Abstract
The aim of this study was to assess whether lonidamine (LND) interferes with some steps in angiogenesis progression. We report here, for the first time, that LND inhibited angiogenic-related endothelial cell functions in a dose-dependent manner (1–50 µg/ml). In particular, LND decreased proliferation, migration, invasion, and morphogenesis on matrigel of different endothelial cell lines. Zymographic and Western blot analysis assays showed that LND treatment produced a reduction in the secretion of matrix metalloproteinase- 2 and metalloproteinase-9 by endothelial cells. Vessel formation in a matrigel plug was also reduced by LND. The viability, migration, invasion, and matrix metalloproteinase production of different tumor cell lines were not affected by low doses of LND (1–10 µg/ml), whereas 50 µg/ml LND, which corresponds to the dose used in clinical management of tumors, triggered apoptosis both in endothelial and tumor cells. Together, these data demonstrate that LND is a compound that interferes with endothelial cell functions, both at low and high doses. Thus, the effect of LND on endothelial cell functions, previously undescribed, may be a significant contributor to the antitumor effect of LND observed for clinical management of solid tumors.
Keywords: Angiogenesis, lonidamine, endothelial cells, metalloproteinases, cancer
Introduction
Lonidamine (LND) is an antineoplastic drug that is effective against a wide range of solid tumors [1–7]. It affects energy metabolism [8], alters the plasma and mitochondrial membranes [9,10], and interferes with DNA repair [11] and cellular acidification [12]. Preclinical studies demonstrated the ability of LND to increase the response of human tumor cells to several antineoplastic drugs such as cisplatin [13], etoposide [14] diazepam [15], adriamycin [16,17], and paclitaxel [18]. We previously reported that LND triggers apoptosis independently of the p53 gene [19] and that bcl-2 overexpression blocks LND-induced apoptosis [20]. LND was also demonstrated to trigger apoptosis through a direct bcl-2-inhibited effect on the mitochondrial permeability transition pore [9], and to favor mitochondrial membrane permeabilization through a direct effect on adenine nucleotide translocase [10]. Thus, unlike conventional antitumor drugs that trigger proapoptotic signal transduction pathways upstream of mitochondria, LND acts directly on mitochondria to induce apoptosis. Because mitochondria play a crucial role in the induction and execution of apoptosis [21], recent suggestions have been made to use agents that directly act on mitochondria to trigger apoptosis so that drug-sensitive as well as drug-resistant tumor cells can be eliminated.
Phase II and phase III trials demonstrated the efficacy of LND on metastatic breast cancer [3], advanced ovarian cancer [2,4], inoperable non small lung carcinoma [1,5], and glioblastoma multiforme [6].
The low toxicity observed after long-term exposure of neoplastic patients to LND [1,2,4,7] and the ability of LND to potentiate drug efficacy led us to investigate the effect of LND on angiogenesis. In recent years, the importance of angiogenesis in tumor growth and the dissemination and use of antiangiogenic agents have been widely discussed [22,23]. Most research works, to date, have concentrated on antiangiogenic drugs that prevent the proliferation of vascular endothelial cells, which are essential for the development of tumor vasculature. The targeting of existing tumor vasculature represents a promising alternative approach [24–26]. Both depend on targeting endothelial cells, rather than tumor cells, for drug action, and destruction of the tumor cell is secondary.
We examined the effect of LND on in vitro endothelial cell functions involved in angiogenesis, and show, for the first time, that LND reduces in vitro endothelial cell proliferation, morphogenesis, migration, invasion, metalloproteinases secretion, and vessel formation in a matrigel plug.
Materials and Methods
Endothelial and Tumor Cells
Human umbilical endothelial cells (HUVECs; PromoCell GmbH, Heidelberg, Germany), were cultured in complete EBM-2 medium (Clonetics Bio Whittaker, Walkersville, MD) containing 2% fetal bovine serum. Human microvascular endothelial cells (HMVECs) were kindly provided by Dr. Gabriella Fibbi (Department of Pathology and Oncology, University of Florence, Florence, Italy) and maintained in MCDB-131 medium (Invitrogen, Milan, Italy) supplemented with 0.3% fetal calf serum (FCS). Immortalized EA.hy926 cells [27] were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with high glucose, supplemented with 10% FCS, hypoxanthine aminopterin thymidine medium, 2 mM l-glutamine, and antibiotics. Bovine aortic endothelial cells (BAECs) were furnished by Dr. Carlo Gaetano (Istituto Dermopatico dell'Immacolata, Rome, Italy) and cultured in DMEM (1 g/l glucose) supplemented with 10% FCS and 1% glutamine and antibiotics. The experiments were performed with early [5–10] cell passages. NIH 3T3 mouse embryo fibroblasts were cultured in DMEM containing 10% FCS and 1% glutamine and antibiotics. The JR8 melanoma, CG5 breast, ADFS glioblastoma, and H460 lung human cell lines were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% FCS and 1% glutamine and antibiotics.
The conditioned media (CM) of endothelial, tumor, and NIH 3T3 cells were prepared by incubating subconfluent cells in serum-free medium (SFM) for 24 hours. The CM were then collected under sterile conditions, centrifuged sequentially at 1200 and 12,000 rpm for 10 minutes to eliminate debris, and stored at −20°C.
LND Preparation
LND ([1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid] (Doridamine; Angelini Spa, Pomezia, Italy) was dissolved in dimethyl sulphoxide at a concentration of 10 mg/ml, adjusted with isoton to x10 concentration, and brought to the final concentration with complete medium. Control experiments demonstrated that the doses of dimethyl sulphoxide used through the study did not have any effect.
Cell Proliferation Assay
Exponentially growing endothelial cells, NIH 3T3 fibroblasts, JR8, CG5, ADFS, and H460 tumor cell lines were seeded in 96-well plates (5 x 103 cells/well) and incubated for 24 hours in complete medium. Then, the medium was replaced with fresh complete medium and the cells were incubated for 48 hours in the presence of increasing doses of LND (ranging from 0.1 to 50 µg/ml). Cells exposed to medium, with or without FCS, were used as positive and negative control, respectively. Cell proliferation was evaluated by a colorimetric assay at the end of treatment as described previously [27]. Each dose of LND was tested in sextuplicate. All the experiments were repeated at least three times.
Analysis of Apoptosis
Approximately 5 x 105 endothelial and tumor cells were plated in complete medium in 100-mm tissue culture Petri dishes and incubated at 37°C. After 24 hours, cells were exposed for 48 hours to LND at doses ranging from 1 to 50 mg/ml. Adherent cells recovered from the plates by phosphate- buffered saline (PBS)-EDTA 0.02% treatment were washed, assayed for cell viability (trypan blue exclusion test), and counted. The percentage of apoptotic cells was detected by annexin V assay. Briefly, 1 x 106 cells were doublestained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide using the Vybrant Apoptosis Kit according to the manufacturer's instruction (Molecular Probes, Eugene, OR) and were immediately analyzed by cytofluorimetric analysis (FACScan; Becton Dickinson, Milan, Italy) as previously reported [28]. The experiments were repeated three times.
Morphogenesis Assay on Matrigel
Morphogenesis on matrigel of endothelial cell lines was evaluated as reported previously [27]. Twenty-four-well microtiter plates were coated with 300 µl/well unpolymerized matrigel (10 mg/ml; Becton Dickinson) and allowed to polymerize for 1 hour at 37°C. Endothelial cells were plated (2 x 105 cells/well) in 1 ml of SFM containing 50% NIH 3T3 CM (positive control), to which LND at different doses (1, 5, and 7.5 µg/ml) was added. Cells plated in SFM served as the negative control. After 8 and 24 hours of incubation in a 5% CO2-humidified atmosphere at 37°C, cell growth was observed through a reverted, phase-contrast photomicroscope and photographed. Experiments were repeated at least three times, and each dose was tested in triplicate.
Chemoinvasion and Chemotaxis Assays
These assays were performed in Boyden chambers as previously described [29]. Briefly, endothelial (1.2 x 105 cells/800 µl) or tumor (2 x 105 cells/800 µl) cells were added to the upper chamber in the absence or presence of increasing concentrations of LND (from 1 to 50 µg/ml). The lower compartment was filled with 200 µl of NIH 3T3 CM, or with DMEM supplemented with 0.1% bovine serum albumin (BSA) to evaluate random migration and invasion (negative control). Vascular endothelial growth factor (VEGF; 30 ng/ml) was also used as chemoattractant for the migration assay on endothelial cells. The compartments were separated by an 8-mm pore size polycarbonate filter (Costar Corp., Cambridge, MA), coated with matrigel (25 µg/filter) for chemoinvasion, or gelatin (5 µg/ml; Sigma, Milan, Italy) for chemotaxis. After incubation for 6 hours in a humidified 5% CO2 atmosphere at 37°C, cells on the upper side of the filter were removed mechanically and cells that had invaded or migrated to the lower surface of the filter were fixed in ethanol and stained with toluidine blue. Cells were counted in at least four high-power fields (HPFs; x200). Each dose was tested in triplicate and experiments were repeated at least three times.
Zymography of Gelatinolytic Activity and Western Blot Analysis
Subconfluent endothelial and tumor cells were incubated for 48 hours in SFM in the absence or presence of increasing doses of LND (1–50 µg/ml). CM of tumor cells were concentrated with Centricon-30 concentrators (Amicon, Danvers, MA). Then CM were analyzed by zymography using gelatin-embedded sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) as previously described [29].
The presence of matrix metalloproteinase-2 (MMP2) and matrix metalloproteinase-9 (MMP9) proteins in CM was also analyzed by Western blot analysis. Twenty microliters of sample dilution buffer was boiled and electrophoresed under reducing conditions on an 11% SDS-PAGE. Anti-MMP2 (NoeMarkers, Fremont, CA) and anti-MMP9 (Oncogene, La Jolla, CA) antibodies were used at 1:1000 and 1:500 dilution, respectively. Red Ponceau staining (0.1% in acetic acid; Sigma) of filter was used to check equal loading of proteins.
The experiments were repeated two times.
In Vivo Matrigel Assay
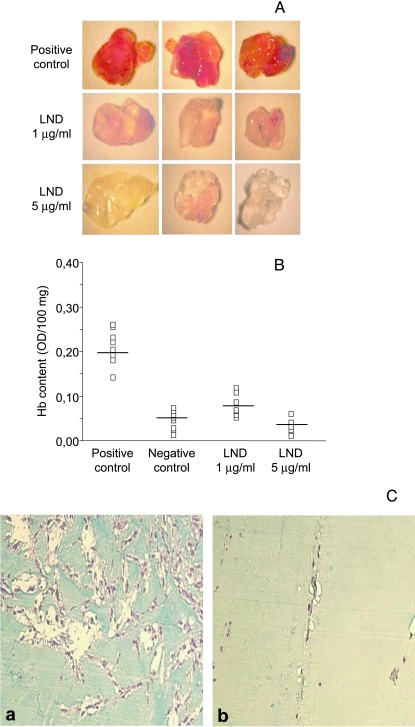
To evaluate the ability of LND to modulate the neovascularization within matrigel plugs, the method described previously was used [30]. Six hundred microliters of matrigel, supplemented with VEGF (60 ng/mice; R&D Systems, Minneapolis, MN), heparin (19.2 U; Schwarz Pharma SpA, Milan, Italy), and 60 µl of LND at two different doses (1 and 5 µg/ml), was injected subcutaneously into the flank of 8-week-old C57BL/6 mice (furnished by the Animal Care Unit of Regina Elena Cancer Institute, Rome, Italy). The negative and positive controls contained heparin alone or heparin plus VEGF, respectively. After 5 days, the angiogenic response was evaluated by macroscopic analysis at autopsy, and by measurement of the hemoglobin (Hb) content into the pellet of matrigel as previously reported [30]. Histologic analysis of matrigel plugs was also performed using Masson Trichrome stain.
The values were expressed as optical density (OD)/100 mg of matrigel. Each group consisted of eight animals. The experiments were repeated four times.
Statistical Analysis
Student's t test analysis was used to compare untreated and LND-treated samples.
Results
LND Inhibits Endothelial Cell Proliferation
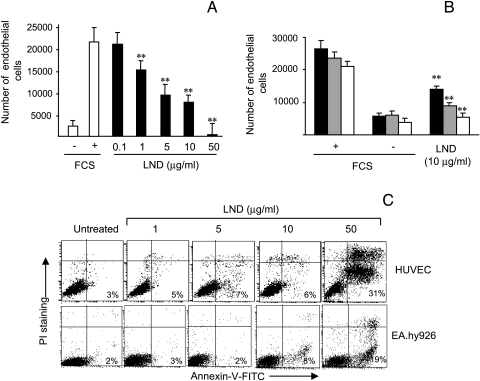
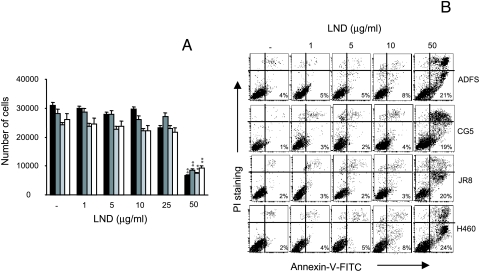
We previously demonstrated that LND induces apoptosis in breast cancer and glioblastoma lines at the dose of 50 µg/ml [19,20], whereas other authors evidenced the ability of LND to trigger the apoptotic program in leukemic cells at the dose of 65 µg/ml [14]. Therefore, with the aim of evaluating the potential antiangiogenic activity of LND, several endothelial cell lines with different origins were exposed to LND doses ranging from 0.1 to 50 µg/ml and cell proliferation was evaluated 48 hours after LND treatment. Cells exposed to medium with or without FCS were used as positive and negative control, respectively. As reported in Figure 1A, LND inhibited the proliferation of HUVEC in a dose-dependent manner. A significant (P < .01) decrease in cell proliferation was observed after exposure of endothelial cells to LND doses ranging from 1 to 50 µg/ml, whereas no effect on proliferation was observed after 0.1 µg/ml LND treatment. The ability of LND to reduce proliferation of other endothelial cells was also evaluated. As reported in Figure 1B, 10 µg/ml LND significantly (P < .01) inhibited the growth of HMVEC, EA.hy926, and BAEC lines by approximately 50%, 30%, and 25%, respectively.
Figure 1.
Low doses of LND inhibit endothelial cell proliferation while not inducing apoptosis. (A) HUVEC proliferation was evaluated after exposure for 48 hours to doses of LND ranging from 0.1 to 50 g/ml (black column). (B) Cell proliferation of HMVEC (dark grey column), EA.hy926 (pale grey column), and BAEC (white column) was evaluated after exposure for 48 hours to 10 g/ml LND. Cells exposed to medium with or without 10% FCS were used as positive (+) and negative (-) control, respectively. (C) Flow cytometric analysis of annexin V was performed by exposing HUVEC and EA.hy926 endothelial lines to doses of LND ranging from 1 to 50 g/ml for 48 hours. The percentage of annexin V- positive cells is reported. Each value is the mean of sextuplicate ±SD, and the data are representative of at least three separate experiments (A and B). A representative experiment out of three is reported (C). Statistical differences between LND-treated versus untreated groups: *P < .05; **P < .01.
To check whether LND exhibits its antiproliferative activity through the induction of apoptosis, HUVEC and EA.hy926 endothelial lines were analyzed for the presence of early apoptotic events on annexin V staining. As reported in Figure 1C, a very low percentage of annexin V-positive cells (less than 8%) was observed after treatment of cells with LND at doses ranging from 1 to 10 µg/ml for 48 hours. On the contrary, 50 µg/ml LND induced apoptosis in the two endothelial cell lines even though a different degree of apoptosis was observed. In particular, about 30% and 20% of HUVECs and EA.hy926 endothelial cells, respectively, were annexin V-positive after LND treatment.
LND Inhibits Endothelial Cell Migration and Invasion
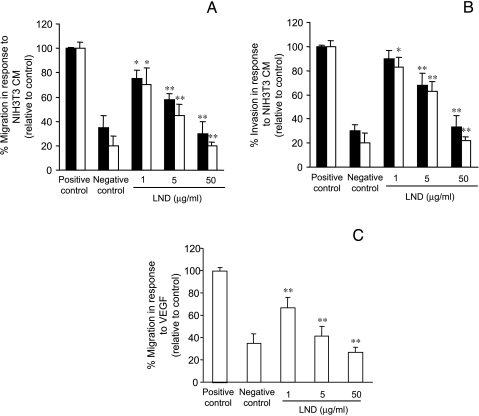
Angiogenesis is highly dependent on endothelial cell motility and invasion. We therefore tested the effect of LND on these two endothelial cell functions. As shown in Figure 2, LND significantly inhibited HUVEC and HMVEC migration (Figure 2A) and invasion (Figure 2B) in a dose-dependent manner. Cells strongly migrated and invaded in response to NIH 3T3 CM (positive control), whereas migration and invasion in the absence of a chemoattractant (negative control) were limited. The presence of 1 µg/ml LND during the migration assay was able to significantly (P < .05) inhibit the migration of the two endothelial lines by about 30% and this inhibition was progressively enhanced with increasing doses (about 50% and 80% inhibition at doses of 5 and 50 µg/ml, respectively; P < .01). Similar results were obtained when VEGF was used as chemoattractant for the migration assay. Inhibition of about 35%, 55%, and 75% at the doses 1, 5, and 50 µg/ml, respectively (P < .01), were observed (Figure 2C). When all the endothelial lines were assayed for invasion in the presence of LND from 1 to 50 µg/ml, the number of invaded cells, compared with the positive control, was reduced by about 20%, 40%, and 80%, respectively. Similar results were obtained using BAEC and EA.hy926 cells (data not shown).
Figure 2.
LND inhibits HUVEC (black column) and HMVEC (white column) migration (A and C) and invasion (B). Cultured cells were dissociated into a single cell suspension, suspended in DMEM supplemented with 0.1% BSA, and incubated for 6 hours on top of gelatin-coated filters (A and C), or on filters coated with matrigel (B) in the absence (positive and negative control) or presence of LND (1, 5, and 50 g/ml). The migration was evaluated by filling the lower compartment with NIH 3T3 CM (A) or VEGF (C). The invasion was evaluated in response to NIH 3T3 CM (B). DMEM supplemented with 0.1% BSA was used as negative control to evaluate random migration and invasion. The migrated or invaded cells were counted in at least four HPFs (x200). Each value is the mean of triplicates ±SD, and the data are representative of at least three separate experiments. Statistical differences between LND-treated versus untreated groups (positive control): *P < .05; **P < .01.
LND Inhibits Endothelial Cell Morphogenesis
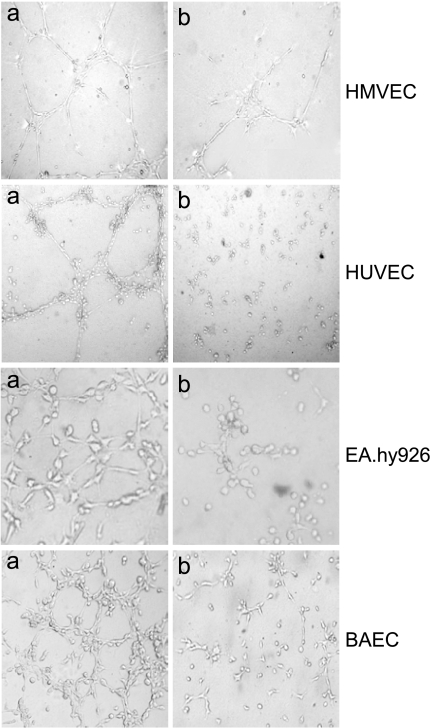
In addition to its effect on endothelial cell proliferation and apoptosis, we tested whether LND prevented the alignment of endothelial cells in a capillary-like structure—an endothelial function crucial to angiogenesis. As shown in Figure 3, when HMVEC, HUVEC, EA.hy926, and BAEC lines were plated on matrigel in the presence of NIH 3T3 CM (positive control, panel a), they aligned with one another and formed tube-like structures resembling a capillary plexus. In contrast, the addition of 5 µg/ml LND resulted in an inhibition of this cord formation (panel b). Cells remained spherical, and isolated and few aggregated cells were observed. This picture resembled that obtained with the negative control (data not shown). Even though HMVECs seem more affected by LND than other endothelial cell lines, all the parameters previously analyzed after LND treatment, such as proliferation, invasion, and migration, are indicative of a superimposable effect of LND in the four endothelial cell lines. Thus, we exclude a different sensitivity to LND treatment between HMVECs and other endothelial cells.
Figure 3.
LND inhibits HMVEC, HUVEC, EA.hy926, and BAEC morphogenesis. Endothelial cells were plated on matrigel in the absence (panel a) or in the presence (panel b) of LND at a dose of 5 g/ml and photographed. Experiments were repeated at least three times, and each dose was tested in triplicate. Representative phase-contrast micrographs are presented.
LND Reduces Metalloproteases Secretion
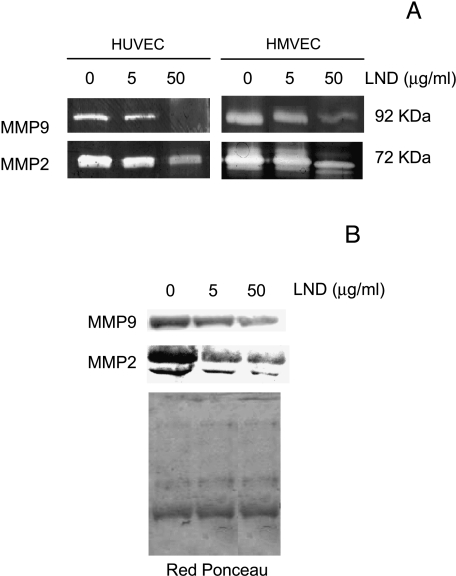
Angiogenesis involves the acquisition by endothelial cells of the capability to degrade the basement membrane and, in general, to remodel the extracellular matrix [31]. Thus, we examined whether treatment with LND at doses of 5 and 50 mg/ml for 48 hours could lead to a decrease in proteases secretion by HUVEC and HMVEC (Figure 4). In the zymographic analysis of the CM from untreated cells, high levels of secreted 72-kDa gelatinase (also designed MMP2) and 92-kDa gelatinase (also designed MMP9) were found in both endothelial lines (Figure 4A). A dose-dependent decrease in the intensity of the bands was observed for both MMP2 and MMP9 in the two endothelial lines. Similar results were obtained when zymographic analysis was performed, both loading CM from equal amounts of viable cells (Figure 4A) and equal amounts of proteins (data not shown).
Figure 4.
LND reduces metalloproteases secretion of HUVEC and HMVEC. CM of endothelial cells incubated for 48 hours in SFM in the absence or presence of LND at doses of 5 and 50 g/ml were collected and analyzed for gelatin zymography (A) and Western blotting analysis (B). Proteins from an equivalent number of viable cells were loaded. Red Ponceau staining of filter was used to check equal loadings of proteins. (A) White bands against a dark background correspond to the gelatinolytic areas of MMP2 (72 kDa) and MMP9 (92 kDa) activity. (B) Western blot analysis of MMP2 and MMP9 proteins in HUVECs. Representatives of two independent experiments are shown.
These results were confirmed by Western blot analysis of HUVEC CM, demonstrating that LND treatment led to a decrease in the expression of the pro-MMP2 (72 kDa) and its active form (68 kDa) and the MMP9 proteins (Figure 4B). Red Ponceau staining of filter was used to check equal loading of proteins.
LND Reduces Vessel Formation in In Vivo Matrigel Assay
The effect of LND on in vivo angiogenesis was evaluated by using the model of matrigel assay [26]. Matrigel plugs containing heparin, VEGF, and two different doses of LND (1 and 5 µg/ml) were injected subcutaneously in mice and the degree of vascularization into matrigel plugs was evaluated after 5 days. Macroscopic analysis of matrigel plugs containing VEGF (positive control) showed intense vascularization (Figure 5A), whereas the matrigel implant containing both VEGF and two different doses of LND were not able to produce such blood vessel formation. Negative control matrigel plugs, in which matrigel was injected with heparin alone, showed only a slight local reaction or angiogenic response (data not shown). The angiogenic response observed by macroscopic analysis was consistent with the quantitative results obtained by measuring the Hb levels in the matrigel. As evident in Figure 5B, Hb content in the matrigel plugs, containing both doses of LND, was significantly lower than in those containing positive control (four and eight times less for 1 and 5 µg/ml LND, respectively). The highest dose of LND (5 µg/ml) showed an angiogenic response similar to that observed for negative control. Histologic examination of vessels into matrigel plugs confirmed the results of quantitative analysis of Hb (Figure 5C). Matrigel plugs from control mice (panel a) revealed a marked capillary vessel proliferation, as compared with animals treated with 5 µg/ml LND (panel b).
Figure 5.
LND reduces vessel formation in a matrigel plug. Vessel formation was assessed after injection of C57BL/6 mice with matrigel plugs containing VEGF alone (positive control) or in combination with two different doses of LND (1 and 5 g/ml). After 5 days, mice were sacrificed, and neovascularization and Hb content of matrigel pellets were evaluated. (A) Macroscopic analysis of matrigels from one representative experiment. (B) Hb content of matrigel plugs. The negative control contained heparin alone. The values were expressed as OD/100 mg of matrigel plug. The OD for each matrigel plug is reported (□). (C) Histologic analysis of matrigel plugs from control mice (panel a) reveals a marked capillary vessel proliferation, as compared with animals treated with LND (panel b). (Masson Trichrome stain; original magnification, x10).
Effect of LND on Tumor Cell Proliferation, Migration, Invasion, and Metalloproteases Production
The ability of LND to modulate tumor cell growth and to trigger the apoptotic program on several tumor cell lines with different histotype was also analyzed. Figure 6A shows the effect of LND on proliferation of ADFS glioblastoma, JR8 melanoma, CG5 breast, and H460 lung carcinoma lines. It is evident that the doses ranging from 1 to 10 µg/ml were completely ineffective on all cell lines used, whereas treatment with 25 µg/ml LND induced a reduction of cell proliferation only in JR8 melanoma (P < .05). A significant reduction (P < .01) in cell numbers (about 60%) was observed when the different lines were exposed to 50 µg/ml LND. Similar results were obtained by exposing NIH 3T3 mouse fibroblasts to LND (data not shown). In addition, although doses of LND ranging from 1 to 10 µg/ml did not induce apoptosis, the 50-µg/ml dose activated the apoptotic program in all the tumor lines by about 20% to 25% of cells (Figure 6B). These data clearly demonstrate that the inhibitory activity of lowdoses LND on cell proliferation was only restricted to endothelial cells, whereas the 50-µg/ml dose triggered apoptosis both in endothelial and tumor cell lines.
Figure 6.
Low doses of LND do not inhibit tumor cell proliferation (A) and do not induce apoptosis (B). ADFS, CG5, JR8, H460, and cells were exposed for 48 hours to doses of LND ranging from 1 to 50 g/ml. (A) Cell proliferation of JR8 (black column), CG5 (dark gray column), ADFS (pale gray column), and H460 (white column) cells was evaluated after incubation of cells in 10% FCS in the absence (control) or in the presence of LND. Each value is the mean of sextuplicates ±SD, and the data are representative of at least three separate experiments. Statistical differences between LND-treated versus untreated groups (positive control): *P < .05; **P < .01. (B) Flow cytometric analysis of annexin V. The percentage of annexin V- positive cells is reported. A representative experiment out of three is reported.
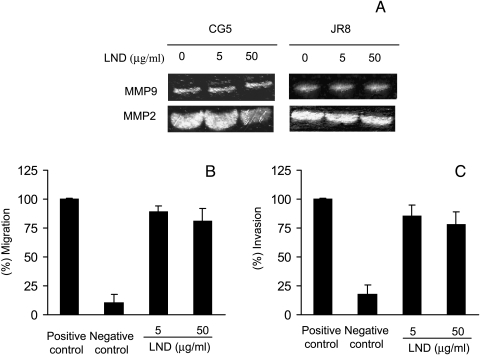
Zymographic analysis of MMP2 and MMP9 was performed in CM of two tumor cell lines with different histotypes: CG5 human breast carcinoma and JR8 human melanoma, untreated or treated with LND for 48 hours (Figure 7A). Low levels of secreted MMP9 and high levels of secreted MMP2 were found in the two untreated tumor cell lines (Figure 7A). No reduction of MMP2 and MMP9 production was observed in either cell lines treated with 5 µg/ml LND, whereas a slight decrease in MMP2 was observed after exposure to 50 µg/ml LND. Western blot analysis of MMP2 protein expression confirmed zymographic results (data not shown).
Figure 7.
LND does not inhibit metalloproteases secretion, migration, and invasion of tumor cells. CM of tumor cells incubated for 48 hours in SFM in the absence or presence of LND at doses of 5 and 50 g/ml were collected and analyzed for MMP9 and MMP2 gelatin zymography (A) at an equivalent number of viable cells. Representative experiments out of two are reported. Migration (B) and invasion (C) of untreated or LND-treated JR8 melanoma cells in response to NIH 3T3 CM. The migrated or invaded cells were counted in at least four HPFs (x200). Each value is the mean of quadruplicates ±SD, and the data are representative of at least three separate experiments.
Migration and invasion of tumor cells in response to different doses of LND (5 and 50 µg/ml) were also evaluated in JR8 human melanoma. As shown in Figure 7, no significant differences in migration (Figure 7B) and invasion (Figure 7C) were observed between untreated and LNDtreated tumor cells.
Discussion
The study described here demonstrated, for the first time, that LND inhibits endothelial cell functions involved in angiogenesis, and vessel formation in an in vivo matrigel assay. We also found that LND does not affect in vitro cell proliferation, migration, invasion, and matrix metalloproteinases production of different tumor lines. Using several endothelial cell lines of different origin, we found that LND inhibits the angiogenic process through effects on both induction and resolution phases of the angiogenic cascade. In the induction phase of angiogenesis, LND inhibited in vitro proliferation, migration, and invasion, whereas in the resolution phase, LND inhibited endothelial cell morphogenesis. The ability of LND to decrease MMP2 and MMP9 secretion by endothelial cells was also demonstrated, indicating that LND causes a shift toward antiproteolysis in these cells. These results are in agreement with observations that matrix metalloproteinases play a key role in angiogenesis, and positive proteolytic balance is required for capillary sprout elongation and lumen formation during angiogenesis [31–35]. In particular, it was found that MMP2 modulates the morphology of endothelial structures, as well as their invasive behavior in in vitro and in vivo assay systems [32], and that MMP9 contributes to angiogenesis and growth of human tumors [35]. The in vitro activity of LND across all phases of the angiogenic process was confirmed by an in vivo angiogenic assay. LND significantly reduces vessel formation and inhibits the propensity of vessels to grow toward the matrigel plug. All these effects were inhibited in a dose-dependent manner. Inhibition of angiogenesis was already observed at low doses of LND, and increased at higher doses. The antiangiogenic activity of LND we observed in vitro and in vivo does not exclude an eventual antivascular property of LND. Antiangiogenic activity and vascular damage have been demonstrated for both old antineoplastic drugs (such as doxorubicin) and new drugs (such as selective cytokine-inhibitory drugs) [36,37]. Further in vivo studies on tumor-bearing animals might evidence whether in vivo LND treatment affects already existing tumor vasculature, leading to damage to tumor blood vessel structure or function such as vascular shutdown, tumor blood flow reduction, and loss of immature tumor vessels.
The results obtained using annexin V assay after LND treatment of endothelial cells show that LND does not induce apoptosis at the lowest doses used (1–10 µg/ml) but produces a cytotoxic effect at the highest dose employed (50 µg/ml), thus indicating that the cytotoxic effect produced by the high doses of LND on proliferating endothelial cells could be due, at least in part, to an induction of apoptosis. Likewise, several endogenous and exogenous angiogenesis inhibitors have been demonstrated to induce endothelial cell apoptosis, which in turn may be responsible for inhibiting angiogenesis [38].
Experiments are in progress to explain how LND affects the proliferation, invasion, migration, and matrix metalloproteinase production in endothelial cells. Because one of the major metabolic changes induced by LND is inhibition of lactate transport and its accumulation, which leads to intracellular acidification, we can hypothesize that LND-induced acidification can be responsible for LND effect on endothelial cell functions [8,12]. In fact, low pH has been suggested to play a role in the relative specificity, efficacy, and mechanism of action of the antiangiogenic factor, angiostatin [39]. In addition, the mitochondrial membrane permeabilization and dysfunction in energy metabolism induced by LND may mediate its downregulation of endothelial cell functions [9,10,15]. The inactivation of adenine nucleotide translocase, a target of LND, has been found to inhibit angiogenesis and proliferating—but not growth-quiescent—endothelial cells [40]. Finally, because LND has been demonstrated to increase cytosolic Ca2+ [41], and because intracellular Ca2+ signaling may initiate or mediate some of the cellular actions of endostatin and angiostatin, Ca2+ modulation by LND may represent another mechanism through which LND exerts its effects on endothelial cell functions [42]. It should be also considered that the effects of LND in terms of cell growth inhibition or induction of apoptosis may not be directly mediated by the drug, but may be a secondary event induced by the drug such as modulation of the expression of genes or proteins involved in proliferation or apoptosis.
We have previously demonstrated that LND induces apoptosis in breast cancer cells at the dose of 50 µg/ml [19,20], and other authors found that LND triggers apoptosis in leukemic cells at the dose of 65 µg/ml [14]. Thus, the effect of different doses of LND was tested on tumor cell proliferation, migration, invasion, and apoptosis. We found that low doses of LND (5 µg/ml) were not able to affect proliferation, invasion, migration, and metalloproteases secretion, or to induce apoptosis of human tumor cell lines (melanoma, glioblastoma, breast, and lung). Thus, the inhibition of endothelial cell functions essential for angiogenesis, by low doses of LND, was not associated with cytotoxicity or induction of endothelial or tumor cell death. This characteristic is an important consideration for the possible therapeutic usefulness of the drug.
In conclusion, our results provide a novel mechanism of action for LND and indicate that LND can be used to treat a wide spectrum of angiogenesis-related neoplasms. The observation that the effect of low doses of LND is restricted to endothelial cells suggests the use of LND at “metronomic” dosing [43] or “antiangiogenic chemotherapy” [44]. Due to the low rate of endothelial cell division compared with tumor cells, and because the damage to the tumor's vasculature can be largely repaired during the long breaks between successive cycles of chemotherapy, standard chemotherapeutic protocols are weakly effective on endothelial cells. Shortening the time between cycles minimizes the efficacy of the repair process, requiring the use of lower doses of drugs. Thus, several studies suggest a potential complementary strategy for rescheduling the administration of classic cytotoxic drugs to target tumor vasculature [44,45]. Most drugs, belonging to virtually every class of anticancer chemotherapeutic agents, showed more efficacy when the regimen was switched to a lower-dose, more frequent schedule [43–49], and the toxicity and rapid development of drug resistance were not observed with this type of treatment [44].
On the contrary, high doses of LND that correspond to the dose employed for clinical management of tumor target multiple cell types of tumors and endothelial cells, not only through inhibition of proliferation but also through induction of apoptosis, thus indicating that the previously undescribed antiangiogenic effect may be a significant contributor to the antitumor effect of LND.
Acknowledgements
We are grateful to Adele Petricca for secretarial assistance in the preparation of the manuscript, to Claudia Travaglini for technical assistance, and to Paula Franke for revising the English language.
Abbreviations
- LND
lonidamine
- MMP2
matrix metalloproteinase-2
- MMP9
matrix metalloproteinase-9
- CM
conditioned media
- HUVEC
human umbilical endothelial cell
- HMVEC
human microvascular endothelial cell
- BAEC
bovine aortic endothelial cell
- FCS
fetal calf serum
- PBS
phosphate-buffered saline
- SFM
serum-free medium
- FITC
fluorescein isothiocyanate
- OD
optical density
- BSA
bovine serum albumin
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- Hb
hemoglobin
Footnotes
This work was supported by the Italian Association for Cancer Research (D.D.B.), Ministero della Salute (D.D.B.), and CNR-MIUR (G.Z.). Angela Iervolino and Daniela Trisciuoglio are the recipients of fellowships from the Italian Foundation for Cancer Research (FIRC).
References
- 1.Ianniello GP, De Cataldis G, Comella P, Scarpati MD, Maiorino A, Brancaccio L, Cioffi R, Lombardi A, Carnicelli P, Tinessa V. Cisplatin, epirubicin, and vindesine with or without lonidamine in the treatment of inoperable nonsmall lung carcinoma: a multicenter randomized clinical trial. Cancer. 1996;78:63–69. doi: 10.1002/(SICI)1097-0142(19960701)78:1<63::AID-CNCR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.De Lena M, Lorusso V, Bottalico C, Brandi M, De Mitrio A, Catino A, Guida M, Latorre A, Leone B, Vallejo C, Gargano G. Revertant and potentiating activity of lonidamine in patients with ovarian cancer previously treated with platinum. J Clin Oncol. 1997;15:3208–3213. doi: 10.1200/JCO.1997.15.10.3208. [DOI] [PubMed] [Google Scholar]
- 3.Nisticò C, Garufi C, Milella M, D'Ottavio AM, Vaccaio A, Fabi A, Terzoli E. Weekly epirubicin plus lonidamine in advanced breast carcinoma. Breast Cancer Res Treat. 1999;56:233–237. doi: 10.1023/a:1006213815195. [DOI] [PubMed] [Google Scholar]
- 4.De Lena M, Lo Russo V, Latorre A, Canizza G, Gargano G, Caporosso L, Guida M, Catino A, Crucitta E, Sambiasi D, Mazzei A. Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37:364–368. doi: 10.1016/s0959-8049(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 5.Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today. 2003;39:157–174. doi: 10.1358/dot.2003.39.3.799451. [DOI] [PubMed] [Google Scholar]
- 6.Oudard S, Carpentier A, Banu E, Fauchon F, Celerier D, Poupon MF, Dutrillaux B, Andrieu JM, Delattre JY. Phase II study of lonidamine and diazepam in the treatment of recurrent glioblastoma multiforme. J Neuro-Oncol. 2003;63:81–86. doi: 10.1023/a:1023756707900. [DOI] [PubMed] [Google Scholar]
- 7.Papaldo P, Lopez M, Cortesi E, Cammilluzzi E, Antimi M, Terzoli E, Lepidini G, Vici P, Barone C, Ferretti G, Di Cosimo S, Nistico C, Carlini P, Conti F, Di Lauro L, Botti C, Vitucci C, Fabi A, Giannarelli D, Marolla P. Addition of either lonidamine or granulocyte colonystimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol. 2003;21:3462–3468. doi: 10.1200/JCO.2003.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Floridi A, Paggi MG, Marcante ML, Silvestrini B, Caputo A, De Martino C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J Natl Cancer Inst. 1981;66:497–499. [PubMed] [Google Scholar]
- 9.Ravagnan L, Marzo I, Costantini P, Susin SA, Zamzami N, Petit PX, Hirsch F, Goulbern M, Poupon MF, Miccoli L, Xie Z, Reed JC, Kroemer G. Lonidamine triggers apoptosis via a direct, Bcl-2 - inhibited effect on the mitochondrial permeability transition pore. Oncogene. 1999;18:2537–2546. doi: 10.1038/sj.onc.1202625. [DOI] [PubMed] [Google Scholar]
- 10.Belzacq A, El Hamel C, Vieira HLA, Cohen I, Haouzi D, Metivier D, Marchetti P, Brenner C, Kroemer G. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, aresen and CD437. Oncogene. 2001;20:7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- 11.Gadducci A, Brunetti I, Muttini MP, Fanucchi A, Dargenio F, Giannessi PG, Conte PF. Epidoxorubicin and lonidamine in refractory or recurrent epithelial ovarian cancer. Eur J Cancer. 1994;30A:1432–1435. doi: 10.1016/0959-8049(94)00231-s. [DOI] [PubMed] [Google Scholar]
- 12.Mardo Y, Kaplan O, Sterin M, Ruiz-Cabello J, Ash E, Roth Y, Ringel I, Cohen JS. Noninvasive real-time monitoring of intracellular cancer cell metabolism and response to lonidamine treatment using diffusion weighted proton magnetic resonance spectroscopy. Cancer Res. 2000;60:5179–5186. [PubMed] [Google Scholar]
- 13.De Cesare M, Pratesi G, Giusti A, Polizzi D. Stimulation of the apoptotic response as a basis for the therapeutic synergism of lonidamine and cisplatin in combination in human tumour xenografts. Br J Cancer. 1998;77:434–439. doi: 10.1038/bjc.1998.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sordet O, Rébe C, Leroy I, Bruey JM, Garrido C, Miguet C, Lizard G, Plenchette S, Corcos L, Solary E. Mitochondria-targeting drugs arsenic trioxide and lonidamine bypass the resistance of TPAdifferentiated leukemic cells to apoptosis. Blood. 2001;97:3931–3940. doi: 10.1182/blood.v97.12.3931. [DOI] [PubMed] [Google Scholar]
- 15.Miccoli L, Poirson-Bichat F, Sureau F, Bras Goncalves R, Bourgeois Y, Dutrillaux B, Poupon MF, Oudard S. Potentiation of lonidamine and diazepam, two agents acting on mitochondria, in human glioblastoma treatment. J Natl Cancer Inst. 1998;90:1400–1406. doi: 10.1093/jnci/90.18.1400. [DOI] [PubMed] [Google Scholar]
- 16.Citro G, Cucco C, Verdina A, Zupi G. Reversal of ADR resistance by lonidamine in a human breast cancer cell line. Br J Cancer. 1991;64:534–536. doi: 10.1038/bjc.1991.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YC, Fung KP, Kwok TT, Lee CY, Suen YK, Kong SK. Mitochondrial targeting drug lonidamine triggered apoptosis in doxorubicin-resistant HepG2 cells. Life Sci. 2002;71:2729–2740. doi: 10.1016/s0024-3205(02)02103-3. [DOI] [PubMed] [Google Scholar]
- 18.Ricotti L, Tesei A, De Paola F, Milandri C, Amadori D, Frassineti GL, Ulivi P, Zoli W. Potentiation of antiproliferative drug activity by lonidamine in hepatocellular carcinoma cells. J Chemother. 2003;15:480–487. doi: 10.1179/joc.2003.15.5.480. [DOI] [PubMed] [Google Scholar]
- 19.Del Bufalo D, Biroccio A, Soddu S, Laudonio N, D'Angelo C, Sacchi A, Zupi G. Lonidamine induces apoptosis in drug-resistant cells independently of the p53 gene. J Clin Invest. 1996;98:1165–1173. doi: 10.1172/JCI118900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biroccio A, Del Bufalo D, Fanciulli M, Bruno T, Zupi G, Floridi A. Bcl-2 inhibits mitochondrial metabolism and lonidamine-induced apoptosis in adriamycin-resistant MCF7 cells. Int J Cancer. 1999;82:125–130. doi: 10.1002/(sici)1097-0215(19990702)82:1<125::aid-ijc21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Solary E, Bettaieb A, Dubrez-Daloz L, Corcos L. Mitochondria as a target for inducing death of malignant hematopoietic cells. Leuk Lymphoma. 2003;44:563–574. doi: 10.1080/1042819021000038001. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Clinical application of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 23.Pepper MS. Manipulating angiogenesis: from basic science to the bedside. Arterioscler Thromb Vasc Biol. 1997;17:605–619. doi: 10.1161/01.atv.17.4.605. [DOI] [PubMed] [Google Scholar]
- 24.Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003;4:141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 25.Logan TF, Jadali F, Egorin MJ, Mintun M, Sashin D, Gooding WE, Choi Y, Bishop H, Trump DL, Gardner D, Kirkwood J, Vlock D, Johnson C. Decreased tumor blood flow as measured by positron emission tomography in cancer patients treated with interleukin-1 and carboplatin on a phase I trial. Cancer Chemother Pharmacol. 2002;50:433–444. doi: 10.1007/s00280-002-0517-4. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, Giantonio B, Zimmer R, Petros WP, Stratford M, Chaplin D, Young SL, Schnall M, O'Dwyer PJ. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21:4428–4438. doi: 10.1200/JCO.2003.12.986. [DOI] [PubMed] [Google Scholar]
- 27.Iervolino A, Trisciuoglio D, Ribatti D, Candiloro A, Biroccio A, Zupi G, Del Bufalo D. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1 - mediated transcriptional activity. FASEB J. 2002;16:1453–1455. doi: 10.1096/fj.02-0122fje. [DOI] [PubMed] [Google Scholar]
- 28.Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosanò L, Trisciuoglio D, Spinella D, Bagnato A. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol. 2002;61:524–532. doi: 10.1124/mol.61.3.524. [DOI] [PubMed] [Google Scholar]
- 29.Del Bufalo D, Biroccio A, Leonetti C, Zupi G. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 1997;11:947–953. doi: 10.1096/fasebj.11.12.9337147. [DOI] [PubMed] [Google Scholar]
- 30.Biroccio A, Candiloro A, Mottolese M, Sapora O, Albini A, Zupi G, Del Bufalo D. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vitro angiogenesis in a breast carcinoma line. FASEB J. 2000;14:652–660. doi: 10.1096/fasebj.14.5.652. [DOI] [PubMed] [Google Scholar]
- 31.Stetler-Stevenson WG. Matrix metalloproteinases in angiogen angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gee MS, Makonnen S, al-Kofahi K, Roysam B, Payvandi F, Man HW, Muller GW, Lee WM. Selective cytokine inhibitory drugs with enhanced antiangiogenic activity control tumor growth through vascular inhibition. Cancer Res. 2003;63:8073–8078. [PubMed] [Google Scholar]
- 33.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel - targeted liposomal chemotherapy. Cancer Res. 2003;63:7400–7409. [PubMed] [Google Scholar]
- 34.Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- 35.Chung AS, Yoon SO, Park SJ, Yun CH. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36:128–137. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 36.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D'Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993;156:235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- 37.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler I. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 38.Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota SJ, Borlat F, Sim BK, Wu Z, Grau GE, Shing Y, Soff GA, Bouck N, Pepper MS. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- 39.Wahl ML, Owen CS, Grant DS. Angiostatin induces intracellular acidosis and anoikis in endothelial cells at a tumor-like low pH. Endothelium. 2002;9:205–216. doi: 10.1080/10623320213633. [DOI] [PubMed] [Google Scholar]
- 40.Don AS, Kisker O, Dilda P, Donoghue N, Zhao X, Decollogne S, Creighton B, Flynn E, Folkman J, Hogg PJ. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell. 2003;3:497–509. doi: 10.1016/s1535-6108(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 41.Castiglione S, Kennedy KA, Floridi A, Fiskum G. Non-ionophoretic elevation of intracellular Ca2+ by Lonidamine. Biochem Pharmacol. 1993;20:330–332. doi: 10.1016/0006-2952(93)90423-t. [DOI] [PubMed] [Google Scholar]
- 42.Lianwei J, Vivekanand J, Mohanraj D, Sukhatme VP, Alper SL. Intracellular Ca2+ signaling in endothelial cells by the angiogenesis inhibitors endostatin and angiostatin. Am J Physiol Cell Physiol. 2001;280:C1140–C1150. doi: 10.1152/ajpcell.2001.280.5.C1140. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 45.Vacca A, Iurlaro M, Ribatti D, Minischetti M, Nico B, Ria R, Pellegrino A, Dammacco F. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1999;94:4143–4155. [PubMed] [Google Scholar]
- 46.Miller KD, Sweeney CJ, Sledge GW. Redefining the target: chemotherapeutics as antiangiogenics. Clin J Oncol. 2001;19:1195–1206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- 47.Bocci G, Nocolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 48.Gately S, Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001;7:427–436. [PubMed] [Google Scholar]
- 49.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel KS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]