 10 ml of 10% zinc-buffered formalin (Medical Chemical Corporation, Torrance, CA), followed by 10 ml of PBS. Subsequently, the pancreases of the mice were dissected, collagenase was digested, and the number of angiogenic islets and small tumors were counted through a dissecting microscope. The intervention trial was scheduled from 11 to 13 weeks of age. The pancreases of the mice were dissected and macroscopic tumors (>1 mm3) were excised and measured, and the number of tumors in each mouse was noted. Tumor volume (v) was calculated by using the formula for a spheroid: v = 0.52 × (width)2 × (length). Tissues were embedded in OCT compound (Sakura Finetek Co., Torrance, CA) and frozen for cryosectioning and histological analysis.
Immunostaining.
Cryosections (5 to 10 μm) were allowed to dry in air before they were equilibrated in PBS. Sections were blocked by using a serum-free protein block (DAKO Cytomation, Carpinteria, CA) for 45 min at room temperature. The primary antibodies were diluted in PBS plus 0.5% blocking reagent (PerkinElmer Life Science, Wellesley, MA) and incubated overnight at 4°C. The primary antibodies used were rabbit anti-MMP-9 (a gift from Zena Werb, University of California, San Francisco), rat anti-mouse CD68 (Serotec, Raleigh, NC), rat anti-mouse 7/4 antigen (22) (Cedarlane, Hornby, ON, Canada), rat anti-mouse Meca-32 and FITC rat anti-mouse CD31 (BD Biosciences, San Jose, CA), and mouse anti-mouse VEGF:VEGF-R2 complex–GV39M (East Coast Biologics, North Berwick, ME). On the next day, the sections were washed with PBS and incubated with FITC or TRITC-labeled secondary antibodies for 2 h at room temperature. After washing in PBS, sections were mounted in Vectashield mounting medium that contained DAPI (Vector Laboratories, Burlingame, CA). For triple staining with CD31, GV39M, and 7/4, the 7/4 and GV39M antibodies were incubated together first overnight. On the next day, the sections were washed incubated with goat anti-mouse IgM biotin for 1 h at room temperature to label the bound GV39M antibodies. After washing, streptavidin-Marina blue (Invitrogen, Carlsbad, CA) and goat anti-rat-TRITC-labeled secondary antibody were applied for 1 h at room temperature. After washing, FITC-labeled CD31 was then incubated with the tissue sections for 2 h at room temperature. After washing, the sections were mounted in 50% glycerol, 0.1 M Tris (pH 8.0), and 0.15 M NaCl. The images were false-colored digitally to match the previous figures.
Flow Cytometric Analysis of RIP1-Tag2 Tumors.
Solid tumors from 13-week-old RIP1-Tag2 mice were dissected away from the exocrine pancreas and digested with collagenase and DNase. The cells were suspended in PBS plus 1% BSA, and after Fc blocking (anti-mouse CD16/32; eBioscience, San Diego, CA), the cells were surface-labeled with FITC-conjugated rat anti-mouse 7/4 antigen (Cedarlane) or rat anti-Gr-1 (BD Biosciences). The cells were then fixed and permeabilized (Cytofix/Cytoperm; BD Biosciences) and incubated with rabbit anti-MMP-9 (a gift from Zena Werb) antibodies and/or FITC-conjugated rat anti-mouse CD68 (Serotec). After washing, cells were incubated with phycoerythrin-labeled anti-rabbit IgG antibody and analyzed by using a FACSCalibur (BD, Franklin Lakes, NJ).
Neutrophil Counts in Systemic Blood.
Peripheral blood was drawn from RIP1-Tag2 mice, and neutrophils were identified and counted by using a Hemavet 850 automated hematological analyzer (Drews Scientific, Oxford, CT).
Statistical Analysis.
Number of tumors and tumor burden were analyzed by using the Mann–Whitney test (InStat version 3.0A, GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank Lewis Lanier for helpful discussions and suggestions, and Lewis Lanier, Eric Brown, Oriol Casanovas, and Lisa Coussens for critical reading of the manuscript. We also thank Zena Werb (University of California, San Francisco, CA) for providing anti-MMP-9 antibody, Eric Brown (University of California, San Francisco, CA) for RB6–8C5 hybridoma cells, and Dale Milfay for technical advice on purification of anti-Gr-1 antibodies. We appreciate access to the University of California, San Francisco, Diabetes Center’s (DERC-funded) Microscopy Core as well as to core facilities maintained by the University of California, San Francisco, Comprehensive Cancer Center. H.N. acknowledges support in part by a fellowship grant from the Sankyo Foundation of Life Science. The research was supported by grants to D.H. from the U.S. National Cancer Institute and by the William F. Bowes Charitable Foundation. D.H. is an American Cancer Society research professor.
Glossary
Abbreviations
- MMP
matrix metalloprotease
- G-CSF
granulocyte colony-stimulating factor
- VEGF-R
VEGF receptor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Coussens L. M., Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin E. Y., Pollard J. W. Br. J. Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn G. P., Old L. J., Schreiber R. D. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Daniel D., Chiu C., Giraudo E., Inoue M., Mizzen L. A., Chu N. R., Hanahan D. Cancer Res. 2005;65:2018–2025. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 5.de Visser K. E., Korets L. V., Coussens L. M. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Adams T. E., Alpert S., Hanahan D. Nature. 1987;325:223–228. doi: 10.1038/325223a0. [DOI] [PubMed] [Google Scholar]
- 7.Welch D. R., Schissel D. J., Howrey R. P., Aeed P. A. Proc. Natl. Acad. Sci. USA. 1989;86:5859–5863. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 9.Bingle L., Brown N. J., Lewis C. E. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A., Allavena P., Sica A. Eur. J. Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Pollard J. W. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G., Brekken R., McMahon G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel D., Meyer-Morse N., Bergsland E. K., Dehne K., Coussens L. M., Hanahan D. J. Exp. Med. 2003;197:1017–1028. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens L. M., Raymond W. W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G. H., Hanahan D. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 16.Casanovas O., Hicklin D. J., Bergers G., Hanahan D. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G., Javaherian K., Lo K. M., Folkman J., Hanahan D. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 18.Coussens L. M., Tinkle C. L., Hanahan D., Werb Z. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudo E., Inoue M., Hanahan D. J. Clin. Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Man A. K., Young L. J., Tynan J. A., Lesperance J., Egeblad M., Werb Z., Hauser C. A., Muller W. J., Cardiff R. D., Oshima R. G. Mol. Cell Biol. 2003;23:8614–8625. doi: 10.1128/MCB.23.23.8614-8625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams T. M., Medina F., Badano I., Hazan R. B., Hutchinson J., Muller W. J., Chopra N. G., Scherer P. E., Pestell R. G., Lisanti M. P. J. Biol. Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch S., Gordon S. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 23.Brown C. R., Blaho V. A., Loiacono C. M. Infect. Immun. 2004;72:4956–4965. doi: 10.1128/IAI.72.9.4956-4965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tazawa H., Okada F., Kobayashi T., Tada M., Mori Y., Une Y., Sendo F., Kobayashi M., Hosokawa M. Am. J. Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce J. A., Baruch A., Chehade K., Meyer-Morse N., Giraudo E., Tsai F. Y., Greenbaum D. C., Hager J. H., Bogyo M., Hanahan D. Cancer Cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 26.Joyce J. A., Freeman C., Meyer-Morse N., Parish C. R., Hanahan D. Oncogene. 2005;24:4037–4051. doi: 10.1038/sj.onc.1208602. [DOI] [PubMed] [Google Scholar]
- 27.Ohki Y., Heissig B., Sato Y., Akiyama H., Zhu Z., Hicklin D. J., Shimada K., Ogawa H., Daida H., Hattori K., Ohsaka A. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki T., Ebihara S., Asada M., Kanda A., Sasaki H., Yamaya M. Int. Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- 29.Di Carlo E., Forni G., Lollini P., Colombo M. P., Modesti A., Musiani P. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 30.Schaider H., Oka M., Bogenrieder T., Nesbit M., Satyamoorthy K., Berking C., Matsushima K., Herlyn M. Int. J. Cancer. 2003;103:335–343. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- 31.Shamamian P., Schwartz J. D., Pocock B. J., Monea S., Whiting D., Marcus S. G., Mignatti P. J. Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 32.Yang L., DeBusk L. M., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L. M., Carbone D. P., Lin P. C. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Iwatsuki K., Kumara E., Yoshimine T., Nakagawa H., Sato M., Hayakawa T. Neurol. Res. 2000;22:465–468. doi: 10.1080/01616412.2000.11740701. [DOI] [PubMed] [Google Scholar]
- 34.Scapini P., Nesi L., Morini M., Tanghetti E., Belleri M., Noonan D., Presta M., Albini A., Cassatella M. A. J. Immunol. 2002;168:5798–5804. doi: 10.4049/jimmunol.168.11.5798. [DOI] [PubMed] [Google Scholar]
- 35.van Rooijen N., Sanders A. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 36.van Rooijen N., van Kesteren-Hendrikx E. J. Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 37.Inoue M., Hager J. H., Ferrara N., Gerber H. P., Hanahan D. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 38.Folkman J., Watson K., Ingber D., Hanahan D. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D., Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.