Abstract
Interactions between extracellular matrix proteins and prostate carcinoma cells change dramatically during prostate tumor progression. We have concentrated on two key modifications that occur in the hemidesmosome in prostate carcinoma: loss of laminin-5 protein expression and altered basal cell polarity of the α6β4 integrin. We previously demonstrated two cell line-specific isoforms (β3A and β3B) of the LAMB3 message. Cells expressing only the β3B isoform did not translate the β3 protein and were unable to assemble the laminin-5 trimer. One such cell line, LNCaP, was selected to determine whether restoration of the laminin-5 β3A isoform would cause expression of a functional laminin-5 β3 chain, assembly and secretion of the laminin-5 trimer, and reversion to a non-neoplastic phenotype. Laminin-5 β3A cDNA was cloned and stably transfected into LNCaP cells. We observed the restoration of the β3 protein, but a laminin-5 trimer was not secreted. Moreover, increased cell migration was demonstrated, and tumorigenicity was increased in SCID mice. A microarray analysis, performed between transfected and nontransfected LNCaP cells, showed most changing genes to be associated with signal transduction. The β3 chain of laminin-5 may thus play an important role in signal transduction, which may enhance cell motility and tumorigenesis.
Keywords: Laminin-5 β3A, prostate carcinoma cells, LNCaP, cell migration, tumorigenicity
Introduction
Intricate connections between the extracellular matrix (ECM), cell surface receptors, and cytoskeleton establish an elaborate, multifaceted adhesion complex and signaling mechanism. Alterations in these relationships are important in the transformation from a normal to malignant phenotype [1,2]. Individual components of the ECM, particularly hemidesmosomal-associated proteins, have been shown to be modified in prostate tumor progression [3,4]. Laminins, a family of ECM glycoproteins expressed in the basal lamina of various tissues, are polymeric proteins composed of three different polypeptide chains (α, β, and γ) arranged in a cruciform structure [5]. Each polypeptide chain is encoded by a separate gene, and different combinations of these chains have led to the identification of various isoforms. Although the distinct biologic functions of several laminins are uncharacterized, some have been shown to play a significant role in cell growth and migration, tissue regeneration, cell differentiation, cell adhesion, and various pathologic conditions [6–14].
Laminin-5 (α3β3γ2) is epithelium-specific and forms anchoring filaments—one of the pivotal hemidesmosomal components involved in a structural relationship between the epithelium and the stroma [15,16]. The C-terminus of the α3 chain of laminin-5 contains a series of globular domains, which constitute the accepted binding site for its receptors: the α6β4 or α3β1 integrins [17–21]. These transmembrane cell surface receptors are well established as mediators of bidirectional signal transduction [22–26]. Recent studies have demonstrated that, depending on the cleavage status of the β3 or γ2 chains, laminin-5 can either cause stable cell adhesion or migration through integrin interaction, or serve as a scatter factor in an integrin-independent manner [27–30]. The pathologic significance of laminin-5 to epithelial attachment was discovered by the identification of mutations in the genes coding for the individual chains in patients affected by Herlitz junctional epidermolysis bullosa (H-JEB), a severe blistering disease with separation within the cutaneous basement membrane at the level of the basal lamina, usually causing neonatal death [9–13,31]. A knockout mouse of laminin-5, through targeted disruption of the α3 chain, resulted in similar blisters and neonatal lethality [32]. The role of laminin-5 in tumorigenesis is less clear [16,33–39]. Recent work has demonstrated that the β3 chain of laminin-5 is specifically cleaved by the metalloproteinase MP1-MPP [40]. We have demonstrated that in prostate carcinoma, protein expression for the β3 and γ2 chains of laminin-5 is absent and the basal cell polarity of the α6β4 integrin is lost [41]. The significance of these defects is presently under investigation in our laboratory.
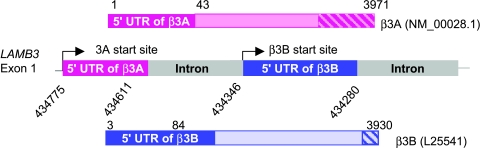
We have previously reported the existence of two isoforms (β3A and β3B) of the message for LAMB3, which was verified by reverse transcription polymerase chain reaction (RT-PCR) analysis [42]. The open reading frames for both forms were homologous. They differed, however, within the 5′ and 3′ untranslated regions, and their expression was cell line-specific (Figure 1). Those cells that expressed only the β3B isoform neither translate the β3 protein, nor assemble nor secrete the laminin-5 trimer. The prostate carcinoma cell line, LNCaP, was one such line, and served as a working model for this present study. LNCaP cells express at least five integrin pairs, including the laminin receptors (α6β1, α3β1, and α6β4). We hypothesized that in vitro restoration of the laminin-5 β3A isoform into LNCaP cells would cause expression of a fully functional β3 protein. We tested whether assembly of an operational heterotrimer would alter cell migration as well as tumorigenicity. In this study, laminin-5 β3A cDNA was cloned from the HaCaT cell line and was stably transfected into LNCaP cells by the liposome-mediated gene transfer method and subsequent G418 selection. We observed the restoration of the β3 protein by Western blot analysis and isolated small amounts of the individual laminin-5 chains from the supernatant. An assembled laminin-5 ECM could not be demonstrated, and focal contacts rather than hemidesmosomes were seen by electron microscopy. Functional studies, however, revealed that the expression of β3 in LNCaP cells increased migration and enhanced tumor formation in SCID mice. We performed a 22,283-human gene microarray analysis to further investigate these findings. A total of 395 genes was found to be significantly changed (greater than two-fold, P ≤ .005). Thirteen of 15 genes selected for validation by real-time RT-PCR agreed with the microarray data. The β3 chain in transfected LNCaP cells seemed to play a putative role in signal transduction, and may explain why transfected LNCaP cells showed increased motility in vitro and increased tumorigenesis in SCID mice.
Figure 1.
β3 Message isoforms. Examination of exon 1 of LAMB3 revealed the 5′ UTR of β3A in the first 43 nucleotides of exon 1 and the 5′ UTR of β3B within the intron. This suggested two transcription start sites with the possibility of differential transcriptional regulation.
Materials and Methods
Cells in Culture
The human keratinocyte cell line, HaCaT, was obtained from Dr. Norman Fusenig's laboratory (German Cancer Center, Heidelberg, Germany).
The LNCaP human prostate carcinoma cell line (passage 36) was obtained from the American Type Culture Collection (Rockville, MD).
The cell lines were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. HaCaT cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Corp., Carlsbad, CA) containing 10% fetal bovine serum, glucose (1 g/l), penicillin G, streptomycin sulfate, and l-glutamine in final concentrations of 100 U/ml, 100 µg/ml, and 0.292 mg/ml, respectively.
LNCaP cells were maintained in RPMI 1640 (Invitrogen Corp.) with supplements as described for HaCaT cells.
Cloning Strategy of LAMB3A
We have previously reported two isoforms of the β3 mRNA, designated β3A and β3B [42]. An examination of exon 1 of LAMB3 revealed the presence of two transcriptional start sites (Figure 1). We demonstrated that both messages were differentially expressed in various cell lines. β3A expression was absent in LNCaP and MCF-7, greatly reduced in PC3-N, but present in eight other epithelial cell lines. β3B was present in all cell lines studied. Only those cells that expressed the β3A message, however, expressed proteins by Western blot and immunohistochemical analyses. Because we previously reported that HaCaT cells contained the β3A message as well as the laminin-5 heterotrimer, we selected this cell line for the cloning of LAMB3A. Total RNA was isolated using the Qiagen RNeasy protocol for animal cells (Qiagen Inc., Valencia, CA) and treated with DNase using the MessageClean Kit (GenHunter Corporation, Nashville, TN) to remove genomic DNA contamination. The enzyme was removed using RNeasy Protocol (Qiagen Inc.) and the RNA was quantitated by spectrophotometry.
Reverse Transcription
DNase-treated HaCaT RNA was reverse-transcribed into cDNA using Omniscript Protocol (Qiagen Inc.). Briefly, 2 µg (6 µl) of RNA was heated to 65°C for 3 minutes and placed on ice, then 1 x buffer RT, 0.5 mM of each dNTP, 1 µm oligo-dT primer, 10 U of RNase inhibitor, and 4 U of Omniscript Reverse Transcriptase were added to the RNA for a final volume of 20 µl and heated to 37°C for 1 hour.
Polymerase Chain Reaction (PCR)
The reverse transcription reaction was verified by using a PCR with β-actin (Actin Primer Pair; Ambion, Inc., Austin, TX) under the following conditions: 95°C, 5 minutes; 30 cycles of 94°C, 1 minute; 59°C, 1 minute; 72°C, 1 minute; and 72°C for 10 minutes.
The primer pairs for β3A spanned the first 20 bases of the unique 5′ UTR as well as the unique base pairs in the 3′ UTR region, generating a 3971-bp product as previously reported [42]:
sense: GCT TTC AGG CGA TCT GGA GA
antisense: TGT GCT TGG TCC AGG ATT CC
β3A was amplified using the Expand High Fidelity PCR System (Roche, Mannheim, Germany) with the following conditions. Master mix 1 was prepared separately and contained 200 µM (1 µl) of each nucleotide, 300 nM (1.5 µl) of each primer, and 1 µl of cDNA template (0.1–0.75 µg) for a final volume of 25 µl. Master mix 2 was prepared separately and contained 19.25 µl of sterile H2O, 1 x Expand High Fidelity buffer with 15 mM MgCl2 (5 µl), and 2.6 U (0.75 µl) of Expand High Fidelity PCR System enzyme mix for a final volume of 25 µl. The master mixes were pipetted together on ice into a thin-walled PCR tube, mixed well, and placed in a PTC-200 Peltier Thermal Cycler (MJ Research, Waltham, MA). β3A was amplified using the following conditions: 94°C, 2 minutes; 10 cycles at 94°C, 15 seconds; 58°C, 30 seconds; 68°C, 3 minutes; 20 cycles at 94°C, 15 seconds; 58°C, 30 seconds; 68°C, 3 minutes, 5 seconds with the addition of 5 sec/cycle; 68°C, 7 minutes. A single 3971-bp product was produced when analyzed by 0.8% agarose gel electrophoresis in 1 x TAE. The band was cut from the gel and extracted using Geneclean Spin Kit (Bio101, Vista, CA).
TOPO TA Cloning
The β3A PCR product was cloned into a pcDNA3.1 vector using the manufacturer's instructions in the TOPO TA Expression Kit (Invitrogen Corp.). Briefly, the PCR product was incubated at room temperature for 30 minutes along with the salt solution, sterile water, and pcDNA 3.1 vector provided. Two microliters of the cloning reaction was added to one vial of One Shot, TOP10 chemically competent Escherichia coli cells and was placed on ice for 30 minutes. The cells were heat-shocked for 30 seconds at 42°C, and were immediately placed on ice. A total of 250 µl of room-temperature SOC medium (Invitrogen Corp.) was then added to the cells and mechanically shaken at 200 rpm for 1 hour at 37°C. Thirty microliters of the reaction was spread evenly in the middle of prewarmed agar plates and incubated overnight at 37°C. Ten colonies were selected and cultured in LB medium containing 100 µg/ml ampicillin at 37°C and mechanically shaken overnight at 400 rpm. Plasmids were isolated using QIAprep Miniprep Kit (Qiagen Inc.) and were analyzed by restriction analysis with EcoRV. Positive samples were verified by automated DNA sequencing using an ABI-Prism 377 automated sequencer, T7 and BGH primers from the TOPO cloning kit, and the 5′ LAMB3A primer used in Expand PCR. This system uses both Taq cycle sequencing and dye deoxy terminator incorporation to optimize throughput and the length of read for sequencing data. Protein production by the LAMB3A gene was demonstrated by the TNT Quick Coupled Transcription/Translation System (Promega Corporation, Madison, WI) using the manufacturer's protocol with [35S]methionine (1000 Ci/mmol at 10 mCi/ml) and Luciferase DNA as a positive control. The proteins were separated by 8% SDS polyacrylamide gel electrophoresis (PAGE) (data not shown).
Transfection of LNCaP Cells
The pcDNA3.1/LAMB3A construct was transfected into LNCaP cells (passage 36) by an enhancement of the lipidmediated gene transfer method [43] using Effectene Transfection Reagent (Qiagen Inc.). Briefly, LNCaP cells were plated into a six-well plate (35 mm/well) to 60% confluence. About 1.6 µg (16 µl) of purified construct or vector alone was diluted into 371.2 µl of buffer EC and 12.8 µl of enhancer for a final volume of 400 µl, and incubated at room temperature for 5 minutes. Twenty microliters of Effectene Transfection Reagent was mixed with the above solution and incubated at room temperature for 10 minutes. The growth medium was aspirated from the cells during this incubation, and 1.6 µl of RPMI 1640 “complete” medium (with 10% serum and antibiotics) was added to the PBS-washed cells. Six hundred microliters of RPMI 1640 “complete” medium was added to the solution containing LAMB3A or empty vector. This solution was added drop by drop to the cells and incubated at 37°C and 5% CO2 for 48 hours. Drug selection using 500 µg/ml G418 (Geneticin; Invitrogen Corp.) was started 48 hours after transfection. Three weeks after drug selection, colonies were harvested by 0.5-mm no. 1 Whatman trypsin-soaked filter papers and expanded to cell lines.
Antibodies
Murine monoclonal antibodies clone 17 against the β3 chain of laminin-5 (Kalinin B1; BD Biosciences, San Jose, CA), clone GB3 specific for the γ2 chain of laminin-5 only when the complete trimer is present (Sera-Lab, Sussex, England, UK), and clone 3E1 against the β4 integrin (Invitrogen Corp.) were purchased. The rat monoclonal antibody clone J1B5 specific for the α6 integrin was generously provided by Dr. Caroline Damsky (University of California, San Francisco, CA).
Mouse monoclonal antibody clone BM165 specific for the α3 chain of laminin-5 was generously provided by Dr. Robert Burgeson (Harvard Medical School, Charlestown, MA).
SDS-PAGE and Western Blot Analysis
Western analysis was performed as described previously with slight modifications [42]. Briefly, total cell lysates, from cultured LNCaP and HaCaT cells and NP40 lysis buffer containing equal amounts of protein (20 µg), were electrophoresed on an 8% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blocked in Tris base saline with Tween 20 (Fisher Scientific, Pittsburgh, PA) (TBS-T) containing 10% nonfat milk powder at 4°C overnight and then incubated for 2 hours with monoclonal antibody clone 17. The membrane was then incubated with secondary antibody [goat anti-mouse horseradish peroxidase (HRP), 1:50,000; Jackson Immuno Research Laboratories, West Grove, PA] for 1 hour and specific proteins were detected using chemiluminescence (Phototype-HRP Detection Kit for Western Blotting; Cell Signaling Technologies, Beverly, MA). Monoclonal antiactin, clone AC40 (Sigma, St. Louis, MO), was used to demonstrate equal loading.
Affinity Column and Silver-Stained SDS-PAGE
Three milligrams of 6F12 anti-β3 chain antibody was mixed together with CNBr-activated Sepharose 4B beads (Amersham Pharmacia Biotech AB, Uppsala, Sweden) and shaken overnight. The next day, the beads were washed with binding buffer (0.1 M NaHCO3 and 0.5 M NaCl, pH 8.3). Tris-HCl (0.1 M) with 0.5 NaCl, pH 8.0, was used for blocking and the column was washed with PBS. Five hundred microliters of transfected LNCaP conditioned media (RPMI in 1% FBS and 50 µg/ml) was placed on the column, washed with PBS, and eluted with 0.1 M glycine elution buffer, pH 2.7. Twenty milliliters was collected, concentrated to 50 µl, and loaded onto a 6% SDS polyacrylamide gel stained with silver under reducing conditions.
Immunohistochemistry and Confocal Microscopy
For immunofluorescence microscopy, cultured cells were grown on glass coverslips until 80% to 90% confluent (usually 48–72 hours). They were fixed by quickly dipping into PBS, then at -20°C methanol for 10 minutes, and six dips in cold acetone, and briefly air-dried. Application of primary and secondary antibodies, as well as observation of immunofluorescence using confocal laser scanning microscopy (LSM 410; Carl Zeiss, Jena, Germany), were as previously reported [44].
Electron Microscopy
For conventional transmission electron microscopy, cells grown on coverslips followed procedures previously reported [45].
Cell Migration Assay Using Time Lapse Video Microscopy
Seventy thousand cultured LNCaP cells transfected with LAMB3A or pcDNA 3.1 vector alone were seeded on a Delta T dish (0.15 mm; BiopTechs, Inc., Butler, PA) in 2 ml of serum-free medium containing 5% penicillin/streptomycin. Prior to the start of the video, cells were allowed to attach to the dish for 5 hours on the microscope stage. Migration was monitored for 18 hours using differential interference contrast optics on an inverted Olympus IMT2 microscope (Olympus America, Melville, NY) equipped with a BiopTechs Delta T live cell system under a humidified (5% CO2 balanced ultrazero air) atmosphere. Images were obtained with a grayscale ORCA-100 CCD camera (Hamamatsu Photonics Sytems, Bridgewater, NJ) and viewed using SimplePCI 4.0 software (Compix Imaging Inc., Cranberry Township, PA). Assays were repeated twice.
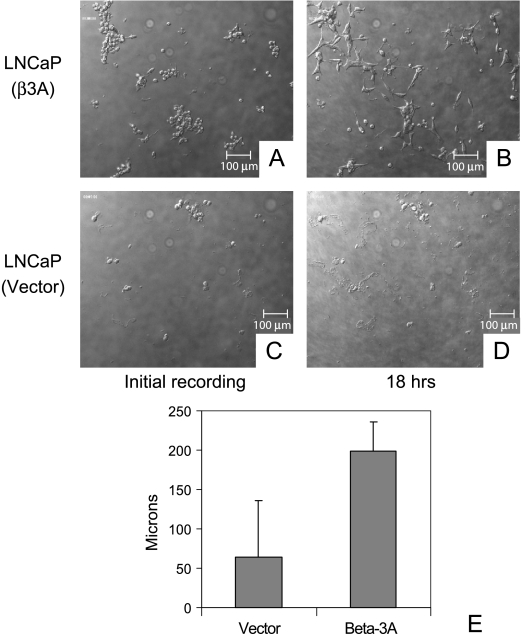
Based on scale measurements provided by SimplePCI 4.0 software, eight cells from each group were independently measured. The distance each cell migrated was measured hourly and a total distance was obtained for the 18-hour period. The mean distance of migration for each group of eight cells (vector alone and β3A transfects) was calculated and the Student's t test (two-tailed, paired) was performed on the data (n = 8, P = .0006).
Tumorigenicity
Two groups of four male SCID mice each were injected subcutaneously with either 10 million clone 2.9β3A cells or LNCaP cells that received vector alone. For each mouse, cells were mixed in equal volumes of matrigel and sterile saline. A second experiment was performed with the same conditions, except that cells were mixed in sterile saline alone. As tumors developed, they were measured for tumor volume estimation (mm3) in accordance with the formula: a2 x b/c, where a is the smallest diameter and b is the largest diameter using Mitutoyo solar hand-held calipers. Animals were sacrificed after 60 days, complete autopsies were performed, and tissues were snap-frozen and formalin-fixed.
Gene Expression Profiles and RT-PCR Analysis
Total RNA from parental LNCaP and those LNCaP cells transfected with empty vector and β3A was isolated using Macherey-Nagel Nucleospin Kit (BD Biosciences Clontech, Palo Alto, CA) and converted into cRNA according to the manufacturer's instructions (Affymetrix, Santa Clara, CA). The Affymetrix HG-U133A microarray (derived from UniGene, dbEST, WUSTL, GenBank, and RefSeq data bases) contained 22,283 unique species and was hybridized, washed, and scanned according to the manufacturer's instructions (Affymetrix). Primary data were processed using Affymetrix Microarray Suite 5.0 software (Affymetrix) to determine the average difference value and to assess signal intensity for each probe set. Briefly, a comparison of gene expression profiles from parental LNCaP and those transfected with empty vector yielded no statistical difference in gene expression, enabling us to compare cells that received empty vector against those transfected with β3A. For genes that increased, sorting was first performed by those that were present in experimental conditions, then by change calls designated “I,” and, finally, by the signal/log ratio ≥ 1.0 (i.e., a fold change of two or greater). Similarly, genes that decreased were first sorted by genes that were present in the control condition, then by a change call designated “D,” and, finally, by the signal/log ratio of ≤ 1.0 (i.e., a two-fold or greater change).
Selected genes were validated by real-time RT-PCR analysis. Briefly, reverse transcription was performed using TaqMan Reverse Transcription Reagents (Roche Molecular Systems, Branchburg, NJ) and 50 ng of total RNA in a 50 µl reaction. The reverse transcription reaction was primed with random hexamers and incubated at 25°C for 10 minutes followed by 48°C for 30 minutes and 95°C for 5 minutes, and chilled at 4°C. Each PCR reaction consisted of 10 µl of cDNA added to 25 µl of TaqMan Universal PCR Master Mix (Roche Molecular Systems), 2.5 µl of gene-specific primer/probe mix (Assays-by-Design; Applied Biosystems, Foster City, CA) and 12.5 µl of PCR water. The PCR conditions were: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds alternating with 60°C for 1 minute. JUN, RASSF1, FGFR2, JAK2, VEGF, FOXM1, BIRC5, HMMR, and GAPDH were performed and the data collected using the ABI Prism 7000 real-time sequence detection system (Applied Biosystems). The gene-specific TaqMan probes were labeled with the 5′ reporter dye, 6-FAM, and a 3′ end that contains a nonfluorescent quencher and a minor groove binder. Differences in expression were determined using the equation 2-ΔΔCt, where the Ct value for each sample was subtracted from the Ct value of the GAPDH control. Primer sequences are available upon request.
Results
Laminin-5 and LAMB3 Expression in Parental LNCaP Cells
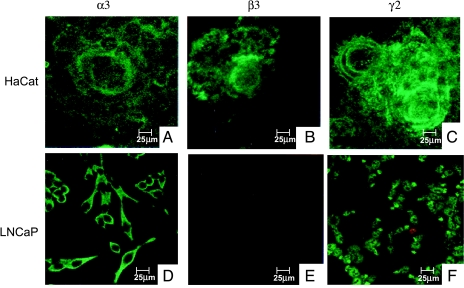
Earlier immunohistochemical analysis in our laboratory revealed that all three chains of laminin-5 were expressed in HaCaT cells (Figure 2, A–C), whereas the β3 chain was absent in LNCaP cells (Figure 2, D–F). The pattern of expression of the α3 and γ2 chains differed between both cell lines. Extracellular secretion and formation of a subcellular matrix were seen in HaCaT cells, whereas only an intracellular pattern was demonstrated in LNCaP cells.
Figure 2.
Laminin-5 expression. Confocal laser scanning micrographs showed the expression of laminin-5 subchains in HaCaT (A–C) and LNCaP cells (D–F). The α3, β3, and γ2 chains were expressed in HaCaT cells, whereas the β3 chain was absent in LNCaP cells (E). Moreover, the pattern of expression differed between both cell lines. Extracellular matrix formation was seen in HaCaT cells for all three chains, whereas the α3 and γ2 chains were intracellular in LNCaP cells.
Generation of LAMB3A LNCaP Cell Lines
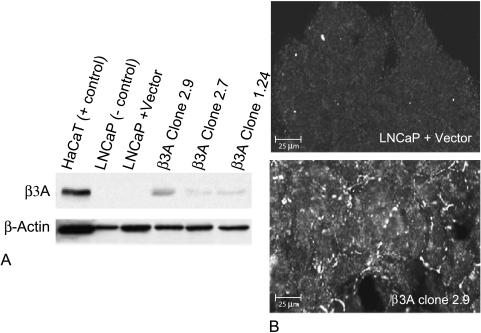
LNCaP cells of low passage number were transfected with the human LAMB3A cDNA expression vector pcDNA3.1/CMV/LAMB3A or the pcDNA3.1/CMV vector alone as a control. After drug selection, various clones were analyzed for LAMB3A gene and protein expression using quantitative (“real-time”) PCR and immunoblotting, respectively. As shown in Figure 3A, varying β3 protein expression was seen in three independent clones (2.9, 2.7, and 1.24) by Western blot analysis, but was absent in both the control LNCaP cell line receiving empty vector and the parental cells. β3 protein expression was also demonstrated by immunohistochemistry (Figure 3B).
Figure 3.
Protein expression of LAMB3 in β3A-transfected LNCaP cells. (A) Western blot analysis of cell lysates from various β3A-transfected clones (2.9, 2.7, and 1.24). Monoclonal antibody clone 17 against the β3 chain of laminin-5 was used. Eight percent acrylamide gel electrophoresis revealed differential β3 protein expression levels in various clones, with clone 2.9 having the strongest expression after controlling for equal loading with β-actin. LNCaP cells that received empty vector demonstrated no β3 protein and behaved like the parental LNCaP cell line used as a negative control. HaCaT cell lysate was used as a positive control. (B) Immunofluorescent analysis using monoclonal antibody clone 17 and confocal laser scanning microscopy of cultured transfected cells grown on coverslips. Note granular deposition of β3 protein on cell surface of β3A-transfected cells and the absence of the β3 protein in LNCaP cells transfected with vector alone.
Heterotrimer Formation and Integrin Expression
Restoration of the laminin-5 trimer and expression of the α6β4 integrin were attempted by immunohistochemistry. For heterotrimer formation detection in LAMB3A-transfected cells, we used a monoclonal antibody (GB3) specific for the γ2 chain of laminin-5 only when the complete trimer is present. Extracellular trimer formation was not detected in parental LNCaP cells or those receiving empty vector. No trimer was apparent surrounding the cells, and an inconclusive staining pattern was seen on the cell surface in the transfected cells. Despite the lack of convincing extracellular trimer, small amounts of the three laminin-5 chains were observed in silver-stained 6% SDS-PAGE gels made from supernatants using an anti-β3 affinity column (data not shown).
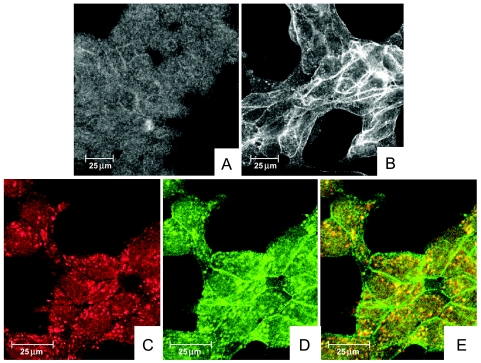
Localization of the α6 integrin along the cytoplasmic membrane was seen in LAMB3A-transfected cells but could barely be detected in parental cells or those receiving empty vector (Figure 4, A and B). α3, β1, and β4 integrin expression could not be detected, and there was no colocalization with either of their respective integrin partners or the β3 chain of laminin-5 (data not shown). In addition to the prominent plasma membrane localization, the α6 integrin was detected in a punctate staining pattern on the surface of the cells. This punctuate α6 integrin (green) colocalized with the α3 chain of laminin-5 (red) in LAMB3A-transfected cells (Figure 4, C–E). This pattern supports the formation of focal contacts, which were seen by electron microscopy.
Figure 4.
Confocal laser scanning micrographs of α6 integrin and colocalization of α6 with the α3 chain of laminin-5. (A and B) Immunofluorescence using clone J1B5 against the α6 integrin. Note an accentuated punctate and intracellular deposition on the cytoplasmic membrane, as well as increased cytoplasmic membrane polarization, of the α6 integrin in β3A-transfected cells (B) compared to LNCaP cells that received vector alone (A). (C–E) Colocalization of clone BM165 against the α3 chain of laminin-5 (C; red) and α6 integrin using J1B5 (D; green) was used in double-labeled immunofluorescence (E). A punctate as well as intracellular distribution of the α6 integrin were noted (D). The punctate α6 integrin staining colocalized (yellow) with the α3 chain of laminin-5 (E). In contrast, the cytoplasmic membrane α6 seen at the juncture of cells (D) did not colocalize with the β3 laminin chain.
Taken together, these results suggest that the introduction of the β3 chain was sufficient for LNCaP cells to produce the β3 protein and small amounts of all three chains in the supernatant. The presence of the ligand apparently effected integrin distribution on the cytoplasmic membrane because these receptors are not typically detected by immunohistochemistry in parental LNCaP cells. The introduction of the β3 chain did not cause the assembly of a heterotrimer or a recognizable ECM. Moreover, hemidesmosomes did not form although focal contacts were observed.
Characterization of Migratory and Adhesive Properties
After 18 hours of growth on plastic, β3A-transfected cells were clearly more migratory than LNCaP cells that received vector alone when viewed with the BiopTechs Delta T live cell system (Figure 5). The β3A-transfected cells demonstrated an ability to spread easily, especially in clusters. Lamellopodia formation was noted in virtually every cell as well as in cell-to-cell attachments (Figure 5, A and B). Cells that received vector alone tended to remain fixed and did not spread. Lamellopodia were absent, with most cells staying rounded and isolated (Figure 5, C and D). The mean distance of migration for eight individual cells receiving β3A was three times greater than for eight cells receiving empty vector (Figure 5E and Table 1).
Figure 5.
Migration assay of LNCaP cells transfected with β3A or vector alone using time lapse video microscopy after 18 hours. (A) β3A-transfected cells were more migratory and demonstrated an ability to spread easily after 18 hours (B). Note lamellopodia formation in virtually every β3A-transfected cell as well as cell-to-cell attachments (B). Cells that received vector alone (C) tended to remain fixed and did not spread (D). Note the absence of lamellopodia, with most cells staying rounded and isolated after 18 hours (D). (E) Mean distance of migration. The mean distance of migration for each group of eight cells (vector alone and β3A transfects) was calculated. Student's t test (two-tailed, paired) was performed (n = 8, P = .0006). Error bars equal 1 SD of the mean.
Table 1.
Migration Distance.
| Vector | β3A-Transfected | |||
| Distance | Rate | Distance | Rate | |
| 156 | 8.6 | 204 | 11.3 | |
| 0 | 0 | 201 | 11.2 | |
| 198 | 11 | 198 | 11 | |
| 0 | 0 | 261 | 14.5 | |
| 0 | 0 | 216 | 12 | |
| 33 | 1.83 | 117 | 6.5 | |
| 30 | 1.67 | 192 | 10.7 | |
| 96 | 5.33 | 198 | 11 | |
| Mean | 64.13 | 3.55 | 198.38 | 11.03 |
| SD | 72.33 | 4.01 | 39.97 | 2.05 |
Clone 2.9 cells adhered better on plastic (n = 6, P = .006) than those that received the empty vector, based on a routine crystal violet absorbance assay (data not shown). The cells, however, required a protracted adhesion time (26–28 hours), which was unusual for this assay because most cell lines studied in our laboratory attach between 30 and 90 minutes. The results were variable and inconclusive at various time points attempted (90 minutes; 4, 6, 18, and 24 hours), and with alternative substrates (e.g., fibronectin and laminin-5) with minimal cell adherence, unless incubated overnight. We noted increased lamellopodia formation after 18 hours and we observed maximal response with cells incubated for over 24 hours.
LAMB3A Increases Tumor Growth In Vivo
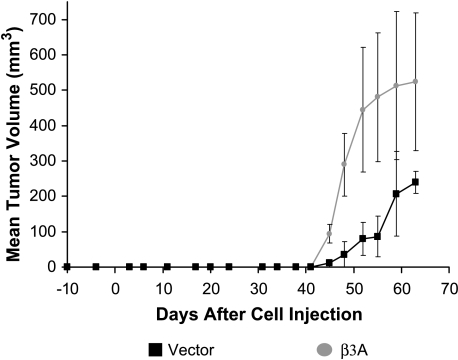
Two groups of four male SCID mice were initially injected subcutaneously with 10 million cells transfected with β3A or empty vector and mixed in equal volumes of matrigel and sterile saline. By day 35, mean tumor volumes were equal for tumors formed by β3A-transfected cells or those receiving vector alone. This closely resembled in vitro growth in which no significant difference was seen in log and plateau phases. After 35 days, however, tumors formed by β3A-transfected cells grew to sizes twice as large as tumors formed by cells receiving vector alone. To remove any effect from matrigel, the experiment was repeated by mixing the transfected cells in sterile saline. Similar results were obtained. After 45 days, the tumors formed by β3A-transfected cells almost tripled in volume (P = .007) in comparison to those that received the empty vector (Figure 6).
Figure 6.
Tumorigenicity. Mean tumor volumes after 10 million cells transfected with β3A or empty vector were mixed in sterile saline and injected subcutaneously into SCID mice. After 45 days, tumors formed by β3A-transfected cells almost tripled in volume (P = .007) in comparison to those that received empty vector. Error bars represented by standard deviation. Similar results were obtained when cells were mixed in equal volumes of matrigel and sterile saline. By 35 days, the β3A-transfected cells had formed tumors smaller than those that received empty vector. After this time, however, as cells apparently adapted to their microenvironment, the tumors formed by β3A-transfected cells doubled in volume and remained twice as large as those formed by cells that received empty vector (data not shown).
These experiments suggest that β3A may play a growthstimulatory role. Morphologically, the tumors formed with transfected cells appeared to be slightly more aggressive than tumors formed by cells receiving empty vector. Ki-67 results showed no difference in counts of positive cells between tumors formed by either β3A-transfected cells or those receiving vector alone.
Gene Expression Profiles
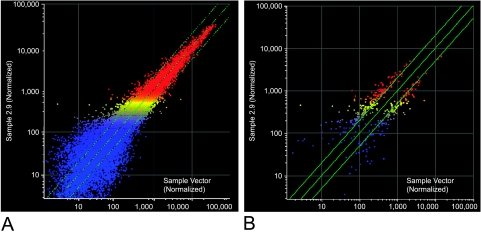
Gene expression profiles were studied using Affymetrix HG-U133A microarrays and Suite 5.0 Software, as described in Materials and Methods section. Of 395 genes that were found to be statistically changed (greater than two-fold change, P ≤ .005), 233 were upregulated and 162 were downregulated (Figure 7) (a complete list can be obtained from http://azcc-microarray.arl.arizona.edu/index.php3). The diverse functions of these genes were known in less than half of the cases and included signal transduction, transcriptional regulation, protein modification, mitosis, cell motility, ion transport, and metabolism. The function of most of the known upregulated genes was related to transcription regulation (21 genes), protein modification (16 genes), and mitosis (16 genes). Signal transduction (13 genes) and cell or vesicle transport (8 genes) were the next most common categories. Protein modification (14 genes), signal transduction (14 genes), and transcription regulation (11 genes) were the most common functions of the downregulated genes.
Figure 7.
Scatter plots from Affymetrix HG-U133A microarray. Microarrays were normalized using the global scaling method recommended by Affymetrix. These data were used to generate fold change. A total of 395 genes, which showed a two-fold change or greater, was selected and analyzed for significant differences using the Affymetrix DMT data mining tool. There were 233 upregulated and 162 downregulated genes. Scatter plots were created using Genespring version 6.1 software (Silicon Genetics, Redwood City, CA). False color representations are depicted showing different levels of signal intensity, with blue being the lowest, yellow being intermediate, and red being the highest. The 395 genes that showed a two-fold change or greater are represented in (A), and the 233 upregulated and 162 downregulated genes are depicted in (B).
Fifteen genes were selected for validation by real-time RT-PCR analysis using GAPDH as a normalizing control (Table 2). Thirteen of 15 genes (87%) concurred with the microarray data. Although FGFR3 (fibroblast growth factor receptor 3) and BIRC1 (baculoviral inhibitor of apoptosis repeat-containing 1) did not agree with the microarray data, other members of these families, FGFR2 and BIRC5 (survivin), substantiated the microarray results.
Table 2.
Genes Selected For Validation.
| Jun |
| Ras association domain family 1 (RASSF1) |
| FGFR2 |
| FGFR3 |
| JAK2 |
| E-cadherin (CDH1) |
| Growth factor receptor-bound protein 10 (GRB10) |
| VEGF |
| Forkhead box M1 (FOXM1) |
| BIRC1 |
| BIRC5 |
| HMMR |
| PI-3 kinase-related kinase (SMG-1) |
| LIM protein |
| GAPDH |
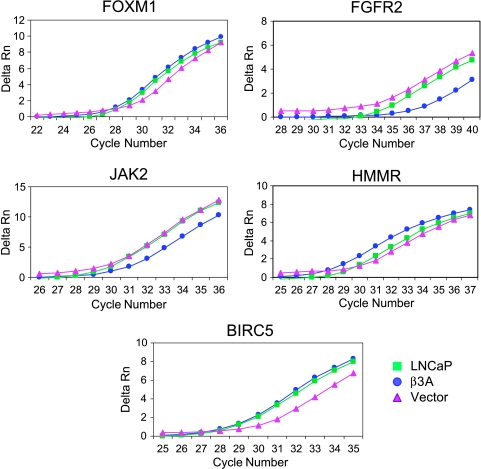
Two of the cell or vesicle transport upregulated genes were directly associated with cell motility, PRC1 (protein regulator of cytokinesis) and HMMR (hyaluronan-mediated motility receptor). PRC1 increased 2.14-fold and HMMR increased 3.25-fold. In contrast, none of the downregulated genes was directly associated with cell motility. Moreover, cells transfected with β3A showed a significant increase in HMMR gene expression compared to those receiving vector alone when validated by real-time RT-PCR (Figure 8). Among the upregulated genes related to signal transduction found in the β3A-transfected cells, LIM protein (LIM kinase, an enzyme involved in cell motility) was increased sevenfold. LYN (a viral-related oncogene homolog), DLG7 (discs, large homolog 7, a tumor-suppressor gene in the Wnt pathway), and ECTZ (epithelial cell transforming sequence 2 oncogene) were related to cell growth. Two genes from the baculoviral inhibitor of apoptosis family, BIRC1 and BIRC5 (survivin), were associated with cell survival. FOXM1 (forkhead box M1), a transcription regulator, and SMG-1, a PI-3 kinase-related kinase, both in the AKT pathway, were also upregulated. LYN, DLG7, and ECTZ were not selected for validation. Cells transfected with β3A also showed a significant increase in gene expression in the remaining five aforementioned genes then those receiving vector alone when validated by real-time RT-PCR (Figure 8).
Figure 8.
Validation by real-time RT-PCR. Real-time RT-PCR was performed as described in Materials and Methods section and the threshold bar for Ct determination was set within the linear range of the PCR amplification. Fold increase was determined by first subtracting out GAPDH expression from each sample before comparing relative expression values (2-ΔΔCt). A representation of 5 of 13 genes that concurred with microarray results is shown with gene expression levels compared between parental, nontransfected (LNCaP) cells, and transfected cells receiving either β3A or empty vector.
The downregulated signal transduction genes were primarily associated with the growth factor-ERK pathway. Jun was decreased 4.6-fold, and RASSF1, FGFR2, FGFR3, VEGF, JAK2, CDH1 (E-cadherin), and GRB10 were decreased two-fold to three-fold in the β3A-transfected cells. All of these were validated by real-time RT-PCR (Figure 8). Taken together, the microarray data appeared to correlate with the in vitro migration data and suggest that the intracellular β3 chain of laminin-5 may play an important role in signal transduction. Specifically, in conjunction with the in vitro cell growth and SCID mice tumor data, the β3 chain may play a role in cell survival.
Discussion
Previous work in our laboratory revealed two isoforms (β3A and β3B) of the message for LAMB3, which were cell line-specific [42]. Although the open reading frames for both forms were homologous, we found differences in the 5′ and 3′ untranslated regions (Figure 1). Cells that expressed only the β3B isoform, such as LNCaP, did not translate the β3 protein nor synthesize the laminin-5 heterotrimer. Our present data indicate that the stable transfection of β3A into these prostate carcinoma cells supports protein production of the β3 chain. In vitro results included focal contact formation, polarization of the α6 integrin to the perimeter of the cell, increased cell spreading, and migration. When injected into SCID mice, in the presence or absence of matrigel, these cells also demonstrated increased tumor growth after a period of 35 to 40 days.
Although the α3 chain has been restored in human fibrosarcoma cells, ours is the first report of restoration of the β3 chain in human carcinoma cells [46]. Other laboratories have successfully restored either the β3 or γ2 chains, and showed laminin-5 heterotrimer formation [47–49]. This work was carried out in keratinocytes taken from junctional epidermolysis bullosa patients who had a mutation in either chain [47–49]. The β3A-transfected LNCaP cells, however, only made small amounts of the individual laminin-5 chains but did not form laminin-5 matrix in comparison to the work done in keratinocytes [47–49]. The lack of a detectable heterotrimer may be suggested by earlier work in our laboratory [50]. In that study, we showed that LNCaP cells retained most of their laminin production and could not secrete various laminin chains owing to abnormal glycosylation [50].
In the current study, transfected cells showed focal contact formation but did not show restoration of an extracellular basal lamina typically lost in prostate carcinoma, nor evidence of hemidesmosome formation. This result is consistent with work done by our group and others [51,52]. We have shown that α6β1 and α3β1 are both involved in the formation of dynamic focal contacts important for cell locomotion [51]. We have found that prostate cell lines able to form invasive tumors in immunocompromised mice have increased expression of the α6β1 integrin [51]. Recently, this integrin has been reported to be more involved in cell spreading than static attachment [52]. We know from previous work that the presence of the α6 integrin in the LNCaP cell can usually not be demonstrated by immunohistochemistry but is present by Western blot analysis. Interestingly, transfection with β3A apparently polarized the α6 integrin to the cytoplasmic membrane and colocalized with the α3 chain of laminin-5 in punctuate focal adhesion contacts. The observed increased lamellopodia formation, spreading, and migration may be related to this polarization of the α6 integrin. We could not demonstrate colocalization of the β4 integrin, suggesting that the presence of overexpression of the β3 laminin chain was insufficient in this cell type to cause hemidesmosome formation.
Based on our microarray results, upregulation of LIM kinase and downregulation of E-cadherin may further explain why the transfected β3A cells showed increased motility. LIM kinase is known to be responsible for cofilin phosphorylation, both of which are key members of a Rac signaling cascade that regulates lamellopodia formation, cell adhesion, and cell motility [53]. Recent evidence suggests that LIM kinase may play an essential role in cancer invasion in general as well as invasive growth of prostate epithelial cells [54,55]. Moreover, the downregulation of E-cadherin agrees with previous work showing a gain of N-cadherin and a loss of E-cadherin (referred to as “cadherin switching”) in human prostate cancer progression [56–58].
Perhaps our most intriguing result was the increased size of tumors when β3A-transfected cells were injected subcutaneously in SCID mice. Prior to 40 days, the in vivo data resembled the in vitro growth curve. After 35 to 40 days, however, as these cells apparently adapted to their microenvironment, tumor volume tripled in comparison to cells receiving empty vector. An Affymetrix microarray experiment was performed to further analyze the effects of β3 transfection. LNCaP cells transfected with β3A showed upregulation of genes associated primarily with transcription regulation, protein modification, mitosis, and signal transduction, as well as cell or vesicle transport. The most common functions of the downregulated genes were also protein modification, signal transduction, and transcription regulation. The upregulated genes were related to survival or the AKT pathway, whereas the downregulated genes were associated with the growth factor-ERK pathway. Preliminarily, the increased tumor volume seems to be attributed to a triggering of cell survival signaling by the intracellular β3 chain. Further work is needed to determine whether the increased tumor volume can be explained by integrin mediation, cell growth signaling, or a combination of these processes, Our results do suggest, however, a putative role for the β3 chain of laminin-5 in signal transduction.
This role is not surprising in light of recent work by Schenk et al. [59], which showed that a proteolytic fragment of the γ2 chain of laminin-5 can serve as a ligand for EGFR. In addition, a novel coculture technique using normal human prostatic fibroblasts and LNCaP cells demonstrated that prostatic fibroblasts promoted tumor formation and retarded the apoptotic pathways in tumor cells [60]. The role of the microenvironment and interactions with neoplastic cells has been well documented perhaps most poignantly by Hanahan and Weinberg in their heterotopic view of cancer biology. In that model, the interplay between carcinoma cells and normal cells, and vessels and stroma is emphasized [61].
In conclusion, we demonstrated that laminin-5 β3A expression in LNCaP cells increased cell migration in vitro and tumorigenicity in vivo. These results were supported by a human microarray experiment, which indicated that the β3 chain of laminin-5 seems to play an important role in signal transduction, particularly cell survival. The β3A-transfected cells, however, remained incapable of secreting a laminin-5 matrix and forming hemidesmosomes, which may suggest a deficiency in the translational machinery unique to LNCaP cells. Whether these findings are unique to prostate cancer or can be replicated in other carcinoma cell lines warrants further investigation. We are presently examining the role of β3A expression in MCF-7 breast cancer cells, which are also incapable of making this protein.
Footnotes
This work was supported by the National Institutes of Health grants K08 CA90662-01, 5 P01 CA56666-05, and 2P30 CA023074-26.
References
- 1.Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci USA. 1981;78:3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 3.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. AmJ Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- 4.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferletta M, Ekblom P. Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J Cell Sci. 1999;112(Pt 1):1–10. doi: 10.1242/jcs.112.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 7.Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sanes JR. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990;1:731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet. 1994;6:293–297. doi: 10.1038/ng0394-293. [DOI] [PubMed] [Google Scholar]
- 10.Pulkkinen L, Christiano AM, Gerecke D, Wagman DW, Burgeson RE, Pittelkow MR, Uitto J. A homozygous nonsense mutation in the beta 3 chain gene of laminin 5 (LAMB3) in Herlitz junctional epidermolysis bullosa. Genomics. 1994;24:357–360. doi: 10.1006/geno.1994.1627. [DOI] [PubMed] [Google Scholar]
- 11.Aberdam D, Galliano MF, Vailly J, Pulkkinen L, Bonifas J, Christiano AM, Tryggvason K, Uitto J, Epstein EH, Jr, Ortonne JP, Meneguzzi G. Herlitz's junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5) Nat Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- 12.Kivirikko S, McGrath JA, Baudoin C, Aberdam D, Ciatti S, Dunnill MG, McMillan JR, Eady RA, Ortonne JP, Meneguzzi G. A homozygous nonsense mutation in the alpha 3 chain gene of laminin 5 (LAMA3) in lethal (Herlitz) junctional epidermolysis bullosa. Hum Mol Genet. 1995;4:959–962. doi: 10.1093/hmg/4.5.959. [DOI] [PubMed] [Google Scholar]
- 13.McGarth JA, Christiano AM, Pulkkinen L, Eady RA, Uitto J. Compound heterozygosity for nonsense and missense mutations in the LAMB3 gene in nonlethal junctional epidermolysis bullosa. J Invest Dermatol. 1996;106:1157–1159. doi: 10.1111/1523-1747.ep12340210. [DOI] [PubMed] [Google Scholar]
- 14.Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- 15.Jones JCR, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin-5 subunits. J Biol Chem. 1995;270:23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- 17.Carter WG, Ryan MC, Gahr P. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 18.Gehlsen KR, Sriramarao P, Furcht LT, Skubitz AP. A synthetic peptide derived from the carboxy terminus of the laminin A chain represents a binding site for the alpha 3 beta 1 integrin. J Cell Biol. 1992;117:449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, Roller PP, Kleinman HK, Yamada Y. Identification of cell binding sites in the laminin α1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270:20583–20590. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 20.Pattaramalai S, Skubitz KM, Skubitz AP. A novel recognition site on laminin for the α3β1 integrin. Exp Cell Res. 1996;222:281–290. doi: 10.1006/excr.1996.0036. [DOI] [PubMed] [Google Scholar]
- 21.Kramer RH, Vu MP, Cheng YF, Ramos DM, Timpl R, Waleh N. Laminin-binding integrin α7β1: functional characterization and expression in normal and malignant melanocytes. Cell Regul. 1991;2:805–817. doi: 10.1091/mbc.2.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the alpha 6 beta 4 integrin: distinct beta 4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumemberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 25.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 26.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 27.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease- 2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 28.Goldfinger LE, Stack MS, Jones JCR. Processing of laminin- 5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JCR. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- 30.Grassi M, Moens G, Rousselle P, Thiéry JP, Jouanneau J. The SFL activity secreted by metastatic carcinoma cells is related to laminin 5 and mediates cell scattering in an integrin-independent manner. J Cell Sci. 1999;112:2511–2520. doi: 10.1242/jcs.112.15.2511. [DOI] [PubMed] [Google Scholar]
- 31.Fine JD, Bauer EA, Briggaman RA, Carter DM, Eady RA, Esterly NB, Holbrook KA, Hurwitz S, Johnson L, Lin A, et al. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa. A consensus report by the Subcommittee on Diagnosis and Classification of the National Epidermolysis Bullosa Registry. J Am Acad Dermatol. 1991;24:119–135. doi: 10.1016/0190-9622(91)70021-s. [DOI] [PubMed] [Google Scholar]
- 32.Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin alpha3beta1 -mediated interaction with laminin-5 stimulates adhesion, migration, and invasion of malignant glioma cells. Int J Cancer. 1998;76:63–72. doi: 10.1002/(sici)1097-0215(19980330)76:1<63::aid-ijc11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am J Pathol. 1997;151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
- 35.Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K. The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- 36.Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol. 1998;185:44–52. doi: 10.1002/(SICI)1096-9896(199805)185:1<44::AID-PATH46>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Bahadoran P, Perrin C, Aberdam D, Spadafora-Pisani A, Meneguzzi G, Ortonne JP. Altered expression of the hemidesmosomeanchoring filament complex proteins in basal cell carcinoma: possible role in the origin of peritumoral lacunae. Br J Dermatol. 1997;136:35–42. [PubMed] [Google Scholar]
- 38.Chopra A, Maitra B, Korman NJ. Decreased mRNA expression of several basement membrane components in basal cell carcinoma. J Invest Dermatol. 1998;110:52–56. doi: 10.1046/j.1523-1747.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 39.Martin KJ, Kwan CP, Nagasaki K, Zhang X, O'Hare MJ, Kaelin CM, Burgeson RE, Pardee AB, Sager R. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4:602–613. [PMC free article] [PubMed] [Google Scholar]
- 40.Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- 41.Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149:1341–1349. [PMC free article] [PubMed] [Google Scholar]
- 42.Hao J, McDaniel K, Weyer C, Barrera J, Nagle RB. Cellline specific translation of two laminin 5 β3 chain isoforms. Gene. 2002;283:237–244. doi: 10.1016/s0378-1119(01)00850-2. [DOI] [PubMed] [Google Scholar]
- 43.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calaluce R, Kunkel MW, Watts GS, Schmelz M, Hao J, Barrera J, Gleason-Guzman M, Isett R, Fitchmun M, Bowden GT, Cress AE, Futscher BW, Nagle RB. Laminin-5 mediated gene expressionhuman prostate carcinoma cells. Mol Carcinog. 2001;30:119–129. doi: 10.1002/1098-2744(200102)30:2<119::aid-mc1020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 45.Schmelz M, Cress AE, Barrera J, McDaniel KM, Davis TL, Fuchs L, Dalkin BL, Nagle RB. PEAZ-1: a new human prostate neoplastic epithelial cell line. Prostate. 2001;48:79–92. doi: 10.1002/pros.1084. [DOI] [PubMed] [Google Scholar]
- 46.Mizushima H, Hirosaki T, Miyata S, Takamura H, Miyagi Y, Miyazaki K. Expression of laminin-5 enhances tumorigenicity of human fibrosarcoma cells in nude mice. Jpn J Cancer Res. 2002;93:652–659. doi: 10.1111/j.1349-7006.2002.tb01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gagnoux-Palacios L, Vailly J, Durand-Clement M, Wagner E, Ortonne J-P, Meneguzzi G. Functional re-expression of laminin-5 in laminin-gamma2-deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem. 1996;271:18437–18444. doi: 10.1074/jbc.271.31.18437. [DOI] [PubMed] [Google Scholar]
- 48.Vailly J, Gagnoux-Palacios L, Dell'Ambra E, Romero C, Pinola M, Zambruno G, DeLuca M, Ortonne J-P, Meneguzzi G. Corrective gene transfer of keratinocytes from patients with junctional epidermolysis bullosa restores assembly of hemidesmosomes in reconstructed epithelia. Gene Ther. 1998;5:1322–1332. doi: 10.1038/sj.gt.3300730. [DOI] [PubMed] [Google Scholar]
- 49.Robbins PB, Lin Q, Goddnough JB, Tian H, Chen X, Khavari PA. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. PNAS. 2001;98:5193–5198. doi: 10.1073/pnas.091484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinowitz I, Cress AE, Nagle RB. Biosynthesis and secretion of laminin and S-laminin by human prostate carcinoma cell lines. Prostate. 1994;25:97–107. doi: 10.1002/pros.2990250207. [DOI] [PubMed] [Google Scholar]
- 51.Rabinovitz I, Nagle RB, Cress AE. Integrin α6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, Otey CA, Zhau HE, Chung LWK. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001;12:99–107. [PubMed] [Google Scholar]
- 53.Lawler S. Regulation of actin dynamics: the LIM kinase connection. Curr Biol. 1999;9:R800–802. doi: 10.1016/s0960-9822(99)80493-x. [DOI] [PubMed] [Google Scholar]
- 54.Yoshioka K, Foletta V, Bernard O, Itoh K. A role for LIM kinase in cancer invasion. Proc Natl Acad Sci USA. 2003;100:7247–7252. doi: 10.1073/pnas.1232344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davila M, Frost AR, Grizzle WE, Chakrabarti R. LIM kinase 1 is essential for the invasive growth of prostate epithelial cells: implications in prostate cancer. J Biol Chem. 2003;278:36868–36875. doi: 10.1074/jbc.M306196200. [DOI] [PubMed] [Google Scholar]
- 56.Tran NL, Nagle RB, Cress AE, Heimark RL. N-cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am J Pathol. 1999;155:787–798. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–3654. [PubMed] [Google Scholar]
- 58.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 59.Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olumi AF, Dazin P, Tlsty TD. A novel coculture technique demonstrates that normal human prostatic fibroblasts contribute to tumor formation of LNCaP cells by retarding cell death. Cancer Res. 1998;58:4525–4530. [PubMed] [Google Scholar]
- 61.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]