Abstract
Although p73 is a structural and functional homologue of the tumor-suppressor gene p53, it is not mutated in many human cancers as p53. Besides, p73 was shown to be activated by only a subset of signals that activate p53, such as γ-irradiation and cisplatin, but not by other common genotoxic stress-inducing agents such as ultraviolet (UV) irradiation, although many of these signals are also capable of inducing p53-independent cell death. Using a p73-specific antibody, we confirmed that c-Abl is required for cisplatin-induced p73 upregulation, and further demonstrate that the p73 protein is upregulated by UV irradiation and other stress stimuli including sorbitol, hydrogen peroxide, nocodazol, and taxol. These stress signals upregulate both p73 mRNA and increases the stability of p73, indicating that p73 is regulated transcriptionally and posttranslationally. Cells stably expressing the dominant-negative p73 inhibitor protein (p73DD) and p73-/- fibroblasts are more resistant than control cells to apoptosis induced by these stress signals, suggesting that p73 contributes to apoptosis induction. Together, the data demonstrate that several stress signals can signal to p73 in vivo, which raises the possibility of eradicating cancers with an unmutated p73 gene by activating them with stress-inducing agents or their mimetics.
Keywords: Apoptosis, p73β, p73DD, stress signals, UV irradiation
Introduction
p73 is a structural and functional homologue of the p53 tumor-suppressor gene that has homology with p53 in the transactivation, tetramerization, and DNA binding domains [1]. However, unlike p53, the p73 protein is expressed as several isoforms due to extensive splicing at the carboxy terminal domain, resulting in at least six splice variants (p73α-φ) [2–4]. In addition, the use of an alternative promoter in intron 3 of the p73 gene leads to the expression of a p73 protein that lacks the transactivation domain (ΔNp73) and acts as a dominant-negative suppressor of p73 [5]. When ectopically overexpressed in cell culture, both p73α and p73β closely mimic p53 and induce programmed cell death [6]. Moreover, both p73α and p73β have been shown to transactivate many p53-responsive promoters, although relative efficiencies vary on different promoters [7]. Cell death induced by overexpression of p73 occurs irrespective of the p53 status and both p73α and p73β have been shown to suppress foci formation [3].
Despite these functional similarities, the p73 gene is rarely mutated in human cancers [8]. Moreover, the ΔNp73 has been shown to be overexpressed in cancers, which could lead to the functional inactivation of the unmutated p73 or p53 in cancer cells [9]. Besides, the p73 protein was also not shown to be induced by all the signals that activate p53. Only a subset of DNA-damaging signals such as γ-irradiation (IR), or anticancer drugs such as cisplatin, camptothecin (CPT), taxol, and doxorubicin have been shown to induce p73 protein expression [10–13]. Other investigators have not been able to observe the induction of p73 expression in response to several other genotoxic stress signals [2], although most of these signals have the ability to induce p53-independent cell death. Detection of endogenous p73 induction has been hampered by the lack of well-characterized antibodies. Currently, there are several p73-specific antibodies that are commercially available, and an overview of some of their specificity is given in Table 1. Most of these antibodies are capable of recognizing the various full-length p73 isoforms and the ΔNp73 isoforms when overexpressed. However, only a few are reported to be able to recognize the endogenous p73 protein (Table 1). The most well characterized among them is the clone ER15, which has been shown by several investigators to be able to recognize the human p73α isoform (Table 1). This antibody has also been used to detect p73β isoform in human and mouse tissues (Table 1). Recently, this antibody was used to show that p73α is upregulated by treatment of cells with several chemotherapeutic agents [13]. The p73β protein was not induced to a similar extent as p73α, when detected with this antibody [13]. Besides this, other antibodies have been less characterized and many reports do not indicate the isoform of p73 that corresponds to the detected band (Table 1). In an attempt to investigate if some of the other stress signals have the ability to induce expression of the p73β protein, we have focused on the induction p73 protein using a p73β-specific antibody—the clone GC15. We report here that p73β can be induced by several stress signals in a p53-independent manner. Detailed results are discussed.
Table 1.
Overview of Some p73-Specific Antibodies Used to Detect Endogenous p73.
| Antibody Clone Number | Epitope | Specificity Described | Reference | |
| Species (Cell Type) | Isoform | |||
| - | 427–636 | Human (IMR32, HT29, SK-N-SH) | p73α | 2 |
| ER15 | 380–495 of p73α | Human/mouse (HCT-116-3, MEFs) | Not mentioned | 11 |
| Mouse (brain tissue) | ΔNp73 | 5 | ||
| Human (HacaT, T98G) | p73α and p73 | 20 | ||
| Human (H1299, HT29, HacaT) | p73α | 25 | ||
| Human (HSC3, ICR31, HN30, H3T6, C339, A431, HacaT) | Not mentioned | 26 | ||
| Human (SW80) | p73α and p73 | 13 | ||
| C17/C20 | C terminus | Human (T47D, SKBR3) | p73α | 27 |
| GC15 | 380–499 of p73 | Human/simian (H1299, COS7) | p73 | 18 |
| Clone 1288 2002 | Not shown* | Human (HCT116-3) | p73α and p73 | 14 |
| Clone 429 | Not shown* | Human (HCT116) | Not mentioned | 19 |
| CJDp73 | 428–599 of p73α | Human (transitional carcinoma cells) | p73α | 28 |
Not described in reference.
Materials and Methods
Cells, Transfections, and Reporter Assays
All cells used in this study were cultured in 10% serum containing DMEM. COS7 cells were transfected with a plasmid expressing the p73DD cDNA or an empty pCDNA vector (1.0 µg), and selected on G418 (1 mg/ml) for 2 to 3 weeks to obtain stable COS7-p73DD (p73DD) clones, which were used for analysis as described. p73-/- mouse embryonic fibroblasts were a kind gift of Dr. Jean Wang.
About 3 x 105 cells (in six-well dishes) and 1 x 106 cells (in 10-cm dishes) were used in transfection experiments using Lipofectamine Plus-Reagent, as per the manufacturer's protocols. H1299 and COS7 cells were transiently transfected with the following amounts of p73 expression plasmids with or without the enhanced green fluorescence protein (Egfp) expression plasmid (Figure 1, A and B: 500 ng of p73; Figures 2A and 3A: 100 ng of p73 and 50 ng of Egfp). Cells were collected 48 hours after transfection and cell extracts were prepared and used for immunoblot analysis. For analysis of p53-independent transactivation, the following plasmids were used in transfections: 0.5 µg of minimal mdm2 promoter luciferase and 0.5 µg of PGK β-galactosidase in COS7 cells or together with 0.5 µg of p73DD in H1299 cells. Cells were ultraviolet (UV)-irradiated (40 J/m2) 24 hours after transfection and the reporter activity was determined after another 2 hours of incubation. COS7 vector and p73DD cells were transfected with 100 ng of p73β expression plasmid together with the reporter plasmids, and the activity was determined 48 hours after transfection. Cells were harvested, washed once in 1 x PBS, and lysed in 150 µl of glycylglycine lysis buffer; β-galactosidase and luciferase assays were performed as described; and the amount of luciferase activity per h-galactosidase unit was calculated [14].
Figure 1.
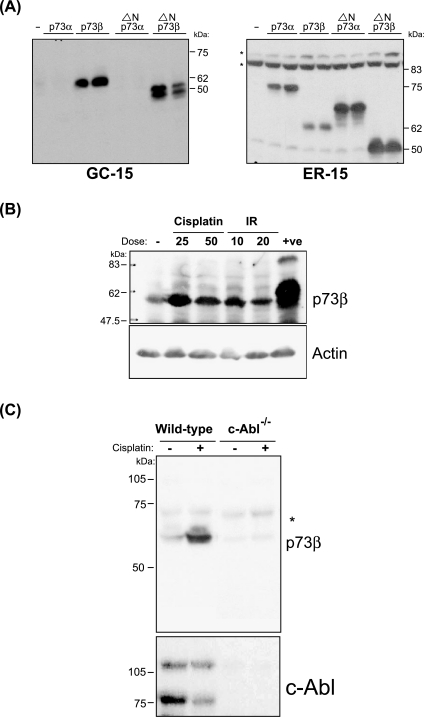
Characterization of p73β-specific antibody. (A) H1299 cells were transfected with 500 ng of the indicated plasmids. One hundred fifty micrograms of cell extracts prepared 48 hours posttransfection were analyzed by Western blot analysis using the GC15 (left panel) or the ER15 (right panel) antibodies. (B) HCT116(3) cells were either cisplatin-treated (25 or 50 µM) or γ-irradiated (IR: 10 or 20 Gy) and 350 µg of cell extracts prepared 24 hours posttreatment was used for immunoblot analysis using the GC15 and anti-actin antibodies. Extracts from H1299 cells transfected with p73β expression plasmid were used as a positive control. (C) Wild-type and c-Abl null immortalized mouse embryonic fibroblasts were treated with 25 µM cisplatin and analyzed 24 hours after treatment using the GC15 and anti-c-Abl antibodies, as in (B). *Indicates a nonspecific band.
Figure 2.
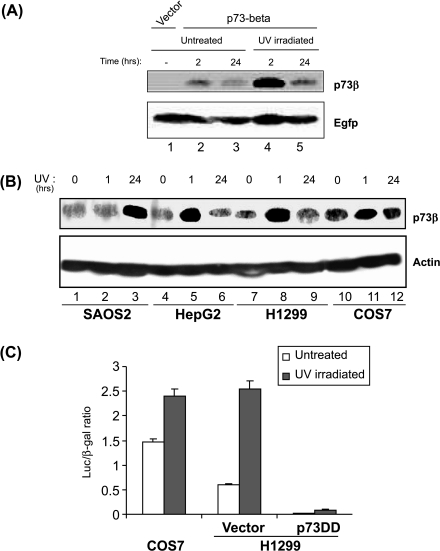
UV irradiation results in p73β upregulation. (A) Accumulation of transfected p73β upon UV irradiation. COS7 cells were transfected with 100 ng of p73β expression plasmid or empty vector together with 50 ng of the Egfp expression vector. Cells were either left untreated or UV-irradiated (40 J/m) 24 hours after transfection, harvested 2 or 24 h postirradiation, and lysed; and lysates were used for Western blot analysis with GC15 and anti-Egfp antibodies. (B) Endogenous p73β is upregulated by UV irradiation. Saos2, HepG2, H1299, and COS7 cells were UV-irradiated (40 J/m) and cells were collected either at 1 or 24 h after irradiation. Three hundred micrograms of total cell lysates was used for Western blot analysis. (C) UV induces p53-like transcriptional activity. COS7 cells were transfected with the plasmid containing the luciferase reporter gene driven by the minimal mdm2 promoter (0.5 µg), together with a plasmid encoding the β-galactosidase gene (0.5 µg) for evaluating and normalizing for the transfection efficiency, and were subjected to UV irradiation (40 J/m) 24 hours posttransfection. H1299 cells were transfected with the above plasmids together with either the empty vector or the dominant-negative p73DD expression plasmid (0.5 µg) where indicated. Cells were harvested 2 hours after irradiation and cell lysates were used for determination of β-galactosidase and luciferase activity. The relative luciferase value per β-galactosidase unit is represented and results are representative of three independent experiments.
Figure 3.
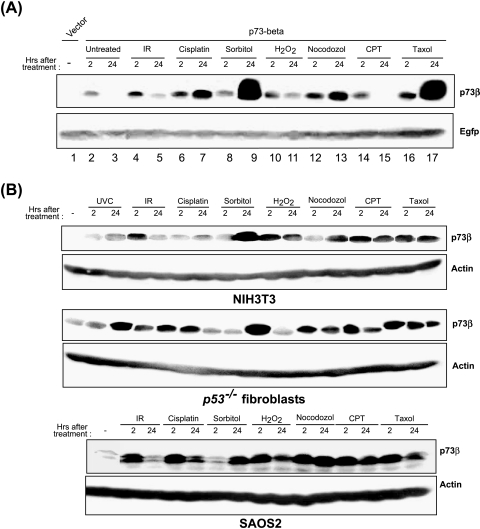
p73β accumulates in response to multiple stress signals. (A) Stress induces accumulation of p73β. COS7 cells transfected with p73β expression plasmid as described above were subjected to the indicated stress signals (IR: 20 Gy; cisplatin: 25 µM; sorbitol: 0.3 M; H2O2: 90 µM; nocodazol: 0.5 µg/ml; CPT: 25 µM; taxol: 100 nM) for either 2 or 24 hours, and cell lysates were analyzed by Western blot analysis. (B) Endogenous p73β is upregulated by stress stimuli. NIH3T3, p53-/- fibroblasts, and Saos2 cells were subjected to the indicated stress stimuli and cells, harvested at 2 and 24 hours after treatment, and analyzed for the endogenous p73β expression by Western blot analysis.
COS7 cells transfected with p73β expression plasmid were treated with the indicated stress-inducing agents 24 hours after transfection and the cells were harvested either 2 or 24 hours later. All other cell types were treated with stress-inducing agents [IR: 20 Gy; cisplatin: 25 µM; sorbitol: 0.3 M; hydrogen peroxide (H2O2): 90 µM; nocodazol: 0.5 µg/ml; CPT: 25 µM; taxol: 100 nM] and cells were harvested 1, 2, or 24 hours later for analysis of endogenous p73β expression.
Apoptosis Assays
Apoptosis assays were performed in duplicates with 1.5 x 105 COS7 vector and p73DD cells in six-well dishes. Twenty-four hours after plating, cells were subjected to the indicated stress-inducing agents, collected 24 hours after treatment, and fixed in 1 ml of 70% ethanol overnight at 4°C. Cells were washed in 1 x PBS, resuspended in 1 x PBS containing 100 µg/ml RNAse A, and incubated for 30 minutes at room temperature. Propidium iodide (20 µg/ml) was then added and the cells were analyzed for DNA content by flow cytometry as described [15]. The assays were performed at least thrice independently. The net amount of apoptotic cells compared to the untreated controls is indicated (i.e., % dead cells after treatment - % dead cells without treatment).
Apoptosis was also determined by staining cells with Annexin V FITC (Pharmingen, San Diego, CA), together with propidium iodide, as per manufacturer's instructions. Annexin V binds to the exposed phosphatidyl serines on the plasma membrane of cells undergoing cell death, and serves as an independent marker for cell death.
RNA Analysis
RNA was prepared by standard procedures from H1299 cells treated with the indicated agents and analyzed by semiquantitative reverse transcription polymerase chain reaction (RT-PCR) analysis, as per manufacturer's recommendations and as described [16]. In brief, full-length p73 PCR reactions were performed using a forward 5′ TCTGGAACCAGACAGCACCT 3′ and a reverse 5′ GTGCTGGACTGCTGGAAAGT 3′ primer under the following conditions: 94°C, 50 seconds; 54°C, 50 seconds; 72°C, 50 seconds, for 34 cycles. The gapdh PCR was carried out using forward 5′ ACCCCTTCATTGACC TCAAC 3′ and reverse 5′ CAGCGCCAGTAGAGGCAG 3′ under the following conditions: 94°C, 50 seconds; 54°C, 50 seconds; 72°C, 50 seconds for 20 cycles. The full-length p73 PCR primers are specific and are not able to detect the ΔNp73 isoforms.
To detect p73DD RNA expression, PCR reactions were performed using a forward 5′ TCTAGGATCCAAGCGTGCCTTCAAG 3′ and a reverse 5′ TAGAGAATTCGTGGATCTCGGCCTC 3′ primer under the following conditions: 94°C, 30 seconds; 50°C, 40 seconds; 72°C, 1 minute, for 30 cycles. The p73DD plasmid was used as a positive control in the PCR reaction.
Protein Analysis
Cells lysates were prepared in lysis buffer containing 0.5% Nonidet P-40 as described [17]. Proteins were separated on SDS polyacrylamide gels and Western-blotted with anti-p73α (ER15; Oncogene, San Diego, CA), anti-p73β (GC15; Oncogene), anti-c-Abl (Cell Signaling Technology, Inc., Beverly, MA), anti-actin (Sigma, St. Louis, MO), anti-p53 (CM-5; Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), and anti-Egfp (Clontech, Palo Alto, CA) antibodies. Generally, 150 µg of lysate was used from transfected cells to monitor steady-state levels of proteins. To determine the endogenous p73β status, between 300 and 500 µg of total cell extracts was used. In addition, immunoblot chemiluminescence was detected with the highly sensitive Super-Signal West Dura detection kit (Pierce-Endogen, Rockford, IL). Conventional ECL and ECL Plus (Amersham Biosciences UK Ltd., Buckinghamshire, England, UK) detection kits were not able to detect endogenous p73, although they were able to detect transfected p73 protein.
For half-life determination, NIH3T3 cells were UV-irradiated (40 J/m2) or treated with sorbitol (0.3M) for 24 hours and the incubated with 20 µg/ml cyclohexamide for indicate time periods. Cells were collected and analyzed by Western blot analysis as described. The percentage of p73β remaining was quantified as pixel values from the intensity of the bands and normalized with respect to actin by using the phosphoimager.
Results
Characterization of p73β-Specific Antibody
We determined the specificity of a commercially available p73β-specific antibody, the clone GC15, which was shown to detect both human and simian endogenous p73β (Table 1) [18]. This antibody was able to only detect transiently overexpressed p73β and ΔNp73β proteins in the human lung cancer cell line H1299, which were migrating at around 62 and 50 kDa, respectively, but not the overexpressed p73α and ΔNp73α proteins (Figure 1A, left panel). In contrast, the p73α-specific ER15 antibody was able to detect both the overexpressed p73α and p73β isoforms (Figure 1A, right panel). We next determined if this p73β-specific antibody could detect endogenous p73β. Treatment of human HCT116(3) cells with cisplatin has been shown to induce p73 [11,19]. Thus, extracts from HCT116(3) cells treated with either cisplatin or exposed to γ-irradiation were subjected to immunoblot analysis with the GC15 antibody. We were able to detect a band that corresponds to p73β and migrates at around 62 kDa, when compared to the overexpressed p73β, which was used as a positive control (Figure 1B). The intensity of this band increased in extracts from cells that were cisplatin-treated or γ-irradiated (Figure 1B), suggesting that this antibody is capable of recognizing endogenous p73β. However, it should be noted that we had to load between 300 and 500 µg of total cell extracts and had to immunodetect with the highly sensitive Supersignal detection kit (see Materials and Methods section). Conventional ECL and ECL Plus immunodetection kits were not able to give clear signals (data not shown). To further confirm if this antibody was specific for p73β, we examined cell extracts from wild-type and c-Abl null mouse fibroblasts that were treated with cisplatin. Absence of c-Abl has been shown to compromise the induction of endogenous p73 [11]. Immunoblot analysis revealed that the intensity of the band at around 62 kDa that was present at low levels in the untreated wild-type cell extracts increased on cisplatin treatment (Figure 1C). By contrast, this band was barely detectable in the c-Abl null cell extracts (Figure 1C), consistent with previous reports [11]. Together, the data suggest that the GC15 antibody is able to specifically recognize the endogenous p73β protein in human and mouse cells.
UV Irradiation Induces p73β
To investigate if multiple stress signals induce p73β, initial experiments were conducted by transiently transfecting monkey COS7 cells with a p73β expression vector and subjected to UV irradiation. We found that UV irradiation resulted in an increase in the amount of transfected p73β protein 2 hours after irradiation compared to unirradiated cells (Figure 2A, compare lanes 2 and 4). The levels of the p73β decreased to basal levels 24 hours after irradiation (Figure 2A, lane 5). In contrast, UV irradiation did not result in an increase of the green fluorescent protein (Egfp) that was used to normalize the transfection efficiency (Figure 2A). Because the expression of the transfected p73β was upregulated by UV irradiation, we next sought to determine if endogenous p73β could also be induced by UV irradiation. To this end, we UV-irradiated several cell lines including Saos2 (p53 null human osteosarcoma), HepG2 (p53 wildtype human liver cancer line), H1299 (p53 null human lung carcinoma), and COS7 (the presence of SV40 antigen resulting in functional inactivation of p53) cells, and collected them at either 1 or 24 hours postirradiation. Accumulation of p73β was observed around an hour after UV irradiation in HepG2, H1299, and COS7 cells, and the levels declined at 24 hours postirradiation in these cells (Figure 2B, compare lanes 4 and 5, 7 and 8, and 10 and 11). However, we noticed that p73β was significantly upregulated at 24 hours postirradiation in Saos2 cells as well as in mouse fibroblasts (Figure 2B, compare lanes 1 and 3; Figure 3B). Although the reason for the difference in kinetics of p73β upregulation by UV irradiation in different cell lines is, at present, unclear, it is evident that the levels of endogenous p73β can be induced by UV irradiation in human, mouse, and monkey cell lines.
We also examined if UV irradiation can lead to induction of p73 transcriptional activity. p73-mediated transcriptional activity was monitored by transiently transfecting both H1299 and COS7 cells with the plasmid containing the luciferase reporter gene driven by the minimal mdm2 promoter construct. UV irradiation resulted in an increase in the levels of luciferase expression in both cell lines (1.7- to 5-fold), correlating with the increase in the levels of p73β protein (Figure 2C). Importantly, the UV-induced p73-like activity was inhibited by the expression of a dominant-negative p73 protein (p73DD), which has been previously demonstrated to inhibit p73 activity [20], suggesting that p73-like transcriptional activity could be induced by UV irradiation in the absence of functional p53. However, it is noteworthy that we were not able to observe a very significant increase in the p73-like activity in the absence of p53, which suggests that this p73-like activity is much weaker than that induced by p53.
Multiple Stress Signals Induce p73β
We next investigated if other stress signals have the ability to upregulate p73. COS7 cells transiently transfected with p73β expression plasmids were treated with various stress-inducing agents as indicated and the amount of p73β was monitored. Analogous to UV irradiation, treatment with other stress stimuli including the DNA-damaging agents IR and cisplatin, topoisomerase inhibitor CPT, osmotic stress-inducing sorbitol, oxidative stress-inducing hydrogen peroxide, and inhibitors of microtubule dynamics such as taxol and nocodazol resulted in the accumulation of transfected p73β protein (Figure 3A). IR, hydrogen peroxide, and CPT treatment resulted in the increase in the levels of p73β at 2 hours posttreatment and the levels returned to basal levels at 24 hours after treatment (Figure 3A, compare lane 4, 10 and 14, to lane 2). By contrast, treatment with cisplatin, sorbitol, nocodazol, and taxol resulted in an increase in the levels of p73 that was maximal at 24 hours posttreatment (Figure 3A, lanes 7, 9, 13, and 17). Nevertheless, all these stress signals resulted in an increase in the levels of the transfected p73β. As such, we examined if the endogenous p73β can be upregulated by these signals. Treatment of immortalized NIH3T3 cells, p53 null mouse fibroblasts, and Saos2 cells with these stress signals resulted in similar upregulation of the p73β protein, further indicating that stress-induced p73 upregulation can occur independent of p53 (Figure 3B), albeit with varying kinetics. As observed with Saos2 cells (Figure 2B), UV irradiation upregulated the p73β protein maximally at 24 hours postirradiation in NIH3T3 and p53 null fibroblasts. By contrast, IR resulted in maximal upregulation of p73β at 2 hours posttreatment in Saos2 and NIH3T3 cells (Figure 3B). However, sorbitol and nocodazol induced maximal p73β protein upregulation at 24 hours posttreatment in all cell lines. Taxol- and hydrogen peroxide-induced p73β expression was observed at both 2 and 24 hours after treatment in NIH3T3 cells, whereas the levels were maximal at 2 hours but decreased at 24 hours in Saos2 cells. The variable pattern of p73β expression among different cell lines probably reflects the inherent differences in the cell types and also the inducing signals. These observations are similar to the variable induction of p53 by different stress signals in different cell types [21], and indicate that all the signals tested here are able to induce p73β as they induce p53 protein.
Stress Signals Induce p73 mRNA and Prolong p73β Half-Life
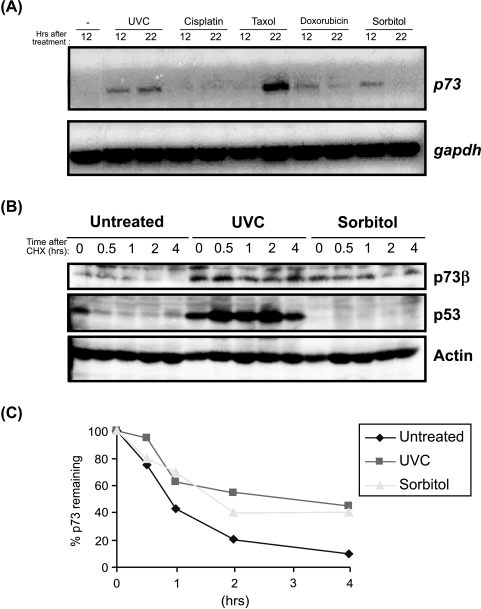
To ascertain if stress-mediated p73 upregulation is dependent on transcriptional activation, we treated H1299 cells with UV and sorbitol as well as the agents that have been shown to activate p73, such as cisplatin and taxol. The status of the full-length p73 mRNA was analyzed by RT-PCR using full-length p73-specific primers that are not able to detect the truncated ΔNp73 (see Materials and Methods section). As shown in Figure 4A, p73 mRNA was upregulated by UV irradiation and treatment with sorbitol and taxol. Cisplatin treatment did not result in the upregulation of p73 mRNA, confirming previous findings demonstrating that cisplatin-mediated upregulation of p73 occurs at the posttranslational level in a c-Abl-dependent manner [10,11]. UV-induced p73 mRNA expression was seen both at 12 and 22 hours postirradiation. In contrast, sorbitol treatment resulted in upregulation of p73 that was observed at 12 hours, but declined at 24 hours (Figure 4A). Taxol treatment resulted in a similar but robust induction of p73 mRNA (Figure 4A). Although the stress signals resulted in an increase in the p73 mRNA levels, this could probably not account for all the accumulation of p73 protein, as the increased levels of p73 mRNA detected 24 hours after UV irradiation do not translate to higher p73 protein levels (Figures 2 and 3). Furthermore, accumulation of the transfected p73 in response to genotoxic stress signals suggested that posttranslational modifications might also play a role in regulating p73 levels. Thus, we evaluated if UV and sorbitol treatment affected the p73β protein half-life. To this end, UV- and sorbitol-treated NIH3T3 cells were incubated with 20 µg/ml cyclohexamide, which results in the inhibition of protein synthesis, and the cells were collected at the indicated time points to determine the amount of remaining p73β. As shown in Figure 4, B and C, the half-life of p73 was about 45 minutes in untreated cells. However, treatment with UV and sorbitol resulted in an increase of the half-life of p73 to about 3–4 and 2 hours, respectively (Figure 4, B and C, compare to actin control). By contrast, only UV irradiation, but not sorbitol treatment, resulted in the increase in the half-life of p53 (Figure 4B). Taken together, the results indicate that p73 is induced transcriptionally as well as posttranslationally by stress signals.
Figure 4.
Transcriptional and posttranslational regulation of p73 by stress stimuli. (A) RT-PCR analysis of full-length p73 in H1299 cells. RNA was prepared by standard procedures from cells treated with the indicated agents and analyzed by semiquantitative RT-PCR analysis. (B and C) Half-life determination. NIH3T3 cells were UV-irradiated (40 J/m) or treated with sorbitol (0.3 M) for 24 hours, and then incubated with 20 µg/m cyclohexamide for the indicated time periods. Cells were collected and analyzed by Western blot analysis as described. The percentage of p73 remaining was quantified by determining the levels of p73 with respect to actin by using the phosphoimager.
p73 Contributes to p53-Independent Cell Death Induced by Stress Signals
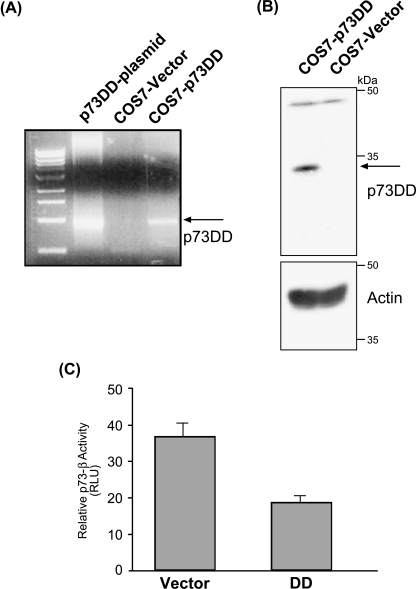
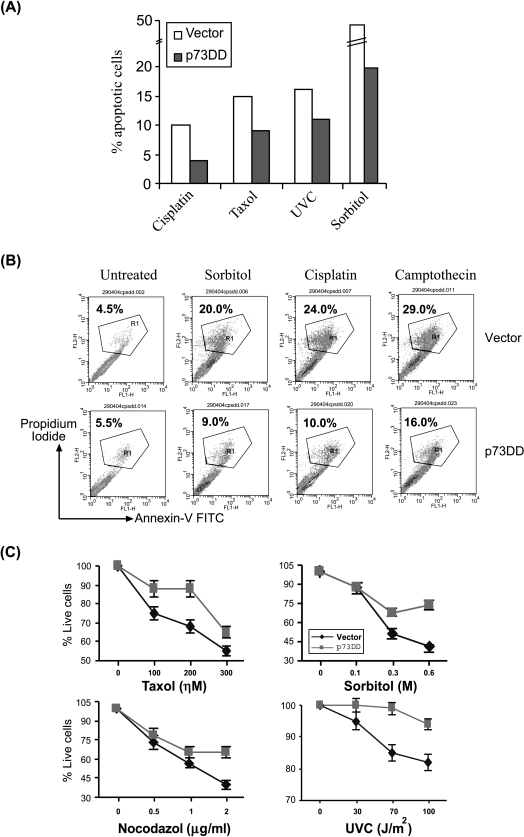
Stress stimuli used in this study can lead to p53-independent cell death. Thus, we examined if inhibition of p73 could affect stress-induced cell death in the absence of p53. To this end, we generated COS7 cells stably expressing the p73DD protein. As shown in Figure 5, A and B, the COS7-p73DD cells expressed both the p73DD RNA and protein, in contrast to the vector-expressing cells. In addition, analysis of p73β-mediated transcriptional activity by transiently transfecting the p73β expression plasmid in these cells indicated that the COS7-p73DD cells exhibited reduced p73β-mediated transcriptional activity (Figure 5C). Hence, we used these cells to analyze the levels of apoptosis induced by the various stress-inducing agents. Treatment of the vector-expressing cells with various stimuli resulted in cell death, when assayed at 24 hours posttreatment for the accumulation of cells with a sub-G1 DNA content (Figure 6A). However, treatment of COS7-p73DD cells with these stress signals resulted in reduced levels of cell death compared to untreated controls (percentage dead cells in vector versus p73DD: cisplatin, 10% vs 4%; taxol, 16% vs 9%; UV, 17% vs 11%; sorbitol, 50% vs 20%) (Figure 6A). Similar results were obtained when apoptosis was determined by another method (i.e., Annexin V staining). p73DD cells were more resistant than vector-expressing cells to apoptosis induced by various signals (percentage of Annexin V+ cells in vector versus p73DD: sorbitol, 20% vs 9%; cisplatin, 24% vs 10%; CPT, 29% vs 16%) (Figure 6B). A more detailed analysis of cell death using varying doses of the stress-inducing agents indicated that the COS7-p73DD cells were generally more resistant to apoptosis than the vector-expressing cells (Figure 6C). Complete resistance to apoptosis was not observed in COS7-p73DD cells probably because the level of inhibition of p73 activity was not complete in these cells, as determined by the magnitude of reduction in the p73-mediated transcriptional activity (Figure 5C). Nevertheless, these findings suggest a critical role for p73β in cell death.
Figure 5.
COS7-p73DD cells. (A) RT-PCR analysis of p73DD expression. COS7 cells were transfected with 1.0 µg of empty vector (pCDNA) or p73DD containing expression plasmid, and were selected on 1 mg/ml G418 for 2 to 3 weeks. Stable cells were collected and analyzed for the expression of p73DD product by RT-PCR analysis. (B) Expression of p73DD by Western blot analysis was performed using 150 µg of total cell lysate and the anti-p73α antibody (clone ER15). (C) Determination of p73 activity in p73DD-expressing cells. Both COS7 vector and p73DD cells were transfected with 100 ng of p73β expression plasmid together with the reporter plasmids, as described in Figure 2C, and analyzed 48 hours after transfection; the relative p73-mediated luciferase activity is indicated.
Figure 6.
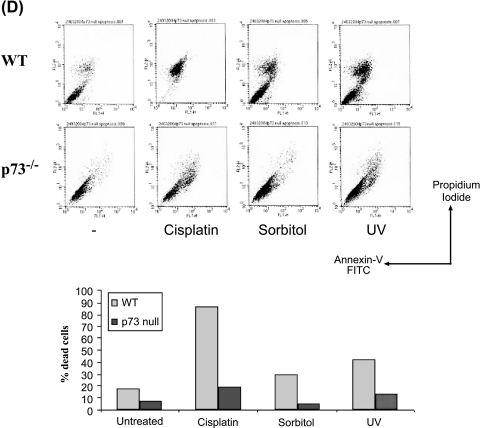
p73DD-expressing cells and p73-/- cells are more resistant to stress-induced apoptosis. (A) Apoptosis is reduced in p73DD-expressing cells. Apoptosis assays were performed in duplicates with COS7 vector (vector) and p73DD (p73DD) cells in six-well dishes with the indicated stress stimuli. Cells were analyzed for the sub-G1 DNA content by flow cytometry. The assays were performed at least thrice independently and representative results are shown. The net amount of apoptotic cells compared to the untreated controls is indicated. (B) The above cells were stained with Annexin V and propidium iodide, and analyzed by flow cytometry to determine apoptotic cells. (C) Analysis of dose-dependent apoptosis. COS7 vector and p73DD cells were treated with the indicated amounts of the various stress-inducing agents and cells were analyzed 24 hours posttreatment as described. The vertical bars represent standard deviations. (D) p73-/- cells are resistant to apoptosis. Wild-type and p73-/- mouse embryonic fibroblasts were treated with the indicated stress signals and apoptotic cells were analyzed by Annexin V staining as described. The percentages of dead cells (Annexin V+) are indicted graphically.
Because p73DD has the ability to inhibit p63, another p53-related protein [20], we further investigated the effect of stress-induced apoptosis using mouse embryonic fibroblasts lacking p73, which were shown to be resistant to doxorubicin and cisplatin treatment [22]. Treatment of wild-type fibroblasts with cisplatin, sorbitol, and UV resulted in massive cell death, as determined Annexin V staining (Figure 6D). However, treatment of p73-/- cells resulted in a dramatic reduction in the apoptotic rates (% Annexin V+ cells in wild type versus p73-/-: cisplatin, 84% vs 19%; sorbitol, 30% vs 5%; UV, 41% vs 13%) (Figure 6D). Taken together, the data indicate that p73 is required for apoptotic induction by various stress signals.
Discussion
The data presented here demonstrate that similar to p53, p73β can also be upregulated by various stress signals, dispelling the notion that p73 is only induced by a subset of agents that induce p53. Subsequent to the report by Kaghad et al. [2] who have not been able to notice an induction of p73 on UV irradiation, it has been generally believed that p73 protein cannot be induced by other stress signals. Kaghad et al. [2] had used a p73α-specific antibody and thus have not been able to detect UV-induced p73β upregulation, although they detected high levels of endogenous p73α in IMR human fibroblasts. However, several groups have subsequently noted the upregulation of endogenous p73 protein by some chemotherapeutic agents such as cisplatin and doxorubicin and by various oncogenes, using different p73-specific antibodies [11,12,18]. Recently, Irwin et al. [13] reported that endogenous p73α can be induced by several chemotherapeutic drugs. We have used a commercially available p73β-specific antibody that has been raised specifically against the p73β protein and that recognizes both transfected and endogenous protein, and found that several common stress signals are able to cause the accumulation of the endogenous p73β protein. It should be noted that endogenous p73β was not easily detectable when lower amounts of cell extracts were used. Very high amounts of total cell extracts (300–500 µg), together with a very sensitive immunodetection system, were required to visualize the endogenous p73β protein, suggesting that the p73β protein is not as abundant and easily detectable as p53. This probably explains hitherto why it has been difficult to detect endogenous p73 protein. Nevertheless, our data together suggest that p73β can be upregulated by several stress signals in human, mouse, and monkey cells.
p73 mRNA was induced by some stress signals such as UV irradiation, sorbitol, taxol, and doxorubicin, indicating that these signals transcriptionally regulate p73. This is in contrast to p53, which is generally thought to be regulated posttranscriptionally by stress signals [23]. Moreover, regulation of p73 mRNA appears to be signal-specific, as cisplatin, a DNA-damaging agent, was not able to upregulate p73 mRNA levels, consistent with previous findings [11]. This suggests that the regulation of p73 is more complex than p53. Moreover, the half-life of p73β is also prolonged by some genotoxic stress signals such as UV irradiation and sorbitol, indicating that these signals regulate p73β both transcriptionally and posttranslationally. Because p73 is less efficient than p53 in inducing some of the p53 target genes [7], it is conceivable that p73 needs perhaps to be regulated by several means for efficient accumulation of the protein.
The induction of p53-independent cell death by many stress signals has been reported previously [11,24]. Although p73 is the functional homologue of p53, its role in cell death induced by other genotoxic stress has been controversial due to the failure to observe an upregulation of p73 protein. However, it has been demonstrated that the well-characterized p73-inducing agents such as cisplatin or doxorubicin and oncogenes such as E2F-1 can result in p73-mediated cell death in a p53-independent manner [18,20]. Our results indicate that p53-like transcriptional activity is induced by UV irradiation in p53 null cells and this can be inhibited by the expression of the dominant-negative p73 inhibitor, p73DD. This suggests that UV irradiation can induce p73 activity, although we cannot exclude the contribution from p63—another p53-related protein that is also inhibited by p73DD [20]. Moreover, treatment of p73DD-expressing cells, as well as p73-/- cells, with several of these stress-inducing agents led to resistance to apoptosis, suggesting that the p73β induced by these agents contributes to p53-independent cell death.
In summary, the data demonstrate that several genotoxic stress signals can activate p73, thus redefining the role of p73 in stress-induced cell death. These findings have far-reaching implications in cancer biology as p73 is currently thought to be activated only by a subset of signals that activate p53. The evidence presented here should intensify research into exploring the possibilities of finding new agents that would activate p73, leading to the inhibition of tumor growth.
Acknowledgements
We thank Y. Shaul, R. Boland, G. Melino, M. Levrero, and J. Wang for c-Abl-/- cells, p73-/- cells, HCT116(3) cells, and the various p73 expression plasmids.
Footnotes
This work was supported by grants from the National Medical Research Council and the Biomedical Research Council of Singapore (to K.S.).
References
- 1.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 2.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, Mckeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;9:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 3.DeLaurenzi V, Costanzo A, Barcaroli D, Terrinoni A, Falco M, Annicchiarico-Petruzzelli M, Levrero M, Melino G. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med. 1998;188:1763–1768. doi: 10.1084/jem.188.9.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 5.Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- 6.Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–194. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Jiang J, Zhou W, Chen X. The potential tumour suppressor p73 differentially regulates cellular p53 target genes. Cancer Res. 1998;58:5061–5065. [PubMed] [Google Scholar]
- 8.Melino G, Lu X, Gasco M, Crook T, Knight RA. Functional regulation of p73 and p63: development and cancer. Trends Biochem Sci. 2003;28:663–670. doi: 10.1016/j.tibs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, Chalas E, Moll UM. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med. 2002;196:765–780. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73 and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 11.Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG Jr, Levrero M, Wang JY. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo A, Merlo P, Pediconi N, Fulco M, Sartorelli V, Cole PA, Fontemaggi G, Fanciulli M, Schiltz L, Blandino G, Balsano C, Levrero M. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol Cell. 2002;9:175–186. doi: 10.1016/s1097-2765(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 13.Irwin M, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WH., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 14.Passegue E, Wagner EF. JunB, suppresses cell proliferation by transcriptional activation of p16(INK4a) expression. EMBO J. 2000;19:2969–2979. doi: 10.1093/emboj/19.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 16.Sabapathy K, Klemm M, Jaenisch R, Wagner EF. Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J. 1997;16:6217–6229. doi: 10.1093/emboj/16.20.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabapathy K, Hu YL, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;11:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 18.Zaika A, Irwin M, Sansome C, Moll UM. Oncogenes induce and activate endogenous p73 protein. J Biol Chem. 2001;276:11310–11316. doi: 10.1074/jbc.M005737200. [DOI] [PubMed] [Google Scholar]
- 19.Shimodaira H, Yoshioka-Yamashita A, Kolodner RD, Wang JY. Interaction of mismatch repair protein PMS2 and the p53-related transcription factor p73 in apoptosis response to cisplatin. Proc Natl Acad Sci USA. 2003;100:2420–2425. doi: 10.1073/pnas.0438031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, Kaelin WH., Jr Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft M, Taya Y, Vousden K. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores ER, Tsai K, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 23.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 24.Baptiste N, Freidlander P, Chen X, Prives C. The proline-rich domain of p53 is required for cooperation with anti-neoplastic agents to promote apoptosis of tumor cells. Oncogene. 2002;21:9–21. doi: 10.1038/sj.onc.1205015. [DOI] [PubMed] [Google Scholar]
- 25.Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;5:1874–1887. doi: 10.1128/MCB.21.5.1874-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, Yulug I, Merlano M, Numico G, Comino A, Attard M, Reelfs O, Gusterson B, Bell AK, Heath V, Tavassoli M, Farrell PJ, Smith P, Lu X, Crook T. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 27.Strano S, Munarriz E, Rossi M, Cristofanelli B, Shaul Y, Castagnoli L, Levine AJ, Sacchi A, Cesareni G, Oren M, Blandino G. Physical and functional interaction between p53 mutants and different isoforms of p73. J Biol Chem. 2000;275:29503–29512. doi: 10.1074/jbc.M003360200. [DOI] [PubMed] [Google Scholar]
- 28.Puig P, Capodieci P, Drobnjak M, Verbel D, Prives C, Cordon-Cardo C, DiComo CJ. p73 Expression in human normal and tumor tissues: loss of p73alpha expression is associated with tumor progression in bladder cancer. Clin Cancer Res. 2003;9:5642–5651. [PubMed] [Google Scholar]