Abstract
Direct tumor injections of oligonucleotides containing unmethylated CpG motifs (CpG ODN) into murine colon tumor 26 (CT-26) tumors can induce a potent antitumor response. Tumor size at the beginning of treatment determines the final therapeutic outcome, with smaller tumors responding favorably to CpG ODN therapy whereas large tumors do not. CpG ODN injections in small tumors resulted in tumor necrosis and extensive inflammatory cell infiltration, with average survival that is significantly higher (48.1 ± 34 days) when compared to control ODN-treated mice (16.1 ± 3.5 days). Cytokines and chemokines are expressed at different levels in small and large CT-26 tumors following intratumoral injections of CpG ODN. We observed that granulocyte-macrophage colony-stimulating factor and interleukin (IL) 6 are the major cytokines that were overexpressed in CpG ODN-treated small tumors but not in large tumors. Similarly, several chemokines (CXCL1, CCL2, and CCL3) were also significantly higher in CpG ODN-treated small tumors compared to control ODN-treated tumors.
Keywords: Colon tumor 26, CpG ODN, intratumoral injection, GM-CSF, IL-6
Introduction
The stimulation of the immune system, which can overcome tumor-mediated immune suppression, may be an effective therapy against cancer. Oligonucleotides containing unmethylated CpG motifs (CpG ODN) stimulate vertebrate immune cells in vitro and clinical trials are currently underway for evaluation of CpG therapies for cancer, allergy, and infectious disease [1]. CpG ODNs are recognized by pattern recognition receptors, a family of receptors known as Toll-like receptors (TLRs) [2]. The CpG ODNs are endocytosed and there they meet their pattern recognition receptor, TLR-9, which is an intracellular receptor [3]. Upon recognition of CpG, TLR-9 recruits the adaptor molecule, myeloid differentiation factor 88 (MyD88), through a common pathway of Toll with interleukin (IL) 1R domains. Subsequent downstream signaling involves IL-1R-associated kinases (IRAK1) and tumor necrosis factor α (TNF-α) receptor-associated factor 6 (TRAF6). Further activation of NF-kB and c-Jun NH2 terminal kinase (JNK) results in transcription from various cytokines and chemokines genes [4]. TLR-9 field is relatively young and, so far, a limited number of differences have been noted between mice and humans. In mice, TLR-9 is expressed on all myeloid cells, dendritic cells, and B cells, whereas in humans, TLR-9 is only expressed on antigen-presenting cells, B cells, and neutrophils [5].
We had hypothesized that manipulation of the tumor microenvironment by introducing immune-stimulating CpG ODNs directly into the tumors might result in an antitumor immune response. This therapy is a form of immunization that uses the in situ tumor as a source of antigen and introduces CpG ODN as an adjuvant to activate an immune response within the tumor. We refer to this as in situ immunization and it has been shown in animal models that in situ immunization with biologic response modifiers (BRMs) like granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-12 [6] or IL-2 [7] can induce tumor regression and stimulate systemic immunity. Clinical studies from our group have also shown that intra-tumoral injections of GM-CSF lead to tumor regression [8]. There are already reports in the literature of CpG stimulating an antitumor response in experimental models [9,10]. We have shown that directly injecting CpG into the tumor results in an antitumor response [11], whereas it was reported that peritumoral injections of CpG ODN were therapeutic for small tumors but not for larger tumors [12].
There is a dynamic relationship between tumor cells, inflammatory cells, cytokines, and chemokines [13]. Cytokines and chemokines within tumors can contribute to the progression of tumors to a more aggressive metastatic phenotype or play a role in tumor therapy [14,15]. The pattern and kinetics of cytokine/chemokine production can be greatly affected by CpG ODNs. The splenic mRNA levels of IL-6, IL-12, and IL-1β, and of serum IL-6, IL-12, MIP-1β, and MCP-1 levels increase significantly following intraperitoneal (i.p.) injections of CpG ODN [16], although intramuscular injection of CpG induced the expression of genes coding for chemokines in myocytes [17]. In vitro studies also demonstrate that macrophage exposed to CpG ODN upregulates expression of mRNA encoding the chemokines MIP-1α, MIP-1β, MIP-2, RANTES, MCP-1, and IP-10 [18]. We hypothesize that CpG ODN injection into tumors might change the patterns of cytokines and chemokines within the tumor microenvironment, contributing to an antitumor response.
It is argued that cancer therapies might only be effective against a low tumor burden. Tumor size determines the antitumor activity of cisplatin or 5-fluorouracil, with decreased antitumor activity against large CT-26 tumors [19]. Larger CT-26 tumors were also resistant to peritumoral injections of CpG ODN [12]. Similarly, intratumor injections of IL-2 in murine mesothelioma were therapeutic in small tumors but not in larger ones [7]. The present study investigates whether the antitumor effects of intratumoral injections of CpG ODN were affected by tumor size. This study also provides quantitative changes in cytokine and chemokine within the tumor microenvironment and in the serum following direct tumor injection of CpG ODN.
Materials and Methods
Mice and Tumor Cell Lines
Female Balb/c mice, 4 to 6 weeks old, were used in the study. All mice were kept according to IACUC guidelines at the animal facility of SUNY at Buffalo. The mice were checked daily and tumors measured three times a week. Colon tumor 26 (CT-26), a colon carcinoma tumor, and Line-1, an adenocarcinoma of Balb/c origin, were used for the present study. CT-26 and Line-1 tumor cell lines were obtained from ATCC (Manassas, VA) and were maintained in RPMI 1640 media supplemented with 10% fetal calf serum, antibiotic-antimycotic solution, and mercaptoethanol at 37°C and 5% CO2 (vol/vol). For implantation of tumor cells, adherent tumor cells were trypsinized and resuspended in phosphate-buffered saline (PBS), and 5 x 105 cells were implanted into the subcutaneous sites in the right flank of the mice. For the study of memory immune response, 5 x 105 CT-26 tumor or Line-1 adenocarcinoma cells were injected on the left flank of the animal.
Reagents
CpG ODNs were a gift from Coley Pharmaceuticals (Wellesley, MA). Immunostimulatory sequence of CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) and control nonstimulatory sequences ODN 1982 (5′-TCCAGGACTTCTCTCAGGTT-3′) were used in the present study. CpG ODNs were phosphorothioate-modified and endotoxin-free.
Animal Experiments
Groups of mice were implanted with CT-26 tumor cells. Tumors were allowed to grow until appropriate tumor size was reached. The tumor size varied from 10 to 32 mm3 for small tumors and from 120 to 450 mm3 for large tumors. Treatment consisted of daily intratumoral injections of a 10-µg dose of CpG ODN or control ODN in 0.1 ml of sterile PBS. Tumor size was measured three times a week with a Vernier caliper and tumor volume [(short arm)2 x long arm/2] was calculated in cubic millimeters. The formula: tumor growth rate = [(final tumor size - initial tumor size)/number of days] was applied to determine tumor growth kinetics. Data are reported in the form of either percent survival or tumor growth rate.
Histology of Tumors Treated with CpG ODN or Control ODN
Small and large tumors were treated with CpG ODN or control ODN for 5 days followed by surgical excision of tumor. Tumors were fixed in 10% buffered formalin and embedded in paraffin blocks for serial sectioning. Tumor sections were stained with hematoxylin-eosin and examined for changes in histology.
Cytokines and Chemokines Quantification in Tumor Microenvironment or in Serum
The tumor was surgically removed and a tumor extract was prepared in T-per extraction buffer (Pierce, Rockland, IL) supplemented with protease inhibitor cocktail (Pierce). Ten milliliters of buffer was used per gram of tumor sample. Supernatant was kept frozen at -80°C until analyzed. Blood was taken from the animal and serum sample was kept in -80°C until analyzed. Levels of cytokines and chemokines in tumor extract and in serum were analyzed by the Luminex-100 flow cytometry assay. A total of 15 cytokines/chemokines was monitored, namely, IL-1b, IL-2, IL-3, IL-4, IL-6, IL-10, IL-12, G-CSF, GM-CSF, IFN-γ, TNF-α, MCP-1 (CCL2), MIP-1α (CCL3), KC (CXCL1), and RANTES (CCL5).
Statistics
All experiments were done in, at least, triplicate and statistical significance of data was determined using a two-tailed Student's t test.
Results
Tumor Size at the Beginning of Treatment Determines the Final Therapeutic Outcome
We have earlier shown that direct tumor injections of low doses of CpG can result in slower tumor growth of established B-16 melanoma and CT-26 murine tumors [11]. It was also reported that peritumoral injection of CpG ODN was found to be effective against small tumors but not against larger tumors [12]. The aim of the present experiment was to determine if antitumor effects of intratumoral injections of CpG ODN were influenced by the tumor size. Animals were divided into two groups depending on tumor size. Mice with small tumors (average tumor size 15–20 mm3) were treated with daily intratumoral injections of either CpG ODN or control ODN. In the other group, treatment began when the tumor size was 125 to 140 mm3. The dose of CpG ODN (10 µg per injection) used in the study was chosen as our results indicated no difference in the therapeutic response at lower doses of CpG ODN (10–20 µg per injection), whereas at higher doses (50 µg per injection or higher), overt signs of toxicity were observed, which includes lethargy, weight loss, and rough coat. Treatment continued until the tumor was completely regressed or tumor burden became greater than 1500 mm3. Mice whose tumors grew slowly were followed for 90 days for calculations of mean survival and tumor growth rate. Mice whose tumors regressed were followed beyond 90 days and were used to determine immune memory.
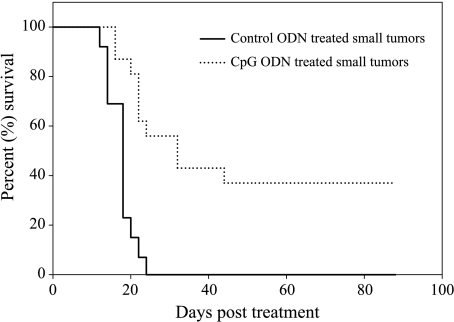
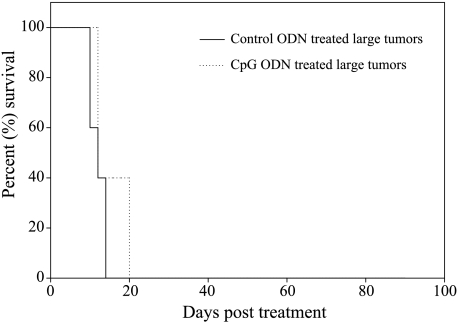
In the group of CpG ODN-treated mice with small tumors (n = 16), the average survival was significantly higher (48.1 ± 34 days) when compared to 16.1 ± 3.5 days for mice with small tumors treated with control ODN (n = 13, P < .001) (Figure 1). The average survival of mice with small tumors treated with CpG ODN was also significantly higher than the average survival of 13.8 ± 4.7 days for large tumor-bearing mice (n = 5) treated with CpG ODN (P < .001). We saw no therapeutic effect of CpG ODN injections into large tumors (Figure 2). This clearly demonstrates that tumor size at the beginning of CpG ODN immune therapy can determine the final therapeutic outcome, with smaller tumors responding favorably to CpG ODN therapy whereas large tumors do not.
Figure 1.
Mice with small CT-26 tumors were treated with daily intratumoral injections of either CpG ODN or control ODN. Figure shows the survival of mice following treatment. Longer survival and tumor regression were seen in small tumors treated with intratumor CpG ODN therapy.
Figure 2.
Mice with large CT-26 tumors were treated with daily intratumoral injections of either CpG ODN or control ODN. Figure shows the survival of mice following treatment. Survival was not significantly different in CpG ODN- or control ODN-treated groups. This shows that CpG ODN treatment had no therapeutic effect in large tumors.
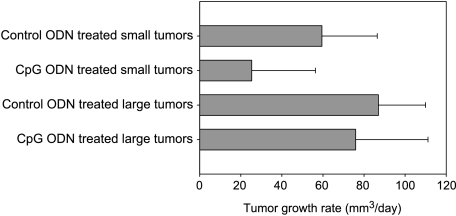
Daily CpG ODN injections resulted in complete tumor regression in 37% (6/16) of treated mice bearing small tumors. No tumor regression was observed in large tumors treated with CpG ODN, or in small or large tumors treated with control ODN. Similarly, the average tumor growth rate was significantly lower in CpG ODN-treated small tumors (25.34 ± 31 mm3/day) compared to 59.5 ± 26.9 mm3/day in control ODN-treated small tumors (P < .01). The average tumor growth rate in CpG ODN-treated large tumors was not significantly different from tumor growth rate in control ODN-treated large tumors (Figure 3). Our earlier results of direct tumor injections of PBS [11] were similar to results obtained with control ODN and served as an additional control (data not shown).
Figure 3.
Mice with small or large CT-26 tumors were treated with daily intratumoral injections of either control ODN or CpG ODN. Figure shows the average tumor growth rate (mm3/day) following treatment. Small tumors treated with CpG ODN show slower tumor growth compared to other groups, which is consistent with longer survival and tumor regression, whereas higher tumor growth rate in other groups is consistent with their therapeutic outcome of no tumor regression and shorter survival.
Histology
To investigate the changes induced by CpG ODN injections, a histologic analysis of tumors was done. Tumors were treated with either CpG ODN or control ODN for 5 days. Histologic evaluation of control ODN-treated tumors looked like untreated tumors: a solid carcinoma with a high mitotic index and no lymphocyte infiltration. The CpG ODN intratumor therapy in small tumors produced areas of extensive necrosis with lots of tumor-infiltrating lymphocytes and poly-morphonuclear cells, whereas no lymphocyte infiltration and necrosis were observed in CpG ODN-treated large tumors.
Treatment with CpG Induces a Memory Immune Response in CT-26 Tumor
To investigate whether CpG ODN-mediated tumor regression induced a memory immune response following complete tumor regression, long-term surviving animals that remained tumor-free for more than 4 months were implanted with a lethal dose (5 x 105) of CT-26 cells in the left flank. No tumor grew in any of the six long-term survivors, showing that a memory immune response can be induced by intratumoral injections of CpG ODN. To further determine the specificity of the memory response, long-term survivors who rejected the CT-26 rechallenge were subsequently injected with a lethal dose (5 x 105) of Line-1 adenocarcinoma. All six mice developed Line-1 adenocarcinoma tumors, indicating that the protective memory immune response resulting from the direct tumor injection of CpG ODN is tumor-specific.
Changes in the Expression of Cytokines and Chemokines in Mice with Small Tumors Following Intratumor Injections of Either CpG ODN or Control ODN
To further investigate differences between small and large tumors, we assayed soluble factors within the tumor microenvironment before and after CpG ODN intratumor therapy. A Luminex-100 bead assay was used to monitor the expression of 15 soluble cytokines and chemokines in the tumor microenvironment. Mice with small tumors (14–28 mm3) had their tumor injected with either CpG ODN or control ODN for 3 days followed by analysis of soluble factors within tumors and in serum.
Table 1 summarizes the significant differences in cytokine and chemokine levels in both the tumor microenvironment and in the serum of mice with small tumors. In CpG ODN-treated tumors, intratumor levels of IL-6 and GM-CSF were higher by 40-fold (P < .05) and 13-fold (P < .05), respectively. Although G-CSF increased by 11-fold, it was not significant due to a high standard deviation. Levels of IL-1β and INF-γ were slightly but not significantly elevated following intratumor injections of CpG ODN, whereas IL-2, IL-10, and TNF-α were slightly but not significantly lower. No change in the levels of expressions was observed for other cytokines.
Table 1.
Key Cytokine and Chemokine Changes (pg/ml) in the Tumor Microenvironment and in the Serum of Small Tumor-Bearing Mice Following Treatment with Either Control ODN or CpG ODN.
| Control Mice | CpG-Treated Mice | Fold Increase | P | ||
| IL-6 | Intratumor | 69 ± 46 | 2807 ± 1836 | 40 | 0.02 |
| Serum | 3 ± 3 | 24 ± 6 | 8 | 0.008 | |
| GM-CSF | Intratumor | 3.5 ± 0.57 | 46.75 ± 32.3 | 13 | 0.03 |
| Serum | 0 | 0 | nd | ||
| CXCL1 | Intratumor | 38 ± 31 | 3379 ± 1436 | 88 | 0.02 |
| Serum | 12 ± 10 | 86 ± 68 | 7 | 0.07 | |
| CCL2 | Intratumor | 560 ± 413 | 8962 ± 3344 | 16 | 0.002 |
| Serum | 71 ± 22 | 159 ± 38 | 2 | 0.009 | |
| CCL3 | Intratumor | 988 ± 114 | 2343 ± 974 | 2 | 0.03 |
| Serum | 543 ± 370 | 1050 ± 246 | 1.9 | 0.09 | |
| CCL5 | Intratumor | 917 ± 393 | 1709 ± 565 | 1.8 | 0.06 |
| Serum | 0 | 70 ± 8 | nd | ||
nd, not determined.
The CpG ODN injections in small tumors also resulted in significantly higher levels of several chemokines (Table 1). Intratumoral expression of CXCL1 (KC) was more than 88-fold higher in CpG ODN-treated tumors (P < .05). Levels of CCL2 (MCP-1) and CCL3 (MIP-1α) were higher by 16-fold (P < .05) and 2-fold (P < .05), respectively. The expression of CCL5 (RANTES) increased 1.8-fold and it was only marginally significant (P > .05).
Changes in the levels of cytokines and chemokines in the serum were also analyzed. Serum levels of IL-6, CXCL1, CCL2, and CCL5 were significantly higher after treatment with CpG ODN (Table 1). CCL5 was only found in the serum after CpG ODN treatment, whereas levels of IL-6 and CXCL1 showed an eight-fold (P < .01) and a seven-fold (P > .05) higher expression compared to mice in control ODN-treated group. IL-10 was present in the serum of some of the CpG ODN-treated mice but not in the serum of any control ODN-treated mice.
Changes in the Expression of Cytokines and Chemokines in Large Tumors After Intratumor Injections of CpG ODN or Control ODN
Mice with large tumors (270–455 mm3) had their tumor injected with either CpG ODN or control ODN for 3 days followed by analysis of cytokines and chemokines in the intratumor microenvironment and in the serum. In the tumor, CCL5 (RANTES) was the only factor that was significantly higher after CpG ODN treatment (Table 2). The intratumoral level of CCL5 was higher by three-fold (P < .05). Intratumoral levels of GM-CSF and G-CSF were more than 13-fold and 2-fold higher after CpG ODN treatment, but they were not significant due to high standard deviations. We also found marginally higher expressions of CCL2 (P > .05) and CCL3 (P > .05) in CpG ODN-treated mice. Unlike in small tumors, CpG ODN did not stimulate IL-6, GM-CSF, or CXCL1 expression. CCL5 was also present in serum of CpG ODN-treated mice, whereas no CCL5 could be detected in serum of mice treated with control ODN. No significant difference in the serum levels of other cytokines and chemokines was observed following intratumor injections of large tumors.
Table 2.
Key Cytokine and Chemokine Changes (pg/ml) in the Tumor Microenvironment and in the Serum of Large Tumor-Bearing Mice Following Treatment with Either Control ODN or CpG ODN.
| Control Mice | CpG-Treated Mice | Fold Increase | P | ||
| CCL5 | Intratumor | 723 ± 344 | 2361 ± 884 | 3 | 0.04 |
| Serum | 0 | 34 ± 6 | nd | ||
nd, not determined.
Discussion
Systemic administration of cytokines and BRMs such as IL-2, TNF-α, GM-CSF, IL-12, or CpG ODN can induce an antitumor response and activate protective immunity. However, systemic administrations of high doses of BRMs are often associated with toxic side effects, preventing the use of curable doses. One possible way of increasing the therapeutic index of BRMs is to use low-dose regional treatment, like peritumoral or intratumoral injection. Intratumoral injections of IL-2 [7] or IL-12 [20] can induce local and systemic antitumor immunity. Regional CpG injections are effective against mouse neuroblastoma [9] and intracranial gliomas [10]. We have also found that intratumoral injections of low doses of CpG ODN can result in a slower tumor growth rate of murine CT-26 and B-16 tumors [11].
Tumor burden or tumor size can be a factor in the outcome of a cancer immune therapy. A CpG ODN immune therapy was shown to be effective against a relatively small tumor, whereas larger CT-26 tumors were resistant [12]. IL-2 intratumor injections induced regression of small murine mesothelioma tumors, whereas large tumors failed to respond [7]. To determine if the antitumor effects of intratumoral injections of CpG ODNs were dependent on the tumor size, we compared intratumoral injections of CpG ODN in small versus large tumors. Injections of 10 µg of CpG ODN into small tumors significantly inhibited tumor growth, increased survival, and resulted in the complete tumor regression of established tumors in 37% of treated mice, whereas CpG ODN treatment of large tumors was completely ineffective.
The pattern and kinetics of cytokine production can be greatly affected by CpG ODNs. The antitumor effects of CpG ODN are likely to be mediated by a series of events. First, CpG ODN induces TLR-9-mediated release of local cytokines and chemokines, which might directly affect tumors cells and recruit inflammatory cells to the site of injection [18]. Intramuscular injection of CpG induced the expression of genes coding for chemokines and MHC class II molecule on myocytes [17], whereas in vitro studies also demonstrate that macrophage exposed to CpG ODN upregulates expression of mRNA encoding the chemokines MIP-1a, MIP-1b, MIP-2, RANTES, MCP-1, and IP-10 [18]. Consistent with the induction of an inflammatory response after CpG ODN treatment, we observed that small tumors become infiltrated with neutrophils. The continued injections of CpG ODN following infiltration of inflammatory cells into the tumor can further change the cytokine profile in the tumor microenvironment as infiltrating cells respond to the CpG. Although there are some in vitro studies showing secretion of certain cytokines and chemokines following CpG ODN exposure [16], no quantitative in vivo data were available for intratumor response.
We found that CpG ODN injections resulted in significant increases in the intratumor levels of GM-CSF and IL-6 in small tumors, whereas injections into large tumors failed to produce any significant changes in the cytokine expressions. GM-CSF levels were significantly higher within small tumors and in serum following CpG ODN treatment. There are several ways that GM-CSF might induce an antitumor response: it stimulates the tumor-lytic activity of cells of the monocyte lineage [21,22], and it also attracts and activates dendritic cells, which can increase the presentation of tumor antigens [23]. In addition, receptors for GM-CSF are also found on endothelial cells, stromal cells, fibroblasts, and tumor cells, so GM-CSF might directly affect nonlymphoid cells within the tumor.
IL-6 levels were also significantly higher in both tumors and serum after CpG ODN injections into small tumors. CpG ODN injection into small tumors results in a 40-fold increase in the intratumor levels of IL-6. IL-6 is also a pleiotropic cytokine and it can influence antigen-specific immune responses and the inflammatory reaction. The transfection of murine tumor cells with a functional IL-6 gene has been shown to lead to the rejection of the genetically modified cells by syngeneic hosts with the antitumor effects of IL-6 dependent on the presence of GM-CSF [24]. Cross-linking of Toll-like receptors can result in the secretion of IL-6, which can block the immune-suppressive effects of regulatory CD4+ CD25+ T cells, allowing activation of the adaptive immune response [25]. The induction of this cytokine, along with GMCSF, creates a permissive cytokine environment for immune activation.
Chemokines can modulate tumor growth by three important mechanisms: regulation of tumor-associated angiogenesis, activation of host antitumor response, and direct stimulation of tumor cell proliferation [26]. Expressions of several chemokines were significantly higher in small tumors following intratumor injections of CpG ODN. Levels of CCL2 (MCP-1), CCL3 (MIP-1α), and CXCL1 (KC) significantly increased, whereas no significant changes were observed in large tumors. CXCL1 levels showed the greatest change, increasing by more than 88-fold within CpG ODN-treated small tumors compared to only a two-fold increase in large tumors. Monocytes, macrophages, and vascular endothelial cells can produce CXCL1, and are involved in neutrophil chemotaxis and activation. In a classic microbial infection, neutrophils are the first immune cells to arrive at the site of infection where they quickly release antimicrobial products and proinflammatory cytokines. CpG ODN injections mimic microbial infections in that the unmethylated CpG ODN can act like the unmethylated CpG motifs in prokaryotic DNA. Consistent with increased level of CXCL1, the predominant tumor-infiltrating cells we see early in the CpG ODN treatment of small tumors are neutrophils. Neutrophils express TLR-9, which is the pattern recognition receptor for CpG motifs [27], and cross-linking TLR-9 on neutrophils might stimulate another cascade of cytokine and chemokine expression.
CCL2 was also significantly higher in CpG ODN-treated small tumors. CCL2 specifically attracts monocytes, memory T cells, and NK cells in vitro and activates tumoricidal activities of macrophage in vivo. Although we did not see significant numbers of these cells infiltrating tumors after 5 days of CpG ODN injections, later time points are being assayed to determine whether CCL2 expression will eventually recruit these cells.
Because small tumors respond to CpG intratumor therapy whereas larger tumors do not, this might be a clinically relevant model as some patients' tumors respond to immune therapies whereas others do not. It would be interesting to know if the cytokines and chemokines that are induced in small, regressing tumors are also expressed in regressing tumors in other animal models of cancer therapy. Additionally, are these patterns of soluble factor expression also shown in regressing human tumors during immune therapy? If so, they may represent new therapeutic targets.
In conclusion, we have found that CpG ODN had significant antitumor and systemic immune stimulatory properties including tumor-specific protective memory immune responses, but there may be limitations on the therapy depending on tumor size or tumor burden. Although the exact molecular mechanisms of the antitumor effects of CpG ODN are still not known, data from our present study demonstrate that overexpression of chemokines, notably CXCL1 and CCL2, and cytokines GM-CSF and IL-6, coupled with neutrophils infiltration, might be involved in CpG ODN-mediated tumor regression. Further studies are currently underway to correlate the temporal appearance of soluble factors with changes in the tumor histology and the appearance and activation of tumor-infiltrating inflammatory cells.
Acknowledgements
We thank Arthur Krieg (Coley Pharmaceuticals) for his generous gift of reagents, and Sibel McGee and Shelley Isaacs for their help in laboratory work.
Footnotes
We thank KST Oncology for financial support, and the Kaleida Foundation and the Margaret Duffy and Cameron Troup Foundation for research grants.
References
- 1.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM. Anti-tumor applications of stimulating Toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 3.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 4.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo S, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 5.Hughes CCW, Mestas J. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 6.Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB. In situ tumor vaccination with interleukin-12 encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60:3832–3837. [PubMed] [Google Scholar]
- 7.Jackaman C, Bundell CS, Kinnear BF, Smith AS, Filion P, Hagen DV, Robinson BWS, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 8.Brooks SP, Takita H, Fang Y, Vaughan M, Sharma S, Karakousis CP. Intra-tumoral injection of GM-CSF in perspective. J Med. 2004 (in press) [PubMed] [Google Scholar]
- 9.Carpentier AF, Chen L, Maltoni F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–5432. [PubMed] [Google Scholar]
- 10.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 11.Sharma S, Karakousis CP, Takita H, Shin K, Brooks SP. Intra-tumoral injection of CpG results in the inhibition of tumor growth in murine colon-26 and B-16 tumors. Biotechnol Lett. 2003;25:149–153. doi: 10.1023/a:1021927621813. [DOI] [PubMed] [Google Scholar]
- 12.Heckelsmiller K, Beck S, Rall K, Sipos B, Schlamp A, Tuma E, Rothenfusser S, Endres S, Hartmann G. Combined dendritic cell- and CpG oligonucleotide-based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol. 2000;32:3235–3245. doi: 10.1002/1521-4141(200211)32:11<3235::AID-IMMU3235>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 14.Yamamoto T, Kimura T, Ueta E, Tatemoto Y, Osaki T. Characteristic cytokine generation patterns in cancer cells and infiltrating lymphocytes in oral squamous cell carcinomas and the influence of chemoradiation combined with immunotherapy on these patterns. Oncology. 2003;64:407–415. doi: 10.1159/000070300. [DOI] [PubMed] [Google Scholar]
- 15.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Temsamani J, Zhou R, Agarwal S. Pattern and kinetics of cytokine production following administration of phosphorothioate oligonucleotides in mice. Antisense Nucleic Acid Drug Dev. 1997;7:495–502. doi: 10.1089/oli.1.1997.7.495. [DOI] [PubMed] [Google Scholar]
- 17.Stan AC, Casares S, Brumeanu TD, Klinman DM, Bona CA. CpG motifs of DNA vaccines induce the expression of chemokines and MHC class II molecules on myocytes. Eur J Immunol. 2001;31:301–310. doi: 10.1002/1521-4141(200101)31:1<301::AID-IMMU301>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita S, Takeshita F, Haddad DE, Ishii KJ, Klinman DM. CpG oligonucleotides induce murine macrophage to up-regulate chemokine mRNA expression. Cell Immunol. 2000;206:101–116. doi: 10.1006/cimm.2000.1735. [DOI] [PubMed] [Google Scholar]
- 19.Van Laar JAM, Rustum YM, Vander Wilt CL, Smid K, Kuiper CM, Pinedo HM, Peters GJ. Tumor size and origin determine the antitumor activity of cisplatin or 5-fluorouracil and its modulation by leucovorin in murine colon carcinomas. Cancer Chemother Pharmacol. 1996;39:79–89. doi: 10.1007/s002800050541. [DOI] [PubMed] [Google Scholar]
- 20.Sabel MS, Hill H, Jong YS, Mathiowitz E, Bankert RB, Egilmez NK. Neoadjuvant therapy with interleukin-12-loaded polylactic acid microspheres reduces local recurrence and distant metastases. Surgery. 2001;130:470–478. doi: 10.1067/msy.2001.115839. [DOI] [PubMed] [Google Scholar]
- 21.Lonsdorf AS, Kuekrek H, Stern BV, Boehm BO, Lehmann PV, Tary-Lehmann M. Intratumor CpG-oligonucleotide injection induces protective antitumor T cell immunity. J Immunol. 2003;171:3941–3946. doi: 10.4049/jimmunol.171.8.3941. [DOI] [PubMed] [Google Scholar]
- 22.Hennemann B, Rehm A, Kottke A, Meidenbauer N, Andreesen R. Adoptive immunotherapy with tumor-cytotoxic macrophages derived from recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF) mobilized peripheral blood monocytes. J Immunother. 1997;20:365–371. doi: 10.1097/00002371-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pan PY, Li Y, Li Q, Gu P, Martinet O, Thung S, Chen SH. In situ recruitment of antigen-presenting cells by intratumoral GM-CSF gene delivery. Cancer Immunol Immunother. 2003;53:17–25. doi: 10.1007/s00262-003-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozbek S, Peters M, Breuhahn K, Mann A, Blessing M, Fischer M, Schirmacher P, Mackiewicz A, Rose-John S. The designer cytokine hyper-IL-6 mediates growth inhibition and GM-CSF-dependent rejection of B16 melanoma cells. Oncogene. 2001;20:972–979. doi: 10.1038/sj.onc.1204180. [DOI] [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell mediated suppression by dendritic cells. Science. 2001;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 26.Frederick MJ, Clayman GL. Chemokines in cancer. Exp Rev Mol Med. 2001 doi: 10.1017/S1462399401003301. (( http://www.ermm.cbcu.cam.ac.uk/01003301h.htm)) [DOI] [PubMed] [Google Scholar]
- 27.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]