Abstract
In eukaryotes, double-stranded (ds) RNA induces sequence-specific inhibition of gene expression referred to as RNA interference (RNAi). We exploited RNAi to define the role of HER2/neu in the neoplastic proliferation of human breast cancer cells. We transfected SK-BR-3, BT-474, MCF-7, and MDA-MB-468 breast cancer cells with short interfering RNA (siRNA) targeted against human HER2/neu and analyzed the specific inhibition of HER2/neu expression by Northern and Western blots. Transfection with HER2/neu-specific siRNA resulted in a sequence-specific decrease in HER2/neu mRNA and protein levels. Moreover, transfection with HER2/neu siRNA caused cell cycle arrest at G0/G1 in the breast cancer cell lines SKBR-3 and BT-474, consistent with a powerful RNA silencing effect. siRNA treatment resulted in an antiproliferative and apoptotic response in cells overexpressing HER2/neu, but had no influence in cells with almost no expression of HER2/neu proteins like MDA-MB-468 cells. These data indicate that HER2/neu function is essential for the proliferation of HER2/neu-overexpressing breast cancer cells. Our observations suggest that siRNA targeted against human HER2/neu may be valuable tools as antiproliferative agents that display activity against neoplastic cells at very low doses.
Keywords: HER2/neu, RNA interference, siRNA, antiproliferative activity, apoptosis
Introduction
The HER2/neu gene encodes a transmembrane tyrosine kinase with close homology to the epidermal growth factor receptor (EGFR) [1]. Previous studies have demonstrated overexpression of the HER2/neu gene and deregulation of the formation and activation of HER2 receptor heterodimers in a high proportion of breast cancer cells [2–6]. Amplified HER2/neu sequences have been detected in early-stage breast cancer cells, and experimental evidence suggests that amplification of HER2/neu sequences leads to malignant transformation [7]. These observations led to the clinical development of trastuzumab (Herceptin; Genentech, South San Francisco, CA), a monoclonal IgG1 class humanized murine antibody that binds specifically to the extracellular domain of the HER2/neu protein [8]. Trastuzumab received US marketing approval in 1998 as a treatment for metastatic breast cancer in patients whose tumors overexpress the HER2 protein [9]. However, there are risks of cardiomyopathy and hypersensitivity reactions associated with the use of trastuzumab [10], and there remains a need for alternative therapies.
RNA interference (RNAi) has become an excellent approach for the targeted silencing of gene expression in plants and invertebrates [11,12]. In this approach, 21-to 23-nucleotide (nt) short interfering RNA (siRNA) complementary to the targeted gene are processed from a long dsRNA precursor by the Dicer enzyme and effectively silence the targeted gene by binding to complementary mRNA and triggering mRNA elimination [12–16]. Elbashir et al. [18] demonstrated that transfection of synthetic 21-nt siRNA duplexes into mammalian cells efficiently inhibits endogenous gene expression in a sequencespecific manner. Strategies based on synthetic siRNA have since been used to silence various cancer-relevant genes such as bcr/abl and polo-like kinase [18–20].
We designed siRNA targeting different regions within the open reading frame of the human HER2/neu mRNA and analyzed their ability to silence the expression of the HER2/neu gene in breast cancer cells overexpressing the HER2/neu gene. In addition, we examined the impact of HER2/neu gene modulation on the biology of HER2/neu-positive cells.
Materials and Methods
siRNA
siRNA were designed according to Elbashir et al. and purchased from Dharmacon Research, Inc. (Lafayette, CO). Four different regions within the HER2/neu gene (NCBI accession no. M11730) were used: siRNA-1 (positions 1255-1277), siRNA-2 (positions 3386-3408), siRNA-3 (positions 3563-3585), and siRNA-4 (positions 3682- 3704). siRNA-2S (scrambled) representing siRNA-2 and siRNA-4S (scrambled) siRNA-4 as random sequences was used as controls. All siRNA contain a 19-bp double-stranded (ds) sequence and symmetric 3′ overhangs of two deoxythymidines.
Antibodies
Monoclonal HER2/neu antibodies for Western blots and indirect immunofluorescence were obtained from Oncogene (Boston). Cyclin B1, cdc2, and caspase 3 monoclonal antibodies for Western blots were from Santa Cruz (Heidelberg, Germany), and β-actin monoclonal antibodies were from Sigma (Deisenhofen, Germany). Goat antimouse secondary antibodies for Western blots were purchased from Santa Cruz.
Cell Culture
Breast cancer cell lines MCF-7, BT-474, and MD-MB-468 cells were purchased from DSMZ (Braunschweig, Germany), and SK-BR-3 was from ATCC (Manassas, VA). Cell culture was performed according to the supplier's instructions. FCS was purchased from PAA Laboratories (Cölbe, Germany). RPMI 1640, phosphate-buffered saline (PBS), OPTI-MEM I (serum-free cell medium), Oligofectamine, glutamine, penicillin/streptomycin, and trypsin were purchased from Invitrogen (Karlsruhe, Germany). McCoy's 5A was from Biochrome (Berlin, Germany).
In Vitro Transfection of siRNA
Cells were transfected with siRNA using the Oligofectamine protocol according to Elbashir et al.'s and the manufacturer's instructions using 0.73% (vol/vol) of Oligofectamine. One day prior to transfection, 2 x 105 cells per six-well plate were seeded without antibiotics, corresponding to a density of 30% to 40% at the time of transfection. In most experiments, cells were treated with siRNA-1–4, siRNA-S2, or siRNA-S4 at a concentration of 56 nM. Control cells were incubated with OPTI-MEM I alone, or with OPTI-MEM I and Oligofectamine (mock-transfected cells), or with OPTI-MEM I and Oligofectamine plus siRNA-2S or siRNA-4S. To evaluate the effect of HER2/neu-specific siRNA, cells were treated with one of the four different HER2/neu-specific siRNA (plus OPTI-MEM I and Oligofectamine). After each treatment, cells were incubated at 37°C for 4 hours followed by addition of fresh culture medium (one third of the transfection volume) with 3x FCS.
Cells were harvested 24 and 48 hours after transfection for protein and mRNA analysis; 24, 48, 72, and 96 hours after transfection for indirect immunofluorescence and determination of proliferation rates; 72 hours after transfection for cell cycle analysis; and 96 hours after transfection for the apoptosis assay.
RNA Preparation and Northern Blots
To isolate total RNA, an RNeasy minikit (Qiagen, Hilden, Germany) was used according to the manufacturer's protocol. Radiolabeling of antisense strands for HER2/neu and β-actin was performed using 250 µCi of [α-P32]dCTP (6000 Ci/ mmol; 1 Ci = 37 GBq) for each reaction, 50 µM of each other dNTP, and 10 pmol (each) of primer HER2/neu-low (5′-caagtgctccatgcccagacc-3′) corresponding to positions 901 to 1200 (NCBI accession no. M11730) within the open reading frame of HER2/neu-or β-actin-2-low (5′-catgaggtagtcagtcaggtc-3′) as described previously [21]. Probes corresponding to positions 901 to 1200 within the HER2/neu gene were generated by polymerase chain reaction (PCR). Northern blotting, hybridizations, and standardizations were carried out as reported [20].
Western Blot Analysis
For Western blotting, cells were lysed with RIPA buffer (150 mM NaCl, 50 mM Tris base pH 8.0, 1 mM EDTA, 0.5% sodium deoxycholate, 1% NP40, 0.1% sodium dodecyl sulfate, 1 mM DTT, 1 mM PMSF, and 1 mM Na3VO4) supplemented with Complete Protease Inhibitor Tablets (Mannheim, Roche, Germany). Lysis and determination of protein concentration were done as described [23]. Twenty-five micrograms of total protein was separated on a 10% sodium dodecyl sulfate polyacrylamide gel followed by transfer (140 mA for 2 hours) to an Immobilon-P membrane (Millipore, Bedford, MA). Membranes were incubated for 1 hour in 5% skim milk in PBS with monoclonal antibodies (HER2/neu: 0.625 µg/ml; β-actin: 1:40,000; cyclin B1: 1:1000; cdc2: 1:1000; caspase 3: 1:1000) followed by incubation with goat antimouse secondary antibodies (1:2.000) for 30 minutes in 5% skim milk in PBS. Proteins were visualized as described previously [23]. Standardization and quantification were carried out as reported previously [19]. Values were given in percentage compared to the mock-transfected cells.
Determination of Cell Proliferation
After siRNA treatment, cell numbers were determined using a hemacytometer. Cell viability was assessed by trypan blue staining. The number of “control” cells (mock-transfected) at each time point was used as reference. Each transfection was carried out at least three times. t-tests were performed with two-sided P values.
Indirect Immunofluorescence
Indirect immunofluorescence, DNA staining, and visualization were carried out as described [23,24]. Monoclonal HER2/neu antibodies (6.25 µg/ml) were used to determine the level of p185HER2/neu.
FACScan Analysis
Analysis of cell cycle distribution after transfection with siRNA was carried out using a Becton Dickinson FACScan (BD Bioscience, Heidelberg, Germany). Cells were harvested, washed with PBS, and probed with CycleTEST PLUS DNA reagent kit (Becton Dickinson) according to the manufacturer's protocol. For each treatment, 30,000 cells were analyzed. Cell cycle distribution in percentage was calculated using ModFit LT for Mac (BD Bioscience).
Apoptosis Assays
Cells were treated with siRNA-2 or siRNA-S2 at 5.6 nM for 96 hours. As apoptosis-positive controls, MCF-7 cells were treated with campothecin (Sigma-Aldrich, Steinheim, Germany) at 10 µM for 16 hours. Apoptosis assay was performed using Vybrant kit according to the manufacturer's instructions (Molecular Probes, Leiden, The Netherlands). Briefly, cells were stained with 3 µl of Annexin V and 1 µl of propidium iodide (PI; 1 µg/ml) in 100 µl of Annexin V binding buffer [10 mM HEPES (pH 7.4), 140 mM NaCl, and 5 mM CaCl2] and incubated for 15 minutes at room temperature in the dark. Subsequently, 400 µl of binding buffer was added and mixed gently. The cells were then analysed by flow cytometry.
Results
Expression of HER2/neu mRNA and Protein in Breast Cancer Cell Lines
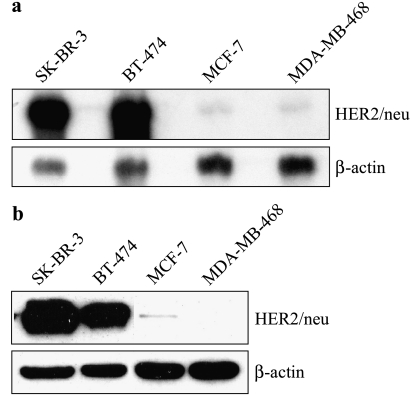
We analyzed HER2/neu expression in four breast cancer cell lines to select the most appropriate cell lines for the experiments. Northern blots showed abundant expression of a 4.5-kb HER2/neu transcript in SK-BR-3 and BT-474 cells and minimal expression of HER2/neu transcripts in MCF-7 and MDA-MB-468 cells (Figure 1a). These findings were corroborated by Western blot analysis of p185HER2/neu expression in these cell lines, standardized to β-actin expression (Figure 1b), which showed high levels of p185HER2/neu in SK-BR-3 cells, markedly lower levels in BT-474 cells, and a faint band in MCF-7 cells. p185HER2/neu expression was below the limit of detection in MDA-MB-468 cells.
Figure 1.
Expression of (a) HER2/neu mRNA and (b) p185HER2/neu, in SK-BR-3, BT-474, MCF-7, and MDA-MB-468 cells. (a) HER2/neu mRNA was subjected to Northern blot analyses. (b) Western blot analyses of p185HER2/neu expression. To control for variability of loading and transfer, Northern and Western blot analysis membranes were reprobed for β-actin, and actin-normalized HER2/neu expression was compared. The exposure time was about 15 seconds.
Specific Inhibition of HER2/neu mRNA and Protein Expression by HER2/neu-Specific siRNA
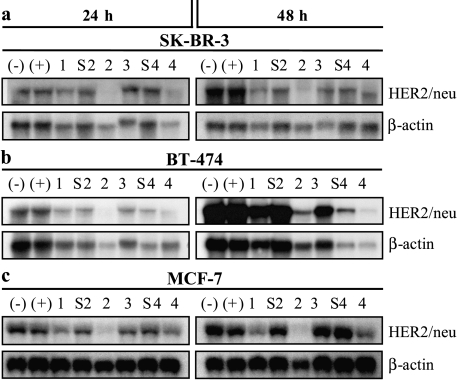
We next tested the ability of HER2/neu-specific siRNA to reduce the endogenous level of HER2/neu mRNA in SK-BR-3, BT-474, and MCF-7 breast cancer cells. We transfected cells with four different HER2/neu-specific siRNA and with scrambled versions of siRNA-2 (siRNA-S2) and siRNA-4 (siRNA-S4) as controls. After 24 hours, HER2/neu mRNA levels were moderately decreased in SK-BR-3 cells transfected with one of the four HER2/neu-specific siRNA (siRNA-1, siRNA-2, siRNA-3, and siRNA-4), and, after 48 hours, HER2/neu mRNA levels were 15% to 85% of the levels seen in mock-transfected cells (Figure 2a). BT-474 and MCF-7 cells transfected with HER2/neu-specific siRNA-1 to siRNA-4 showed also reductions in HER2/neu transcripts at both time points (Figure 2, b and c). The four HER2/neu-specific siRNA had different abilities to alter the levels of the corresponding transcripts, with reductions in BT-474 cells or MCF-7 cells transfected with the different siRNA ranging from 20% to 42%, or from 7% to 60%, respectively, of the transcript levels seen in mock-treated cells after 48 hours. In all cell lines examined, strong reduction of the HER2/neu mRNA was achieved by transfection with siRNA-2 and, to a lesser extent, with siRNA-4. Mock-transfected cells or cells transfected with the scrambled siRNA-S2 or siRNA-S4 exhibited only a slight reduction of HER2/neu mRNA.
Figure 2.
Northern blot analyses of HER2/neu mRNA expression after transfection with HER2/neu-specific siRNA in (a) SK-BR-3, (b) BT-474, and (c) MCF-7 cells. HER2/neu mRNA was subjected to Northern blot analysis 24 and 48 hours after the beginning of transfection with 56 nM siRNA. To control for variability of loading and transfer, membranes were reprobed for human β-actin, and β-actin-normalized HER2/neu mRNA levels were compared. Control cells (-) were incubated with serum-free medium. OPTI-MEM I alone and control cells (+) were incubated with OPTI-MEM I plus Oligofectamine but no siRNA. Breast cancer cells were treated with siRNA-1, siRNA-2, siRNA-3, or siRNA-4. Furthermore, cells were transfected with control siRNA-S2 or siRNA-S4.
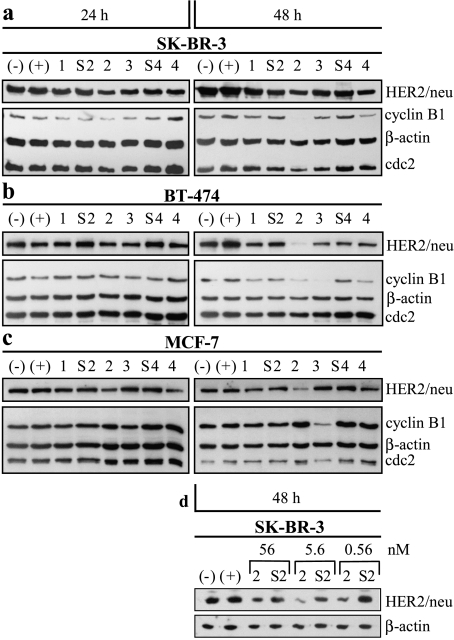
Western blot analysis of p185HER2/neu levels in transfected SK-BR-3, BT-474, and MCF-7 cells showed decreased protein levels consistent with the results of Northern blot analyses in these cells (Figure 3). SK-BR-3 cells transfected with siRNA-1 to siRNA-4 showed reductions of p185HER2/neu compared to the expression in mocktransfected cells 48 hours after transfection, with a clear reduction observed after transfection with siRNA-2 (Figure 3a). With all transfections, downregulation of p185HER2/neu in SK-BR-3 cells increased over time. In BT-474 and MCF-7 cells, reduction of transcript levels correlated also with a decrease of p185HER2/neu levels (Figure 3, b and c). Depending on the HER2/neu-specific siRNA administered, a reduction of p185HER2/neu levels to 3% to 50% in BT-474 cells and to 29% to 83% in MCF-7 cells compared to the expression in mock-transfected cells was observed. siRNA-2 at a concentration of 56 nm reduced strongly p185HER2/neu expression in MCF-7 cells (Figure 3c), which constitutively express only a low level of HER2/neu expression.
Figure 3.
Western blot analyses of p185HER2/neu, cyclin B1, and cdc2 expression in (a) SK-BR-3, (b) BT-474, and (c) MCF-7 cells after transfection with HER2/neu-specific siRNA. Western blot analyses were carried out 24 and 48 hours after the beginning of transfection with 56 nM siRNA. The exposure time for (c) was about 20 minutes due to low expression of HER2/ neu in MCF-7 cells. (d) Western blot of SK-BR-3 cells 48 hours after transfection with different concentrations (0.56, 5.6, and 56 nM) of siRNA-2 or its equivalent control siRNA-S2. β-Actin was used to control for variability of loading and transfer in (a)–(d). Subsequently, β-actin-normalized protein levels were compared.
To improve the uptake of siRNA at a concentration of 56 nM, we increased the Oligofectamine concentration from 0.73% to 2.9% (vol/vol) per transfection and observed greater inhibition of HER2/neu-specific siRNA in BT-474 cells compared to mock-transfected cell (data not shown). This result suggests that optimal liposomal encapsulation is critical for efficient RNAi-mediated gene silencing. We then examined lower concentrations of siRNA-2 (5.6 and 0.56 nM) in SK-BR-3 cells and found that transfection with siRNA-2 at a concentration of 5.6 nM [and with 0.73% (vol/ vol) Oligofectamine] resulted in inhibition of p185HER2/neu expression that was at least as strong as the inhibition achieved with 56 nM siRNA-2 (Figure 3d). The least effective of the three siRNA-2 concentrations tested for the down-regulation of HER2/neu expression was 0.56 nM.
Analysis of HER2/neu Expression by Immunofluorescence Staining
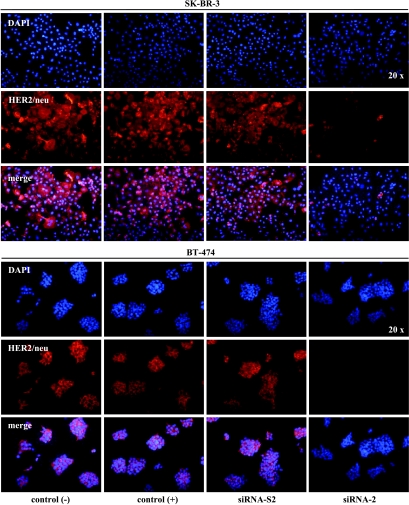
To evaluate the data from our Western blot experiments, we further performed an immunofluorescence analysis of different breast cancer cell lines. As illustrated in Figure 4, untreated SK-BR-3 and BT-474 cells showed strong levels of p185HER2/neu staining, whereas signal intensity in MCF-7 cells was weak (data not shown). We tested the ability of siRNA to reduce the endogenous level of HER2/neu immunofluorescence in SK-BR-3 and BT-474 cells. Twenty-four hours after transfection of SK-BR-3 and BT-474 cells with siRNA-2, HER2/neu-specific immunofluorescence was diminished, with complete disappearance of staining in SKBR- 3 and BT-474 cells 96 hours after transfection (Figure 4). By contrast, no reductions in expression of HER2/neu protein in vitro were detected in SK-BR-3 or BT-474 cells between 24 and 96 hours after mock transfection or transfection with the scrambled siRNA-2S.
Figure 4.
Immunofluorescence analyses of SK-BR-3 and BT-474 cells treated with 56 nM siRNA-2. Control cells (-) were incubated with OPTI-MEM I alone and control cells (+) were incubated with OPTI-MEM I plus Oligofectamine but no siRNA. Cells were treated with siRNA-2 or with siRNA-S2. Ninety-six hours after the beginning of transfection, cells were analyzed for p185HER2/neu (red) and subsequently for DNA by DAPI staining (blue). Lower panels show the merging of p185HER2/neu (red) and DNA staining (blue).
The Antiproliferative Effect of HER2/neu-Specific siRNA Is Pronounced in HER2/neu-Overexpressing Breast Cancer Cells
Because inhibition of HER/neu mRNA expression in human breast cancer cells can result in a significant dose-dependent suppression of p185HER2/neu levels, we next investigated the impact of siRNA transfection on cell proliferation in SK-BR-3, MCF-7, and MDA-MB-468 breast cancer cells. Considering the limited half-life of siRNA in living cells, we did not test the biologic activity of HER2/neu-specific siRNA in BT-474 cells, which have a doubling time of approximately 100 hours.
We observed a positive correlation between the transfection of siRNA on HER2/neu expression and biologic effects in SK-BR-3 breast cancer cell proliferation: SK-BR-3 cells transfected with one of the HER2-targeted siRNA showed significant inhibition of proliferation 96 hours after transfection (siRNA-1: 66.8%; siRNA-2: 56.0%; siRNA-3: 82.9%; siRNA-4: 70.1%) compared to mock-transfected cells (P ≤ .01) (Figure 5). The data indicated that the antiproliferative effects of HER2/neu-directed siRNA were sequence-specific.
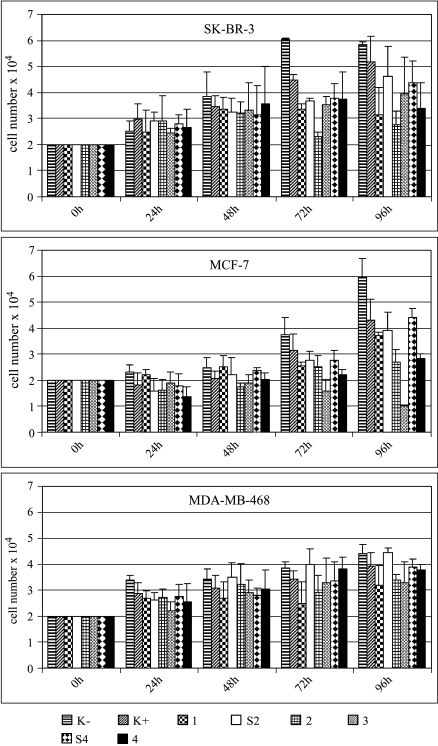
Figure 5.
Effect of HER2/neu-specific siRNA on proliferation of SK-BR-3, MCF-7, and MDA-MB-468 cells. Cells were treated with 56 nM siRNA-1, siRNA-2, siRNA-3, and siRNA-4 or with one of the control siRNA-S2 or siRNA-S4 and cell numbers were counted at indicated time points. Control (K-) and control (K+) cells were incubated as described in Figure 4.
Most of the siRNA tested had also an effect on the proliferation of MCF-7 cells, which have low constitutive levels of HER2/neu expression. After transfection with siRNA-1, siRNA-2, or siRNA-4, proliferation of MCF-7 cells was reduced to 85.9%, 67.0%, or 69.5% of that of mock-transfected control cells (Figure 5). It was noted that transfection with siRNA-3 exerted a strong antiproliferative effect in MCF-7 cells, reducing the proliferation to 24.8% compared to mock-transfected cells, despite a lack of efficacy in silencing HER2/neu expression in MCF-7 cells (Figures 2 and 3).
In contrast to SK-BR-3 cells, transfection with different HER2/neu-specific siRNA did not affect cellular proliferation of MDA-MB-468 cells, which express almost no HER2/neu (Figure 5).
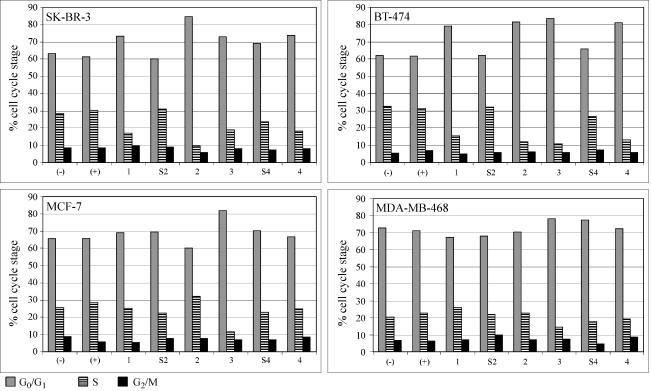
Treatment of p185HER2/neu-Overxpressing Cells with HER2/neu-Specific siRNA Induces G0/G1 Arrest and Correlates with Downregulation of Cyclin B1
To study the antiproliferative effect exerted by HER2/neu-specific siRNA in more detail, we analyzed the cell cycle distribution of siRNA-treated cells. Seventy-two hours of transfection of SK-BR-3 and BT-474 cells with siRNA-2 resulted in an increase in the proportion of cells in G0/G1 phase, compared to mock-transfected control cells (Figure 6). MCF-7 cells transfected with siRNA-2 did not show a similar antiproliferative response. As expected from the previous proliferation assays, transfection with siRNA-S2 or siRNA-S4 did not alter the cell cycle distribution compared to mock-transfected cells (Figure 6).
Figure 6.
Effect of HER2/neu-specific siRNA on cell cycle distribution of SK-BR-3, BT-474, MCF-7, and MDA-MB-468 cells. FACScan analyses 72 hours after treatment of cells. Control cells (-) were incubated with serum-free medium. OPTI-MEM I alone and control cells (+) were incubated with OPTI-MEM I plus Oligofectamine but no siRNA. Cells were treated with 56 nM siRNA-1, siRNA-2, siRNA-3, and siRNA-4 or with one of the control siRNAs-S2 or siRNA-S4.
To verify that cells were blocked at stage G0/G1, we used Western blot analysis to screen for markers of later stages in cell cycle progression (Figure 3). As a component of the maturation-promoting factor, cyclin B1 is a marker of G2/M progression and was strongly downregulated in SK-BR-3 and BT-474 cells treated with siRNA-2 (Figure 3, a and b). By contrast, MCF-7 cells treated with siRNA-2 showed almost complete depletion of p185HER2/neu, but levels of cyclin B1 were unchanged relative to levels of β-actin. A Western blot analysis of all three cell lines subsequently demonstrated constitutive expression of cdc2 independent of p185HER2/neu or cyclin B1 downregulation (Figure 3).
An unexpected finding was that siRNA-3 had a strong inhibitory effect on the proliferation of MCF-7 cells (Figure 5), despite having only a very weak ability to downregulate HER2/neu expression in these cells (Figures 2 and 3). Furthermore, siRNA-3 treatment resulted in an elevation of the percentage of cells in G0/G1 after 72 hours (Figure 6) together with reduced levels of cyclin B1 protein (Figure 3).
Treatment With HER2/neu-Specific siRNA Induces Apoptosis in p185HER2/neu-Overxpressing Cells
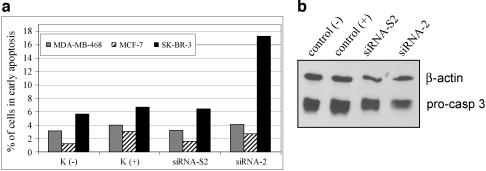
To explore whether the cells arrested in G0/G1 then progressed to apoptosis, we stained siRNA-2-transfected cells with Annexin V and PI and analyzed the cells using flow cytometry. As illustrated in Figure 7, more than 17% of SKBR-3 cells transfected with siRNA-2 were Annexin-positive compared to only 6% of SK-BR-3 cells transfected with the scrambled siRNA-S2. Distribution of mock-transfected SK-BR-3 was similar to that of siRNA-S2-transfected cells. Late stage of apoptosis (both Annexin-positive and PI-positive) was not observed in SK-BR-3 cells even 96 hours posttransfection, which is in line with the date from a recent publication [44]. In contrast to the results seen with SK-BR-3 cells, no Annexin-positive cells were observed following transfection of siRNA-2 into MDA-MB-468, without detectable HER2/neu expression, or MCF-7 cells, with low constitutive levels of HER2/neu expression (Figure 7a). To show that apoptosis could be induced in those two cell lines, which were not affected with the treatment of siRNA-2, MCF-7 cells were selected and treated with exogenic apoptotic stimulator campothecin. After exposure to campothecin for 16 hours, MCF-7 cells displayed 7% of Annexin-positive cells and 10% of PI-positive cells, compare to 3% and 6%, respectively, in control nontreated MCF-7 cells (data not shown). Furthermore, apoptosis after siRNA treatment in SK-BR-3 cells was corroborated by Western blot analysis using specific antibodies against pro-caspase 3 (32 kDa), which is a precursor of caspase 3. The cellular extracts from siRNA-2-treated SK-BR-3 cells showed less pro-caspase 3 compared to mock-treated cellular lysates [control(+)], whereas scrambled siRNA-S2 scarcely exhibited effects (Figure 7b). Further quantification of the bands from the Western blot standardized with loading control β-actin showed a reduction of 18% of pro-caspase 3 protein in siRNA-2-treated cells compared to control (+) cells. The results suggested that the down-regulation of HER2/neu with siRNA-2 in SK-BR-3 cells led to an activation of apoptosis pathway by showing the cleavage of pro-caspase 3 to its active form caspase 3. Collectively, the data from Annexin staining and reduction of pro-caspase 3 protein demonstrated that siRNA-2 targeting of HER2/neu induced specific apoptosis in SK-BR-3 cells.
Figure 7.
Effect HER2/neu-specific siRNA on apoptosis induction in MDA-MB-468, MCF-7, and SK-BR-3 cells. (a) Cells were treated with 5.6 nM siRNA-2 or siRNA-S2. Control cells C (-) were incubated with serum-free medium OPTI-MEM I alone. C (+) cells were additionally treated with Oligofectamine but without siRNA. Ninety-six hours after transfection, cells were analysed for early apoptosis by Annexin staining (Vybrant apoptosis kit). (b) SK-BR-3 cells were treated with siRNA-2 or its scrambled form for 96 hours. Western blot analysis was performed by using specific antibodies against pro-caspase 3. The same membrane was stained with antibodies against β-actin as loading controls.
Discussion
Amplification and overexpression of the HER/neu protooncogene is found in a number of human malignancies and human cancer cell lines, and has been correlated with decreased time to relapse and decreased overall survival in subsets of patients with breast, ovarian, or certain other cancers [2–6]. As a transmembrane growth factor receptor with an extracellular ligand-binding domain, p185HER2/neu is an appropriate target for monoclonal antibody therapy of human cancers characterized by p185HER2/neu overexpression. Clinical studies using monoclonal antibodies to target p185HER2/neu in human breast cancer represent the first example of the successful translation of results from the molecular analysis of cancer cells into clinical trials [25–28].
Antibody-based therapeutic interventions have certain limitations in hindering HER2/neu signalling pathways. The inhibitory effect of antibodies is restricted to transmembrane forms of p185HER2/neu, and thus cannot inhibit intracellular receptors, which may contribute to the increased proliferation of HER/neu-overexpressing cells prior to exposure of the p185HER2/neu receptors on the cell membrane [30]. p185HER2/neu-specific antibodies might be neutralized by the extracellular domain of the receptor shed from the membrane into the surrounding medium. Brodowicz et al. [30] have demonstrated that this mechanism seems to interfere with the impact of monoclonal antibodies in vitro, and the presence of antigenic p185HER2/neu-specific fragments found in patient serum correlates with a poor clinical response to p185HER2/neu-specific antibodies [31]. Trastuzumab, the only monoclonal antibody currently approved for treatment of breast cancer, has a cytostatic—rather than a cytotoxic—action on breast cancer cells in vitro [26,32]. The activity of trastuzumab was found to depend in vivo on the engagement of Fc receptor-expressing lymphocytes [33], indicating antibody-dependent cell cytotoxicity as a major determinant of antibody action. Because monoclonal antibodies targeted to p185HER2/neu may not be effective tools for the therapeutic manipulation of HER2/neu signalling in every case, alternative approaches need to be explored for the inhibition of neoplastic proliferation.
An alternative method of selectively inhibiting gene expression is the use of antisense oligonucleotides (ASOs) [34]. Various studies using ASOs to inhibit HER2/neu gene expression in human breast cancer cells showed significant dose-dependent suppression of p185HER2/neu levels and suppression of cancer cell growth [35–39]. Antiproliferative effects of HER2/neu-specific ASOs were seen in HER2/neu-overexpressing breast cancer cells transfected with ASOs at concentrations between 1 and 20 µM [36,39]. In addition, HER2/neu antisense treatment inhibited cellular proliferation in p185HER2/neu-overexpressing cancer cells of different histologic origin, such as ovarian, lung, and pancreatic cells [38]. Still, ASOs have limited therapeutic potential due to toxicities and side effects likely arising from inhibition through other mechanisms [40]. Currently, the only approved ASO therapy is the one for CMV retinitis because ASOs are confined to a very small compartment (intraocular injection) to reach relatively high concentrations. Whether high plasma levels of ASOs, which are needed to inhibit HER2/neu, can be systemically reached in breast cancer patients without severe side effects remains to be elucidated.
Gene expression can also be regulated by exploiting elements of the RNAi machinery. Elbashir et al. [17] have shown that transient transfection of synthetic siRNA can downregulate target genes in mammalian cells. siRNA as tools for therapeutic interventions must be specific for the gene of interest, but for a given gene, only certain siRNA are efficient for gene silencing. We exploited this technique to study the role of HER2/neu in the proliferation of human cancer cells exhibiting different levels of HER2/neu overexpression.
Our analysis revealed that transfection of human breast cancer cells with siRNA targeted to HER2/neu downregulates corresponding transcripts in cell culture. In our study, siRNA-2 and siRNA-4 exerted the most pronounced effect on the level of HER2/neu expression. However, all cell lines tested in our study underwent gene silencing of HER2/neu after treatment with HER2/neu-specific siRNA, regardless of baseline HER2/neu expression. As expected, the scrambled forms of siRNA-2 and siRNA-4 had very little effect on HER2/neu expression.
siRNA-2 had an antiproliferative effect in breast cancer cells showing high levels of HER2/neu expression (BT-474 and SK-BR-3). siRNA-2 exerted also antiproliferative effect to a lesser extent in MCF-7 cells, a cell line with low levels of HER2/neu expression. By contrast, we could not detect inhibitory impacts of proliferation in MDA-MB-468 cells, which express scarcely HER/neu. Taken together, the data suggest that the antiproliferative effect of siRNA is associated with the expression level of HER2/neu in breast cancer cells.
HER2/neu-independent mechanisms may explain the unexpected observation that siRNA-3 had only a very weak potential to inhibit HER/neu expression in MCF-7 cells yet still inhibits cell proliferation. A recent report described offtarget effects exerted by a core of sequence similarity to the 3V end of the siRNA sense strand [41]. To address the question of whether short stretches of identity to siRNA-3 exerted siRNA-specific rather than target-specific signatures in our analysis, we aligned both sense and antisense strands, as well as subfragments thereof, to the databank (GenBank, EMBL, April 2004), but no significant homologies were detectable. Thus, there is no obvious explanation for the effect of siRNA-3 in MCF-7 cells including G0/G1 arrest, downregulation of cyclin B1, and its inhibition of proliferation. We are going to reduce the concentration of siRNA-3 and further examine the impact on G1/S molecules in MCF-7 cells. The observation also suggested that further studies are required to explore the working mechanisms of siRNA in more detail, and a careful selection and examination of siRNA for gene silencing is a cardinal requirement for a target-specific gene silencing through RNAi.
During the preparation of this report, two studies evaluating the RNAi-based gene silencing of the HER2/neu gene using synthetic siRNA or a retrovirus-mediated expression of hairpin RNA, both directed against the HER2/neu gene, were published [42,43]. In agreement with our results, both studies suggest that HER2/neu depletion in HER2/neu-overexpressing breast cancer cells inhibits their proliferation. Still, these reports provide a different view on the cell cycle distribution of treated cells: Although Choudhury et al. [42] describe growth arrest at G1/S, Yang et al. [43] observed increased G0/G1 arrest at a level similar to that seen in our studies. Consistent with the our results seen with G0/G1 arrest, transfection with siRNA-2 promoted HER2/neu-induced apoptosis only in high-expressing SK-BR-3 cells, and not in MCF-7 or MD-MB-468 cells, suggesting that the induced apoptosis was specific and dependent on the reduction of HER2/neu in HER2/neu-overexpressing cells.
Taken together, compared to antibody-or antisensemediated methods for the inhibition of HER2/neu signaling, gene silencing through siRNA combines two major advantages: 1) strong and specific downregulation of HER2/neu expression at very low concentrations; and 2) siRNA treatment, which exerts a cytotoxic effect in HER2/neu-overexpressing cells. Translation of these promising in vitro results into therapies for human disease faces some obstacles. Despite the success of siRNA-mediated strategies in silencing gene expression in cultured cells, unmodified siRNA does not seem to be effective at reducing target gene expression in murine tissues after systemic administration, unless applied by hydrodynamic injection in relatively large nonphysiological volumes of saline [44]. siRNA-mediated effects in living animals described in early reports are restricted to hepatocytes. Until chemical modifications can be developed to improve the utility of siRNA, alternative strategies such as vector-driven expression of hairpin RNA are required for the inhibition of tumor growth in vivo [45].
Footnotes
This work is supported, in part, by the Nationales Genomforschungsnetz (grant KR-S07T01 to K.S.), Deutsche Krebshilfe (grant 10-1212-St 1 to K.S.), Deutsche Forschungsgemeinschaft (grant STR/8-1 to K.S.), Messer Stiftung, Sander Stiftung, and Dresdner Bank.
References
- 1.Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 2.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 3.Berchuck A, Rodriguez G, Kinney RB, Soper JT, Dodge RK, Clarke-Pearson DL, Blast RC., Kr Overexpression of HER-2/neu in endometrial cancer is associated with advanced stage disease. Am J Obstet Gynecol. 1991;64:15–21. doi: 10.1016/0002-9378(91)90615-x. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri R, Matthews C, Dobrovic A, Horsfall DJ. The significance of oncogene amplification in primary breast cancer. Int J Cancer. 1989;43:270–272. doi: 10.1002/ijc.2910430218. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989;7:1120–1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- 7.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifenresistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 8.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117–123. doi: 10.1016/s1535-6108(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 11.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 12.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 13.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 14.Nykanen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 15.Parrish S, Fleenor J, Xu S, Mello C, Fire A. Functional anatomy of a dsRNA trigger: differential requirement for the two trigger strands in RNA interference. Mol Cell. 2000;6:1077–1087. doi: 10.1016/s1097-2765(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 16.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 17.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100:5789–5794. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spänkuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–1877. doi: 10.1093/jnci/94.24.1863. [DOI] [PubMed] [Google Scholar]
- 20.Wilda M, Fuchs U, Wossmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi) Oncogene. 2002;22:5716–5724. doi: 10.1038/sj.onc.1205653. [DOI] [PubMed] [Google Scholar]
- 21.Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–549. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 22.Böhme B, VandenBos T, Cerretti DP, Park LS, Holtrich U, Rübsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;271:24747–24752. doi: 10.1074/jbc.271.40.24747. [DOI] [PubMed] [Google Scholar]
- 23.Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, Staubli U, Bereiter-Hahn J, Strebhardt K, Kuhl D. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)-and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 2002;21:8282–8292. doi: 10.1038/sj.onc.1206011. [DOI] [PubMed] [Google Scholar]
- 25.Drebin JA, Stern DF, Link VC, Weinberg RA, Greene MI. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature. 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 26.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 28.Stancovski I, Hurwitz E, Leitner O, Ullrich A, Yarden Y, Sela M. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci USA. 1991;88:8691–8695. doi: 10.1073/pnas.88.19.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibodymediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodowicz T, Wiltschke C, Budinsky AC, Krainer M, Steger GG, Zielinski CC. Soluble HER-2/neu neutralizes biologic effects of anti-HER-2/neu antibody on breast cancer cells in vitro. Int J Cancer. 1997;73:875–879. doi: 10.1002/(sici)1097-0215(19971210)73:6<875::aid-ijc19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 32.Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 34.Dean NM, Bennett CF. Antisense oligonucleotide-based therapeutics for cancer. Oncogene. 2003;22:9087–9096. doi: 10.1038/sj.onc.1207231. [DOI] [PubMed] [Google Scholar]
- 35.Bertram J, Killian M, Brysch W, Schlingensiepen KH, Kneba M. Reduction of erbB2 gene product in mamma carcinoma cell lines by erbB2 mRNA-specific and tyrosine kinase consensus phosphorothioate antisense oligonucleotides. Biochem Biophys Res Commun. 1994;200:661–667. doi: 10.1006/bbrc.1994.1499. [DOI] [PubMed] [Google Scholar]
- 36.Colomer R, Lupu R, Bacus SS, Gelmann EP. erbB-2 antisense oligonucleotides inhibit the proliferation of breast carcinoma cells with erbB-2 oncogene amplification. Br J Cancer. 1994;70:819–825. doi: 10.1038/bjc.1994.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roh H, Pippin J, Boswell C, Drebin JA. Antisense oligonucleotides specific for the HER2/neu oncogene inhibit the growth of human breast carcinoma cells that overexpress HER2/neu. J Surg Res. 1998;77:85–90. doi: 10.1006/jsre.1998.5353. [DOI] [PubMed] [Google Scholar]
- 38.Roh H, Pippin J, Drebin JA. Down-regulation of HER2/neu expression induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2000;60:560–565. [PubMed] [Google Scholar]
- 39.Roh H, Pippin JA, Green DW, Boswell CB, Hirose CT, Mokadam N, Drebin JA. HER2/neu antisense targeting of human breast carcinoma. Oncogene. 2000;19:6138–6143. doi: 10.1038/sj.onc.1204001. [DOI] [PubMed] [Google Scholar]
- 40.Stein CA. Does antisense exist? Nat Med. 1995;1:1119–1121. doi: 10.1038/nm1195-1119. [DOI] [PubMed] [Google Scholar]
- 41.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals offtarget gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 42.Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B, Kiessling R. Small interfering RNA (siRNA) inhibits the expression of the HER2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;108:71–77. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 43.Yang G, Cai KQ, Thompson-Lanza JA, Bast RC, Jr, Liu J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem. 2004;279:4339–4345. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- 44.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 45.Spänkuch-Schmitt B, Mattess Y, Knecht R, Zimmer B, Kaufmann M, Strebhardt K. RNA silencing of PLK1 expression induces apoptosis and abrogates spindle formation in cancer cells. J Natl Cancer Inst. 2004;96:862–872. [Google Scholar]