Abstract
The epidermal growth factor receptor (EGFR) is expressed in a variety of human solid tumors, including malignant mesothelioma. EGFR has been implicated in regulation of cell proliferation, survival, angiogenesis, and metastasis, making it an ideal target for drug development. ZD1839 (gefitinib) and OSI-774 (erlotinib) are new, low-molecular-weight, EGFR-selective tyrosine kinase (TK) inhibitors, whereas CI-1033 is a pan- EGFR family TK inhibitor. In the present study, we used ZD1839, OSI-774, and CI-1033 and investigated the effect of these drugs on proliferation, migration, and matrix metalloprotease (MMP) production in three malignant mesothelioma cell lines (M14K, ZL34, and SPC212). Using [3H]thymidine incorporation, DNA synthesis assay, we found that all three drugs inhibited transforming growth factor-alpha (TGF-α)-induced cellular proliferation in a dose-dependent manner. In addition, all three drugs induced apoptosis in ZL34 cells as determined by flow cytometry using annexin-V staining. Furthermore, all three drugs inhibited TGFA-α-induced cell migration (chemotaxis) in a dose-dependent manner as determined by Boyden chamber assay. TGF-α-induced MMP-9 production was also inhibited in a dose-dependent manner as determined by gelatin zymography in three cell lines tested. In conclusion, our study demonstrates inhibitory effectiveness of EGFR-TK inhibitors in malignant mesothelioma cells and suggests that these drugs may be an effective treatment strategy for malignant mesothelioma.
Keywords: Malignant mesothelioma, epidermal growth factor receptor (EGFR), tyrosine kinase (TK) inhibitors, migration, matrix metalloproteases (MMPs)
Introduction
Malignant mesothelioma is a highly aggressive tumor, which arises from mesothelial-lined surfaces, most often the pleural cavities, but also from the peritoneum, pericardium, and paratesticular soft tissues [1]. Malignant mesotheliomas spread through the underlying basement membrane or along serosal surfaces. Typically, mesothelioma spreads to the pericardium, contralateral hemithorax, and/or peritoneal cavity by invasion through the diaphragm. Invasion through needle biopsy tracts and incisions in the thoracic wall are common features in malignant mesothelioma [6,7]. The resulting tumor often forms diffuse thickening of involved surfaces rather than solitary rounded lesions seen in other neoplasms. Metastases occur in up to 75% of mesothelioma patients [31]. Three histologic subtypes of malignant mesothelioma are distinguished: epithelial, fibrous (sarcomatoid), and mixed (biphasic). There is no effective standard therapy for malignant mesothelioma regardless of the treatment modality used. Although various chemotherapies have been evaluated in malignant mesothelioma, few have shown response rates better than 20% [3]. Notably, these therapies did not prolong patient survival. Therefore, more effective chemotherapy agents are needed.
The epidermal growth factor receptor (EGFR)2 is a transmembrane glycoprotein (Mr 170 kDa) with an external binding domain and an intracellular tyrosine kinase (TK) domain. The binding of EGFR ligands, such as transforming growth factoralpha (TGF-α), to EGFR induces receptor dimerization, followed by activation of the intrinsic TK, leading to receptor tyrosine autophosphorylation [35,38]. Activation of EGFR-TK has been identified as an important feature that initiates the cascade of intracellular signaling events that regulate cell proliferation, survival, angiogenesis, cell movement, and metastasis [14,45]. The EGFR is expressed in a variety of human solid tumors, including malignant mesothelioma [15], and is associated with disease progression, poor survival, and poor response to traditional cytostatic therapy [17,18,33,43]. A previous study suggested that EGFR might be involved in asbestos-induced pathogenesis of malignant mesothelioma [46]. In addition, our previous study has also shown that EGFR is functionally active and that ligation of this receptor induces mesothelioma cell migration and matrix metalloprotease (MMP)-9 production [29]. For these reasons, the blockade of EGFR-activating pathway may provide a potential therapeutic target for treatment of malignant mesothelioma.
ZD1839 (gefitinib) and OSI-774 (erlotinib) are low-molecular-weight EGFR-selective TK inhibitors, whereas CI-1033 is a pan-EGFR family TK inhibitor that blocks signal transduction pathway implicated in cancer cell proliferation, survival, and other host-dependent processes promoting cancer progression [5,19,41]. ZD1839, OSI-774, and CI-1033 are currently under evaluation in clinical trials in patients with lung cancer and other tumors.
In the present study, we examined if these three TK inhibitors affect cell proliferation, cell migration, and MMP production, and induce apoptosis in three established malignant mesothelioma cell lines representing different histologic subtypes of this tumor.
Materials and Methods
Cells
Three human malignant mesothelioma cell lines were used: M14K (epithelial type), ZL34 (fibrous type), and SPC212 (mixed type) [37,39]. All malignant mesothelioma cell lines were cultured in RPMI 1640 supplemented with 5% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 µg/ml), and 2 mM l-glutamine. The cells were maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
Chemicals
ZD1839 was provided by AstraZeneca (Macclesfield, UK). OSI-774 was provided by OSI/Genentech (Melville, NY) and CI-1033 was provided by Pfizer (New York, NY). All drugs were dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C. TGF-α was purchased from R&D Systems (Abingdon, UK). RPMI 1640, FCS, penicillin, streptomycin, and l-glutamine were obtained from Life Technologies (Paisley, UK). Gelatin, BSA, and collagen type IV were purchased from Sigma (St. Louis, MO).
RNA Isolation, cDNA Synthesis, Primers, and RT-PCR
Total RNA was isolated from cell lines using the guanidinium isothiocyanate method as previously described [11]. cDNA synthesis was performed using a first-strand cDNA synthesis kit (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. For cDNA synthesis, 5 µg of total RNA were taken and Not- (T) 18 primers were used for cDNA generation. The reverse transcript (1 µl in a final volume of 25 µl) was subjected to PCR. The primers used and the PCR procedures have been described previously [42].
Reverse transcription-polymerase chain reaction (RT-PCR) was performed on RNA samples from three malignant mesothelioma cell lines and a MCF-7 cell line (positive control) [13]. PCR products were visualized by ethidium bromide staining of 1% agarose gels. Integrity of RNA and cDNA synthesis was monitored by amplification of β-actin mRNA.
Growth-Inhibition Assay
[3H]Thymidine incorporation assays were performed in triplicate in 96-well microtiter plates. Each well was filled with malignant mesothelioma cells (2 x 104) in serum-containing medium. After 24 hours, the medium was changed and the cells were cultured for additional 6 hours in serum-free medium. Finally, fresh serum-free medium with indicated concentrations of different drugs and/or TGF-α were added, and the cells incubated for 48 hours. Control wells were filled with serum-free medium. [3H]Thymidine (1 µCi/well) (Amersham Life Science, Uppsala, Sweden) was added during the last 18 hours of culture. The cells were harvested onto filters with a plate harvester (Harvester 996; Tomtec, Hamden, CT) according to the manufacture's instructions and counted in an automated counter (1450 MicroBeta Trilux; Wallac, Sweden AB, Stockholm, Sweden); results were expressed as counts per minute (cpm).
In addition, growth-inhibition assays were also performed by counting the number of viable cells after different treatments.
Flow Cytometric Analysis of Apoptosis
To investigate whether different drugs induce apoptosis in mesothelioma cells, flow cytometry was performed using a FACScan analyzer after staining with annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI). The mesothelioma cells were cultured in six-well culture plates in the presence of different drugs and/or TGF-α. After 24 hours treatment, the cells were examined for phosphatidylserine expression on the cell surface with the Annexin-V FITC apoptosis detection kit (BioSource Europé, S.A., Nivelles, Belgium) according to the manufacturer's instructions. The cells that stained positive for annexin-V and negative for PI were defined as in the early apoptosis.
Cell Migration Assay
The migration of mesothelioma cells was assayed using 48-well Boyden chambers (Neuro Probe, Cabin John, MD) [8] fitted with 12-µm polyvinylpyrrolidone-free polycarbonate filters (Poretics, Livermore, CA). For haptotaxis assays, the lower side of filters were coated overnight at 4°C with 10 µg/ml of collagen type IV [25]. For determination of chemotaxis, the filters were coated on both sides with 10 µg/ml of collagen type IV and the indicated concentrations of TGF-α diluted in RPMI 1640 containing 1 mg/ml of BSA were placed in the lower compartments of Boyden chambers. Mesothelioma cells (5 x 104 cells, preincubated overnight with the indicated concentrations of different drugs and remained with the cells throughout the assay) were placed in the upper compartments of Boyden chambers and incubated for 5 hours in a humidified incubator at 37°C. At the end of the assays, the filters were removed, fixed in methanol, and stained with Giemsa stain. The filters were then placed onto glass slides and the cell pellets corresponding to the upper wells were wiped off with cotton swabs. Cells that have migrated through the pores to the lower side of the filters were counted in a blinded fashion using light microscopy under high-power field (400x). For each triplicate, the number of cells in three high-power fields was counted and the average was determined.
Gelatin Zymography
For determination of MMP production, mesothelioma cells were cultured until subconfluent, serum-supplemented medium was removed, and the cell monolayer was extensively washed with PBS to remove remaining serum proteins. The cells were then cultured in serum-free RPMI 1640 for 6 hours. TGF-α and/or different drugs were then added and the cells were cultured for 36 hours. Aliquots of serumfree conditioned medium (adjusted to the same number of cells) were solubilized in sample buffer containing 2% sodium dodecyl sulfate (SDS) without reducing agents, then the samples were applied without boiling on 6% polyacrylamide gels containing 2 mg/ml of gelatin. After electrophoresis, the gels were washed in Hanks' balanced salt solution (HBSS) in the presence of 2.5% Triton X-100 for 2 x 20 minutes to remove SDS, then briefly rinsed in HBSS. The gels were then incubated in buffer (20 mM glycine, 10 mM CaCl2, and 1 µM ZnCl2, pH 8.3) for 48 hours at 37°C. After incubation, the gels were stained in 0.1% Coomassie brilliant blue for 1 hour and destained. The substrate degrading enzymes were identified as clear bands in the blue background. The area and intensity of the bands was quantified by NIH Image V.1.62 software (National Institutes of Health, Bethesda, MD).
Results
Expression of EGFR in Mesothelioma Cells by RT-PCR
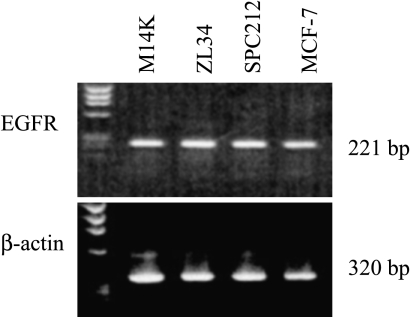
To characterize EGFR expressed by mesothelioma cells, RT-PCR with specific primers was performed. As shown in Figure 1, all three mesothelioma cell lines expressed EGFR.
Figure 1.
Expression of EGFR in human malignant mesothelioma cells. Total RNA from each sample was reverse-transcribed, amplified for 30 cycles, and analyzed on 1.5% agarose gel. The 221- and 320-bp DNA fragments were produced using primers designed to amplify EGFR and β-actin cDNA sequences, respectively. Positive control was MCF-7 for EGFR. The bands were visualized using UV light and photographed.
Effect of TK Inhibitors on Proliferation of Mesothelioma Cells
To determine the effect of different drugs on proliferation in mesothelioma cells, we performed DNA synthesis assay using [3H]thymidine incorporation. Cell counts after incubation of mesothelioma cells with TGF-µ and TK inhibitors were also performed.
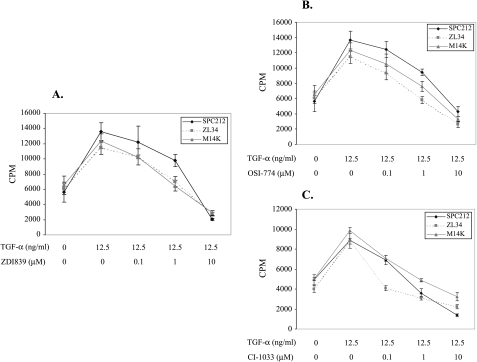
First, we investigated the effect of ZD1839, OSI-774, and CI-1033 on cell proliferation at 24, 48, and 72 hours, respectively. All three drugs inhibited TGF-α-induced cellular proliferation in a time-dependent manner, reaching maximum inhibitory effect at 48 hours compared with controls cultured without TK inhibitors (data not shown). We then investigated the effect of different concentrations of TK inhibitors on cell proliferation after 48 hours of incubation. As shown in Figure 2 (A–C), three drugs inhibited cellular proliferation in a dose-dependent manner and had significant inhibitory effect at 10 µM concentration. In addition, three drugs inhibited cellular proliferation at 10 µM concentration in three mesothelioma cell lines as determined by cell counting (data not shown).
Figure 2.
(A–C) DNA synthesis assay. DNA synthesis was determined by incorporation of [3H]thymidine into mesothelioma cells. Different drugs (A, ZD1839; B, OSI-774; C, CI-1033) and/or TGF-α (12.5 ng/ml) were added and cells incubated for 48 hours. The results are expressed as counts per minute (cpm) and presented as the mean ± SD triplicates for each TGF-α and/or drug concentrations.
Induction of Apoptosis by TK Inhibitors in Mesothelioma Cells
To determine whether ZD1839-, OSI-774-, and CI-1033-inhibited cellular proliferation was accompanied by apoptosis in mesothelioma cells, we performed flow cytometric analysis of mesothelioma cells after annexin-V and PI staining for the detection of the early stage of apoptosis.
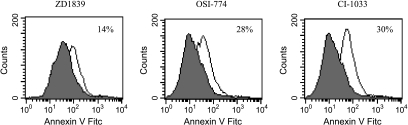
As shown in Figure 3, early stage of apoptosis was observed in 14%, 28%, and 30% of the ZL34 cells treated with 10 µM ZD1839, OSI-774, and CI-1033, respectively. However, all three drugs failed to induce apoptosis in the other two mesothelioma cell lines (M14K and SPC212) (data not shown).
Figure 3.
Induction of early-stage apoptosis by TK inhibitors in malignant mesothelioma cells. The apoptosis was determined by Annexin-V FITC and PI staining and analyzed by flow cytometry. Histograms of cells versus log fluorescence intensity were generated. Shaded histograms represent fluorescence of cells treated with TGF-α only; open histograms indicate fluorescence of cells treated with different drugs and TGF-α. The early stage of apoptosis was observed in 14%, 28%, and 30% of the ZL34 cells treated with 10 µM of ZD1839, OSI-774, and CI-1033, respectively. Data shown are representative of three independent experiments. For each sample, 10,000 cells were analyzed.
Inhibition of TGF-α-Induced Chemotaxis by TK Inhibitors
To determine whether ZD1839, OSI-774, and CI-1033 inhibited migration of mesothelioma cells, we performed the Boyden chamber assay. Two different assays were performed: chemotaxis, using TGF-α as chemoattractant, and haptotaxis, where the lower side of the filter was coated with collagen type IV [25].
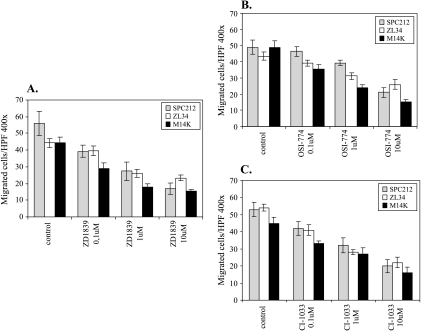
As shown in Figure 4 (A–C), TK inhibitors inhibited TGF-α-induced cell migration in a dose-dependent manner in three investigated mesothelioma cell lines. In contrast, the same drugs failed to inhibit collagen type IV-induced haptotaxis, indicating that TK inhibitors act specifically on TGF-α-induced motile events but not collagen type IV-induced haptotaxis (data not shown).
Figure 4.
(A–C) Inhibition of TGF-α-induced chemotaxis by TK inhibitors in malignant mesothelioma cells. Lower wells of Boyden chambers were filled with TGF-α (12.5 ng/ml) diluted in serum-free RPMI supplemented with 1 µg/ml BSA. The membranes were coated on both sides with 10 µg/ml of collagen type IV. Mesothelioma cells (preincubated overnight with indicated concentrations of different drugs that remained with the cells throughout the assay) were placed in the upper wells of Boyden chambers. The number of migrated cells is the mean ± SD of triplicates for each data point.
Inhibition of MMP-9 Production by TK Inhibitors in Mesothelioma Cells
To determine whether different drugs inhibited TGF-α-induced production of MMPs, we performed gelatin zymography and quantified the production of MMPs by using densitometry.
Aliquots of serum-free conditioned medium from mesothelioma cells treated with TGF-α in the presence or absence of TK inhibitors were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) gelatin zymography. As shown in Figure 5 (A–C), all three drugs inhibited the production of MMP-9 induced by TGF-α in a dose-dependent manner in the three investigated mesothelioma cell lines. In contrast, the level of MMP-2 production was not affected by the same drugs. Notably, stable levels of MMP-2 can be viewed as an internal control for loading of equal amounts of protein. The human fibrosarcoma cell line HT-1080 was used as positive control for demonstration of MMP-9 and MMP-2 production [9].
Figure 5.
(A–C) Inhibition of MMP-9 production by TK inhibitors in malignant mesothelioma cells. The gels show proteolytic activity in serum-free conditioned medium in the presence or absence of different drugs and/or TGF-α. Bars summarize the relative activity of the area and intensity of MMP-9 and MMP-2, as determined by image analysis, with 0 ng/ml of TGF-α and 0 µM of drugs of MMP-9 band set as 1. Data shown are representative of three independent experiments.
Discussion
Inhibition of EGFR signaling represents a promising new strategy in the development of selective anticancer therapies. ZD1839 and OSI-774 are selective EGFR-TK inhibitors, whereas CI-1033 is a pan-EGFR family TK inhibitor. In the present study, we evaluated the effect of these three TK inhibitors on cell proliferation, induction of apoptosis, cell migration, and production of MMPs in cell lines representing all three histologic subtypes of malignant mesothelioma.
Exposure of mesothelioma cells to TK inhibitors produced a dose-dependent growth inhibition in all cell lines tested. Although previous studies have shown that TK inhibitors may induce apoptosis in some cancer cell lines [12,32,34], in the present study the growth inhibition induced by three TK inhibitors was mainly cytostatic. The apoptosis induced by three TK inhibitors was observed in only one cell line (ZL34) with higher drug concentration (10 µM). The failure to induce apoptosis in two mesothelioma cell lines was probably because most cancer cell lines secrete several autocrine growth factors, some of which are likely to maintain survival in the absence of serum [36]. In addition, in the present study apoptosis was examined at only one time point (24 hours), any transient increase in apoptosis may have gone undetected. Other studies reported a similar observation that TK inhibitor treatment leads to primarily cytostatic rather than cytotoxic effects in malignant mesothelioma cells [21]. Similar findings have also been observed in in vitro studies using ovarian, breast, and colon cancer cell lines [12].
Another important finding in the present study was that all three TK inhibitors inhibited TGF-α-induced chemotaxis and production of MMP-9. It is widely accepted that cell migration and enzymic degradation of extracellular matrix (ECM) play an essential role in tumor invasion and metastasis [27]. Our previous studies and unpublished results have shown that mesothelioma cells migrate to different ECM components, such as type IV collagen, and different growth factors, including ligands of EGFR [23–26]. In the present study, ZD1839, OSI-774, and CI-1033 inhibited TGF-α-induced chemotaxis but failed to inhibit type IV collagen-induced haptotaxis. This difference may be the fact that these two motile processes are governed by a distinct signal transduction pathway [2]. Our previous studies have also shown that mesothelioma cells express several members of the MMP family and that different growth factors regulate production of MMP-9 [28,29]. Furthermore, EGFR activation initiates complex signaling cascades, which can result in various cellular effects, including regulation of cell invasion and metastasis by several downstream signaling mechanisms. For instance, EGFR-phospholipase C-γ signaling is required for enhanced cell motility in prostate carcinoma [22]; phosphatidylinositol 3-kinase/Akt signaling is involved in colorectal carcinoma cell migration [10]; and mitogen-activated protein kinase/p21- activated kinase 1 signaling mediated cell motility in skin cancer cells [4]. Previous studies of metastases of bladder carcinoma xenografts have shown that treatment of tumorbearing mice with anti-EGFR MAb 225 results in prevention of metastases formation, accompanied by a decrease in tumor production of MMP-9 [20]. In addition, the study of metastases of murine hepatocellular carcinoma has also shown that TK inhibitor ZD1839 inhibited EGFR-induced chemotactic migration and production of active MMP-9 in vitro and introhepatic metastasis in vivo [30]. These studies provide evidence that cellular activities potentiating metastasis can be regulated by different EGFR downstream signaling pathways. These studies and our present results indicate that EGFR signaling plays an important role in tumor metastasis and EGFR inhibitors are effective at inhibiting tumor metastasis in vitro and in vivo.
Malignant mesothelioma is an asbestos-associated tumor. Previous studies suggested that EGFR signaling may be involved in asbestos-induced pathogenesis of malignant mesothelioma. For instance, asbestos-transformed rat mesothelial cells express EGFR and TGF-α [44], and asbestos fiber can induce the phosphorylation of EGFR, which appears to correlate with the carcinogenicity of asbestos fibers in rat pleural mesothelial cells [46]. A specific inhibitor of EGFR, tyrphostin AG 1478, can significantly inhibit activator protein-1 DNA binding activity and EGFR phosphorylation after exposure of mesothelial cells to crocidolite, a fibrous asbestos preparation [16]. In addition, a recent study has demonstrated that TK inhibitor ZD1839 significantly enhanced the antitumor activity of radiation in a mesothelioma model [40]. Many other studies have also revealed a potentiation of the antitumor effects of cytotoxic chemotherapy agents by TK inhibitor ZD1839 in various human tumor models [12]. Taken together, these studies strongly suggest that EGFR signaling is involved in the pathogenesis of malignant mesothelioma and the blockade of EGFR signaling pathway may provide a potential therapeutic target for treatment of patients with malignant mesothelioma.
In conclusion, the present study demonstrates that the selective EGFR-TK inhibitors ZD1839 and OSI-774 and the pan-EGFR family TK inhibitor CI-1033 are effective at inhibiting not only proliferation, but also migration and MMP-9 production of all three histologic types of mesothelioma cells. Therefore, these three TK inhibitors can be considered as new targets for further therapeutic interventions in patients with malignant mesothelioma.
Abbreviations
- EGFR
epidermal growth factor receptor
- TGF-α
transforming growth factor-α
- TK
tyrosine kinase
- MMPs
matrix metalloproteases
- FCS
fetal calf serum
- BSA
bovine serum albumin
- PI
propidium iodide
- ECM
extracellular matrix
Footnotes
This work was supported by grants to J. Klominek from the Swedish Cancer Society and the Swedish Heart and Lung foundation.
References
- 1.Antman KH. Clinical presentation and natural history of benign and malignant mesothelioma. Semin Oncol. 1981;8:313–320. [PubMed] [Google Scholar]
- 2.Aznavoorian S, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baas P. Chemotherapy for malignant mesothelioma: from doxorubicin to vinorelbine. Semin Oncol. 2002;29:62–69. doi: 10.1053/sonc.2002.30231. [DOI] [PubMed] [Google Scholar]
- 4.Barnes CJ, Bagheri-Yarmand R, Mandal M, Yang Z, Clayman GL, Hong WK, Kumar R. Suppression of epidermal growth factor receptor, mitogen-activated protein kinase, and Pak1 pathways and invasiveness of human cutaneous squamous cancer cells by the tyrosine kinase inhibitor ZD1839 (Iressa) Mol Cancer Ther. 2003;2:345–351. [PubMed] [Google Scholar]
- 5.Baselga J, Averbuch SD. ZD1839 (‘Iressa’) as an anticancer agent. Drugs. 2000;60(Suppl 1):33–40. doi: 10.2165/00003495-200060001-00004. discussion 41 – 32. [DOI] [PubMed] [Google Scholar]
- 6.Boutin C, Rey F, Gouvernet J, Viallat JR, Astoul P, Ledoray V. Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer. 1993;72:394–404. doi: 10.1002/1097-0142(19930715)72:2<394::aid-cncr2820720214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest. 1995;108:754–758. doi: 10.1378/chest.108.3.754. [DOI] [PubMed] [Google Scholar]
- 8.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Kleiner DE, Unsworth EJ, Stetler-Stevenson WG. Cellular activation of the 72 kDa type IV procollagenase/TIMP-2 complex. Kidney Int. 1993;43:163–170. doi: 10.1038/ki.1993.27. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, DePlacido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- 13.Ciardiello F, Caputo R, Borriello G, Del Bufalo D, Biroccio A, Zupi G, Bianco AR, Tortora G. ZD1839 (IRESSA), an EGFR-selective tyrosine kinase inhibitor, enhances taxane activity in bcl-2 overexpressing, multidrug-resistant MCF-7 ADR human breast cancer cells. Int J Cancer. 2002;98:463–469. doi: 10.1002/ijc.10230. [DOI] [PubMed] [Google Scholar]
- 14.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 15.Dazzi H, Hasleton PS, Thatcher N, Wilkes S, Swindell R, Chatterjee AK. Malignant pleural mesothelioma and epidermal growth factor receptor (EGF-R). Relationship of EGF-R with histology and survival using fixed paraffin embedded tissue and the F4, monoclonal antibody. Br J Cancer. 1990;61:924–926. doi: 10.1038/bjc.1990.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faux SP, Houghton CE, Hubbard A, Patrick G. Increased expression of epidermal growth factor receptor in rat pleural mesothelial cells correlates with carcinogenicity of mineral fibres. Carcinogenesis. 2000;21:2275–2280. doi: 10.1093/carcin/21.12.2275. [DOI] [PubMed] [Google Scholar]
- 17.Fox SB, Smith K, Hollyer J, Greenall M, Hastrich D, Harris AL. The epidermal growth factor receptor as a prognostic marker: results of 370 patients and review of 3009 patients. Breast Cancer Res Treat. 1994;29:41–49. doi: 10.1007/BF00666180. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Slaton JW, Perrotte P, Davis DW, Bruns CJ, Hicklin DJ, McConkey DJ, Sweeney P, Radinsky R, Dinney CP. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6:4874–4884. [PubMed] [Google Scholar]
- 21.Janne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res. 2002;62:5242–5247. [PubMed] [Google Scholar]
- 22.Kassis J, Moellinger J, Lo H, Greenberg NM, Kim HG, Wells A. A role for phospholipase C-gamma-mediated signaling in tumor cell invasion. Clin Cancer Res. 1999;5:2251–2260. [PubMed] [Google Scholar]
- 23.Klominek J, Baskin B, Hauzenberger D. Platelet-derived growth factor (PDGF) BB acts as a chemoattractant for human malignant mesothelioma cells via PDGF receptor beta-integrin alpha3beta 1 interaction. Clin Exp Metastasis. 1998;16:529–539. doi: 10.1023/a:1006542301794. [DOI] [PubMed] [Google Scholar]
- 24.Klominek J, Baskin B, Liu Z, Hauzenberger D. Hepatocyte growth factor/scatter factor stimulates chemotaxis and growth of malignant mesothelioma cells through c-met receptor. Int J Cancer. 1998;76:240–249. doi: 10.1002/(sici)1097-0215(19980413)76:2<240::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Klominek J, Robert KH, Sundqvist KG. Chemotaxis and haptotaxis of human malignant mesothelioma cells: effects of fibronectin, laminin, type IV collagen, and an autocrine motility factor-like substance. Cancer Res. 1993;53:4376–4382. [PubMed] [Google Scholar]
- 26.Klominek J, Sumitran Karuppan S, Hauzenberger D. Differential motile response of human malignant mesothelioma cells to fibronectin, laminin and collagen type IV: the role of beta1 integrins. Int J Cancer. 1997;72:1034–1044. doi: 10.1002/(sici)1097-0215(19970917)72:6<1034::aid-ijc19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990;1:99–106. [PubMed] [Google Scholar]
- 28.Liu Z, Ivanoff A, Klominek J. Expression and activity of matrix metalloproteases in human malignant mesothelioma cell lines. Int J Cancer. 2001;91:638–643. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1102>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Klominek J. Regulation of matrix metalloprotease activity in malignant mesothelioma cell lines by growth factors. Thorax. 2003;58:198–203. doi: 10.1136/thorax.58.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo M, Sakurai H, Saiki I. ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, shows antimetastatic activity using a hepatocellular carcinoma model. Mol Cancer Ther. 2003;2:557–561. [PubMed] [Google Scholar]
- 31.Moskal TL, Urschel JD, Anderson TM, Antkowiak JG, Takita H. Malignant pleural mesothelioma: a problematic review. Surg Oncol. 1998;7:5–12. doi: 10.1016/s0960-7404(98)00019-x. [DOI] [PubMed] [Google Scholar]
- 32.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 33.Neal DE, Sharples L, Smith K, Fennelly J, Hall RR, Harris AL. The pidermal growth factor receptor and the prognosis of bladder cancer. Cancer. 1990;65:1619–1625. doi: 10.1002/1097-0142(19900401)65:7<1619::aid-cncr2820650728>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Normanno N, Campiglio M, De LA, Somenzi G, Maiello M, Ciardiello F, Gianni L, Salomon DS, Menard S. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann Oncol. 2002;13:65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 35.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pederson L, Winding B, Foged NT, Spelsberg TC, Oursler MJ. Identification of breast cancer cell line-derived paracrine factors that stimulate osteoclast activity. Cancer Res. 1999;59:5849–5855. [PubMed] [Google Scholar]
- 37.Pelin-Enlund K, Husgafvel-Pursiainen K, Tammilehto L, Klockars M, Jantunen K, Gerwin BI, Harris CC, Tuomi T, Vanhala E, Mattson K. Asbestos-related malignant mesothelioma: growth, cytology, tumorigenicity and consistent chromosome findings in cell lines from five patients. Carcinogenesis. 1990;11:673–681. doi: 10.1093/carcin/11.4.673. [DOI] [PubMed] [Google Scholar]
- 38.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 39.Schmitter D, Lauber B, Fagg B, Stahel RA. Hematopoietic growth factors secreted by seven human pleural mesothelioma cell lines: interleukin-6 production as a common feature. Int J Cancer. 1992;51:296–301. doi: 10.1002/ijc.2910510220. [DOI] [PubMed] [Google Scholar]
- 40.She Y, Lee F, Chen J, Haimovitz-Friedman A, Miller VA, Rusch VR, Kris MG, Sirotnak FM. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 selectively potentiates radiation response of human tumors in nude mice, with a marked improvement in therapeutic index. Clin Cancer Res. 2003;9:3773–3778. [PubMed] [Google Scholar]
- 41.Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin Oncol. 2001;28:80–85. doi: 10.1016/s0093-7754(01)90285-4. [DOI] [PubMed] [Google Scholar]
- 42.Tahara M, Tasaka K, Masumoto N, Adachi K, Adachi H, Ikebuchi Y, Kurachi H, Miyake A. Expression of messenger ribonucleic acid for epidermal growth factor (EGF), transforming growth factoralpha (TGF alpha), and EGF receptor in human amnion cells: possible role of TGF alpha in prostaglandin E2 synthesis and cell proliferation. J Clin Endocrinol Metab. 1995;80:138–146. doi: 10.1210/jcem.80.1.7829602. [DOI] [PubMed] [Google Scholar]
- 43.Uhlman DL, Nguyen P, Manivel JC, Zhang G, Hagen K, Fraley E, Aeppli D, Niehans GA. Epidermal growth factor receptor and transforming growth factor alpha expression in papillary and nonpapillary renal cell carcinoma: correlation with metastatic behavior and prognosis. Clin Cancer Res. 1995;1:913–920. [PubMed] [Google Scholar]
- 44.Walker C, Everitt J, Ferriola PC, Stewart W, Mangum J, Bermudez E. Autocrine growth stimulation by transforming growth factor alpha in asbestos-transformed rat mesothelial cells. Cancer Res. 1995;55:530–536. [PubMed] [Google Scholar]
- 45.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 46.Zanella CL, Posada J, Tritton TR, Mossman BT. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]