Abstract
Infiltration of leukocytes into premalignant tissue is a common feature of many epithelial neoplasms and is thought to contribute to cancer development. However, the molecular and cellular regulatory mechanisms underlying activation of innate host responses to enhanced neoplastic cell proliferation are largely unknown. Considering the importance of the complement system in regulating inflammation during acute pathologic tissue remodeling, we examined the functional significance of complement component 3 (C3) as a regulator of inflammatory cell infiltration and activation during malignant progression by using a transgenic mouse model of multistage epithelial carcinogenesis, e.g., HPV16 mice. Whereas abundant deposition of C3 is a characteristic feature of premalignant hyperplasias and dysplasias coincident with leukocyte infiltration in neoplastic tissue, genetic elimination of C3 neither affects inflammatory cell recruitment toward neoplastic skin nor impacts responding pathways downstream of inflammatory cell activation, e.g., keratinocyte hyperproliferation or angiogenesis. Taken together, these data suggest that complementindependent pathways are critical for leukocyte recruitment into neoplastic tissue and leukocytemediated potentiation of tumorigenesis.
Keywords: Cancer, inflammation, complement, immunity, angiogenesis
Introduction
Tumors are composed of genetically altered cells, a variety of responding host cells, including fibroblasts, vascular cells (endothelial and mural cells), and a diverse array of inflammatory leukocytes embedded in a dynamic extracellular matrix (ECM) microenvironment [1,2]. Accumulating clinical and experimental data suggest that inflammatory leukocytes provide essential regulatory roles for developing tumors where their innate activities are conscripted by neoplastic cells to make significant contributions to malignant phenotypes [1–3]. The significance of inflammation associated with cancer development is underscored by the realization that tissues afflicted with chronic inflammatory states, e.g., Crohn's disease, ulcerative colitis, pancreatitis, etc., exhibit increased risk for malignancy [4–7]. Moreover, long-term users of anti-inflammatory drugs demonstrate reduced cancer risk [8–14]. Taken together, it seems reasonable to suggest that therapeutic strategies targeting inflammatory cell recruitment, activation, or response represent tractable anticancer opportunities.
Complement activation is a central event during innate immune defense after pathogenic tissue assault [15,16]. Three different pathways of complement activation have been identified, namely, the classic, the alternative, and the lectin pathways [17–19]. Complement component C3 is a central protein of the complement cascade, expression of which is essential for activation of all three complement pathways [19]. Foreign antigens and immune complexes activate the proteolytic complement activation cascade, resulting in formation of lytic membrane attack complexes (MAC) [17–19] and formation and liberation of anaphylatoxins, e.g., C3a and C5a, potent proinflammatory factors that induce recruitment and activation of various inflammatory cells, in particular mast cells, eosinophils, and neutrophils [20–23]. Deposition of complement component proteins is a common occurrence at sites of inflammation [24–28]. Studies utilizing complement-depleted mice and C3- or C5-deficient mice have identified crucial roles for complement in mast cell recruitment and activation during airway hyperresponsiveness [25,29,30], IgG-antigen complex-mediated inflammation [31], intestinal ischemia/reperfusion [32], delayed-type hypersensitivity [33], and subepidermal blistering disease [34], thus underscoring the central role complement plays in regulating disease pathogenesis.
Using a transgenic mouse model of multistage epithelial carcinogenesis, e.g., HPV16 mice [35], we have investigated the functional role of complement component 3 (C3) as a regulator of inflammatory cell recruitment and premalignant progression. HPV16 mice express the early region genes of human papillomavirus type 16 (HPV16) as transgenes under control of the human keratin 14 promotor/enhancer [36]. By 1 month of age, HPV16 mice develop epidermal hyperplasias with 100% penetrance that are characterized by a terminally differentiating hyperproliferative epidermis atop a dermal compartment rich in recruited activated mast cells [37]. Hyperplastic lesions advance focally into angiogenic dysplasias between 3 and 6 months of age and are distinct from hyperplasias based on the prominent hyperproliferative epidermis that fails to undergo terminal differentiation and a dermis where intense mast cell and neutrophil infiltration occurs proximal to dilated and enlarged angiogenic vasculature [37–39]. By 1 year of age, 60% of HPV16 mice develop malignant skin carcinomas, 50% of which are squamous cell carcinomas (SCCs) that metastasize to regional lymph nodes with an ∼ 30% frequency, and ∼ 10% of which represent nonmetastatic microcystic adnexal carcinomas (MACs) [38,40]. Mast cell deficiency in HPV16 mice significantly attenuates keratinocyte hyperproliferation and activation of angiogenesis, thus supporting the concept that inflammation, and in particular activation of sentinel mast cells and release of their factors, functionally contribute to early neoplastic development in HPV16 mice [37].
In the present study, we report abundant deposition of C3 in neoplastic skin of HPV16 mice. To determine if C3 deposition represented a functionally significant parameter of mast cell recruitment, activation, or response, we generated HPV16 mice harboring a homozygous null mutation in the C3 gene, e.g., HPV16/C3-/- mice [15,16]. Despite abundant C3 deposition into the neoplastic microenviroment of HPV16 mice, our data suggest that activation of the complement system does not functionally contribute to recruitment of inflammatory cells, induction of keratinocyte hyperproliferation, or activation of angiogenesis during epithelial neoplastic progression. Because abundant deposition of IgG antibodies is an early event that is sustained throughout neoplastic progression and coincides with inflammatory cell infiltration, we propose an antibody-dependent but complement- independent pathway regulating mast cell recruitment into neoplastic skin.
Materials and Methods
Transgenic Mice
Generation and characterization of HPV16 transgenic mice and neoplastic staging based on keratin intermediate filament expression has been described previously [35,36]. The generation of C3-deficient mice has been described [15,16]. C3-/- mice were kindly provided by Dr. M. C. Carroll (Harvard Medical School, Boston, MA). To generate HPV16 mice in the C3-deficient backgrounds, C3+/- mice were individually backcrossed into the FVB/n strain to N19, where they were then intercrossed with HPV16 mice to generate a breeding colony of HPV16/C3-/- and C3-/- animals. All mice were maintained within the University of California, San Francisco, Laboratory for Animal Care facility according to IACUC procedures. Genotyping of C3-/- mice was based on a C3 ELISA where C3 levels in sera (or tissue) were assayed using a polyclonal goat anti-mouse C3 antiserum (1:500; ICN/Cappel, Irvine, CA). Because the C3 locus is located near the MHC region, we confirmed H-2Kq expression and absence of H-2Db expression after backcrossing into the FVB/n strain by FACS analysis using mouse anti-mouse H-2Db and mouse anti-mouse H-2Kq mAbs (BD Biosciences, San Diego, CA).
Immunofluorescence Detection of Antibody and C3 Depositions
Age-matched tissue samples from transgenic and control animals were frozen directly in glycerol-based freezing medium (OCT). For immunofluorescence (IF) detection of antibody and C3 deposition, 10-µm OCT-embedded tissue sections were cut with a Leica CM1900 cryostat. Sections were air-dried, fixed in acetone for 5 minutes, and subjected to IF staining as follows: sections were blocked for 30 minutes in blocking buffer (5% goat serum/2.5% BSA/PBS). For IF detection of antibodies, a 1:50 dilution of rat anti-mouse CD16/CD32 mAb (BD Biosciences) was added to the blocking buffer to prevent nonspecific antibody binding. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (γ-chain specific, 1:100; Sigma, St. Louis, MO), and rat anti-mouse C3 (1:100; Caltag, Burlingame, CA) were diluted in 0.5 x blocking buffer and incubated with tissue sections for 1 hour at room temperature, protected from light. Sections were then extensively washed with PBS. For IF detection of C3, sections were blocked for 5 minutes in blocking buffer and incubated with FITC-conjugated goat anti-rat (1:100, Jackson ImmunoResearch, West Grove, PA) for 45 minutes at room temperature, protected from light. Slides were then extensively washed with PBS before mounting using Vectashield containing 4′,6 diamidino-2-phenylindole (DAPI) (Vector, Burlingame, CA). All IF experiments included negative controls for determination of background staining, which was negligible. Data shown are representative of results obtained after examination of tissues removed from a minimum of four mice per group.
Immuno- and Enzyme Histochemistry
Age-matched tissue samples from transgenic and control animals were immersion-fixed in 10% neutral-buffered formalin followed by dehydration through graded alcohols and xylene, and embedded in paraffin. Five-micrometer-thick paraffin sections were cut using a Leica 2135 microtome. Sections were deparaffinized and subjected to immunohistochemical detection as previously described [41]. Primary antibodies were diluted in blocking buffer (5% goat serum/2.5% BSA/PBS) at 1:50 dilution for rat anti-mouse CD31 (BD Biosciences) and 1:500 for rat anti-mouse neutrophilspecific primary antibody (Cedarlane Labs, Hornby, Ontario, Canada). Sections were incubated with primary antibody for 2 to 4 hours at room temperature, followed by PBS washing, brief (5 minutes) incubation in blocking buffer, and subsequent incubation with biotinylated secondary antibody (rabbit anti-rat IgG 1:200, Vector) for 45 minutes at room temperature. After PBS washing, Vectastain Elite ABC reagent (Vector) was applied for 30 minutes. Sections were then washed in PBS and endogenous peroxidase activity blocked by incubation in 30% H2O2 in methanol for 20 minutes. Antibodies were visualized by treatment with Fast 3,3′-diaminobenzodine (Sigma), dehydrated in graded alcohols (70%, 95%, and 100% ethanol), and mounted in Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI).
Chloroacetate esterase (CAE) histochemistry was performed to visualize serine esterase activity in mast cells as previously described [37]. Briefly, 1 mg of naphthol AS-D chloroacetate (Sigma) was dissolved in 20 µl of N,N-dimethyl formamide (DMF) and 1 ml buffer (8% DMF, 20% ethylene glycol monoethylether in 80 mM Tris-maleate, pH 7.5). Subsequently, 1 mg Fast Blue BB salt (Fluka, Buchs, Switzerland) was added, the solution filtered using a 0.45-µm filter and applied to deparaffinized paraffin sections for 5 minutes. Sections were rinsed in PBS, dehydrated, and mounted in glycerol. Quantitative analysis of mast cells and neutrophils was performed by counting cells in five highpower fields (40 x) per age/staged tissue section from five mice per neoplastic stage. Data presented reflect the average total cell count per field from the ventral ear leaflet. Statistical analysis was performed using GraphPad InStat version 3.0a for Macintosh (GraphPad Software, San Diego, CA). The specific tests that were used were the Mann- Whitney (unpaired, nonparametric two-tailed) test and the Fishers (unpaired) t test. P < .05 was considered statistically significant. All immunolocalization experiments were repeated on multiple tissue sections and included negative controls for determination of background staining, which was negligible. Data shown are representative of results obtained after examination of tissues removed from a minimum of four different mice per distinctive stage of neoplastic progression.
Determination of Keratinocyte Proliferation Index
To detect bromodeoxyuridine (BrdU)-positive keratinocytes in neoplastic and control tissues, animals received intraperitoneal injections of BrdU (Roche, Indianapolis, IN) dissolved in PBS at 50 µg/g total body weight 90 minutes before sacrifice and preparation of tissue samples. Fivemicrometer-thick paraffin sections were deparaffinized in xylenes, rehydrated in graded ethanol, boiled in Citra antigen retrieval solution (BioGenex, San Ramon, CA), washed in PBS, and blocked in blocking buffer and BrdU-positive cells were detected essentially as described by the manufacturer's recommendations using the BrdU Labeling Kit II (Roche), developed by Vector Red Alkaline Phosphatase Kit (Vector), counterstained with eosin, dehydrated through graded ethanol and xylenes, and mounted in Cytoseal 60 (Richard-Allan Scientific). Images used for quantification were captured at high magnification (40 x) on a Leica DMRXA microscope attached to a Leica digital camera operated by OpenLab software (Improvision, Lexington, MA). The proliferative index was quantified from five high-power (40 x) images per neoplastically staged tissue and included four mice per category as the percentage of BrdU-positive nuclei over the total number of keratinocytes. Statistical analysis was performed using GraphPad InStat version 3.0a for Macintosh (GraphPad Software). The specific tests that were used were the Mann-Whitney (unpaired, non-parametric two-tailed) test, and the Fishers (unpaired) t test. P < .05 was considered statistically significant.
Flow Cytometry
The magnitude of inflammatory infiltrate in neoplastic tissues was analyzed by flow cytometry. Ear skin biopsies from (-)LM, HPV16 mice, and HPV16/C3-/- mice at specific time points were manually minced using a scalpel, followed by a 13-minute enzymatic digestion with 2.5 mg/ml collagenase Type II (Worthington, Lakewood, NJ), 2.5 mg/ml collagenase Type IV (Gibco, Carlsbad, CA), and 0.5 mg/ml DNase (Sigma) in PBS containing 1% BSA (Sigma) (PBS/BSA) at 37°C under continuous stirring conditions. The digest was quenched by adding Dulbecco's modified Eagle's medium (DMEM) (Gibco) containing 10% FBS (Gibco) and was filtered through a 70-µm nylon filter (Falcon). Single-cell suspensions were treated with PharM Lyse ammonium chloride lysing reagent (BD Biosciences) for 10 minutes to remove erythrocytes. Cells were washed with DMEM containing 10% FBS, followed by a wash with PBS/BSA. Cells were incubated for 10 minutes at 4°C with rat anti-mouse CD16/CD32 mAb (BD Biosciences) at a 1:50 dilution in PBS/BSA to prevent nonspecific antibody binding. Subsequently, cells were washed and incubated for 20 minutes with 50 ml of 1:100 dilution of APC-conjugated anti-mouse CD45 (eBioscience, San Diego, CA). Cells were washed twice with PBS/BSA and 7-AAD (BD Biosciences) was added at a dilution of 1:10 to discriminate between viable and dead cells. Data acquisition and analysis were performed on a FACSCalibur using CellQuestPro software (BD Biosciences). Statistical analysis was performed using GraphPad InStat version 3.0a for Macintosh (GraphPad Software, San Diego, CA). The specific tests that were used were the Mann-Whitney (unpaired, non parametric two-tailed) test, and the Fishers (unpaired) t test. P < 0.05 was considered statistically significant.
Results
Neoplastic Progression in HPV16 Mice Is Characterized by C3 Deposition
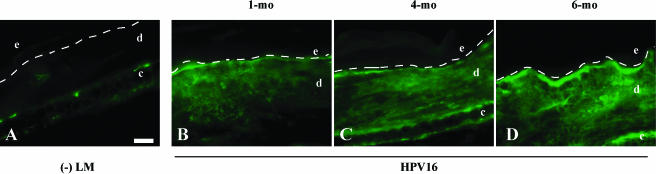
Early neoplastic progression in HPV16 mice is characterized by a dramatic influx of cells of the innate immune system, in particular mast cells and neutrophils [37,38]. To investigate whether the observed accumulation of inflammatory cells in the neoplastic microenvironment was accompanied by activation of the complement system, C3 deposition was examined by IF microscopy on tissue sections and ELISA with tissue lysates (Figure 1). Whereas only minimal detection of C3 was observed in nontransgenic skin, abundant dermal deposits of C3 were prominent in hyperplastic and dysplastic skin of HPV16 mice (Figure 1). Using a C3 ELISA, we found a statistically significant increase in C3 levels in hyperplastic skin lysates from 1-month-old HPV16 mice compared with nontransgenic controls (P < .05, Fishers (unpaired) t test; data not shown). As expected, C3 staining and immunoreactivity (based on ELISA) was not observed in C3-/- mice (data not shown). These data suggest that neoplastic progression in HPV16 mice is characterized by early activation of the complement system and C3 accumulation coincident with initial development of hyperplasias. Moreover, C3 accumulation becomes more pronounced as neoplastic progression to dysplasia ensues, suggesting that activation of complement represents an early parameter of premalignant progression in HPV16 mice.
Figure 1.
Deposition of C3 in the neoplastic microenvironment. Immunofluorescence for C3 (FITC signal) on 10-µm OCT-embedded frozen tissue sections in (A) negative littermate (-LM) ear skin, (B) hyperplastic (1 month of age), (C) early dysplastic (4 months of age), and (D) late dysplastic (6 months of age) ear tissue from HPV16 mice. Dashed line indicates epidermal-dermal interface. The epidermis (e), dermis (d), and cartilage (c) are indicated. Scale bar: 50 µm (A–D).
Infiltration of Inflammatory Cells during Neoplastic Progression Is Not Reduced in the Absence of C3
Previous studies using HPV16 mice have revealed an important role for bone-marrow-derived inflammatory cells during skin carcinogenesis [37–39,42]. Given the critical role of the complement system in recruitment and activation of inflammatory cells during pathologic tissue remodeling [25,29,30,32–34,43,44], in combination with the observation that deposition of C3 is already detectable in hyperplastic skin lesions of HPV16 mice (Figure 1), we hypothesized that complement mediates recruitment of inflammatory cells toward neoplastic skin. To test this hypothesis, we took a genetic approach and generated HPV16/C3-/- mice [15,16] and examined several key characteristics of neoplastic progression in the absence and presence of C3.
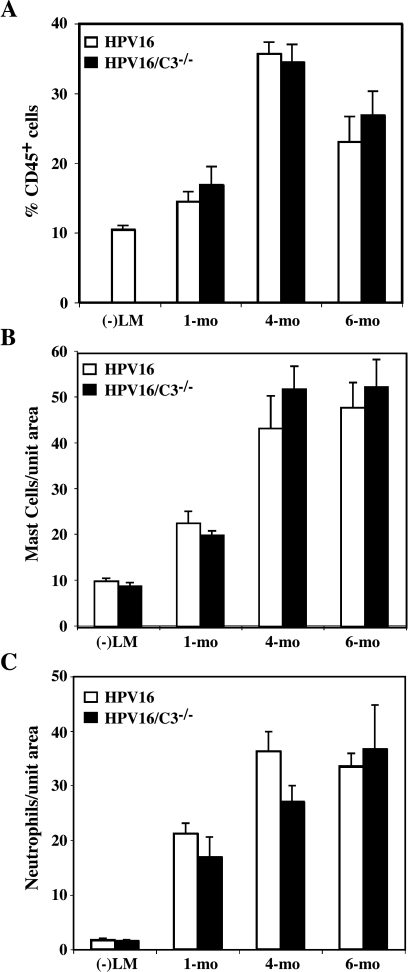
To determine whether absence of C3 in HPV16/C3-/- mice altered characteristic inflammatory cell recruitment into early neoplastic lesions, we first profiled infiltration of leukocytes by quantitative detection of CD45 (leukocyte common antigen)-positive cells in age-matched neoplastic tissue from HPV16/C3-/- and HPV16 mice by flow cytometry (Figure 2A). This analysis revealed that the magnitude of the inflammatory infiltrate is most striking in early dysplasias (4 months of age), where ∼35% of all cells are CD45+ inflammatory cells (Figure 2A). Surprisingly, the absence of C3 in HPV16/C3-/- mice did not result in any quantitative changes in this profile of infiltrating CD45-positive leukocytes (Figure 2A, Mann-Whitney test).
Figure 2.
Infiltration of inflammatory cells during neoplastic progression is not reduced in the absence of C3. (A) Single-cell suspensions derived from negative littermate ear tissue (-LM) and hyperplastic (1 month of age), early dysplastic (4 months of age), and late dysplastic (6 months of age) ear tissue from HPV16 mice and HPV16/C3-/- mice were analyzed by flow cytometry to determine the percentage of CD45+ cells as a percentage of total viable cells (n = 3). Results are shown as mean percentages ± SEM. No statistically significant differences were observed between age-matched neoplastic tissues of HPV16 and HPV16/C3-/- mice (Mann-Whitney unpaired t test). (B) Quantitative analysis of mast cells at distinct stages of neoplastic progression in ear tissue from HPV16, HPV16/C3-/- mice, and negative littermates (-LM). Mast cells were visualized by CAE histochemistry on 5-µm paraffin-embedded tissue sections. Values represent number of mast cells, averaged from five high-power fields per mouse and five mice per category. Error bars represent SEM. No statistically significant differences were observed between age-matched neoplastic tissues between HPV16 and HPV16/C3-/- mice (Mann-Whitney unpaired t test). (C) Quantitative analysis of neutrophils at distinct stages of neoplastic progression in ear tissue from negative littermate (-)LM, HPV16, and HPV16/C3-/- mice. Neutrophils were visualized by immunohistochemistry on 5-µm paraffin-embedded tissue sections. Values represent number of neutrophils, averaged from five highpower fields per mouse and five mice per category. Error bars represent SEM. No statistically significant differences were observed between agematched neoplastic tissues between HPV16 and HPV16/C3-/- mice (Mann-Whitney unpaired t test).
To investigate the possibility that C3 deficiency resulted in a shift in immune cell profile, we quantitatively assessed the two major immune cell populations infiltrating HPV16 neoplastic skin, namely, mast cells and neutrophils [37,38]. We used CAE histochemistry on paraffin-embedded agematched tissue sections to detect serine esterase activity in mast cells in situ. As described previously [37], normal nontransgenic skin contains few mast cells distributed broadly throughout the dermis (Figure 2B). Mast cell numbers increase beginning in hyperplasias (1 month of age) and further increase with the onset of intense angiogenesis in dysplastic lesions (Figure 2B). Quantitative analysis of the number of mast cells in situ revealed that the number of infiltrating mast cells in skin from age-matched HPV16/C3-/- mice was indistinguishable from that detected in agematched HPV16 controls (Figure 2B, Mann-Whitney test), suggesting that C3 is not required for recruitment of mast cells into premalignant skin lesions.
To assess if C3 instead regulated neutrophil recruitment, we quantitatively compared neutrophils in age-matched HPV16 and HPV16/C3-/- mice and found no significant differences (Figure 2C, Mann-Whitney test), suggesting that C3 is not involved in the recruitment of either mast cell or neutrophils into premalignant skin. Thus, although deposition of C3 in neoplastic skin coincides with infiltration of inflammatory cells, C3 does not mediate the recruitment of inflammatory cells into the neoplastic microenvironment.
Activation of Keratinocyte Hyperproliferation and Angiogenesis during Neoplastic Progression Are not Regulated by C3
Although C3 does not appear to be essential for recruitment of inflammatory cells toward neoplastic lesions, we reasoned that C3 could instead be critical for activation of inflammatory cells in the neoplastic microenvironment. Mast cells secrete a variety of mitogenic factors affecting a wide range of cell types, including keratinocytes and endothelial cells [37,42,45–49]. Using mast-cell-deficient/HPV16 mice, we previously reported that mast cells contribute to neoplastic progression by potentiating keratinocyte hyperproliferation and activating angiogenesis [37], in part by release of matrix metalloproteinase-9 (MMP9) [38,42]. Together, these data suggest that activation of mast cells is an important contributing event to tumorigenesis in HPV16 mice. To assess whether C3 was involved in mast cell activation we compared keratinocyte proliferation indices and activation of angiogenic vasculature as readouts for mast cell activation in HPV16 and HPV16/C3-/- mice.
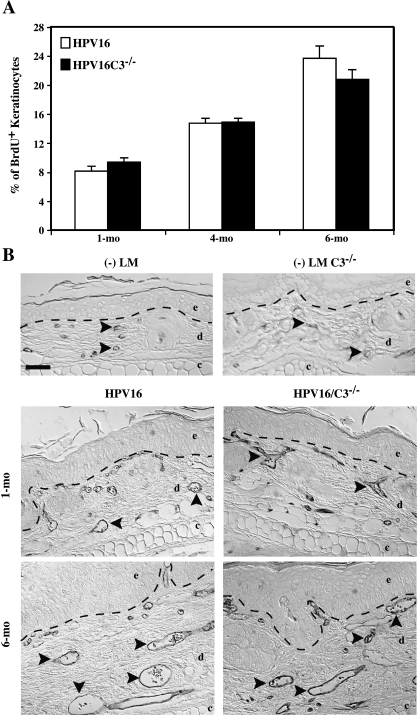
Hyperproliferation is an intrinsic property of neoplastic cells. Accordingly, we previously reported that keratinocyte hyperproliferation incrementally increases during neoplastic progression in HPV16 mice and, in part, characterizes progression between premalignant stages [35,36]. Keratinocyte proliferative indices were determined in age-matched skin from HPV16 and HPV16/C3-/- mice and were found to be similar at each neoplastic stage examined (Figure 3A, Mann-Whitney test), suggesting that C3 is not involved in regulating epithelial hyperproliferation downstream of mast cell activation.
Figure 3.
Activation of keratinocyte hyperproliferation and angiogenesis during neoplastic progression is not affected in the absence of C3. (A) Quantitative analysis of the percentage of BrdU-positive keratinocytes (keratinocyte proliferative index) at distinct stages of neoplastic progression in ear tissue of HPV16 and HPV16/C3-/- mice at 1, 4, and 6 months of age. BrdU+ cells were visualized by immunohistochemical detection on 5-µm paraffin-embedded tissue sections. Values represent keratinocyte proliferative indices, averaged from five high-power fields per mouse and four to eight mice per category. Error bars represent SEM. No statistically significant differences were observed between age-matched neoplastic tissues between HPV16 and HPV16/C3-/- mice (Mann-Whitney unpaired t test). (B) Immunolocalization of CD31/PECAM-1 (black staining) in 5-µm paraffin-embedded sections of age-matched neoplastic ear tissue of HPV16 and HPV16/C3-/- mice and in ear tissue of negative littermate (-LM) mice reveals no difference between dilated and enlarged capillaries comparing HPV16 and HPV16/C3-/- mice at all ages tested. Dashed line indicates epidermal-dermal interface. Arrowheads indicate representative blood vessels. The epidermal (e), dermal (d), and cartilage (c) regions of the tissues are indicated. Scale bar: 50 µm.
The vascular architecture changes in a characteristic manner during neoplastic progression in HPV16 mice [37,38] and can be monitored by immunodection of the endothelial cell-specific marker CD31. Normal nontransgenic mouse skin contains infrequent capillaries located deep within the dermis (Figure 3B). Neoplastic progression involves an early increase in capillary density first evident subjacent to hyperplastic epithelium, whereas dysplastic lesions contain dilated and enlarged capillaries that are increased in number indicative of an angiogenic switch from vascular quiescence to modest neovascularization in early low-grade lesions (hyperplasias) to a striking upregulation of angiogenesis in high-grade lesions (dysplasias) (Figure 3B). Comparison of vascular architecture, density, and patterning during premalignant progression in HPV16 and HPV16/C3-/- mice failed to reveal any differences (Figure 3B), suggesting that C3 does not contribute to inflammatory cell-mediated activation of angiogenic vasculature.
IgG Deposition Suggests Antibody-Dependent, but Complement- Independent Regulation of Neoplasia-Associated Inflammation
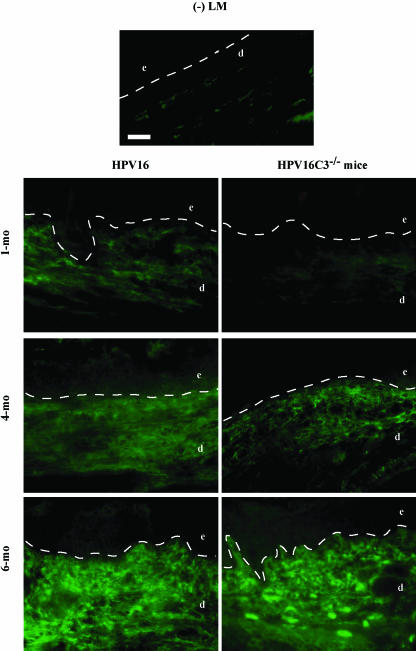
Despite the fact that complement activation plays a prominent role in regulating many immunologic injuries [25,29–34,50] and significant deposition of C3 is observed in dermal compartments of neoplastic skin, the absence of C3 did not functionally alter leukocyte recruitment or activation during premalignant progression in HPV16 mice. Inflammatory cells can, however, be recruited and activated directly by antibodies through cross-linking of antibody surface receptors, e.g., Fcγ and/or Fcε receptors [51,52]. To study whether the humoral immune response is involved in regulating inflammation in HPV16 mice, the spatial and temporal deposition patterns of IgG antibodies in neoplastic skin were analyzed by IF (Figure 4). In negative littermate nontransgenic skin, low IgG immunoreactivity was detectable in the dermis (Figure 4). In HPV16 hyperplastic skin (1 month of age), extensive regions of IgG antibodies were visualized throughout the dermis that was further expanded and intensified in dysplastic lesions (4 to 6 months of age) (Figure 4). HPV16/C3-/- mice displayed similar IgG deposition patterns during neoplastic progression (Figure 4), suggesting that an antibody-mediated immune response independent of complement activation and C3 deposition might therefore be involved in mediating recruitment of inflammatory cells during malignant progression in HPV16 mice.
Figure 4.
IgG deposition in the neoplastic microenvironment of HPV16 mice. Direct immunofluorescence on 10-µm OCT-embedded frozen tissue sections detects IgG antibodies (FITC signal) in negative littermate (-LM) ear skin, hyperplastic (1 month of age), early dysplastic (4 months of age), and late dysplastic (6 months of age) ear tissue from HPV16 and HPV16/C3-/- mice. Dashed line indicates epidermal-dermal interface. The epidermis (e), dermis (d), and cartilage (c) are indicated. Scale bar: 50 µm.
Discussion
In the present study we examined the functional significance of C3 deposition and complement activation in a mouse model of epithelial carcinogenesis characterized, in part, by prominent inflammatory cell infiltrates in premalignant lesions. Results from this study suggest that C3 is neither required for the dramatic accumulation of inflammatory cells in premalignant skin nor influences the contributory role of inflammatory cells to squamous carcinogenesis, e.g., keratinocyte hyperproliferation or activation of angiogenic vasculature. These data stand in contrast to studies showing a crucial role for complement in recruitment and activation of inflammatory cells in other states of pathologic remodeling, e.g., airway hyperresponsiveness [25,29,30], IgG-antigen complex-mediated inflammation [31], intestinal ischemia/reperfusion [32,50], delayed-type hypersensitivity [33], and subepidermal blistering disease [34].
The experimental model of IgG-mediated subepidermal blistering disease [34,43,44] has many characteristics in common with the HPV16 mouse model of de novo multistage skin carcinogenesis, namely, deposition of IgG and complement at the dermal-epidermal junction and recruitment of mast cells and neutrophils [34]. Moreover, subepidermal blister formation is mediated by mast cell activation; mastcell-deficient mice are resistant to experimental subepidermal blistering disease [44]. Likewise, we have previously reported that mast-cell-deficient/HPV16 mice display attenuated characteristics of neoplastic progression [37]. However, whereas subepidermal blistering disease depends on complement activation, as complement component C5-deficient mice failed to develop cutaneous blisters [34], no functional significance of the complement system was observed in our study. To our knowledge, this is the first report in which the role of the complement system in a model of de novo neoplastic progression has been investigated.
Why is neoplastic progression and its accompanied inflammation not altered by deficient complement activation? The major difference between inflammation associated with malignant progression and that associated with other states of pathologic remodeling, e.g., subepidermal blistering disease, airway hyperresponsiveness, intestinal ischemia/reperfustion, and delayed hypersensitivity, is the duration of inflammatory response. Inflammation during neoplastic progression is not abated because the “wound does not heal” [2]; thus, inflammatory cells fail to egress out of neoplastic tissue, therefore representing a chronic disease state. Moreover, antigens that are likely important for activation of the adaptive immune system similarly are present chronically and likely induce chronic B-cell responses, resulting in significant antibody deposition into neoplastic tissue.
If complement is not required for recruitment of inflammatory cells toward premalignant lesions, what other complement- independent recruitment pathways might be involved? Inflammatory cells can be recruited and activated through a complement-independent, antibody-dependent pathway. Antibodies can interact directly with inflammatory cells through antibody binding surface receptors, e.g., Fcγ and/or Fcε receptors [51,52]. Fc receptors are multimeric cell-surface receptors that bind the Fc portion of antibodies and are expressed by many hematopoietic cells [53]. Antibody- mediated cross-linking of Fc receptors results in recruitment and activation of inflammatory cells. For example, in IgE-associated hypersensitivity reactions and allergic disorders, mast cell activation is initiated by cross-linking of IgE bound to the high-affinity FcεRI receptors, resulting in secretion of products that can have effector, immunoregulatory, or autocrine effects [51]. It has become clear that in addition to IgE-dependent recruitment mechanisms, IgG-dependent mechanisms can regulate recruitment and activation of mast cells through Fcγ receptors [52]. In vitro incubation of human mast cells through Fcγ RI with IgG1 antibodies resulted in degranulation, increased production of arachidonic acid metabolites, and tumor necrosis factor-α release [52]. Fcγ receptor-deficient mice are resistant to development of experimental immune hemolytic anemia and immune thrombocytopenia [54]. Likewise, Fcγ receptor expression on mast cells has been reported to promote the course of experimental allergic encephalomyelitis (EAE) [55]. In addition, antibody- mediated experimental cutaneous Arthus reaction requires expression of Fcγ receptors on inflammatory cells and develops normally in complement-deficient mice [56,57]. Thus, activation of cell-surface Fcγ receptors can result in activation of an inflammatory response independent of complement activation. We observed early and abundant deposition of IgG antibodies in premalignant skin lesions (Figure 4) and the spatial and temporal deposition profile of IgG antibodies coincided with the inflammatory infiltrate; thus we are currently investigating whether antibodies localized in the neoplastic microenvironment trigger complementindependent recruitment and activation of mast cells. Because inflammatory cells, and in particular mast cells, are important contributors to the early stages of carcinogenesis in HPV16 mice as well as during human epithelial carcinogenesis, a detailed understanding of the molecules and mechanisms involved in activating the dramatic recruitment will eventually facilitate the rational design of novel therapies against human cancer.
Acknowledgements
We thank M. C. Carroll for kindly providing C3-/- mice. We also thank Alexandra Eichten for critically reading the manuscript, members of the Coussens laboratory for insightful discussion, Evelyn Galenski for administrative assistance, and Eva Soliven, William Hyun, and Sarah Elmes for technical assistance. K. E. dV. is supported by a fellowship from the Dutch Cancer Society. LMC is supported by grants from the National Institutes of Health, National Cancer Institute, and Department of Defense.
Abbreviations
- BrdU
bromodeoxyuridine
- CAE
chloroacetate esterase
- C3
complement component 3
- IF
immunofluorescence
- HPV16
human papillomavirus 16
References
- 1.Bissell MJ, Radisky D. Putting tumors in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 5.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 6.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. [PubMed] [Google Scholar]
- 7.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 8.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Meier CR, Schmitz S, Jick H. Association between acetaminophen or nonsteroidal antiinflammatory drugs and risk of developing ovarian, breast, or colon cancer. Pharmacotherapy. 2002;22:303–309. doi: 10.1592/phco.22.5.303.33189. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested case-control study of the effects of non-steroidal antiinflammatory drugs on breast cancer risk and stage. Br J Cancer. 2000;83:112–120. doi: 10.1054/bjoc.2000.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1213–1217. [PubMed] [Google Scholar]
- 13.Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer. 2001;84:965–968. doi: 10.1054/bjoc.2001.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Perez A, Rodriguez L, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:1–12. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3- deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 16.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 18.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 19.Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- 21.Johnson AR, Hugli TE, Muller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067. [PMC free article] [PubMed] [Google Scholar]
- 22.DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127–1136. [PubMed] [Google Scholar]
- 23.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- 24.Lapiere JC, Guitart J, Ettlin DA, Chen DM, Amagai M, Chan LS. Preferential activation of the complement system in the lower epidermis of patients with pemphigus vulgaris. Br J Dermatol. 1998;139:851–854. doi: 10.1046/j.1365-2133.1998.02512.x. [DOI] [PubMed] [Google Scholar]
- 25.Walters DM, Breysse PN, Schofield B, Wills-Karp M. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2002;27:413–418. doi: 10.1165/rcmb.4844. [DOI] [PubMed] [Google Scholar]
- 26.Berstad AE, Brandtzaeg P, Stave R, Halstensen TS. Epithelium related deposition of activated complement in Helicobacter pylori associated gastritis. Gut. 1997;40:196–203. doi: 10.1136/gut.40.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugita Y, Morita E, Kawamoto H, Horiuchi K, Yamada S, Koro O, Yamamoto S. Correlation between deposition of immunocomponents and infiltration pattern of polymorphonuclear leukocytes in the lesions of chronic urticaria. J Dermatol. 2000;27:157–162. doi: 10.1111/j.1346-8138.2000.tb02142.x. [DOI] [PubMed] [Google Scholar]
- 28.Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–1271. doi: 10.1016/0016-5085(90)90343-y. [DOI] [PubMed] [Google Scholar]
- 29.Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2001;167:4141–4145. doi: 10.4049/jimmunol.167.8.4141. [DOI] [PubMed] [Google Scholar]
- 30.Bautsch W, Hoymann HG, Zhang Q, Meier-Wiedenbach I, Raschke U, Ames RS, Sohns B, Flemme N, Meyer zu Vilsendorf A, Grove M, et al. Cutting edge: guinea pigs with a natural C3a-receptor defect exhibit decreased bronchoconstriction in allergic airway disease: evidence for an involvement of the C3a anaphylatoxin in the pathogenesis of asthma. J Immunol. 2000;165:5401–5405. doi: 10.4049/jimmunol.165.10.5401. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Ramos BF, Jakschik BA. The role of mast cells and their mediators in IgG-antigen complex-mediated inflammation. Am J Ther. 1995;2:761–767. doi: 10.1097/00045391-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Andoh A, Fujiyama Y, Araki Y, Kimura T, Tsujikawa T, Bamba T. Role of complement activation and mast cell degranulation in the pathogenesis of rapid intestinal ischemia/reperfusion injury in rats. Digestion. 2001;63(Suppl 1):103–107. doi: 10.1159/000051920. [DOI] [PubMed] [Google Scholar]
- 33.Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003;171:6225–6235. doi: 10.4049/jimmunol.171.11.6225. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, Diaz LA. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coussens LM, Hanahan D, Arbeit JM. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- 36.Arbeit JM, Munger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol. 1994;68:4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kempen LCL, Rhee JS, Dehne K, Lee J, Edwards DR, Coussens LM. Epithelial carcinogenesis: dynamic interplay between neoplastic cells and their microenvironment. Differentiation. 2002;70:501–623. doi: 10.1046/j.1432-0436.2002.700914.x. [DOI] [PubMed] [Google Scholar]
- 39.Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, Hanahan D. Immune enhancement of skin carcinogenesis by CD4+ T cells. J Exp Med. 2003;197:1017–1028. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee JS, Diaz R, Korets L, Hodgson JG, Coussens LM. TIMP-1 alters susceptibility to carcinogenesis. Cancer Res. 2004;64:952–961. doi: 10.1158/0008-5472.can-03-2445. [DOI] [PubMed] [Google Scholar]
- 41.Coussens LM, Hanahan D, Arbeit J. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- 42.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, Fairley JA, Zhao ML, Giudice GJ, Zillikens D, Diaz LA, Liu Z. Macrophages, but not T and B lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: macrophage-mediated neutrophil infiltration depends on mast cell activation. J Immunol. 2002;169:3987–3992. doi: 10.4049/jimmunol.169.7.3987. [DOI] [PubMed] [Google Scholar]
- 45.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- 48.Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci USA. 1994;91:128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci USA. 1990;87:3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Andoh A, Fujiyama Y, Saotome T, Bamba T. A blockade of complement activation prevents rapid intestinal ischaemia-reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin Exp Immunol. 1998;111:484–490. doi: 10.1046/j.1365-2249.1998.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 52.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 53.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th ed. New York: Garland Publishing; 2001. [Google Scholar]
- 54.Clynes R, Ravetch JV. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995;3:21–26. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 55.Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- 56.Sylvestre DL, Ravetch JV. A dominant role for mast cell Fc receptors in the Arthus reaction. Immunity. 1996;5:387–390. doi: 10.1016/s1074-7613(00)80264-2. [DOI] [PubMed] [Google Scholar]
- 57.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]