Abstract
Barrett's esophagus (BE) epithelium is the precursor lesion for esophageal adenocarcinoma. Cell cycle proteins have been advocated as biomarkers to predict the malignant potential in BE. However, whether disruption of the cell cycle plays a causal role in Barrett's carcinogenesis is not clear. Specimens from the Barrett's dysplasia-carcinoma sequence were immunostained for cell cycle phase markers (cyclin D1 for G1; cyclin A for S, G2, and M; cytoplasmic cyclin B1 for G2; and phosphorylated histone 3 for M phase) and expressed as a proportion of proliferating cells. Flow cytometric analysis of the cell cycle phase of prospective biopsies was also performed. The proliferation status of nondysplastic BE was similar to gastric antrum and D2, but the proliferative compartment extended to the luminal surface. In dysplastic samples, the number of proliferating cells correlated with the degree of dysplasia (P < .001). The overall levels of cyclins A and B1 correlated with the degree of dysplasia (P < .001). However, the cell cycle phase distribution measured with both immunostaining and flow cytometry was conserved during all stages of BE, dysplasia, and cancer. Hence, the increased proliferation seen in Barrett's carcinogenesis is due to abnormal cell cycle entry or exit, rather than a primary abnormality within the cell cycle.
Keywords: Cell proliferation, dysplasia, adenocarcinoma, cyclin, cell cycle
Background
Cancer is characterized by the loss of proliferative and apoptotic control, leading to a growth advantage of malignant cells compared to their nonmalignant counterparts. These abnormalities in growth control mechanisms accumulate gradually because most epithelial malignancies have a premalignant stage that may progress to cancer over a period of years [1]. Barrett's esophagus (BE) is a premalignant, metaplastic condition in which the normal squamous epithelium lining the esophagus is replaced by a glandular epithelium akin to that found in gastric and intestinal mucosa. Esophageal adenocarcinoma (AC) gradually develops from BE through a dysplasia-carcinoma sequence in an estimated 5% to 10% of patients [2,3]. The grading of dysplastic changes within BE by histopathologic criteria is highly subjective [4], and we are very limited in our ability to predict the point at which progression to AC is inevitable. To develop prognostic tests and curative intervention strategies for patients with BE, it is necessary to understand the stage at which normal growth controls are abrogated and the molecular mechanisms that are involved.
In the quest for predictive markers of progression to cancer, cell cycle stage and cell cycle-related proteins have been investigated in BE over the last 20 years. Several retrospective studies using flow cytometry demonstrated that the number of cells in gap 1 (G1), DNA synthesis (S), gap 2 (G2), and mitosis (M) phases increased with progression from BE to AC [5,6]. In addition, a prospective study linked increased G2/tetraploid phases fraction with progression from nondysplastic and dysplastic BE to AC [7–10]. Apart from these flow cytometry studies, most of the works done on cell cycle-related molecules in BE have been performed by immunohistochemistry because paraffin-embedded tissue is readily available and permits the determination of the distribution of these markers. Overexpression of cyclin D1, expressed in mid- to late-G1 [11], has been shown to have a predictive value for progression to neoplasia in a retrospective, longitudinal, case-control study [12]. The increased expression of cyclins E and B1 along the progression from BE to AC has been suggested to result from an increased entry into S [13] and G2/M phases, respectively [14]. Hence, all of these studies led to the conclusion that there is an overexpression of cell cycle proteins, or deregulation of cell cycle phase distribution as BE progresses toward AC. These studies were generally conducted with the aim of identifying a predictive marker for cancer progression and, in most cases, the study was restricted to a single protein marker. By studying an individual cell cycle marker independently of the proliferation status, it is not possible to determine whether the findings are due to overexpression of a specific protein, or are a consequence of the overall increase in proliferation.
Recently, with an increased understanding of the key regulators of cell cycle progression and proliferation, cell cycle markers with a greater specificity have become available [15]. Minichromosome maintenance (MCM) proteins are expressed in all cells in a cycle [16,17], whereas the timing of expression of Ki-67 in cycling cells is subject to controversy [18]. Cyclins, proteins involved in the tight regulation of cell cycle progression, are markers of choice as they are expressed at specific phases of the cell cycle. Cyclin D1 is expressed in mid- to late-G1 phase, and is translocated to the cytoplasm in S phase where it is degraded [11]. Cyclin A is expressed in early S phase and is degraded during metaphase [19]. Cyclin B1 accumulates in the cytoplasm of cells in the G2 phase, and is then translocated to the nucleus in early mitosis to be degraded at the metaphase- anaphase transition [20,21]. Histone H3, one of the four histones composing the nucleosome, is phosphorylated in late G2 to early M to allow chromosome condensation [22]. The phosphorylation levels peak during metaphase and start to decrease in anaphase, and histone H3 is dephosphorylated during telophase before chromosomes decondense [23]. The use of those molecules as cell cycle markers has been previously validated in the context of colorectal neoplasia [15].
The aim of this study was to perform a comprehensive analysis of the overall proliferation status and cell cycle stage distribution in BE-associated carcinogenesis. We demonstrate that although cell proliferation increases during the progression to BE dysplasia and cancer with a disordered proliferation compartment, the distribution of the cell cycle phases is conserved.
Methods
Patients and Tissue Collection
Samples for immunostaining Archival blocks were obtained from patients who had attended Addenbrooke's Hospital (Cambridge, UK) and Middlesex Hospital (London, UK). We studied 35 cases of nondysplastic BE, 26 low-grade dysplasia (LGD), 11 high-grade dysplasia (HGD), and 16 invasive AC from different patients. Sections of nonmalignant, normal tissues from BE and non-BE patients were used as control specimens [10 from the second part of the duodenum (D2) and 20 from the gastric antrum].
Samples for flow cytometry Two biopsies per patient (6 D2, 4 gastric, 13 BE, 8 LGD, and 6 AC) were placed in Dulbecco's modified Eagle's medium (Invitrogen, Paisley, UK) containing 10% fetal calf serum and 10% dimethyl sulfoxide (Sigma, Gillingham, UK) at the time of endoscopy. Samples were transported on ice, then stored at -80°c until processed. D2 and gastric antrum control samples were taken from patients with no endoscopic or histopathologic abnormality in these anatomic areas. Biopsies were called LGD only if patients had multifocal LGD throughout the BE segment to avoid sample bias.
All patientswithBEhadanendoscopicallyvisiblecolumnarlined segment and a histopathologic diagnosis of specialized intestinal metaplasia. Approval for this study was obtained from the Local Research Ethics Committees.
Immunostaining
Specificity of all the antibodies was confirmed by Western blot analysis (data not shown). For all antibodies except cyclin D1, tissue sections were deparaffinized in xylene and rehydrated through alcohol solutions, water, and, finally, Tris-buffered saline-Tween (1.4 mol/l NaCl, 0.25 mol/l Tris-base, and 0.025% Tween; TBS-Tween). Washes using TBS-Tween were performed between each step. An antigen retrieval step was performed by pressure-cooking samples for 3 minutes in 0.01 M Tris-sodium citrate buffer at pH 6.0. Staining was performed using the Dako autostainer Dako ChemMale (DakoCytomation Ltd., Ely, UK) and the staining kit Dako ChemMate for increased reproducibility. Blocking of nonspecific binding was performed using 10% normal goat serum and 10% bovine serum albumin for 30 minutes at room temperature. The samples were incubated for 1 hour at room temperature with each of the following antibodies diluted in antibody diluent (DakoCytomation Ltd.): monoclonal Mcm2 antibody (dilution 1/10; gift from Steve DilworthandRonLaskey),anti-Ki-67antibody(dilution1/100, MIB-1; DakoCytomation Ltd.), anti-cyclin A antibody (dilution 1/20; Novocastra, Newcastle upon Tyne, UK), anti-cyclin B1 antibody (dilution 1/400; DakoCytomation Ltd.), anti-phosphorylated histone H3 (pH3) antibody (dilution 1/300; Upstate Biotechnology, Buckingham, UK). Endogenous peroxidase activity was blocked with peroxidase blocking solution (DakoCytomation Ltd.) for 5 minutes. The secondary and peroxidase-linked antibody were both incubated subsequently for 30 minutes at room temperature followed by DAB substrate (DakoCytomation Ltd.) for 10 minutes. Sections were counterstained with hematoxylin. A negative control was performed by omission of the primary antibody.
Cyclin D1 antibody (dilution 1/50; DakoCytomation Ltd.) staining was performed by hand using Dako Target Retrieval Solution High pH (DakoCytomation Ltd.) to perform the antigen retrieval step in a microwave for 30 minutes at 98°C. The staining procedure was performed using the Dako EnVisionTM + System. This kit replaces the peroxidase block and the secondary and peroxidase-linked antibody with a peroxidase-labeled polymer applied for 30 minutes. The DAB solution was then added for 10 minutes. Washing steps were performed between each step with TBS-Tween.
Validation of Cyclin A Expression in the Context of BE
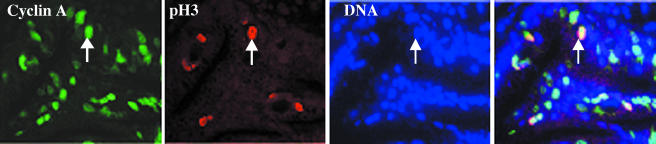
It is generally accepted that cyclin A is expressed in S and G2/M phases [19]; however, it has been demonstrated that for colorectal cancer, immunohistochemical expression of cyclin A might only be detectable in S phase [15]. To test this in the context of BE, we performed colocalization studies between cyclin A and pH3. A fraction of epithelial cells staining for both cyclin A and pH3 was seen in BE mucosa (Figure 1), suggesting that cyclin A is indeed present in G2/M phases and that cyclin A is a marker of S-G2 and M.
Figure 1.
Verification of expression of cyclin in BE mucosa. Colocalization of cyclin A (green), pH3 (red), and DNA (blue) in Barrett's mucosa. The last panel represents merging of the three pictures.
Scoring Immunohistochemistry
For glandular epithelia, the mucosal compartment of each well-oriented section was divided into the following three zones: 1) luminal surface (the most superficial layer of the columnar epithelium); 2) upper crypt (upper half of the crypt); and 3) proliferative compartment (lower half of the crypt and underlying glands) [24]. The upper and lower crypts were divided halfway between the surface and the bottom of the crypt. The number of immunopositive epithelial cells was calculated as a percentage of the total number of epithelial cells. The percentage of proliferative cells positive for each marker refers to the percentage of cells positive for a given marker divided by the percentage of cells positive for one of the proliferation markers from the same biopsy sample (either Mcm2 or Ki-67) multiplied by 100. The whole surface, and as many well-oriented crypts and glands as possible, were counted. Twenty-five percent of the sections stained for Mcm2 and Ki-67 were also analyzed by an independent pathologist (R.B.).
Flow Cytometry
Biopsies were processed for cell cycle analysis by flow cytometry using a modification of the technique described by Reid et al. [6]. Briefly, biopsies were minced in NST buffer (146 mM NaCl, 10 mM Tris base, 1 mM CaCl2, 0.5 mM MgSO4, 21 mM MgCl2, 0.05% bovine serum albumin, and 0.2% IGEPAL CA-630; Sigma) using a scalpel blade and passed several times through a 25-gauge needle to break up clumps. Samples were spun down and resuspended in NST buffer containing 10% normal goat serum (DakoCytomation Ltd.) and DAPI at 10 µg/ml. Each sample was separated into two tubes to which were added anti-Ki-67-RPE (positive sample; DakoCytomation Ltd.) at a dilution of 1/20 and mouse IgG1-RPE (negative control; DakoCytomation Ltd.) at a dilution of 1/25. Samples were then incubated for 1 hour at 4°C. SEG-1 cells were used as a positive control. The samples were spun down, resuspended in NST containing DAPI (10 µg/ml), and passed through a 70-µm cell strainer before being captured for cell cycle distribution on the flow cytometer BE LSR2 (BD Biosciences, Oxford, UK) using a FACSDIVA software and analyzed using ModFit (Verity Software House, Topsham, ME). Previous data by Reid et al. showed that although proliferating inflammatory cells were present in the lamina propria, the proliferating cells detected using Ki-67 immunostaining were predominantly epithelial and, hence, microdissection of the specimen was not deemed necessary [6].
Statistical Analysis
The paired Kruskal-Wallis test was used to identify differences between the two observers for the scores from Mcm2 and Ki-67 from. The Jonckheere-Terpstra test [25] and the chi-square analysis for trend were used to assess the correlation between the expression of individual markers and the degree of dysplasia. The Jonckheere-Terpstra test is a nonparametric statistic test used to identify a shift in ordered distributions (Mcm2 surface expression) when stratified by ordered categories (BE, LGD, HGD, and AC). The Kruskal-Wallis test was used to identify specific differences within a population, and the Mann-Whitney test was used to identify specific differences between groups. In all cases, P < .05 was required for significance to identify specific differences [26].
Results
Proliferative Status of BE
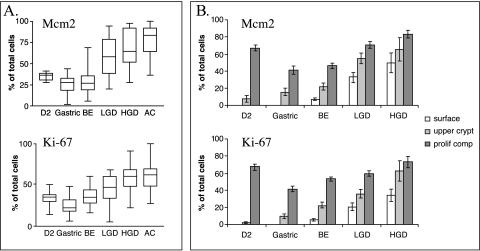
The overall proliferation status of nondysplastic BE was similar to that of gastric antrum and D2 for both Mcm2 (29.9 ± 2.5%, 25.2 ± 2.5%, and 34.7 ± 1.6%, respectively) and Ki-67 (34.7 ± 2.1%, 23.9 ± 2.6%, and 32.8 ± 2.6%) antibodies (Figure 2A) (P = ns between groups). Overall, 41% (gastric antrum) and 67% (duodenum) of the cells within the proliferative compartment are Ki-67-positive and Mcm2- positive (Figure 2B). Over 75% of these proliferating cells in the gastric antrum and duodenum are confined to the proliferative compartment (Figures 2B and 3). In contrast, in BE samples, 40% of the proliferative cells were found in the upper crypt and at the luminal surface even prior to the development of dysplasia (Figures 2B and 3). This suggests that outside of the normal proliferative compartment, differentiating cells re-enter the cell cycle, or cells do not differentiate as they migrate toward the lumen.
Figure 2.
Cumulative data for proliferation indices and epithelial compartments in the upper gastrointestinal mucosa. Mcm2 and Ki-67 expression is shown as a percentage of total epithelial cells (panel A) and according to the localization of proliferating cells (panel B) in the second part of the duodenum (D2), gastric antrum (gastric), BE, LGD, HGD, and AC.
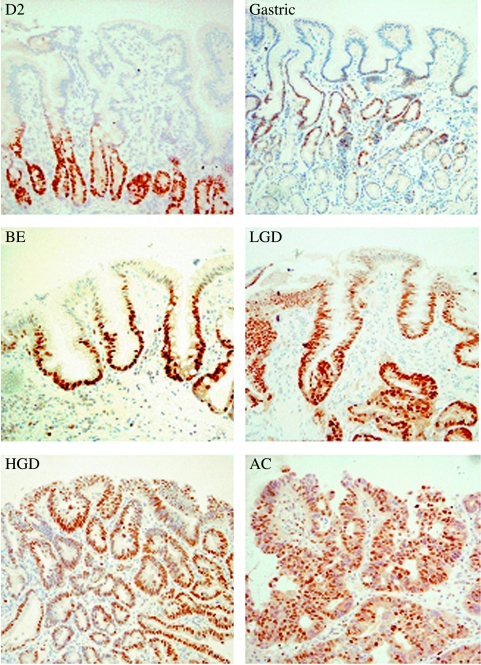
Figure 3.
Expression of Mcm2 (brown immunostain, with blue hematoxylin counterstain) in representative tissue sections from the second part of the duodenum (D2), gastric antrum (gastric), BE, LGD, HGD, and AC.
The total number of proliferative cells in the epithelial mucosa increased as BE progressed to AC (Figures 2A and 3). Furthermore, the proliferation levels as judged by Mcm2 and Ki-67 staining correlated with the grade of dysplasia (P < .0001 in both cases). As BE progresses to LGD and, subsequently, to HGD, the percentage of proliferating cells increases equally in each compartment (Figure 2B), suggesting again an abnormal cell cycle entry/exit or a decrease of cell cycle length. The levels of Mcm2 staining were generally higher than Ki-67 staining for each tissue with the exception of BE, where there were wide confidence intervals for Mcm2 staining (Figure 2B).
Cell Cycle Distribution
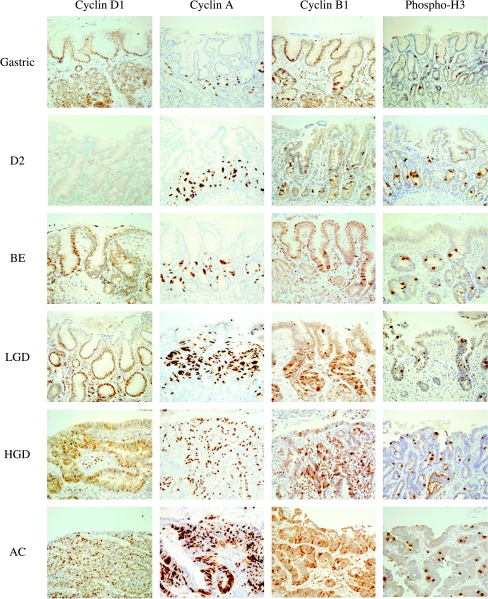
Immunohistochemistry The cell cycle markers (cyclins D1, A, and B1, and pH3) were expressed almost exclusively in the proliferative compartment of the gastric antrum and D2 similar to Mcm2 and Ki-67 (Figure 4). Cyclin D1 staining was absent from the epithelial cells and intramucosal lymphocytes of the D2 by immunostaining, but present in small amounts by immunoblotting (Figure 4; data not shown), suggesting that the antibody is unable to detect low levels of cyclin D1 by immunohistochemistry. In all stages of BE carcinogenesis, the expression of cell cycle markers extended to the luminal surface to an extent depending on the degree of dysplasia (Figure 4), similar to the findings for Mcm2 and Ki-67 (Figure 2B).
Figure 4.
Immunohistochemical staining for cyclins D1, A, and B1, and pH3 in a representative tissue section from the gastric antrum (gastric), second part of the duodenum (D2), BE, LGD, HGD, and AC.
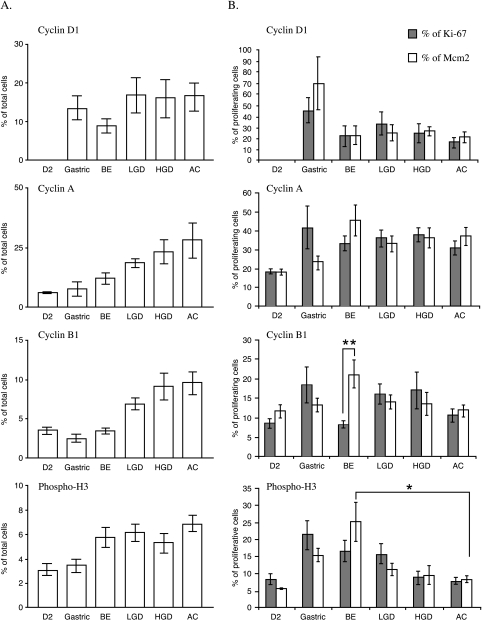
When expressed as a percentage of total epithelial cells, the overall expression of cyclins A and B1 correlated with the degree of dysplasia (12.9 ± 1.8% and 3.5 ± 0.4% in BE, 18.3 ± 1.7% and 6.9 ± 0.8% in LGD, 23.1 ± 2.3% and 9.1 ± 1.7 in HGD, and 29.9 ± 3.4% and 9.6 ± 1.4% in AC, respectively, for cyclins A and B1, P < .001). The levels of pH3 increased moderately as the tissue progressed to AC, but no statistical significance was reached. No specific trend was seen with regards to the expression of cyclin D1 (Figure 5A). In contrast, when expressed as a percentage of the proliferating cells (i.e., those staining positive for Mcm2 or Ki-67), cyclins D1, A, and B1 levels remain consistent as the tissue progresses from BE to AC (Figure 5B). It was observed that there was a statistically significant difference in the level of cyclin B1 in BE when expressed as a percentage of Mcm2 compared with Ki-67 (Figure 5B) (P < .005). pH3 levels also remained consistent along the progression from BE to AC when expressed as a proportion of Ki-67-positive cells (Figure 5B). However, the level of pH3 as a percentage of Mcm2 staining is statistically significant between BE and AC (P < .03). These statistical differences for pH3 and cyclin B1 may reflect the lower expression level of Mcm2 compared to Ki-67 expression in BE (Figure 1B). However, taken together, the data for Mcm2 and Ki-67 suggest that for a given level of proliferation, the cell cycle distribution is conserved as BE progresses to AC.
Figure 5.
Expression of the cell cycle markers (cyclins D1, A, and cyclin B1, and pH3) in the gastric antrum (gastric), second part of the duodenum (D2), BE, LGD, HGD, and AC. Panel A shows data as a percentage of total cells. Panel B shows data as a percentage of the proliferative fraction. The percentage of the proliferative fraction was calculated by dividing the percentage of expression of each marker in a given sample by the percentage of proliferating cells (either Mcm2 or Ki-67) in the same slide. *P < .03; **P < .005.
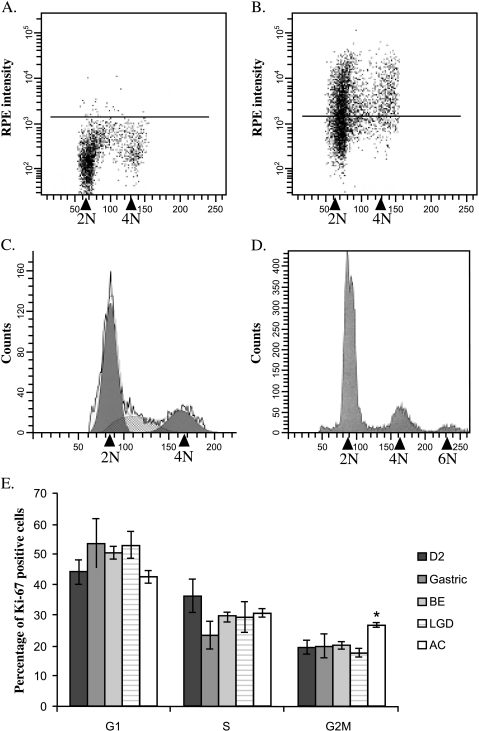
Flow cytometry Flow cytometric analysis of cell cycle phase using endoscopic biopsies was then performed to confirm the results obtained by immunohistochemistry. Figure 6A demonstrates the results when nuclei were incubated with mouse IgG1-RPE (negative control) to set the limit between Ki-67-RPE-positive (above black line) and Ki- 67-RPE-negative nuclei (below, black line) (Figure 6A). The cell cycle profile of Ki-67-positive cells (Figure 6C) was derived from the results shown in Figure 6B. An aneuploid population at 6N chromosomes was found in 66.7% (4/6) of AC (Figure 6D). Interestingly, there was a statistically significant increase of 7% in the G2/tetraploid phase of the Ki-67-positive cells in AC when compared to the other mucosa (P < .02) (Figure 6E). The cells from this increased G2/tetraploid fraction possibly represent the G1 fraction of the aneuploid population found in AC. Cumulative data from cell cycle profiling demonstrated that the percentage of cells positive for Ki-67 in G1 and S phases was conserved in D2, gastric, BE, LGD, and AC, consistent with immunostaining (no statistical significance in any case; Figure 6E).
Figure 6.
Flow cytometry results for upper gastrointestinal biopsy samples. Panels A–C are representative examples of analysis from a BE biopsy where 2N refers to diploid cells (G0/G1 phase of the cell cycle) and 4N refers to tetraploid cells (G2/M cells). The black line in the negative control (panel A) represents the maximum RPE intensity of unstained cells. In the positive samples (panel B), Ki-67 - negative cells (G0) are below the line and Ki-67 - positive cells are above. Ki-67 - positive nuclei only are used for the cell cycle profile (panel C). The rough black line represents raw data. The black areas at 2N and 4N denote cells in G1 and G2/M, respectively, and the hashed area is the S phase. The components were fitted using ModFit. The cell cycle profile of a cancer sample exhibiting a tetraploid population is shown for comparison (panel D). The 2N and 4N peaks only were used to obtain the cell cycle fractions. Panel E shows the cumulative data, which demonstrated that the percentages of cells in G1 and S phases are similar for all tissue samples (D2, gastric antrum, BE, LGD, and AC). The percentage of Ki-67 cells in the G2/tetraploid phase is statistically increased in AC when compared to BE and LGD (*P < .02).
Discussion
In this study, we have demonstrated that the overall proliferative index of nondysplastic BE is not increased when compared to other glandular gastrointestinal mucosa. Nevertheless, nondysplastic BE cannot be considered as a normal mucosa because there are proliferating cells present in the upper crypt and luminal surface of this epithelium. These cells may have retained their proliferative status as they migrated toward the region of the crypt normally occupied by differentiated cells. There is an overall increase in the number of proliferating cells observed as BE progresses to LGD, HGD, and AC, as expected. However, using two techniques, we have shown that the cell cycle phase distribution is conserved throughout the progression from nondysplastic BE to LGD, HGD, and AC.
To assess the degree of cell proliferation, both Mcm2 and Ki-67 were used (Figures 2 and 3). Ki-67 is a well-known proliferation marker [27], although its precise role is not understood. In contrast, Mcm2 is known to be part of a heteromeric-hexameric complex that binds to the origins of replication to ensure a single round of DNA replication. Unlike Ki-67, MCMs are expressed in all cycling cells throughout the cell cycle and are degraded when mammalian cells exit the cell cycle into quiescent, differentiated, and senescent states [16,17,28]. This expression profile increases the sensitivity of MCM antibodies to detect cycling cells; therefore, MCM proteins have been proposed as candidate markers for cancer screening and surveillance, and as prognostic markers [29–34].
In the published literature, the proliferative status of BE is controversial. Two independent studies have demonstrated that BE proliferates more than squamous epithelium [35,36], but Iftikhar et al. [37] concluded on the contrary. We did not compare the proliferative indices of BE with squamous esophageal mucosa because their different architectures make direct comparisons impossible. In previous studies, which have compared the proliferative index of BE with other glandular tissues, the results are conflicting. Studies using tritiated thymidine incorporation reported similar indices between BE, gastric mucosa, and duodenum [36,38]; whereas increased proliferation has been reported for BE compared with gastric tissue by immunohistochemical analysis of Ki-67 [24]. These discrepancies may be due to the different techniques used and the subjective interpretation of immunostaining. It is possible that the proliferative status of nondysplastic BE reflects the change to a metaplastic, glandular phenotype. However, in keeping with our data, there is general agreement in the literature that the overall proliferative index increases as BE progresses to AC [6,24,39,40], with an expansion of the proliferative compartment toward the luminal surface [24,34,39,40].
The shift of the proliferative compartment, usually confined to the lower crypt and glands, to the upper crypt and the luminal surface has critical implications because we have demonstrated previously that surface proliferation was of prognostic value for the development of AC [34]. Overstimulation of proliferation by growth factors, luminal factors, and mutations in oncogenes [41] would be expected to increase proliferation. The Wnt pathway is a tight regulator of crypt cell fate and patterning in the intestinal mucosa [42] . Knockout mice models of members of the Wnt pathway, Fkh6 (forkhead homologue transcription factor) [43], and Nkx2-3 (NK 2 homeobox transcription factor) [44] can result in hyperproliferative, expanded crypt compartments. It is therefore possible that these pathways are disrupted early in BE.
Most cancer cells have impaired cell cycle checkpoints, resulting in the accumulation of genetic aberrations [45]. Studies of cell cycle molecules in BE have suggested an array of abnormalities, but the work was not performed in the context of overall proliferation status. If there is an increase in cell proliferation, then more cells will be entering the cell cycle (increased G1 fraction and cyclin D1 expression). Most of the cells in G1 will progress into S phase (increased S phase fraction) and then enter G2 (G2 accumulation and cyclin B1 increased expression). Unless these cell cycle markers are expressed as a fraction of the denominator (proliferation), then there will be an apparent disruption of cell cycle control. Our data suggest that the cell cycle phase distribution is conserved throughout Barrett's metaplasia- dysplasia sequence.
In addition, the flow cytometry analysis demonstrated an apparent increase in the G2/tetraploid fraction in AC (Figure 6, D and E) and an aneuploidy 6N peak in 66.7% of the AC patients. It is therefore possible that the cells from the G2/tetraploid fraction are tetraploid cells in G1, as flow cytometry does not allow discriminating between tetraploid G1 cells and true G2/M cells. A 7% increase in the cyclin D1 fraction should have been observed, but the variability of the cyclin D1 staining makes this difficult to deduce. A more sensitive technique to measure the G1 fraction is required to resolve this question. Furthermore, four patients in our study had an increased G2/tetraploid content early in the metaplasia- dysplasia sequence [2/13 BE (15%), and 2/8 LGD (20%)]. This finding is in keeping with previous data, which also suggest that G2/tetraploidy status was of prognostic value [7–10]. However, the sample size and cross-sectional nature of our study mean that the prognostic significance of these findings cannot be established.
Despite our findings that the cell cycle phase distribution is conserved during carcinogenesis, proliferation and cell cycle proteins may still be useful prognostic markers by virtue that their overall expression level increases coincidentally with the increased proliferation index. Both cyclin D1 and Mcm2 have proven valuable in a phase 3 longitudinal case-control study design [12,34]. In our hands, despite the use of different cyclin D1 antibodies, the results were found to be heterogeneous both within and between the different tissues examined. These practical difficulties would limit its application to clinical practice. Phases 3 and 4 prospective studies are required to accurately determine the clinical utility of any of these molecular markers [46].
In conclusion, we have demonstrated that there is no primary abnormality in the cell cycle stage that could account for the increased and disordered cell proliferation during the Barrett's metaplasia-dysplasia sequence. This suggests that abnormal cell cycle entry or exit and possibly a shortened cell cycle length may be responsible for the increased proliferative index. A better understanding of the molecular mechanisms controlling cell cycle entry may pave the way for clinically valid prognostic markers and novel therapeutic targets.
Acknowledgements
We thank the patients and the staff in the endoscopy unit at Addenbrooke's Hospital and University College London, as well as William Gage, Angela Wong, and Selvasekar Chelliah for their cooperation with the sample collection. We are grateful to Sarah Vowler who helped us with the statistical analysis, Carissa Sanchez and Lesley Morris for their technical assistance, and Mark Madine for his constructive comments on the manuscript.
Abbreviations
- AC
adenocarcinoma
- BE
Barrett's esophagus
- D2
second part of the duodenum
- HGD
high-grade dysplasia
- LGD
low-grade dysplasia
References
- 1.O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, Sigman CC, Bertagnolli MM, Stratton SP, Lam S, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 2.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 3.Haggitt RC, Dean PJ. Adenocarcinoma in Barrett's epithelium. In: Goyal RK, editor. Barrett's Esophagus: Pathophysiology, Diagnosis and Management. New York: Elsevier; 1977. pp. 153–166. [Google Scholar]
- 4.Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H, et al. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 5.Sciallero S, Giaretti W, Bonelli L, Geido E, Rapallo A, Conio M, Ravelli P, Lombardo L, Briglia R, Lapertosa G, et al. DNA content analysis of Barrett's esophagus by flow cytometry. Endoscopy. 1993;25:648–651. doi: 10.1055/s-2007-1010424. [DOI] [PubMed] [Google Scholar]
- 6.Reid BJ, Sanchez CA, Blount PL, Levine DS. Barrett's esophagus: cell cycle abnormalities in advancing stages of neoplastic progression. Gastroenterology. 1993;105:119–129. doi: 10.1016/0016-5085(93)90017-7. [DOI] [PubMed] [Google Scholar]
- 7.Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, Rabinovitch PS. Flow-cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–1219. [PubMed] [Google Scholar]
- 8.Rabinovitch PS, Longton G, Blount PL, Levine DS, Reid BJ. Predictors of progression in Barrett's esophagus: III. Baseline flow cytometric variables. Am J Gastroenterol. 2001;96:3071–3083. doi: 10.1111/j.1572-0241.2001.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett MT, Sanchez CA, Prevo LJ, Wong DJ, Galipeau PC, Paulson TG, Rabinovitch PS, Reid BJ. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet. 1999;22:106–109. doi: 10.1038/8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett MT, Pritchard D, Palanca-Wessels C, Anderson J, Reid BJ, Rabinovitch PS. Molecular phenotype of spontaneously arising 4N (G2-tetraploid) intermediates of neoplastic progression in Barrett's esophagus. Cancer Res. 2003;63:4211–4217. [PubMed] [Google Scholar]
- 11.Pines J. Four-dimensional control of the cell cycle. Nat Cell Biol. 1999;1:E73–E79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 12.Bani-Hani K, Martin IG, Hardie LJ, Mapstone N, Briggs JA, Forman D, Wild CP. Prospective study of cyclin D1 overexpression in Barrett's esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92:1316–1321. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 13.Sarbia M, Bektas N, Muller W, Heep H, Borchard F, Gabbert HE. Expression of cyclin E in dysplasia, carcinoma, and nonmalignant lesions of Barrett esophagus. Cancer. 1999;86:2597–2601. [PubMed] [Google Scholar]
- 14.Geddert H, Heep HJ, Gabbert HE, Sarbia M. Expression of cyclin B1 in the metaplasia-dysplasia-carcinoma sequence of Barrett esophagus. Cancer. 2002;94:212–218. doi: 10.1002/cncr.10152. [DOI] [PubMed] [Google Scholar]
- 15.Scott IS, Morris LS, Bird K, Davies RJ, Vowler SL, Rushbrook SM, Marshall AE, Laskey RA, Miller R, Arends MJ, et al. A novel immunohistochemical method to estimate cell-cycle phase distribution in archival tissue: implications for the prediction of outcome in colorectal cancer. J Pathol. 2003;201:187–197. doi: 10.1002/path.1444. [DOI] [PubMed] [Google Scholar]
- 16.Tye BK. MCM proteins in DNA replication. Annu Rev Biochem. 1999;68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Todorov IT, Werness BA, Wang HQ, Buddharaju LN, Todorova PD, Slocum HK, Brooks JS, Huberman JA. HsMCM2/BM28: a novel proliferation marker for human tumors and normal tissues. Lab Invest. 1998;78:73–78. [PubMed] [Google Scholar]
- 18.Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res. 2000;257:231–237. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 19.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 20.Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 23.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 24.Hong MK, Laskin WB, Herman BE, Johnston MH, Vargo JJ, Steinberg SM, Allegra CJ, Johnston PG. Expansion of the Ki-67 proliferative compartment correlates with degree of dysplasia in Barrett's esophagus. Cancer. 1995;75:423–429. doi: 10.1002/1097-0142(19950115)75:2<423::aid-cncr2820750202>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Hodges JL, Lehmann EL. Nonparametric Statistical Methods. New York: John Wiley and Sons; 1963. [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 27.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Madine MA, Swietlik M, Pelizon C, Romanowski P, Mills AD, Laskey RA. The roles of the ORC, MCM, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J Struct Biol. 2000;129:198–210. doi: 10.1006/jsbi.2000.4218. [DOI] [PubMed] [Google Scholar]
- 29.Hunt DP, Freeman A, Morris LS, Burnet NG, Bird K, Davies TW, Laskey RA, Coleman N. Early recurrence of benign meningioma correlates with expression of mini-chromosome maintenance-2 protein. Br J Neurosurg. 2002;16:10–15. doi: 10.1080/02688690120114174. [DOI] [PubMed] [Google Scholar]
- 30.Rodins K, Cheale M, Coleman N, Fox SB. Minichromosome maintenance protein 2 expression in normal kidney and renal cell carcinomas: relationship to tumor dormancy and potential clinical utility. Clin Cancer Res. 2002;8:1075–1081. [PubMed] [Google Scholar]
- 31.Davies RJ, Freeman A, Morris LS, Bingham S, Dilworth S, Scott I, Laskey RA, Miller R, Coleman N. Analysis of minichromosome maintenance proteins as a novel method for detection of colorectal cancer in stool. Lancet. 2002;359:1917–1919. doi: 10.1016/S0140-6736(02)08739-1. [DOI] [PubMed] [Google Scholar]
- 32.Williams GH, Romanowski P, Morris L, Madine M, Mills AD, Stoeber K, Marr J, Laskey RA, Coleman N. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA. 1998;95:14932–14937. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, Coleman N. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res. 1999;5:2121–2132. [PubMed] [Google Scholar]
- 34.Sirieix PS, O'Donovan M, Brown J, Save V, Coleman N, Fitzgerald RC. Surface expression of minichromosome maintenance proteins provides a novel method for detecting patients at risk for developing adenocarcinoma in Barrett's esophagus. Clin Cancer Res. 2003;9:2560–2566. [PubMed] [Google Scholar]
- 35.Herbst JJ, Berenson MM, McCloskey DW, Wiser WC. Cell proliferation in esophageal columnar epithelium (Barrett's esophagus) Gastroenterology. 1978;75:683–687. [PubMed] [Google Scholar]
- 36.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus: an ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120–2127. doi: 10.1172/JCI119018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iftikhar SY, Steele RJ, Watson S, James PD, Dilks K, Hardcastle JD. Assessment of proliferation of squamous, Barrett's and gastric mucosa in patients with columnar lined Barrett's oesophagus. Gut. 1992;33:733–737. doi: 10.1136/gut.33.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellish LJ, Hjaae GL. Cell proliferation in three types of Barrett's epithelium. Gut. 1980;21:26–31. doi: 10.1136/gut.21.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillen P, McDermott M, Grehan D, Hourihane DO, Hennessy TP. Proliferating cell nuclear antigen in the assessment of Barrett's mucosa. Br J Surg. 1994;81:1766–1768. doi: 10.1002/bjs.1800811219. [DOI] [PubMed] [Google Scholar]
- 40.Going JJ, Keith WN, Neilson L, Stoeber K, Stuart RC, Williams GH. Aberrant expression of minichromosome maintenance proteins 2 and 5, and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett's mucosa. Gut. 2002;50:373–377. doi: 10.1136/gut.50.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza RF, Morales CP, Spechler SJ. Review article: a conceptual approach to understanding the molecular mechanisms of cancer development in Barrett's oesophagus. Aliment Pharmacol Ther. 2001;15:1087–1100. doi: 10.1046/j.1365-2036.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 42.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–770. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Kaestner KH, Silberg DG, Traber PG, Schutz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- 44.Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126:2215–2225. doi: 10.1242/dev.126.10.2215. [DOI] [PubMed] [Google Scholar]
- 45.Malumbres M, Carnero A. Cell cycle deregulation: a common motif in cancer. Prog Cell Cycle Res. 2003;5:5–18. [PubMed] [Google Scholar]
- 46.Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–397. doi: 10.1016/s1052-5157(03)00006-0. [DOI] [PubMed] [Google Scholar]