Abstract
Colorectal carcinoma occurs in 1 of 20 individuals in most developed countries. The relapse after resection with metastatic liver disease is a major cause of death. 7-t-Butyldimethylsilyl-10-hydroxycamptothecin (DB67) has been incorporated into liposomes allowing for intravenous (i.v.) administration. A preclinical efficacy study of liposomal DB67 was performed using the colon carcinoma CT-26 cell line. The therapeutic dose for DB67 and liposomal DB67 was found to be 7 mg/kg per day using the qdx5/1 schedule. The results are compared with those obtained with irinotecan. The treatment with liposomal DB67 administered intravenously was more effective in reducing the weight and volume of primary spleen tumors and the weight and extent of liver metastases than free DB67 or liposomal DB67 administered intraperitoneally, but less effective than irinotecan. When the primary tumor was resected, treatment with liposomal DB67 administered intravenously was more effective in reducing the weight and extent of liver metastases than DB67 or liposomal DB67 administered intraperitoneally, and irinotecan. DB67 showed a higher accumulation in spleen and liver after its i.v. administration in liposomal form compared with its free or liposomal form administered intraperitoneally. DB67 and liposomal DB67 are more effective than irinotecan in the treatment of liver metastases after resection of the primary tumor.
Keywords: Colorectal carcinoma, CT-26, DB67, irinotecan, liposome
Introduction
Colorectal cancer occurs in 1 of 20 individuals in most developed countries. Over 80% of colorectal carcinomas are resectable at the time of diagnosis, but 50% of patients subsequently relapse with metastatic disease caused by the presence of micrometastases not detected at diagnosis [1]. The liver is the most common site for metastases, occurring in approximately 40% of cases. Surgery is the standard therapy when liver metastases are resectable. However, in most cases, resection is not possible and chemotherapy is the only therapy available. Therefore, designing better systemic therapy for metastatic disease is a major challenge to oncologists.
In phase II clinical trials in patients with metastatic colorectal carcinoma, irinotecan (Pfizer, Inc., New York, NY) has demonstrated activity in chemotherapy naïve patients and patients previously treated with 5-fluorouracil (5-FU) [2,3]. 5-FU has been the only cytotoxic agent with significant activity in advanced colorectal cancer for more than 30 years. More recently, compared with 5-FU and calcium folinate (leucovorin, or LV) alone, the combination of irinotecan with 5-FU/LV has demonstrated increased response rate, time to progression, and survival as a first-line treatment in metastatic colorectal cancer [4]. As a second-line treatment, after failure of 5-FU/LV therapy, irinotecan prolongs survival of the patients compared with classical supportive care or 5-FU by continuous infusion [5,6].
Irinotecan and the other camptothecin derivatives belong to the family of topoisomerase I (topo I) inhibitors. Topo I is a nuclear enzyme with the function of relaxing supercoiled DNA. Topo I inhibitors intercalate with DNA during replication, transcription, recombination, and chromosomal decondensation. Their mechanism of action depends on the formed DNA-topo I intermediate, termed “cleavable complex.” They interfere with the DNA breakage-reunion reaction catalyzed by the enzyme stabilizing the new ternary complex (DNA-topo I inhibitor) [7]. As a result, DNA suffers single-strand breaks. If exposed for a long time to topo I inhibitors, cellular DNA in replication is double-strand broken, leading the cell to apoptosis. Due to this particular mechanism of action, topo I inhibitors are specially cytotoxic to tumor cells because of their rapid division.
Camptothecin, the first topo I inhibitor tested, had poor solubility, which limited its administration. Its structure contains an α-hydroxy-δ-lactone pharmacophore, which exists in two distinct forms at physiologic pH of 7.0 and above: one is the “ring-closed” lactone form, which is fully inhibitory of topo I, and the second is the “ring-open” carboxylate form, which is inactive with respect to inhibition of topo I [8]. Human serum albumin (HSA) preferentially binds to the carboxylate form of camptothecin with a higher affinity than the lactone form; these interations result in camptothecin opening more rapidly and completely in the presence of HSA than in the absence of the protein [9]. This shifts the lactone/carboxylate equilibrium toward the inactive carboxylate form, limiting its bioavailability.
To overcome this major problem, two major rational approaches were used [10]. The first one was the introduction of substituents in its chemical structure to interfere with the bonding of the camptothecin carboxylate form to HSA. Two examples are the compounds topotecan (Hycamtin; SmithKline Beecham, Philadelphia, PA) (a 9,10-disubstituted camptothecin) and irinotecan (a 7,10-disubstituted camptothecin), which do not bind with high affinity to HSA [11,12]. In addition to that, both have improved aqueous solubility and better bioavailability than camptothecin [13]. Moreover, irinotecan itself is synthesized as an inactive prodrug. To become significantly active in vivo, it has to be metabolized to form SN-38 by a carboxyesterase [14]. Unfortunately, irinotecan has a complex metabolism and the large interpatient variability in carboxyesterase activity directly affects its activation to SN-38, which may compromise the toxicity and efficacy of the drug [15–17]. The second approach was the introduction of substituents in the analog's chemical structure, promoting the partition of camptothecin into lipid bilayers, thereby stabilizing the lactone form [18]. Lipophilic camptothecins, like 9-amino-camptothecin, have shown a higher intrinsic potency against topo I and an improved in vivo antitumor activity in murine models [19,20]. It has also been demonstrated that lipid bilayers, like liposomal membranes, stabilize the lactone form of camptothecins [21]. Continuing with this chemical development, 7-silylcamptothecins (silatecans) have shown significant in vitro activity against tumor cell lines [22]. Among them, DB67 (NSC 708298) showed high activity, forming very stable topo I cleavable complexes; a high lipophilicity; and an intrinsic topo I-inhibitory activity [23]. Also, DB67 remained in the lactone form at high levels in the presence of HSA [24] and was readily incorporated in liposomal bilayers, maintaining its active lactone form [25]. In addition, DB67 presented a potent antitumoral activity against glioma [26].
In this study, we have performed a preclinical efficacy study of liposomal DB67 using the murine colon carcinoma CT-26 cell line. We have observed that the therapeutic dose for DB67 and liposomal DB67 in tumor-bearing mice was found to be 7 mg/kg per day using a schedule of daily injection for 5 days/1 week (qdx5/1) both for intraperitoneal (i.p.) and intravenous (i.v.) routes of administration. Our results demonstrate that the incorporation of DB67 in liposomes made possible the injection of the lipophilic drug DB67 intravenously, thereby increasing liposomal DB67 therapeutic effectiveness, as compared with itself and its free form administered intraperitoneally against liver metastases of a murine colon carcinoma after resection of the primary tumor. This enhanced efficacy is due to a higher liver drug accumulation after using the i.v. route of injection.
Materials and Methods
Materials
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dimyristoyl-sn-glycero-3-phospho-sn-1-glycerol (DMPG) ammonium salt were from Avanti Polar Lipids (Alabaster, AL) and were used without further purification. Irinotecan was Camptosar (irinotecan hydrochloride injection) from Yakult Honska Co. Ltd. (Tokyo, Japan). Triethylamine and high-performance liquid chromatography (HPLC)-grade acetonitrile were from Fisher Scientific (Fair Lawn, NJ). Sucrose and chloroform were analytic grade from Sigma Chemical Co. (St. Louis, MO). Polycarbonate membranes with a pore size of 100 nm were obtained from Whatman International Ltd. (Maidstone, England, UK). Lipo- Fast-Pneumatic was from Avestin (Ottawa, Canada).
Synthesis of DB67
The synthesis of 7-t-butyldimethylsilyl-10-hydroxycamptothecin (DB67) has been previously described [23]. DB67 was prepared from a 1 mg/ml stock solution dissolved in dimethylsulfoxide (DMSO) (ACS, spectrophotometric grade; Aldrich, Milwaukee, WI). For in vivo use, DB67 was diluted with 70% DMSO and 30% sterile NaCl 0.9% solution (Abbott Laboratories, North Chicago, IL) prior to use.
Liposome Preparation
For the preparation of liposomal DB67 (lipo-DB67), mixtures of DB67 in free form and phospholipids (DMPC:DMPG, 7:3 molar ratio) at a molar ratio of 1:30 were dissolved in chloroform. The solvent was removed by rotary evaporation at 40°C to 45°C, and the residual chloroform was removed by keeping the flask in vacuum for 24 hours. The film was hydrated with 10% sucrose. The suspension was extruded 10 times at 40°C to 45°C through a polycarbonate membrane with a pore size of 100 nm, using Lipo Fast-Pneumatic. The liposomal DB67 suspension was frozen in liquid nitrogen and kept for 10 minutes, then transferred to a freeze-drying chamber and freeze-dried. The binding of DB67 to liposomal membranes was close to 100%. For in vivo use, lipo-DB67 was resuspended in sterile NaCl 0.9% solution to a final concentration of 1.3 mg/ml. The resulting suspension was shaken at 115 rpm at room temperature protected from light for 1 hour. The average size of lipo-DB67 after suspension was 185 ± 30 nm, measured by dynamic light scattering with a submicron particle sizer (Model 370; Nicomp, Santa Barbara, CA).
In Vitro Studies
Cell culture The murine CT-26 colon carcinoma cell line wasprovided by Dr. I. J. Fidler (M.D. Anderson Cancer Center, TX). This cell line was maintained in growth medium consisting of Dulbecco's modified Eagle's medium/nutrient mixture F-12 ham (1:1) (Sigma Chemical Co.), 5%heat-inactivated fetal bovine serum (Biowhittaker, Walkersville, MD), 2% l-glutamine(GibcoBRL, GrandIsland, NY), and 1% sodium pyruvate (Gibco BRL). Cultures were established in 75-cm2 flasks (Costar, Corning, NY), maintained at 37°C in a humidified atmosphere with 5% CO2 in air, and subcultured every 2 to 3 days with trypsin-versene mixture (Biowhittaker). In all experiments, exponentially growing cultures and only singlecell suspensions of viability greater than 90% (determined by trypan blue dye exclusion) were used. Cells were examined and found to be free of Mycoplasma (assayed by Gene-Probe Mycoplasma TC; Gene-Probe, Inc., Gaithersburg, MD) and the following pathogenic murine viruses: Sendai virus, pneumonia virus, mouse hepatitis virus, minute virus, mouse poliovirus, reovirus type 3, polyomavirus, mouse adenovirus, lymphocytic choriomeningitis virus, ectromelia, lactate dehydrogenase virus, and epizootic diarrhea of infant mice (assayed by Charles River Laboratories, Wilmington, MA).
Cytotoxicity assays Cytotoxicity was determined by the MTT assay as described [27]. The initial cell plating density was chosen to ensure a linear relationship between cell number and absorbance at the end of the experiment. Briefly, CT-26 cells growing in the culture flasks (Costar) were trypsinized, and 100 µl of growth medium containing 2.5 x 103 cells was plated in each well of 96-well flat-bottomed microtiter plates (Costar), 24 hours prior to the assay. On the second day, 100 µl of growth medium, containing serial dilutions of assayed drugs, was added to each well. After 30 minutes or 2 hours (not for 48 hours) at 37°C, the cells were washed twice and incubated with growth medium for a further 48 hours. In experiments evaluating the effect of the continuous drug effect, cells were incubated directly with drugs for 48 hours. At this point, 50 µl of 1 mg/ml 3-(4,5-dimethylazol-2-yl)2,5-diphenyltetrazolium (MTT) (Sigma Chemical Co.) dissolved in PBS was added to each well and the cells were incubated for another 4 hours at 37°C. The medium was removed and the cells were solubilized in 150 µl of DMSO (Aldrich). The number of viable cells in each well was then determined by absorbance at 540 nm measured on an automated ELISA microplate reader model MRX (Dynex Technologies, Chantilly, VA). Background absorbance of the medium was measured in a triplicate set of control wells that contained the medium and the MTT solution without cells, and was subtracted from the absorbance measured in each of the sample wells. IC50 is defined as the concentration of drug that caused a 50% inhibition of the control growth.
In Vivo Studies
Animals Female 6- to 8-week-old mice Hsd:ICR (CD-1) were used for toxicity experiments (maximum tolerated dose, or MTD) and Balb/C AnNHsd were used for therapy experiments and tissue accumulation studies. Both were obtained from Harlan (Indianapolis, IN). Mice maintained in a specific pathogen-free environment were allowed to acclimatize to their new environment for 1 week prior to the beginning of experimental procedures. Experiments were conducted under the auspices of the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine (AECOM) for humane treatment of animals.
Determination of MTD For the determination of the MTD, drugs were suspended and given intraperitoneally or intravenously using the (qdx5/1) schedule. For DB67 and liposomal DB67, the doses were 10 and 40 mg/kg per day, whereas for irinotecan, the doses were 55 and 70 mg/kg per day. Body weight (BW) in each group was monitored twice weekly. Irinotecan at a concentration of 20 mg/ml was further diluted in NaCl 0.9% and used within 1 hour from preparation. The loss of 15% BW or greater is considered lethal and the MTD was determined. Changes in BW were calculated by the formula: BW (%) = (A–B)/B x 100, where A is the mean BW of mice at that day and B is the mean BW of mice on day 0. Each treatment and control group consisted of five mice.
Tumor models
Liver metastases. Mice were anesthesized with an i.p. injection of Nembutal (pentobarbital) (Abbott Laboratories) at a dose of 70 mg/kg. After shaving, a small incision was made through the skin over the spleen. The spleen, visible through the abdominal wall, was grasped with tissue forceps, and a small incision was made over the tip. The 30-gauge needle was inserted, at a shallow angle, into the spleen to a depth of 5 mm, and 5 x 104 viable CT-26 cells in 100 µl of serum-free medium were injected at a slow rate. The syringe was slowly removed, the spleen was released, and the incision in the skin was closed with an autoclip. Seven days later, depending on the experimental design, mice were anesthesized and the primary tumor was surgically resected.
Subcutaneous (s.c.) primary tumor. When the tumor was induced subcutaneously, 5 x 104 viable CT-26 cells in 100 µl of serum-free medium were injected in the left flank of Balb/C mice at a slow rate with a 30-gauge needle. The growth of the tumor was monitored every other day, and tumor volumes were calculated from Vernier calipers using the following formula: tumor volume = 0.5 x length x width2. When tumor volume reached 0.1 cm3, drug treatment was initiated. Animal weights were recorded twice weekly.
Therapy experiments Therapy started depending on experimental design: 1) on day 10 after intrasplenic cell inoculation; 2) 7 days after primary tumor resection; or 3) when s.c. primary tumor reached a size of nearly 0.1 cm3. Mice were randomly assigned to be injected intravenously or intraperitoneally with the drugs using the (qdx5/1) schedule. The dose was 7 mg/kg per day for DB67 or liposomal DB67, and 55 mg/kg per day for irinotecan. All treatments were given within less than 1 hour after drug solution preparation to avoid drug hydrolysis [28]. Each group of mice was weighed three to five times weekly until the weight nadir was reached. The groups were weighed once or twice weekly until the end of the experiment. In the liver metastases model, when the increase in liver volume was visible and mice were moribund (as determined by a blinded observer), mice were sacrificed and their livers were weighed. For the s.c. tumor model, the experiment was ended when mice tumors reached a volume of 2.0 cm3. The incidence of s.c. tumors and liver metastases was 100%. In this set of experiments, the group size was 7 to 10 mice per group. In survival experiments, mice were kept alive until moribund to determine survival time, and the increase in lifespan (ILS) was determined (median survival treated mice/median tumor control mice) x 100-100. Mice that survived to 21 days without requiring sacrifice were retreated with up to three additional 21-day cycles of therapy. We define a 21-day cycle of therapy as a treatment that consists of a daily injection of drug for 5 days following 16 days without treatment. In this other set of experiments, the group size was six to seven mice per group. In all cases, mice were necropsied to determine tumor load when moribund.
Necropsy procedures and histologic studies Mice were sacrificed by cervical dislocation after sedation with Nembutal, and their primary tumors and/or livers were excised and weighed. For hematoxylin and eosin staining procedures, a section of the tumor tissue was formalin-fixed and paraffin-embedded. Macroscopically visible liver metastases were counted under a dissecting microscope.
Tissue accumulation of DB67 Healthy female Balb/C mice were injected intraperitoneally with DB67, and intraperitoneally and intravenously with liposomal DB67, in all cases at a single dose of 7 mg/kg. Samples were collected 0.5 and 2 hours after injection. Prior to this, mice were anesthesized by i.p. administration of Nembutal and blood was collected by cardiac puncture, placed in microtainer tubes with heparin lock flush solution (100 USP U/ml; Wyeth Laboratories Inc., Philadelphia, PA), and centrifuged at 1500g for 10 minutes to isolate the serum. Internal organs, liver, and spleen were carefully removed, washed, blotted to remove attached blood, weighed, and stored at -70°C. Tissue accumulation of DB67 was measured as previously described [29]. Briefly, after the liver and spleen tissue samples were thawed, phosphate-buffered saline (PBS) buffer, pH 7.4, was added for tissue extraction. The amount of PBS buffer was nine times the amount of tissue. Tissues were homogenized using a Brinkmann Polytron model PT1035 tissue homogenizer. After homogenization, samples were vortexed and a volume of 100 µl was transferred into a new microcentrifuge tube. Following this, 3 µl of 1 M NaOH was added and vortexed for 5 seconds, and the mixture was incubated for 5 minutes. Then, 400 µl of cold methanol was added and vortexed for 5 seconds. Finally, the samples were centrifuged for 1 minute at 8000g. A volume of 200 µl from the supernatant was removed and added to the same volume of PBS, pH 12.0. The mixture was gently shaken and injected into the HPLC. The HPLC system consisted of a Waters Alliance 2690 separation module with a Waters 474 scanning fluorescence detector. Separations were carried out on a Waters Symmetry C18 5-µm column. The mobile phase consisted of a mixture of 41% acetonitrile and 59% of an aqueous buffer containing triethylamine and acetate. The buffer (pH 5.5) contained 2% triethylamine added to distilled deionized water with pH adjustment made with concentrated acetic acid. Triethylamine acts as the ion pairing reagent, masks underivatized silanols, and also serves as the major buffer component. At this pH (5.5), the mobile phase provides adequate retention time of the carboxylate species. A fluorescence detector was used with a λex = 380 nm and λem = 560 nm, with a gain of 100. The flow rates used were 1 ml/min. DB67 calibration curve from controlled tissues was performed by pipetting 250 µl of blank homogenized tissue suspension into the test tubes. In the following step, standard concentrations of DB67 were added to each specimen to obtain a range of concentrations from 50 to 10,000 ng/ml. Subsequently, each blank sample was processed to analyze DB67 as previously described for treated tissues. The concentration of DB67 (carboxylate form) was determined from peak area ratios of the compound by reference to a calibration curve run daily. The results of DB67 tissue accumulation are expressed as percentage of the injected dose ±SD per gram of tissue of five mice per group.
Statistical analysis The statistical significance of the differences was determined by the paired Student's t test. The significance of the differences between treatments in the survival experiments was determined using the log-rank test.
Results
Determination of MTDs for DB67 and Liposomal DB67: Effect of Different Injection Routes
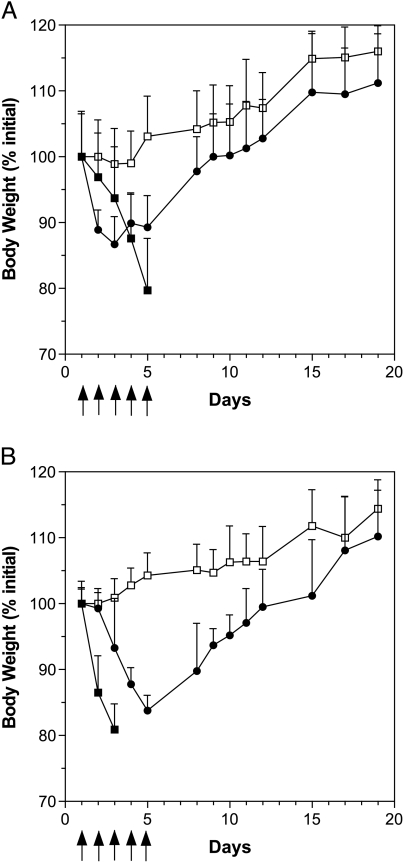
Camptothecins are S-phase-specific drugs. Optimal topo I inhibition is obtained when the tumors are exposed to the drug for continuous periods of time. The schedule of administration selected for topo I inhibitors was daily injections during 5 days (qdx5/1), best mimicking the clinical situation [30]. The MTD of DB67 for the daily i.p. injection during 5 days was first determined in healthy Hsd:ICR mice (Figure 1A). Doses used were 10 and 40 mg/kg per day. At 40 mg/kg per day, mice had lost 20.3 ± 1.6% of their BW (mean ± SD) and the dose had to be reduced to 10 mg/kg per day. At this dose, the nadir of BW loss occurred on day 3 and reached 13.4 ± 0.7%, followed by an increase in BW from that day on to a complete recovery by day 10. The MTD of liposomal DB67 was also determined. At 40 mg/kg per day, mice had lost 19.1 ± 3.9% of their BW and the dose had to be reduced to 10 mg/kg per day (Figure 1B). At this dose, the nadir of BW loss occurred on day 5 and reached 16.2 ± 2.8%, followed by an increase in BW from that day on to a complete recovery by day 12. In view of the excessive weight loss caused by DB67 in both of its forms, free and liposomal, at a dose of 10 mg/kg per day, the dose was reduced to 7 mg/kg per day in subsequent therapy experiments with Balb/C tumor-bearing mice. In this case, the maximum weight loss did not exceed 10%, which showed that the dose was welltolerated and treatment-related diarrhea was not observed in our experiments with mice. DB67, in both of its forms, caused similar parameters of acute toxicity such as ruffed fur, hunched posture, and lethargy.
Figure 1.
Toxicity of DB67 and liposomal DB67. Hsd:ICR mice were injected intraperitoneally on a schedule of daily injection for 5 days (qdx5/1). (A) DB67: 10 mg/kg (●), 40 mg/kg (■), control (not treated) (□). (B) Liposomal DB67: 10 mg/kg (●), 40 mg/kg (■), or control (not treated) (□). The data are represented as mean weights ±SD with five mice per group. Arrows indicate the day of treatment.
The MTD of irinotecan for the daily i.p. injection during 5 days was determined using a starting dose of 70 mg/kg per day. Two days after the last injection (day 7), mice had lost 20.9 ± 1.9% of their BW (data not shown); therefore, the dose was reduced to 55 mg/kg per day. At this dose, the maximum weight loss in all cases did not exceed more than 10%. Similar results were found in therapy experiments with Balb/C bearing-tumor mice. The dose of 52.5 mg/kg per day has been previously defined as the higher nontoxic dose [31].
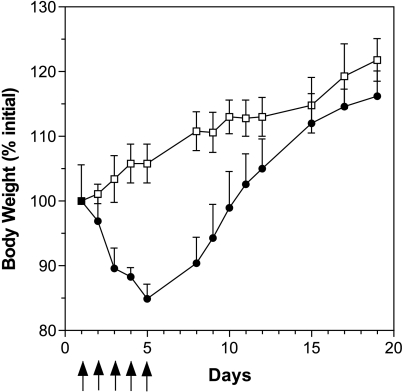
Thereafter, we examined the effect of the injection route on the toxicity of liposomal DB67. The MTD of liposomal DB67 for the daily i.v. injection during 5 days was determined using a starting dose of 10 mg/kg per day; by day 5, mice had lost 15.1 ± 0.4% of their BW, followed by an increase in BW from that day on to a complete recovery by day 11 (Figure 2). In view of the excessive weight loss, in the subsequent therapy experiments, the dose was reduced to 7 mg/kg per day. In this case, the maximum weight loss in all cases did not exceed 10%, which showed that the dose was welltolerated. For i.v. injection of irinotecan, the results obtained were similar to those found with the i.p. injection route. At a dose of 55 mg/kg per day, the maximum weight loss in all cases did not exceed 10% and was the dose used in therapy experiments with Balb/C tumor-bearing mice. No differences in the acute toxicity of irinotecan using different injection routes and a daily treatment schedule have been previously described [32]. Treatments with DB67, liposomal DB67, and irinotecan may be considered as equitoxic as required for analyzing differences in therapy experiments. Signs of acute toxicity such as ruffed fur, hunched posture, or lethargy were evaluated. No toxic deaths occurred in any of the experiments that followed.
Figure 2.
Toxicity of liposomal DB67. Hsd:ICR mice were injected intravenously on a schedule of daily injection for 5 days (qdx5/1) with 10 mg/kg liposomal DB67 (●) and control (not treated) (■). The data are represented as mean weights ±SD with five mice per group. Arrows indicate the day of treatment.
Therapeutic Effect of DB67 and Liposomal DB67 on Primary Tumor-Bearing Mice: Effect of Different Injection Routes
CT-26 is a murine colon carcinoma that metastasizes to the liver when injected intrasplenically. However, after a sufficient period of time, the spleen can be removed without affecting the ability to grow tumor cells already in the liver. Balb/C mice were given intrasplenic injections of 5 x 104 viable CT-26 cells on day 0. On day 10 after tumor cell inoculation, mice were given i.p. administrations of DB67 or liposomal DB67, both at a dose of 7 mg/kg per day, and irinotecan at a dose of 55 mg/kg per day. Mice were euthanized and necropsied when the control (vehicle) group became moribund (on day 20 after tumor cell inoculation). Necropsy confirmed that 100% of the control mice had formed splenic primary tumor and metastasized to the liver. The results of this set of experiments are shown in Table 1. The treatments with DB67 and liposomal DB67 did not produce a statistically significant reduction in the weight or tumor volume of spleen tumors as compared with the control (vehicle). On the contrary, irinotecan produced a significant reduction in the weight and tumor volume of spleen tumors as compared with the control (vehicle), DB67, and liposomal DB67 (P < .0001, P < .01, and P < .01, respectively). The weight and tumor volume of spleen tumors in these mice were less than those of the control mice on the day of the first injection.
Table 1.
Effect of Topo I Inhibitors on Spleen Tumors and Experimental Liver Metastases of Murine CT-26 Colon Carcinoma: Effect of Intraperitoneal Injection Route.
| Treatment | Spleen Tumor | Liver Metastasis | ||||
| Incidence | Spleen Weight (g) | Mean Tumor Volume (cm3) | Incidence | Liver Weight (g) | Median (range) | |
| Control | 8/8 | 2.31 ± 0.45 | 2.74 ± 0.58 | 8/8 | 4.54 ± 0.52 | 59 (50–70) |
| Control* | 5/5 | 1.45 ± 0.24 | 1.50 ± 0.32 | 5/5 | 1.50 ± 0.32 | 11 (9–17) |
| DB67 | 7/7 | 2.06 ± 0.66 | 1.98 ± 0.71 | 7/7 | 2.57 ± 0.92† | 33 (16–50)† |
| Lipo-DB67 | 7/7 | 1.97 ± 0.51 | 2.61 ± 1.03 | 7/7 | 2.53 ± 0.79‡ | 27 (4–45)‡ |
| Irinotecan | 8/8 | 0.86 ± 0.23§,¶,# | 0.49 ± 0.26§,¶,# | 8/8 | 1.45 ± 0.18§,¶,# | 10 (4–21)§,¶,** |
Balb/C mice were injected in the spleen with 5 x 104 viable CT-26 cells on day 0. On day 10 after cell inoculation, groups of mice were treated on a schedule of daily injection for 5 days (qdx5/1) with i.p. injections of 7 mg/kg DB67, 7 mg/kg liposomal DB67, or 55 mg/kg irinotecan. Mice were sacrificed on day 20.
Median is the value of the variable that has an equal number of items on either side and divides a frequency distribution into two halves.
On day 10 after cell injection.
P < .005 as compared to control (vehicle).
P < .002 as compared to control (vehicle).
P < .0001 as compared to control (vehicle).
P < .01 as compared to DB67.
P < .01 as compared to liposomal DB67.
P < .03 as compared to liposomal DB67.
On the other hand, the weight and extent of liver metastases were statistically significantly reduced by the treatment with DB67 and liposomal DB67 as compared with the control (vehicle) (P < .005 and P < .002, respectively). Irinotecan, as well, produced a significant reduction in the weight and extent of liver metastases as compared with the control (vehicle) and DB67 (P < .001 and P < .01, respectively). In addition, irinotecan produced a significant reduction in the weight and extent of liver metastasis (P < .01 and P < .03, respectively) as compared with liposomal DB67. The weight and extent of liver metastases in mice treated with irinotecan did not significantly differ from those of the control mice on the day of the first injection. It must be emphasized that, although there is an apparent absence of effect of DB67 and liposomal DB67 on weight and tumor volume of spleen tumors, on the other hand, they have a significant effect on weight and extent of liver metastases as compared with the control (vehicle).
The next step was to examine the effect of the variation in the injection route for the same experimental model. On day 10, mice were given i.v. administrations of liposomal DB67 or irinotecan. The results of this last set of experiments are shown in Table 2. The treatment with liposomal DB67 produced a statistically significant reduction in the weight and tumor volume of spleen tumors as compared with the untreated controls (P < .002 and P < .025, respectively). The treatment with irinotecan also produced a significant reduction in the weight and tumor volume of spleen tumors as compared with the untreated controls (P < .0001 and P < .002, respectively) and as compared with liposomal DB67 (P < .025). Again, the weight and tumor volume of spleen tumors of these mice were less than those of the control mice on the day of the first injection. In addition, the weight and extent of liver metastases were significantly reduced by the treatment with liposomal DB67 as compared with the untreated control (P < .025 and P < .002, respectively). Irinotecan produced a significant reduction in the weight and extent of liver metastases as compared with the untreated control (P < .001) and as compared with liposomal DB67 (P < .02 and P < .025, respectively). The weight and extent of liver metastases in mice treated with irinotecan did not significantly differ from those of the control mice on the day of the first injection. The weight and volume of spleen tumors, and the weight and extent of liver metastases in mice treated with irinotecan did not differ significantly compared by the route through which the treatment is given, either intraperitoneally or intravenously.
Table 2.
Effect of Topo I Inhibitors on Spleen Tumors and Experimental Liver Metastases of Murine CT-26 Colon Carcinoma: Effect of Intravenous Injection Route.
| Treatment | Spleen Tumor | Liver Metastasis | ||||
| Incidence | Spleen Weight (g) | Mean Tumor Volume (cm3) | Incidence | Liver Weight (g) | Median (range) | |
| Control | 7/7 | 2.33 ± 0.46 | 2.04 ± 0.65 | 7/7 | 3.87 ± 1.25 | 50 (43–70) |
| Control* | 5/5 | 1.45 ± 0.24 | 1.50 ± 0.32 | 5/5 | 1.50 ± 0.32 | 11 (9–17) |
| Lipo-DB67 | 8/8 | 1.24 ± 0.36† | 0.91 ± 0.45‡ | 8/8 | 2.31 ± 0.59‡ | 16 (3–27)§ |
| Irinotecan | 7/7 | 0.77 ± 0.24¶,# | 0.32 ± 0.19†,# | 7/7 | 1.30 ± 0.13†,** | 7 (2–17)#,§ |
Balb/C mice were injected in the spleen with 5 x 104 viable CT-26 cells on day 0. On day 10 after cell inoculation, groups of mice were treated on a schedule of daily injection for 5 days (qdx5/1) with i.v. injections of 7 mg/kg liposomal DB67 or 55 mg/kg irinotecan. Mice were sacrificed on day 20.
Median is the value of the variable that has an equal number of items on either side and divides a frequency distribution into two halves.
On day 10 after cell injection.
P < .002 as compared to control (no treatment).
P < .025 as compared to control (no treatment).
P < .001 as compared to control (no treatment).
P < .0001 as compared to control (no treatment).
P < .025 as compared to liposomal DB67.
P < .02 as compared to liposomal DB67.
Therapeutic Effect of Liposomal DB67 on Splenectomized Mice: Effect of Different Injection Routes
Following the previous experiments, Balb/C mice were given intrasplenic injections of 5 x 104 viable CT-26 cells on day 0. Seven days after tumor cell inoculation, mice were splenectomized. On day 14, mice were given i.p. administrations of DB67 and liposomal DB67, both at a dose of 7 mg/kg per day, and irinotecan at a dose of 55 mg/kg per day. Mice were euthanized and necropsied when the control (vehicle) group became moribund (on day 21 after tumor cell inoculation). The results of this set of experiments are shown in Table 3. The treatments with DB67, liposomal DB67, and irinotecan statistically significantly reduced the liver weight as compared with the control (vehicle) (P < .005, P < .001, and P < .01, respectively). In addition, all treatments significantly reduced the extent of liver metastases as compared with the control (vehicle) (P < .001). More importantly, the treatment with DB67 and liposomal DB67 produced a significant reduction in the liver weight as compared with irinotecan (P < .05).
Table 3.
Effect of Topo I Inhibitors on Experimental Liver Metastases of Murine CT-26 Colon Carcinoma When Injected i.p.
| Treatment | Liver Metastasis | ||
| Incidence | Liver Weight (g) | Median (range) | |
| Control | 9/9 | 5.00 ± 0.54 | 58 (45–80) |
| Control* | 5/5 | 1.56 ± 0.49 | 9 (5–12) |
| DB67 | 9/9 | 2.55 ± 1.05†,‡ | 25 (5–55)§ |
| Lipo-DB67 | 9/9 | 2.52 ± 1.04†,‡ | 16 (9–35)§ |
| Irinotecan | 9/9 | 3.45 ± 1.13¶ | 35 (6–55)§ |
Balb/C mice were injected in the spleen with 5 x 104 viable CT-26 cells on day 0. Seven days later, mice were splenectomized. On day 14 after cell injection, groups of mice were treated on a schedule of daily infection for 5 days (qdx5/1) with i.p. injections of 7 mg/kg DB67, 7 mg/kg liposomal DB67, or 55 mg/kg irinotecan. Mice were sacrificed on day 21.
Median is the value of the variable that has an equal number of items on either side and divides a frequency distribution into two halves.
On day 10 after cell injection.
P < .005 as compared to control (vehicle).
P < .05 as compared to irinotecan.
P < .001 as compared to control (vehicle).
P < .001 as compared to control (vehicle).
Then, we examined the effect of the variation in the injection route for the same experimental model. On day 14, mice were given i.v. administrations of liposomal DB67 or irinotecan. The results of this set of experiments are shown in Table 4. The treatment with liposomal DB67 and irinotecan significantly reduced the liver weight as compared with the untreated controls (P < .005). In addition, both treatments significantly reduced the extent of liver metastases as compared with the untreated controls (P < .001). More importantly, the treatment with liposomal DB67 produced a significant reduction in the liver weight and extent of liver metastases as compared with irinotecan (P < .01 and P < .001, respectively). The liver weight of mice treated with liposomal DB67 did not significantly differ from those of the control mice on the day of the first injection. The weight and extent of liver metastases in mice treated with irinotecan did not differ significantly by the route through which the treatment is given, either intraperitoneally or intravenously.
Table 4.
Effect of Topo I Inhibitors on Experimental Liver Metastases of Murine CT-26 Colon Carcinoma When Injected Intravenously.
| Treatment | Liver Metastasis | ||
| Incidence | Liver Weight (g) | Median, n (range) | |
| Control | 9/9 | 5.26 ± 0.54 | 55 (45–80) |
| Control* | 5/5 | 1.56 ± 0.49 | 9 (5–12) |
| Lipo-DB67 | 10/10 | 1.85 ± 0.65†,‡ | 7 (0–14)§,¶ |
| Irinotecan | 10/10 | 3.51 ± 1.27† | 34 (10–55)§ |
Balb/C mice were injected in the spleen with 5 x 104 viable CT-26 cells on day 0. Seven days later, mice were splenectomized. On day 14 after cell injection, groups of mice were treated on a schedule of daily injection for 5 days (qdx5/1) with i.v. injections of 7 mg/kg liposomal DB67 or 55 mg/kg irinotecan. Mice were sacrificed on day 21.
Median is the value of the variable that has an equal number of items on either side and divides a frequency distribution into two halves.
On day 14 after cell injection.
P < .005 as compared to control (no treatment).
P < .01 as compared to irinotecan.
P < .001 as compared to control.
P < .001 as compared to irinotecan.
Survival of Splenectomized Mice Treated with Liposomal DB67
To further evaluate the antimetastatic effect of liposomal DB67, survival of Balb/C mice bearing CT-26 liver metastases was determined. The treatment consisted of i.v. administrations starting 7 days after splenectomy, 14 days after cell inoculation. The results of this set of experiments are shown in Table 5. In experiment 1, the mean survival times ±SD of the untreated control and liposomal DB67-treated group at a dose of 7 mg/kg per day, and irinotecan-treated group at a dose of 55 mg/kg per day were 21.0 ± 1.0, 30.5 ± 2.7, and 26.7 ± 3.6 days, respectively. Thus, the increase in the lifespan was 45% for the liposomal DB67-treated group, and 27% for the irinotecan-treated group, both compared with the control (Table 5). In experiment 2, the mean survival times ±SD of the untreated control, liposomal DB67-treated group, and irinotecan-treated group were 19.0, 30.8 ± 3.9, and 26.5 ± 3.9 days, respectively. Thus, the increase in the lifespan was 62% for the liposomal DB67-treated group, and 39% for the irinotecan-treated group, both compared with the untreated control (Table 5). In both experiments, comparing to the untreated control, the treatment with liposomal DB67 and irinotecan statistically significantly extended the survival of mice with CT-26 liver metastases (P < .01 and P < .05, respectively). Furthermore, in each experiment, one long-term survivor for more than 90 days was found after three 21-day cycle treatments with liposomal DB67. The results clearly show that liposomal DB67 is more effective than irinotecan in prolonging the life of liver metastases-bearing mice.
Table 5.
Effect of Liposomal DB67 and Irinotecan on Survival in a Murine CT-26 Colon Carcinoma.
| Experiment | Treatment | Dose (mg/kg) | Mean Survival Time* (day ± SD) | ILS (%)† | Long-Term Survivors‡/Total Mice |
| 1 | Control | - | 21.0 ± 1.0 | - | 0/7 |
| Lipo-DB67 | 7 x 5 | 30.5 ± 2.7§,¶,# | 45 | 1/7 | |
| Irinotecan | 55 x 5 | 26.7 ± 3.6** | 27 | 0/7 | |
| 2 | Control | - | 19.0 ± 0.0 | - | 0/6 |
| Lipo-DB67 | 7 x 5 | 30.8 ± 3.9§,¶,# | 62 | 1/6 | |
| Irinotecan | 55 x 5 | 26.5 ± 4.1** | 39 | 0/6 | |
Balb/C mice were injected in the spleen with 5 x 104 viable CT- 26 cells on day 0. Seven days later, mice were splenectomized. On day 14 after cell injection, groups of mice were treated on a schedule of daily i.v. injection for 5 days (qdx5/1), by 21-day cycles when necessary, with doses as follows.
Days after cell injection.
Was obtained using the formula: (T x 100/C) - 100, where T is median survival of treated mice (in days) over C control.
For more than 90 days.
Mean survival time results include only dead mice, not survivors.
P < .01 as compared to control (no treatment) using the log-rank test.
P < .05 as compared to irinotecan using the log-rank test.
P < .05 as compared to control (no treatment) using the log-rank test.
Tissue Accumulation of DB67 After Its Administration in Free or Liposomal Form: Effect of Different Injection Routes
To find out whether the fact, that liposomal DB67 administered intravenously has a higher therapeutic effect than either DB67 or liposomal DB67 when administered intraperitoneally, could be correlated with a higher tissue accumulation, we studied DB67 accumulation in spleen and liver of Balb/C mice after its injection in free or liposomal form, using both administration routes. The results are expressed as percentages of the injected dose ±SD per gram of tissue and were determined in mice 0.5 and 2 hours after injection of a dose of 7 mg/kg. The results of this set of experiments are shown in Table 6. The results obtained for spleen and liver show that higher percentages of the injected dose of DB67 are found 0.5 hour after injection than 2 hours after in any of its forms. At 2 hours, in the spleen, less than 1% of the injected dose is found for DB67 injected in any of its forms. On the other hand, nearly 5% of the injected dose was found in the liver at 2 hours after the injection of DB67 in any of its forms. When DB67 in free or liposomal form was injected intraperitoneally, similar percentages of the injected dose were found in the spleen and liver at any time point. On the contrary, when liposomal DB67 was injected intravenously, a higher percentage of the injected dose was found in the spleen and liver compared to itself or to DB67 in free form injected intraperitoneally. Therefore, the fraction of the injected dose of DB67 found in the spleen and liver was related to the injection route.
Table 6.
Tissue Accumulation of DB67 After Its Injection in Free or Liposomal Form.
| Treatment | Time (hours) |
Percent Injected Dose Per Gram of Tissue | |||
| Spleen | Liver | ||||
| i.p. | i.v. | i.p. | i.v. | ||
| DB67 | 0.5 | 1.95 ± 0.43 | - | 11.02 ± 1.05 | - |
| 2 | 0.69 ± 0.10 | - | 4.80 ± 1.27 | - | |
| Lipo-DB67 | 0.5 | 2.21 ± 0.33 | 3.50 ± 0.45 | 12.01 ± 1.27 | 15.98 ± 1.00 |
| 2 | 0.23 ± 0.03 | 0.23 ± 0.05 | 4.80 ± 0.42 | 4.60 ± 0.45 | |
In both cases, DB67 was injected intraperitoneally or intravenously as a single bolus of 7 mg/kg. Balb/c mice were sacrificed at indicated time points after injection. Tissue samples were collected and analysed for drug content (see Materials and Methods section). The values are expressed as percentage of the injected dose ±SD per gram of tissue of five mice per group.
Cytotoxicity of DB67 and Liposomal DB67 as a Function of Time
The cytotoxicity of DB67 and liposomal DB67 to murine CT-26 colon carcinoma cell line was compared as a function of time. As shown in Table 7, when the incubation time was continuous for 48 hours, IC50 values of DB67 and liposomal DB67 were 0.024 ± 0.004 and 0.019 ± 0.002 µg/ml, respectively. When the incubation time was limited to 2 hours, IC50 values of DB67 and liposomal DB67 were 1.40 ± 0.13 and 1.40 ± 0.15 µg/ml, respectively. No statistically significantly differences were found between the IC50 values of these two treatments and for both incubation times studied. As expected, the reduction of the incubation time of the drug with the cells is related with the increase in the IC50 values. The IC50 values of free and liposomal DB67, as a function of time, are very similar and produce the same cytotoxic effect when cells are treated with free or liposomal DB67 even at 2 hours of incubation time. The comparison of IC50 values between free and liposomal DB67, as a function of time, suggests that the cells have the same availability for DB67 when treated with DB67 either in free or liposomal form.
Table 7.
Cytotoxicity of DB67 and Liposomal DB67 as a Function of Time.
| Topo I Inhibitor | IC50 (µg/ml)† | |
| 2 hours | 48 hours | |
| DB 67 | 1.40 ± 0.13 | 0.024 ± 0.004 |
| Lipo-DB67 | 1.40 ± 0.15 | 0.019 ± 0.002 |
CT-26 colon carcinoma cells at a density of 2.5 x 103 viable cells were plated in 96-well microtiter plates. On the second day, various concentrations of DB67 and liposomal DB67 were added for 2 or 48 hours. Cytotoxicity was determined using the MTT assay.*
The data are represented as mean ± SD of three independent experiments done in triplicate.
IC50 is the concentration of drug that caused a 50% inhibition of the control growth.
Discussion
In this study, we have shown that liposomes allow DB67 to be administered intravenously and, using this route of injection, liposomal DB67 is more effective than DB67 in free form or even itself administered intraperitoneally in the treatment of liver metastases of a colorectal carcinoma after resection of the primary tumor, due to a higher liver accumulation. Using the same model, liposomal DB67 is also more effective than irinotecan.
DB67 belongs to the family of topo I inhibitors, which are termed S-phase-specific because when DNA replicates, it is more susceptible to single-strand breaks in their presence [7,33]. Consequently, a continuous exposure to a topo I inhibitor is lethal to a larger proportion of tumor cells. Preclinical and clinical data suggest that frequent administration of lower doses of irinotecan, ensuring a prolonged exposure time, may be more effective than higher doses given at longer intervals [32,34,35]. Under this rationale, we used the qdx5/1 schedule in all experiments. Intraperitoneal administration of DB67, as well as i.p. and i.v. administration of liposomal DB67, in all cases at a dose of 7 mg/kg per day caused less than 10% of BW loss in mice and were well tolerated, and therefore this dose was chosen in the following therapy experiments.
Due to its high lipophilicity, DB67 cannot be dissolved in aqueous media, and therefore is not suitable for i.v. administration. The incorporation of DB67 in liposomes overcomes this difficulty, allowing liposomal DB67 to be resuspended in aqueous media and be used for frequent i.v. administration. Otherwise, DB67 would have to be resuspended in organic compounds that are toxic when administered intravenously. The results demonstrate that DB67 injected intraperitoneally and liposomal DB67 injected intraperitoneally and intravenously were equally toxic in the daily x 5 schedule. The routes of administration used, i.p. and i.v., do not play an important role in the toxicity of DB67 in any of its forms. However, in therapy experiments with mice bearing ectotopic or orthotopic malignant glioma tumors, animals were treated using the same schedule used here, but at a dose of 30 mg/kg per day with DB67 in free form and using the s.c. route [26]. Our toxicity results demonstrate that, using this schedule, the therapeutic dose of DB67 or liposomal DB67 administered intraperitoneally or intravenously is 7 mg/kg per day. This difference in the dose used is related to the route of administration: s.c. injection versus i.p. or i.v. injection [36].
In the therapy experiments, we used two approaches: first, when the primary tumor is present (in this case, the treatment started 10 days after cell inoculation); and, second, after the primary tumor was surgically resected. This second approach was done to mimic the clinical situation when the primary tumor is surgically removed and the effect of the therapy against liver metastases is studied. In this latter case, the treatment started 7 days after the splenectomy. In both cases, the treatment started when the disease was at an advanced stage [37].
In the first approach, when the treatment was given intraperitoneally, DB67 and liposomal DB67 caused a reduction of less than 27% in the splenic primary tumor volume and 44% in the liver weight, both compared with the control. Tissue accumulation experiments showed that after injection of DB67 or liposomal DB67, there is a similar fraction of the injected dose of DB67 in the spleen for the time period studied. In the liver, a higher fraction of the injected dose was found, but it was again similar for both treatments. That explains the similar antitumor effect found between DB67 and liposomal DB67 in reducing the splenic primary tumor and liver metastasis. When the treatment was given intravenously, liposomal DB67 caused a reduction of 55% in the splenic primary tumor volume and of 40% in the liver weight, both compared with the control. The primary tumor volume reduction was then higher than that obtained when liposomal DB67 was given intraperitoneally. In this case, for spleen and liver, the fraction of the injected dose was 58% higher at 0.5 hour compared with the fraction found when the treatment was given intraperitoneally. The schedule used, daily for 5 days, contributes to increase this difference at the end of the therapeutic period. When liposomes are injected intraperitoneally or intravenously, a preferential uptake by organs of the mononuclear phagocytic system (MPS), principally liver and spleen, has been described [36]. In addition, the fast and easy accessibility to these organs once the liposomes are in the bloodstream after their i.v. injection can explain the higher accumulation found after i.v. injection. In contrast, irinotecan administered intraperitoneally caused a reduction of 82% in the splenic primary tumor volume, and of 68% in the liver weight, both compared with the control. Similar results were found when it was administered intravenously. The route of administration does not therefore play an important role in the efficacy of irinotecan because it was equally effective given i.p. or i.v. administrations in the daily x 5 days schedule as previously demonstrated [31,32]. The high efficacy of irinotecan reducing spleen primary tumor volume is surprising because we have not found anything in the literature data describing this effect. In addition, we have not found either data regarding levels of accumulation of irinotecan or SN-38 in spleen and liver that could explain this effect nor any validated method for that quantification.
The higher fraction of the injected dose that reaches the liver after liposomal DB67 injected intravenously does not cause a reduction in the liver weight, compared with the lower fraction found after i.p. injection. This higher fraction of DB67 may not be enough to suppress the growth of the tumor cells that are continuously leaving the primary tumor and locating in the liver. Metastatic cells leave the primary tumor to colonize other organs [38]. In particular, CT-26 in the splenic primary tumor metastasize to the liver [39]. Irinotecan caused a more effective reduction in splenic primary tumor volume (82%), which probably reduced the number of tumor cells available to leave the spleen and able to metastasize to the liver.
In the second approach, the primary tumor is removed. When the treatment was given intraperitoneally, DB67 and liposomal DB67 caused nearly a 50% reduction in the liver weight compared with the control. The fraction of the injected dose that reaches the liver is similar for both treatments at the time points studied. The data give support to the previous therapy results. When the treatment was given intravenously, liposomal DB67 caused the more effective reduction in the liver weight (65%) compared with the control. The fraction of the injected dose found at 0.5 hour in the liver after the i.v. injection of liposomal DB67 is 45% higher than that found when it was injected intraperitoneally.
Surprisingly, the treatment with irinotecan given intraperitoneally or intravenously caused a reduction of less than 33% in the liver weight. Such reduction is half of that found for irinotecan in the presence of the primary tumor and may be an indicator of irinotecan liver accumulation. Under this latter approach, the number of cells continuously leaving the splenic primary tumor and reaching the liver is abrogated on day 7 after cell inoculation. The subsequent growth of liver metastases will be from those already present at that time in the liver tissue. In the presence of the primary tumor, irinotecan reduced by 82% the splenic primary tumor volume and probably at the same time caused an important reduction in the number of tumor cells available to leave the primary tumor and able to colonize the liver. That may result in a reduction in liver colonies formation and finally in the liver weight.
Continuing in this second approach, survival experiments confirmed the results of previous experiments. Liposomal DB67 injected intravenously with a multiple dosing schedule was more effective, increasing by about 2.7 times the median lifespan of treated mice, as compared with irinotecan. Of greater importance is the presence of one long-term survivor for more than 90 days in each of two experiments after treatment with liposomal DB67. The liposomal carrier system of DB67 made i.v. injection of DB67 possible, thus increasing the survival of mice treated with it.
When CT-26 tumor was induced subcutaneously, the treatment with DB67, liposomal DB67, and irinotecan using the i.p. or i.v. route of administration caused only a short delay in tumor growth, compared with the control (results not shown). The cause of this result could be due to the short circulation time once in the bloodstream described for DB67, liposomal DB67, and irinotecan, which enabled only little amounts of the drug to enter the growing s.c. tumor [14,40]. The close relationship between s.c. tumor drug accumulation and blood circulation time has been previously demonstrated [41,42]. Encapsulation of antitumor drugs in liposomes has been used to increase their antitumor activity and to decrease undesired side effects, compared to those of free drug. Irinotecan has been encapsulated in conventional rigid [43] and peguilated liposomes [44], thus acting as a slowrelease drug delivery system to achieve an increased circulation time of the drug itself and SN-38 in plasma and a reduction in the uptake by the MPS in the liver and spleen. That increased circulation time in plasma leads to a passive targeting to the subcutaneously induced tumor, which brings an increase in irinotecan and SN-38 accumulation in tumor. This result is a reduction in tumor weight [44] and an enhanced antitumor activity [43]. However, in our tumor model, our objective was the treatment of the metastatic cells growing specifically in the liver. In that case, DB67 and liposomal DB67 caused a significant reduction in the liver weight, compared to irinotecan, when the primary tumor was surgically removed. The increased activity of liposomal DB67 is dependent on enhanced tumor accumulation in the liver, and thus DB67 is a serious candidate for the treatment of liver metastases as an adjuvant to chemotherapy. Also, it is important to note that liposomal DB67 is not a slow-release drug system, as cytotoxicity assays demonstrate. DB67 is lipid-bound to the liposome bilayers [24]. This type of interaction may cause, firstly, the same toxicity found in vitro, and, secondly, a similar tissue accumulation in liver and spleen than after its administration using the i.p. route of injection.
Two reasons encourage the continuation of experimentation in humans with liposomal DB67: first, DB67 exhibits higher human blood stability, measured as a percentage of lactone form (30%), as compared with SN-38 (20%) or irinotecan (21%) [23]; and, second, the observation that mice convert irinotecan to SN-38 to a much greater extent than humans [31].
As a conclusion, the incorporation of DB67 in liposomes made possible the injection of the lipophilic drug DB67 intravenously, thereby increasing liposomal DB67 therapeutic effectiveness, as compared with itself and its free form administered intraperitoneally against liver metastases of a murine colon carcinoma after resection of the primary tumor. This enhanced efficacy is due to a higher liver drug accumulation after using the i.v. route of injection. Using this same model, it is also more effective than irinotecan.
Abbreviations
- 5-FU
5-fluorouracil
- LV
leucovorin
- Topo I
topoisomerase I
- HSA
human serum albumin
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-dimyristoylsn-glycero-3-phospho-sn-1-glycerol
- HPLC
high-performance liquid chromatography
- DMSO
dimethylsulfoxide
- MTT
3-(4,5-dimethylazol-2-yl)2,5-diphenyltetrazolium
- MTD
maximum tolerated dose
- qdx5/1
daily injection for 5 days/1 week
- BW
body weight
- ILS
increase in lifespan
- PBS
phosphate-buffered saline
Footnotes
This work was supported by grants NIH CA63653 and CA75761.
References
- 1.Cunningham D, Findlay M. The chemotherapy of colon cancer can no longer be ignored. Eur J Cancer. 1996;29A:2077–2079. doi: 10.1016/0959-8049(93)90036-f. [DOI] [PubMed] [Google Scholar]
- 2.Rougier P, Paillot B, LaPlanche A, Morvan F, Seitz JF, Rekacewicz C, Laplaige P, Jacob J, Grandjowan S, Tigaud JM, Fabri MC, Luboinski M, Ducreux M. 5-Fluorouracil (5-FU) continuous intravenous infusion compared with bolus administration. Final results of a randomised trial in metastatic colorectal cancer. Eur J Cancer. 1997;33:1789–1793. doi: 10.1016/s0959-8049(97)00175-5. [DOI] [PubMed] [Google Scholar]
- 3.Pitot HC, Wender DB, O'Connel MJ, Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Kirschling RJ, Lewitt R, Windschitl HE. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol. 1997;15:2910–2919. doi: 10.1200/JCO.1997.15.8.2910. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson P, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;25:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Strarkhammar H, Topham CA, Awad L, Jacques C, Herait P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 6.Rougier P, Van Gutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal carcinoma. Lancet. 1998;352:1407–1412. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu LA. DNA topoisomerase poison as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 8.Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Structure-activity of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res. 1989;49:1465–1469. [PubMed] [Google Scholar]
- 9.Mi Z, Burke TG. Marked interspecies variations concerning the interactions of camptothecin with serum albumins: a frequency-domain fluorescence spectroscopic study. Biochemistry. 1994;33:12540–12545. doi: 10.1021/bi00208a002. [DOI] [PubMed] [Google Scholar]
- 10.Curran DP, Liu H, Josien H, Ko SB. Tandem radical reactions of isonitriles with 2-pyridonyl and other aryl radicals: scope and limitations, and a first generation synthesis of (racemic) camptothecin. Tetrahedron. 1996;52:11385–11404. [Google Scholar]
- 11.Burke TG, Mi Z. The structural basis of camptothecin interaction with human serum albumin: impact on drug stability. J Med Chem. 1994;37:40–46. doi: 10.1021/jm00027a005. [DOI] [PubMed] [Google Scholar]
- 12.Mi Z, Malak H, Burke TG. Reduced albumin binding promotes the stability and activity of topotecan in human blood. Biochemistry. 1995;34:13722–13728. doi: 10.1021/bi00042a002. [DOI] [PubMed] [Google Scholar]
- 13.Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, Gandia D. Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol. 1995;13:210–221. doi: 10.1200/JCO.1995.13.1.210. [DOI] [PubMed] [Google Scholar]
- 14.Kaneda N, Nagata H, Furuta T, Yokokura T. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res. 1990;50:1715–1720. [PubMed] [Google Scholar]
- 15.Houghton PJ, Steward CF, Thompson J, Santana VH, Furman WL, Friedman HS. Extending principles learned in model systems to clinical trials design. Oncology. 1998;12:84–93. [PubMed] [Google Scholar]
- 16.Mathijssen RHJ, Vermeij J, de Jonge MJA, Booter K, Stoter G, Sparreboom A. Impact of body-size measures on irinotecan clearance: alternative dosing recommendations. J Clin Oncol. 2002;20:81–87. doi: 10.1200/JCO.2002.20.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke TG, Mishra AK, Wani MC, Wall ME. Lipid bilayer partitioning and stability of camptothecin drugs. Biochemistry. 1993;32:5352–5364. doi: 10.1021/bi00071a010. [DOI] [PubMed] [Google Scholar]
- 19.Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86:836–842. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- 20.Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M. DNA topoisomerase I-targeted chemotherapy of human colon cancer xenografts. Science (Washington, DC) 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 21.Burke TG, Staubus AE, Mishra AK. Liposomal stabilization of camptothecin's lactone ring. J Am Chem Soc. 1992;114:8318–8319. [Google Scholar]
- 22.Josien H, Bom D, Curran DP, Zheng YH, Chou TC. 7-Silylcamptothecins (Silatecans): a new family of camptothecin antitumor agents. Bioorg Med Chem Lett. 1997;7:3189–3194. [Google Scholar]
- 23.Bom D, Curran DP, Kruszewski S, Zimmer SG, Strode JT, Kohlhangen G, Du W, Chavan AJ, Fraley KA, Bingcang AL, Latus LJ, Pommier Y, Burke TG. The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J Med Chem. 2000;43:3970–3980. doi: 10.1021/jm000144o. [DOI] [PubMed] [Google Scholar]
- 24.Bom D, Curran DP, Zhang J, Zimmer SG, Bevins R, Kruszewski JN, Howe JN, Bingcang A, Latus LJ, Burke TG. The highly lipophilic DNA topoisomerase I inhibitor DB-67 displays elevated lactone levels in human blood and potent anticancer activity. J Control Release. 2001;74:325–333. doi: 10.1016/s0168-3659(01)00343-1. [DOI] [PubMed] [Google Scholar]
- 25.Bom D, Curran DP, Chavan AJ, Kruszewski S, Zimmer SG, Fraley KA, Burke TG. Novel A,B,E-ring - modified camptothecins displaying high lipophilicity and markedly improved human blood stabilities. J Med Chem. 1999;42:3018–3022. doi: 10.1021/jm9902279. [DOI] [PubMed] [Google Scholar]
- 26.Pollack IF, Erff M, Bom D, Burke TG, Strode BJT, Curran DP. Potent topoisomerase I inhibition by novel silatecans eliminates glioma proliferation in vitro and in vivo. Cancer Res. 1999;59:4898–4905. [PubMed] [Google Scholar]
- 27.Alley MC, Scudeiro DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 28.Li WY, Koda RT. Stability of irinotecan hydrochloride in aqueous solutions. Am J Health-Syst Pharm. 2002;59:539–544. doi: 10.1093/ajhp/59.6.539. [DOI] [PubMed] [Google Scholar]
- 29.Warner DL, Burke TG. Simple and versatile high-performance liquid chromatographic method for the simultaneous quantitation of lactone and carboxylate forms of camptothecin anticancer drugs. J Chromatogr B. 1997;691:161–171. doi: 10.1016/s0378-4347(96)00426-4. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J, Stewart CF, Hougton PJ. Animal models for studying the action of topoisomerase I targeted drugs. Biochim Biophys Acta. 1998;1400:301–319. doi: 10.1016/s0167-4781(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 31.Bissery MC, Vrignaud P, Lavelle F, Chabot GG. Experimental anti-tumor activity and pharmacokinetics of the camptothecin analog irinotecan (CPT-11) in mice. Anti-Cancer Drugs. 1996;7:437–460. doi: 10.1097/00001813-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Jansen WJM, Kolfschofen GM, Erkelens CAM, Van Ark-Otte J, Pinedo HB, Boven E. Anti-tumor activity of CPT-11 in experimental human ovarian cancer and human soft-tissue sarcoma. Int J Cancer. 1997;73:891–896. doi: 10.1002/(sici)1097-0215(19971210)73:6<891::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.DelBino G, Lassota P, Darzynkiewicz Z. The S-phase cytotoxicity of camptothecin. Exp Cell Res. 1991;103:27–35. doi: 10.1016/0014-4827(91)90534-2. [DOI] [PubMed] [Google Scholar]
- 34.Houghton PJ, Cheshire PJ, Hallman JD, Lotz L, Friedman HS, Danks MK, Houghton JA. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 35.Poon MA, O'Connell MJ, Wiland HS, Kroog JE, Gerstrom JA, Tschetter LK, Levitt R, Kardinal CG, Mailliard JA. Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol. 1991;9:1967–1972. doi: 10.1200/JCO.1991.9.11.1967. [DOI] [PubMed] [Google Scholar]
- 36.Allen TM, Hansen C, Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989;981:27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- 37.Mayhew EG, Lasic D, Babbar S, Martin FJ. Pharmacokinetics and antitumor activity of epirubicin encapsulation in long-circulating liposomes incorporating a polyethyleneglycol-derivatized phospholipid. Int J Cancer. 1992;51:302–309. doi: 10.1002/ijc.2910510221. [DOI] [PubMed] [Google Scholar]
- 38.Fidler IJ. Metastases: quantitative analysis of the distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1990;45:773–782. [PubMed] [Google Scholar]
- 39.Guba M, Cernaianov G, Koehl G, Geissler EK, Janch KW, Anthuber M, Falk W, Steinbauer M. A primary tumor promotes dormancy of solitary tumor cells before inhibiting angiogenesis. Cancer Res. 2001;61:5575–5579. [PubMed] [Google Scholar]
- 40.Noker PE, Lin TH, Page JG, Burke TG, Schweikart KM, Rhie JK. Pharmacokinetics of the camptothecin analog DB-67, in mice, rats, and dogs. Proc Am Assoc Cancer Res. 2002;43:S425. [Google Scholar]
- 41.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA. 1998;85:6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, Martin FJ. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen Zhang J, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, Ahmad I. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270:93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Sadzuka Y, Hirotsu S, Hirota S. Effect of liposomalization on the antitumor activity, side-effects and tissue distribution of CPT-11. Cancer Lett. 1998;127:99–106. doi: 10.1016/s0304-3835(98)00031-7. [DOI] [PubMed] [Google Scholar]