Abstract
Feto-acinar pancreatic protein (FAPP) characterized by mAbJ28 reactivity is a specific component associated with ontogenesis and behaves as an oncodevelopment-associated antigen. We attempted to determine whether pancreatic tumoral SOJ-6 cells are expressed at their surface FAPP antigens and to examine if specific antibodies directed against these FAPP epitopes could decrease the growth of pancreatic tumors in a mice model. For this purpose, we used specific antibodies against either the whole FAPP, the O-glycosylated C-terminal domain, or the N-terminal domain of the protein. Our results indicate that SOJ-6 cells expressed at their surface a 32-kDa peptide corresponding to the C-terminal domain of the FAPP. Furthermore, we show, by using endoproteinase Lys-C or geldanamycin, a drug able to impair the FAPP secretion, that this 32-kDa peptide expressed on the SOJ-6 cell surface comes from the degradation of the FAPP. Finally, an in vivo prospective study using a preventative tumor model in nude mice indicates that targeting this peptide by the use of mAb16D10 inhibits the growth of SOJ-6 xenografts. The specificity of mAb16D10 for pancreatic tumors and the possibility to obtain recombinant structures of mucin-like peptides recognized by mAb16D10 and mAbJ28 are promising tools in immunologic approaches to cure pancreatic cancers.
Keywords: Pancreas, cancer, bile salt-dependent lipase (BSDL), feto-acinar pancreatic protein (FAPP), monoclonal antibody
Introduction
The bile salt-dependent lipase (BSDL; EC 3.1.1.13) is found in the pancreatic secretions of all species examined up to now, from fish to humans [1]. This enzyme plays an essential role in the intestinal processing of cholesterol [2–4]. During its transport from the endoplasmic reticulum to the trans-Golgi network, BSDL is associated with intracellular membranes by means of a complex that involves the glucose-related protein of 94 kDa or Grp94 [5,6]. This chaperone controls the late folding steps and the sorting of the active enzyme toward secretion [7]. Once in the trans-Golgi network and after completion of glycosylation [8,9], the complex is released from membranes upon the phosphorylation of the Thr residue at position 340 [10] by a protein kinase CKII [11,12]. Once released from intracellular membranes, BSDL enters the secretion route.
The feto-acinar pancreatic protein (FAPP) is a specific component of acinar cells of the human pancreas, which is associated with the ontogenesis and development of the gland [13]. FAPP has been characterized using the patented monoclonal antibody mAbJ28 [14]. Maximum synthesis of FAPP, determined as the emergence of the J28 epitope, occurs when acinar cell proliferation is maximal between 20 and 22 weeks gestation; it then declines to parturition [15]. Thereafter, FAPP, defined by the expression of the J28 epitope, behaves as an oncodevelopment-associated antigen [13]. FAPP (a 100- to 120-kDa protein) presents many homologies with BSDL (a 100- kDa protein) [16], and its cloning from human pancreatic tumoral cells [17] indicates that the N-terminal domain encoded by exons 1 to 10 is identical to that of BSDL. However, the sequence corresponding to exon 11, which encodes for 16 identical repeated sequences (C-terminal domain) of BSDL, is deleted by 330 bp and encodes only six of these repeated sequences on FAPP. Albeit, this latter protein is poorly secreted by pancreatic tumoral cells [13,18,19]; its low rate of secretion might not result from inherent properties of the protein, which is a priori normally N-glycosylated and O-glycosylated [16,17] and phosphorylated [10]. The retention of FAPP in human pancreatic tumoral SOJ-6 cells could result from a defect of Rab6 cycling due to the expression of a defective Rab GDIβ [20]. The localization of FAPP in tumoral cells indicates that the protein, which is mainly membrane-associated like its normal counterpart BSDL, distributes within the endoplasmic reticulum and the Golgi [18]. Such retention of the protein associated with modifications in glycosyltransferase expression due to pancreatic neoplastic processes [21] could be responsible for the formation on the C-terminal mucin-like domain of FAPP [22] of the J28 epitope that requires the core 2 β(1–6) N-acetylglucosaminyltransferase and the α(1–3/4) fucosyltransferase [23,24]—two glycosyltransferases normally located within the late Golgi compartment [25].
It is likely that the retained enzyme cannot indefinitely accumulate within tumor cells and should be degraded following the same way as aberrant BSDL molecules [26]. The question is how to determine what could be the future of degradation products, in part the C-terminal domain that carries out oncofetal epitopes. Antigenic peptides issued from FAPP degradation could be presented at the surface of tumoral cells. Supporting this is the report that 125I mAbJ28 administrated to hamsters treated with nitrosamines to induce pancreatic cancer accumulated at the level of the pancreas, and that the maximal accumulation is associated with pleomorphic alterations of the acinar tissues at pretumoral stage [27]. This suggests that peptides or proteins that carry out the J28 epitope can be presented at the surface of tumoral cells. In the present study, we effectively described that a 32-kDa peptide issued from the FAPP degradation is presented at the surface of human pancreatic SOJ-6 cells. This peptide is specifically recognized by mAbJ28 and mAb16D10, two monoclonal antibodies directed against the O-glycosylated C-terminal domain of FAPP. The specificity of mAb16D10 allowed us to investigate its efficacy in pancreatic cancer models. In a prospective study, we showed that the growth of xenografted SOJ-6 cells in nude mice was significantly decreased by preventative injections of mAb16D10.
Materials and Methods
Chemicals and Reagents
RPMI 1640, Ham F12, Opti-MEM media, glutamine, penicillin, streptomycin, trypsin-EDTA, fungizone, zeocin, and fetal calf serum (FCS) were purchased from Invitrogen (Cergy-Pontoise, France). Paraformaldehyde was from Fluka (Buchs, Switzerland). Bovine serum albumin (BSA), streptavidin-agarose, geldanamycin, and cell dissociation solution were obtained from Sigma (St. Louis, MO). Complete EDTA-free (a mix of protease inhibitors) and endoproteinase Lys-C were purchased from Roche Diagnostic (Meylan, France). Isoflow buffer was purchased from Coulter (Hialeah, FL). Sulfo-NHS-LC-biotin was from PerbioScience (Helsiborg, Sweden).
Antibodies
Polyclonal antibodies raised against purified human BSDL were obtained in our laboratory and isolated by affinity chromatography on protein A Sepharose [28]. These antibodies, referred to as pAbL64, were able to react with nonglycosylated and glycosylated domains of BSDL obtained by in vitro translation using human pancreatic mRNA and rabbit reticulocytes [17]. The patented monoclonal antibody (mAbJ28) specific for the fucosylated J28 glycotope carried by repeated C-terminal sequences of the oncofetal glycoisoform of BSDL (i.e., FAPP) was a generous gift from Dr. M. J. Escribano (INSERM, Marseilles, France). The mouse monoclonal antibody (mAb16D10) also directed against O-glycosylated repeated C-terminal sequences of FAPP (unpublished observation) and a polyclonal rabbit antibody (pAbAntipeptide) directed against a synthetic peptide of FAPP located within the N-terminal domain of the protein from Ser326 to Glu350 [29] were obtained from our laboratory. All the details concerning the specificity of these antibodies are given in Table 1. Polyclonal antibodies to the glucose transporter Glut-1 came from Chemicon International (Temecula, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-mouse goat IgG, FITC-conjugated anti-mouse goat IgM, FITC-conjugated anti-rabbit goat IgG, alkaline phosphatase-conjugated anti-mouse goat IgG, alkaline phosphate-conjugated anti-rat goat IgG, and alkaline phosphatase-conjugated anti-rabbit goat IgG were from Sigma. Peroxidase-conjugated anti-mouse and anti-rabbit goat IgG were from Roche Diagnostic.
Table 1.
Antibody Specificity.
| Antibody Name | Specificity | Particularity |
| pAbL64 | BSDL and FAPP | Obtained by rabbit immunization with a mix of BSDL and FAPP [28] Binds to nonglycosylated and glycosylated BSDL and FAPP [16] |
| pAbAntipeptide | BSDL and FAPP | Obtained by immunization of rabbits with a peptide sequence from Leu348 to Ser371 located in the N-terminal domain of BSDL and FAPP |
| mAbJ28 | FAPP | Binds to a fucosylated glycotope located in the C-terminal domain of FAPP [23] Binds to fetal and tumoral pancreatic human tissues [14,15] Does not recognize other fetal, normal, or tumoral human tissues [14,15] |
| mAb16D10 | FAPP | Binds to the C-terminal domain of FAPP (unpublished observation) Binds to human pancreatic tumoral tissue (unpublished observation) Does not recognize human pancreatic normal tissues (unpublished observation) |
Cell Culture
The SOJ-6 [30] cell line that constitutively expresses FAPP [17] was kindly provided by Dr. M. J. Escribano. SOJ-6 cells were grown in RPMI 1640 medium supplemented with 10% FCS, glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), and fungizone (0.1%). The CHO-C2-F3 cell line, issued from the CHO-K1 cell line (ATCC, Rockville, MD), was transfected with cDNA encoding glycosyltransferases, core 2 β(1–6) N-acetylglucosaminyltransferase, and α(1–3/4) fucosyltransferase [23]. CHO-C2-F3 cells were grown in Ham F12 medium supplemented with 10% FCS, 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 µg/ml), fungizone (0.1%), geneticin (500 µg/ml), and hygromycin (200 µg/ml). Cells were kept at 37°C in an humidified atmosphere of 95% air and 5% CO2.
Preparation of Cell Extracts
Cells were washed in PBS (10 mM sodium phosphate buffer, pH 7.4, 150 mM NaCl), harvested with a rubber policeman, washed once again in PBS, and pelleted by centrifugation. Pellets were washed twice with PBS and lysed for 30 minutes at 4°C in 100 µl of buffer (pH = 7.5; 10 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, and complete). Cell debris and insoluble materials were eliminated by centrifugation (14,000g, 30 minutes, 4°C) and soluble fractions were either immediately assayed or stored at -20°C until use.
Construction of Plasmids
The cDNA encoding the C-terminal tandem repeated sequence of FAPP (Cter-cDNA) was obtained by reverse transcription of mRNA extracted from SOJ-6 cells. The cDNA(-) pool was amplified by performing a polymerase chain reaction (PCR) using a pair of primers designed to cover this sequence [17] from nucleotide 1089 (Phe364) to nucleotide 1839 (termination codon) (Cter FAPP-5; 5′-CGTCTAaagcttTTTGATGTCTACACCGAGTCC and Cter FAPP-3; 5′-TTTCGTgaattcACGCTAAAACCTAATGACTGCAGGCATCTG) and using the GC-rich PCR kit from Clontech (Palo Alto, CA). These two primers include HindIII and EcoRI overhangs (lower case letters) for the ligation into the pSecTag vector (Invitrogen), which carries the gene for resistance to zeocin. Bases were randomly added in 5′ of these primers to allow efficient restrictive cleavage of PCR fragments. To eliminate the myc epitope and the sequence coding the six histidine residues in the 3′-end of the multicloning site of the pSecTag vector, a stop codon was introduced in the primer hybridizing with the 3′-end of the Cter-cDNA. The DNA was amplified using a 35-reaction cycle program as follows: denaturation (94°C, 1 minute), annealing (52°C, 1 minute), and extension (68°C, 4 minutes). The reaction was terminated by an incubation at 68°C for 10 minutes. PCR fragments were analyzed on 1% agarose gel. After purification using the nucleospin extract (Macherey-Nagel, Hoerdt, France), transcripts were subcloned into pCR2.1 TOPO vector (Invitrogen) and sequenced using M13 forward and reverse primers. Once sequenced, the transcript referred to as Cter-cDNA was excised by HindIII and EcoRI digestion and ligated into the pSecTag expression/secretion vector to give the pSecCterFAPP. PCR experiments were performed with a Gene AmpPCR System 2400 (PE Applied Biosystem, Roissy, France).
Transfection
Stable transfection of CHO-C2-F3 cells [24] was performed with pSecCterFAPP and using the Lipofectaminemediated transfection procedure according to the manufacturer (Invitrogen). CHO-C2-F3 cells were grown as described above. Semiconfluent cells were typically transfected with 1 µg of pSecCterFAPP plasmid. Antibiotic-resistant cells were selected using several medium exchanges with Ham F12 containing 10% FCS, geneticin (500 µg/ml), hygromycin (200 µg/ml), and zeocin (500 µg/ml) during a time period of at least 4 weeks. Zeocin-resistant clones secreting the recombinant C-terminal peptide of FAPP (CterFAPP) were isolated using cloning cylinders and visualized by fluorescence microscopy using pAbL64.
Fluorescence Microscopy
Cells grown to 80% confluence on microscope coverslips were washed three times with PBS buffer and fixed with 3% (vol/vol) paraformaldehyde for 20 minutes. The excess of paraformaldehyde was eliminated by washing the slides in 1 M glycine (pH 8.5). Between each step, cells were exhaustively rinsed with PBS. Then cells were treated for immunofluorescence as already described [17] using adequate primary antibodies and conjugates.
Flow Cytometry
Detection of antigens at the surface of SOJ-6 cells was carried out by indirect fluorescence under the following conditions: cells were released from culture plates by treatment with a nonenzymatic cell dissociation solution for 15 minutes at 37°C. All subsequent steps were carried out at 4°C. The cells were washed with PBS, fixed with 2% paraformaldehyde in PBS for 10 minutes, and extensively washed with 1% BSA in PBS. Antigens were exposed for 1 hour to specific antibodies, washed with PBS, and finally incubated for 30 minutes with adequate FITC-labeled secondary antibodies. Cells were then washed, resuspended in Isoflow buffer, and analyzed on an EPICS Profile II flow cytometer (Coulter). Detection of antigens located intracellularly in SOJ-6 cells was carried out by indirect fluorescence with modified conditions: the cells were released from culture plates by treatment with trypsin-EDTA solution for 5 minutes. After fixation with 2% paraformaldehyde, cells were permeabilized with 0.05% saponin in PBS for 15 minutes. Then the protocol was the same as described above.
Polyacrylamide Gel Electrophoresis and Western Blot Analysis
Gel electrophoreses (sodium dodecyl sulfate polyacrylamide gel electrophoresis, or SDS-PAGE) were performed on slab gels of polyacrylamide (10% acrylamide) and 0.1% sodium dodecyl sulfate [31]. Proteins were electrophoretically transferred onto a nitrocellulose membrane [32] in 0.2M Tris-HCl (pH 9.2) buffer (10% methanol) at 0.3 mA/cm2 overnight in a cold room. Transfer was verified by staining membranes with Ponceau red and replicas were developed using polyclonal antibodies to pancreatic BSDL (pAbL64) and the glucose transporter Glut-1, or the monoclonal antibody specific for the J28 glycotope (mAbJ28) or mAb16D10 or pAbAntipeptide. Quantification was performed using the NIH-Image program (developed at the U.S. National Institutes of Health, http://rsb.info.nih.gov/nih-image/).
Sequence Determination
The SDS-PAGE gel area corresponding to the pAbL64-reactive material was excised and electroeluted (Biotrap device; Schleicher and Schuell, Dassel, Germany). After dialysis/concentration (Centricon; Millipore, Billerica, MA), the electroeluted material was sequenced (IBSM-CNRS, Marseille, France).
Biotinylation and Streptavidin Precipitation of Cell Surface Proteins
The protocol is based on a method developed by Altin and Pagler [33] and modified in our laboratory. The SOJ-6 cells were washed three times with PBS, then were released from culture plates by treatment with a nonenzymatic cell dissociation solution for 15 minutes at 37°C. The cells were suspended at a concentration of 25 x 106 cells/ml in ice-cold PBS (pH 8.0) and 0.5 mg of sulfo-NHS-LC biotin was added per milliliter of reaction volume including complete EDTA-free mix to avoid degradation of proteins. The mixture was incubated at 4°C for 2 to 3 hours. Then the cells were washed three times with ice-cold PBS (pH 8.0) to remove the remaining biotinylation reagent. After centrifugation, the cell pellet was resuspended in an adequate volume of ice-cold PBS, sonicated twice for 15 seconds, and, after a 30-minute centrifugation at 13,000g, the supernatant containing cell surface-biotinylated proteins was saved. Biotinylated proteins (300 µg) were loaded on streptavidin-agarose beads (150 µl) and were incubated overnight at 4°C under agitation. Beads were exhaustively washed with PBS, then complexes formed of biotinylated proteins bound to streptavidin-agarose were dissociated by 0.1 M acetic acid (pH 2.0) for 30 minutes at 37°C, and centrifuged to collect the biotinylated proteins that were found in the supernatant. Alternatively biotinylated surface peptides were purified by steptavidin precipitation, then immunoprecipitated with pAbL64 or mAbJ28 using the Seize primary immunoprecipitation kit (PerbioScience). Immunoprecipitated and biotinylated peptides were separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes. Membranes were probed with adequate primary and secondary antibodies to detect immunoprecipitated biotinylated peptides using Supersignal West Pico (PerbioScience).
Protein Purification, Protein Concentration, and Activity Determinations
BSDL was purified from normal human pancreatic juice [1]. Proteins were quantified using the bicinchoninic acid assay from PerbioScience using BSA as standard. FAPP activity was determined on 4-nitrophenyl hexanoate in the presence of 4 mM sodium taurocholate as specific activator of the enzyme [8].
Enzymatic Degradation Using Endoproteinase Lys-C
Enzymatic degradation was performed in 25 mM Tris-HCl, 1 mM EDTA pH 8.5 buffer. The recombinant C-terminal peptide of FAPP (120 µg), which arborated the J28 glycotope, pure human pancreatic BSDL (120 µg), and SOJ-6 cell culture supernatant containing FAPP (120 µg), was specifically degraded by addition of endoproteinase Lys-C (2% by weight) at 37°C overnight. The reaction was stopped by freezing, and the reaction medium was then lyophilized and suspended in an adequate volume of Laemmli's buffer [31] before separation on SDS-PAGE and analysis by Western blot analysis.
Animals and Tumor Induction
Female NMRI Nu/Nu mice (6 weeks old) were purchased from Janvier (Le Genest-St-Isle, France) and were kept under pathogen-free conditions. All tissue removal procedures and animal care were carried out according to accreditation Nb 04333 given by the French Ministère de l'Agriculture. SOJ-6 human pancreatic tumoral cells were cultured to 90% confluence, washed twice with cold PBS, and harvested with the cell dissociation solution. The cells were pelleted and washed three times with PBS and kept on ice until injection. The animals were anesthesized with Foren inhalations (Abbott Laboratories, Pasadena, CA) and the tumor cell suspension (2 x 106 cells in 150 l of PBS) was inoculated subcutaneously in the right flank of each animal. Two groups of five mice each were constituted. One day before (day -1) tumoral cell inoculation (day 0), the control group received an intraperitoneal injection of 150 l of PBS (vehicle), whereas the assay group received 0.5 mg of mAb16D10 in 150 µl of PBS. Vehicle buffer or mAb16D10 was then injected in animals at days +1, +3, +6, +8, and + 10. When the tumor became palpable, measurements in two dimensions with Vernier calipers were performed once a week and the volume of tumors was calculated according to the formula of ellipsoid volume: (П/6)(ab2), where a is the largest and b is the smallest diameter of the tumor.
Results
Antibodies to FAPP Recognize 28- to 32-kDa Peptides in SOJ-6 Cell Lysates
It has been previously shown that FAPP but not BDSL was expressed by SOJ-6 cells [18]. The first goal of the present study was to determine whether the entire FAPP or a peptide derived from the FAPP was expressed at the surface of the SOJ-6 cell line. To demonstrate this proposal, we first attempted to detect degradation products of FAPP within SOJ-6 cell lysates by using an antibody directed against FAPP (pAbL64). Human pancreatic SOJ-6 tumoral cells were cultured in Opti-MEM medium in the absence of FCS for 24 hours. Cells were harvested, lysed in the presence of Triton X-100, and centrifuged to isolate solubilized material. Proteins present in the serum-free culture medium and the soluble fraction of cell lysate were separated on SDS-PAGE, electrotransferred onto nitrocellulose membranes, and probed with the polyclonal antibody pAbL64 [16] (Table 1). The results indicated that a protein associated with an Mr of around 120 kDa corresponding to the FAPP [18] was present in the cell-free culture medium of SOJ-6 cells (Figure 1, lane 1). Interestingly, in cell lysate, pAbL64 mainly recognizes peptides around 30 kDa (lane 2, arrows), which could be degradation products of FAPP. The 120-kDa protein is also present in cell lysates but in a very low amount as a result of degradation, or because FAPP in SOJ-6 cells is mainly associated with insoluble materials [17].
Figure 1.
Immunodetection of proteins present in extracellular medium and cell lysate of SOJ-6 cells. Subconfluent SOJ-6 cells were cultured in Opti-MEM medium for 24 hours. Then the cell culture medium was withdrawn and the cells were harvested, lysed, and centrifuged to eliminate insoluble materials. Proteins present in the soluble fraction were separated on SDSPAGE and electrotransferred onto nitrocellulose membranes. The membranes were finally probed with the polyclonal antibody to BSDL, pAbL64, in cell culture medium (lane 1) and in the soluble fraction of cell lysate (lane 2). Arrows indicate the apparent molecular mass of detected proteins or peptides.
Antibodies to FAPP Recognize the SOJ-6 Cell Surface
We next determined whether antibodies to FAPP were able to recognize the surface of SOJ-6 cells. For this purpose, these cells were cultured to subconfluence on coverslips and incubated with primary antibodies and then with the corresponding FITC-labeled secondary antibodies. As shown in Figure 2 (panel A), antigenic sites recognized by pAbL64 and two monoclonal antibodies mAbJ28 and mAb16D10 directed against the C-terminal domain of FAPP [22] (unpublished results) decorated the plasma membrane of the SOJ-6 cells (Figure 2, panels A2–A4). The polyclonal antibodies, pAbAntipeptide, obtained from a peptide sequence of FAPP located within the N-terminal domain of the protein poorly reacted with SOJ-6 plasma membranes (Figure 2, panel A5). In control experiments omitting either primary antibodies, no significant labeling of cell membranes was obtained (Figure 2, panel A1). These results were confirmed by fluorescence-activated cell sorter (FACS) analyses (Figure 2, panel B). Ten to 100 times more fluorescence than control was associated with cells probed with pAbL64, mAbJ28, and mAb16D10. A low fluorescence, albeit higher than that of control, was associated with cells incubated with the pAbAntipeptide. These results show that antibodies to the C-terminal domain of FAPP bound to structures that were present at SOJ-6 cell surface.
Figure 2.
SOJ-6 surface reactivity with antibodies to FAPP. Panel A: Subconfluent SOJ-6 cells were fixed with paraformaldehyde (3%) then probed with different antibodies pAbL64 (lane 2), mAbJ28 (lane 3) mAb16D10 (lane 4), and pAbAntipeptide (lane 5) before immunofluorescence microscopy analyses. Lane 1 shows the nonspecific binding of secondary FITC-labeled antibody. Panel B: Cell surface antigen expression was analyzed by flow cytometry using pAbL64 (lane 2), mAbJ28 (lane 3), mAb16D10 (lane 4), and pAbAntipeptide (lane 5) and compared to controls (lane 1), which shows the nonspecific binding of secondary FITC-labeled antibodies.
Antibodies to FAPP Recognize a 32-kDa Surface Peptide
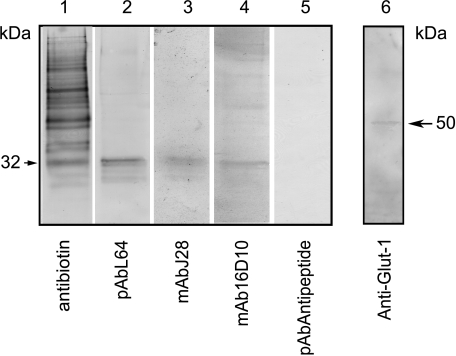
We next determined whether surface antigenic structures detected by antibodies to FAPP were associated with the 120-kDa protein or with 28- to 32-kDa peptides. For this purpose, SOJ-6 cells were grown until confluence, then membrane proteins and peptides were biotinylated, and cells were ultimately lysed. Biotinylated membrane proteins and peptides were further purified on steptavidin-agarose beads. The material eluted by acetic acid was separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes. Membranes were finally probed with antibodies. We showed that many proteins were reactive to antibodies to biotin (Figure 3, lane 1; 0.5 µg of protein loaded), but only one peptide of 32 kDa was probed with pAbL64 (Figure 3, lane 2; 5 µg of protein loaded), mAbJ28 (Figure 3, lane 3; 5 µg of protein loaded), and mAb16D10 (Figure 3, lane 4; 5 µg of protein loaded). Furthermore, antibodies to an N-terminal sequence of FAPP (pAbAntipeptide) did react neither with this 32-kDa peptide nor with any other biotinylated material (Figure 3, lane 5). The presence of the glucose transporter Glut-1 migrating as the mature membrane form of the protein [34] suggested that this material is representative of membrane proteins (Figure 3, lane 6). Taken as a whole, those results demonstrated that antibodies to the C-terminal domain of FAPP recognized antigenic structures present at the plasma membrane of human tumoral pancreatic SOJ-6 cells. These antigenic structures are associated with a 32-kDa peptide.
Figure 3.
Immunodetection of biotinylated cell surface antigens in SOJ-6 cells. SOJ-6 cells were grown until confluence, and membrane proteins and peptides were biotinylated and lysed. Then biotinylated membrane proteins were purified on streptavidin-agarose affinity column. The material eluted with 0.1 M acetic acid was separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes, which were finally probed with antibodies: antibiotin (lane 1; 0.5 µg of protein), pAbL64 (lane 2; 5 µg of protein), mAbJ28 (lane 3; 5 µg of protein), mAb16D10 (lane 4; 5 µg of protein), pAbAntipeptide (lane 5; 5 µg of protein), and antibodies to Glut-1 (lane 5; 5 µg of protein). Arrow indicates the apparent molecular mass of detected peptides.
Polyclonal and Monoclonal Antibodies to FAPP Recognize the Same 32-kDa Peptide
From this point, it was essential to determine whether antigenic structures recognized by polyclonal and monoclonal antibodies to FAPP were carried out by the same peptide. To solve this, we have first purified biotinylated surface peptides by streptavidin-agarose as described in the Materials and Methods section. Secondly, we have isolated biotinylated peptides reactive to pAbL64 and to mAbJ28 by means of affinity chromatography on respective agaroseimmobilized antibodies column, then the material eluted from each immobilized antibodies column was separated on SDSPAGE and electrotransferred onto nitrocellulose membranes. Membranes were then probed with the counterpart antibodies. As shown in Figure 4A, the material eluted from the immobilized pAbL64 affinity column was reactive on Western blot analysis with the mAbJ28. Of course, the material eluted from the agarose-immobilized mAbJ28 was also recognized by pAbL64 (Figure 4B). In each case, a 32-kDa peptide was detected following affinity column and Western blot analysis. Therefore, the same peptide was recognized by polyclonal antibodies to FAPP and mAbJ28, a monoclonal antibody that binds specifically to a glycotope present on the C-terminal domain of FAPP [22].
Figure 4.
Immunodetection of biotinylated cell surface antigens purified on agarose-immobilized antibodies to FAPP. Membrane peptides of SOJ-6 cells were first biotinylated and purified on streptavidin-agarose affinity column (Figure 3). (A) The material eluted with acetic acid was purified on agaroseimmobilized pAbL64 affinity column and the material eluted was separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes. Then membranes were probed with mAbJ28. (B) Conversely, the material eluted with acetic acid was purified on agarose-immobilized mAbJ28 affinity column and the material eluted was separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes. Then membranes were probed with pAbL64. The detection of immunoprecipitated biotinylated peptides was performed using Lumi-Lightplus Western Blotting kit. Arrow indicates the apparent molecular mass of detected peptide.
Geldanamycin Increases the Amount of Peptides Presented at the Cell Surface
In a recent report, we showed that the 94-kDa glucoseregulated protein or Grp94 assisted the sorting of the BSDL, and that the fraction of the protein that cannot be folded by Grp94 was addressed to a degradative compartment implicating the proteasome [26]. We further showed that geldanamycin, a drug that impaired the interaction of Grp94 with its substrate, decreased the secretion of BSDL and enhanced the amount of ubiquitinylated BSDL that was further addressed to the proteasomal degradation [7]. Therefore, we reasoned that if the 32-kDa peptide that was presented at the surface of pancreatic tumoral SOJ-6 cells is actually the end product of the degradation of FAPP, geldanamycin should increase the amount of the 32-kDa peptide, which is in fine presented at the cell surface. To verify this point, SOJ-6 cells were cultured to subconfluence then the medium was replaced with fresh medium supplemented or not with geldanamycin (3 µM) and further incubated for 6 hours. At the end of the incubation period, the culture medium was withdrawn and cells were washed, harvested, and lysed. Compared to control cells incubated in the absence of geldanamycin, the secretion of FAPP was decreased by approximately 70%, independently of the way to record the protein [i.e., either by quantification of Western blot using pAbL64 (Figure 5, A and B) or by determination of FAPP activity (Figure 5C)]. These results agreed with those already found using the rat pancreatic AR4-2J cell line [7]. In another set of experiments, SOJ-6 cells, either treated with geldanamycin under abovedescribed conditions or mock-treated, were harvested using a nonenzymatic cell dissociation solution for extracellular FACS staining and trypsin-EDTA solution for intracellular FACS staining. As shown in Table 2, the amount of antigenic structures presented at the surface of SOJ-6 cells and recognized by the pAbAntipeptide was not increased by geldanamycin when compared to control cells. However, the presentation of antigenic structures recognized by monoclonal antibodies mAbJ28 and mAb16D10 to the C-terminal domain of FAPP was significantly increased by 42% and 101%, respectively. Conversely, intracellular FACS staining (Table 2) showed that the antigenic structures recognized by monoclonal antibodies mAb16D10 to the C-terminal domain of FAPP were significantly decreased by 42%, whereas the structures recognized by the pAbAntipeptide are only slightly modified. These results suggested that the presentation of the 32-kDa peptide at the plasma membrane of SOJ-6 cells is increased under conditions promoting the dissociation of Grp94/FAPP complexes by geldanamycin [7]. Therefore, the 32-kDa peptide presented at the tumoral cell surface is probably the C-terminal domain of FAPP, which could be the end product of the intracellular degradation of this latter protein.
Figure 5.
Effect of geldanamycin on the rate of FAPP secretion by SOJ-6 cells. Subconfluent SOJ-6 cells were incubated in fresh RPMI in the absence (empty column) or presence (dashed column) of 3 µM geldanamycin and further incubated for 6 hours. At the end of incubation, the culture medium was removed. The secretion of FAPP was analyzed by SDS-PAGE and Western blot analysis using pAbL64 (A), and the quantification of this Western blot analysis is performed by the NIH Program (B). The FAPP activity was also determined (C) using 4-nitrophenyl hexanoate as substrate. This experiment was done in triplicate and results are presented as mean ± SD.
Table 2.
Cell Expression of Antigenic Structures on SOJ-6 Cell Line after Geldanamycin Treatment.
| Antigen | ||||||
| Cell Surface Expression | Intracellular Expression | |||||
| Control | Geldanamycin (3 µM) | Variation* (%) | Control | Geldanamycin (3 µM) | Variation* (%) | |
| Median Fluorescence Intensity† | Median Fluorescence Intensity† | |||||
| mAbJ28 | 8.3 | 11.8 | +42 | 7.7 | 7.3 | -5 |
| mAb16D10 | 36.9 | 74.4 | +101 | 81.8 | 47.3 | -42 |
| pAbAntipeptide | 5.1 | 5.1 | 0 | 3.2 | 3.7 | -15 |
*Percentage of variation versus control; average of two independent experiments.
Median fluorescence intensity of cells stained with the respective antibodies as determined by FACScan analysis.
The 32-kDa Peptide Is the C-terminal Domain of FAPP
As suggested above by antibody reactivity, the 32-kDa peptide could be representative of the C-terminal domain of FAPP. On FAPP, this domain is composed of six identical repeated sequences of 11 amino acids, whereas on BSDL, it is formed by 16 of these identical repeated sequences. A survey of the literature suggested that classic trypsin-like, chymotrypsin-like, and peptidyl glutamyl-peptide-hydrolyzing activities of the proteasome [35] can degrade N-glycosylated FAPP and BSDL [26,36], leaving undegraded the C-terminal repeated sequences. Cytosolic ubiquitous calpain activities [37] that promote restricted cleavages of proteins [38,39] might also leave repeated sequences intact. Although these repeated sequences have built-in PEST degradation signals [40], they can further be protected from intracellular proteolysis by their high O-glycosylation [21,22]. The N-terminal domain of FAPP and that of BSDL are sensitive to proteolysis by either proteasome or calpains, and by any other proteases such as endoproteinase Lys-C (unpublished observations). Therefore, FAPP obtained from the serum-free culture medium of SOJ-6 cells and BSDL isolated from human pancreatic juice were treated with endoproteinase Lys-C, overnight at 37°C, then analyzed on SDS-PAGE and Western blot analysis using pAbL64, mAbJ28, and mAb16D10 to detect any antigenic structure generated by the proteolysis. As shown in Figure 6A, the mock-treated FAPP (lane 2) was a little degraded compared to untreated (i.e., without incubation overnight at 37°C) FAPP (lane 1), likely by proteases cosecreted along with FAPP by SOJ-6 cells. However, FAPP (six C-terminal repeated identical sequences) treated with endoproteinase Lys-C (lanes 3 and 4) generated many peptides, among which the one associated with an Mr of 32 kDa was reactive with pAbL64 (lane 3), mAbJ28 (lane 4), and mAb16D10 (lane 5). Following the same treatment, pure human pancreatic BSDL (Figure 6B) gave peptides with a lower migration (around 50–60 kDa) due to the presence of 16 repeated sequences and, overall, no peptide associated with a 32-kDa size and no material reactive with mAbJ28 were detected. To confirm that the 32-kDa peptide corresponded to tandemly repeated C-terminal sequences of FAPP, the sequence encoding this domain has been cloned and inserted into an eukaryotic vector named pSecCterFAPP. This vector allows the expression and secretion of the translated product. CHOC2-F3 cells equipped with glycosyltransferases essential for the establishment of the J28 epitopic structure were transfected with the pSecCterFAPP vector and selected as already described [23]. Cells were cultured in Opti-MEM medium and allowed to secrete a translation product of approximately 42 kDa (Figure 6C, lanes 1 and 2), which arborated the J28 epitope [23]. Once treated with endoproteinase Lys-C, this product generated a 32-kDa peptide that was recognized by pAbL64 (Figure 6C, lane 3), mAbJ28 (Figure 6C, lane 4), and mAb16D10 (Figure 6C, lane 5).
Figure 6.
Endoproteinase Lys-C treatment of BSDL, secreted FAPP, and recombinant C-terminal peptide of FAPP. (A) FAPP obtained from the serum-free culture medium of SOJ-6 cells was treated (+) or mock-treated (-) with endoproteinase Lys-C (2% by weight), overnight at 37C, then analyzed on SDS-PAGE and Western blot analysis using pAbL64. Lane 1 represents untreated FAPP present in the SOJ-6 cell culture medium. Lane 2 represents mock-treated FAPP of the SOJ-6 cell culture medium. Lane 3 represents FAPP of the SOJ-6 cell culture medium treated with endoproteinase Lys-C. Lanes 4 and 5 represent FAPP of the SOJ-6 cell culture medium treated with endoproteinase Lys-C and immunodetection using mAbJ28 and mAb16D10, respectively. (B) Pure human pancreatic BSDL was treated and analyzed under identical conditions. Lane 1 represents native BSDL, lane 2 represents mock-treated BSDL, and lane 3 represents BSDL treated with endoproteinase Lys-C. Lane 4 represents BSDL treated with endoproteinase Lys-C and immunodetection using mAbJ28. (C) The recombinant C-terminal peptide of FAPP obtained from CHO-C2-F3 cells transfected with the pSecCterFAPP was treated (+) or mocked-treated (-) with endoproteinase Lys-C, overnight at 37°C, then analyzed on SDS-PAGE and Western blot analysis using pAbL64. Lane 1 represents recombinant C-terminal peptide, lane 2 represents mocktreated recombinant C-terminal peptide, and lane 3 represents recombinant C-terminal peptide treated with endoproteinase Lys-C. Lanes 4 and 5 represent recombinant C-terminal peptide treated with endoproteinase Lys-C and immunodetection using mAbJ28 and mAb16D10, respectively. Arrows indicate the apparent molecular mass of detected peptides.
Finally, the 32-kDa surface peptide was sequenced to demonstrate that it is actually issued from FAPP degradation. For this purpose, SOJ-6 cell surface proteins were biotinylated and biotin-labeled material was further purified on steptavidin-agarose beads. The material eluted from beads was freeze-dried and treated for SDS-PAGE. The gel area corresponding to pAbL64-reactive material was excised, electroeluted, and sequenced. The N-terminal sequence of this peptide was Thr-Gly-Asp-Pro and corresponded to residues 474 to 477 of FAPP located some 100 residues to the first tandemly C-terminal repeated sequence of FAPP [17]. Therefore, we must conclude that the 32-kDa peptide reactive to pAbL64 and mAbJ28 and presented at the SOJ-6 cell surface corresponds to the C-terminal mucin-like domain of FAPP consecutive to its intracellular degradation.
mAb to C-terminal Domain of FAPP Decreases the Growth Rate of Inoculated SOJ-6 Pancreatic Tumoral Cells
At that point, our results indicate that pancreatic tumoral cells SOJ-6 expressed at their surface the 32-kDa C-terminal peptide of the FAPP. Because BSDL is not expressed in tumoral cell lines [18], targeting the C-terminal domain of the FAPP using specific antibodies could be an interesting strategy in vivo to diminish the growth of the pancreatic tumor. Therefore, the efficacy of the monoclonal antibody mAb16D10 against SOJ-6 tumors has been examined in preventative xenograft model using nude mice. For this purpose, a prospective study was performed and mAb16D10 or PBS vehicle was administered intraperitoneally on the day before tumor cell inoculation in the flank of each animal and was given three times per week for 2 weeks. At a dose of 0.5 mg per injection (assay group), mAb16D10 largely decreases the growth of xenografted SOJ-6 cells (Figure 7). The rate of tumor growth in the assay group stays very low for a period up to 10 weeks. At this time, the range of tumor volume was 0.62 to 3.45 cm3 (mean volume 1.59 ± 0.55 cm3, n = 5) for the control group (vehicle), which is higher than the tumor size range in the assay group (0.5 mg per injection; i.e., 0–0.76 cm3; mean volume 0.41 ± 0.,15 cm3). To determine whether the antibody blocks tumor cell proliferation, we have recorded in vitro the proliferation of SOJ-6 cells by [3H]thymidine incorporation. Our results indicate that the SOJ-6 cell proliferation was not modified by the presence of mAb16D10 at 0.5 mg/ml (data not shown), suggesting that, in vivo, the antibody may not affect directly SOJ-6 cells proliferation in the tumor. In conclusion, mAb16D10 binds to the C-terminal domain of FAPP presented at the surface of SOJ-6 cells and decreases the rate of growth of xenografted pancreatic tumor.
Figure 7.
Antitumor effect of mAb16D10 on SOJ-6 xenograft growth rates in a preventative model. SOJ-6 cells were injected subcutaneously into the right flank of 6-week-old nude mice (n = 5) at day 0. Mice were injected intraperitoneally with 0.5 mg of mAb16D10 (assay group, triangle) or with PBS vehicle only (control group, diamond) starting 1 day prior to tumor cell inoculation. Data are expressed as mean tumor volume ± SEM.
Discussion
In the last decade, the incidence of cancers of the exocrine pancreas has been increasing [41]. Despite advances in the treatment of gastrointestinal malignancies, no significant progress has been made against pancreatic cancer, although chemotherapy associated with radiotherapy has some positive effects on the survival of patients [42]. The curative resectability rate is very low because the cancer is asymptomatic and its diagnosis in most patients occurs once the tumor has spread into lymph nodes or has formed distant metastases [43]. Consequently, exocrine pancreatic cancer still represents a virtual death sentence for the patient and new therapies are promising. Specific markers to target pancreatic tumoral cells are spare, albeit many monoclonal antibodies were generated against malignant pancreatic epithelial cells. These include Span-1 [44], Du-Pan-2 [45], CA19-9 [46], CA50 [47], and CAR-3 [48]. These antibodies generally displayed distinct patterns of tissue staining, often being more highly expressed in certain tissues and tumors than others. However, the reactivity of these antibodies depends upon the genotype of patients [49,50].
FAPP is an oncofetal glycovariant of the BSDL [16], a lipolytic enzyme secreted as a component of the pancreatic juice and involved in the duodenal hydrolysis of cholesterol esters [2]. FAPP is a specific constituent of pancreatic acinar cells, which are associated with the ontogenesis and development of the human pancreas. The protein is defined as a member of the oncodevelopment-associated pancreatic antigens, the earliest expression of which is seen in undifferentiated mesenchymal cells and nascent acini. This expression is concomitant to the beginning of the morphologic differentiation of the pancreas, which occurs after 9 to 10 weeks of gestation [15]. Maximal synthesis of FAPP appears at the time of intense acinar cell proliferation, between 15 and 25 weeks gestation. Then the expression of FAPP declines progressively to reach a very low level in adults [15]. FAPP has never been detected in any other fetal tissues [15] and in any adult tissues, except the pancreas of patients suffering from cancer [14]. FAPP was first identified using polyclonal antiserum and further characterized in human pancreas with mAbJ28 [14]. The reactivity of this monoclonal antibody with serum is significantly increased in pancreatic adenocarcinoma, and mAbJ28 recognized a structure found in human tumoral pancreas and human pancreatic tumor cell lines, SOJ-6 and BxPC-3 [18,51], but not in normal tissues [14]. Furthermore, it has been shown that mAbJ28 recognizes a carbohydrate antigenic structure [13,22] located within O-glycosylated mucin-like C-terminal domains of FAPP [16,22,23].
Many works suggested that epitopic structures recognized by monoclonal antibodies to FAPP are present at the surface of tumoral pancreatic cells. These studies included immunocytologic localization of FAPP within human pancreatic tumoral tissues compared to normal tissues [14], and the specific targeting of nitrosamine-induced pancreatic tumor in hamsters (which expressed the J28 epitope) with the radiolabeled mAbJ28 [27]. Furthermore, in diabetic patients, specific antibodies to the C-terminal domain of BSDL were found in the blood circulation, suggesting that this domain could be presented at the surface of pancreatic cells under inflammatory or pathologic conditions [24] to activate the immune system.
In this paper, we showed that two monoclonal antibodies mAbJ28 and mAb16D10 directed against the O-glycosylated C-terminal domain of FAPP recognized structures present at the surface of human pancreatic tumoral SOJ-6 cells. We further demonstrated that these epitopic structures are carried out by a 32-kDa peptide located at the surface of SOJ-6 cells and that this peptide corresponded to the O-glycosylated repeated sequences of FAPP as it is demonstrated by: 1) the reactivity of mAbJ28, which recognized fucosylated O-glycan structures linked to the C-terminal domain of FAPP [22,23]; 2) FAPP and its recombinant C-terminal domain expressed by CHO-C2-F3 cells equipped with glycosyltransferases involved in the formation of the J28 epitope [23], which is generated upon proteolysis of a peptide with an electrophoretic migration identical to that of the peptide detected at the cell membrane level. Under identical conditions, BSDL leads to a 50- to 60-kDa peptide; 3) geldanamycin, a drug that impaired the folding of the normal glycovariant of FAPP and addressed the protein toward the proteasome degradation [7], which enhanced the reactivity of tumoral cell membranes with mAbJ28 and mAb16D10; 4) polyclonal antibodies directed against an N-terminal peptidic sequence of FAPP located outside repeated sequences (pAbAntipeptide), which were ineffective in membrane-binding studies and Western blot analysis of biotinylated membrane proteins; and 5) finally, the N-terminal sequence of the SOJ-6 cell surface peptide, which corresponded to that of a sequence starting at Thr474 of the FAPP sequence and located just before repeated sequences of the C-terminal domain of the protein [17].
How the C-terminal peptide of FAPP constituted of O-glycosylated tandemly repeated sequences is presented at the surface of tumoral pancreatic cells is an open question. The Grp94, which plays a pivotal role in the secretion of the normal variant of FAPP [5,7], has gained attention for its ability to elicit CD8+ cytotoxic T-cell responses to its bound peptide [52]. A minor fraction of Grp94 localizes to the cell surface, albeit a built-in canonical endoplasmic reticulum retention signal is present on Grp94 [53]. Interestingly, this altered localization has, for instance, only been seen in transformed cells [52]. The presentation of peptides to the immune system by oligomers of Grp94 [54] explains the tumor rejection activity of Grp94 [55], which is mediated by means of CD8+ T-cell cytotoxic response [56]. However, data obtained with geldanamycin seem a priori to disagree with the involvement of Grp94 in the presentation to the cell surface of the C-terminal domain of FAPP. Furthermore, the size of this peptide does not correlate with that of peptides usually presented to the cell surface through major histocompatibility complexes [57]. More investigations will be necessary to elucidate this mechanism. The oncodevelopmental self-character of FAPP might have led to immunologic tolerance and no circulating antibodies to FAPP were detected in the blood of patients suffering from pancreatic carcinoma [24]. It is also possible that T cells reactive to the pancreatic tumor may be present at insufficient level, do not have enough avidity for tumor cells, or do not produce the appropriate cytokines to mediate the destruction of the tumor. The tumor itself may also actively participate in immune suppression [58]. All these mechanisms may limit the effectiveness of natural immune responses against tumoral cells. The achievements of active immunization against infectious diseases have provided hope that cancer patients could be actively immunized against their own cancers to prevent or treat the disease. New approaches in the development of cancer vaccines require the identification of human cancer antigens. But these approaches are limited by minute quantities of cancer antigenic molecules present at the surface of tumoral cells. New findings presented in this study demonstrate the presence of specific antigens, originating from intracellular degradation of FAPP, at the surface of pancreatic tumoral SOJ-6 cells. The possibility to obtain recombinant structures reactive with monoclonal antibodies to O-glycan structures of the C-terminal repeated sequences of FAPP [23,24] may be promising tools in active immunotherapy of pancreatic cancers. However, antibodies to these structures may be interesting therapeutic agents for passive immunization as described with Herceptin, a monoclonal antibody against the tyrosine kinase receptor HER-2 and breast cancer [59]. The prospective study as presented here suggests that mAb16D10 has in vivo a preventative antitumor activity against SOJ-6 cells expressing the C-terminal domain of FAPP at their surface. Intraperitoneal injections of mAb16D10 in mice xenografted with human pancreatic tumoral SOJ-6 cells decreased the growth rate of the tumor. This may have resulted from the recognition of C-terminal peptide of FAPP at the surface of tumoral SOJ-6 cells by mAb16D10 followed by a possible activation of complement cascade and/or macrophage-mediated cytotoxicity. Further studies will be necessary to confirm these data and to understand the mechanism by which mAb16D10 inhibits pancreatic tumor growth. Furthermore, the unique specificity of mAb16D10, which recognizes SOJ-6 cell membrane antigens and also binds to pancreatic adenocarcinomatous tissues but not to normal pancreatic tissues (some studies are in progress), could be effective in passive immunologic therapy of exocrine pancreatic tumors.
Acknowledgements
We are indebted to A. Valette for her technical help. M.-J. Escribano is greatly acknowledged for her kind release of mAbJ28 and SOJ-6 cells.
Abbreviations
- mAb
monoclonal antibody
- pAb
polyclonal antibodies
- BSA
bovine serum albumin
- BSDL
bile salt-dependent lipase
- FACS
fluorescence-activated cell sorter
- FAPP
feto-acinar pancreatic protein
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- Grp94
glucose-regulated protein of 94 kDa
- PCR
polymerase chain reaction
Footnotes
This work was supported by grant 4473 from the Association pour la Recherche contre le Cancer (ARC, Paris, France) and the Conseil Général des Bouches du Rhône (Marseilles, France), and by institutional funding from INSERM (Paris) and the Université de la Méditerranée (Marseilles).
Present address: Center for Hemostasis, Thrombosis, and Vascular Biology, Beth Israel Deaconness Medical Center, Harvard Medical School, Research East, 41 Avenue Louis Pasteur, Boston, MA, 02115, USA.
References
- 1.Lombardo D. Bile salt-dependent lipase: its pathophysiological implications. Biochim Biophys Acta. 2001;1533:1–28. doi: 10.1016/s1388-1981(01)00130-5. [DOI] [PubMed] [Google Scholar]
- 2.Lombardo D, Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice: II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim Biophys Acta. 1980;611:147–155. doi: 10.1016/0005-2744(80)90050-9. [DOI] [PubMed] [Google Scholar]
- 3.Shamir R, Johnson WJ, Zolfaghari R, Lee HS, Fisher EA. Role of bile salt-dependent cholesteryl ester hydrolase in the uptake of micellar cholesterol by intestinal cells. Biochemistry. 1995;34:6351–6358. doi: 10.1021/bi00019a013. [DOI] [PubMed] [Google Scholar]
- 4.Howles P, Carter C, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–7202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 5.Bruneau N, Lombardo D. Chaperone function of a Grp 94-related protein for folding and transport of the pancreatic bile salt-dependent lipase. J Biol Chem. 1995;270:13524–13533. doi: 10.1074/jbc.270.22.13524. [DOI] [PubMed] [Google Scholar]
- 6.Bruneau N, Lechene de la Porte P, Sbarra V, Lombardo D. Association of bile salt-dependent lipase with membranes of human pancreatic microsomes. Eur J Biochem. 1995;233:209–218. doi: 10.1111/j.1432-1033.1995.209_1.x. [DOI] [PubMed] [Google Scholar]
- 7.Nganga A, Bruneau N, Sbarra V, Lombardo D, Le Petit-Thévenin J. Control of the pancreatic bile salt-dependent lipase secretion by Grp94. Biochem J. 2000;352:865–874. [PMC free article] [PubMed] [Google Scholar]
- 8.Abouakil N, Mas E, Bruneau N, Benajiba A, Lombardo D. Bile salt-dependent lipase biosynthesis in rat pancreatic AR 4-2 J cells. Essential requirement of N-linked oligosaccharide for secretion and expression of a fully active enzyme. J Biol Chem. 1993;268:25755–25763. [PubMed] [Google Scholar]
- 9.Bruneau N, Nganga A, Fisher EA, Lombardo D. O-glycosylation of C-terminal tandem-repeated sequences regulates the secretion of rat pancreatic bile salt-dependent lipase. J Biol Chem. 1997;272:27353–27361. doi: 10.1074/jbc.272.43.27353. [DOI] [PubMed] [Google Scholar]
- 10.Vérine A, Le Petit-Thévenin J, Panicot-Dubois L, Valette A, Lombardo D. Phosphorylation of human bile salt-dependent lipase: identification of phosphorylation site and relation with secretion process. J Biol Chem. 2000;276:12356–12361. doi: 10.1074/jbc.M008658200. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualini E, Caillol N, Mas E, Bruneau N, Lexa D, Lombardo D. Association of bile salt-dependent lipase with membranes of human pancreatic microsomes is under the control of ATP and phosphorylation. Biochem J. 1997;327:527–537. doi: 10.1042/bj3270527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasqualini E, Caillol N, Valette A, Lloubes R, Vérine A, Lombardo D. Phosphorylation of the rat pancreatic bile salt-dependent lipase by casein-kinase II is essential for secretion. Biochem J. 2000;345:121–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Escribano MJ, Imperial S. Purification and molecular characterization of FAP, a feto-acinar protein associated with the differentiation of human pancreas. J Biol Chem. 1989;264:21865–21871. [PubMed] [Google Scholar]
- 14.Escribano MJ, Cordier J, Nap M, Ten Kate FJW, Burtin P. Differentiation antigens in fetal human pancreas. Int J Cancer. 1986;38:155–160. doi: 10.1002/ijc.2910380202. [DOI] [PubMed] [Google Scholar]
- 15.Albers GHR, Escribano MJ, Gonzalez M, Mulliez M, Nap M. Fetoacinar pancreatic protein in the developing human pancreas. Differentiation. 1987;34:210–215. doi: 10.1111/j.1432-0436.1987.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 16.Mas E, Abouakil N, Roudani S, Miralles F, Guy-Crotte O, Figarella C, Escribano MJ, Lombardo D. Human fetoacinar pancreatic protein: an oncofetal glycoform of the normally secreted pancreatic bile salt-dependent lipase. Biochem J. 1993;269:609–615. doi: 10.1042/bj2890609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasqualini E, Caillol N, Panicot L, Mas E, Lloubes R, Lombardo D. Molecular cloning of the oncofetal isoform of the human pancreatic bile salt-dependent lipase. J Biol Chem. 1998;273:28208–28218. doi: 10.1074/jbc.273.43.28208. [DOI] [PubMed] [Google Scholar]
- 18.Miralles F, Langa F, Mazo A, Escribano MJ. Retention of the fetoacinar pancreatic (FAP) protein to the endoplasmic reticulum of tumor cells. Eur J Cell Biol. 1993;60:115–121. [PubMed] [Google Scholar]
- 19.Roudani S, Pasqualini E, Margotat A, Gastaldi M, Sbarra V, Malezet-Desmoulin C, Lombardo D. Expression of a 46 kDa protein in human pancreatic tumors and its possible relationship with the bile salt-dependent lipase. Eur J Cell Biol. 1994;64:132–144. [PubMed] [Google Scholar]
- 20.Caillol N, Pasqualini E, Lloubes R, Lombardo D. Impairment of bile salt-dependent lipase secretion in human pancreatic tumoral SOJ-6 cells. J Cell Biochem. 2000;79:628–647. doi: 10.1002/1097-4644(20001215)79:4<628::aid-jcb120>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Mas E, Pasqualini E, Caillol N, El Battari A, Lombardo D, Sadoulet MO. Fucosyltransferase activities in human pancreatic tissue: comparative study between cancer tissues and established tumoral cell lines. Glycobiology. 1998;8:605–613. doi: 10.1093/glycob/8.6.605. [DOI] [PubMed] [Google Scholar]
- 22.Mas E, Crotte C, Lecestre D, Michalski JC, scribano MJ, Lombardo D, Sadoulet MO. Investigation of two glycosylated forms of bile salt-dependent lipase in human pancreatic juice. Glycobiology. 1997;7:745–752. doi: 10.1111/j.1432-1033.1997.0299a.x. [DOI] [PubMed] [Google Scholar]
- 23.Panicot L, Mas E, Pasqualini E, Zerfaoui M, Lombardo D, Sadoulet MO, El Battari A. The formation of the oncofetal J28 glycotope involves core-2 beta 6-N-acetylglucosaminyltransferase and alpha 3/4-fucosyltransferase activities. Glycobiology. 1999;9:935–946. doi: 10.1093/glycob/9.9.935. [DOI] [PubMed] [Google Scholar]
- 24.Panicot L, Mas E, Thivolet C, Lombardo D. Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes. 1999;48:2316–2323. doi: 10.2337/diabetes.48.12.2316. [DOI] [PubMed] [Google Scholar]
- 25.Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LePetit-Theévenin J, Verine A, Nganga A, Nobili O, Lombardo D, Bruneau N. Impairment of bile salt-dependent lipase secretion in AR4-2J rat pancreatic cells induces its degradation by the proteasome. Biochim Biophys Acta. 2001;1530:184–198. doi: 10.1016/s1388-1981(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 27.Takeda Y, Miralles F, Daher N, Escribano MJ. Radioimmunolocalization of the monoclonal antibody J28 in early transformation stages in N-nitrosobis(2-hydroxypropyl)amine-induced pancreatic tumors in the Syrian golden hamster. J Cancer Res Clin Oncol. 1992;118:377–385. doi: 10.1007/BF01294443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouakil N, Rogalska E, Bonicel J, Lombardo D. Purification of pancreatic carboxylic ester hydrolase by immunoaffinity and its application to the human bile salt-stimulated lipase. Biochim Biophys Acta. 1988;961:299–308. doi: 10.1016/0005-2760(88)90077-x. [DOI] [PubMed] [Google Scholar]
- 29.Sbarra V, Bruneau N, Mas E, Hamosh M, Lombardo D, Hamosh P. Molecular cloning of the bile salt-dependent lipase of ferret lactating mammary gland: an overview of functional residues. Biochim Biophys Acta. 1998;1393:80–89. doi: 10.1016/s0005-2760(98)00067-8. [DOI] [PubMed] [Google Scholar]
- 30.Fujii Y, Sekiguchi M, Shiroko Y, Shimizu H, Sugawara J, Hasumi K, Eriguchi M, Ikeuchi T, Uchida H. Establishment and characterisation of human pancreatic adenocarcinoma cell line SOJ producing carcinoembryonic antigen and carbohydrate antigen 19-9. Hum Cell. 1990;3:31–36. [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 33.Altin JG, Pagler EB. A one-step procedure for biotinylation and chemical cross-linking of lymphocyte surface and intracellular membrane-associated molecules. Anal Biochem. 1995;224:382–389. doi: 10.1006/abio.1995.1054. [DOI] [PubMed] [Google Scholar]
- 34.Samih N, Hovsepian S, Notel F, Prorok M, Zattara-Cannoni H, Mathieu S, Lombardo D, Fayet G, El Battari A. The impact of N- and O-glycosylation on the functions of Glut-1 transporter in human thyroid anaplastic cells. Biochim Biophys Acta. 2003;1621:92–101. doi: 10.1016/s0304-4165(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 35.Orlowski M, Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys. 2000;383:1–16. doi: 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- 36.Ermonval M, Kitzmuller C, Mir AM, Cacan R, Ivessa NE. N-glycan structure of a short-lived variant of ribophorin I expressed in the MadIA214 glycosylation-defective cell line reveals the role of a mannosidase that is not ER mannosidase I in the process of glycoprotein degradation. Glycobiology. 2001;11:565–576. doi: 10.1093/glycob/11.7.565. [DOI] [PubMed] [Google Scholar]
- 37.Banik NL, Chou CH, Deibler GE, Krutzch HC, Hogan EL. Peptide bond specificity of calpain: proteolysis of human myelin basic protein. J Neurosci Res. 1994;37:489–496. doi: 10.1002/jnr.490370408. [DOI] [PubMed] [Google Scholar]
- 38.Matsushima-Nishiwaki R, Shidoji Y, Nishiwaki S, Moriwaki H, Muto Y. Limited degradation of retinoid X receptor by calpain. Biochem Biophys Res Commun. 1996;225:946–951. doi: 10.1006/bbrc.1996.1276. [DOI] [PubMed] [Google Scholar]
- 39.Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 40.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 41.Huguier M, Mason NP. Treatment of cancer of the exocrine pancreas. Am J Surg. 1999;177:257–265. doi: 10.1016/s0002-9610(99)00003-3. [DOI] [PubMed] [Google Scholar]
- 42.Abbruzzese JL. Past and present treatment of pancreatic adenocarcinoma: chemotherapy as a standard treatment modality. Semin Oncol. 2002;29:2–8. doi: 10.1053/sonc.2002.37382. [DOI] [PubMed] [Google Scholar]
- 43.Ellenrieder V, Adler G, Gress TM. Invasion and metastasis in pancreatic cancer. Ann Oncol. 1999;10:46–50. [PubMed] [Google Scholar]
- 44.Kobayashi T, Kawa S, Tokoo M, Oguchi H, Kiyosawa K, Furuta S, Kanai M, Homma T. Comparative study of CA-50 (timeresolved fluoroimmunoassay), Span-1, and CA 19-9 in the diagnosis of pancreatic cancer. Scand J Gastroenterol. 1991;26:787–797. doi: 10.3109/00365529108998600. [DOI] [PubMed] [Google Scholar]
- 45.Lan MS, Khorrani A, Kaufman B, Metzgar RS. Molecular characterization of a mucin-type antigen associated with human pancreatic cancers: the Du-Pan 2 antigen. J Biol Chem. 1987;262:12863–12870. [PubMed] [Google Scholar]
- 46.Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patient as mucin. Cancer Res. 1983;43:5489–5492. [PubMed] [Google Scholar]
- 47.Masson P, Palsson B, Andren-Sandberg A. Cancer-associated tumour markers CA 19-9 and CA-50 in patients with pancreatic cancer with special reference to the Lewis blood cell status. Br J Cancer. 1990;62:118–121. doi: 10.1038/bjc.1990.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prat M, Medico E, Rossino P, Garrino C, Comogho PM. Biochemical and immunological properties of the human carcinomaassociated CAR-3 epitope defined by the monoclonal antibody AR-3. Cancer Res. 1989;49:1415–1421. [PubMed] [Google Scholar]
- 49.Kawa S, Tokoo M, Oguchi H, Furuta S, Homma T, Hasekawa Y, Ogata H, Sakata K. Epitope analysis of Span-1 and DUPAN-2 using synthesized glycoconjugates sialyllact-N-fucopentaose II and sialyllact-N-tetraose. Pancreas. 1994;9:692–697. doi: 10.1097/00006676-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, Nishihara S, Sugano K, Okura H, Fujita S, Hirohashi S. Lewis and secretor gene dosages affect CA 19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58:512–518. [PubMed] [Google Scholar]
- 51.Mazo A, Fujii Y, Shimotake J, Escribano MJ. Expression of fetoacinar pancreatic (FAP) protein in the pancreatic human tumor cell line BxPC-3. Pancreas. 1991;6:37–45. doi: 10.1097/00006676-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Nicchitta CV. Biochemical, cell biological and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/gp96. Curr Opin Immunol. 1998;10:103–109. doi: 10.1016/s0952-7915(98)80039-3. [DOI] [PubMed] [Google Scholar]
- 54.Linderoth NA, Simon MN, Hainfeld JF, Sastry S. Binding of antigenic peptide to the endoplasmic reticulum-resident protein gp96/GRP94 heat shock chaperone occurs in higher order complexes. Essential role of some aromatic amino acid residues in the peptidebinding site. J Biol Chem. 2001;276:11049–11054. doi: 10.1074/jbc.M010059200. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Admon A, Barnea E, Ziv T. Tumor antigens and proteomics from the point of view of the major histocompatibility complex peptides. Mol Cell Proteomics. 2003;2:388–398. doi: 10.1074/mcp.R300004-MCP200. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 59.Walker RA. The significance of histological determination of HER-2 status in breast cancer. Breast. 2000;9:130–133. doi: 10.1054/brst.2000.0167. [DOI] [PubMed] [Google Scholar]