Abstract
The potential of noninvasive laser Doppler flowmetry (LDF) and near infrared spectroscopy (NIRS) to detect acute effects of different vascular-modifying agents on perfusion and blood volume in tumors was evaluated. C3H mouse mammary carcinomas (∼200 mm3) in the rear foot of CDF1 mice were treated with flavone acetic acid (FAA, 150 mg/kg), 5,6-dimethylxanthenone-4-acetic acid (DMXAA, 20 mg/kg), combretastatin A-4 disodium phosphate (CA4DP, 250 mg/kg), hydralazine (HDZ, 5 mg/kg), or nicotinamide (NTA, 500 mg/kg). Tumor perfusion before and after treatment was evaluated by noninvasive LDF, using a 41°C heated custom-built LDF probe with four integrated laser/receiver units, and tumor blood volume was estimated by NIRS, using light guide coupled reflectance measurements at 800±10 nm. FAA, DMXAA, CA4DP, and HDZ significantly decreased tumor perfusion by 50%, 47%, 73%, and 78%, respectively. In addition, FAA, DMXAA, and HDZ significantly reduced the blood volume within the tumor, indicating that these compounds to some degree shunted blood from the tumor to adjacent tissue, HDZ being most potent. CA4DP caused no change in the tumor blood volume, indicating that the mechanism of action of CA4DP was vascular shut down with the blood pool trapped in the tumor. NTA caused no change in either tumor perfusion or tumor blood volume. We conclude that noninvasive LDF and NIRS can determine acute effects of vascular modifying agents on tumor perfusion and blood volume.
Keywords: laser Doppler flow (LDF), near infrared spectroscopy (NIRS), combretastatin A4 disodium phosphate, hydralazine, C3H mouse mammary carcinoma
Introduction
Because tumor survival is critically dependent on a functional vasculature, selective damaging of the vasculature of tumors has been proposed as an attractive strategy against cancer [1,2]. This treatment strategy is based on differences in the endothelial cells between quiescent normal vessels in organs and angiogenic active vessels in tumors. Today, several vascular-damaging agents selectively targeting tumor vessels have been developed. The vascular effect of these agents has been documented by using various destructive and invasive methods, including the 86RbCl extraction technique, histologic assessment, and invasive laser Doppler flowmetry (LDF) procedure [3–5].
Flavone acetic acid (FAA) and its analogue 5,6-dimethylxanthenone-4-acetic acid (DMXAA) are synthetic flavonoids with antitumor activity in a variety of experimental tumors [6–8], mediated through a large and persistent reduction in blood flow. This vascular effect is thought to be mediated by induction of TNF-α [9,10]. Combretastatin A-4 disodium phosphate (CA4DP) belongs to the group of combretastatins initially isolated from the South African tree Combretum caffrum. CA4DP is a tubulin-binding agent, which causes pronounced vascular shutdown in experimental tumors [3,4,11–13]. It has been hypothesized that the inhibition of tubulin polymerization affects endothelial cell shape, leading to thrombus formation or changes in permeability of the endothelium, but the exact mechanism of action by CA4DP in vivo is still unknown [14,15]. The peripheral vasodilator hydralazine (HDZ) and the amide derivative of vitamin B3, nicotinamide (NTA), are useful control compounds in tumor perfusion studies [16–19]. HDZ reduces tumor perfusion by shunting blood from the tumor to adjacent normal tissue [16], whereas NTA has been shown to increase tumor blood flow in some but not all murine animal models [17]. The exact mechanism of action of NTA is still unknown.
LDF provides noninvasive estimates of local blood perfusion in superficial tissue [20]. The principle of this technique is based on the change in wavelength (Doppler shift) of laser light reflected from moving red blood cells [21]. The Doppler-shifted light is converted into an arbitrary perfusion signal, which is approximately proportional to the mean blood cell velocity multiplied by the concentration of moving blood cells within the sampling volume.
Comparison of LDF with the 133xenon-clearance method has shown high correlation [22–24]. Near infrared spectroscopy (NIRS) provides noninvasive estimates of the hemoglobin concentration, because hemoglobin is a strong absorber of near infrared light [25]. At 800 nm (the isosbestic point of hemoglobin) the light absorption of a tissue is proportional to the total hemoglobin concentration, disturbed only marginally by other chromophores of the tissue [26,27]. The total hemoglobin concentration reflects the blood volume, because the source of hemoglobin is the red blood cells.
We have previously shown that LDF and NIRS can detect angiogenic and antiangiogenic activity in a simple subcutaneous bFGF-pellet implantation model [28], and that NIRS alone can provide estimates of the degree of vascularization in solid tumors similar to the information obtained by histologic Chalkley count analysis [29]. Here we describe a novel, noninvasive, in vivo model to evaluate acute effects of vascular targeting agents on tumor perfusion and blood volume, using LDF and NIRS.
Materials and Methods
Animal and Tumor Model
Female 10- to 14-week-old CDF1 mice were used. A C3H mouse mammary carcinoma was transplanted and grown on the right rear foot. Experiments were carried out when tumors had reached approximately 200 mm3 in size. Institutional guidelines for animal welfare and experimental conduct were followed.
Drug Preparation
DMXAA (20 mg/kg), CA4DP (250 mg/kg), NTA (500 mg/kg), and HDZ (5 mg/kg) were dissolved in saline and injected intraperitoneally (i.p.) at a volume of 0.02 ml/g mouse body weight. FAA (150 mg/kg) was dissolved in 1% Na2CO3 saline and injected i.p. at a volume of 0.02 ml/g mouse body weight. NTA was purchased from Sigma Chemical Co. (St. Louis, MO). All the remaining drugs were generously supplied to us as follow: CA4DP (Oxigene Europa AB, Lund, Sweden), DMXAA (Dr. William Denny, University of Auckland, New Zealand), FAA (LIPHA, Lyon, France), and HDZ (Ciba-Geigy, Copenhagen, Denmark) All drugs were freshly prepared before each experiment. The dose of each agent was chosen from previous experience [17,30,31].
Experimental Design
All experiments were performed in a temperature-controlled room (25±1°C). A micromanipulator was used to maintain a reproducible localization of the LDF and NIRS probes perpendicular to and in close contact to the skin. Both LDF and NIRS recordings were performed before treatment and after 1 minute (clamped), 1/2 hour (HDZ), 1 hour (CA4DP and NTA), or 3 hours (FAA, DMXAA, and control). During LDF and NIRS recordings, animals were anesthetized with ketamine/xylazine (5/50 mg/kg BW s.c.) for immobilization.
NIRS recordings The NIRS instrument was custom-built (NMR Center, University of Copenhagen, Denmark) including a xenon flash as the light source (I4633 Hammamatsu, near infrared spectrometer, Burlingame, CA) and a photo diode (Siemens BPW21 photo diode, Hamburg, Germany) as the light detector. Briefly, an optical filter (800±10 nm interference filter) in front of the xenon flash unit results in emission of light with a wavelength of 800±10 nm. A branched light guide (Y-shaped) accomplished the coupling between the instrument and the animal. Backscattered light is transmitted through the other branch of the Y-shaped optical fiber to a second optical filter in front of the photodiode. The 800±10-nm optical filters ensure detection of light absorption of this specific wavelength only. In the fused end of the light guide, the emitting and transmitting fibers are randomly mixed, and the diameter of that part of the probe is 3.0 mm. The NIRS instrument was calibrated before each experimental session. The calibration was stable over time and the calibration was controlled after use. Full absorption, i.e., zero signal, was set to 0 reflectance units (RU) and motility standard (Perimed, Stockholm, Sweden) was calibrated to 70 RU. The NIRS value was calculated as the mean of two recordings.
LDF recordings A Periflux laser Doppler flowmeter 4001 was utilized. Laser light with the wavelength 780 nm is transmitted to the skin by a 41°C heated custom-built probe (6 mm o.d., 250 µm fiber separation, time constant 0.2 second, PF415-175, Perimed). The probe (PF-415-175) has four integrated lasers/receiver units, which increase the measuring volume and improve the reproducibility in tissues with spatial variations in perfusion. The LDF apparatus was calibrated to 250 perfusion units (PU) in motility standard (Perimed) before each recording session. The calibration was stable over time and the calibration was controlled after use. The LDF signal was recorded continuously until a stable plateau was obtained after approximately one minute. The LDF probe was retracted if a sufficient total backscatter was not obtained. The PU value was calculated as mean PU on the plateau.
Results
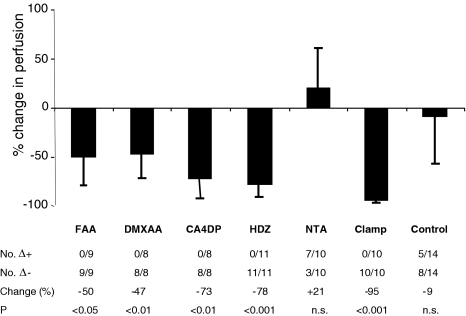
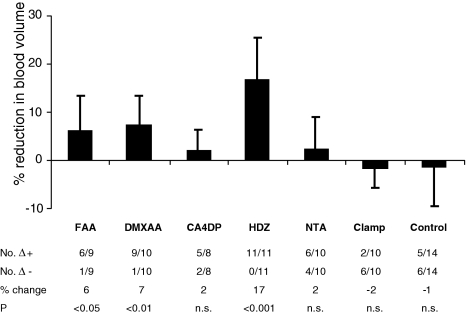
Figure 1 shows the effect of vascular-targeting agents on tumor perfusion measured by noninvasive LDF, while the effect on tumor blood volume measured by noninvasive reflectance NIRS is shown in Figure 2. Changes induced by treatment were calculated for each individual tumor relative to its pre-therapeutic value. The number of positive and negative differences in each group is listed in both figures.
Figure 1.
Effects of vascular modifying agents on perfusion in a CH3 mouse mammary carcinoma measured by noninvasive LDF. (Top) Mean percent change (±SD) in relative perfusion. (Bottom) No. Δ+ indicates the number of tumors in which the perfusion was increased after treatment. No. Δ-indicates the number of tumors in which the perfusion was decreased after treatment. Change (%) indicates the mean relative change in perfusion. Statistical evaluations were performed using a paired two-tailed t test.
Figure 2.
Effects of vascular-targeting agents on blood volume in a CH3 mouse mammary carcinoma measured by noninvasive NIRS. (Top) Mean percent change (±SD) in relative reflectance. Notice that an increase in reflectance is due to less blood volume. (Bottom) No. Δ+ indicates the number of tumors in which the reflectance was increased after treatment. No. Δ-indicates the number of tumors in which the reflectance was decreased after treatment. Change (%) indicates the mean relative change in reflectance. Statistical evaluations were performed using a paired two-tailed t test.
The vascular-damaging agents FAA, DMXAA, and CA4DP and the peripheral vasodilator HDZ significantly decreased perfusion in C3H mouse mammary carcinoma by 50%, 47%, 73%, and 78%, respectively. In addition, FAA, DMXAA, and HDZ significantly reduced the blood volume within the tumor, indicating that these compounds to some degree shunted blood from the tumor to adjacent tissue, HDZ being most potent. CA4DP caused no change in the tumor blood volume, indicating that the action of CA4DP was a vascular shut down with the blood pool trapped in the tumor. NTA caused no change in either tumor perfusion or tumor blood volume.
Occlusion of the rear foot blood flow by an occlusive clamp blocked tumor perfusion by 95%, without affecting the blood volume, because the blood was trapped in the tumor. The residual perfusion after clamping, median 8 PU (range 5–15 PU) higher than the instrument zero, defined the biological zero of the C3H mouse mammary carcinoma. In the present work, the biological zero was disregarded as a component in all measurements. The controls showed no change in tumor perfusion or blood volume.
Discussion
In the present study we demonstrated that LDF and NIRS are useful tools to detect acute effects of different vascular-targeting agents on tumor perfusion and blood volume in the C3H mouse mammary carcinoma. In line with earlier findings, we found that noninvasive LDF could determine the rapid and extensive decrease in tumor perfusion by the vascular-damaging agents FAA, DMXAA, and CA4DP and the peripheral vasodilator HDZ. In fact, we found very similar reduction in tumor perfusion by DMXAA (-50% vs -50%), CA4DP (-73% vs 66%), and HDZ (-78% vs -70%) as found using the same tumor model and same time intervals in recent studies using an invasive LDF procedure and the 86RbCl extraction technique [4,18].
By combining noninvasive LDF with noninvasive reflectance NIRS we also obtained information about the blood volume within the tumor. In accordance with studies using magnetic resonance spectroscopy [32,33], we found that HDZ significantly reduced the tumor blood volume. This effect is due to a direct action of HDZ on vascular smooth muscle cells in the vessels of normal tissues without dilating tumor blood vessels, because they lack smooth muscle cells. This results in a redistribution of the blood away from the tumor, which is described as the vascular steal phenomenon [16,34]. FAA and DMXAA also reduced tumor perfusion by partially shunting the blood to adjacent tissue. The action of FAA and DMXAA is thought to be a result of an induction of TNF-α in the tumor [8]. TNF-α is known to decrease tumor perfusion and cause hemorrhagic necrosis [35]. In the ex vivo perfused tissue-isolated tumor model, TNF-α has been shown to increase the vascular resistance in tumors [36]. In addition, histologic examination revealed that the vessel lumen was reduced after TNF-α treatment. Other studies report a considerable swelling of endothelial cells and significant intravascular recruitment of polymorphonuclear cells in biopsies from patients after TNF-α treatment [37]. The decrease in blood volume measured by NIRS following FAA and DMXAA treatment indicates that less blood has access to the tumor, which is likely to be the result of an increased geometric resistance in the tumor.
In contrast, we showed that the vascular-damaging agent CA4DP shut down perfusion while trapping the blood pool inside the tumor volume. CA4DP does not induce TNF-α production in the tumor [12], but is believed to induce its antivascular action in tumors by changing the shape or detachment of endothelial cells, leading to thrombus formation [14,15]. However, the exact mechanism of action of CA4DP is still unknown. We found no significant effect of NTA on either perfusion or blood volume. The lack of effect on perfusion by NTA in C3H mouse mammary carcinoma has been shown before by Horsman et al. using the RbCl uptake procedure [38]. Furthermore, we obtained high LDF values in untreated C3H mouse mammary carcinomas (161±66 PU, mean±SD), indicating that the tumors initially were well perfused, which makes a further increase in perfusion more difficult.
The noninvasive LDF and NIRS methods as applied here are topologically limited to superficial tumors, like on the rear foot, because both reflectance NIRS and especially LDF have limited measuring depths [39]. However, the present study shows that our noninvasive LDF and NIRS methods can determine the effects of vascular modifying agents on tumor perfusion and blood volume. The LDF and NIRS recordings in the control group showed considerable variation, but in large groups of animals, the results were consistent, as previously shown [28].
The nondestructive and noninvasive nature of our LDF and NIRS equipment makes it attractive to use these techniques to study different time-points of the activity of vascular targeting agents in the future. We conclude that LDF and NIRS are useful, noninvasive methods for evaluation of acute effects of vascular modifying agents on tumor perfusion and blood volume in C3H mammary carcinomas.
Abbreviations
- LDF
laser Doppler flowmetry
- NIRS
near infrared spectroscopy
- NTA
nicotinamide
- DMXAA
5,6-dimethylxanthenone-4-acetic acid
- FAA
flavone acetic acid
- HDZ
hydralazine
- CA4DP
combretastatin A-4 disodium phosphate
Footnotes
Financial support from the IMK Charitable Foundation, the Danish Medical Research Council (grant no. 9702250) and the Danish Cancer Society (grant no. 9810034) is gratefully acknowledged.
References
- 1.Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 1990;9(3):267–282. doi: 10.1007/BF00046365. [DOI] [PubMed] [Google Scholar]
- 2.Chaplin DJ, Dougherty GJ. Tumour vasculature as a target for cancer therapy. Br J Cancer. 1999;80(Suppl 1):57–64. [PubMed] [Google Scholar]
- 3.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57(10):1829–1834. [PubMed] [Google Scholar]
- 4.Murata R, Overgaard J, Horsman MR. Comparative effects of combretastatin A-4 disodium phosphate and 5,6-dimethylxanthenone-4-acetic acid on blood perfusion in a murine tumour and normal tissues. Int J Radiat Biol. 2001;77(2):195–204. doi: 10.1080/09553000010007695. [DOI] [PubMed] [Google Scholar]
- 5.Malcontenti-Wilson C, Muralidharan V, Skinner S, Christophi C, Sherris D, O'Brien PE. Combretastatin a4 prodrug study of effect on the growth and the microvasculature of colorectal liver metastases in a murine model. Clin Cancer Res. 2001;7(4):1052–1060. [PubMed] [Google Scholar]
- 6.Plowman J, Narayanan VL, Dykes D, Szarvasi E, Briet P, Yoder OC, Paull KD. Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep. 1986;70(5):631–635. [PubMed] [Google Scholar]
- 7.Bibby MC, Double JA, Phillips RM, Loadman PM. Factors involved in the anti-cancer activity of the investigational agents LM985 (flavone acetic acid ester) and LM975 (flavone acetic acid) Br J Cancer. 1987;55(2):159–163. doi: 10.1038/bjc.1987.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lash CJ, Li AE, Rutland M, Baguley BC, Zwi LJ, Wilson WR. Enhancement of the anti-tumour effects of the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) by combination with 5-hydroxytryptamine and bioreductive drugs. Br J Cancer. 1998;78(4):439–445. doi: 10.1038/bjc.1998.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph WR, Cao Z, Mountjoy KG, Marshall ES, Baguley BC, Ching LM. Stimulation of tumors to synthesize tumor necrosis factor-alpha in situ using 5,6-dimethylxanthenone-4-acetic acid: a novel approach to cancer therapy. Cancer Res. 1999;59(3):633–638. [PubMed] [Google Scholar]
- 10.Ching LM, Goldsmith D, Joseph WR, Korner H, Sedgwick JD, Baguley BC. Induction of intratumoral tumor necrosis factor (TNF) synthesis and hemorrhagic necrosis by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF knockout mice. Cancer Res. 1999;59(14):3304–3307. [PubMed] [Google Scholar]
- 11.Horsman MR, Ehmrooth E, Ladekarl M, Overgaard J. The effect of combretastatin A-4 disodium phosphate in a C3H mouse mammary carcinoma and a variety of murine spontaneous tumors. Int J Radiat Oncol Biol Phys. 1998;42(4):895–898. doi: 10.1016/s0360-3016(98)00299-5. [DOI] [PubMed] [Google Scholar]
- 12.Chaplin DJ, Pettit GR, Hill SA. Anti-vascular approaches to solid tumour therapy: evaluation of combretastatin A4 phosphate. Anticancer Res. 1999;19(1A):189–195. [PubMed] [Google Scholar]
- 13.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, Stratford MR, Dennis MF, Chaplin DJ. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 1999;59(7):1626–1634. [PubMed] [Google Scholar]
- 14.Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81(8):1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galbraith SM, Chaplin DJ, Lee F, Stratford MR, Locke RJ, Vojnovic B, Tozer GM. Effects of combretastatin A4 phosphate on endothelial cell morphology in vitro and relationship to tumour vascular targeting activity in vivo. Anticancer Res. 2001;21(1A):93–102. [PubMed] [Google Scholar]
- 16.Belfi CA, Paul CR, Shan S, Ngo FQ. Comparison of the effects of hydralazine on tumor and normal tissue blood perfusion by MRI. Int J Radiat Oncol Biol Phys. 1994;29(3):473–479. doi: 10.1016/0360-3016(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 17.Horsman MR. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. Acta Oncol. 1995;34(5):571–587. doi: 10.3109/02841869509094031. [DOI] [PubMed] [Google Scholar]
- 18.Bentzen L, Horsman MR, Daugaard P, Maxwell RJ. Noninvasive tumour blood perfusion measurement by 2H magnetic resonance. NMR Biomed. 2000;13(8):429–437. doi: 10.1002/1099-1492(200012)13:8<429::aid-nbm663>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Robinson SP, Howe FA, Stubbs M, Griffiths JR. Effects of nicotinamide and carbogen on tumour oxygenation, blood flow, energetics and blood glucose levels. Br J Cancer. 2000;82(12):2007–2014. doi: 10.1054/bjoc.2000.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254:56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson GE, Tenland T, Öberg PÅ. Evaluation of laser Doppler flowmetry for measurement of tissue blood flow. IEEE Trans Biomed Eng BME. 1980;27:597–604. doi: 10.1109/TBME.1980.326582. [DOI] [PubMed] [Google Scholar]
- 22.Holloway GA, Watkins DW., Jr Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol. 1977;69:306–309. doi: 10.1111/1523-1747.ep12507665. [DOI] [PubMed] [Google Scholar]
- 23.Petersen LJ, Kristensen JK. Simultaneous assessment of blood flow in UVB-inflamed human skin by laser Doppler flowmetry and the 133-xenon washout technique. Acta Physiol Scand Suppl. 1991;603:67–73. [PubMed] [Google Scholar]
- 24.Lees VC, Fan TP. A freeze-injured skin graft model for the quantitative study of basic fibroblast growth factor and other promoters of angiogenesis in wound healing. Br J Plast Surg. 1994;47:349–359. doi: 10.1016/0007-1226(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Millikan GA. The oximeter, an instrument for measuring continuously the oxygen saturation of arterial blood in man. Rev Sci Instrum. 1942;13:343–352. [Google Scholar]
- 26.Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 27.Chance B, Nioka B, Kent J, McCully K, Fountain M, Greenfeld R, Holtom G. Time resolved spectroscopy of hemoglobin in resting and ischaemic muscle. Anal Biochem. 1988;174:698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 28.Kragh M, Quistorff B, Kristjansen PEG. Quantitative estimates of angiogenic and anti-angiogenic activity by laser Doppler flowmetry (LDF) and near infra-red spectroscopy (NIRS) Eur J Cancer. 2001;37(7):924–929. doi: 10.1016/s0959-8049(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 29.Kragh M, Quistorff B, Lund EL, Kristjansen PEG. Quantitative estimates of vascularity in solid tumors by non-invasive near infrared spectroscopy (NIRS) Neoplasia. 2001;3(3):1–7. doi: 10.1038/sj.neo.7900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsman MR, Christensen KL, Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- 31.Horsman MR, Sampson LE, Chaplin DJ, Overgaard J. The in vivo interaction between flavone acetic acid and hyperthermia. Int J Hyperthermia. 1996;12(6):779–789. doi: 10.3109/02656739609027684. [DOI] [PubMed] [Google Scholar]
- 32.Thomas C, Counsell CJ, Wood P, Adams GE. Relative change in blood volume following administration of hydralazine as monitored by 19F NMR spectroscopy. Int J Radiat Biol. 1991;60(1–2):219–223. doi: 10.1080/09553009114551871. [DOI] [PubMed] [Google Scholar]
- 33.Adams GE, Bremner JC, Counsell CJ, Stratford IJ, Thomas C, Wood PJ. Magnetic resonance spectroscopy studies on experimental murine and human tumors: comparison of changes in phosphorus metabolism with induced changes in vascular volume. Int J Radiat Oncol Biol Phys. 1992;22(3):467–471. doi: 10.1016/0360-3016(92)90855-c. [DOI] [PubMed] [Google Scholar]
- 34.Zlotecki RA, Baxter LT, Boucher Y, Jain RK. Pharmacologic modification of tumor blood flow and interstitial fluid pressure in a human tumor xenograft: network analysis and mechanistic interpretation. Microvasc Res. 1995;50(3):429–443. doi: 10.1006/mvre.1995.1069. [DOI] [PubMed] [Google Scholar]
- 35.Jimbo T, Akimoto T, Tohgo A. Systemic administration of a synthetic lipid A derivative, DT-5461a, reduces tumor blood flow through endogenous TNF production in hepatic cancer model of VX2 carcinoma in rabbits. Anticancer Res. 1996;16(1):359–364. [PubMed] [Google Scholar]
- 36.Kristensen CA, Roberge S, Jain RK. Effect of tumor necrosis factor alpha on vascular resistance, nitric oxide production, and glucose and oxygen consumption in perfused tissue-isolated human melanoma xenografts. Clin Cancer Res. 1997;3(3):319–324. [PubMed] [Google Scholar]
- 37.Renard N, Lienard D, Lespagnard L, Eggermont A, Heimann R, Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha) Int J Cancer. 1994;57(5):656–663. doi: 10.1002/ijc.2910570508. [DOI] [PubMed] [Google Scholar]
- 38.Horsman MR, Kristjansen PEG, Mizuna M, Christensen KL, Chaplin DJ, Quistorff B, Overgaard J. Biochemical and physiological changes induced by nicotinamide in a C3H mouse mammary carcinoma and CDF1 mice. Int J Radiat Oncol Biol Phys. 1992;22:451–454. doi: 10.1016/0360-3016(92)90851-8. [DOI] [PubMed] [Google Scholar]
- 39.Jakobsson A, Nilsson GE. Prediction of sampling depth and photon path length in laser Doppler flowmetry. Med Biol Eng Comput. 1993;31:301–307. doi: 10.1007/BF02458050. [DOI] [PubMed] [Google Scholar]