Abstract
Malignant meningiomas (MMs) are aggressive intracranial neoplasms with a 75% 5-year recurrence rate. Verotoxin 1 (VT1) is an Escherichia coli toxin, which has recently been shown to have anti-neoplastic action by targeting the globotriosylceramide (Gb3) glycolipid on tumor cells and tumor neovasculature. To investigate the potential use of VT1 as a clinical agent for MM, we initially tested 16 meningiomas for Gb3 expression. Nine of 11 MMs (82%), but only one of five benign meningiomas (20%), were positive for Gb3. An orthotopic xenograft model was used to test the efficacy of VT1 treatment for MM. We first demonstrated that Gb3 was highly expressed by the MM cell line, IOMM-Lee, and that this cell line was highly sensitive to VT1 treatment in vitro. A single intratumoral injection of VT1 significantly improved survival in nude mice harboring intracranial tumours (P<.0001). Factor-eight immunostaining of tumours harvested from VT1-treated animals revealed a marked reduction in the tumour microvascular density. In addition, the tumors of VT1-treated animals displayed increased apoptosis by TUNEL analysis and showed a significant decrease in cell proliferation, as determined by MIB-5 immunostaining. VT1 treatment of MM is effective in our orthotopic xenograft model, and warrants further exploration as a potential treatment for these highly anaplastic and aggressive neoplasms.
Keywords: apoptosis, IOMM-Lee, meningioma, microvascular density, verotoxin
Introduction
Meningiomas are common tumours of the central nervous system that have an annual incidence as high as 6/100,000 and account for approximately 20% of all intracranial neoplasms [1–5]. Pathological studies suggest that these tumours most often arise form the arachnoidal cap cells of the inner meninges. Most meningiomas are benign lesions (World Health Organization, WHO, grade 1), with surgical extirpation providing a cure for most patients with accessible tumours [1,4–6]. However, a large number of benign meningiomas occur in locations such as the skull base where complete resection is difficult to achieve [4,6]. For incompletely resected meningiomas, the 10-year recurrence rate increases dramatically to 78% [4,6]. Even after complete resection, the recurrence rate for meningiomas is still 20% at 10 years [4–6]. Although rare, meningiomas may harbour atypical or anaplastic features. Malignant (WHO grade III) and atypical meningiomas (WHO grade II) are more prone to recurrence and aggressive growth than their benign counterparts. Patients with malignant meningiomas (MMs) have a median survival time of less than 2 years after diagnosis [1,4–7].
Most patients with MMs will receive radiation therapy after surgery [6,7]. However, radiation therapy alone has proven ineffective [8]. Conventional anti-neoplastic agents have been administered to small numbers of patients with meningiomas with minimal benefit [8]. Most recently, hormonal therapy has been attempted, using agents that block estrogen or progesterone receptors [8,9]. However, trials with tamoxifen, medroxyprogesterone acetate, megestrol acetate, and the progesterone receptor antagonist, RU486 [8,9], have also been disappointing. Clearly, our current treatment protocols for the management of patients with malignant and atypical meningiomas are inadequate.
Verotoxin (VT) has been examined recently as a viable, new agent for the treatment of certain forms of cancer [10–14]. VTs, also referred to as Shiga-like toxins, are a family of Escherichia coli-derived subunit protein toxins that are associated with the pathogenesis of hemolytic uremic syndrome, hemorrhagic colitis, and microangiopathies [15–17]. Currently, there are four known members, verotoxin 1 (VT1), VT2, VT2c, and VT2e [15–17]. The VTs consist of a 30-kDa enzymatic A subunit, noncovalently linked to a pentameric 7-kDa B subunit array, which recognizes and binds to a specific glycolipid in sensitive cells [15–17]. The VT receptor is globotriosylceramide (Gb3; galα1–4galβ1–4glc ceramide), which is known to be elevated in several human neoplasms [10,11,13,14]. Cancer cell lines established from such tumours are sensitive to VT in vitro. All the VTs have similar biological activities but differ in their antigenicity, presumably due to their differing B subunits [15,16]. Despite the involvement of VT in clinical disease, this property of Gb3 upregulation and VT1 sensitivity found in human cancers have been exploited to investigate the possible role of VT as an anti-neoplastic agent. In the case of astrocytomas, we have previously demonstrated a VT1-related anti-neoplastic effect using a subcutaneous astrocytoma xenotransplant model [11]. In the present study, we extend this work to show that VT1 is effective against the Gb3-positive IOMM-Lee MM cell line when grown intracranially in immunocompromised mice.
Materials and Methods
Analysis of GB3 Levels in Human Meningiomas
To detect the VT receptor, Gb3, in surgical specimens of human meningiomas, 16 frozen tumour specimens were obtained from the University of Toronto Nervous System Tissue Bank. Of those, 11 were MMs and five were benign meningiomas. An immunohistochemical-based method [18] was performed on 5-µm-thick cryostat sections. Briefly, frozen sections were blocked in 1 mM sodium azide, 10 mM glucose, and 1 U/ml glucose oxidase (all from Sigma, St. Louis, MO) to quench endogenous peroxidases. Sections were subsequently incubated in biotin blocker (kit from Vector Laboratories, Burlingame, CA) and then in 1% normal goat serum. In order to mark Gb3, sections were incubated with 200 ng/ml purified VT1, prepared fresh [10]. After high-affinity binding of purified VT to Gb3, sections were washed with phosphate-buffered saline (PBS), incubated with a mouse monoclonal anti-VT antibody (PH1, dilution 1:1000), and then treated with a biotinylated goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories, West Grove, PA). Detection was through the ABC (Vector)-DAB (VectaStain Elite, Vector Laboratories, Burlingame, CA) system, which was followed by counterstaining with Meyer's Hematoxylin, Lillies Modification (Dako, Carpintera, CA). Tissue sections were dehydrated and then mounted with paramount (Sigma). In negative controls (one for each section), incubation with purified VT1 was emitted.
Cell Culture, Gb3 Expression, and VT1 Cytotoxicity of IOMM-Lee
IOMM-Lee is a MM cell line derived from a 61-year-old man with repeated recurrent MM of the skull (a kind gift from Dr. Ian McCutcheon, Department of Neurosurgery, MD Anderson, Houston, TX) [3]. Cells were maintained in Dulbecco's modified Eagle's medium with low glucose and supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin sulfate. Cells were grown in monolayer cultures and kept in a humidified environment at 37°C and 5% CO2.
To determine the status of Gb3 in IOMM-Lee cells in vitro, the glycolipid was extracted from cultured cells with 20 vol of a 2:1 by volume chloroform:methanol solution [10,18]. The extract was partitioned against water and the lower phase partitioned again against theoretical upper phase. The lower phase was dried completely and dissolved in a known volume of 2:1 chloroform:methanol. The extract and a standard [consisting of glucosylceramide, lactosylceramide, Gb3, and globotetraosylceramide (Gb4)] were separated by thin-layer chromatography (TLC) [10,18] with chloroform:methanol:water; 65:25:4 by volume. The TLC plate was dried and blocked with 1.5% gelatin in 50 mM TBS at 37°C overnight. The plate was washed with TBS and subsequently incubated with 0.1 µg/ml VT1 and immunodetected using monoclonal PH1 anti-VT1 antibody for 1 hour and then goat anti-mouse antibody conjugated to peroxidase (Sigma). Alternatively, the same preparation was sprayed with orcinol for straight detection of Gb3.
To evaluate the in vitro efficacy of VT1 on IOMM-Lee cells, a VT1 cell viability assay was performed [10]. Cells grown to confluence were trypsinized and seeded in 96-well dishes at a density of 5000 cells/well for 24 hours at 37°C prior to treatment with VT1. Ten-fold serial dilutions of VT1 or sodium azide as a positive control were prepared and added to cells. Each dilution was tested in triplicate. Following 1-, 2-, 3-, and 5-day incubations at 37°C, the cells were fixed in 2% formaldehyde in PBS, stained with crystal violet, and then added to a 10% acetic acid solution to solubilize the crystal violet. The percentage of live cells was calculated by absorbance at 590 nm using a microtiter plate reader (Dynatech Laboratories, Chantilly, VA). The apoptotic effects of VT1 on IOMM-Lee cells in culture were also tested. Briefly, approximately 5000 IOMM-Lee cells were plated on Teflon-masked 10-well glass slides (Creative Scientific Methods, Phoenix, AZ) coated with poly l-lysine (Sigma). Cells were then incubated with varying concentrations of VT1 at 37°C. After several time points, cells were washed and then incubated for 30 minutes at room temperature with 20 µl/ml annexin V Alexa 568 (Roche Biochemicals, Mannheim, Germany) and 0.5 µg/ml DAPI (in 10 mM HEPES, pH 7.4, 140 mM NaCl, and 5 mM CaCl2, all from Sigma) for the detection of phosphatidylserine on the outer leaflet of apoptotic cells and cell nuclei, respectively. All doses and time points were done in duplicate. Cells were analyzed by fluorescence microscopy.
Orthotopic Intracranial MM Model
A total of 75 six- to eight-week-old female athymic nude mice (CD1, nu/nu; Charles River Breeding Laboratory, Wilmington, MA) weighing 22±3 g were used for tumour inoculation. All animals were housed in a closed colony in the animal care facility at The Hospital for Sick Children. The protocol followed the guidelines and regulations of the Animal Care Committee. Animals were anesthesized intraperitoneally with a 1:1 combination of Hypnorm (composition: 0.315 mg fentanyl citrate/ml, 10 mg fluanisone/ml, diluted 1:4 in sterile water; Jansen Pharmaceutica, Beerse, Belgium) and Midazolam (5 mg/ml, diluted 1:6.6 in sterile water; Roche Diagnostics, Mississauga, Canada) at a dose of 0.1 ml/10 g.
Mice were stabilized in a stereotactic head frame (ASI instrument, small animal stereotactic frame). The head was prepped with betadine iodine and a subperostial injection of Lidocaine (Abbott Laboratories, Abbott Park, IL) was given. A linear skin incision was subsequently made at the midline, skin was retracted, and the periosteum elevated. A burr hole (Dremel Drill, Racine, WI) was drilled in the anterior right frontal region of the cranium, lateral to the midline and anterior to the bregma suture. A nylon guide screw (Department of Biomedical Engineering, The Hospital for Sick Children, Toronto, Canada) [19] was placed securely in the burr hole and the incision was closed with interrupted Dexon 5-0 sutures. Animals were given a subcutaneous injection of saline and kept warm until recovery.
One week following placement of the guide screw, the animals were reanesthesized and their wounds reopened. Confluent cultures of IOMM-Lee were harvested using 0.05% trypsin for 2 minutes at 37°C. After neutralization of trypsin with media, cells were pelleted by centrifugation and then washed with sterile PBS. The cell pellet was resuspended in sterile PBS. Approximately 1 x 106 cells (3 µl vol) were drawn with the Hamilton syringe and were injected slowly to a depth of 5 mm through the guide screw. The skin was closed and the animals were allowed to recover as above.
VT1 Treatment of Mice Harboring Intracranial Meningiomas and Survival Analysis
Six days following tumor implantation, animals were weighed and randomized to receive one of three treatments: 1) VT1, 2) heat-inactivated VT (HI-VT), or 3) PBS — the latter two serving as controls. Active VT1 was prepared for administration as described [10], or it was inactivated by heating at 80°C or higher for 1 hour. VT1 was prepared fresh each time in sterile PBS to a concentration that would administer 2 µg/kg VT1 or HI-VT toxin in a single dose. Mice were once again anesthesized and their wounds reopened. The Hamilton syringe was used to inject VT1 or controls to precisely determine the same depth of the previously implanted tumor cells through the guide screw in a 2-µl injection volume. After the wounds were closed, the animals were monitored daily for neurological changes and weighed every other day. Survival was measured in days post tumour cell inoculation. Animals were sacrificed by placing them in a mixed CO2 and O2 gas chamber. Immediately after sacrifice, the brains of animals were removed and embedded in OCT (Tissue Tek) embedding medium on dry ice and stored at -80°C until further processing.
Immunohistochemical Analysis of Proliferation, Microvascular Density (MVD), and Apoptosis
Coronal, 5-µm cryostat sections were fixed in acetone and immunohistochemical procedures were performed for immunodetection of MIB-5 and Factor VIII. The procedure was carried out on the NEXES autoimmunostainer (Ventana Medical Systems, Tuscon, AZ) with either a mouse monoclonal anti-MIB-5 antibody (dilution: 1:20; Dako), or a polyclonal anti-Factor VIII antibody (dilution: 1:400; Dako). MIB-5 reactivity was visualized using a modification of the MOM ABC (Vector Laboratories; cat no. Pk-2200)-DAB system. For Factor VIII, visualization of positive staining was through the ABC system employing the Ventana DAB detection system.
Apoptosis was examined by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP biotin end labeling) method using the In Situ Cell Death Detection POD kit (Roche Diagnostics; cat no. 1684817) with some modifications. Briefly, sections were fixed in 4% paraformaldehyde and washed in PBS. Endogenous peroxidases were blocked in 0.3% hydrogen peroxide in methanol. The TUNEL reaction mixture was added to the tissue and incubated at 37°C for 60 minutes. After washing in PBS, sections were treated with a protein block and incubated with the Converter POD (anti-fluorescein antibody conjugated to horseradish peroxidase) at 37°C for 30 minutes. Detection of incorporation of dUTP was achieved by treating the sections with DAB in hydrogen peroxide (Sigma). All sections were counterstained with haematoxylin, dehydrated, and mounted.
Semiquantitative and Statistical Analyses
The apoptotic index of IOMM-Lee cells in culture was obtained by averaging the percentage of annexin V-positive cells in five high-powered fields (HPFs) (x400 magnification). Cells negative for annexin V were identified by DAPI. For immunohistochemical quantitation, entire tissue sections were analyzed for the strength of positive Gb3 staining obtained and scored on a scale ranging from “+” (very little staining) to “+ + +” (very strong staining). Negative control slides were also scored. The mouse brain sections were analyzed by Scion Image Beta 4.02 (downloaded from http://www.scioncorp.com, NIH image). First, five HPFs (x400 magnification) were acquired from representative and random sites in the tumour using a digital CCD CoolSnap camera (Photometrics, Trenton, NJ) mounted on a DMLB Leica light microscope (Photometrics). The MIB-5 and TUNEL labeling indices were obtained by averaging the percentage of positively stained nuclei in five HPFs [20]. MVD was calculated by averaging the number of Factor VIII-positive vessels per five HPFs [21]. In order to determine statistical significance (P<.05) between groups, multiple comparisons of the data set were performed using the analysis of variance (ANOVA) statistical test. Survival data of animals were plotted on a Kaplan-Meier curve and the log-rank (Manley-Cox) statistical test for survival was performed.
Results
VT Receptor Expression in Human Meningiomas
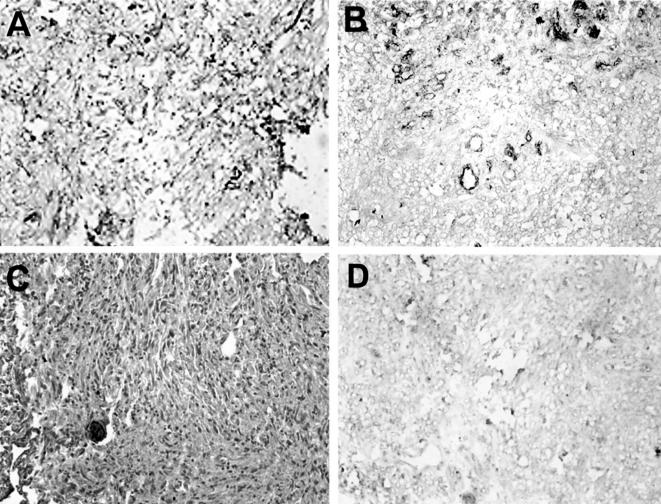
The VT receptor, Gb3, was elevated in MMs when compared to benign meningiomas (Table 1). Of 11 MMs examined, nine were positive for Gb3; interestingly, the degree of Gb3 immunoreactivity was variable (Table 1, Figure 1). Four of nine Gb3-positive tumours demonstrated strong expression of the glycolipid. In all positive samples, Gb3 was primarily localized to the microvasculature with immunoreactivity often demarcating the presence of many vessels (Figure 1, A and B). In two tumors with strong staining, immunoreactivity was found not only within the vasculature, but also within tumour cells (Figure 1A). Only one of five benign meningiomas demonstrated any Gb3 positivity, and this was extremely faint (Figure 1C). In tissue sections serving as control (VT1 emitted) no Gb3 immunoreactivity was detected (Figure 1D).
Table 1.
Semiquantitative Analysis of Gb3 Expression in Human Meningiomas (n=16).
| Strength of Gb3 Staining | Number of MMs Staining Positive | Number of Benign Meningiomas Staining Positive |
| + + + + | 2 | |
| + + + | 2 | |
| + + | 2 | |
| + | 3 | 1 |
| 0 | 2 | 4 |
Figure 1.
Photomicrograph (original magnification, x200) illustrating immunohistochemical staining of Gb3 in human meningiomas. (A) A malignant meningioma representing very strong (+ + + +) Gb3 immunoreactivity (brown) where staining is both vascular and extravascular. (B) A MM semiquantitated as strong staining (+ + +) primarily due to the predominantly vascular localization of Gb3. (C) A benign meningioma showing no evidence of Gb3 positivity. (D) A MM (same as in B) showing no Gb3 immunoreactivity when VT1 is omitted.
IOMM-Lee Cells Express Gb3 and are Sensitive to VT1
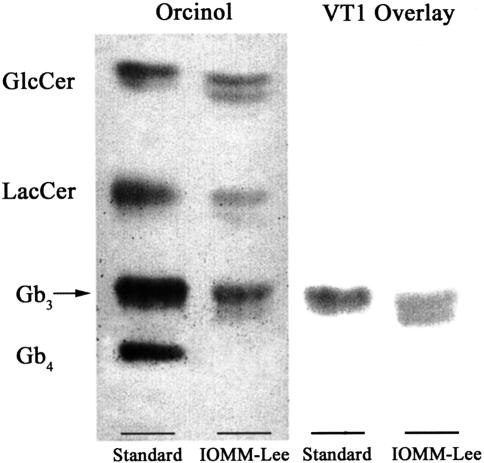
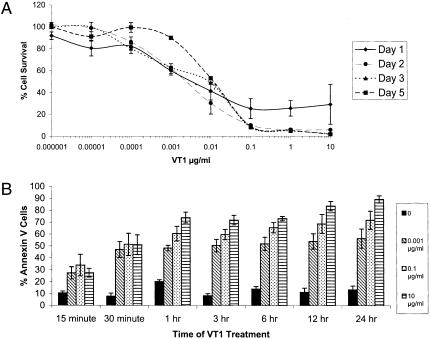
The Gb3 status of IOMM-Lee cells was determined by TLC of the glycolipid extract and was followed by orcinol spray or a VT1 overlay to detect Gb3 binding to VT. Consistent with the demonstration of Gb3 within IOMM-Lee cells (Figure 2), the cell viability assay demonstrated that IOMM-Lee was sensitive to VT1 in a dose-dependent manner (Figure 3). However, our results suggest that the effects of VT1-mediated toxicity are a sustained effect over the time period studied (Figure 3A). The CD50 of cells at day 3 post VT1 treatment was 0.01 µg/ml. The other time points were within 1log difference of the CD50 at this time point. The cell growth seen at day 5 post VT1 treatment can be attributed to cells beginning to repopulate the wells. Treatment of cells with 10 mM sodium azide resulted in 100% kill of cells (data not shown). Similarly, the results of our annexin V study suggest that cells were inducing apoptosis in a dose-dependent fashion with no significant evidence of a time-dependent response (Figure 3B).
Figure 2.
Detection of the VT receptor glycolipid, Gb3, in IOMM-Lee cell line. A TLC separation of the glycolipid visualized by orcinol (left). The right panel is the same preparation assayed by VT1 overlay. As seen from the TLC plates, Gb3 is expressed (left) and binds to VT1 (right) in IOMM-Lee.
Figure 3.
VT1 sensitivity of IOMM-Lee cells. (A) Increasing concentration of toxin by 10-fold dilutions leads to a decrease in the number of viable cells (CD50 of 0.01 µg/ml) and this dose response is sustained over the course of 1, 2, 3, and 5 days after initial treatment with VT1 (n=3 for each point on the graph). (B) The percentage of annexin V-positive cells, an indicator of cell death, increases with higher concentrations of VT1 (0.001, 0.1, and 10 µg/ml). Each bar graph represents average percentages of five HPFs taken from two samples.
Survival Studies
Four of 75 animals studied died immediately postoperatively and were not included in survival studies. There were a total of 28 animals treated with VT1, but one animal was sacrificed prematurely due to severe conjunctivitis and was not included in any further analysis. Twenty-five and 15 animals were used in the HI-VT and PBS groups, respectively, and three animals were included in a sham surgery arm in which they received the anesthetic, a scalp incision, and burr hole but received neither cells nor treatment.
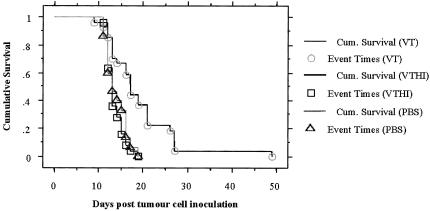
A single intratumoral VT1 injection was sufficient to increase the survival of nude mice harboring IOMM-Lee tumours intracerebrally by a significant factor (P<.0001, log-rank test for survival; Figure 4). A total of 27 nude mice received a single dose (2 µg/kg) of active VT1. The median survival for VT1-treated animals was 17 days and the survival ranged from 9 to 49 days. For control, a total of 25 animals received a single injection of either HI-VT1 (2 µg/kg) or 15 animals received a single injection of PBS. The median survival for animals treated with HI-VT or PBS was 13 days for both groups, and in these groups, survival ranged from 11 to 19 days. The three animals that underwent sham surgery (no cell inoculation, or treatment) outlived all experimental mice and showed no evidence of disease symptoms (data not shown). Their brains were harvested and used as normal brain control for later immunohistochemical studies. Tumor take was 100% as was evident from histological assessment of mouse brains. A critical endpoint criterion in the survival study involved monitoring weight loss and ensuring that animals were sacrificed within a narrow margin of a 20% loss in body weight. All three treatment groups were sacrificed within this parameter. The average weight of VT1-treated animals at sacrifice was 17.3 g (18.4% loss of body weight); for HI-VT-treated animals, the average weight was 21.2 g (19.1% loss of body weight); and for PBS-treated animals, the average weight was 21.4 g (22.7% loss of body weight). The differences in weight between the groups were not statistically significant.
Figure 4.
Survival analysis of mice treated with VT1, HI-VT, or PBS. Kaplan-Meier curve demonstrates that VT1-treated mice survive significantly longer, up to 49 days post tumour cell inoculation, than either of the control groups which only had mice live up to a maximum of 19 days (P < .0001, log-rank test for survival).
Effects of VT1 on IOMM-Lee Proliferative Index, Vascularity, and Apoptosis
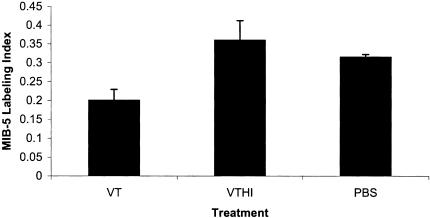
The MIB-5 labeling index of VT1-treated animals was 20±0.029%, a significant reduction in the percentage of proliferating cells when compared to control-treated animals [HI-VT, 36±0.05% (P<.0043) and PBS, 31±0.007% (P<.0248)] (Figures 5 and 6). No significant different was found between HI-VT and PBS (P<.6543).
Figure 5.
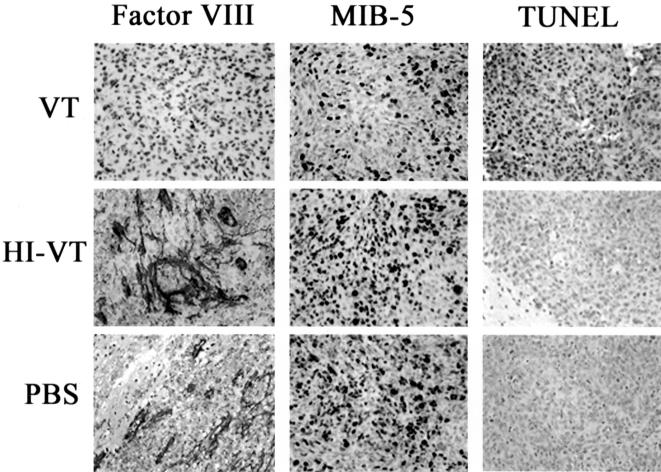
Immunohistochemical analysis of VT1-associated anti-neoplastic effects in IOMM-Lee tumours. Photomicrographs (original magnification, x400) illustrates the marked reduction in MVD (Factor VIII) and the proliferation index (MIB-5) in VT1-treated animals when compared to control groups. Additionally, this figure depicts the remarkable increase in the percentage of cells undergoing apoptosis (TUNEL) in animals treated with VT1. The brown staining indicates immunoreactivity.
Figure 6.
Anti-proliferative effects of VT1 on IOMM-Lee-derived tumours. Graph illustrates a reduction in the percentage of cells stained with the proliferation marker, MIB-5, in VT1-treated animals (20±0.029%). Compared to HI-VT (36±0.05%, P<.0043) and PBS (31±0.007%, P<.0248), no significant different was found between HI-VT and PBS (P<.6543).
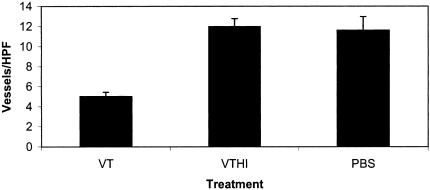
To assess the anti-angiogenic effects of VT1, an anti-Factor VIII antibody was used to stain the microvasculature of IOMM-Lee-derived tumours. The number of Factor VIII-positive blood vessels was compared among the three experimental groups. There was a marked reduction in the MVD of VT1-treated animals when compared to HI-VT- and PBS-treated animals (Figures 5 and 7). This is best exemplified by MVD raw counts of 5±0.42 for VT1-treated animals and 11.9±0.79 or 11.6±1.35 for HI-VT and PBS groups, respectively. In addition, blood vessels associated with either of the control groups appeared larger and more robust than the blood vessels of the VT1-treated animals (Figure 5). ANOVA demonstrated statistical significance between the VT1 and HI-VT groups (P<.0001) and between the VT1- and PBS-treated groups (P<.0001). There was no significant difference between the PBS- and HI-VT-treated groups (P<.693).
Figure 7.
Angiogenesis in IOMM-Lee tumours of treated animals. The graph depicts a decrease in the MVD of animals treated with VT1 (5±0.42). This is compared to HI-VT-treated (11.9±0.79, P<.0001) and PBS-treated (11.6±1.35, P<.0001) animals. These values were obtained by averaging the number of Factor VIII-stained vessels in five HPFs. There was no significant difference between the PBS- and HI-VT-treated groups (P<.693).
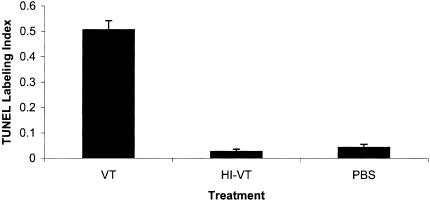
The level of apoptosis within the tumour xenograft following VT1 injection was determined by TUNEL staining. In animals treated with VT1, 51±0.04% of cells was undergoing apoptosis, which is remarkably higher than the apoptotic indices of either HI-VT-treated (2.6±0.009%, P<.0001) or PBS-treated (4.3±0.012%, P<.0001) animals (Figures 5 and 8). There was no statistical significance between the levels of apoptosis in HI-VT- and PBS-treated animals (P<.6254).
Figure 8.
Treatment with VT1 leads to a proapoptotic effect. TUNEL staining of IOMM-Lee tumours treated with VT1 (51±0.04%) shows a remarkable increase in the number of cells undergoing apoptosis. This is contrasted with the apoptotic indices of HI-VT-treated (2.6±0.009%, P<.0001) and PBS-treated (4.3± 0.012%, P<.0001) animals. There was no statistical significance between the levels of apoptosis in HI-VT- and PBS-treated animals (P<.6254).
Discussion
In this study, we have shown that a single intratumoral injection of VT1 was sufficient to significantly improve survival in nude mice bearing intracranial MM. These results are particularly promising when one considers the upregulation of Gb3 observed in 9/11 human cases of MM. On the other hand, the benign meningiomas examined here were virtually all negative for expression of the glycolipid. As such, we would predict that VT1 may not be an effective agent for this histologic subtype of meningioma. Based on the percentage of VT1-treated animals that outlived the median survival of animals in control groups, approximately 70% of animals treated with VT1 has shown a response to treatment.
VT1 is a type II ribosome-inactivating protein produced by pathogenic strains of E. coli and only targets cells that express the glycolipid, Gb3, otherwise known as CD77 [16]. Based on the observations that Gb3 is upregulated in many human neoplasias including cancers of breast, ovarian, testicular, hematologic, and astrocytic origin, several studies to date have exploited the notion that VT may be a viable approach to cancer treatment [10–14,22,23]. Additionally, VT1 treatment of mice bearing Gb3-positive tumours was found to prevent metastasis [12] and reduce tumour burden in mice harboring subcutaneous astrocytic tumours [11]. Furthermore, drug-resistant ovarian tumours were also found to express higher levels of Gb3 than the primary tumor [22]. Interestingly, multiple drug-resistant variants of such cell lines were found to be hypersensitive to VT1 and cell transfection with MDR1 has been found to dramatically increase Gb3 and susceptibility to VT [11]. This suggests a particularly attractive potential that VT could be used as a therapeutic against drug-resistant tumours where prior chemotherapy had failed, as is the case for many brain tumours including MMs [8] and astrocytomas [11].
Expectedly, not all animals in our study treated with VT1 responded to treatment. One explanation could simply be related to the intrinsic biological variation among mice. Additionally, whereas a significant effect on survival was achieved with only a minimal dose of VT1 (2 µg/kg; LD50 is 40 µg/kg) [11], a better response to treatment is likely attainable if the administration of VT1 is further optimized, namely, by increasing the dose or possibly by giving multiple injections. An intriguing observation is the relative insensitivity of human cerebral capillary endothelial cells to VT [10]. Thus, increasing the dose of VT1 administered is likely not to be toxic to the normal brain vasculature. Capillary endothelial cells derived from kidney and the large bowel appear to be the primary targets of VT-associated injury in hemolytic uremic syndrome and hemorrhagic colitis [14–16]. This has not been a concern with direct intracranial, intratumoral administration of VT1. Fortunately, we have performed previous murine toxicology studies in our laboratory, which have indicated that mice are able to tolerate 10 times the LD50 dose of VT1 when given intracerebrally without demonstrating any symptoms or signs affecting either the renal or gastrointestinal systems (unpublished data).
Gb3 levels in endothelial cell cultures, and thereby their sensitivity to VT, have been shown to be modulated in the presence of various cytokines [14,24]. It is possible that cytokines secreted by the tumour cells in meningiomas [25,26] act in a paracrine fashion to upregulate Gb3 expression in tumour-derived endothelial cells, in turn rendering them susceptible to VT1. This hypothesis is consistent with our data, which have clearly demonstrated increased Gb3 expression in the vasculature of human MMs. This is further supported by the fact that VT1 led to an anti-angiogenic effect, as seen by the reduction in the MVD of IOMM-Lee tumours. Meningiomas are known to be highly vascularized tumours and known to induce angiogenesis by paracrine mechanisms involving one or more molecules like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [27,28]. Therefore, molecules such as VT1, which are known to inhibit angiogenesis, may prove to be clinically efficacious.
Homeostatic control of cell number is the result of a dynamic balance between cell proliferation and cell death. It is evident from our study that VT1 targets both components of this equation by inhibiting cell proliferation through the inhibition of protein synthesis and increasing cell death by inducing apoptosis. It has been shown that the B subunit is capable of inducing apoptosis independent of the enzymatic actions of the A subunit [29]. Therefore, in addition to cell death by the inhibition of protein synthesis, VT holotoxin is capable of inducing apoptosis. For example, it has been shown that Gb3-positive Burkitt lymphoma cells and germinal B lymphocytes are highly susceptible to apoptosis by the VT1-B subunit [29]. Apoptosis is an important cellular process within multicellular organisms and is clearly a target in the design of anti-neoplastic regimes [11].
The use of VT in an in vivo therapeutic setting appears challenging at the present time because of its association with hemolytic uremic syndrome and hemorrhagic colitis. However, we believe that a therapeutic window of opportunity exists for VT1 in cancer therapy. For example, hemolytic uremic syndrome and hemorrhagic colitis are diseases at the extremes of age, under age 3 and over age 70, respectively. This indicates that the general adult population is resistant to VT-induced pathology [15]. In addition, topical application or local deposition (intratumoral) of the toxin into a confined body compartment, such as in the current study, represents possible options. The brain is especially privileged to local treatment because the diffusion of macromolecules to and from the brain is especially limited by the blood-brain barrier [13].
Our current studies have described the Gb3 receptor status and VT1 sensitivity in the MM cell line, IOMM-Lee, and has shown the potency of VT1 treatment in vivo by employing an orthotopic mouse model of IOMM-Lee. Clearly, a plethora of potent anti-neoplastic effects on intracerebral IOMM-Lee tumours was evident. These anti-neoplastic mechanisms effectively increased the survival of nude mice with only a single injection of the toxin intratumorally. Taken together, it is not unreasonable to believe that MMs and other brain tumours alike, which express Gb3, could be the target for VT1 therapy.
Acknowledgements
The authors would like to thank Michael Ho and Lily Morikawa, from the Department of Pathology at the Hospital for Sick Children, Toronto, Canada, for their expertise in immunohistochemical and tissue processing procedures.
Abbreviations
- Gb3
globotriosylceramide
- I-VT
heat-inactivated verotoxin
- HPF
high-powered field
- MVD
microvascular density
- BS
phosphate-buffered saline
- VT1
verotoxin 1
- WHO
World Health Organization
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the National Brain Tumor Foundation, Brainchild, and the Dutch Cancer Foundation (Wilhelmina Kanker Fonds). J.T. Rutka is a scientist of the CIHR.
References
- 1.Buschges R, Bostrom J, Wolter M, Blaschke B, Weber R, Lichter P, Collins V, Reifenberger G. Analysis of human meningiomas for aberrations of the MADH2, MADH4, APM-1, and DCC tumour suppressor genes on the long arm of chromosome 18. Int J Cancer. 2001;92:551–554. doi: 10.1002/ijc.1219. [DOI] [PubMed] [Google Scholar]
- 2.Joseph E, Sandhyamani S, Rao M, Nair S, Radhakrishnan V. Atypical meningioma: a clinicopathological analysis. Neurol India. 2000;48:338–342. [PubMed] [Google Scholar]
- 3.Lee W. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27:389–396. [PubMed] [Google Scholar]
- 4.McCutcheon I, Friend K, Gerdes T, Zhang B, Wildrick D, Fuller G. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg. 2000;92:306–314. doi: 10.3171/jns.2000.92.2.0306. [DOI] [PubMed] [Google Scholar]
- 5.Perry A, Cai D, Scheithauer B, Swanson P, Lohse C, Newsham I, Weaver A, Gutmann D. Merlin, DAL-1 and progesterone receptor expression in clinicopathologic subsets of meningioma: a correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59:872–879. doi: 10.1093/jnen/59.10.872. [DOI] [PubMed] [Google Scholar]
- 6.Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 7.Hug E, DeVries A, Thornton A, Munzenrider J, Pardo F, Hedley-Whyte E, Bussiere M, Ojemann R. Management of atypical and malignant meningiomas: role of high dose 3D-conformal radiation therapy. J Neuro-Oncol. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 8.Newton H, Slivka M, Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neuro-Oncol. 2000;49:165–170. doi: 10.1023/a:1026770624783. [DOI] [PubMed] [Google Scholar]
- 9.Blankenstein M, Verheijen F, Jacobs J, Donker T, Duijnhoven MV, Thijssen J. Occurrence, regulation and significance of progesterone receptors in human meningioma. Steroids. 2000;65:795–800. doi: 10.1016/s0039-128x(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 10.Arab S, Murakami M, Dirks P, Boyd B, Hubbard S, Lingwood C, Rutka J. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J Neuro-Oncol. 1998;40:137–150. doi: 10.1023/a:1006010019064. [DOI] [PubMed] [Google Scholar]
- 11.Arab S, Rutka J, Lingwood C. Verotoxin induces apoptosis and the complete, rapid and long-term elimination of human astrocytoma xenografts in nude mice. Oncol Res. 1999;11:33–39. [PubMed] [Google Scholar]
- 12.Farkas-Himsley H, Hill R, Rosen B, Arab S, Lingwood A. The bacterial colicin active against tumour cells in vitro and in vivo is verotoxin 1. Proc Natl Acad Sci. 1995;92:6996–7000. doi: 10.1073/pnas.92.15.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gariepy J. The use of Shiga-like toxin 1 in cancer therapy. Crit Rev Oncol/Hematol. 2001;39:99–106. doi: 10.1016/s1040-8428(01)00126-3. [DOI] [PubMed] [Google Scholar]
- 14.Lingwood C. Verotoxin/globotriaosyl ceramide recognition: angiopathy, angiogenesis and antineoplasia. Biosci Rep. 1999;19:345–354. doi: 10.1023/a:1020299819637. [DOI] [PubMed] [Google Scholar]
- 15.Lingwood C. Glycolipid receptors for verotoxin and Heliobacter pylori: role in pathology. Biochim Biophys Acta. 1999;1455:375–386. doi: 10.1016/s0925-4439(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 16.Lingwood C. The role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 17.O'Loughlin E, Robinson-Browne R. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect. 2001;3:493–507. doi: 10.1016/s1286-4579(01)01405-8. [DOI] [PubMed] [Google Scholar]
- 18.Nuttika A, Boyd BB, Lingwood Ca. Methods for the identification of host receptors for verotoxin. Methods Mol Med in press. 2002 doi: 10.1385/1-59259-316-x:197. [DOI] [PubMed] [Google Scholar]
- 19.Bampoe J, Glen J, Hubbard S, Salhia B, Shannon P, Rutka J, Bernstein M. Adenoviral vector-mediated gene transfer: timing of wild-type p53 gene expression in vivo and effect of tumor transduction on survival in a rat glioma brachytherapy model. J Neuro-Oncol. 2000;49:27–39. doi: 10.1023/a:1006476608036. [DOI] [PubMed] [Google Scholar]
- 20.Carlotti C, Salhia B, Weitzman S, Greenberg M, Dirks P, Becker L, Rutka J. Evaluation of proliferative index and cell cycle protein expression in choroid plexus tumors in children. Acta Neuropathol. 2002;103:1–10. doi: 10.1007/s004010100419. [DOI] [PubMed] [Google Scholar]
- 21.Salhia B, Angelov L, Roncari L, Wu X, Shannon P, Guha A. Expression of vascular endothelial growth factor by reactive astrocytes and associated neoangiogenesis. Brain Res. 2000;883:87–97. doi: 10.1016/s0006-8993(00)02825-0. [DOI] [PubMed] [Google Scholar]
- 22.Arab S, Russel E, Chapman W, Rosen B, Lingwood C. Expression of the verotoxin receptor glycolipid, globotriaosylceramide, in ovarian hyperplasias. Oncol Res. 1997;9:553–563. [PubMed] [Google Scholar]
- 23.LaCasse E, Bray M, Patterson B, Lim W, Perampalam S, Radvanyi L, Keating A, Stewart A, Buckstein R, Sandhu J, Miller N, Banerjee D, Singh D, Belch A, Pilarski L, Gariepy J. Shiga-like toxin-1 receptor on human breast cancer, lymphoma, and myeloma and absence from CD34+ hematopoietic stem cells: implications for ex vivo tumour purging and autologous stem cell transplantation. Blood. 1999;94:2901–2910. [PubMed] [Google Scholar]
- 24.Molostvov G, Morris A, Rose P, Basu S. Interaction of cytokines and growth factor in the regulation of verotoxin-induced apoptosis in cultured human endothelial cells. Br J Haematol. 2001;113:891–897. doi: 10.1046/j.1365-2141.2001.02835.x. [DOI] [PubMed] [Google Scholar]
- 25.Merlo A, Juretic A, Zuber M, Filgueira L, Luscher U, Caetano V, Ulrich J, Gratzl O, Heberer M, Spagnoli G. Cytokine gene expression in primary brain tumours, metastases and meningiomas suggests specific transcription patterns. Eur J Cancer. 1993;29A:2118–2125. doi: 10.1016/0959-8049(93)90046-i. [DOI] [PubMed] [Google Scholar]
- 26.Boyle-Walsh E, Birch M, Gallagher J, Speirs V, White M, Shenkin A, Fraser W. RT-PCR detection of cytokine transcripts in a series of cultured human meningiomas. J Pathol. 1996;178:442–446. doi: 10.1002/(SICI)1096-9896(199604)178:4<442::AID-PATH521>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Sanson M, Cornu P. Biology of meningiomas. Acta Neurochir. 2000;142:493–505. doi: 10.1007/s007010050462. [DOI] [PubMed] [Google Scholar]
- 28.Bitzer M, Opitz H, Popp J, Morgalla M, Gruber A, Heiss E, Voigt K. Angiogenesis and brain oedema in intracranial meningiomas: influence of vascular endothelial growth factor. Acta Neurochir. 1998;140:333–340. doi: 10.1007/s007010050106. [DOI] [PubMed] [Google Scholar]
- 29.Taga S, Carlier K, Mishal Z, Capoulade C, Mangeney M, Lecluse Y, Coulaud D, Tetaud C, Pritchard L, Tursz T, Wiels J. Intracellular signaling events in CD77-mediated apoptosis of Burktitt's lymphoma cells. Blood. 1997;90:2757–2767. [PubMed] [Google Scholar]