Abstract
The etiology and pathogenesis of male breast cancer (MBC) are poorly known. This is due to the fact that the disease is rare, and large-scale genetic epidemiologic studies have been difficult to carry out. Here, we studied the frequency of eight recurrent Finnish BRCA2 founder mutations in a large cohort of 154 MBC patients (65% diagnosed in Finland from 1967 to 1996). Founder mutations were detected in 10 patients (6.5%), eight of whom carried the 9346(-2) A>G mutation. Two novel mutations (4075 delGT and 5808 del5) were discovered in a screening of the entire BRCA2 coding region in 34 samples. However, these mutations were not found in the rest of the 120 patients studied. Patients with positive family history of breast and/or ovarian cancer were often BRCA2 mutation carriers (44%), whereas those with no family history showed a low frequency of involvement (3.6%; P < .0001). Finally, we found only one Finnish MBC patient with 999 del5, the most common founder mutation in Finnish female breast cancer (FBC) patients, and one that explains most of the hereditary FBC and MBC cases in Iceland. The variation in BRCA2 mutation spectrum between Finnish MBC patients and FBC patients in Finland and breast cancer patients in Iceland suggests that modifying genetic and environmental factors may significantly influence the penetrance of MBC and FBC in individuals carrying germline BRCA2 mutations in some populations.
Keywords: BRCA2, mutation, male breast cancer, population, penetrance
Introduction
In men, breast cancer is approximately 100 times less common than in women. Breast cancer accounts for approximately 1% of all cancers in men. The incidence rates of male breast cancer (MBC) are lower than one case per 100,000 man-years in most populations [1]. In Finland, the age-adjusted incidence of MBC was 0.4 per 100,000 person-years in 2001, with about 13 cases diagnosed annually [2].
Genetic risk factors of MBC include Klinefelter's syndrome [3,4], as well as mutations of the androgen receptor [5,6], BRCA1 [7,8], and CHEK2 genes [9]. The strongest known risk factors for MBC are germline mutations in the BRCA2 gene [7,10–22]. The reported percentage of BRCA2 mutations in MBC patients has varied significantly (4–40%) among the published studies [13,21] (Table 1). Some of this variation may be due to the small numbers of the study subjects, different patient ascertainment criteria, as well as the variability in mutation detection methodology.
Table 1.
Frequency of BRCA2 Mutations in MBC Patients According to Family History of Breast-Ovarian Cancer.
| Study Population | Number of Patients | Screening Strategy | Percentage of BRCA2 Mutation Carriers* | Reference | |
| All Patients | Patients with Positive Family History | ||||
| Canada | 14 | Entire gene | 14 (2/14) | 29 (2/7) | [10] |
| France | 12† | Entire gene | 25 (3/12) | 25 (3/12) | [11] |
| Hungary | 18 | Entire gene | 33 (6/18) | 0 (0/4) | [12] |
| Iceland | 30 | Founder | 40 (12/30) | 90 (9/10) | [13] |
| Israel | 124 | Founder | 12 (15/124) | - | [7] |
| Italy | 25 | Entire gene | 12 (3/25) | 29 (2/7) | [14] |
| Poland | 37 | Entire gene | 11 (4/37) | 20 (1/5) | [15] |
| Spain | 17‡ | Entire gene | 18 (3/17) | 33 (3/9) | [16] |
| Sweden | 34 | Entire gene | 21 (7/34) | 20 (1/5) | [17] |
| UK | 28 | Entire gene | 7 (2/28) | 10 (1/10) | [18] |
| UK | 33§ | Entire gene | 36 (12/33) | 40 (12/30) | [19] |
| UK | 94 | Entire gene | 5 (5/94) | 16 (3/19) | [20] |
| USA | 54 | Entire gene | 4 (2/54) | 6 (1/16) | [21] |
| USA | 50 | Entire gene | 14 (7/50) | 15 (6/40) | [22] |
| Total | 570 | 7 (83/570) | |||
| Finland | 154 | Founder/entire gene | 8 (12/154) | 44 (7/16) | Present study |
Missense mutations with unknown significance excluded.
Only cases with positive family history were included.
Six females with breast cancer and a first-degree relative with MBC included.
Seventeen females with breast cancer and a first-degree or second-degree relative with MBC included.
In Finland, 15 BRCA1 and 8 BRCA2 mutations have been reported in FBC patients. Fourteen of these, seven in both genes, represent recurrent founder mutations that account for the majority of all detected mutations [23–30]. However, the frequency of BRCA2 germline mutations in Finnish MBC patients has remained unknown.
Here, we determined the frequency of eight previously described Finnish BRCA2 mutations in 154 MBC patients collected in Finland over a 30-year period using both blood and paraffin-embedded samples. This represents the largest study so far reported on MBC and BRCA2 mutations. In addition, in a subset of 34 patients for whom a blood specimen was available, we analyzed the entire coding region of the BRCA2 gene using protein truncation test (PTT) and denaturing high-performance liquid chromatography (DHPLC).
Materials and Methods
Sample Collection
We reviewed all men with an ICD code for breast cancer notified to the nationwide, population-based Finnish Cancer Registry between 1967 and 1996, and those 237 with microscopically confirmed diagnosis of carcinoma were eligible for the study. We linked the personal identification codes of these MBC patients with data from the Finnish Population Register Centre, providing us with additional information on vital status, possible date of death, or emigration. All first-degree relatives of the MBC patients, and second-degree relatives when possible, were then identified from the population and parish registries. Cancer diagnoses of the relatives were also identified by linking the personal identification codes of the relatives back to the Cancer Registry. The study protocol was approved by the Ethical Committee of the Tampere University Hospital and the Ministry of Social Affairs and Health in Finland.
Of 237 patients, 79 (33%) were alive, and they were approached through the attending physicians. We obtained a written informed consent to participate in the study and a blood sample from 37 patients. A questionnaire on malignancies in the family was also received. Paraffin-embedded tissue samples were obtained from 130 patients; most of these patients had died of the disease. In 122 of the paraffin-embedded tumors, breast cancer diagnosis was confirmed by a pathologist (T.K.), leading to abandonment of eight samples.
Mutation Detection
DNA was extracted from blood samples using Puregene (Gentra Systems, Minneapolis, MN) or from paraffin-embedded nonmalignant tissues using QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Sufficient amount of DNA was obtained from 156 samples. Eight BRCA2 mutations, previously found to be present in the Finnish FBC patients, were analyzed in all 156 samples. The mutations screened were 999 del5, 4081 insA, 5797 G>T, 6495/6496 G>C, delCA, 6503 delTT, 7708 C>T, 8555 T>G, and 9346(-2) A>G (NCBI RefSeq mRNA accession no. NM 000059). Short (80–150 bp) fragments around the possible mutation sites were amplified by polymerase chain reaction (PCR) using genomic DNA. For each primer set, one primer was 5′-biotinylated.
Mutation detection was done using solid-phase minisequencing (single-base extension reaction) as previously described [31] using streptavidin-coated microtiter plates manufactured from scintillating plastic (Wallac; Perkin Elmer, Turku, Finland) and 1450 MicroBeta PLUS Liquid scintillation counter by the same manufacturer. PCR primers and minisequencing detection primers are available upon request. Mutation-positive and mutation-negative controls as well as a negative control for the PCR reaction were included in each analysis. The results were confirmed by direct sequencing using ABI PRISM 310 Genetic Analyzer and Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer) according to the manufacturer's instructions. Two DNA samples from paraffin-embedded tissues were excluded because of conflicting mutation results.
BRCA2 founder mutation was found in 3 of 37 patients from whom DNA samples from whole blood were available, and thus 34 samples were screened for the presence of additional BRCA2 mutations. The entire coding region of BRCA2 was analyzed using PTT for exons 10 and 11, and DHPLC for exons 2 to 9 and 12 to 27, including also the first 300 bp of exons 10 and 11. Samples with truncated PTT bands or aberrant DHPLC chromatograms were sequenced, using a new PCR product as a template [32,33].
Statistical Analyses
Statistical analyses were performed using GraphPad InStat version 2.04a (GraphPad Software, San Diego, CA). Continuous parametric variables were shown as mean and standard deviation, and comparisons were made with Student's t test. Associations between categorical variables were analyzed by Fisher's exact test and chi-square analysis.
Results
Mutations
We screened eight previously described Finnish BRCA2 founder mutations in 154 MBC patients, and three different mutations [999 del5, 7708 C>T, and 9346(-2) A>G] were found. Altogether, 10 (6.5%) patients were mutation-positive (Table 2). Eight (5.2%) patients carried the 9346(-2) A>G mutation.
Table 2.
Characteristics of MBC Patients with BRCA2 Mutations.
| Patient | Mutation | Effect | Age at Diagnosis (years) | Histologic Type (Grade) | Family History of Cancer Among First-Degree and Second-Degree Relatives | |
| Type of Relative (Age at Diagnosis) | ||||||
| Breast Cancer | Other Cancers | |||||
| Y170 | 999 del5 | Frameshift | 46 | IDC (3) | S (25), S (45), S (54), S (65, 67)* | M stomach (76), F prostate (63), B lung (64), PA uterine (61), PA fallopian tube (79) |
| Y047 | 4075 delGT | Frameshift | 58 | ILC | B (78) | S ovary (67), S cervical (71) |
| Y099 | 5808 del5 | Frameshift | 66 | IDC (3) | MA (76), PA (83) | MA skin (97), GD leukemia (5), B skin (74), BD chorion (30) |
| Y021 | 7708 C>T | Nonsense | 71 | IDC (3) | M (80), S (50) | F skin (NA), S liver (78), SD brain (8) |
| Y063-17 | 9346(-2) A>G | Splice site | 67 | IDC (2) | SS (71), SD (36), SD (49), SD (50) a, SD (54) b |
SD ovarian (58), D uterine (55), So prostate (60), B colon (87), S laryngeal (NA), SD cervical (39), SD cervical (47) a, SD lymphoma (69) b |
| Y076 | 9346(-2) A>G | Splice site | 64 | IDC (3) | ||
| Y084 | 9346(-2) A>G | Splice site | 49 | IDC (2) | S (73) | F prostate (69), F skin (47), B prostate (58) |
| Y089 | 9346(-2) A>G | Splice site | 66 | IDC (2) | ||
| Y095 | 9346(-2) A>G | Splice site | 69 | IDC (3) | D (44) c, S (80) | D chorion (33) c, B prostate (69) |
| Y103 | 9346(-2) A>G | Splice site | 91 | IDC (1) | ||
| Y115 | 9346(-2) A>G | Splice site | 57 | ILC | ||
| Y157 | 9346(-2) A>G | Splice site | 75 | IDC (2) | B stomach (84), So prostate (56) | |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
M, mother; F, father; S, sister; B, brother; D, daughter; So, son; GD, granddaughter; PA, paternal aunt; PU, paternal uncle; MA, maternal aunt; SD, sister's daughter; SS, sister's son; BD, brother's daughter; NA, not available.
a, b, c, same individuals.
Bilateral breast cancer.
PTT and DHPLC screening of 34 patients revealed two novel mutations (5.9%): 4075 delGT and 5808 del5. Additionally, 12 different silent polymorphisms were detected (data not shown). Subsequently, the 4075 delGT and 5808 del5 mutations were studied in all 154 samples, but no additional mutation carriers were found. Taken together, BRCA2 mutations were present in 12 (7.8%) MBC patients.
Characteristics of Mutation Carriers
MBC patients with BRCA2 mutations were only slightly younger (mean 64.9 years; SD 11.9; range 46–91) than noncarriers (mean 65.7 years; SD 12.4; range 30–94) at the time of diagnosis; this difference did not reach statistical significance. None of the four patients under 40 years, and only two of the 17 patients under 50 years of age at the time of diagnosis, carried a mutation.
Sixteen MBC patients had at least one first-degree or second-degree female relative with breast cancer. Seven of these MBC patients (44%) carried a BRCA2 mutation, whereas only 5 of 138 (3.6%) patients without family history of breast cancer had a BRCA2 mutation. This difference was statistically highly significant [P < .0001; OR = 20 (95% CI 5–76)].
Three MBC patients had a first-degree or second-degree relative with ovarian cancer, and two of these MBC patients carried BRCA2 mutations: 4075 delGT and 9346(-2) A>G (Table 2). Two mutation-positive MBC patients were found to have relatives with MBC. Additionally, two initially unrelated MBC patients with no BRCA2 mutations were found to be related with each other (second-degree relatives) in more detailed pedigree analysis. Unfortunately, only tissue samples were available from these patients, and we were not able to screen the entire BRCA2 gene for mutations.
Discussion
We report here the frequency of eight different, previously found Finnish BRCA2 mutations in 154 Finnish MBC patients, as well as the screening of the entire coding sequence of the BRCA2 gene in 34 MBC patients for whom blood samples were available. To our knowledge, this is the largest set of MBC patients included in a systematic screening of BRCA2 mutations. The patients comprised 65% of all MBC patients identified in Finland over a 30-year period, providing a higher coverage of cases in the source population than in many previous studies.
The overall BRCA2 mutation frequency was 7.8% in the present study, which is slightly lower than BRCA2 mutation frequencies in MBC in most previously published studies [7,10–22] (Table 1). However, if we expect the rate of sporadic mutations among the 154 to be the same as among the 34 patients screened comprehensively for BRCA2 mutations, we could estimate that the total mutation burden, including both recurrent and sporadic mutations, is approximately 12% to 13% (6.5% + 5.9%). This is well in line with those found in previous studies, where the entire coding sequence of all patients was analyzed [7,10–22] (Table 1).
In our study, 10.4% (16/154) of the MBC cases had a positive family history of breast cancer, and as many as seven of these 16 patients (44%) carried a BRCA2 mutation. The percentage of family history-positive MBC patients with BRCA2 mutations has been found to range from 0% to 90% [7,10–22] (Table 1). In Iceland and Finland where strong founder effects can be found, a larger proportion of family history-positive patients carried a BRCA2 mutation compared to other populations [13]. In contrast, BRCA2 mutations were rare (3.6%) among Finnish MBC patients with negative family history of breast and/or ovarian cancer. Many of the other MBC studies have reported similar results, with a range of BRCA2 positivity in family history-negative cases from 0% to 15% [7,10–22] (Table 1). On the other hand, up to 21% of family-negative MBCs carried a BRCA2 mutation in Sweden (6/29) and 43% (6/14) in Hungary [17,12].
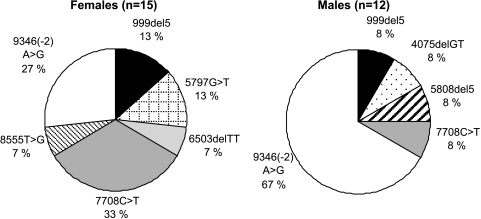
The frequencies of different BRCA2 mutations varied greatly among Finnish MBC and female breast cancer (FBC) populations (Figure 1). Among 1035 unselected Finnish FBC patients, 1.8% carried one of the BRCA1 (0.4%) or BRCA2 (1.4%) founder mutations [27]. In MBC patients, the most common BRCA2 mutation was 9346(-2) A>G, with a frequency of 67% (8/12 BRCA2 mutation-positive cases). In unselected FBC population, the 9346(-2) A>G mutation was the second most common mutation with a much lower frequency (27%; 4/15) [28]. The difference in 9346(-2) A>G mutation frequencies was significant between males and females (P = .038; OR = 5.5; 95% CI 1–29). In contrast, only one MBC patient carried the 999 del5 mutation, which is the most common BRCA1/2 founder mutation in Finland [29]. Up to 40% of all Icelandic MBC patients carries this mutation [13]. All 10 MBC founder mutation carriers originated from the same regions in Finland as the FBC patients carrying the corresponding founder mutations [28]. The low number of 999 del5 mutations cannot be explained by simple selection bias because dozens of samples were available from the two founder areas of this mutation [13]. Our findings and those by Thorlacius et al. [13] therefore point to additional genetic modifier loci, or possibly environmental factors that differentially influence MBC and FBC penetrance among different BRCA2 mutation carriers.
Figure 1.
BRCA2 mutation spectrum among mutation-positive Finnish FBC [27] and MBC populations.
In summary, we report here a large genetic epidemiologic study that suggested an approximately 13% frequency of BRCA2 mutations in unselected Finnish MBC patients. Founder mutations account for the majority of these, with one particular mutation [9346(-2) A>G] showing a very high frequency among MBC patients. In contrast, the most common Finnish founder mutation in FBC patients (999 del5) was seen only in one MBC patient. Interestingly, the same 999 del5 founder mutation accounts for the majority of both male and female hereditary breast cancers in Iceland [13]. Although the numbers of BRCA2-positive MBC cases are still relatively small, our results point to some frequency differences of individual BRCA2 founder mutations when compared to analogous nationwide study of FBC. This may reflect differences in modifier loci, or, equally likely, environmental differences (such as hormonal and dietary) that influence the penetrance of different BRCA2 mutations between female and male populations as well as between different populations.
Acknowledgements
We thank the patients and doctors who participated in the study, Kati Rouhento for technical assistance, and Kristiina Selkee for her help in patient contacts.
Abbreviations
- DHPLC
denaturing high-performance liquid chromatography
- FBC
female breast cancer
- MBC
male breast cancer
- PTT
protein truncation test
Footnotes
This study was supported, in part, by grants from the Tampere University Hospital, Finnish Cancer Institute, Finnish Cancer Society, and Sigrid Juselius Foundation.
References
- 1.Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53:538–549. doi: 10.1002/ijc.2910530403. [DOI] [PubMed] [Google Scholar]
- 2.The Finnish Cancer Registry, author. Cancer Statistics (Last updated on November 21, 2002 ( http://www.cancerregistry.fi)) 2002.
- 3.Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter's syndrome: is there an association? CA Cancer J Clin. 1987;37:246–251. doi: 10.3322/canjclin.37.4.246. [DOI] [PubMed] [Google Scholar]
- 4.Hultborn R, Hanson C, Kopf I, Verbiene I, Warnhammar E, Weimarck A. Prevalence of Klinefelter's syndrome in male breast cancer patients. Anticancer Res. 1997;17:4293–4297. [PubMed] [Google Scholar]
- 5.Wooster R, Mangion J, Eeles R, Smith S, Dowsett M, Averill D, Barrett-Lee P, Easton DF, Ponder BA, Stratton MR. A germline mutation in the androgen receptor gene in two brothers with breast cancer and Reifenstein syndrome. Nat Genet. 1992;2:132–134. doi: 10.1038/ng1092-132. [DOI] [PubMed] [Google Scholar]
- 6.Lobaccaro JM, Lumbroso S, Belon C, Galtier-Dereure F, Bringer J, Lesimple T, Heron JF, Pujol H, Sultan C. Male breast cancer and the androgen receptor gene. Nat Genet. 1993;5:109–110. doi: 10.1038/ng1093-109. [DOI] [PubMed] [Google Scholar]
- 7.Struewing JP, Coriaty ZM, Ron E, Livoff A, Konichezky M, Cohen P, Resnick MB, Lifzchiz-Mercerl B, Lew S, Iscovich J. Founder BRCA1/2 mutations among male patients with breast cancer in Israel. Am J Hum Genet. 1999;65:1800–1802. doi: 10.1086/302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 9.Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, Houben M, Crepin E, van Veghel-Plandsoen M, Elstrodt F, vanDuijn C, Bartels C, Meijers C, Schutte M, McGuffog L, Thompson D, Easton D, Sodha N, Seal S, Barfoot R, Mangion J, Chang-Claude J, Eccles D, Eeles R, Evans DG, Houlston R, Murday V, Narod S, Peretz T, Peto J, Phelan C, Zhang HX, Szabo C, Devilee P, Goldgar D, Futreal PA, Nathanson KL, Weber B, Rahman N, Stratton MR CHEK2-Breast Cancer Consortium MR, author. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–59. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 10.Wolpert N, Warner E, Seminsky MF, Futreal A, Narod SA. Prevalence of BRCA1 and BRCA2 mutations in male breast cancer patients in Canada. Clin Breast Cancer. 2000;1:57–63. doi: 10.3816/CBC.2000.n.005. [DOI] [PubMed] [Google Scholar]
- 11.Pages S, Caux V, Stoppa-Lyonnet D, Tosi M. Screening of male breast cancer and of breast-ovarian cancer families for BRCA2 mutations using large bifluorescent amplicons. Br J Cancer. 2001;84:482–488. doi: 10.1054/bjoc.2000.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csokay B, Udvarhelyi N, Sulyok Z, Besznyak I, Ramus S, Ponder B, Olah E. High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Res. 1999;59:995–998. [PubMed] [Google Scholar]
- 13.Thorlacius S, Olafsdottir G, Tryggvadottir L, Neuhausen S, Jonasson JG, Tavtigian SV, Tulinius H, Ogmundsdottir HM, Eyfjord JE. A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet. 1996;13:117–119. doi: 10.1038/ng0596-117. [DOI] [PubMed] [Google Scholar]
- 14.Ottini L, Masala G, D'Amico C, Mancini B, Saieva C, Aceto G, Gestri D, Vezzosi V, Falchetti M, De Marco M, Paglierani M, Cama A, Bianchi S, Mariani-Costantini R, Palli D. BRCA1 and BRCA2 mutation status and tumor characteristics in male breast cancer: a population-based study in Italy. Cancer Res. 2003;63:342–347. [PubMed] [Google Scholar]
- 15.Kwiatkowska E, Teresiak M, Lamperska KM, Karczewska A, Breborowicz D, Stawicka M, Godlewski D, Krzyzosiak WJ, Mackiewicz A. BRCA2 germline mutations in male breast cancer patients in the Polish population. Hum Mutat. 2001;17:73. doi: 10.1002/1098-1004(2001)17:1<73::AID-HUMU12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Diez O, Cortes J, Domenech M, Pericay C, Brunet J, Alonso C, Baiget M. BRCA2 germ-line mutations in Spanish male breast cancer patients. Ann Oncol. 2000;11:81–84. doi: 10.1023/a:1008339009528. [DOI] [PubMed] [Google Scholar]
- 17.Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, Borg Å. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58:1367–1371. [PubMed] [Google Scholar]
- 18.Mavraki E, Gray IC, Bishop DT, Spurr NK. Germline BRCA2 mutations in men with breast cancer. Br J Cancer. 1997;76:1428–1431. doi: 10.1038/bjc.1997.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans DG, Bulman M, Young K, Gokhale D, Lalloo F. High detection rate for BRCA2 mutations in male breast cancer families from North West England. Fam Cancer. 2001;1:131–133. doi: 10.1023/a:1021165031643. [DOI] [PubMed] [Google Scholar]
- 20.Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, Pharoah PD. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002;4:R2. doi: 10.1186/bcr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman LS, Gayther SA, Kurosaki T, Gordon D, Noble B, Casey G, Ponder BA, Anton-Culver H. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60:313–319. [PMC free article] [PubMed] [Google Scholar]
- 22.Couch FJ, Farid LM, DeShano ML, Tavtigian SV, Calzone K, Campeau L, Peng Y, Bogden B, Chen Q, Neuhausen S, Shattuck-Eidens D, Godwin AK, Daly M, Radford DM, Sedlacek S, Rommens J, Simard J, Garber J, Merajver S, Weber BL. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13:123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- 23.Vehmanen P, Friedman LS, Eerola H, McClure M, Ward B, Sarantaus L, Kainu T, Syrjäkoski K, Pyrhönen S, Kallioniemi OP, Muhonen I, Luce M, Frank TS, Nevanlinna H. Low proportion of BRCA1 and BRCA2 mutations in Finnish breast cancer families: evidence for additional susceptibility genes. Hum Mol Genet. 1997;6:2309–2315. doi: 10.1093/hmg/6.13.2309. [DOI] [PubMed] [Google Scholar]
- 24.Vehmanen P, Friedman LS, Eerola H, Sarantaus L, Pyrhonen S, Ponder BA, Muhonen T, Nevanlinna H. A low proportion of BRCA2 mutations in Finnish breast cancer families. Am J Hum Genet. 1997;60:1050–1058. [PMC free article] [PubMed] [Google Scholar]
- 25.Huusko P, Pääkkönen K, Launonen V, Pöyhönen M, Blanco G, Kauppila A, Puistola U, Kiviniemi H, Kujala M, Leisti J, Winqvist R. Evidence of founder mutations in Finnish BRCA1 and BRCA2 families. Am J Hum Genet. 1998;62:1544–1548. doi: 10.1086/301880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarantaus L, Auranen A, Nevanlinna H. BRCA1 and BRCA2 mutations among Finnish ovarian carcinoma families. Int J Oncol. 2001;18:831–835. doi: 10.3892/ijo.18.4.831. [DOI] [PubMed] [Google Scholar]
- 27.Syrjäkoski K, Vahteristo P, Eerola H, Tamminen A, Kivinummi K, Sarantaus L, Holli K, Blomqvist C, Kallioniemi OP, Kainu T, Nevanlinna H. Population-based study of BRCA1 and BRCA2 mutations in 1035 unselected Finnish breast cancer patients. J Natl Cancer Inst. 2000;92:1529–1531. doi: 10.1093/jnci/92.18.1529. [DOI] [PubMed] [Google Scholar]
- 28.Sarantaus L, Huusko P, Eerola H, Launonen V, Vehmanen P, Rapakko K, Gillanders E, Syrjäkoski K, Kainu T, Vahteristo P, Krahe R, Pääkkönen K, Hartikainen J, Blomqvist C, Löppönen T, Holli K, Ryynänen M, Bützow R, Borg Å, Wasteson Arver B, Holmberg E, Mannermaa A, Kere J, Kallioniemi OP, Winqvist R, Nevanlinna H. Multiple founder effects and geographical clustering of BRCA1 and BRCA2 families in Finland. Eur J Hum Genet. 2000;8:757–763. doi: 10.1038/sj.ejhg.5200529. [DOI] [PubMed] [Google Scholar]
- 29.Barkardottir RB, Sarantaus L, Arason A, Vehmanen P, Bendahl PO, Kainu T, Syrjäkoski K, Krahe R, Huusko P, Pyrhönen S, Holli K, Kallioniemi OP, Egilsson V, Kere J, Nevanlinna H. Haplotype analysis in Icelandic and Finnish BRCA2. Eur J Hum Genet. 2001;9:773–779. doi: 10.1038/sj.ejhg.5200717. [DOI] [PubMed] [Google Scholar]
- 30.Vahteristo P, Eerola H, Tamminen A, Blomqvist C, Nevanlinna H. A probability model for predicting BRCA1 and BRCA2 mutations in breast and breast-ovarian cancer families. Br J Cancer. 2001;84:704–708. doi: 10.1054/bjoc.2000.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syvänen AC. Solid-phase minisequencing as a tool to detect DNA polymorphism. Methods Mol Biol. 1998;98:291–298. doi: 10.1385/0-89603-443-7:291. [DOI] [PubMed] [Google Scholar]
- 32.Håkansson S, Johannsson O, Johansson U, Sellberg G, Loman N, Gerdes AM, Holmberg E, Dahl N, Pandis N, Kristoffersson U, Olsson H, Borg Å. Moderate frequency of BRCA1 and BRCA2 germ-line mutations in Scandinavian familial breast cancer. Am J Hum Genet. 1997;60:1068–1078. [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner T, Stoppa-Lyonnet D, Fleischmann E, Muhr D, Pages S, Sandberg T, Caux V, Moeslinger R, Langbauer G, Borg A, Oefner P. Denaturing high-performance liquid chromatography detects reliably BRCA1 and BRCA2 mutations. Genomics. 1999;62:369–376. doi: 10.1006/geno.1999.6026. [DOI] [PubMed] [Google Scholar]