Abstract
The term mucositis is coined to describe the adverse effects of radiation and chemotherapy treatments. Mucositis is one of the most common adverse reactions encountered in radiation therapy for head and neck cancers, as well as in chemotherapy, in particular with drugs affecting DNA synthesis (S-phase-specific agents such as fluorouracil, methotrexate, and cytarabine). Mucositis may limit the patient's ability to tolerate chemotherapy or radiation therapy, and nutritional status is compromised. It may drastically affect cancer treatment as well as the patient's quality of life. The incidence and severity of mucositis will vary from patient to patient. It will also vary from treatment to treatment. It is estimated that there is 40% incidence of mucositis in patients treated with standard chemotherapy and this will not only increase with the number of treatment cycles but also with previous episodes. Similarly, patients who undergo bone marrow transplantation and who receive high doses of chemotherapy have a 76% chance of getting mucositis. Patients receiving radiation, in particular to head and neck cancers, have a 30% to 60% chance. The exact pathophysiology of development is not known, but it is thought to be divided into direct and indirect mucositis. Chemotherapy and/or radiation therapy will interfere with the normal turnover of epithelial, cells leading to mucosal injury; subsequently, it can also occur due to indirect invasion of Gram-negative bacteria and fungal species because most of the cancer drugs will cause changes in blood counts. With the advancement in cytology, a more precise mechanism has been established. With this understanding, we can select and target particular mediators responsible for the mucositis. Risk factors such as age, nutritional status, type of malignancy, and oral care during treatment will play important roles in the development of mucositis. Many treatment options are available to prevent and treat this condition, but none of them can completely prevent or treat mucositis. More and more pathological methods are being developed to understand this condition so that better therapeutic regimens can be selected. Emphasis also should be made in assessing the patient's psychologic condition, particular depressive disorders. This is important because treatment with antidepressants will not only contribute in lifting depression but also reduces pain somatization. Although mucositis is rarely life-threatening, it will interfere with treatment of cancer to a great extent.
Keywords: Mucositis, head and neck cancers, cell viability, somatization, chemotherapy
Introduction
Chemotherapy and radiation therapy are the most widely used interventions for the treatment of cancer. Although these treatments are employed to improve the patient's quality of life, they are associated with several side effects. Severe adverse reactions due to these therapies result in patient morbidity and mortality. In addition, they also contribute to economic ramifications of the affected patient. Annually, there are approximately 400,000 cases of treatment-induced damage to the oral cavity [1]. Oral complications that arise with chemotherapy and/or radiation therapy include mucositis (stomatitis); xerostomia (dry mouth); bacterial, fungal, or viral infection (particularly in neutropenic patients); dental caries; loss of taste; and osteoradionecrosis [2]. Oral mucositis also represents a major nonhematologic complication of cytotoxic chemotherapy and radiotherapy associated with significant morbidity, pain, odynodysphagia, dyseugia, and subsequent dehydration and malnutrition [3]. Severe oral toxicities can also compromise the delivery of optimal cancer therapy protocols. For example, dose reduction or treatment schedule modifications may be necessary to allow for resolution of oral lesions. In cases of severe oral morbidity, the patient may no longer be able to continue cancer therapy; treatment is then usually discontinued. These disruptions in dosing due to oral complications can directly affect patient survivorship.
The term oral mucositis emerged in the late 1980s to describe the adverse effects of chemotherapy-induced and radiation therapy-induced inflammation of the oral mucosa. Symptoms of mucositis vary from pain and discomfort to an inability to tolerate food or fluids. Mucositis may also limit the patient's inability to tolerate either chemotherapy or radiation therapy, resulting in dose-limiting toxicity and hence drastically affecting cancer treatment and outcome.
Epidemiology
Incidence as well as severity may vary from patient to patient. The probability of developing mucositis is dependent upon the treatment. It is estimated that about 40% of patients treated with standard chemotherapy develop mucositis [4]. The risk of developing mucosal injury increases with the number of chemotherapy cycles and previous episodes of chemotherapy-induced mucositis. To our knowledge, there should be a qualitative difference between the severity of oral mucositis induced by radiation and that induced by chemotherapy. But we have no supporting literature to confirm this. Drugs affecting DNA synthesis (S-phase-specific agents such as fluorouracil, methotrexate, and cytarabine) exhibit more pronounced stomatotoxic effects [5]. It is estimated that there is an increased risk of mucositis development with bolus and continuous infusions compared to prolonged or repetitive administration of lower doses of cytotoxic agents [5,6]. In patients who undergo bone marrow transplantation and receive high-dose chemotherapy, the incidence is approximately 76%. Between 30% and 60% of patients receiving radiation therapy for cancer of the head and neck may develop oral mucositis, and greater than 90% of patients receiving concomitant chemotherapy and localized radiation therapy will be affected [4,7]. The degree and duration of mucositis in patients treated with radiation therapy are related to radiation source, cumulative dose, dose intensity, volume of radiated mucosa, smoking, alcohol consumption, and oral hygiene [8,9]. Mucosal erythema occurs in the first week in patients treated with standard 200 cGy of daily fractionated radiotherapy programs. Patchy or confluent mucositis peaks during the fourth to fifth weeks of treatment with the same dose of radiation. With daily fractionated programs of <200 cGy, the severity of mucositis is expected to be low. However, in accelerated radiotherapy programs, mucositis peaks within 3 weeks of the radiation therapy. Mucositis caused by interstitial radioactive implants usually appears in 7 to 10 days and peaks after 2 weeks [10].
A variety of patient-related factors are responsible for the increased potential for developing mucositis after chemotherapy or radiation therapy. It is stated that up to 75% of the general population has chronic periodontal disease, and it is also hypothesized that many acute bacterial superinfections may follow chemotherapy. Patients with improved oral hygiene who can abstain from smoking can definitely reduce the incidence and severity of mucositis [11].
Pathophysiology
The exact pathophysiology of mucositis is not fully elucidated, but it is thought to have two mechanisms: direct mucositis and indirect mucositis, caused by chemotherapy and/or radiation therapy.
Direct Mucositis
The epithelial cells of the oral mucosa undergo rapid turnover, usually every 7 to 14 days, which makes these cells susceptible to the effects of cytotoxic therapy. Both chemotherapy and radiation therapy can interfere with the maturity and cellular growth of epithelial cells, causing changes to normal turnover and cell death [4].
Indirect Mucositis
Oral mucositis can also be caused by the indirect invasion of Gram-negative bacteria and fungal species. Patients are at increased risk for oral infections when they are neutropinic, and this usually happens when indirect stomatotoxicity appears. The onset of mucositis secondary to myelosuppression varies, depending upon the timing of the neutrophil nadir associated with the chemotherapy agent administered, but typically develops anywhere from 10 to 21 days after chemotherapy administration [12].
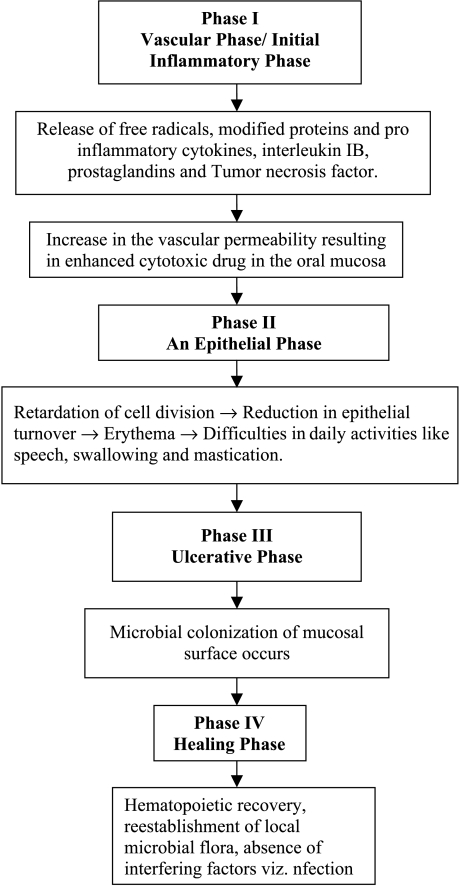
Based on the above consideration, new pathophysiology concepts have emerged, and the mechanism of mucositis involves four phases as described below [13] (Figure 1):
Figure 1.
Flow chart representing the different phases of mucositis development with chemotherapy and/or radiation therapy.
Phase I: Initial inflammatory/vascular phase: During this phase, exposed cells (epithelial, endothelial, and connective tissue cells) in the buccal mucosa release free radicals, modified proteins, and proinflammatory cytokines, including interleukin-1B, prostaglandins, and tumor necrosis factor (TNF). These inflammatory mediators cause further damage either directly or indirectly by increasing vascular permeability, thereby enhancing cytotoxic drug uptake into the oral mucosa [14].
Phase II: Epithelial phase: In this phase, chemotherapy and/or radiation retards cell division in the oral mucosal epithelium, leading to reduced epithelial turnover and renewal, resulting in epithelial breakdown. This results in erythema from increased vascularity and epithelial atrophy 4 to 5 days after the initiation of chemotherapy. At this stage, microtrauma from day-to-day activities such as speech, swallowing, and mastication leads to ulceration.
Phase III: Ulcerative/bacteriological phase (pseudomembraneous): Epithelial breakdown ultimately results in the ulcerative phase, which occurs within 1 week of therapy. Loss of epithelia and furious exudation lead to the formation of pseudomembranes and ulcers. In this phase, microbial colonization of damaged mucosal surfaces by Gram-negative organisms and yeast occurs, and this may be exacerbated by concomitant neutropenia. Infectious complications arising in neutropinic bone marrow transplantation recipients are among the most challenging aspects of aggressive myelosuppressive antineoplastic drug therapy. There are numerous reports that demonstrate the importance of ulcerative mucositis as an etiologic factor in the development of systemic α-hemolytic streptococcal infections in the neutropinic cancer patients [15].
Phase IV: Healing phase: The duration of this phase usually lasts from 12 to 16 days, and mainly depends on factors such as epithelial proliferation rate, hematopoietic recovery, reestablishment of the local microbial flora, and absence of factors interfering with wound healing viz. infection and mechanical irritation [13].
Risk Factors for the Development of Mucosal Injury
Some of the major risk factors include age, nutritional status, type of malignancy, oral care during treatment, and neutrophil count before treatment. In general, younger patients are more prone to mucositis because of rapid epithelial mitotic rate, or the presence of more epidermal growth factor receptors [16]. Similarly, physiological decline in renal function with aging may also contribute to the development of mucositis in the elderly [17]. There are reports stating that poor oral hygiene can also contribute to the development of mucositis following chemotherapy and/or radiation therapy [18]. The repair of ill-fitting dental prostheses, elimination of periodontal disease, and extraction of offending teeth, combined with effective oral hygiene during treatment, has demonstrated a reduction in the incidence and severity of mucositis [16]. In patients who are nutritionally compromised, there is always a chance for poor mucosal regeneration, which will in turn contribute to the development of severe mucositis [18]. Chemotherapy-induced mucositis is more common in hematological cancers because of more prolonged and intense myelosuppression (depending also on the chemotherapeutic agent given) and radiation-induced mucositis is common in head and neck cancers because of direct irradiation on the oral cavity (depending also on the dose and type of radiation) [16]. Polypharmacy is more common in cancer patients because of underlying comorbidities. Depressive disorders are one of the most common psychiatric complications in cancer patients and most of the antidepressants have anticholinergic activities, which contribute to xerostomia leading to mucositis. Other drugs that are most commonly overlooked are opiates, phenothiazines, antihypertensives, antihistamines, sedatives, and so on [19]. The drugs that are commonly responsible for oral mucositis are shown in Table 1. The exact incidences of different chemotherapeutic agents are lacking. Still, adverse drug reaction (ADR) monitoring studies have to be carried out to understand the exact incidence of oral mucositis. Certain chemotherapeutic agents such as methotrexate and etoposide may also be secreted in saliva, leading to increased risk of direct mucositis [20]. Recent studies have hypothesized that decreased neutrophil count is the risk factor for the development of mucositis [18]. Drugs causing mucosal damage are given in Table 1.
Table 1.
Different Chemotherapeutic Agents Known to Cause Mucosal Injury.
| Chemotherapeutic Agents Causing Mucositis [24] | |
| Alkylating agents | |
| Busulfan | |
| Cyclophosphamide, thiotepa, procarbazine | |
| Anthracyclines | |
| Doxorubicin, epirubicin, daunorubicin | |
| Antimetabolites | |
| 5-FU, methotrexate, hydroxyurea | |
| Antitumor agents | |
| Actinomycin D, bleomycin, mitomycin | |
| Taxanes | |
| Paclitaxel | |
| Vinca alkaloids | |
| Vincristine, vinblastine | |
Assessment of Mucosal Injury
In routine clinical practice as well in the area of research, proper assessment of oral mucosa is of paramount importance before initiating radiation therapy to the head and neck regions, as well as chemotherapy. A variety of protocols and grading systems have been introduced, but only a few of them are standardized and validated. A good scoring system is that which will consider all patient-related factors viz. the patient's physical and nutritional status combined with a detailed inspection of the oral cavity. Clinical severity of mucosal injury will vary from mild, to moderate, to severe. Standardized criteria that are routinely used for clinical and research purposes are described below:
World Health Organization (WHO) grading of mucositis: This scoring system is widely used in routine clinical practice and clinical trials for the evaluation of mucositis. It is graded from 0 to 4. If the patient has no signs and symptoms, it is graded as 0. If the patient has painless ulcers, edema, or mild soreness, it is graded as 1. If there is painful erythema, edema, or ulcers but able to eat, it is graded as 2. If there is painful erythema, edema, or ulcers but unable eat, it is graded as 3. If there a requirement for parenteral or enteral support, it is graded as 4 [7].
National Cancer Institute common toxicity criteria for grading of stomatitis [7]: This scale is also graded from 1 to 4. Painless ulcers, erythema, or mild soreness is graded as 1. When the patient has painful erythema, edema, and ulcer but is able to eat, it is graded as 2. When there is inability to eat, it is graded as 3. A patient requiring parenteral or enteral support is graded as 4.
Radiation therapy oncology oral mucositis grading system: This scale is divided into gross and functional. Gross is the one which is assessed by the attending physician or a research personal, and functional is assessed by the patient. It is scored from 0 to 4, wherein the presence of erythematous sores is scored as 1, patchy mucositis (<1/2 mucosa) is scored as 2, fibrinous mucositis (>1/2 mucosa) is scored as 3, and, similarly, hemorrhage and necrosis are scored as 4 [7].
Oral assessment guide (OAG): This is a very important tool for evaluating mucositis. Its validity and interreliability testing have been established. When oral cavity status changes secondary to chemotherapy and/or radiation therapy are quantified through the use of reliable and valid OAG, researchers can study the effectiveness of different oral care protocols and can identify the individuals at risk for problems secondary to stomatitis. It mainly consists of eight items, which are rated from 1 to 3 [21].
Objective scoring system for site assessment: This is a more commonly used scoring system for assessing site involvement in oral mucositis induced by chemotherapy with or without radiation therapy. It consists of nine items wherein one can assess the site and size of ulcers and the severity of erythema. It is scored from 0 to 3. Zero indicates no lesions; if the size is less than 1 cm2, then it is scored as 1. If the size is between 1 and 2 cm2, it is scored as 2. If it is greater than 3 cm2, it is scored as 3. Similarly, depending on the severity of erythema, it is scored from 0 to 2. Zero indicates no erythema, 1 indicates nonsevere erythema, and 2 indicates severe erythema [22].
Although the grading of mucositis is necessary to document its degree and to evaluate the effect of prevention or intervention, most of these scoring systems can be applied only for clinically visible mucositis. It may not correlate with complaints of patients. However, all these scoring systems remain subjective; a lot of intervariability is possible. Thus, recently, new in vitro assays for quantitation of mucosal injury have been established. Following are some of the objective pathological parameters that define mucosal injury induced by radiation or chemotherapy [23].
Estimation of Cell Viability in Mouthwashes
Trypan blue dye exclusion method is a method commonly used in cytology to assess the number of living cells and dead cells in the oral mucosa. The main principle involved in the test is that trypan blue will stain dead or dying cells. Viable cells are able to repel the dye and do not stain. In general, this test is used regularly in tissue cultures to determine the number of live cells for planting. This test is also being used to assess cell viability in patients with mucositis. Machteld et al. conducted a phase I study of transforming growth for the prevention of chemotherapy-induced mucositis wherein authors used the assessment of cell viability as a secondary outcome measure to study the effect of TGF-β3. Authors have concluded that there is an increase in the total viable cells in patients treated with different standard chemotherapeutic regimens [23].
Estimation of Neutrophil Levels in Mouthwashes
One of the significant causes of morbidity with respect to mucositis in patients treated with high-dose chemotherapeutic agents is infection. It is well established that the risk of infection increases with the severity and duration of neutropinic episodes. Graham et al. [24] conducted a study on healthy volunteers to establish the normal count of neutrophils in oral rinses. It was found to be 472 ± 329 x 103 compared to undetectable levels found in patients on chemotherapy. In this assay, mouthwash with normal saline is collected in centrifuge tubes containing acridine orange. Counting of orange granular cytoplasm is done on a hemocytometer. This method is an important tool to monitor neutrophil levels in patients who are at high risk for mucositis [24].
Epithelial Cell Morphology and Differentiation/Maturation
The oral mucosa will respond to physical, chemical, or biologic agents, leading to ulcerations and inflammations. Scrapings from these lesions may reveal cells whose cytoplasm is abnormally acidophilic, with enlarged nuclei and scanty surrounding cytoplasm, or both [25]. A study conducted by Wymenga et al. reveled that the number of viable cells increases from baseline in patients receiving chemotherapy. In an attempt to understand the maturity of these epithelial cells, Papanicolaou staining technique had been performed. According to this technique, cells with orange color are considered to be mature cells; green or blue indicate immature cells; and partial orange and partial green/blue are considered to be intermediate cells [23,24,26].
Quality of Life
Quality of life is defined as an individual's perceptions of his/her position in life in the context of culture and value systems in which he/she lives in relation to his/her goals, expectations, and concerns [27]. Over the past decade, there has been a dramatic increase in the use of quality-of-life measurements in clinical trials. Improving the quality of patients' lives has become as important as extending the quantity of life. Radiation-induced mucositis is a very severe complication wherein patients' daily living habits are compromised. Thus, the treatment aimed to reduce the symptoms of mucositis should also aim to improve the quality of life. To our knowledge, very few studies have been carried out in this area.
Treatment Options Available for Oral Mucositis
Preventive Treatment
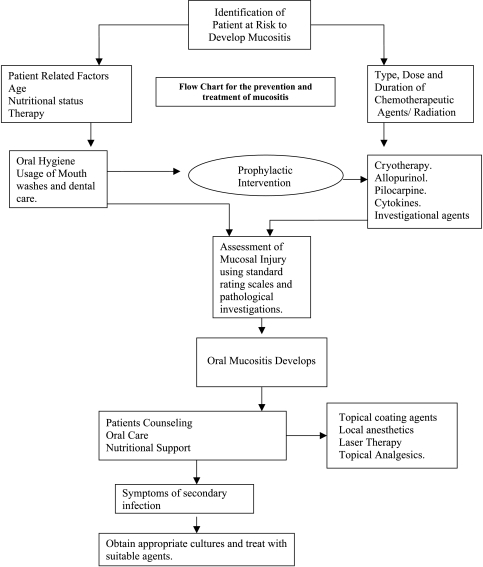
Currently, no intervention that is completely successful at preventing oral mucositis exists [7]. Despite the availability of many therapeutic agents that claim to prevent or reduce severity, oral mucositis often takes a therapeutically refractory turn, necessitating the use of topical and systemic analgesics. This following part of the paper attempts to review different treatment options available to prevent and treat this condition. However, evaluations of different modalities are difficult because of polypharmacy, heterogeneity, and relatively small patient populations in clinical trials. On top of this, most of the trials that have been conducted were based on subjective evaluation systems. There are very few studies evaluated using pathological tests as objective measures. Prevention measures play important roles because the incidence of mucositis with radiation therapy to the oral cavity in head and neck cancer patients with or without chemotherapy is well established [7] (Figure 2).
Figure 2.
Flow chart of management.
Oral Hygiene
Patients should be referred to the dentist for a comprehensive examination to identify and correct any potential complication before cancer therapy is initiated. This includes the identification of infections requiring prompt antibiotic therapy to prevent systemic infection. Patients are encouraged to seek professional dental care throughout cancer therapy, as necessary. Most importantly patients, are instructed to brush their teeth with soft toothbrush and fluoridated toothpaste after every meal and before bedtime everyday, and toothbrush should be changed monthly [7]. Patients should be encouraged to take a nutritious and balanced diet.
Cryotherapy
It has been hypothesized that cooling of oral mucosa using ice chips will reduce the blood flow to the oral mucosa, thus reducing the availability of chemotherapeutic agents to the oral mucosa [28].The North Central Cancer Treatment Group (NCCTG) conducted studies on 95 patients receiving their first course of 5FU-based chemotherapy versus non-therapy. The patients who received oral cryotherapy had approximately 50% reduction in stomatitis [29]. But to our knowledge, there are no confirmatory studies.
Allopurinol
Oxypurinol, which is the metabolite of allopurinol, inhibits an enzyme involved in pyrimidine synthesis, leading to intracellular accumulation of orotic acid. This compound, in turn, inhibits the activation of 5FU to fluorouracil monophosphate, thus diminishing 5FU toxicity. Although initial pilot studies conducted by Bleyer [30] showed beneficial effects of allopurinol mouthwashes, studies conducted by the NCCTG showed an increase in 5FU-induced mucositis for patients receiving the allopurinol mouthwash.
Propantheline
Propantheline is an anticholinergic agent that is known to produce dry mouth and xerostomia. Chemotherapeutic agent etiposide is known to be excreted in the saliva; it was hypothesized that propantheline may reduce dose-limiting toxicity in patients receiving etiposide. Randomized trials have shown a reduction in incidence and severity of mucositis [31].
Pilocarpine
Pilocarpine is a cholinergic agonist that has demonstrated efficacy in relieving symptoms of radiation-induced mucositis. Johnson et al., who conducted preliminary studies, suggest that administration of pilocarpine will preserve salivary gland function when used for patients receiving radiotherapy. Still double-blind randomized studies are required to conclude its efficacy in preventing mucositis [32].
Cytokines
Oral mucosal defense mechanism is enhanced by the local accumulation of activated neutrophils subsequent to systemic administration of granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). In addition to this, topical administration of G-CSF and GM-CSF has promising effects on the oral mucosa [33].
GM-CSF mouthwashes have been shown to cause marked alleviation of existing oral mucositis in several studies without detectable systemic accumulation of GM-CSF or effects upon systemic neutrophil counts [34]. Reduction in chemotherapy-induced mucositis was observed coincidentally with amelioration of neutropenia after chemotherapy. It was first reported by Antman et al. that there is a 75% decrease in the incidence of oral mucositis when G-CSF was included in the M-VAC (methotrexate, vinblastine, dosorubicin, and cisplatin) chemotherapy regimen. A similar effect was observed when GM-CSF was given with doxorubicin, ifosfamide, and dacarbazine chemotherapy [35]. But all studies that have been conducted were nonrandomized studies. Kwan-Hawa et al. have conducted a first randomized, prospective controlled study to evaluate the effect of GM-CSF versus no treatment on the reduction of salivary-induced oral mucositis and duration of chemotherapy-induced oral mucositis. In this study, 20 patients with (PFL) cisplatin, fluorouracil, and leucovorin chemotherapy regimen (20 mg/mm per day cisplatin, 800 mg/mm per day 5FU, 90 mg/mm per day leucovorin) was selected because it is effective for the treatment of head and neck cancers and often induces oral mucositis. The results of this study indicated that GM-CSF has reduced the severity and duration of mucosal injury [34,36]. The effects of these agents also vary with the route and time of administration. But it still remains uncertain whether GM-CSF or G-CSF administered topically or intravenously has any beneficial effects [37]. Machteld et al. have conducted a phase I trial on transforming growth factor-β3 (TGF-β3) mouthwashes for the prevention of chemo-induced mucositis. Evaluations of both subjective and objective parameters were performed on 11 patients. The primary aim was to evaluate the safety and tolerability of the mouthwash and, secondary, was to evaluate the efficacy. Out of 11 patients, three of them had increases in WHO scores (>grade 0 and maximum grade 3). No unexpected adverse events were observed at the dose of 100 µg/ml per day. Still controlled clinical trials have to be performed to conclude the efficacy of the preparation [23].
Amifostine
Amifostine in a local application (250 mg) form has shown mixed response in the preventive treatment of radiochemotherapy-induced mucositis. A study conducted by Vacha et al. has shown significant radioprotective effects on salivary glands and a potential effect on oral mucosa by amifostine in postoperative radiotherapy combined with carboplatin. But to conclude, further randomized trials should be conducted on the beneficial effects of amifostine in patients receiving radiation and chemotherapy [38].
Dinoprostone
Prostaglandin E2 (PGE2), which is a naturally occurring cytoprotective agent, has been reported to be beneficial in healing gastric ulcers and chronic leg ulcers. Studies that have been conducted have shown promising results as preventive treatment to chemotherapy-induced and radiation therapy-induced mucositis [7]. A randomized double-blind trial comparing topically applied PGE2 versus placebo in 60 patients undergoing bone marrow transplantation for acute leukemia has not shown promising results in reducing the severity and incidence of mucosal injury [39].
Antimicrobial/Antifungal/Antiviral Agents
The oral mucosa of cancer patients is colonized by a variety of potentially pathogenic microorganisms such as Gram-positive and Gram-negative opportunistic bacteria and fungi. Antibiotic lozenges containing polymyxin E and tobramycin (and amphotericin B) have successfully eliminated microbial flora and prevented severe forms of oral mucositis, compared to controls in patients with radiation treatment in head and neck cancers. Although fungi are not primarily involved in the development of oral mucositis, they account for the most frequent infections of the damaged mucosa in the immunosuppressed patients. The frequently used polyene antifungals such as nystatin have been evaluated in clinical trials and failed to show impressive results. Viruses, particularly herpes simplex virus type I (HSV) and varicella zoster virus (VZV), represent the most common pathogens aggravating oral mucositis in the course of antineoplastic therapy. For seropositive and myelosuppressed patients, topical and systemic acyclovir treatment is effective in the management of oral herpetic infections [40].
Chamomile
The chamomile plant (Matricaria chamomilla) has been used for medicinal purposes for centuries. This plant contains many active constituents including chamazulene, a-bisabolol, bisabolol oxides, spiroethers, and flavanoids. Data pertaining to this suggest that these compounds have anti-inflammatory, antibacterial, and antifungal properties. Initial preliminary uncontrolled studies suggested that this compound has shown good results in reducing the severity and duration of mucositis in a smaller group of population [41]. However, later phase III trials have failed to conclude that the chamomile given in mouthwash formulations is effective in patients with chemotherapy-induced mucositis [42].
Traumeel S (Homeopathic Medication)
Traumeel S, a homeopathic medication in the form of mouth rinse, has been tried on patients undergoing allogeneic or autologous stem cell transplantation. This study indicated that Traumeel S may reduce significantly the severity and duration of chemotherapy-induced stomatitis; however, a limited number of patients in the trial may not be sufficient to prove its efficacy [43].
Glutamine
Suspension of glutamine has been tried in different trials with inconclusive results. Huang et al. have conducted a pilot study in radiation-induced oral mucositis, and authors concluded that glutamine may significantly reduce the duration and severity of oral mucositis. However, the number of patients who received the active drug was only eight [44]. In another study conducted by Jebb et al., which evaluated 5FU-induced and folinic acid-induced mucositis in 28 patients, it was concluded that there is no effect of oral glutamine supplementation [45].
Keratinocyte Growth Factor-2 (KGF-2)
KGF-2 selectively induces epithelial cell proliferation, differentiation, and migration. KGF-2 has no in vitro and in vivo proliferative effects on human epithelial-like tumors. This failure to stimulate tumor cell growth highlights the ability of this drug to specifically target normal epithelial tissue [46]. In a study wherein KGF-2 was given as an intravenous formulation, it was well tolerated by patients and it reduced the severity of mucosal injury in patients receiving intravenous bolus 5-FU [47].
Treatment Options of Established Mucositis
Patient education is very important in managing chemotherapy-induced and/or radiation-induced mucositis. Patients should be motivated to follow guidelines indicated below to reduce the discomfort caused by mucositis:
Patients are encouraged to sit upright at a 90° angle and lean their head slightly forward.
Eat slowly. Food should be cut in to small pieces and chewed completely.
Eat small frequent meals instead of heavy meals.
Food taken should be warm, or at room temperature. Hot food and drinks should be avoided. Similarly, crunchy foods such as potato chips and nuts should also be avoided.
Soft food is always encouraged. Finely chopped cooked meat, fruits, and vegetables should be taken. Patients can also try commercial baby foods, which are nutritious, convenient, and very easy to swallow. Milk shakes that have very high proteins can also be tried.
Usage of straw will not only make drinking easy but will also avoid direct contact with the affected portion.
Do not talk while food is in the mouth.
Acidic foods such as tomatoes, grapes, apple fruits or juices, alcohol and tobacco, and spicy foods should be avoided.
In order to relieve discomfort of dry mouth, patients are asked to rinse mouth with water before and after every meal [48].
Oral Care
Routine mouth care should be performed every 4 days. Patient should be counseled to rinse mouth thoroughly after every meal so that the food particles do not remain in the mouth. The routine oral care of patients includes removal of dentures, debridement of necrotic tissues, and oral rinse with saline regularly. Antibiotic rinses such as chlorohexidine may also be used; however, their efficacy remains unproven according to the double-blind placebo-controlled study conducted by Worthington et al. [48].
Topical Coating Agents
Sucralfate, magnesium hydroxide, and hydroxypropyl cellulose are some of the film-forming or coating agents which may be beneficial in treating established mucositis [49]. Sucralfate is a basic albumin salt of sucrose octasulfate that has been approved by the US FDA for its usage in patients with active duodenal ulcers. A preliminary randomized trial showed good results in reducing the severity of mucositis in a patient population. But later, a phase III trial in 131 patients did not support the prestudy hypothesis stating that sucralfate is beneficial in chemotherapy-induced mucositis [50]. Hydroxypropyl cellulose (MGI 209), which is a bioadhesive, may serve as a protective barrier over mucosal ulceration, allowing pain relief and improved healing. In this pilot study, investigators concluded that MGI 209 could relieve oral ulcer discomfort for at least 3 hours even with exposure to an acidic, irritating beverage [51].
Topical Anesthetics
Local anesthetics such as lidocaine, cocaine, and capsaicin have shown mixed results [7].
Conclusion
Oral mucositis is an extremely serious and challenging complication of both radiation and chemotherapy in cancer patients. Because the treatment of mucositis is limited, prophylaxis is emphasized. Patient education with regard to oral hygiene is stressed. Pretreatment should be aimed to reduce systemic infection, patient's nutritional status should not be compromised, and, most importantly, patient's quality of life should not be affected. Assessment of oral pathology is essential to minimize acute and chronic oral and systemic sequelae of antineoplastic and radiation therapy. A number of agents have been evaluated in clinical trials, but currently none of them has succeeded in reaching clinical practice. The probable reasons behind this is that most of the studies used scoring systems that were not validated and whose interreliability was not established; all these scoring systems are absolutely subjective. Because now in vitro pathological methods have been established, usage of these methods has to be encouraged to reduce the bias of subjective methodology. Some of the parameters to be evaluated include the release of free radicals, modified proteins, and proinflammatory cytokines including interleukin-1B, prostaglandins, and TNF by epithelial, endothelial, and connective tissue cells. These mediators cause further damage either directly or indirectly by increasing vascular permeability, enhancing the cytotoxic drug in the oral mucosa. Intervention with new antioxidants may be helpful in preventing mucosal damage and improving quality of life. Emphasis also should be made in assessing the patient's psychologic condition, in particular depressive disorders. This is important because treatments with antidepressants will not only contribute to lifting depression but also to reducing pain somatization. Although mucositis is rarely life-threatening, it will interfere, to a great extent, with the outcome of cancer treatment.
References
- 1.Dose AM. The symptoms experience of mucositis, stomatitis and xerostomia. Semin Oncol Nurs. 1995;11:248–255. doi: 10.1016/s0749-2081(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 2.Zlotolow IM. General consideration in prevention and treatment of oral manifestation of cancer therapies. In: Berget AP, Weissman DE, editors. Principles and Practice of Supportive Oncology. Philadelphia, PA: Lippincott Raven; 1998. p. 237. [Google Scholar]
- 3.Bitran JD, Samuel B, Klein L, Hanauer S, Johnson L, Martinec J, Harris E, Kempler J, White L. Random high dose chemotherapy supported by hematopoietic progenitor cells yields prolonged survival in stage IV breast cancer. Bone Marrow Transplant. 1996;17:157–162. [PubMed] [Google Scholar]
- 4.Berger AM, Kilroy TJ. In: Oral complications: Principles and Practice of Oncology. 5th ed. DeVita VJ Jr, Hellmen S, Rosenberg SA, editors. Philadelphia, PA: Lippincott Raven; 1997. p. 2714. [Google Scholar]
- 5.Peterson DE. Research advances in oral mucositis. Curr Opin Oncol. 1999;11:261–266. doi: 10.1097/00001622-199907000-00005. [Medline] [DOI] [PubMed] [Google Scholar]
- 6.The Joanna Brigs Institute, author. Best Practice, prevention and treatment of oral mucositis in cancer patients. [July 7, 2004]. Available at: http://www.joannabriggs.edu.au/best_practice/bp5.php.
- 7.Wilkes JD. Prevention and treatment of oral mucositis following cancer therapy. Semin Oncol. 1998;25:538–551. [PubMed] [Google Scholar]
- 8.Franzen L, Funegard U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. Eur J Cancer. 1992;28:457–462. doi: 10.1016/s0959-8049(05)80076-0. [Medline] [DOI] [PubMed] [Google Scholar]
- 9.Verdi CJ. Cancer therapy and oral mucositis—an approval of drug prophylaxis. Drug Saf. 1993;9:185–195. doi: 10.2165/00002018-199309030-00004. [Medline] [DOI] [PubMed] [Google Scholar]
- 10.Baker DG. The radiobiological basis for tissue reactions in the oral cavity following therapeutic X-irradiation. Arch Otolaryngol. 1982;108:21–24. doi: 10.1001/archotol.1982.00790490023005. [DOI] [PubMed] [Google Scholar]
- 11.Sonis S, Leung SW, Wang CJ. Prevention and management of oral mucositis induced by antineoplastic therapy. Oncology. 1999;5:11–18. [PubMed] [Google Scholar]
- 12.Verdi CJ. Cancer therapy and oral mucositis. Drug Saf. 1993;9:185–195. doi: 10.2165/00002018-199309030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sonis ST. Mucositis as a biological process—a new hypothesis for the development of chemotherapy induced stomatotoxicity. Oral Oncol. 1998;34:39–43. doi: 10.1016/s1368-8375(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 14.Sonis ST, Lindquist L, Van Vugt A, Stewart AA, Stam K, Qu GY, Iwata KK, Haley JD. Prevention of chemotherapy- induced ulcerative mucositis by transforming growth factor beta-3. Cancer Res. 1994;54:1135–1138. [PubMed] [Google Scholar]
- 15.Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST. The impact of mucositis on alpha hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer. 1998;82(11):2275–2281. [PubMed] [Google Scholar]
- 16.Sonis S, Costa JW, Jr, Evitts SM, Lindquist LE, Nicolson M. Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive common chemotherapy. Oral Surg Oral Med Oral Pathol. 1992;74:749–755. doi: 10.1016/0030-4220(92)90402-c. [Medline] [DOI] [PubMed] [Google Scholar]
- 17.Lichtman SM. Physiological aspects of aging—implication for the treatment of cancer. Drugs Aging. 1995;7:212–225. doi: 10.2165/00002512-199507030-00006. [DOI] [PubMed] [Google Scholar]
- 18.Sonis ST. In: Oral complication of cancer therapy: Principles and Practices of Oncology. Devita VT, Hellman R, Rosenberg, SA, editors. Philadelphia, PA: Lippincott; 1993. pp. 2385–2394. [Google Scholar]
- 19.Navajesh M. Xerostomia—diagnosis and treatment. Am J Otolaryngol. 1983;4:283–292. doi: 10.1016/s0196-0709(83)80072-6. [DOI] [PubMed] [Google Scholar]
- 20.Olif A, Blayer WA, Poplak DG. Methotrexate induced oral mucositis and salivary methotrexate concentration. Cancer Chemother Pharmacol. 1979;2:225–226. doi: 10.1007/BF00258300. [DOI] [PubMed] [Google Scholar]
- 21.Eilers J, Berger AM, Petersen MC. Development, testing and application of oral assessment guide. Oncol Nurs Forum. 1988;15:325–330. [PubMed] [Google Scholar]
- 22.Sonis ST. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer. 1999;10:2103. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Wymenga AN, van der Graaf WT, Hofstra LS, Spijkervet FK, Timens W, Timmer-Bosscha H, Sluiter WJ, van Buuren AH, Mulder NH, de Vries EG. Phase I study of transforming growth factor-beta 3 mouthwashes for prevention of chemotherapy induced mucositis. Clin Cancer Res. 1999;5:1363–1368. [PubMed] [Google Scholar]
- 24.Graham GJ, Ramenghi U, O'Connor MP, Sheridan W, Szer J, Morstyn G. Studies of oral neutrophil levels in patients receiving G-CSF after autologus marrow transplantation. Br J Haematol. 1992;82:589–595. doi: 10.1111/j.1365-2141.1992.tb06472.x. [DOI] [PubMed] [Google Scholar]
- 25.Wymenga AN, van der Graaf WT, Spijkervet FL, Timens W, Timmer-Bosscha H, Sluiter WJ, de Vries EG, Mulder NH. A new in vitro assay of quantitation of chemotherapy induced mucositis. Br J Cancer. 1997;8:1062–1066. doi: 10.1038/bjc.1997.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman S., Jr . In: Oral cavity: comprehensive cytopathology. 2nd ed. Bibbo S, editor. Philadelphia, PA: WB Saunders Company; 1997. pp. 403–412. [Google Scholar]
- 27.WHOQOL-BREF, Introduction, administration, scoring and generic version of the assessment, Field trial version December 1996; Programme on Mental Health, WHOQOL Group, WHO; Geneva 27 CH 1211, Switzerland. [Google Scholar]
- 28.Rocke LK. A randomized clinical trial of two different durations of oral cryotherapy for prevention of 5FU-related stomatitis. Cancer. 1993;7:224. doi: 10.1002/1097-0142(19931001)72:7<2234::aid-cncr2820720728>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Mahood DJ, Dose AM, Loprinzi CL. Inhibition of 5FU induced mucositis by oral cryotherapy. J Clin Oncol. 1991;9:449–452. doi: 10.1200/JCO.1991.9.3.449. [DOI] [PubMed] [Google Scholar]
- 30.Bleyer JP. A controlled evaluation of an allopurinol mouthwashes as prophylaxis against 5FU induced stomatitis. Cancer. 1990:1879. doi: 10.1002/1097-0142(19900415)65:8<1879::aid-cncr2820650834>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed T, Engelking C, Szalyga J, Helson L, Coombe N, Cook P, Corbi D, Puccio C, Chun H, Mittelman A. Propantheline prevention of mucositis from etiposide. BMT. 1993;12:131–132. [PubMed] [Google Scholar]
- 32.Johnson JT, Ferretti GA, Nethery WJ, Valdez IH, Fox PC, Ng D, Muscoplat CC, Gallagher SC. Oral pilocarpine for post irradiation xerostomia in patients with head and neck cancers. N Engl J Med. 1993;327:390–395. doi: 10.1056/NEJM199308053290603. [DOI] [PubMed] [Google Scholar]
- 33.Raderer M, Kornek G, Hejna M, Koperna K, Scheithauer W, Base W. Topical granulocyte-macrophage colony-stimulating factor in patients with cancer and impaired wound healing. J Natl Cancer Inst. 1997;89:263. doi: 10.1093/jnci/89.3.263. [DOI] [PubMed] [Google Scholar]
- 34.Bez C, Demorasi F, Sardella A, Lodi G, Bertolli VG, Annaloro C, Rimondini L, Porter SR, Carrassi A. GM-CSF mouthrinses in the treatment of severe oral mucositis—a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:311–315. doi: 10.1016/s1079-2104(99)70034-x. [Medline] [DOI] [PubMed] [Google Scholar]
- 35.Antman KS, Griffin JD, Elias A, Socinski MA, Ryan L, Cannistra SA, Oette D, Whitley M, Frei E, 3rd, Schnipper LE. Effect of recombinant human GMCSF factor on chemotherapy induced myelosuppression. N Engl J Med. 1988;319:593–598. doi: 10.1056/NEJM198809083191001. [DOI] [PubMed] [Google Scholar]
- 36.Chi KH, Chen CH, Chan WK, Chow KC, Chen SY, Yen SH, Chao JY, Chang CY, Chen KY. Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil and leucovorin chemotherapy. J Clin Oncol. 1995;13:2620–2628. doi: 10.1200/JCO.1995.13.10.2620. [DOI] [PubMed] [Google Scholar]
- 37.Duncan M. Oral and intestinal mucositis—causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853–874. doi: 10.1046/j.1365-2036.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 38.Vacha P, Fehlauer F, Mahlmann B, Marx M, Hinke A, Sommer K, Richter E, Feyerabend T. Randomized phase III trial of postoperative radiochemotherapy +/- amifostine in head and neck cancer. Is there evidence for radioprotection? Strahlenther Onkol. 2003;179:385–389. doi: 10.1007/s00066-003-1016-1. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin JS, Cerppins J. Regulation of immune response by prostaglandin. J Clin Immunol. 1983;4:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 40.Wolfgang JK, Kostler Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51:290–315. doi: 10.3322/canjclin.51.5.290. [DOI] [PubMed] [Google Scholar]
- 41.Klaschka F, Modiano MR. Chamomile: mode of action and formulation. Interdisciplinary chamomile symposium. West Germany: Frankfurt/Main; 1987. [Google Scholar]
- 42.Fidler P, Loprinzi CL, O'Fallon JR, Leitch JM, Lee JK, Hayes DL, Novotny P, Clemens-Schutjer D, Bartel J, Michalak JC. Prospective evaluation of chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer. 1996;77:522–525. doi: 10.1002/(SICI)1097-0142(19960201)77:3<522::AID-CNCR14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Menachem O. A randomized controlled clinical trial of the homeopathic medication Traumeel SR in the treatment of chemotherapy induced stomatitis in children undergoing stem cell transplantation. Cancer. 2001;92:684–690. doi: 10.1002/1097-0142(20010801)92:3<684::aid-cncr1371>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Huang EY, Leung SW, Wang CJ, Chen HC, Sun LM, Fang FM, Yeh SA, Hsu HC, Hsiung CY. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–539. doi: 10.1016/s0360-3016(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 45.Jebb SA, Osborne RJ, Maughan TS, Mohideen N, Mack P, Mort D, Shelley MD, Elia M. 5-Fluorouracil and folinic acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer. 1994;70:732–735. doi: 10.1038/bjc.1994.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alderson R, Gohari-Fritsch S, Olsen H, Roschke V, Vance C, Connolly K. In vitro and in vivo effects of repifermin (keratinocyte growth factor-2, KGF-2) on human carcinoma cells. Cancer Chemother Pharmacol. 2002;50:202–212. doi: 10.1007/s00280-002-0493-8. [DOI] [PubMed] [Google Scholar]
- 47.Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, Rothenberg ML, Redding SW, Serdar CM, Yao B, Heard R, Rosen LS. Randomized phase I trial of recombinant keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. J Clin Oncol. 2003;21:1452–1458. doi: 10.1200/JCO.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 48.Worthington HV, Clarkson JE, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2004;2:CD001973. 2001 doi: 10.1002/14651858.CD001973.pub3. [DOI] [PubMed] [Google Scholar]
- 49.Footer RL. Randomized trial of a chlorohexidine mouthwash for alleviation of radiation induced mucositis. J Clin Oncol. 1994;12:2650–2653. doi: 10.1200/JCO.1994.12.12.2630. [DOI] [PubMed] [Google Scholar]
- 50.Loprinzi CL, Ghosh C, Camoriano J, Sloan J, Steen PD, Michalak JC, Schaefer PL, Novotny PJ, Gerstner JB, White DF, Hatfield AK, Quella SK. Phase III controlled evaluation of sucralfate to alleviate stomatitis inpatients receiving fluorouracil based chemotherapy. J Clin Oncol. 1997;3:1235–1238. doi: 10.1200/JCO.1997.15.3.1235. [DOI] [PubMed] [Google Scholar]
- 51.LeVeque FG, Parzuchowski JB, Farinacci GC, Redding SW, Rodu B, Johnson JT, Ferretti GA, Eisenberg PD, Zimmer MB. Clinical evaluation of MGI 209 an anesthetic film forming agent for relief from painful oral ulcers associated with chemotherapy. J Clin Oncol. 1992;12:1963–1968. doi: 10.1200/JCO.1992.10.12.1963. [DOI] [PubMed] [Google Scholar]