Abstract
Microtubule inhibitor-induced Bcl2 phosphorylation is detrimental to its antiapoptotic function. Phosphorylation of Bcl2 predominantly occurs on two serine residues (70 and 87) in cells arrested at G2-M phase by microtubule disarraying agents. Phospho Bcl2 can associate with a cis-trans peptidyl prolyl isomerase, Pin1. Pin1 and its homologues are known to target the proline residue carboxyl terminal to the phosphorylated threonine or serine residue of mitotic phosphoproteins, such as Bcl2. However, it was not clear how an extranuclear protein could associate with nuclear Pin1. The confocal images of the immunofluorescence studies employing phospho Bcl2-specific antibody developed in the laboratory demonstrated the translocation of phospho Bcl2 inside the nucleus. Interestingly, proteasomal degradation of Pin1 facilitates dephosphorylation of phospho Bcl2 due to longer exposure of Taxol. Here we show for the first time that proteasomal degradation of Pin1 is the key factor to determine the fate of phosphoforms of Bcl2. When Pin1 is degraded by proteasomes, phospho Bcl2 is converted to its native form. Thus, transient conformational change of Bcl2 due to association with peptidyl prolyl isomerase can contribute to irreversible apoptotic signaling.
Keywords: apoptosis, Bcl2, phosphorylation, Pin1, proteasomes
Introduction
Apoptosis is an intrinsic cell death program necessary for development and tissue homeostasis within all multicellular organisms [1–4]. The induction of the cell death program can be helpful for evading the potential malignant outcome of genomic damage. On the contrary, the deregulated expression of a family of antiapoptotic oncogenes such as Bcl2 can be a substantial factor for the development of human malignancies [5,6]. The family of these oncoproteins is comprised of both pro- and antiapoptotic members and their ratio critically determines the apoptotic threshold [1,2,7].
In recent years, studies on the posttranslational modifications of these proteins have revealed some interesting facts. In the presence of survival factor IL-3, a proapoptotic protein, BAD loses its biologic function by phosphorylation on two different serine residues [8]. The pioneer of this family, the antiapoptotic member Bcl2, is phosphorylated and is inactivated by microtubule disarraying agents or in the presence of phosphatase inhibitors [9–24]. The ASK1/Jun N-Terminal protein kinase pathway phosphorylates Bcl2 during cell cycle progression as a normal physiologic process to inactivate Bcl2 at G2-M phase [10,11,13,15,20].
Intracellular signal transduction pathways play a crucial role in a variety of cellular processes such as differentiation, proliferation, or apoptosis, and the reversible phosphorylation of their components is a major regulatory mechanism used to control their activities. Bcl2, a 26-kDa integral membrane oncoprotein contains a membrane-anchoring domain near its carboxyl terminus that causes its insertion into intracellular membranes of mitochondria and other organelles such as ER [25–27]. Microtubule-damaging drug-induced phosphorylation of Bcl2 at G2-M phase predominantly occurs on two serine residues: serine 70 and 87 and possibly to a small extent on threonine 69. All of these phosphorylation sites have a consensus motif consisting of serine/threonine residue followed by proline. Phospho Bcl2 can associate with a cis-trans peptidyl prolyl isomerase Pin1 [28]. Pin1 and its homologues [29–31] can target the proline residue carboxyl terminal to the phosphorylated threonine or serine residue of mitotic phosphoproteins. However, it was not clear why and how an extranuclear protein like Bcl2 could associate with nuclear Pin1.
Here we show that phospho Bcl2 is translocated inside the nucleus and that its association with Pin1 can facilitate dephosphorylation of phospho Bcl2 in a proteasome-dependent manner [32–34]. Apparently, the association of Pin1 with phospho Bcl2 can modulate its antiapoptotic function by inducing a transient conformational change. Subsequent proteasomal degradation of Pin1 destabilizes Pin1/P-Bcl2 association, which may result in the enhanced access of phosphatase for phospho Bcl2, thereby converting it to its native form.
Materials and Methods
Reagents
Mouse monoclonal antibodies against Bcl2 and PARP were available from Upstate Biotechnology (Lake Placid,NY) and Pharmingen (San Diego, CA), respectively. Proteasome inhibitors MG132, lactacystin, or PSI were purchased from Calbiochem (San Diego, CA). Taxol was obtained from Sigma Chemical (St. Louis, MO). ECL detection reagent was the product of Amersham Life Sciences (Piscataway, NJ). All other reagents were of molecular biology grade. Polyclonal antibody against Pin1 was obtained from Oncogene Research Products (Cambridge, MA).
Cells and Culture Conditions
Human prostate cancer cells PC-3 were maintained in minimum essential medium (MEM) supplemented with 10% FBS and 50 µg/ml gentamicin. Pre B leukemic cells 697 were grown in RPMI supplemented with 10% FBS and 50 µg/ml gentamicin.
Stable transfection of wild-type Bcl2 Wild-type Bcl2 cDNA cloned into pcDNA3 was stably transfected in PC-3 cells using calcium phosphate coprecipitation method as described before [35,36]. Cells were allowed to grow for 48 hours for the expression of the resistance gene. Subsequently, cells were propagated in selective medium containing 1 mg/ml geneticin until clones appear. The positive clones were identified by immunoblot using monoclonal antibody against human Bcl2. Among the selected clones, W-34 cells that overexpressed Bcl2 protein several fold excess compared to the parental PC-3 cells were used for further investigation.
Assessment of Bcl2 Phosphorylation or Pin1 Level
Cells were treated with specified concentrations of Taxol or proteasome inhibitors (freshly prepared) for varying periods of time in a 5% CO2 incubator. Equal amounts of protein extracted from cells were subjected to SDS-PAGE followed by electrophoretic transfer onto nitrocellulose membranes. Transferred proteins on nitrocellulose membranes were routinely visualized by Ponceau S staining to check equal loading and further processed for immunoblotting using monoclonal antibody against Bcl2 or rabbit polyclonal antibody against Pin1. The phosphorylated forms of Bcl2 protein were detected as slower migrating forms as described previously [9–24]. The slower migrating forms of Bcl2 were shown to be phosphorylated forms either by λ-phosphatase treatment or P32 labeling previously by others and by our laboratory [9–24].
Assessment of Apoptosis
Apoptosis was determined by DAPI staining and PARP cleavage. For DAPI staining, control and treated cells were fixed and permeabilized with 0.5% Tween 20. The slides containing the cells were mounted with a fluid containing 2 µg/ml DAPI to stain nuclear DNA. A Nikon Eclipse E600 fluorescence microscope (Columbia, MD) was used for fluorescence microscopy. For PARP degradation, the cells were harvested following the scheduled exposure to the designated concentration of paclitaxel. Equivalent amounts of protein from each sample were electrophoresed on 5% to 15% gradient SDS-PAGE, transferred to membranes, and followed by immunoblotting with monoclonal antibody against PARP.
Cell Sorting by Flow Cytometry
Cells following designated drug treatment were stained with Hoechst 33342 (Sigma) at a concentration of 15 µg/ml for 1 hour at 37°C. 3,3′-Dipentyloxacarbocyanine iodide (Molecular Probes, Eugene, OR) was added at a concentration of 0.2 µg/ml at the same time with Hoechst stain to increase resolution of DNA distribution. Cells were sorted by a fluorescence activated cell sorter at Ireland Cancer Center's core facility.
Development of Phospho Bcl2-Specific Antibody
Serine-70 residue of Bcl2 protein is the evolutionarily conserved phosphorylation site [15]. To develop phospho Bcl2-specific antibody the 15-mer Bcl2 peptide, DPVARTS* PLQTPAAP (S* indicates phosphorylated serine-70 residue), was synthesized. HPLC-purified peptide was coupled to KLH (Keyhole limpet hemocyanin) by the glutaraldehyde method [37]. The peptide conjugate was injected into rabbits to raise polyclonal antibody using Freund's adjuvant. Blood was collected before the first injection to be used as preimmune sera. Following the third injection, antibody titer was tested by Western blot. Serum fractionated from whole blood of the immune animals was used throughout these studies. The specificity of the antibody was tested by blocking with preincubation of the respective peptide.
Immunofluorescence Microscopy
Control and Taxol-treated cells were fixed in chilled methanol/acetone (1:1) for 10 minutes at room temperature and permeabilized with 0.5% Tween 20 in PBS for 5 minutes. Permeabilized cells were blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in PBS for 30 minutes at room temperature. Both groups of cells were then incubated with 1:100 dilution of phosphospecific rabbit Bcl2 polyclonal antiserum or mouse monoclonal Bcl2 antibody at room temperature for 2 hours. Following brief washes with PBS containing 1% BSA and 0.1% Tween 20, slides were incubated with fluorescein-conjugated goat anti-rabbit IgG (Boehringer Mannheim, Indianapolis, IN) or CY3-conjugated anti-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) at a 1:200 dilution for 30 minutes at room temperature. Subsequently, slides were washed and mounted with a fluid containing DAPI (Vector Laboratories) to visualize the nuclear stain [23]. A Nikon Eclipse E600 fluorescence microscope was used for fluorescence microscopy. The computerized merged images were taken using “SPOT RT” software program. For confocal microscopy, 20 nM TO-PRO (Molecular Probes) was used for nuclear staining. Images were captured on a Leica SP2 confocal microscope at the Institute's core facility.
Results
Phospho Bcl2 Is Dephosphorylated due to Longer Exposure of Taxol
Previous studies performed by us in regard to Bcl2 phosphorylation were limited to 16 to 24 hours exposure of Taxol. Initially, we were interested in testing the effect of Taxol on the phosphorylation status of Bcl2 for longer periods of treatment. Parental PC-3 or W-34 cells (PC-3 ectopically overexpressed with wild-type Bcl2) were challenged with 1 µM Taxol for different time periods (8 to 72 hours). As evident in Figure 1, A and B, the extent of Bcl2 phosphorylation reaches a peak at 16 hours (Figure 1, A, lane 3and B, lane 4) whereas longer periods of exposure of Taxol such as 48 or 72 hours lead to the decline of phosphoforms of Bcl2 (Figure 1, A, lanes 5 and 6 and B, lanes 8 and 10). Of note, in endogenously low Bcl2-expressing PC-3 cells only one phosphorylated form is detectable. However, when it is ectopically overexpressed in the same cells (W-34 clone), additional hyperphosphorylated forms of Bcl2 are evident following the same period of Taxol exposure. Bcl2 gets predominantly phosphorylated on Serine 70 residue following treatment with microtubule damaging agents. Phosphorylation on other weak sites is visible only when it is overexpressed, as in the case of W-34 cells.
Figure 1.
Phospho Bcl2 is dephosphorylated due to longer exposure of Taxol. PC-3 (panel A) or W-34 (panel B) cells were challenged with 1 µM Taxol for indicated periods. Total protein extracts were immunoblotted using monoclonal antibody against Bcl2. The slower mobility forms of Bcl2 indicate phosphorylated Bcl2. It is worth mentioning that whereas only 25 µg protein was loaded in the case of W-34 cells, PC-3 cells required loading of 250 µg protein to visualize the phosphorylated form of Bcl2. Panel C: the extent of Taxol-induced apoptosis determined by DAPI staining of nuclei [23,26]. PC-3 cells treated with 1 µM Taxol for indicated periods were subjected to nuclear staining with DAPI (2 µg/ml). Approximately 150 cells were scored for each data point. All results are exhibited as means±SD of three experiments. Panel D: PARP degradation by Western blot of Taxol-treated PC-3 cells [15,20]. Note that the intensity of 85-kDa PARP cleavage product increased with the longer period of exposure to Taxol.
Bcl2 is phosphorylated by ASK1/Jun N-terminal protein kinase pathway that is normally activated at G2-M phase of the cell cycle [10]. However, the phospho Bcl2 cannot be sustained in G2-M-arrested cells, but apoptosis continues even if dephosphorylated native Bcl2 returns after longer exposure of Taxol. As evident in Figure 1, C and D, 16-hour exposure of Taxol to PC-3 cells can lead to significantly less DNA fragmentation and PARP cleavage than that of 48-hour exposure. Our data are in concordance with the previous observation [38]. Apparently, dephosphorylation of Bcl2 cannot cause the reversion of cells from the executioner stage of apoptosis.
Phospho Bcl2 and Pin1 Decline at the Same Period of Taxol Exposure and Proteasome Inhibitors Can Stabilize This Decline
Recent observation implicates the association of phospho Bcl2 with Pin1 in M phase arrested cells [28]. Pin1-induced cis-trans isomerization of the peptide bond at the amino side of proline residue develops tangible structural changes in the target protein [29–31]. These conformational changes could alter protein stability or cellular architecture. We asked whether the association of Pin1 is the key mechanism to sustain phosphoforms of Bcl2. Thus, we were interested in testing the status of Pin1 when phospho Bcl2 disappears. The decline of Pin1 was evident following 48-hour Taxol treatment (Figure 2A, lane 3). This event led us to think that their concomitant decline might be interdependent. We considered that perhaps the downregulation of Pin1 is proteasome mediated [32–34]. Interestingly, cell permeable proteasome inhibitor MG132 can prevent this decrease of Pin1 in Taxol-arrested mitotic cells (Figure 2A, lanes 4 and 6). Similarly, the disappearance of phospho Bcl2 can be blocked under conditions when proteasomes are not in the activated state (Figure 2B).
Figure 2.
Phospho Bcl2 and Pin1 decline at the same period of Taxol exposure and proteasome inhibitors can stabilize this decline. Panel A: Peptidyl prolyl isomerase Pin1 is also down regulated at 48 hours exposure of Taxol and proteasome inhibitor MG132 can prevent this decline. W-34 cells were exposed to 1 µM Taxol in the presence or absence of proteasome inhibitor MG132 (20 µM). Total protein extracts were subjected to Western blot using rabbit polyclonal antibody against Pin1. Lane 1: Vehicle solvent for 24 hours; lane 2: 24 hours Taxol; lane 3: 48 hours Taxol; lanes 4 and 6: 48 hours Taxol and last 24 hours MG132; lane 5:24 hours MG132 alone. Panel B: Proteasome inhibitor MG132 can also stabilize the decline of phospho Bcl2 under identical conditions. Lane 1: Vehicle solvent for 16 hours; lane 2: 16 hours Taxol; lane 3: vehicle solvent for 24 hours; lane 4: 24 hours Taxol; lane 5: coincubation of MG132 and Taxol for 24 hours; lane 6: 24 hours Taxol and last 16 hours MG132; lane 7: 24 hours vehicle solvent and last 16 hours MG132; lane 8: 24 hours vehicle solvent and MG132; lane 9: vehicle solvent for 48 hours; lane 10: 48 hours Taxol; lane 11: 48 hours Taxol and last 24 hours MG132; lane 12: 48 hours Taxol and last 16 hours MG132; lane 13: 48 hours vehicle solvent and last 24 hours MG132; lane 14: 48 hours vehicle solvent and last 16 hours MG132.
The data presented in Figure 2B clearly documents that the gradual decline of phospho Bcl2 in prostate cancer cells can be prevented in the presence of 20 µM MG132 (Figure 2B, lanes 11and 12). Nevertheless, in all cases, pretreatment of Taxol was always necessary before the addition of MG132. Usually, 16 to 24 hours of Taxol treatment leads to peak accumulation of phospho Bcl2, but no accumulation of phospho Bcl2 was evident if MG132 is added together (Figure 2B, lane 5). Some accumulation of phospho Bcl2 is noted if MG132 is added after 8 hours of Taxol treatment (Figure 2B, lane 6). As shown in Figure 2B (lane 10), no or little accumulation of phosphoforms of Bcl2 is noted due to 48 hours of Taxol exposure. Interestingly, if we add MG132 for the last 24 and 16 hours of the total 48-hour incubation period with Taxol, phospho Bcl2 can be stabilized to a different extent (Figure 2B, lanes 11 and 12). Of note, incubation of MG132 during the last 24 hours can accumulate more than that of 16 hours (Figure 2B, lane 11 vs. lane 12). The inability of Taxol to accumulate phosphoforms of Bcl2 in the presence of MG132 can be explained if MG132 can block cell growth at G1 phase. Because Taxol-induced Bcl2 phosphorylation predominantly occurs at G2-M phase of the cell cycle, MG132-induced G1 arrest might be a contributing factor to hinder Bcl2 phosphorylation. That MG132 can arrest cells at G1 phase is reported in the literature [39]. Because Pin1 is expressed in mitotic phase, we also find diminished expression of Pin1 in cells treated with MG132 only (Figure 2A, lane 5) for the same reason.
Phospho Bcl2 and Pin1 Have Similar Half-Lives (t{1/2}) and Proteasome Inhibitors Can Restore Their Destabilization
To determine whether phosphorylated forms of Bcl2 disappear faster than the nonphosphoform, Bcl2-overexpressing PC-3 cells were first treated with 1 µM Taxol for 16 hours. The levels of phospho Bcl2 were monitored in the presence or absence of 20 µg/ml cycloheximide for several time periods (2 to 6 hours). Similar approaches using cycloheximide were undertaken to determine the half-lives (t{1/2}) of other proteins such as p53 and survivin [40,41]. In the presence of protein synthesis inhibitor, cycloheximide (CHX), phospho Bcl2 starts to disappear significantly at 4 hours with almost complete decline after 6 hours addition of CHX (Figure 3A, lanes 8 and 10). However, simultaneous treatment with 20 µM MG132 prevents this decline of phospho Bcl2 (Figure 3A, lanes 9 and 11). Figure 3B indicates a similar observation in the presence of another proteasome inhibitor, lactacystin. We also examined the half-life of native Bcl2 by a similar approach. In the presence of CHX (20 µg/ml), native Bcl2 partially declines after 16 to 24 hours addition of CHX (Figure 3C). Clearly, the half-life of native Bcl2 is much longer than that of phospho Bcl2. Additionally, the status of Pin1 was examined in the presence of cycloheximide in Taxol-treated cells. Sixteen-hour Taxol-treated cells were exposed to CHX for different time periods (2 to 6 hours). Strikingly, from 4-hour CHX treatment the level of Pin1 starts to decline (Figure 4A, lane 4) and the significant reduction of Pin1 was observed following 6-hour CHX treatment (Figure 4A, lane 6). The kinetics is identical to that of phospho Bcl2 decline. More interestingly, coincubation with specific proteasome inhibitor, MG132, results in the stabilization of Pin1 (Figure 4A, lanes 5 and 7). Not only MG132 but also other proteasome inhibitors lactacystin and PSI behave in a similar fashion to stabilize Pin1 (Figure 4B). Notably, due to G2-M arrest following initial Taxol exposure, the level of mitotic protein Pin1 was increased (Figure 4, A and B, lane 2). Our data strongly suggest that proteasome-mediated destabilization of Pin1 plays a major role in the decline of phospho Bcl2.
Figure 3.
Phospho Bcl2 has shorter half-life (t{1/2}) than that of native Bcl2 and proteasome inhibitors can stabilize phospho Bcl2 even in the presence of cycloheximide (CHX). Panels A and B: 16-hour 1 µM Taxol-treated W-34 cells were incubated with or without proteasome inhibitors (20 µM MG132/10 µM lactacystin) in the presence or absence of CHX (20 µg/ml) for the indicated periods. Panel A: 16-hour Taxol-treated cells were further incubated. Lane1: no CHX (0 hours); lanes 2 and 7: 2 hours without or with CHX treatment, respectively; lanes 3 and 8: 4 hours without or with CHX, respectively; lanes 4 and 9: coincubation for 4 hours with either MG132 (lane 4) or CHX and MG132 (lane 9); lanes 5 and 10: 6 hours without or with CHX, respectively; lanes 6 and 11: coincubation for 6 hours with either MG132 (lane 6) or CHX and MG132 (lane 11). Panel B represents identical experiments except another proteasome inhibitor lactacystin was used instead of MG132. Panel C: W-34 cells were incubated with or without 20 µg/ml CHX for indicated time periods. Equal amounts of proteins were subjected to immunoblotting using Bcl2 monoclonal antibody.
Figure 4.
Phospho Bcl2 and Pin1 have similar half-life (t{1/2}). Panel A: 16-hour 1 µM Taxol-treated W-34 cells were incubated with CHX in the absence or presence of proteasome inhibitor MG132. Cell lysates were subjected to Western blot with Pin1 antibody. Lane1: control; lane 2: 16-hour Taxol treatment; lanes 3, 4, 6: 16 hours Taxol followed by 2, 4, and 6 hours CHX treatment, respectively; lanes 5 and 7: 16 hours Taxol followed by coincubation with CHX and MG132 for 4 and 6 hours, respectively. Panel B: 16-hour 1 µM Taxol-treated W-34 cells were incubated with CHX in the absence or presence of other proteasome inhibitors lactacystin/PSI for 6 hours. Lane 1: control; lane 2: 16 hours Taxol treatment; lane 3: 16 hours Taxol followed by 6 hours CHX treatment; lanes 4 and 6: 16 hours Taxol followed by 6 hours coincubation with CHX and lactacystin; lanes 5 and 7: 16 hours Taxol followed by 6 hours coincubation with CHX and PSI.
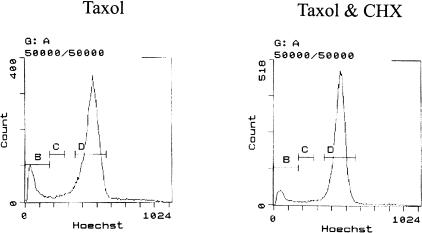
Cycloheximide Treatment Does Not Alter G2-M Profile Induced by Taxol
Because the protein synthesis inhibitor cycloheximide (CHX) can arrest cells at G1 phase [42], it has been shown that Taxol-induced Bcl2 phosphorylation can be blocked by the pretreatment with this agent (Basu et al., unpublished data). As shown in Figure 3, A and B, Taxol -induced phosphoforms of Bcl2 gradually decline in the presence of CHX. To assess whether such decline of phospho Bcl2 in the presence of CHX is due to G1 phase arrest, the following groups of cells were chosen: (a) Taxol treated for 22 hours; (b) Taxol treated for 22 hours with CHX for the last 4.5 hours. These groups of cells were sorted by flow cytometry into two major populations: (i) G0-G1-S and (ii) G2-M. Interestingly, Taxol-induced G2-M arrest remains unaffected if CHX addition follows Taxol treatment (Figure 5). This indicates that once the cells are in G2-M phase of the cell cycle, CHX cannot revert them to G1 phase of growth arrest. Thus, the disappearance of phospho Bcl2 as observed in Figure 3, A and B, cannot be attributed to the ability of CHX to cause G1 phase arrest.
Figure 5.
Taxol-induced G2-M phase arrest is unaltered by posttreatment with CHX. W-34 cells were either treated with (i) 1 µM Taxol for 22 hours or (ii) 22 hours Taxol (1 µM) with CHX (20 µg/ml) for the last 4.5 hours. Cell cycle distribution was analyzed by flow cytometry.
Phospho Bcl2 Is Translocated to the Nucleus
Because Bcl2 is exclusively localized in mitochondrial membrane with trace amounts found in the ER and nuclear membranes [25–27], we asked the question how phospho Bcl2 interacts with a nuclear protein Pin1. One postulation was that phospho Bcl2 is translocated from other organelles to inside of the nucleus. For this purpose, a phospho Bcl2-specific peptide antibody was developed. Initially, studies were undertaken to characterize the specificity of this antibody.
Nitrocellulose strips containing control and Taxol-treated W-34 cell lysate were probed with preimmune rabbit serum (Figure 6A, lanes 1 and 2), phospho Bcl2-specific antiserum (Figure 6A, lanes 3 and 4) as well as mouse monoclonal antibody against Bcl2 (Figure 6A, lane 5). Preimmune rabbit serum did not react with any form of Bcl2 as evident from Figure 6A (lanes 1 and 2). Phospho Bcl2-specific antibody specifically reacted with only two slower migrating phosphoforms of Bcl2 (Figure 6A, lane 4). Preincubation of the antiserum with the specific peptide used as immunogen abolished this reaction (Basu et al., unpublished observation). Mouse monoclonal antibody used to probe lane 5 is known to recognize both native and phosphoforms of Bcl2. Thus, the phospho Bcl2-specific antibody specifically recognizes phosphoforms of Bcl2 on Western blots.
Figure 6.
Characterization of phospho Bcl2-specific antibody by Western blot and immunofluorescence microscopy. Panel A: Phospho Bcl2-specific antiserum specifically recognizes phosphoforms of Bcl2 on Western blot. Lanes 1 and 3: Untreated W-34 cell lysate; lanes 2, 4, 5: Taxol-treated W-34 cell lysate. Panel B: Immunofluorescence microscopy with phosphosBcl2-specific antiserum. Pre B cells 697 expressing endogenous Bcl2 as well as W-34 cells (Bcl2-transfected PC-3) were treated with DMSO or 1 µM Taxol for 16 hours. Both control and treated cells were double labeled for DAPI and FITC. Note that merged images indicate the presence of FITC-labeled phospho Bcl2 inside the nucleus in the case of Taxol treatment. Images shown are representative of typical cells most commonly observed in these experiments.
Next, we were interested in determining whether this antibody can specifically recognize phospho Bcl2 inside the cells. For this purpose, two cell lines were chosen: (a) Pre B leukemic cells 697, which endogenously express Bcl2, and (b) W-34 cells (PC-3 cells genetically engineered to overexpress Bcl2). Control and Taxol-treated cells were subjected to immunofluorescence microscopy using phospho Bcl2-specific antibody as primary. Phospho Bcl2 was detected by using FITC-conjugated anti-rabbit IgG. FITC-labeled phosphoforms of Bcl2 were clearly evident in only Taxol-treated cells Figure 6B. Normally, native Bcl2 is localized on the intracellular membranes, predominantly on mitochondrial membrane [25–27]. To ascertain whether any change in localization occurred following posttranslational modification, we stained the cells with both FITC and a nuclear-specific stain (DAPI). Our dual-labeling studies clearly indicate that phospho Bcl2 is translocated inside the nucleus (uppermost panels of Figure 6B).
The above immunofluorescence data was confirmed by confocal microscopy. Figure 7B represents the merging of FITC-labeled phospho Bcl2 inside the nucleus as defined by the single optical section of differential interference contrast images (Figure 7C).
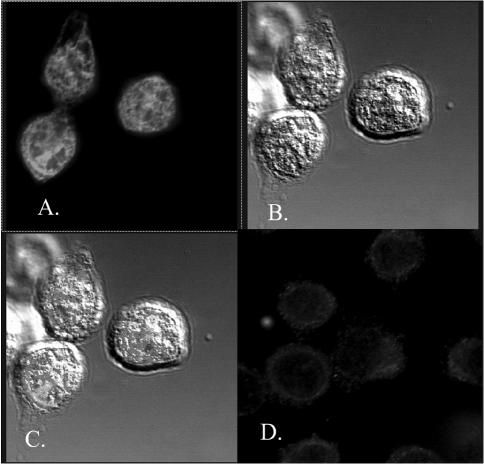
Figure 7.
Confocal microscopy with phospho Bcl2-specific antibody. (A) FITC-labeled single optical section. (B) Differential interference contrast images. (C) Merged images of (A) and (B). (A, B, C) Taxol-treated W-34 cells. Panel D indicates no FITC labeling of untreated W-34 cells.
The confocal images were also taken using mouse monoclonal antibody that recognizes both phospho and nonphosphoforms of Bcl2. In untreated PC-3 cells, the localization of Bcl2 is extranuclear as evident in Figure 8F whereas Taxol treatment resulted in CY3 fluorescence distribution inside the TO-PRO-stained nuclei. It was interesting to note that phospho Bcl2 cannot specifically enter the intact nuclei (Figure 8, C and E). As noted in Figure 8, B and D, the intranuclear localization of phospho Bcl2 is evident only in the cells with destabilized nuclear envelope. It is known that Taxol can induce mitotic arrest and during mitosis, nuclear envelope breakdown occurs shortly after the centrosome separation [28]. Undoubtedly, the single optical sections obtained by confocal microscopy enabled us to resolve the puzzle how phospho Bcl2 can associate with nuclear Pin1.
Figure 8.
Confocal microscopy of PC-3 cells with Bcl2 monoclonal antibody. (A–E) Taxol-treated. (A) CY3-labeled single optical section; (B) TO-PRO staining; (C) merged images of (A) and (B); (D) differential interference contrast images; (E) merged images of (A) and (D); (F) double staining of untreated cells with CY3 and TO-PRO.
Discussion
Microtubule disarraying agents can trigger cell death following G2-M arrest [43–47]. Previous studies have demonstrated that Bcl2 is normally phosphorylated during mitosis [10,48]. Our flow cytometry studies with wild-type and S70, 87 A (phosphorylation defective mutant) Bcl2 transfected cells exhibited similar extent of mitotic arrest (Basu et al., unpublished observation). Thus, Taxol-mediated Bcl2 phosphorylation is the consequence of prolonged G2-M arrest due to drug exposure. But the phosphorylation defective mutant of Bcl2 exhibited enhanced antiapoptotic activity in comparison with the wild-type counterpart [10,13]. The ASK1/JNK1 pathway is activated at G2-M phase of the cell cycle to inactivate the antiapoptotic protein Bcl2 [10,13]. PKCα, however, has been implicated in the phosphorylation of Bcl2 on serine 70 following IL-3 treatment of hematopoietic cells [49]. Whereas Ito et al. [49] observed that the S70A mutant of Bcl2 can exert less antiapoptotic function in the IL-3-dependent cell line NSF/N1.H7, Yamamoto et al. [10] noted enhanced antiapoptotic effect of the same mutant in another IL-3-dependent cell line, FL5.12. Interestingly, in contrary to PKCα, another isoform of protein kinase C, PKC delta, can translocate to mitochondria to trigger apoptosis [50].
Ling et al. [38] observed that Bcl2 phosphorylation reaches a peak after 24 hours Taxol treatment and phosphoforms gradually disappear to be converted to dephosphorylated or native Bcl2. Our results are in agreement with these studies. However, the mechanism of regulation of phosphorylation-dephosphorylation was not clear. Very recent observation describing the specific association of a mitosis specific nuclear protein Pin1 with phospho Bcl2 was also puzzling to us. We thought about one possibility, that following Taxol treatment phospho Bcl2 is being translocated inside the nucleus. Indeed, phospho Bcl2-specific antibody could recognize Bcl2 inside the nucleus of Taxol-treated cells (Figure 6B). To our knowledge, this is the very first report of translocation of phospho Bcl2 into the nucleus employing such unique reagent. Bcl2 does not contain typical nuclear localization signal (NLS) sequences. The most plausible mechanism of translocation seems to be passage through the dissolved nuclear envelope during mitosis as discussed earlier [28].
Another novel finding of our studies is the proteasomal degradation of Pin1, a member of the peptidyl prolyl isomerase family. The sequence-specific and phosphorylation-dependent proline isomerization by Pin1 is well established [51–54]. Prolyl isomerases catalyze relatively slower peptidyl-prolyl isomerization of proteins and thus can allow the relaxation of local energetically unfavorable conformational states [31]. Pin1 is known to promote conformational changes to many proteins including cdc25 [51]. Very recently, Pin1 homolog DoDo was found to target a transcription factor to proteasomal degradation [29], but the self-decline of Pin1 itself by proteasomes represents an unprecedented event.
To summarize, our data clearly indicate a novel mechanism by which phospho Bcl2 alters the destiny of cells by its transient conformational modification. Initially, phospho Bcl2 is translocated inside the nucleus. The association of Pin1, a nuclear peptidyl prolyl isomerase, with phospho Bcl2 induces transient conformational change in such a way that Bcl2-specific phosphatase loses its access. Subsequently Pin1 is degraded by proteasomes resulting in accelerated dephosphorylation of phospho Bcl2 by Bcl2-specific phosphatase. However, transient conformational change due to Pin1 association can lead cells to a point of no return (effector phase) of apoptotic cascade. Apparently, dephosphorylated Bcl2 inside the nucleus lacks antiapoptotic function and this may be due to its inability to relocate to the mitochondria. This explains why the cells still continue to die by apoptosis even in the presence of the native (dephosphorylated) form of Bcl2 (Figure 1, C and D).
Both Bcl2 and Pin1 exert important roles in tumor cell survival. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis [54]. The combined effect of loss of antiapoptotic function of Bcl2 and the subsequent elimination of Pin1 might play a major role in irreversible demise of cancer cells following Taxol treatment. In this respect, the report on the increased affinity of phosphorylated survivin for active Caspase-9 is quite interesting [55]. Phosphorylated survivin can modulate the function of Caspase-9 by stabilizing a complex between them.
Our investigation has prospective clinical significance because Taxol is being used for treatment of several malignancies including metastatic breast cancer [56]. Because of the overexpression of Bcl2 in cancer but not in normal tissues, the elucidation of in-depth mechanism of inactivation of Bcl2 by phosphorylation [9–24] will help us to identify another molecular target for therapy. Nonetheless, it is true that other antiapoptotic molecules such as Bcl-xL [57] and survivin [55] are also known to hinder resistance to therapy. At least the mechanism of inactivation of Bcl2 can be determined to develop a prospective therapy for a subpopulation of cancer cells overexpressing Bcl2. The differential level of expression of one particular protein in similar type of cancer cells is reported in the literature. For instance, the epigenetic inactivation of Apaf-1, a downstream proapoptotic effector molecule is reported only in a subpopulation of metastatic melanoma cells with functional p53 [58,59]. Thus, the inactivating mechanism of Bcl2 by posttranslational modification might be of paramount importance to therapy for malignancies in which Bcl2 or Pin1 overexpression [60] plays a key role.
Acknowledgements
We thank Michael Sramkoski of Ireland Cancer Center Core facility for FACS analysis, Patricia Glazebrook of confocal microscopy core facility at MetroHealth Medical Center, Kay Haas for technical assistance and the National Institutes of Health (CA77328) for generous support.
Abbreviations
- Bcl2
B-cell leukemia and lymphoma-2
- CHX
cycloheximide
- DAPI
4, 6-diamidino-2-phenylindole
- FITC
fluorescein isothiocyanate
- PARP
poly (ADP-ribose) polymerase
- PSI
proteasome inhibitor I
References
- 1.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Thornberry NA, Lazebnik Y. Caspases enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.White E. Life, death and pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the Bcl2 gene in follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ. Cloning the chromosomal breakpoint of the t (14; 18) human lymphomas, clustering around JH on chromosome 14 and near a transcriptional unit 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 7.Oltvai ZN, Korsmeyer SJ. Checkpointing of dueling dimers foil death wishes. Cell. 1994;79:189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 8.Zha J, Harada H, Yang J, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X (L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 9.Basu A, You SA, Haldar S. Regulation of Bcl2 phosphorylation by stress response kinase pathway. Int J Oncol. 2000;16:497–500. doi: 10.3892/ijo.16.3.497. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Ichijo H, Korsmeyer SJ. Bcl2 is phosphorylated and inactivated by an Ask1/Jun N-terminal protein kinase pathway. Mol Cell Biol. 19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Wang Z, Boise L, Dent P, Grant S. Loss of the bcl-2 phosphorylation loop domain increases resistance of human leukemia cells (U937) to paclitaxel-mediated mitochondrial dysfunction and apoptosis. Biochem Biophys Res Commun. 1999;259:67–72. doi: 10.1006/bbrc.1999.0669. [DOI] [PubMed] [Google Scholar]
- 12.Domina AM, Smith JH, Craig RW. Myeloid cell leukemia 1 is phosphorylated through two distinct pathways, one associated with extracellular signal-regulated kinase activation and the other with G2/M accumulation or protein phosphatase 1/2A inhibition. J Biol Chem. 2000;275:21688–21694. doi: 10.1074/jbc.M000915200. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava RK, Mi Q-S, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava RK, Srivastava AR, Korsmeyer SJ, Nesterova M, Cho-Chung Y, Longo DL. Involvement of microtubules in the regulation of Bcl-2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol. 1998;18:3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldar S, Basu A, Croce CM. Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Res. 1998;58:1609–1615. [PubMed] [Google Scholar]
- 16.Basu A, Haldar S. Microtubule-damaging drugs triggered Bcl2 phosphorylation-requirement of phosphorylation on both serine-70 and serine-87 residues of Bcl2 protein. Int J Oncol. 1998;13:659–664. doi: 10.3892/ijo.13.4.659. [DOI] [PubMed] [Google Scholar]
- 17.Poruchynsky MS, Wang EE, Rudin CM, Blagosklonny MV, Fozo T. Bcl-xL is phosphorylated in malignant cells following microtubule disruption. Cancer Res. 1998;58:3331–3338. [PubMed] [Google Scholar]
- 18.Blagosklonny MV, Giannakakou P, El-Deiry WS, Kingston DGI, Higgs PI, Neckers L, Fozo T. Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res. 1997;57:130–135. [PubMed] [Google Scholar]
- 19.Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson C. Identification of a novel regulatory domain in Bcl-X (L) and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 21.Haldar S, Chintapalli J, Croce CM. Taxol induces Bcl2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 22.Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 transduction pathway. Cancer Res. 1996;56:1851–1854. [PubMed] [Google Scholar]
- 23.Haldar S, Jena N, Croce CM. Inactivation of Bcl2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92(10):4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Guo CY, Castillo A, Dent P, Grant S. Effect of bryostatin 1 on taxol-induced apoptosis and cytotoxicity in human leukemia cells (U937) Biochem Pharmacol. 1998;56:635–644. doi: 10.1016/s0006-2952(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 25.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 26.Haldar S, Jena N, Coss RW, Sedar AW, Wachsberger P, Beatty C, Croce CM. Cellular localization of bcl2 protein and response to glucocorticoid stress. Cell Death Differ. 1994;1:109–115. [PubMed] [Google Scholar]
- 27.Krajewski S, Tanaka S, Takayama S, Schibler M, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 28.Pathan N, Aime-Sempe C, Kitada S, Basu A, Haldar S, Reed JC. Microtubule-targeting drugs induce Bcl-2 phosphorylation and association with Pin1. Neoplasia. 2001;3:550–559. doi: 10.1038/sj.neo.7900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu T, McRackan D, Vincent TS, Ger De Couet H. Drosophila Pin1 prolyl isomerase Dodo is a MAP kinase signal responder during oogenesis. Nat Cell Biol. 2001;3:538–543. doi: 10.1038/35078508. [DOI] [PubMed] [Google Scholar]
- 30.Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld J-U, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 32.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NFkappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 34.Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- 35.Graham F, van der Eb A. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;52:456–457. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 36.Basu A, Lenka N, Mullick J, Avadhani NG. Regulation of murine cytochrome oxidase Vb gene expression in different tissues and during myogenesis. Role of a YY-1 factor-binding negative enhancer. J Biol Chem. 1997;272:5899–5908. doi: 10.1074/jbc.272.9.5899. [DOI] [PubMed] [Google Scholar]
- 37.Harlow E, Lane D. Antibodies, A Laboratory Manual, Cold Spring Harbor Laboratory. New York: Cold Spring Harbor; 1988. pp. 78–81. Chapter 5. [Google Scholar]
- 38.Ling YH, Tornos C, Perez-Soler R. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 39.Kumeda SI, Deguchi A, Toi M, Omura S, Umezawa K. Induction of G1 arrest and selective growth inhibition by lactacystin in human umbilical vein endothelial cells. Anticancer Res. 1999;19:3961–3968. [PubMed] [Google Scholar]
- 40.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113:4363–4371. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 41.Persons DL, Yazlovitaskay EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem. 2000;275:35778–35785. doi: 10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 42.Liebmann J, Cook JA, Teague D, Fisher J, Mitchell JB. Cycloheximide inhibits the cytotoxicity of paclitaxel (Taxol) Anticancer Drugs. 1994;5(3):287–292. [PubMed] [Google Scholar]
- 43.Sherwood SW, Sheridan JP, Schimke RT. Induction of apoptosis by the anti-tubulin drug colcemid: relationship of mitotic checkpoint control to the induction of apoptosis in HeLa S3 cells. Exp Cell Res. 1994;215:373–379. doi: 10.1006/excr.1994.1354. [DOI] [PubMed] [Google Scholar]
- 44.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;22:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 45.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan M, Toso RJ, Thrower D, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King KL, Cidlowski JA. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995;58:175–180. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- 48.Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, Pietenpol JA. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest. J Biol Chem. 1998;273:30777–30784. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- 49.Ito T, Deng X, Carr B, May SW. Bcl2 phosphorylation required for its antiapoptotic function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 50.Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- 51.Stukenberg PT, Kirschner MW. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol Cell. 2001;7:1071–1083. doi: 10.1016/s1097-2765(01)00245-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhou XZ, Lu PJ, Wulf G, Lu KP. Phosphorylation-dependent prolyl isomerization: a novel signaling regulatory mechanism. Cell Mol Life Sci. 1999;56:788–806. doi: 10.1007/s000180050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Kullertz G, Stark M, Fisher G, Lu KP. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- 54.Rippmann JF, Hobbie S, Daiber C, Guillard B, Bauer M, Birk J, Nar H, Garin-Chesa P, Rettig WJ, Schnapp A. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell Growth Differ. 2000;11:409–416. [PubMed] [Google Scholar]
- 55.O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Alteiri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2001;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 57.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 58.Soengas M, Capodiecl P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 59.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and Caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 60.Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, Lu KP. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 2001;20:3459–3472. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]