Abstract
Changes in the expression and activity of lipidmetabolizing enzymes, including the linoleic acid (LA)-metabolizing enzyme 15-lipoxygenase-1 (15-LO-1), may play a role in the development and progression of human prostate carcinoma (PCa). We reported that human 15-LO-1 (designated as leukocyte type 12-LO or 12/15-LO in mouse) is expressed in human prostate and increased in PCa, particularly high-grade PCa. Genetically engineered mouse (GEM) models of PCa could facilitate the study of this gene and its regulation and function in PCa progression. In this study, we examine the protein expression and enzyme activity levels of 12/15-LO associated with PCa progression in the TRansgenic Adenocarcinoma of Mouse Prostate (TRAMP) model of PCa. This GEM model develops prostatic intraepithelial neoplasia (PIN), followed by invasive gland-forming PCa and invasive and metastatic less differentiated PCa, with neuroendocrine (NE) differentiation (NE Ca). In the wild-type and TRAMP prostates, the most prominent LA metabolite was 13-hydroxyoctadecadienoic acid (13-HODE). Lesser amounts of 12-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid (HETE) were made from arachidonic acid (AA). In TRAMP prostates, 12/15-LO activity was increased compared to wild type at 20, 29, 39, and 49 weeks, as assessed by LA conversion to 13-HODE, and by AA conversion to 12/15-HETE, respectively. Immunostaining demonstrated that the increased capacity to generate 13-HODE was paralleled by an increase in neoplastic epithelial expression of 12/15-LO in PIN and invasive carcinomas. In conclusion, although there is a basal 12/15-LO activity in the wild-type mouse prostate, there is a marked increase in the expression of 12/15-LO with TRAMP PCa progression, paralleling our previously reported increased expression of the ortholog 15-LO-1 in high-grade human PCa. Thus, 12/15-LO and LA metabolism in the TRAMP model shares similarities to human PCa, and may allow to confirm a role for LA metabolism and other biologic functions of 15-LO-1 in human PCa. In addition, the TRAMP model will serve as a tool for testing the suitability of 12/15-LO—and ultimately human 15-LO—as a therapeutic target during PCa progression.
Keywords: Lipoxygenase, prostate cancer, mouse model, TRAMP, linoleic acid
Introduction
In the development and progression of prostate carcinoma (PCa), precursor lesions such as high-grade prostatic intraepithelial neoplasia (HGPIN) in the peripheral zone evolve into invasive carcinoma, with progression to more extensive organconfined tumors, and then to tumors that can penetrate the prostatic capsule and invade locally or metastasize [1]. An estimated 230,110 new cases of PCa will be detected in the United States in 2004, and an estimated 29,900 PCa deaths will occur (American Cancer Society, Facts and Figures 2004). All currently adopted therapies for PCa have limitations, and there is no cure for PCa leading to metastatic disease. Advanced and metastatic PCa commonly progresses to clinically hormone-refractory disease (androgen insensitivity), limiting treatment options in advanced disease (Ref. [1] and references therein). Investigations to more completely understand the development and progression of PCa have been difficult in human patients because the cancer typically develops at an extremely slow pace, and, along with difficulties in tissue sampling over a time period, this makes it practically impossible to track the molecular changes that are associated with the disease progression in patients. These characteristics also pose a challenge for therapeutic trials, including those based on targeting desirable genes or pathways that are predicted to be causal during the earlier stages of the disease.
Hence, genetically engineered mouse (GEM) models of PCa are beginning to emerge as potentially powerful tools in PCa research, allowing for correlation of molecular alterations with tumor progression and design of appropriate interventional trials based on blocking of stage-specific disease progression [2–4]. GEM models [5–9] [such as the TRansgenic Adenocarcinoma of Mouse Prostate (TRAMP) model generated using a prostate-selective short probasin promoter and the SV40 early region [6,7], and long probasin promoter SV40-large T antigen (LPB-Tag; LADY) generated using the LPB-Tag [8,9] recapitulate certain key aspects of human PCa [1]. These particular models, designed to potentially mimic certain major initiating and secondary molecular changes in PCa, progress from PIN to invasive, metastatic, and hormone-refractory PCa. Therefore, it is reasonable to hypothesize that demonstrated therapeutic interventions [2–4] that are effective in GEM models validated for molecular and pathologic changes similar to those in human PCa may also be beneficial in human PCa patients.
Alterations in genes, such as lipoxygenases [5-LO [10], 12-LO [11], 15-lipoxygenase-1 (15-LO-1) [12–14], and 15-LO-2 [15–18]] and cyclooxygenases (COX-1 and/or COX-2 [19–21]) that metabolize dietary lipids, have been postulated to be key factors in PCa development or progression, and represent attractive chemoprevention or therapeutic targets. The precise role(s) of these enzymes (or enzymatic products) in PCa is an area of active investigation. Studies have also suggested that one of the major reasons why Americans suffer from high rates of cancers may be due to an imbalance in the ratio of omega (ω-3 to ω-6) fatty acids [22–24]. An important member of the ω-6 group is linoleic acid [LA; polyunsaturated fatty acid (PUFA), C18: 2n–6], which is an essential PUFA found in sunflower, safflower, canola, and peanut oils. 15-LO-1 preferentially metabolizes LA [25]. The human 15-LO-1 enzyme forms 13-hydroxyoctadecadienoic acid (13-HODE) from LA, and its ortholog in mouse [i.e., 12/15-LO enzyme (also known as leukocyte type 12-LO)] forms a mixture of 12-HETE and 15-HETE from AA, respectively [25,26]. Human 15-LO-1-derived 13-S-hydroxyoctadecadienoic acid (13-S-HODE) can exert several proneoplastic effects, including enhanced cellular proliferation [13,14,27–36]. We previously observed that 15-LO-1 expression is increased in human PCa compared to benign prostate, particularly in high-grade PCa, further suggesting a possible etiologic role in PCa progression [13]. We subsequently demonstrated that overexpression of 15-LO-1 in a human PCa cell line, PC-3, produced aggressive tumors in athymic mice, at least in part by promoting angiogenesis [14]. It should be noted that Shappell et al. [15,16] and Jack et al. [17] have demonstrated that a distinct 15-lipoxygenase (15-LO-2) is highly expressed in benign prostate, and reduced in HGPIN and PCa. 15-LO-2 converts its preferred substrate AA to 15-S-hydroxyeicosatetraenoic acid (15-S-HETE) [25], which could potentially inhibit the growth of PCa cells by activating the anti-tumorigenic peroxisome proliferator -activated receptor γ (PPARγ) activity [37]. More recently, 15-LO-2 was shown to be a negative cell cycle regulator in normal prostate [18], and others have shown that 15-LO-1 and 15-LO-2 have opposing pro-neoplastic effects and anti-neoplastic effects, due at least in part to the preferred formation of 13-HODE and 15-HETE, respectively, by differential effects on MAPK and PPARγ [36,38]. Thus, although a role for 15-LO-1 in other organ systems remains to be more fully defined [39], in prostate, 15-LO-1 appears to behave distinctly different from 15-LO-2 in that 15-LO-1 specifically appears to be increased in PCa and may contribute to PCa by promoting cell proliferation and angiogenesis [14].
Although the ortholog of the human 15-LO-1 gene in the mouse is 12/15-LO, for the human 15-LO-2 in mouse, it is 8-LO [25]. Surprisingly, 8-LO is absent in the mouse prostate, but is specifically expressed in the skin [41]. As the murine ortholog of 15-LO-1 enzyme has been identified [26], TRAMP and LPB-Tag mouse models of PCa may allow for: 1) elucidation of the role(s) of 15-LO-1 (human)/12/15-LO (mouse) in the development and progression of PCa; and 2) suitability of 15-LO-1 as a potential therapeutic target in PCa. In the current study, expression of 12/15-LO was investigated in wild-type mice, with PCa progression in TRAMP mice achieved through a combination of enzyme activity assays and immunohistochemistry analyses; results compared with those that were previously observed in human PCa.
Materials and Methods
Transgenic Mouse and Tissue Procurement
TRAMP mice hemizygous for the probasin promoter SV40-large T antigen (PB-Tag) transgene were maintained in a pure C57BL/6 background (Harlan Sprague Dawley, Inc., Indianapolis, IN). TRAMP mice were bred to nontransgenic FVB mice (Harlan Sprague Dawley) to obtain nontransgenic (wild type) and transgenic (C57BL/6 TRAMP FVB) F1 (i.e., TRAMP) males. Experimental protocols were reviewed and approved by the animal protocol review committee of the University of Pittsburgh. Fresh prostate tissues procured from the wild-type (controls) and TRAMP mice were placed in liquid nitrogen or immediately placed in -80°C freezer for subsequent protein extraction and enzyme activity assays. The remaining prostate tissue was fixed in 10% formalin for routine histologic processing and paraffin embedding for immunohistochemistry. Enzyme activity was assessed in protein extracts of frozen prostate tissues of wild-type and TRAMP mice at different ages, corresponding to tumor progression in TRAMP. For this study, prostates from wild-type and TRAMP mice at time points of 20 weeks (n = 5), 29 weeks (n = 5), 39 weeks (n = 5), and 49 weeks (n = 3) were used.
Tissue Incubations and High-Performance Liquid Chromatography (HPLC) Analysis
The 12/15-LO enzyme activity analyses were similarly performed as previously described [13]. Briefly, 100 to 200 mg of mouse prostate tissues was individually homogenized in 4 vol of buffer (50 mmol/l Tris with 100 mmol/l NaCl, 100 mol/l CaCl2 pH 7.4, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 µg/ml pepstatin A). Cell homogenates (total protein, 100 µg) were incubated at 30°C for 15 minutes in 600 µl of 50 mM Tris-HCl (pH 7.4) and 5 mM CaCl2 with 25 µM [14C] arachidonic acid (AA) or [14C]LA (1 x 06 cpm, 15 nmol; DuPont-New England Nuclear, Boston,MA) added from stock solutions in ethanol (2.5% of final volume). Fatty acids were isolated from the incubation buffer by a C18-PrepSep solid phase extraction column (Waters, Milford, MA) after acidification to pH 3.5 with acetic acid. The column was then washed with acidified water, and the metabolite was eluted with methanol, evaporated to dryness, and reconstituted with HPLC solvent.
Reverse-phase HPLC analysis was performed using an Ultrasphere ODS column (5 mm; 4.6 x 250 mm; Beckman, Philadelphia, PA). All solvents were of HPLC grade and were from Baker (Phillipsburg, NJ). The solvent system consisted of a methanol/acetic acid/water gradient (80/1/19 vol/vol) at a flow rate of 1.1 ml/min. Radioactivity was monitored using a Flow Scintillation Analyzer (Packard, Palo Alto, CA) with EcoLume (ICN Biochemicals, Costa Mesa, CA) as the liquid scintillation cocktail. UV analysis was performed by monitoring absorbance at 234 nm with a Waters 486 detector. Authentic standards of 12-S-HETE, 15-S-HETE, and 13-S-HODE (Cayman Chemical, Ann Arbor, MI) were simultaneously injected to monitor retention times. Percent (%) conversion of [14C]LA and [14C]AA to their respective products was used to quantify the enzyme activity.
Pathology
The pathology of progressively severe neoplastic lesions in TRAMP, including temporal progression in individual lobes, has been previously described [1,6]. In the current study, histopathologic descriptions (by hematoxylin and eosin as well as immunohistochemistry) of neoplastic lesions for the purpose of reporting immunostaining are in accord with the recently reported pathologic criteria of the National Cancer Institute (NCI) Mouse Models of Human Cancer Consortium (MMHCC) [1]. Lesions previously reported as HGPIN in TRAMP based on degree of nuclear atypia [1] are herein referred to as PIN (with capacity to progress to invasive carcinoma in MMHCC classification scheme, as MMHCC guidelines currently recommend a natural history classification over histologic PIN grading schemes).
Many of the lesions in TRAMP mice previously referred to as well-differentiated adenocarcinoma were regarded by an NCI-sponsored consensus conference as to more likely represent an in situ lesion, and are referred to herein as PIN [1]. Against this more uniform background lesion, architecturally distinct foci of atypical gland formations occasionally extending into periprostatic stroma and having an associated desmoplastic response are herein referred to as adenocarcinoma, in accord with MMHCC guidelines [1]. In sections immunostained in the current series, such foci of more unequivocal adenocarcinoma were uncommon and focal compared to lesions more conservatively regarded as PIN. Such foci generally showed immunostaining similar to PIN, and are thus not separately reported in results (with lesions at this stage of tumor progression referred to as PIN/adenocarcinoma for the purpose of correlation with results of immunostaining). Lesions composed of polypoid proliferations of atypical epithelium and hypercellular stroma with or without atypia and referred to previously as “phyllodes-like” [1] (R. Barrios, N. Greenberg, personal communication) remain to be more completely characterized and are referred to herein as epithelial and stromal hyperplasia, in keeping with recent MMHCC recommendations [1]. Lesions previously reported as moderately and poorly differentiated carcinoma in TRAMP [6,7] were typically seen to coexist in larger unequivocally invasive carcinoma foci [1]. These lesions in TRAMP were regarded by an NCI-sponsored consensus conference as showing morphologic features of neuroendocrine (NE) carcinoma, including small cell carcinoma [1], as confirmed by immunohistochemistry and electron microscopy in other models showing similar histologic and cytologic features. In the current study, such foci are referred to as invasive poorly differentiated carcinoma with NE features of invasive NE carcinoma, in keeping with MMHCC guidelines [1].
12/15-LO Immunohistochemistry
Paraffin-based immunohistochemical staining for the murine leukocyte 12-LO (12/15-LO) was performed as described [26], and was reviewed and classified by the same pathologist (Scott Shappell), who was blinded to the age of the mice. The rabbit polyclonal antibody for murine L12-LO was a generous gift from Dr. Colin Funk (University of Pennsylvania) [23], and was used at a 1:50 dilution with citrate steam antigen retrieval. Known expression in bronchial epithelium was used in mouse lung sections as a positive control. Specificity of the immunostaining has previously been confirmed by performing immunostaining on lung sections of a 12/15-LO (L12-LO) knockout mouse [40]. The extent of immunostaining was assessed semiquantitatively as 0 to 4+ as follows: 0, negative; 1+, rare immunostaining, < 5% of cells in the section; 2+, focal, with immunostaining in 5 to 25 of cells or up to 50% of epithelial cells within 5% to 25% of gland profiles; 3+, immunostaining in 25% to 50% of cells or extensive immunostaining in 25% to 50% of gland profiles; 4+, diffuse, with immunostaining in > 50% of cells or extensive immunostaining in > 50% of gland profiles.
Statistical Analyses
All experimental data are representative of at least five different experiments (animals), except wherever noted. Data are expressed as mean ± standard error (SE), and were compared by one-way ANOVA. The criterion for statistical significance was taken as P < .05.
Results
Enzymatic Activity of 12/15-LO and Conversion of LA and AA in Wild-Type and TRAMP Prostates
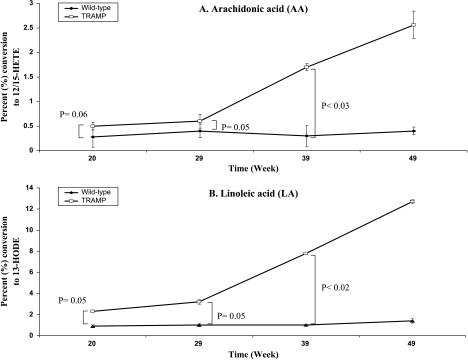
Incubation of wild-type prostates with [14C]LA resulted in the formation of [14C]13-HODE (Figures 1A and 2A), and incubation with [14C]AA resulted in the formation of [14C]12-HETE (a small amount of [14C]15-HETE) (Figures 1B and 2B), as determined by reverse-phase HPLC. LA was converted to one major metabolite, which coeluted with standard 13-S-HODE at ∼20 minutes on reverse-phase HPLC. AA was converted to one major metabolite, which coeluted at ∼18 minutes with standard 12-HETE, as well as a minor metabolite, which coeluted at ∼19 minutes with standard 15-HETE. Although not incompatible with the platelet 12-LO, this enzymatic activity is compatible with the leukocyte type (L) 12-LO or 12/15-LO, which forms 13-HODE from LA [25], and forms much more 12-HETE than 15-HETE from AA [25]. The 12/15-LO activity in the wild-type and TRAMP mouse prostate tissues was assessed by the respective percent (%) metabolite conversion of LA to 13-HODE and of AA to 12/15-HETE, respectively (Figure 3). Although LA was more efficiently metabolized (≈3-fold, averaged for all time points) compared to AA in wild-type mice, the individual percentages for AA and LA conversions did not appear to significantly change at all the different ages analyzed (Figure 3). On the other hand, LA was more efficiently metabolized (≈4.9-fold, averaged for all time points) compared to AA in TRAMP. The individual percentages for AA conversion remained constant at 20 and 29 weeks and increased at 39 and 49 weeks, whereas LA conversions appeared to increase gradually from 20 to 49 weeks (P < .03 for AA and P < .02 for LA at 39 weeks) at all the different ages analyzed (Figure 3).
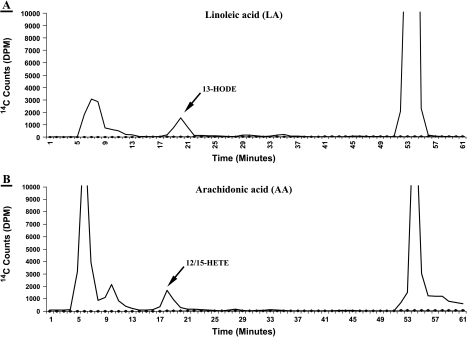
Figure 1.
Representative LA and AA metabolism in wild-type nontransgenic 20-week mouse prostate. [14C]HODE and [14C]12/15-HETE formation in wild-type mouse prostate (control) from 15-minute incubation with 25 µM [14C]LA or [14C]AA. The solvent system consisted of a methanol/water gradient at a flow rate of 1.1 ml/min for reverse-phase HPLC analysis, with in-line radiodetection (Y-axis as disintegrations per minute, or DPM). A prominent 13-HODE peak (panel A) elutes at ≈ 20 minutes, and a mixture of two metabolites coelutes at ∼18 to 19 minutes with standard 12-HETE and 12-HETE, respectively (12/15-HETE peak, panel B) (X-axis as minutes). The unmetabolized LA and AA elutes are the prominent peaks at far right (in both panels A and B) eluting at ≈ 54 and 55 minutes, respectively. Also, a prominent peak of a mixture of prostaglandins (PGs) is seen eluting at ≈ 6 to 7 minutes.
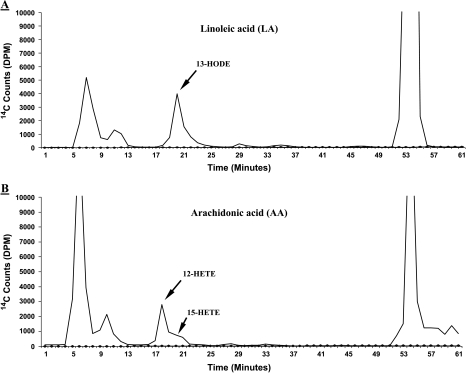
Figure 2.
Representative LA and AA metabolism in 20-week TRAMP mouse prostate. [14C]HODE and [14C]12/15-HETE formation in wild-type mouse prostate (control) from 15-minute incubation with 25 µM [14C]LA or [14C]AA. The solvent system consisted of a methanol/water gradient at a flow rate of 1.1 ml/min for reverse-phase HPLC analysis, with in-line radiodetection ( Y-axis as DPM). A prominent 13-HODE peak (panel A) elutes at ≈20 minutes in a mixture of two metabolites: one major metabolite coelutes at ∼18 minutes with standard 12-HETE, as well as a minor metabolite that coelutes at ∼19 minutes with standard 15-HETE (panel B) (X-axis as minutes). The unmetabolized LA and AA elutes are the prominent peaks at far right (in both panels A and B) eluting at ≈54 and 55 minutes, respectively. Also, a prominent peak of a mixture of prostaglandins (PGs) is seen eluting at ≈ 6 to 7 minutes.
Figure 3.
12/15-LO enzyme activity (% conversion to metabolic product) at different time points [20 weeks (n = 5), 29 weeks (n = 5), 39 weeks (n = 5), and 49 weeks (n = 3)] with AA and LA in wild-type nontransgenic and TRAMP mouse prostates. The values for 12/15-LO are the sum of 12-HETE and 15-HETE, respectively.
Increased Leukocyte 12/15-LO Immunostaining in Aging TRAMP Mice
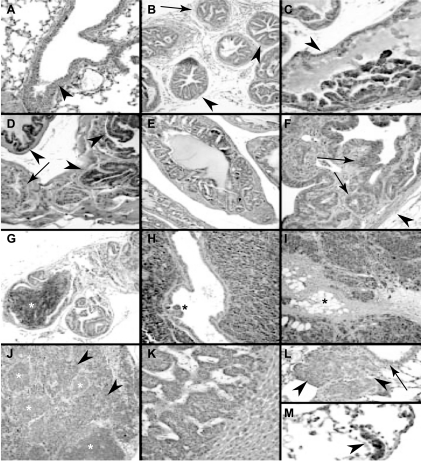
Consistent with the results of LA and AA incubations, immunostaining indicated that the leukocyte 12-LO (12/15-LO) was increased in the neoplastic prostate of TRAMP mice (Figure 4). Similar to previous observations in wild-type mouse prostate [40], no significant 12/15-LO immunostaining was noted in the normal prostate of an 18-week wild-type mouse (not shown). In contrast, focal to extensive 12/15-LO immunostaining was present in the atypical epithelial cells of PIN lesions in three of six TRAMP mice 18 to 29 weeks of age, particularly in that of the oldest (i.e., 29-week TRAMP mouse) (Figure 4). 12/15-LO immunostaining was predominantly cytoplasmic (Figure 4), as also noted previously in human PCa [12] and other GEM mouse lesions [5]. In sections where PIN was focal, increased 12/15-LO immunostaining was particularly correlated with areas of neoplastic involvement (Figure 4). Increased expression of 12/15-LO with tumor progression was suggested by the presence of stronger and more uniform immunostaining in foci of unequivocal invasive, poorly differentiated (NE) carcinoma (Figure 4). Such invasive poorly differentiated foci showed 4+ immunostaining in four of five mice at 18 to 29 weeks, but one mouse without prominent immunostaining in such invasive carcinoma (and in a lung metastasis) showed weaker immunostaining than normal in bronchial epithelium, raising technical concerns regarding antigen preservation (Table 1).
Figure 4.
Representative 12/15-LO immunostaining in TRAMP mice. (A) Positive control, showing normal cytoplasmic immunostaining in the bronchial epithelium in the lung, with clean background in surrounding alveolated parenchyma. (B) Negative immunostaining in prostate ducts with PIN lesions (arrowheads) in 24-week TRAMP mouse. Included are occasional foci (arrowhead) in which distinct smaller gland profiles do not as obviously connect up to the central duct lumen and conform less obviously to the normal duct lining, raising considerations of well-differentiated adenocarcinoma. (C) Strong cytoplasmic 12/15-LO immunostaining in stratified atypical cells in focus of PIN (*) in the prostate of 18-week TRAMP mouse. Residual (but immunonegative) normal-appearing prostate-secretory epithelium is noted (arrowhead). (D) 12/15-LO immunostaining in focal PIN (arrowheads) in the prostate of 29-week TRAMP mouse. Residual normal prostatic epithelium is noted (arrowhead). (E) Strong uniform 12/15-LO immunostaining in focus of PIN of 18-week TRAMP mouse. Although there are small and apparently increased gland spaces, the connection of these to the central normal duct lining, the uniformity of this lesion, and the preservation of the normal surrounding contractile periprostatic stroma support the designation of PIN. Other similarly involved duct/gland profiles with less prominent or absent immunostaining (e.g., bottom left). (F) Strong extensive 12/15-LO immunostaining in atypical epithelium (arrows) of polypoid focus of combined epithelial and stromal hyperplasia (“phyllodes like tumor”) of 29-week TRAMP mouse. Duct lumen is toward top left. Surrounding rim of pre-existing prostatic contractile stroma is at bottom right (arrowhead). (G) Strong, uniform 12/15-LO immunostaining in microscopic focus of invasive, poorly differentiated (NE) carcinoma (white*) in a background of focally but less prominently immunostaining PIN in 29-week TRAMP mouse. (H) Strong, uniform, diffuse 12/15-LO immunostaining in invasive, poorly differentiated (NE) carcinoma in the prostate of 29-week TRAMP mouse. A residual entrapped PIN containing the prostate duct is noted (*). (I) Strong, uniform, diffuse 12/15-LO immunostaining in extensively invading, poorly differentiated (NE) carcinoma in 39-week TRAMP mouse. Tumor focus comprises areas previously referred to as moderately differentiated and compatible with NE carcinoma in the current designation (top right), as well as areas previously referred to as poorly differentiated carcinoma that are morphologically compatible with small cell carcinoma (bottom left), showing focal “crush” artifact typical of such tumors. Tumor massively infiltrates surrounding pelvic soft tissues (adipose tissue noted*). (J) Strong, uniform 12/15-LO immunostaining in metastatic poorly differentiated (NE) carcinoma (arrowheads) in retroperitoneal lymph node from 39-week TRAMP mouse. Immunonegative residual normal lymphoid germinal centers are noted (white*). (K) 12/15-LO immunonegative liver metastasis in 39-week TRAMP mouse. Benign liver parenchyma is seen at bottom right. (L) 12/15-LO negative lung metastasis of poorly differentiated (NE) carcinoma (arrowheads) in 20-week TRAMP mouse. Note moderately strong positive control immunostaining in adjacent normal bronchial epithelium (arrow). (M) Strong 12/15-LO immunostaining in lung micrometastasis (arrowhead) in 29-week TRAMP mouse.
Table 1.
Immunohistochemistry with 12-LO Antibody, and Pathologic Description in Prostates of Aging TRAMP Mice.
| Mouse Age (weeks) | PIN | Epithelial Stromal Hyperplasia | Invasive Poorly Differentiated (NE) Carcinoma | Lymph Node Metastases | Liver Metastases | Lung Metastases |
| 18 | 0 | 0 | -* | - | - | - |
| 18 | 1–2+† | - | - | - | - | - |
| 18 | 1+ | - | 4+ | - | - | - |
| 20 | 0-1+ | - | 0 | - | - | 0‡ |
| 22 | - | - | 4+ | 4+ | - | - |
| 24 | 0 | - | 4+ | - | - | - |
| 29 | 3+ | 3+ | 4+ | 4+ | 2+§ | 4+ |
| 39 | - | 0 | - | - | 0¶ | 2+# |
(+) Positive 12-LO staining; (-) either negative 12-LO staining, or staining is not observed.
Not present in sections examined, thus was not evaluated for mmunostaining.
Ranges reflect difference in separate prostatic lobes examined.
Weaker than usual immunostaining of internal control benign bronchial epithelium in section with lung metastasis.
Foci consistent with focally positive liver micrometastases.
Four metastatic foci, all negative.
Seven of nine essentially negative micrometastases; two were weekly positive.
This poorly differentiated carcinoma with NE features [1] is the lesion most correlated with metastatic potential in TRAMP [6,7]. Similar to the 12/15-LO immunostaining in such invasive foci in the prostate, lymph node metastases with the same NE carcinoma morphology showed uniform, diffuse, strong 12/15-LO immunostaining in two of two TRAMP animals examined, one of which also showed similarly strong expression in lung metastases. However, 12/15-LO immunostaining was weaker and somewhat more variable in liver metastases (Table 1, Figure 4). In summary, compared to wild-type prostate, 12/15-LO is increased in the neoplastic epithelium of PIN in TRAMP mice, and even more profoundly increased in the invasive and metastatic poorly differentiated carcinoma.
Discussion
LA, followed by AA, is the major component of high-fat western diet [24]. These dietary lipids are metabolized in humans by cyclooxygenases (COX-1 and/or COX-2) or lipoxygenases (5-LO, 12-LO, 15-LO-1, and 15-LO-2). The mouse, on the other hand, has three different 12S-lipoxygenases: 1) an epidermal type (E12-LO); 2) a platelet type (P12-LO) that is homologous to human platelet type 12-LO; and 3) a leukocyte type (L12-LO or 12/15-LO) homologous to human 15-LO-1 [25,26]. We previously demonstrated that the fatty acid LA metabolizing 15-LO-1 enzyme is upregulated in human PCa compared to benign prostate [13]. In vitro studies and studies with 15-LO-1 overexpressing the cell line PC-3 in nude mice indicate that 15-LO-1 can contribute to PCa through multiple mechanisms, including increased cell proliferation and promotion of angiogenesis [14].
Although altered lipid metabolism by lipoxygenases and cyclooxygenases has particularly focused exclusively on the role of AA in human PCa development and progression, there is now a new growing interest in the possible role of LA, especially since our discovery that the LA-metabolizing 15-LO-1 enzyme is high in human PCa. However, determining the role of one or more candidate genes in PCa development and progression and their suitability as candidate targets for therapeutic intervention is complicated by issues such as the difficulty in obtaining appropriate tissues from the same patients over the long time course of PCa progression, and the necessary duration and other complexities of conducting clinical trials. GEM models validated for potential relevance to at least some subsets of human PCa patients are potentially extremely useful reagents used to study delineating etiologic pathways in PCa development and progression, and to test for effective treatments that can finally be exploited for human applications. However, the meaningful use of such models for translating results to human PCa will definitely require cautious and careful validation—work that is now ongoing in multiple laboratories. Validation criteria include comparison of histopathologic, genetic, biochemical, and natural history features of GEM models compared to known aspects of human PCa, including relevant changes at different stages of progression of this complex and heterogeneous neoplasm [1,3,42,43].
The widely used TRAMP model was developed by placing the expression of the SV40 early genes under the temporally and spatially restricted prostate-specific control of the minimal rat probasin promoter [6]. The prostate epithelial expression of T antigen sequesters p53 and pRB, which have been implicated in human PCa, includes early, lowstage PCa and subsets of HGPIN, as well as advanced PCa [1,6]. PCa development and progression in TRAMP mice mimic certain key aspects of the natural history of the human clinical disease. In TRAMP, as early as 6 to 12 weeks of age, the prostate glands display mild to severe hyperplasia of the prostate epithelium, resembling PIN, and by 30 weeks of age, 100% of TRAMP mice display primary and metastatic PCa [1,6]. In the current study, we demonstrate that similar to human PCa, the development and progression of PCa in TRAMP are accompanied by increased expression of the murine 12/15-LO homologue of the lipid-metabolizing enzyme, 15-LO-1. Given the multiple potential mechanisms whereby this attractive pharmacologic target may contribute to PCa [13,14], these results further support the potential use of GEM in elucidating the role of LA- and AA-metabolizing enzymes in PCa development and progression, and specifically the use of TRAMP to further determine the etiologic contribution of the 12/15-LO to PCa progression. This is further significant, in part, because pharmacologic inhibitors and other strategies for reducing 12/15-LO (15-LO-1) exist, and because the TRAMP model has a reproducible natural history that allows for histopathologic and other relevant natural history parameters to be readily employed in intervention studies [2,3,4,7].
Kasper et al. [8] recently showed that PIN and PCa development in the LPB-Tag GEM model was accompanied by increased expression of the murine 12/15-LO or leukocyte 12-LO [40]. LPB-Tag prostates made substantially more 12-HETE from AA than wild-type prostates, with enzymatic properties typical of the 12/15-LO (leukocyte 12-LO), and mRNA and immunostaining showed increased expression of the leukocyte 12-LO, but not the platelet 12-LO. The 12/15-LO was increased in PIN, and invasive and metastatic carcinoma [40]. This murine enzyme, demonstrated to be increased in TRAMP tumors in the current study, differs from the human 15-LO-1 by apparently making much more 12-HETE than 15-HETE from AA [40]. In contrast to putative tumor-suppressive properties of 15-HETE in the human prostate [18,36,37], 12-HETE has been shown to possibly contribute to PCa progression, including by promotion of angiogenesis [11]. Although 15-LO-2 is found in the normal human prostate, its mouse ortholog, 8-LO, is absent in the normal mouse prostate [25,40]. Interestingly, the most preferred substrate for 15-LO-2 in humans and 8-LO in the mice is AA versus LA [40].
Thus, it is now becoming increasingly clear that that LA, and not AA, plays a major role in the process of carcinogenesis particularly in humans, wherein alterations in the pro-carcinogeniclipoxygenases (i.e., 15-LO-1) and anti-carcinogenic lipoxygenases (i.e., 15-LO-2) could contribute to PCa [13,14,15,37]. Therefore, it is reasonable to conclude that the overexpression of 12/15-LO in GEM models of PCa, and 15-LO-1 in human PCa, in conjunction with LA-rich fat diet, can cause prostate tumorigenesis.
Although 12/15-LO-derived 12-HETE could contribute to PCa development and progression in TRAMP, recent studies showing that the preferred substrate for 15-LO-1 is LA rather than AA [13,22] suggest the need for increased attention on the possible pro-neoplastic role of 15-LO-1 metabolism of LA to 13-HODE. Similar to the human 15-LO-1, the murine 12/15-LO makes 13-HODE from LA [22]. To our knowledge, our current study is the first to demonstrate the increased capacity for 13-HODE synthesis in a neoplastic GEM versus wild-type mouse prostate. Multiple possible mechanisms have been proposed for the contribution of (human) 15-LO-1 and 13-HODE to tumor progression, including promotion of tumor growth and spread by induction of angiogenesis through increased vascular endothelial growth factor [13] and upregulation of insulin-like growth factor receptor (IGF-1R) activated by the mitogenactivated protein kinase pathway [44]. Studies have also suggested a pro-neoplastic effect of 15-LO-1-derived 13-HODE (but not the 15-LO-2-derived AA metabolite, 15-HETE) through downregulation of PPARγ by interaction with epidermal growth factor (EGF) and phosphorylation of PPARγ mediated by activated mitogen-activated protein kinase [36]. Hence, the results of our study suggest that GEM models of PCa and the TRAMP model specifically may be useful for delineating how dietary lipids implicated in human PCa and their metabolism by 15-LO-1 can contribute to PCa development and progression.
One long-term goal of these and related studies is to provide a mechanism that explains the observation that diets high in fat and aberrant expression of 15-LO-1 are associated with an increased risk of PCa. Although our studies do not exclude a role for E12-LO and P12-LO in the increased AA-derived 12-HETE, the results of our enzyme and immunohistochemical studies certainly demonstrate the increased expression of the 12/15-LO and its ability to form LA-derived 13-HODE. Although altered lipid metabolism by LOs and COXs has particularly focused on the role of AA in human PCa development and progression, there is a new growing interest in the possible role of LA metabolism in PCa, especially since our discovery of high levels of 15-LO-1 associated with PCa [13], followed by the biologic role [44] and evidence of its pro-proliferative role in vivo using a human PCa cell line [14]. In this regard, it is extremely interesting that this gene is increased in two different GEM models of PCa: the LPB-Tag model [40] and TRAMP model (this study). In both models, 12/15-LO appears to be increased in PIN, and invasive and metastatic carcinoma. This is potentially significant, at least in part, as these are two fundamentally different models [1]. Likely as a consequence of these fundamental model differences, the GEM models develop distinct phenotypes and natural histories. Hence, that the 12/15-LO homologue of the human 15-LO-1 is increased with PIN and invasive and metastatic PCa in both models strongly suggests that it may contribute fundamentally to PCa development and progression.
In summary, our initial studies characterizing LA and AA metabolism in the TRAMP mouse have demonstrated an increased expression of 12/15-LO in TRAMP PCa development, similar to that noted in human PCa. Regardless of the precise relationship between 15-LO-1 overexpression in HGPIN and/or invasive PCa in human PCa and in PIN and PCa in the TRAMP model, the increased expression of 12/15-LO in this model will allow for additional elucidation of the mechanisms whereby this enzyme and its metabolites may contribute to tumor development and progression. Given the availability of inhibitors and/or knockout mice for this specific gene, GEM models may be useful for elucidating the contribution of 15-LO-1 to ascertain its role in human PCa and its suitability as a target for therapy or chemoprevention.
Acknowledgements
We sincerely thank Colin Funk for his generous gift of 12/15-LO polyclonal antibody, and Beth Pflug for providing the frozen prostate tissues from the TRAMP mice.
Abbreviations
- HRP
horseradish peroxidase
- 13-S-HODE
13-S-hydroxyoctadecadienoic acid
- 15-LO
15-lipoxygenase
- COX
cyclooxygenase
- LA
linoleic acid
- AA
arachidonic acid
- IGF
insulin-like growth factor
- PBS
phosphate-buffered saline
- PC-3
prostate cancer cell line-3
- HGPIN
high-grade prostatic intraepithelial neoplasia
- PCa
prostate carcinoma
- LO
lipoxygenase
- 12/15
leukocyte type 12
- P-12
platelet type 12
- HETE
hydroxyeicosatetraenoic acid
- GEM
genetically engineered mouse
- TRAMP
TRansgenic Adenocarcinoma of Mouse Prostate
- LPB-Tag
long probasin promoter SV40-large T antigen
- PIN
prostatic intraepithelial neoplasia
- PG
prostaglandin
- p53
tumor protein 53
- Rb
retinoblastoma
- NE
neuroendocrine
- MMHCC
Mouse Models of Human Cancer Consortium
- HPLC
highperformance liquid chromatography
- IHC
immunohistochemistry
Footnotes
Grant funding from both the National Institutes of Health (R21-CA098657) and the American Cancer Society (RSG-03-022-01) to U.P.K. supported this work.
References
- 1.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechter WJ, Leipold DD, Murray ED, Jr, Quiggle D, McCracken JD, Barrios RS, Greenberg NM. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203–2208. [PubMed] [Google Scholar]
- 4.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–67782. [PubMed] [Google Scholar]
- 5.Abate-Shen C, Shen MM. Mouse models of prostate carcinogenesis. Trends Genet. 2002;18:S1–S5. doi: 10.1016/s0168-9525(02)02683-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 8.Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ. Development, progression, and androgen-dependence of prostate tumors in probasinlarge T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:319–333. [PubMed] [Google Scholar]
- 9.Masumori N, Tsuchiya K, Tu WH, Lee C, Kasper S, Tsukamoto T, Shappell SB, Matusik RJ. An allograft model of androgen independent prostatic neuroendocrine carcinoma derived from a large probasin promoter-T antigen transgenic mouse line. J Urol. 2004;171:439–442. doi: 10.1097/01.ju.0000099826.63103.94. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Grignon DJ, Chbihi T, Zacharek A, Chen YQ, Sakr W, Porter AT, Crissman JD, Pontes JE, Powell IJ, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 12.Spindler SA, Sarkar FH, Sakr WA, Blackburn ML, Bull AW, LaGattuta M, Reddy RG. Production of 13-hydroxyoctadecadienoic acid (13-HODE) by prostate tumors and cell lines. Biochem Biophys Res Commun. 1997;239:775–781. doi: 10.1006/bbrc.1997.7471. [DOI] [PubMed] [Google Scholar]
- 13.Kelavkar UP, Cohen C, Kamitani H, Eling TE, Badr KF. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis. 2000;21:1777–1787. doi: 10.1093/carcin/21.10.1777. [DOI] [PubMed] [Google Scholar]
- 14.Kelavkar UP, Nixon J, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 (15-LO-1) in PC-3 human prostate cancer cells causes aggressive tumorigenesis. Carcinogenesis. 2001;22:1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 15.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-Lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;155:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shappell SB, Manning S, Boeglin WE, Guan YF, Roberts RL, Davis L, Olson SJ, Jack GS, Coffey CS, Wheeler TM, Breyer MD, Brash AR. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack GS, Brash AR, Olson SJ, Manning S, Coffey CS, Smith JA, Jr, Shappell SB. Reduced 15-lipoxygenase-2 immunostaining in prostate adenocarcinoma: correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum Pathol. 2000;31:1146–1154. doi: 10.1053/hupa.2000.16670. [DOI] [PubMed] [Google Scholar]
- 18.Tang S, Bhatia B, Maldonado CJ, Yang P, Newman RA, Liu J, Chandra D, Traag J, Klein RD, Fischer SM, Chopra D, Shen J, Zhau H, Chung LW, Tang DG. Evidence that Arachidonate 15-lipoxygenase 2 is a negative cell-cycle regulator in normal prostate epithelial cells. J Biol Chem. 2002;277:16189–16201. doi: 10.1074/jbc.M111936200. [DOI] [PubMed] [Google Scholar]
- 19.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 21.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 22.Dunn JE. Cancer epidemiology in populations of the United States—with emphasis on Hawaii and California—and Japan. Cancer Res. 1975;35:3240–3245. [PubMed] [Google Scholar]
- 23.Haenszel W, Kurihara M. Studies of Japanese migrants: I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 24.Fleshner N, Bagnell PS, Klotz L, Venkateswaran V. Dietary fat and prostate cancer. J Urol. 2004;171:S19–S24. doi: 10.1097/01.ju.0000107838.33623.19. [DOI] [PubMed] [Google Scholar]
- 25.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 26.Chen X-S, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J Biol Chem. 1994;269:13979–13987. [PubMed] [Google Scholar]
- 27.Reddy N, Everhart A, Eling T, Glasgow W. Characterization of a 15-lipoxygenase in human breast carcinoma BT-20 cells stimulation of 13-HODE formation by TGF alpha/EGF. Biochem Biophys Res Commun. 1997;231:111–116. doi: 10.1006/bbrc.1997.6048. [DOI] [PubMed] [Google Scholar]
- 28.Bertomeu MC, Gallo S, Lauri D, Haas TA, Orr FW, Bastida E, Buchanan MR. Interleukin 1-induced cancer cell/endothelial cell adhesion in vitro and its relationship to metastasis in vivo role of vessel wall 13-HODE synthesis and integrin expression. Clin Exp Metastasis. 1993;11:243–250. doi: 10.1007/BF00121167. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan MR, Horsewood P, Brister SJ. Regulation of endothelial cell and platelet receptor-ligand binding by the 12- and 15-lipoxygenase monohydroxides. 12-15-HETE and 13-HODE. Prostaglandins Leukot Essent Fat Acids. 1998;58:339–346. doi: 10.1016/s0952-3278(98)90069-2. [DOI] [PubMed] [Google Scholar]
- 30.Ikawa H, Kamitani H, Calvo BF, Foley JF, Eling TE. Expression of 15-lipoxygenase-1 in human colorectal cancer. Cancer Res. 1999;59:360–366. [PubMed] [Google Scholar]
- 31.Kamitani H, Geller M, Eling T. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569–21577. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan R, Nadler J. Role of lipoxygenases in breast cancer. Front Biosci. 1998;3:E81–E88. doi: 10.2741/a369. [DOI] [PubMed] [Google Scholar]
- 33.Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429–1434. [PubMed] [Google Scholar]
- 34.Zock PL, Katan MB. Linoleic acid intake and cancer risk a review and meta-analysis. Am J Clin Nutr. 1998;68:142–153. doi: 10.1093/ajcn/68.1.142. [DOI] [PubMed] [Google Scholar]
- 35.Kelavkar U, Badr K. Effects of mutant p53 expression on human 15-lipoxygenase-promoter activity and murine 12/15-lipoxygenase gene expression: evidence that 15-lipoxygenase is a mutator gene. Proc Natl Acad Sci USA. 1999;96:4378–4783. doi: 10.1073/pnas.96.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsi LC, Wilson L, Nixon J, Eling TE. 15-Lipoxygenase-1 metabolites down-regulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J Biol Chem. 2001;276:34545–34552. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- 37.Shappell SB, Gupta RA, Manning S, Whitehead R, Boeglin WE, Schneider C, Case T, Price J, Jack GS, Wheeler TM, Matusik RJ, Brash AR, Dubois RN. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- 38.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate: alteration in PPARgamma. J Biol Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 39.Shureiqi I, Wojno KJ, Poore JA, Reddy RG, Moussalli MJ, Spindler SA, Greenson JK, Normolle D, Hasan AK, Lawrence TS, Brenner DE. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis. 1999;20:1985–1995. doi: 10.1093/carcin/20.10.1985. [DOI] [PubMed] [Google Scholar]
- 40.Shappell SB, Olson SJ, Hannah SE, Manning S, Roberts RL, Masumori N, Jisaka M, Boeglin WE, Vader V, Dave DS, Shook MF, Thomas TZ, Funk CD, Brash AR, Matusik RJ. Elevated expression of 12/15-lipoxygenase and cyclooxygenase-2 in a transgenic mouse model of prostate carcinoma. Cancer Res. 2003;63:2256–2267. [PubMed] [Google Scholar]
- 41.Jisaka M, Kim RB, Boeglin WE, Nanney LB, Brash AR. Molecular cloning and functional expression of a phorbol ester-inducible 8S-lipoxygenase from mouse skin. J Biol Chem. 1997;272:24410–24416. doi: 10.1074/jbc.272.39.24410. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the TRansgenic Adenocarcinoma of Mouse Prostate (TRAMP) model. Cancer Res. 1999;59:2203–2209. [PubMed] [Google Scholar]
- 43.Ruijter E, Van De Kaa C, Miller G, Ruiter D, DeBruyne F, Schalken J. Molecular genetics and epidemiology of prostate carcinoma. Endocrinol Rev. 1999;20:22–45. doi: 10.1210/edrv.20.1.0356. [DOI] [PubMed] [Google Scholar]
- 44.Kelavkar U, Cohen C. 15-Lipoxygenase-1 expression upregulates and activates insulin-like growth factor-1 receptor in prostate cancer cells. Neoplasia. 2004;6:41–52. doi: 10.1016/s1476-5586(04)80052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]