Abstract
The distribution of α6/α3 integrin in adhesion complexes at the basal membrane in human normal and cancer prostate glands was analyzed in 135 biopsies from 61 patients. The levels of the polarized α6/α3 integrin expression at the basal membrane of prostate tumor glands were determined by quantitative immunohistochemistry. The α6/α3 integrin expression was compared with Gleason sum score, pathological stage, and preoperative serum prostate -specific antigen (PSA). The associations were assessed by statistical methods. Eighty percent of the tumors expressed the α6 or α3 integrin and 20% was integrin-negative. Gleason sum score, but not serum PSA, was associated with the integrin expression. Low Gleason sum score correlated with increased integrin expression, high Gleason sum score with low and negative integrin expression. Three prostate tumor phenotypes were distinguished based on differential integrin expression. Type I coexpressed both α6 and α3 subunits, type II exclusively expressed α6 integrin, and type III expressed α3 integrin only. Fifteen cases were further examined for the codistribution of vinculin, paxillin, and CD 151 on frozen serial sections using confocal laser scanning microscopy. The α6/α3 integrins, CD151, paxillin, and vinculin were present within normal glands. In prostate carcinoma, α6 integrin was colocalized with CD 151, but not with vinculin or paxillin. In tumor phenotype I, the α6 subunit did not colocalize with the α3 subunit indicating the existence of two different adhesion complexes. Human prostate tumors display on their cell surface the α6β1 and/or α3β1 integrins. Three tumor phenotypes associated with two different adhesion complexes were identified, suggesting a reorganization of cell adhesion structures in prostate cancer.
Keywords: prostate cancer, focal adhesion proteins, α6/α3 integrin, CD 151
Introduction
Prostate carcinoma is the most commonly diagnosed visceral neoplasm in men and has become one of the nation's major health problems. However, its basic biology is still not well understood [1].
A great biological variability in the rate of clinical progression is seen in prostate cancer patients. In addition, in most men afflicted with prostate cancer, there are multiple tumors showing variations in the morphologic pattern. DNA analysis and in situ hybridization studies showed that multiple tumors from the same prostate were genetically different and smaller tumors may give rise to distant lymph node metastasis [2,3]. The hypothesis of the independent origin of multiple tumors in prostate cancer was also supported by the demonstration of discordant allelic deletion patterns on chromosomes 8p12-21 and 17q21 in distinctly separate tumors [3–5].
A hallmark feature in all prostate cancers is the progressive loss of basal cells that are attached to the underlying extracellular matrix (ECM) in normal glands by two major junctional structures, the hemidesmosomes (HDs) and focal adhesions (FAs) [6,7]. In neoplastic prostate cells, the formation of HDs and the expression of their components including laminin 5 [6,7], which is the extracellular ligand of the α6β4 integrin complex in HDs [8,9], are not observed. Thus, neoplastic cells do not express β4 integrin, a hemidesmosomal protein, at the basal membrane. Although integrins are downregulated in most human carcinoma, invasive prostate carcinoma shows a persistent expression of α3 and α6 integrins [10].
In human skin, mutations in both alleles of either α6 or β4 integrins cause an inherited recessive blistering disorder called junctional epidermolysis bullosa associated with pyloric atresia (PA-JEB) [11,12]. In cultured keratinocytes from a patient exhibiting a complete absence of the β4 integrin subunit, the α6 integrin subunit lacking its β4 partner formed a complex with the β1 subunit and redistributed with vinculin to FAs [12], whereas in normal human keratinocytes, the α3β1 complex generally is recruited to FAs and the α6β4 integrins to HDs [13]. Both α6 and β4 null-mutant mice lacking HDs showed a widespread subepidermal blistering [14–16]. Both α3-null and α6-null mutations were shown to be lethal [17], whereas heterozygote α3±/α6± mice showed multiple defects in organ development including the urogenital tract revealing synergistic activities for these integrins during murine embryo development [16]. These observations indicate that the α6β4 and α3β1 integrins play an essential role in cell adhesion.
In addition, the adhesion structures in skin contain the tetraspan TM4 protein designated CD151. CD 151 forms complexes with the α3β1 or α6β4 laminin-binding integrins and colocalizes with α6β4 in HDs in human skin [18]. HDs and FAs were shown to be dynamic and to change their molecular composition during their maturation [18–21].
Cell adhesion plays a crucial role in migration and invasion of malignant cells to distant tissues. It is suspected that the integrin expression is related to the invasive and migratory potential of tumors [22–26]. Because prostate carcinoma shows a persistent expression of α6 and α3 integrins, we therefore examined the relationship of Gleason sum score, pathological stage, and preoperative serum prostate-specific antigen (PSA) with the α6 and α3 integrin expression. In this study, we observed three different prostate tumor phenotypes reflecting different adhesion structures in prostate cancer.
Materials and Methods
Clinical Material
For this study, 135 biopsy cores containing prostate carcinoma were analyzed from 61 men seen at the University of Arizona Health Sciences Center between 1993 and 1999. These biopsies were collected with IRB approval (Assurance of Compliance no. M-1233). Men with a suspicious nodule on digital rectal examination or serum PSA levels greater than 4 ng/dl were biopsied transrectally under ultrasound guidance using a Biopty gun with an 18-gauge spring loaded Trucut needle (Biopty, Bard Urological Division, Covington, GA). Sextant samples were typically obtained from the apex, midbase, and peripheral zones bilaterally. Immediately following biopsy, the tissue cores were oriented apex-to-base flat in the bottom of a metal embedding mold. The mold was carefully filled with OCT compound embedding media (Miles, Diagnostic Division, Elkhart, IN) and snap-frozen by immersion in a Freon cooled bath of isopentane using a Lipshaw Shandon Histobath (Detroit, MI). The tissues were stored at -80°C.
In this study, 56 men underwent subsequent radical retropubic prostatectomy (RRP). Five men elected not to have surgery. The removed prostates were totally embedded and used to accurately record Gleason sum scores [27] as well as pathologic stage. Serum PSA was presurgically obtained and at 6-month intervals following RRP and was used to assess chemical recurrence. Two men died — one due to disseminated coccidiomycosis, and another one of his disseminated disease. Four men were lost to follow-up. Of the remaining 55 men, 7 men (8%) developed evidence of residual disease with return of positive serum PSA greater than 0.02 ng/dl.
Immunohistochemistry
Samples of frozen biopsies were serially sectioned (3–4 µm) with a cryostat. Serial sections were picked up on sequentially labeled, charged glass slides. For diagnosis and grading of the carcinoma lesions, sections of all 135 biopsies were stained with hematoxylin/eosin (H&E), and reacted with antibodies against cytokeratins 5 and 14 specific for basal cells, and with antibodies specific for α6, α3, and β4 integrins. For immunoperoxidase staining, frozen sections were fixed for 5 minutes in -20°C cold acetone, incubated in primary antibody diluted in PBS containing 1% BSA for 30 minutes at room temperature, and rinsed in three changes of PBS. Biotinylated secondary antibodies to mouse and rabbit were localized with biotin-streptavidin conjugated to horseradish peroxidase (LSAB 2 kit; Dako, Carpinteria, CA). The final color reaction was developed in diaminobenzidine substrate. Tissue sections were counterstained with hematoxylin.
Immunohistochemical Quantitation
H&E-stained sections of each biopsy core were examined for the presence of tumor and graded using the Gleason sum score system. Additional sequential sections were immunolabeled with antibodies specific for α3, α6, and β4 integrins and visualized by streptavidin-biotin DAB (Dako). Two observers using a double-headed scope viewed the H&E-stained sections together in order to agree on the areas of cancer. Then the scoring of the intensity of the integrin staining in each tumor was done separately by these two observers. Only integrin immunostaining polarized at the interface with the ECM of the carcinoma cells was considered positive. Diffuse cytoplasmic integrin staining was not scored. The α6, α3, and β4 integrin stainings were scored separately from each other. Thus, each biopsy obtained three scores. The immunostaining intensity was scored as follows: 1=negative; 2=weak; 3=strong. The percentage of cancerous glands within each biopsy section showing clustering of the α6/α3 integrin subunits at the basal membranes per tumor was scored as follows: 1=0% to 25%; 2=25% to 50%; 3=50% to 75%; and 4=75% to 100%. The final score of the staining in each biopsy was determined by multiplying the intensity score times the estimated percentage of the cancerous glands stained. Thus, a biopsy without polarized integrin staining at the interface to the ECM in cancerous glands would receive a final score of 1x1=1. A biopsy showing strong polarized immunostaining at the interface to the ECM (intensity score 3) in 75% to 100% of the cancerous glands (percentage score 4) would obtain a final score of 3x4=12. The “within-patient variability,” which was not measured specifically, was taken into account by the percentage of glands showing this special integrin staining pattern. The combined score reflected both the intensity of staining and the extent of staining.
Confocal Laser Scanning Microscopy
For immunofluorescence microscopy, 15 selected frozen biopsies from 5 patients were serial sectioned with a cryostat, mounted on glass slides, fixed in -20°C acetone for 10 minutes, and then briefly air-dried. Primary antibodies were directly applied for 30 minutes, followed by three 5-minute washes in PBS. Secondary antibodies were incubated for 30 minutes, and then rinsed off with three 5-minute washes in PBS. Prior to mounting, the sections were rinsed in water, and then postfixed in 100% ethanol for 3 minutes.
Immunofluorescence was observed with a confocal laser scanning immunofluorescence microscope (LSM 410; Carl Zeiss, Jena, Germany). For simultaneous double-label fluorescence, an argon/krypton ion laser operating at 488 and 568 nm was used, together with a long-pass filter of 590 for visualization of Alexa 568 fluorescence and a band-pass filter of 515 to 540 for visualization of Alexa 488 fluorescence, respectively. RGB images were taken in highresolution mode using 1024x1024 image points (pixels) and 8-second scan time. Noise levels were reduced by line averaging of the scans.
Antibodies
Mouse monoclonal antibodies against cytokeratins 5 and 14 (clones KA1 and KA2) were generated and characterized according to standard protocols [28]. The following murine monoclonal antibodies were purchased: alpha 3 integrin clone P1B5 and beta 4 integrin clone 3E1 (Gibco BRL Life Technologies, Rockville, MD), CD 151 clone 14A2.H1 (Research Diagnostics, Flanders, NJ), vinculin clone 11-5 (Sigma, St. Louis, MO), paxillin clone 349 (Transduction Laboratories, Lexington, KY), and laminin 5 clone GB3 (Sera-Lab, Sussex, England, UK). Mouse monoclonal antibody 4C7 specific for the laminin α5 chain (laminin 10) was kindly provided by Dr. Eva Engvall (The Burnham Institute, La Jolla, CA) [29]. The rat monoclonal antibody specific for alpha 6 integrin clone J1B5 was generously provided by Dr. Caroline Damsky (University of California, San Francisco, CA) [30].
Secondary antibodies used were Alexa Fluor 488 goat antimouse IgG and Alexa Fluor 568 goat antimouse or antirat IgG (Molecular Probes, Eugene, OR).
To reduce background and nonspecific reaction, the monoclonal antibody (clone J1B5) specific for alpha 6 integrin was purified as follows: 100 ml of JIB5 conditioned medium was loaded onto a 5-ml Hi-Trap protein G Sepharose column (Amersham Pharmacia Biotech, Piscataway, NJ). The column was washed with three column volumes of 100 mM Tris/HCl (pH 7.9) and three column volumes of 10 mM Tris/HCl (pH 7.9) followed by washing with two column volumes of 100 mM glycine (pH 3.7). JIB5 was eluted with 15 column volumes of 100 mM glycine (pH 2.8). The pH of the eluate was readjusted to pH 7.9 by adding 50 µl of 1 M Tris/HCl (pH 8.5)/ml eluate.
Statistical Method
An estimation of the intraclass correlation coefficient, which is defined as ST2/(ST2+Se2), where ST2 is estimation of between-patient variability and Se2 is within-patient variability, was used to explore the intrapatient variability for measurement of Gleason sum score, α3, α6, or α3+α6 integrins. Because the Gleason sum scores were correlated with each patient, the Generalized Estimating Equations (GEE) model was used to analyze the association between Gleason score and α3, α6, α3+α6 integrins [31]. For the association between preoperative serum PSA and α3, α6, α3+α6 integrins, the Spearman rank-order correlation was used [32]. The difference in expression between the phenotypes was evaluated by the Kruskal-Wallis test [33].
Results
For this study, 135 biopsy cores containing prostate carcinoma from 61 men were analyzed for the polarized expression of α6, α3, and β4 integrins at the basal membrane of malignant prostate epithelium. The expression level concentrated at the basal membrane was scored (1–12; 1=negative, 2=low, 12=high). In prostate carcinoma, both a diffuse surface pattern and a clustering of α6 and α3 integrins at the basal membrane were observed (Figure 1, C and D). The α6 and β4 integrins were also positive in blood vessels (Figure 1, B and D). Consistent with our previous results [10], the polarization of β4 integrin at the basal membrane was not detected in any prostate tumor gland in which α6 and/or α3 integrins were clustered at the basal membrane (Figure 1B). Laminin 5, the extracellular ligand of the α6β4 integrin complex, was also found to be absent in these glands (data not shown).
Figure 1.
Immunoperoxidase localization of β4, α3, and α6 integrins in normal and malignant prostate glands. Frozen serial sections of prostate tissue containing normal glands (N) and Gleason sum score (3+3=6) infiltrating carcinoma were stained with H&E (A) or immunoreacted with specific antibodies to β4 (B), α3 (C), or α6 (D) integrins as indicated. All three integrins were expressed in the epithelial basal membrane in the normal glands. Some weak staining with anti-β4 integrin antibody was detected within the malignant epithelial cytoplasm, but none in the basal membrane of malignant epithelia. Note β4 expression in periglandular vascular basement membranes. Both antibodies specific for α3 and α6 integrins focally outlined the basal membranes (arrows) of malignant glands. Arrowheads: blood vessels; N: normal glands. Bar, 20 µm.
The Expression Levels of α6/α3 Integrins and Association with Gleason Sum Score and PSA Levels
The patients' preoperative PSA levels, Gleason sum score, and pathological stage of each tumor at the time of prostatectomy were recorded and compared to the integrin staining pattern.
Analysis of serial sections by immunohistochemistry revealed that 80% of the tumors examined expressed either α6 or α3 integrin (Figure 2A). A total of 70% of the tumors expressed α6 integrin and 54% expressed α3 integrin within a range from low to high expression levels (2–12). Within the group of tumor glands showing high expression levels (9–12) at the basal membrane (Figure 2A), 37% highly expressed either α6 or α3 integrin, 34% showed high levels of α6 integrin, 10% showed high levels of α3 integrin. Approximately 20% of the tumors examined showed no expression of either α6 or α3 integrin at the basal membrane.
Figure 2.
Expression profile of α6 and α3 integrins polarized at the basal membranes of human prostate carcinoma epithelium. One hundred and thirty-five needle biopsy containing carcinoma obtained from 61 patients were analysed for the expression of the α6 and/or α3 integrin subunit and the intensity of the immunoreaction was scored: 2 to 12=all expression; 2 to 8=low expression; 9 to 12=high expression. Graph (A) demonstrates the percentage of all biopsies analyzed (135=100%) that expressed α6 and/or α3 integrin. The group of cases with the expression levels 2 to 12 included the cases with high expression levels 9 to 12, which also were analyzed separately (A). Graph (B) shows the expression profile of the integrin subunits in association with the tumor grading (Gleason sum score). The definitions of the descriptors are: α6, all cases which expressed α6 integrin; α3, all cases which expressed α3 integrin; integrin-negative, all cases which were integrin-negative. None of the tumors expressed polarized β4 integrin. The Gleason sum score was found to be inversely associated with the integrin expression.
Interestingly, three different phenotypes of prostate tumors were observed: 1) 44% of the tumors coexpressed both α6 and α3 subunits (type I), with 4.4% showing high-level expression (9–12) of both subunits (Figure 2A); 2) 26% of the tumors exclusively expressed the α6 subunit alone (type II), with 12.5% showing high expression levels (9–12) of α6 integrin only (Figure 2A); and 3) 10% of the tumors exclusively expressed the α3 subunit (type III) at the interface of the cell-ECM site (Figure 2A), with only a few tumors (0.74%; Figure 2A) expressing high levels (9–12) of the α3 subunit without expressing the α6 subunit. Because the expression values were not normally distributed, the Kruskal-Wallis test was performed to test the difference among the three tumor phenotypes. The differences for these three tumor phenotypes were statistically significant (see Table 1).
Table 1.
Association between Integrin Expression and the Three Tumor Phenotypes.
| (A) The Median of Expression Level for Each Tumor Phenotype | |
| Median | |
| α3 integrin only | 2 |
| α6 integrin only | 8 |
| α3+α6 | 12 |
| (B) The Statistical Significance (P Value) of Difference | ||
| α3 only versus α6 only | α3 only versus α3+α6 | α6 only versus α3+α6 |
| .0012 | .0003 | .0003 |
Whether the group of integrin -negative tumors represent a fourth phenotype or is a result of poor preservation will require further examination. Because these specimens were snap-frozen immediately after biopsy, it is likely that these are truly integrin -negative.
The biopsies then were arranged into two groups according to the Gleason sum score (≤6, ≥7) and the integrin expression profile analyzed for these two groups (Figure 2B). Within the group of tumors with Gleason sum score ≤6, a total of 83% expressed α6 integrin, and 66% expressed α3 integrin (Figure 2B). Neither of the two subunits was expressed in 5% of tumors in this group. From the tumors with Gleason sum score ≥7, a total of 29% of the tumors expressed α6 integrin, 27% expressed α3 integrin, and 54% showed no expression at all of either subunit at the basal membrane (Figure 2B). We questioned whether there was an association between Gleason sum score and PSA with α6, α3, or α6+α3 integrin expression. The estimation of the intraclass correlation coefficient was computed for the Gleason sum score, α6, α3, and α6+α3 (Table 2A). This test also included the group of integrin negative biopsies. The Gleason sum score was inversely associated with the α6 integrin expression (P value .0272; Table 2B). Low Gleason sum scores were significantly related to increased α6 expression levels at the basal membrane (Figure 2B). Likewise, the Gleason sum score and α3 integrin expression (P value .0001), and the Gleason sum score and both α6+α3 integrin expression (P value .0001) were inversely associated (Table 2B), Figure 2B). There was also a significant correlation between Gleason sum score and the group of integrin-negative biopsies (Figure 2B). These integrin-negative biopsies were predominantly seen in the group of less well-differentiated samples, with high Gleason sum scores suggesting that in these cases a nonintegrin adhesion receptor may be involved in cell-substrate attachment.
Table 2.
Association between Gleason Sum Score and α6, α3, α6, and α3 Integrin Expression.
| (A) The Intraclass Correlation Coefficient for Gleason, α6, α3, α6+α3 Integrin | ||||
| Gleason | α6 | α3 | α6+α3 | |
| Within-patient variability: Se2 | 0.4986 | 6.9700 | 3.5033 | 11.8756 |
| Between-patient variability: ST2 | 1.1092 | 12.4917 | 4.5184 | 21.5735 |
| Intraclass correlation coefficient: R | 0.6986 | 0.6418 | 0.5633 | 0.6449 |
| (B) The Statistical Significance for the Association between Gleason Sum Score and α6, α3, α6+α3 Integrin | |||
| α6 | α3 | α6+α3 | |
| Gleason sum score (P values) | .0272 | .0001 | .0001 |
No association between serum PSA (Table 3) and α6, α3, α6, and α3 integrin expression was observed.
Table 3.
The Association between Serum PSA and α6, α3, α6, and α3 Expression.
| The Spearman Rank-order Correlation Between PSA and α6, α3, α6+α3 Expression | |||
| α6 | α3 | α6+α3 | |
| Correlation | -0.0108 | 0.06831 | 0.0452 |
| P value for correlation | .9373 | .6169 | .7429 |
The correlation between disease-free survival rate in patients with any of the three phenotypes could not be tested because no significant association between disease-free survival rate in patients and pathological stage or Gleason sum score was found in this study.
The Correlation of Phenotypes with Gleason Sum Score and Pathological Stage
The distribution of the three prostate tumor phenotypes in relation to the Gleason sum score was observed as follows: for Gleason sum score ≤6, 55% coexpressed both α6+α3 integrin (type I), 29% of the tumors in this group expressed α6 integrin only (type II), whereas only 6.3% exclusively expressed α3 integrin (type III; Figure 3A). For Gleason sum score ≥7, 17% coexpressed both subunits (type I), 17% expressed α6 integrin only (type II), whereas 10% expressed α3 integrin only (type III; Figure 3A). Taken together, these data suggest that increasing Gleason sum score selects against the predominance of a type I adhesion phenotype. Interestingly, 44% of the prostate tumors graded with Gleason sum score 7 and higher, which is correlated with decreased differentiation, was equally likely to express either phenotype I, II, or III (Figure 3A).
Figure 3.
Distribution pattern of tumor phenotypes as compared to Gleason sum score (A) and pathological stage (B). (A) The distribution pattern of the three phenotypes in relation to the Gleason sum score. A total number of 94 biopsies (100%) were graded with Gleason sum score ≤6, and 41 biopsies (100%) with Gleason sum score ≥7. (B) The distribution of the three phenotypes in relation to the pathological staging within the biopsy group with Gleason sum score ≤6. Of the 135 biopsies examined, 94 were graded with Gleason sum score ≤6 and were correlated after prostatectomy to pathological staging as follows: T1a to T2c=29 biopsies; T3a to T3b=38 biopsies; and T3c=27 biopsies. (C) The average expression intensity (2–12) of the individual integrin subunits in each phenotype compared to the pathological stage and Gleason sum score ≤6. (D) The distribution of the three phenotypes in relation to the pathological stage T3a to T3b within the biopsy group (26 biopsies) with Gleason sum score ≥7 (for pathological stages T1a to T2c and T3c, the number of cases available for examination was too low to be representative).
A subset of tumors graded with Gleason sum score ≤6 (94 biopsies) was analyzed for the expression pattern of the different phenotypes in relation to pathological staging. For stage T1a–T2c, 48% coexpressed α6 and α3 integrins, 28% expressed α6 integrin only, and 10% expressed α3 integrin only (Figure 3B). For stage T3a–T3b, 63% coexpressed α6 and α3 integrins, 21% expressed α6 only, and 15% expressed α3 integrin only (Figure 3B). For stage T3c, 52% coexpressed both α6 and α3 integrin, 41% expressed α6 integrin only, and none expressed α3 integrin only (Figure 3B).
In the subset of tumors (41 biopsies) graded with Gleason sum score ≥7, the pathological stages were unevenly represented. For example, only two biopsies with stage T1a–T2c were available for the examination of the integrin expression; of these, one case expressed the α6 integrin only (type II), and the other was integrin-negative. For pathological stage T3c, eight biopsies were available. Of these, two tumors still expressed α6 integrin only (type II), one tumor expressed α3 only (type III), and the expression of α6+α3 integrin (type I) was not observed. In stage T3a–T3b (26 biopsies; Figure 3D), 22% expressed α6+α3 integrin (type I), 19% expressed α6 integrin only (type II), and 11% expressed α3 integrin only (type III). Five biopsies were not staged because prostatectomy was not performed. Overall, these data indicate that in higher pathological stages, a selection for the expression of α6 integrin adhesion may occur and that the phenotype III (α3 integrin only) may be suppressed.
When analyzing the average expression intensities of the integrin subunits in each phenotype (Figure 3C), it appeared that α6 integrin showed the highest intensity levels compared with the α3 integrin expression. Within the phenotype I, the average α6 and α3 expression levels were similar in the pathological stage T1a–T2c, whereas in stages T3–T3b and T3c, the expression intensities for the α6 subunit were two-fold higher than for the α3 subunit (Figure 3C).
Immunofluorescence Localization of α6 and α3 Integrins in Normal and Neoplastic Prostate Glands
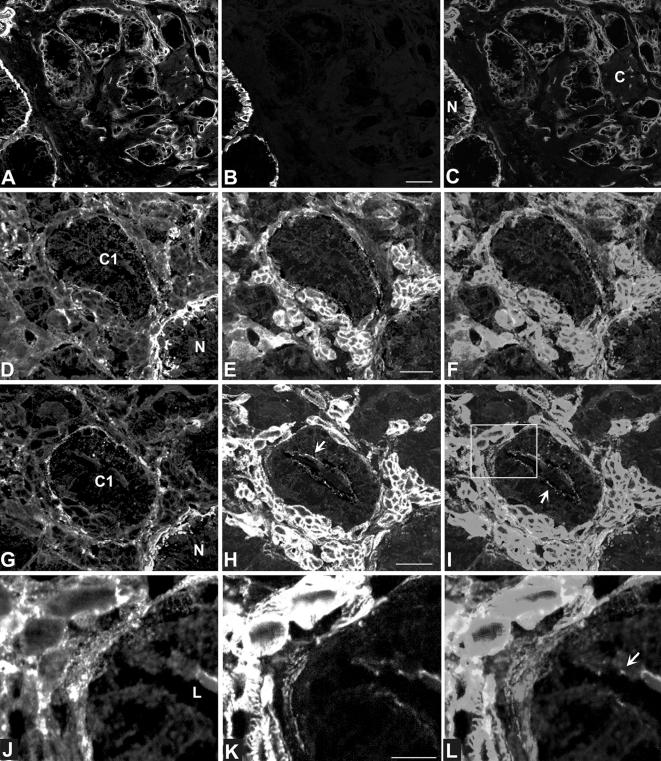
In normal prostate glands, the majority of the α6 integrin subunits pair with the β4 subunit, consistent with the formation of a hemidesmosomal structure. A minor amount of the α6 integrin subunit pairs with β1 integrin. The α6β1 integrin in cell cultures is associated with FAs containing, in part, vinculin and paxillin [34]. In prostate cancer however, the β4 integrin subunit and the majority of other integrin subunits (with the exception of β1, α6, and α3) are no longer observed on the tumor cell surfaces [10]. The assembly of alpha-beta integrin complexes is required for the transport through the Golgi apparatus to the plasma membrane and for the exposure of integrin subunits on the cell surface [35]. In prostate tumor cells consequently, the α6 and the α3 subunits have to be expressed on the cell surface in a complex with the β1 subunit. To determine the α6 integrin location with respect to FAs in prostate cancer, we performed a double-staining immunofluorescence comparison of α6 integrin with vinculin and paxillin in frozen sections of normal and neoplastic prostate (Figures 4 and 5). In the normal prostate gland, α6 integrin, vinculin, and paxillin were intensely expressed at the basal membrane (Figure 4). The α6 integrin staining overlapped in some areas with both the vinculin and paxillin staining (Figure 4, C and F). In other areas, the α6 integrin staining could be resolved from the vinculin or paxillin staining (Figure 4, C and F), reflecting that they did not colocalize. These data indicated heterogeneity of these adhesion complexes in the normal prostate gland. Vinculin, which is also known to contribute to E-cadherin containing cell-cell junctions in certain cell types, was expressed in the apical junctional region of the Luminal cells in normal glands (not shown). In addition to glands, vinculin and paxillin were also prominently expressed in the surrounding smooth muscle cells (Figures 4A and 5, E and H). The α6-integrin was found to be strongly expressed in blood vessels (Figures 1D and 7, A and E).
Figure 5.
Confocal laser scanning micrographs of simultaneous detection of α6 integrin (A, D, G, J; red) and cytokeratins 5 and 14 (B; green), paxillin (E; green) or vinculin (H, K; green) in the malignant prostate epithelium of two patients (patient 1: Gleason grade 3 +3, pathological stage T3c, shown in A–C; patient 2: Gleason grade 3+3, pathological stage T3c, shown in D–L). Overlays are shown in (C, F, I, L). Malignant glands were distinguished from normal glands by the absence of cytokeratins 5 and 14 (A–C). Note the abundant staining of the basal membrane in normal glands, whereas the basal membranes in malignant glands were outlined by an attenuated staining. In serial sections (D–I), the distribution of α6 was compared to paxillin (D–F) and vinculin (G–L). The field within the white frame in (I) is shown at high magnification in (J–L), revealing that α6 was not distributed to focal adhesions in malignant prostate glands lacking the β4 integrin subunit. Both paxillin and vinculin strongly stained smooth muscle cells. Vinculin was also expressed in the apical junctional zone of the luminal cells (arrows). N, normal gland; C, cancer; C1, cancer gland 1 indicating the same gland in two serial sections; L, lumen of gland. Bars, 10 µm (B), 5 µm (E, F), 2 µm (K).
Figure 4.
Confocal laser scanning micrographs of double-label immunofluorescence microscopy of α6 integrin (B, C, E, F; green) with vinculin (A, C; red) and paxillin (D, F; red) in human normal prostate glands. Overlays are shown in (C) and (F). Note that in most of the areas, the red was resolved from the green. A few areas showed yellow indicating some colocalization of the α6 integrin with either vinculin or paxillin. Note that vinculin is also present in smooth muscle cells. Bars, 10 µm (A), 2 µm (D).
Figure 7.
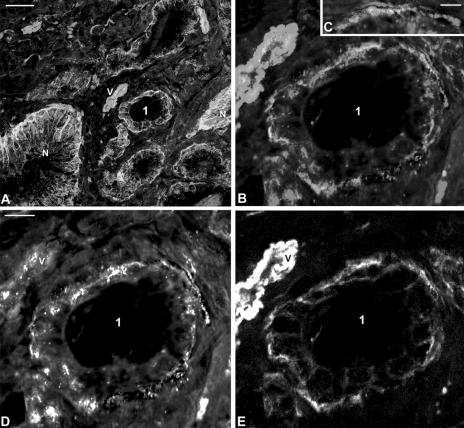
Confocal laser scanning micrographs of α6 (green; E) and α3 integrin (red; D) in human normal (A) and neoplastic prostate (Gleason grade 3+3, pathological stage T3a; A–E). (A) Field with normal (N) and cancer glands. Cancer gland 1 is shown at higher magnification in (B, D, and E). (C) Shows high resolution field of basal membrane in cancer gland 1. The monoclonal antibody specific for α3 integrin subunit showed some cross-reaction with the nuclei. N, normal glands; V, vessel. Bars, 50 µm (A), 20 µm (D), 2 µm (C).
In prostate cancer, the hemidesmosomal structures (HDs) and the clustered expression of hemidesmosomal proteins, especially the β4 integrin, are not observed. We questioned whether α6 integrin would colocalize with vinculin or paxillin to FAs in the absence of its HD partner β4 integrin in malignant prostate glands. For identification of cancerous and normal glands, sections were first analyzed by detecting simultaneously the α6 subunit and cytokeratins 5 and 14 (Figure 4, A–C). In normal cytokeratin 5- and 14-positive glands (Figure 5, A–C), the α6 integrin staining of the basal membrane appeared as a strong wide band (Figure 5, A, D, G), whereas in the cytokeratin 5- and 14-negative cancer glands, the α6 integrin staining appeared less intensely in a fine line (Figure 5A). Sections then were processed for simultaneous detection of α6 integrin and either paxillin (Figure 5, D–F) or vinculin (Figure 5, G–L). Both paxillin and vinculin were abundantly expressed in the smooth muscle cells, and less intensely at the glandular basal membranes. In addition, vinculin was also expressed in the apical junctional region of the luminal cells in the neoplastic glands (Figure 5, H, I, K, L). In the absence of the β4 integrin subunit, we were surprised to discover that the α6 integrin did not colocalize with either paxillin (Figure 5, D–F) or vinculin (Figure 5, J–L) in the FAs of the malignant prostate glands. To determine whether α6 integrin colocalized with the tetraspan molecule CD151 in these malignant glands, we performed a double staining with antibodies specific for these two antigens. As shown in Figure 6, A–D), α6 integrin colocalized with CD151 at the basal membranes of the malignant glands in most areas. CD 151 was also present in the basal membrane of normal prostate glands (Figure 6A). In addition, CD 151 was observed on the surface of epithelial cells in normal and neoplastic glands (Figure 6A).
Figure 6.
Simultaneous detection of α6 integrin (red) with CD151 (green) in human malignant prostate glands (Gleason grade 3+3, pathological stage T3c) as viewed by indirect immunofluorescence and laser scanning confocal microscopy. CD151 (A, B, D: green) and α6 integrin (A, B, C: red) colocalized (yellow) at the interface of epithelium to basal membrane. (A) Shows field of malignant prostate glands. Malignant gland 1 is shown at higher magnification in (B–D). N, normal gland. Bars, 10 µm (A), 5 µm (B).
To determine whether α6 and α3 integrins were involved in the same or in different adhesion complexes at the basal membranes in the type I cancer phenotype (i.e., cancers expressing both subunits), we performed a comparison of the two antigens in normal and in malignant prostate glands. In the normal prostate gland, the α6 and α3 integrins were strongly expressed in the basal membrane (Figure 7A), but were not found to be colocalizing when analyzed by high resolution (not shown). In prostate cancer glands expressing both subunits (type I), α6 and α3 integrins were not colocalized at the basal membranes (Figure 7, C–E). At high magnification, the α6 integrin staining could be resolved from the α3 integrin staining at the basal membrane (Figure 7C), suggesting the existence of two different cell-to-basal membrane adhesion sites and complexes in prostate cancer glands.
Discussion
In this study, we have identified three prostate tumor phenotypes expressing α6 and α3 integrins (type I), α6 integrin only (type II), or α3 integrin only (type III). Prostate carcinoma is a heterogeneous disease with a wide spectrum of pathologic and clinical manifestations. Most prostates with cancer were shown to contain spatially and distinctly separate tumors by the time of clinical diagnosis [36,37]. Multiple tumors from the same prostate were shown by in situ hybridization and DNA analysis to be genetically different and that even smaller tumors may give rise to distant lymph node metastasis [2,3]. The demonstration of discordant allelic deletion patterns on chromosomes 8p12-21 and 17q21 in distinctly separate tumors supported the hypothesis of the independent origin of multiple tumors in prostate cancer [3–5]. Our findings of the expression of different prostate tumor phenotypes were consistent with these previous observations showing the heterogeneity of prostate tumors.
In contrast to the findings in the β4-negative PA-JEB skin keratinocytes [11], we described in this study that the α6 integrin interestingly was not colocalized with vinculin or paxillin in primary prostate carcinoma. Because paxillin is a scaffold protein that provides docking sites for an array of signaling and structural proteins and also provides linkage to the actin cytoskeleton [38], these data suggest the existence of different cell-ECM adhesion complexes in prostate tumors. Recently, cross linkers of the actin-filament systems with plasma membrane receptors other than paxillin have been described, such as the ERM (ezrin, radixin, and moesin) proteins [39]. These newer members of the ERM family were reported to play key roles in morphology, motility, signal transduction, and apoptosis [40,41]. Furthermore, the finding in type I tumors — that the α6 integrin was not colocalized with the α3 integrin subunit — is consistent with a recent study, which demonstrated a heterogeneity in the composition of adhesion complexes [42]. It will be of great interest to isolate by laser capture microscopy techniques the proteins present within the α6-containing adhesion sites in the tissue and determine their identity.
There is evidence that the morphology and molecular composition of FAs are dependent on laminin isoforms and on the integrins present in the complexes [43]. The activation of the α6β1 integrin alone or the α6β1 with α3β1 integrin leads to the formation of different adhesion complexes [43,44]. The formation of adhesion complexes is crucial for signal transduction from the ECM into the cell and vice versa [45,46]. Binding of integrins to extracellular ligands leads to a series of events including clustering of receptors, recruitment of FA linker proteins, actin polymerization, and a phosphorylation cascade [46]. A different ultrastructural arrangement of the molecules within the adhesion complexes could be linked to the transmission of different distinct signals. Consistent with this idea is our observation that CD 151, a molecule laterally associated with the α6 integrin, was present in prostate cancer, whereas vinculin and paxillin were absent. Recently, CD 151 has been implicated in novel signaling complexes inducing protein kinase C [47].
The association of the integrin expression with the Gleason sum score was consistent with the fact that many human carcinomas have a decreased expression of integrins when compared with normal tissue [22]. However, for human pancreatic carcinoma, no correlation of integrin expression with tumor differentiation was observed [48]. Interestingly, 44% of the prostate tumors with a Gleason sum score ≥7 expressed either one of the described phenotypes. However, in differentiated tumors (Gleason sum score ≤6) invading into the seminal vesicles (pathological stage T3c), only the phenotypes I (α6 +α3 integrin) and II (α6 integrin only) with high expression levels of the α6 subunit were observed. This suggests that the clustered expression of α6 integrin may be correlated with invasion and the failure to express α3 integrin may be related to more aggressive tumors (T3c). There is evidence that the interaction of α3β1 with the adjacent basal membrane might transduce signals that inhibit the invasive behavior of epithelial cells [49,50], although its functional role is still poorly understood. In contrast, a role for the α6β1 integrin in invasion was suggested by previous studies [23,51,52]. Recently, the existence of a new subtype of α6 integrin lacking the extracellular “beta-barrel” domain was demonstrated in various cell types and was upregulated in cancer cells that exhibited migratory-enhancing properties [53]. This study also showed that the α6-specific antibody (GoH3) used here will not distinguish between the two α6 integrin forms [53]. Therefore, a switch between the α6 integrin subtypes from normal to cancer may have occurred. This is the subject of further study to determine the distribution of this novel structural variant within the three phenotypes.
The invasive potential of the tumor phenotypes expressing different adhesion complexes may also be determined by the availability or composition of their extracellular ligands. Laminins 5, 10, and 11 were shown to be preferred ligands for α3β1 [54]. The α6β1 integrin also was reported to be a major receptor for laminin 10/11 in human pancreatic carcinoma cells [55] and mouse hematopoietic cells [56]. A recent study showed that fibroblast adhesion to laminin 10/11 was mediated by both α3β1 and α6β1 integrins [57]. It will be of interest to determine the composition and localization of the different laminin forms within the three different integrin tumor phenotypes. Understanding the factors contributing to the invasive potential of prostate carcinoma is of fundamental importance in this disease.
Prostate cancer is unique in that latent and aggressive phenotypes exist. Currently used prognostic factors for the management of the disease include the histopathological evaluation of tumor size, grade, and localization and distribution within the gland [58]. For example, if invasion to the seminal vesicle had occurred, the disease-free 10-year survival rate in patients decreased by approximately 50% as compared to the one in patients without seminal vesicle invasion [59]. Other studies showed that prostate cancers with established capsular penetration had a higher risk of progression than those with focal capsular penetration [60]. Capsular penetration is correlated with lymph node metastasis [61]. The preferred sites of metastasis in prostate cancer are lymph nodes and bone marrow — sites enriched with laminin 10/11 [62]. Taken together, these data suggest that retention of laminin 10/11 receptors by the cancer cells may play a role in prostate cancer metastasis.
The further understanding of integrin laminin receptors and the different integrin -containing adhesion phenotypes will contribute to determining their role in prostate cancer metastasis. We propose that the three tumor phenotypes, which are distinguished by differential integrin expression, will be useful for the separation of cases with organ-confined disease from cases with capsular penetration or with seminal vesicle invasion.
Acknowledgement
We thank Virginia Clark for expert technical assistance.
Abbreviations
- HDs
hemidesmosomes
- FAs
focal adhesions
- PSA
prostate-specific antigen
- ECM
extracellular matrix
- PA-JEB
junctional epidermolysis bullosa associated with pyloric atresia
- RRP
radical retropubic prostatectomy
Footnotes
National Institutes of Health Grants PO1 CA 56666, CA 75152, CA 23074, and ACS IRG-110T.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Qian J, Bostwick DG, Takahashi S, Borell TJ, Herath JF, Lieber MM, Jenkins RB. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res. 1995;55:5408–5414. [PubMed] [Google Scholar]
- 3.Cheng L, Song SY, Pretlow TG, Abdul-Karim FW, Kung HJ, Dawson DV, Park WS, Moon YW, Tsai ML, Linehan WM, Emmert-Buck MR, Liotta LA, Zhuang Z. Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst. 1998;90:233–237. doi: 10.1093/jnci/90.3.233. [DOI] [PubMed] [Google Scholar]
- 4.Emmert-Buck MR, Vocke CD, Pozzatti RO, Duray PH, Jennings SB, Florence CD, Zhuang Z, Bostwick DG, Liotta LA, Linehan WM. Allelic loss on chromosome 8p12–21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55:2959–2962. [PubMed] [Google Scholar]
- 5.Sakr WA, Macoska JA, Benson P, Grignon DJ, Wolman SR, Pontes JE, Crissman JD. Allelic loss in locally metastatic, multisampled prostate cancer. Cancer Res. 1994;54:3273–3277. [PubMed] [Google Scholar]
- 6.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 7.Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostate. Am J Pathol. 1996;149:1341–1349. [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell -basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JC, Kurpakus MA, Cooper HM, Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 11.Pulkkinen L, Kimonis VE, Xu Y, Spanou EN, McLean WH, Uitto J. Homozygous alpha 6 integrin mutation in junctional epidermolysis bullosa with congenital duodenal atresia. Hum Mol Genet. 1997;6:669–674. doi: 10.1093/hmg/6.5.669. [DOI] [PubMed] [Google Scholar]
- 12.Niessen CM, van der Raaij-Helmer MH, Hulsman EH, van der Neut R, Jonkman MF, Sonnenberg A. Deficiency of the integrin beta 4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. Cell Sci. 1996;109:1695–1706. doi: 10.1242/jcs.109.7.1695. [DOI] [PubMed] [Google Scholar]
- 13.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. Alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 16.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 17.De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- 18.Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6-beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaapveld RQ, Borradori L, Geerts D, van Leusden MR, Kuikman I, Nievers MG, Niessen CM, Steenbergen RD, Snijders PJ, Sonnenberg A. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borradori L, Chavanas S, Schaapveld RQ, Gagnoux-Palacios L, Calafat J, Meneguzzi G, Sonnenberg A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion — reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp Cell Res. 1998;239:463–476. doi: 10.1006/excr.1997.3923. [DOI] [PubMed] [Google Scholar]
- 21.Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68:4–17. [PubMed] [Google Scholar]
- 23.Vogelmann R, Kreuser ED, Adler G, Lutz MP. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999;80:791–795. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–342. [PubMed] [Google Scholar]
- 25.Mukhopadhyay R, Theriault RL, Price JE. Increased levels of alpha6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin Exp Metastasis. 1999;17:325–332. doi: 10.1023/a:1006659230585. [DOI] [PubMed] [Google Scholar]
- 26.Cooper CR, Chay C, Pienta KJ. The role of avb3 in prostate cancer progression. Neoplasia. doi: 10.1038/sj.neo.7900224. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 28.Schmelz M, Cress AE, Barrera J, McDaniel KM, Davis TL, Fuchs L, Dalkin BL, Nagle RB. PEAZ-1: a new human prostate neoplastic epithelial cell line. Prostate. 2001;48:79–92. doi: 10.1002/pros.1084. [DOI] [PubMed] [Google Scholar]
- 29.Tiger CF, Champliaud MF, Pedrosa-Domellof F, Thornell LE, Ekblom P, Gullberg D. Presence of laminin alpha5 chain and lack of laminin alpha1 chain during human muscle development and in muscular dystrophies. J Biol Chem. 1997;272:28590–28595. doi: 10.1074/jbc.272.45.28590. [DOI] [PubMed] [Google Scholar]
- 30.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 31.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 32.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences Wiley, New York. 1987 [Google Scholar]
- 33.Hollander M, Wolfe DA. Nonparametric Statistical Methods Wiley, New York. 1973 [Google Scholar]
- 34.Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2:E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 35.Heino J, Ignotz RA, Hemler ME, Crouse C, Massague J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989;264:380–388. [PubMed] [Google Scholar]
- 36.Bastacky SI, Wojno KJ, Walsh PC, Carmichael MJ, Epstein JI. Pathological features of hereditary prostate cancer. J Urol. 1995;153:987–992. [PubMed] [Google Scholar]
- 37.Greene DR, Wheeler TM, Egawa S, Weaver RP, Scardino PT. Relationship between clinical stage and histological zone of origin in early prostate cancer: morphometric analysis. Br J Urol. 1991;68:499–509. doi: 10.1111/j.1464-410x.1991.tb15394.x. [DOI] [PubMed] [Google Scholar]
- 38.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 39.Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics: authors' correction. Trends Cell Biol. 1999;9:289. doi: 10.1016/s0962-8924(99)01607-4. [DOI] [PubMed] [Google Scholar]
- 40.Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11:109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- 41.Vaheri A, Carpen O, Heiska L, Helander TS, Jaaskelainen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O. The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Opin Cell Biol. 1997;9:659–666. doi: 10.1016/s0955-0674(97)80119-6. [DOI] [PubMed] [Google Scholar]
- 42.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 43.Dogic D, Rousselle P, Aumailley M. Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J Cell Sci. 1998;111:793–802. doi: 10.1242/jcs.111.6.793. [DOI] [PubMed] [Google Scholar]
- 44.Sondermann H, Dogic D, Pesch M, Aumailley M. Targeting of cytoskeletal linker proteins to focal adhesion complexes is reduced in fibroblasts adhering to laminin-1 when compared to fibronectin. Cell Adhes Commun. 1999;7:43–56. doi: 10.3109/15419069909034391. [DOI] [PubMed] [Google Scholar]
- 45.Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra-and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- 46.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem. 2001;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- 48.Lohr M, Trautmann B, Gottler M, Peters S, Zauner I, Maier A, Kloppel G, Liebe S, Kreuser ED. Expression and function of receptors for extracellular matrix proteins in human ductal adenocarcinomas of the pancreas. Pancreas. 1996;12:248–259. doi: 10.1097/00006676-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Dedhar S, Saulnier R, Nagle R, Overall CM. Specific alterations in the expression of alpha 3 beta 1 and alpha 6 beta 4 integrins in highly invasive and metastatic variants of human prostate carcinoma cells selected by in vitro invasion through reconstituted basement membrane. Clin Exp Metastasis. 1993;11:391–400. doi: 10.1007/BF00132982. [DOI] [PubMed] [Google Scholar]
- 50.Pignatelli M, Hanby AM, Stamp GW. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- 51.Witkowski CM, Rabinovitz I, Nagle RB, Affinito KS, Cress AE. Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J Cancer Res Clin Oncol. 1993;119:637–644. doi: 10.1007/BF01215981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis TL, Rabinovitz I, Futscher BW, Schnolzer M, Burger F, Liu Y, Kulesz-Martin M, Cress AE. Identification of a novel structural variant of the alpha 6 integrin. J Biol Chem. 2001;276:26099–26106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. Laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 55.Tani T, Lehto VP, Virtanen I. Expression of laminins 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp Cell Res. 1999;248:115–121. doi: 10.1006/excr.1999.4399. [DOI] [PubMed] [Google Scholar]
- 56.Gu Y, Sorokin L, Durbeej M, Hjalt T, Jonsson JI, Ekblom M. Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood. 1999;93:2533–2542. [PubMed] [Google Scholar]
- 57.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 58.Murphy GP, Busch C, Abrahamsson PA. Consensus Conference on Diagnosis and Prognostic Parameters in Localized Prostate Cancer. Stockholm, Sweden: 1993. Histopathology of localized prostate cancer. [PubMed] [Google Scholar]
- 59.Hering F, Schmid HP, Graber P. Influence of microinvasion of the capsule and/or micrometastasis of regional lymph nodes on disease free survival after radical prostatectomy. Ann Urol (Paris) 1994;28:196–201. [PubMed] [Google Scholar]
- 60.Epstein JI, Carmichael MJ, Pizov G, Walsh PC. Influence of capsular penetration on progression following radical prostatectomy: a study of 196 cases with long-term follow up. J Urol. 1993;150:135–141. doi: 10.1016/s0022-5347(17)35415-0. [DOI] [PubMed] [Google Scholar]
- 61.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–1233. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 62.Siler U, Seiffert M, Puch S, Richards A, Torok-Storb B, Muller CA, Sorokin L, Klein G. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood. 2000;96:4194–4203. [PubMed] [Google Scholar]