Abstract
To identify a set of genes related to radiosensitivity of cervical squamous cell carcinomas and to establish a predictive method, we compared expression profiles of 9 radiosensitive and 10 radioresistant tumors obtained by biopsy before treatment, on a cDNA microarray consisting of 23,040 human genes. We identified 121 genes whose expression was significantly greater in radiosensitive cells than in radioresistant cells, and 50 genes that showed higher levels of expression in radioresistant cells than in radiosensitive cells. Some of these genes had already known to be associated with the radiation response, such as aldehyde dehydrogenase 1 (ALDH1) and X-ray repair cross-complementing 5 (XRCC5) (P<.05, Mann-Whitney test). The validity of the total of 171 genes as radiosensitivity related genes were certified by permutation test (P<.05). Furthermore, we selected 62 genes on the basis of a clustering analysis, and confirmed the validity of these genes with cross-validation test. The cross-validation test also indicates the possibility of making prediction of radiosensitivity for discriminating radiation-sensitive from radiation resistant biopsy samples by predicting score (PS) values calculated from expression values of 62 genes in 19 samples, because the prediction successfully and unequivocally discriminated the radiosensitive phenotype from the radioresistant phenotype in our test panel of 19 cervical carcinomas. The extensive list of genes identified in these experiments provides a large body of potentially valuable information for studying the mechanism(s) of radiosensitivity, and selected 62 genes opens the possibility of providing appropriate and effective radiotherapy to cancer patients.
Keywords: cervical cancer, radiosensitivity, cDNA microarray, gene expression profiles, classification
Introduction
Although proper diagnosis and effective treatments for cervical cancers are widely available now [1], this disease is still a leading cause of death for women worldwide [2]. Radiotherapy is a generally effective therapeutic method, particularly for patients with cancers at an advanced stage. However, individual patients may show quite different patterns of response against radiotherapy; some can be cured, but others cannot, and the latter may therefore suffer needlessly from severe side effects. Hence, if treatment is to become more patient specific, the molecular mechanism(s) of radiosensitivity need to be clarified.
Several molecular markers that reflect radiosensitivity have been proposed as the result of studies that have involved, for example, transfection of oncogenes such as N-ras, v-myc with H-ras, and v-fos into cultured cells to induce a radioresistant phenotype [3,4]. Activation of c-raf-1 has been positively correlated with radioresistance of head and neck squamous cell carcinomas [5], and certain cell cycle-and apoptosis-related genes also have been correlated with radiosensitivity; e.g., loss or dysfunction of p16 renders melanoma cells resistant to ionizing radiation, whereas expression of exogenous wild-type p16 and p21 in glioblastoma cells can induce radiosensitivity [6,7]. Transfection- or radiation-induced expression of Bcl-2 proteins, which regulate apoptosis, into pro-myeloid cells has introduced a radioresistant phenotype [8,9]; furthermore, expression of Bax, when induced by gamma irradiation, confers radiosensitivity on lymphoid cells, small intestinal epithelial cells [10] and cervical cancer cells [9]. However, although such discoveries have brought partial understanding of the molecular mechanisms responsible for cellular radiosensitivity, the whole picture remains to be clarified.
Because the complex mechanism of radiosensitivity cannot be explained by a small number of genes, we need to collect genome-wide information about all the genes involved. To that end, we recruited a newly developed technique, cDNA microarray [11], which provides high-throughput analysis of expression profiles by means of small-array slides spotted with cDNAs [12–15].
Here we report a genome-wide cDNA microarray analysis of 23,040 human cDNAs, in biopsy samples from 19 cervical squamous carcinomas (9 of them radiosensitive and 10 radioresistant, classified according to tumor-suppression ratios). We identified 171 genes that were differentially expressed between the two groups; of those, 121 showed elevated expression and 50 showed decreased expression in radiosensitive tumor cells relative to their expression levels in radioresistant cells. In addition, further selected 62 genes showed feasibility of predicting the radiosensitivity of cervical squamous cell carcinomas. These results not only disclose the complex nature of radiosensitivity as regards the response of cervical squamous cell carcinomas to ionizing radiation, but also provide information that should identify novel targets for efforts to expand the effectiveness of radiotherapy.
Materials and Methods
Tissue Samples
Cervical squamous cell carcinoma tissues were obtained with informed consent from 19 patients who underwent biopsy before radiotherapy at Kansai Medical University, and snap-frozen at -80°C. All cases were at stages IIB to IVB, and were papillomavirus (HPV)-positive. We observed the population of tumor cells in all biopsy samples was over 90% by hematoxylin and eosin staining. Methods for typing of HPV and establishing p53 status were described previously [9].
Radiation Treatments
All 19 patients were treated with radiotherapy after sampling. A total of 30.6 Gy was provided to the whole pelvis, plus an additional dose to parametria with central shielding to complete 52.2 Gy, along with 192Ir high doserate intracavitary brachytherapy. Details have been described elsewhere [9]. Effects of the therapy, including local failure, were checked a month after treatment. Nine patients revealed 100% reduction in tumor size and the remaining 10 showed 0% to 40% reduction (Table 1). The former were classified as a radiosensitive group (complete response; [CR]) and the latter as a radioresistant group (no change [NC]).
Table 1.
Clinical Characteristics of 19 Cervical Squamous Carcinoma Samples.
| Sample No. | Stage at Diagnosis* | Initial Response† | Tumor Suppression Ratio in Size (%) | Age | Status at p53‡ |
| 16 | IIIB | CR | 100 | 69 | WT |
| 17 | IIIB | CR | 100 | 65 | WT |
| 23 | IIIB | CR | 100 | 67 | WT |
| 47 | IVA | CR | 100 | 67 | WT |
| 74 | IIIB | CR | 100 | 77 | WT |
| 75 | IIIB | CR | 100 | 32 | WT |
| 81 | IVB | CR | 100 | 55 | WT |
| 83 | IIIB | CR | 100 | 59 | WT |
| 89 | IIIB | CR | 100 | 63 | WT |
| 31 | IIIB | NC | 40 | 53 | WT |
| 35 | IIIB | NC | 19 | 47 | WT |
| 39 | IIIB | NC | 0 | 70 | WT |
| 45 | IIIB | NC | 40 | 59 | WT |
| 52 | IVB | NC | 5 | 56 | WT |
| 53 | IVB | NC | 5 | 63 | WT |
| 55 | IVB | NC | 0 | 76 | WT |
| 85 | IVB | NC | 0 | 52 | WT |
| 87 | IVB | NC | 0 | 84 | WT |
| 96 | IVB | NC | 10 | 53 | WT |
Tumors were staged according to International Federation of Gynecology and Obstetrics criteria.
CR, tumor suppression ratio of 100%. NC, 50% suppression to +25% growth a month after radiotherapy.
Mutational status of p53. WT, wild type.
RNA Extraction and Amplification
Total RNAs were extracted from each specimen by TRIZOL (Invitrogen, Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. The extracted RNAs were treated for 1 hour at 37°C with 10 U of DNase I (Nippon Gene, Japan) in the presence of 1 U of RNase inhibitor (TOYOBO, Osaka, Japan), to remove any contaminating genomic DNA. After inactivation of DNase at 70°C for 10 minutes, the RNAs were purified with phenol-chloroform-isoamyl alcohol (Gibco BRL, Grand Island, NY) and then precipitated by ethanol. Next, all DNase I-treated RNAs were subjected to T7-based RNA amplification as described previously [16]. Two rounds of amplification yielded 65 to 152 µof amplified RNA (aRNA) from each sample. As a control for comparing gene expression profiles between the CR and NC groups on the microarray, we performed two rounds of T7-RNA amplification using a mixture of poly A+ RNAs from tissues of 12 normal human organs (brain, heart, liver, skeletal muscle, small intestine, spleen, placenta, thyroid, fetal brain, fetal kidney, fetal lung, and fetal liver) purchased from Clontech (Palo Alto, CA) as a control.
Microarray Design, Production, and Hybridization
We selected 23,040 cDNA clones from the UniGene database of the National Center for Biotechnology Information (Bethesda, MD) (build #131). Our cDNA microarray was constructed essentially as described previously [13]; 2.5 µg of aRNA from each cervical carcinoma was labeled with Cy5-dCTP and the control aRNA was labeled with Cy3-dCTP by a protocol described elsewhere [13]. Hybridization, washing, and scanning were carried out according to published methods [13].
Data Analysis and Selection of Differentially Expressed Genes
Signal intensities of Cy3 and Cy5 from the 23,040 spots were quantified by the Array vision software (Amersham Biosciences, Piscataway, NJ) and normalized as described previously [12]. In the quantification step, local background correction method was adopted. Because the data were unreliable when intensities fell below 2.5x105 relative fluorescent units or signal to noise ratios were below 3.0 for both Cy3 and Cy5, genes corresponding to those spots were not investigated any further. To investigate genes that were clearly expressed differently between CR and NC tumors, the Mann-Whitney test was applied based on geneexpression values of X, where X=the Cy5/Cy3 signal intensity ratio for each gene and for each sample. U values for Mann-Whitney test were calculated for each gene. Genes with U values lower than 20 or greater than 70 were selected (P<.05 for comparing 9 CR samples vs. 10 NC samples). Because the U values were calculated for each sample in the CR group against each sample in the NC group for each gene based on each X value, genes that have U value lower than 20 indicate upregulated in the CR group compared to the NC group. However, genes that have U value more than 70 indicate upregulated in the NC group compared to the CR group. 297 genes were upregulated in the CR group and 132 genes were upregulated the in NC group. However, because more than half of these genes have small differences in expression level between CR and NC group, the difference might be caused by data fluctuation. Therefore, genes showing differences more than double the median expression value between the two groups (µXCR/µXNC≦0.5 or ≧2.0, where µXCR and µXNC indicate median X values for the CR or NC group, respectively) were defined as radiosensitivity (or radioresistance) related genes. A total of 171 genes were selected (121 were significantly greater in radiosensitive cells than in radioresistant cells, and 50 were higher levels of expression in radioresistant cells than in radiosensitive cells).
Permutation Test
To further evaluate the validity of 171 genes selected by Mann-Whitney tests, permutation test was performed as described previously [18], and the probabilities of the genes to be correlated to group distinction, Ps, were also estimated. When each gene was represented by expression vector v(g)=(X1, X2, ..., X19), where Xi denotes the expression level of gene of the ith sample in the initial set of samples, idealized expression patterns were represented by c=(c1, c2, ..., c19), where ci= +1 or 0 according to whether the ith sample belongs to the CR or NC group. The correlation between a gene and a group distinction Pgc was defined as follows: i.e., Pgc=(µCR-µNC)/(σCR+σNC), where µCR (µNC) and σCR (σNC) indicate the means and the standard deviations of log2 X of the gene “g” for each sample in newly defined CR (NC) group. Permutation test was conducted by permuting the coordinates of c 10,000 times. During every permutation, the correlation values, Pgcs, were calculated. These procedures were performed 10,000 times, repeatedly. On the hypothesis that these obtained 10,000 Ag values show ideal normal distribution, P values, which imply probability of the genes to classify the two groups by chance was estimated for each selected 171 gene.
Hierarchical Clustering
These 171 genes were subjected to a hierarchical-clustering protocol using “Cluster” and “Tree view” software written by M. Eisen [17]. Before applying the clustering algorithm, gene-expression values (X) for each gene in each of the 19 samples were log-transformed (log2 X). After that all values in each row and/or column of data were multiplied by scale factor S, so that the sum of the squares of the values in each row and column was 1.0 (a separate S is computed for each row/column). Next, row-wise and column-wise median values were subtracted from the values in each row and/or column data, so that the median value of each row and/or column is 0. Hierarchical clustering was performed using distance metrics based on Pearson correlation and adopting Average Linkage Clustering method.
Cross-Validation Test
The selected 62 genes from 171 genes by clustering experiment were subjected to cross-validation test. Among the total 19 samples tested above, one sample was withheld as test sample and the other 18 samples were used for building predictor according to the method as described previously [18]. Next, predictive score (PS) for test sample was calculated as follow; PS=ΣVg, where Vg=Ag′(Xg-Bg), Ag′ = (µCR′- µNC′)/(σCR′+σNC′), and Bg=(µCR′+µNC′)/2; µCR′ (µNC′) and σCR′(σNC′) indicates the means and standard deviations of log2 X of the gene “g” for each sample in the CR (NC) group defined as predictor samples. Xg denotes the log2 X of the gene “g” for test sample. Finally, the predictor sample and test sample were changed and then PS for new test sample was calculated. This process was performed 19 times, repeatedly.
Results
Identification of Genes Responding to Radiation
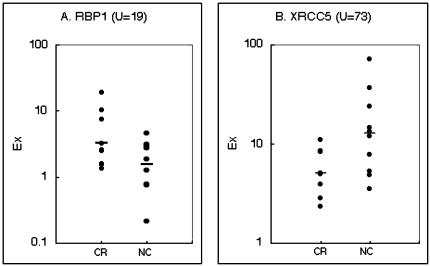
We performed cDNA microarray analysis of gene expression in 19 cervical-cancer materials, of which 9 were radiation sensitive and 10 were radiation resistant on clinical grounds. By means of the Mann-Whitney test (P<.05) and subsequent procedures (see Materials and Methods section ), we selected a total of 171 genes (including 74 ESTs) as being differently expressed between CR (complete response) and NC (no change) groups. Of those 171 genes, 121 (including 62 ESTs) revealed increased expression, and 50 (including 12 ESTs) showed decreased expression, in carcinomas belonging to the CR group compared with the NC group (Table 2, A and B). Genes involved in adipogenesis and in the MAP kinase pathway were significantly upregulated in the CR group compared to the NC group; the former included aldehyde dehydrogenase 1 (ALDH1), and retinol-binding protein 1 (RBP1; Figure 1A). The latter included mitogen-activated protein kinase kinase kinase 2 (MAP3K2), G protein beta subunit-like (GBL), and RAB5C (a member of the RAS oncogene family). However, genes that are considered to be associated with repair of breaks in double-stranded DNA, including X-ray repair cross-complementing 5 (XRCC5; Figure 1B) were downregulated in the CR group relative to the NR group. In addition, a number of genes related to DNA repair, transcription, signal transduction, cell skeleton, and adhesion were among those expressed differently in the two groups.
Table 2.
Genes Showing Different Expression Between CR and NC Groups.
| A. Genes Showing Relatively Higher Expression in Radiosensitive Carcinoma Cells than in Radioresistant Cells | |||||||
| Category | Unigene ID (build #131) | Gene Symbol | Gene Name | U | CR/NC | P | Locus |
| DNA repair | Hs.3248 | MSH6 | mutS (E. coli) homolog 6 | 13 | 2.0 | 0.003 | 2p16 |
| Signal transduction | Hs.76578 | PIAS3 | protein inhibitor of activated STAT3 | 10 | 4.5 | 0.000 | 1q21 |
| Hs.29203 | GBL | G protein beta subunit-like | 11 | 2.8 | 0.044 | 16 | |
| Hs.479 | RAB5C | RAB5C, member RAS oncogene family | 12 | 2.8 | 0.001 | 17q21.2 | |
| Hs.28827 | MAP3K2 | mitogen-activated protein kinase kinase kinase 2 | 12 | 2.4 | 0.002 | 2 | |
| Hs.85155 | BRF1 | butyrate response factor 1 (EGF-response factor 1) | 14 | 2.4 | 0.003 | 14q22-q24 | |
| Hs.74615 | PDGFRA | platelet-derived growth factor receptor, alpha polypeptide | 14.5 | 3.3 | 0.023 | 4q11-q13 | |
| Hs.77439 | PRKAR2B | protein kinase, cAMP-dependent, regulatory, type II, beta | 15 | 5.9 | 0.009 | 7q22-q31.1 | |
| Hs.83070 | GRB14 | growth factor receptor-bound protein 14 | 17 | 9.8 | 0.042 | 2q22-q24 | |
| Transcription | Hs.66394 | RNF4 | ring finger protein 4 | 6 | 5.1 | 0.000 | 4p16.3 |
| Hs.8858 | BAZ1A | bromodomain adjacent to zinc finger domain, 1A | 9 | 5.0 | 0.031 | 14q12-q13 | |
| Hs.155321 | SRF | serum response factor (c-fos serum response element-binding transcription factor) | 9 | 2.0 | 0.038 | 6pter-6q15 | |
| Hs.289068 | TCF4 | transcription factor 4 | 15 | >2.2 | 0.048 | 18q21.1 | |
| Hs.760 | GATA2 | GATA-binding protein 2 | 15 | 2.0 | 0.011 | 3q21 | |
| Hs.316 | DDX6 | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 6 | 16 | 3.3 | 0.009 | 11q23.3 | |
| Hs.301963 | HOXD8 | homeo box D8 | 17 | 3.3 | 0.038 | 2q31-q37 | |
| Hs.228059 | TIF1B | KRAB-associated protein 1 | 19 | 2.0 | 0.029 | 5 | |
| Adipogenesis | Hs.76392 | ALDH1 | aldehyde dehydrogenase 1, soluble | 15.5 | 14.1 | 0.018 | 9q21 |
| Hs.101850 | RBP1 | retinol-binding protein 1, cellular | 19 | 2.1 | 0.018 | 3q23 | |
| Cytoskeleton | Hs.7645 | FGB | fibrinogen, B beta polypeptide | 9 | 4.4 | 0.018 | 4q28 |
| Hs.75279 | LAMA2 | laminin, alpha 2 (merosin, congenital muscular dystrophy) | 10 | 5.2 | 0.040 | 6q22-q23 | |
| Hs.75445 | SPARCL1 | SPARC-like 1 (mast9, hevin) | 15 | 4.0 | 0.004 | 7 | |
| Hs.97266 | PCDH18 | protocadherin 18 | 16 | 5.9 | 0.006 | ||
| Hs.11494 | FBLN5 | fibulin 5 | 16 | 4.0 | 0.008 | 14q32.1 | |
| Hs.6441 | TIMP2 | tissue inhibitor of metalloproteinase 2 | 17 | 2.8 | 0.033 | 17q25 | |
| Hs.79914 | LUM | lumican | 17 | 2.2 | 0.035 | 12q21.3-q22 | |
| Hs.108896 | LOC51084 | lambda-crystallin | 18 | 4.7 | 0.022 | 13cen3q14.2 | |
| Hs.20072 | MIR | myosin regulatory light-chain interacting protein | 19 | 2.4 | 0.015 | 6p23-p22.3 | |
| Immune system | Hs.1244 | CD9 | CD9 antigen (p24) | 8 | 2.2 | 0.001 | 12p13 |
| Hs.74631 | BSG | basigin | 8 | 2.2 | 0.001 | 19p13.3 | |
| Hs.502 | ABCB3 | ATP-binding cassette, subfamily B (MDR/TAP), member 3 | 11 | 2.1 | 0.009 | 6p21.3 | |
| Hs.24395 | SCYB14 | small inducible cytokine subfamily B (Cys-X-Cys), member 14 (BRAK) | 17 | 5.0 | 0.007 | 5q31 | |
| Proteolysis | Hs.173091 | UBL3 | ubiquitin-like 3 | 15 | 2.5 | 0.016 | 13q12-q13 |
| Hs.75275 | UBE4A | ubiquitination factor E4A (homologous to yeast UFD2) | 19 | 3.8 | 0.047 | 11 | |
| Tumor related | Hs.81988 | DAB2 | disabled (Drosophila) homolog 2 (mitogen-responsive phosphoprotein) | 16 | 2.4 | 0.003 | 5p13 |
| Hs.75462 | BTG2 | BTG family, member 2 | 16 | 2.1 | 0.028 | 1q32 | |
| Peptide hormone | Hs.134932 | UCN | urocortin | 9 | 11.3 | 0.017 | 2p23-p21 |
| Others and ESTs | Hs.150926 | FPGT | fucose-phosphate guanylyltransferase | 10 | 2.1 | 0.009 | 1 |
| Hs.74566 | DPYSL3 | dihydropyrimidinase-like 3 | 18 | 2.6 | 0.015 | 5q32 | |
| Hs.101735 | DKFZP564J102 | DKFZP564J102 protein | 19 | 3.0 | 0.042 | 4 | |
| Hs.74571 | ARF1 | ADP-ribosylation factor 1 | 19 | 2.9 | 0.015 | 1q42 | |
| Hs.56874 | HSPB7 | heat shock 27-kDa protein family, member 7 (cardiovascular) | 4 | 2.5 | 0.005 | 1p36.23-p34.3 | |
| Hs.74376 | NOE1 | olfactomedin-related ER localized protein | 7 | 22.4 | 0.028 | 9 | |
| Hs.112569 | GAN | giant axonal neuropathy (gigaxonin) | 12 | 2.6 | 0.001 | 16q24.1 | |
| Hs.7535 | LOC55871 | COBW-like protein | 13 | 2.3 | 0.001 | 2 | |
| Hs.20597 | LCP | host cell factor homolog | 13 | 2.0 | 0.002 | ||
| Hs.24948 | SNCAIP | synuclein, alpha interacting protein (synphilin) | 14 | 7.0 | 0.003 | 5q23.1-q23.3 | |
| Hs.108725 | LOC51660 | HSPC040 protein | 15 | 3.0 | 0.005 | 6 | |
| Hs.49912 | LOC55895 | 22-kDa peroxisomal membrane protein-like | 15 | 2.7 | 0.015 | ||
| Hs.78103 | NAP1L4 | nucleosome assembly protein 1-like 4 | 17 | 7.0 | 0.009 | 11p15.5 | |
| Hs.111779 | SPARC | secreted protein, acidic, cys align="char"teine-rich (osteonectin) | 17 | 2.2 | 0.020 | 5q31.3-q32 | |
| Hs.75887 | COPA | coatomer protein complex, subunit alpha | 17 | 2.0 | 0.028 | 1q23-q25 | |
| Hs.129872 | SPAG9 | sperm-associated antigen 9 | 17 | 2.9 | 0.001 | 17 | |
| Hs.62041 | NID | nidogen (enactin) | 17 | 11.3 | 0.032 | 1q43 | |
| Hs.83834 | CYB5 | cytochrome b-5 | 18 | 3.6 | 0.040 | 18q23 | |
| Hs.29981 | SLC26A2 | solute carrier family 26 (sulfate transporter), member 2 | 19 | 2.8 | 0.001 | 5q31-q34 | |
| Hs.17930 | BING4 | BING4 | 19 | 2.9 | 0.000 | 6p21.3 | |
| Hs.6113 | STAU | staufen (Drosophila, RNA-binding protein) | 19 | 2.0 | 0.002 | 20q13.1 | |
| Hs.11951 | ENPP1 | ectonucleotide pyrophosphatase/phosphodiesterase 1 | 19.5 | >50 | 0.047 | 6q22-q23 | |
| Hs.172870 | ESTs | 4 | >50 | 0.000 | |||
| Hs.29664 | Human DNA sequence from clone 682J15 on chromosome 6p11.22.3. | 6 | 15.1 | 0.001 | |||
| Hs.12365 | KIAA1427 | KIAA1427 protein | 7 | >50 | 0.004 | ||
| Hs.107515 | ESTs | 7 | 3.5 | 0.000 | |||
| Hs.40583 | Homo sapiens clone TCBAP1028 mRNA sequence | 7 | 4.3 | 0.004 | |||
| Hs.115315 | ESTs | 7 | 2.2 | 0.037 | |||
| Hs.176092 | ESTs, moderately similar to myosin-binding protein H [H. sapiens] | 7 | 7.5 | 0.000 | |||
| Hs.125291 | ESTs | 7 | 10.0 | 0.000 | |||
| Hs.85053 | H. sapiens clone 24440 mRNA sequence | 8 | 11.2 | 0.000 | |||
| Hs.188228 | H. sapiens cDNA FLJ11003 fis, clone PLACE1002851 | 9 | 4.0 | 0.002 | |||
| Hs.296772 | Human DNA sequence from clone RP1-292B18 | 10 | 4.1 | 0.001 | |||
| Hs.83724 | Human clone 23773 mRNA sequence | 10 | 6.8 | 0.033 | |||
| Hs.124558 | EST | 10 | 2.8 | 0.008 | |||
| Hs.120399 | ESTs | 10 | 8.2 | 0.016 | |||
| Hs.116464 | ESTs | 11 | 3.4 | 0.014 | |||
| Hs.281434 | H. sapiens cDNA FLJ14028 fis, clone HEMBA1003838 | 11 | 2.9 | 0.031 | |||
| Hs.191271 | ESTs | 11 | 5.5 | 0.003 | |||
| Hs.26676 | FLJ10850 | hypothetical protein FLJ10850 | 11 | 4.8 | 0.004 | 20pter-20q12 | |
| Hs.292162 | ESTs | 11 | >50 | 0.007 | |||
| Hs.181304 | 13CDNA73 | putative gene product | 11 | >50 | 0.000 | 13 | |
| Hs.98265 | ESTs | 11 | 10.9 | 0.000 | |||
| Hs.126857 | H. sapiens cDNA FLJ12936 fis, clone NT2RP2005018 | 11 | 20.5 | 0.014 | |||
| Hs.95071 | ESTs | 11.5 | >50 | 0.001 | |||
| Hs.110373 | ESTs | 12 | 4.7 | 0.007 | |||
| Hs.114453 | ESTs | 12 | 2.6 | 0.005 | |||
| Hs.15725 | LOC51278 | hypothetical protein SBBI48 | 12 | 2.7 | 0.003 | 1p36.13q41 | |
| Hs.291979 | ESTs, Highly similar to pre-mRNA splicing SR protein rA4 | 12 | 4.6 | 0.019 | |||
| Hs.113082 | KIAA0443 | KIAA0443 gene product | 13 | 6.3 | 0.019 | X | |
| Hs.116117 | EST | 13 | 2.2 | 0.003 | |||
| Hs.24391 | H. sapiens cDNA FLJ13612 fis, clone PLACE1010833 | 13 | 5.9 | 0.034 | |||
| Hs.23120 | H. sapiens cDNA: FLJ21421 fis, clone COL04123 | 13 | 2.1 | 0.007 | |||
| Hs.116585 | ESTs | 13 | 2.0 | 0.014 | |||
| Hs.49476 | H. sapiens clone TUA8 Cri-du-chat region mRNA | 14 | 10.8 | 0.010 | |||
| Hs.112745 | EST | 14 | 2.1 | 0.029 | |||
| Hs.21851 | H. sapiens cDNA FLJ12900 fis, clone NT2RP2004321 | 14 | 2.7 | 0.001 | |||
| Hs.13809 | ESTs | 14 | 2.8 | 0.007 | |||
| Hs.61268 | ESTs | 14 | >50 | 0.032 | |||
| Hs.8469 | ESTs | 15 | 2.0 | 0.049 | |||
| Hs.11365 | H. sapiens cDNA FLJ12145 fis, clone MAMMA1000395 | 15 | 2.1 | 0.006 | |||
| Hs.107812 | ESTs, Weakly similar to SPOP [H. sapiens] | 15 | 2.8 | 0.027 | |||
| Hs.25329 | ESTs | 15 | 4.4 | 0.033 | |||
| Hs.178730 | ESTs | 16 | 2.3 | 0.000 | |||
| Hs.112607 | ESTs | 16 | 2.6 | 0.023 | |||
| Hs.27497 | H. sapiens cDNA FLJ11756 fis, clone HEMBA1005595 | 16 | 2.5 | 0.002 | |||
| Hs.29356 | ESTs | 16 | 2.0 | 0.024 | |||
| Hs.158688 | IF2 | KIAA0741 gene product | 16 | 4.6 | 0.042 | 2 | |
| Hs.23617 | FLJ20531 | hypothetical protein FLJ20531 | 17 | 2.8 | 0.041 | ||
| Hs.44159 | LOC51105 | CGI-72 protein | 17 | 13.7 | 0.003 | 8 | |
| Hs.23650 | ESTs, Weakly similar to AAB47496 NG5 [H. sapiens] | 17 | 3.3 | 0.041 | |||
| Hs.133081 | ESTs, Weakly similar to hypothetical protein [H. sapiens] | 17 | 24.6 | 0.011 | |||
| Hs.191379 | ESTs | 17 | 6.1 | 0.003 | |||
| Hs.72363 | H. sapiens mRNA for FLJ00116 protein, partial cds | 17.5 | >50 | 0.038 | |||
| Hs.173094 | H. sapiensmRNA; cDNA DKFZp564H142 (from clone DKFZp564H142) | 17.5 | 6.0 | 0.006 | |||
| Hs.179891 | ESTs, Weakly similar to prolyl 4-hydroxylase alpha subunit [H. sapiens] | 18 | 2.5 | 0.005 | |||
| Hs.22860 | ESTs | 18 | 2.3 | 0.011 | |||
| Hs.12867 | ESTs | 18 | 2.3 | 0.005 | |||
| Hs.200332 | FLJ20651 | hypothetical protein FLJ20651 | 18 | 3.8 | 0.004 | 9p24.1-9q22.33 | |
| Hs.273186 | LOC56997 | hypothetical protein, clone Telethon(Italy_B41)_Strait02270_FL142 | 18 | 2.8 | 0.041 | 1 | |
| Hs.30643 | ESTs | 18 | 5.7 | 0.018 | |||
| Hs.11805 | ESTs | 19 | 3.1 | 0.038 | |||
| Hs.22505 | FLJ10159 | hypothetical protein FLJ10159 | 19 | 2.7 | 0.025 | 6 | |
| Hs.127407 | ESTs | 19 | 5.6 | 0.011 | |||
| B. Genes Showing Relatively Higher Expression in Radioresistant Carcinoma Cells than in Radiosensitive Cells | |||||||
| Category | Unigene ID (build #131) | Gene Symbold | Gene Name | U | CR/NC | P | Locus |
| DNA repair | Hs.84981 | XRCC5 | X-ray repair complementing defective repair in Chinese hamster cells 5 | 73 | 0.39 | 0.034 | 2q35 |
| Signal transduction | Hs.155924 | CREM | cAMP responsive element modulator | 71 | 0.48 | 0.020 | 10p12.1-p11.1 |
| Hs.118520 | LOC55970 | G-protein gamma2 subunit | 73 | 0.40 | 0.015 | 1 | |
| Hs.34780 | DCX | doublecortex; lissencephaly, X-linked (doublecortin) | 74 | 0.48 | 0.012 | Xq22.3-q23 | |
| Hs.7138 | CHRM3 | cholinergic receptor, muscarinic 3 | 77 | 0.50 | 0.034 | 1q41-q44 | |
| Hs.250857 | CAMK2G | calcium/calmodulin-dependent protein kinase II gamma | 85 | 0.41 | 0.009 | 10q22 | |
| Transcription | Hs.168005 | TIF1GAMMA | transcriptional intermediary factor 1 gamma | 72 | 0.38 | 0.000 | 1p13.1 |
| Hs.21771 | WHSC2 | Wolf-Hirschhorn syndrome candidate 2 | 73 | 0.47 | 0.021 | 4p16.3 | |
| Hs.172280 | SMARCC1 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin | 74 | 0.38 | 0.005 | 3p23-p21 | |
| Hs.110457 | WHSC1 | Wolf-Hirschhorn syndrome candidate 1 | 78 | 0.45 | 0.005 | 4p16.3 | |
| Hs.78580 | DDX1 | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 1 | 73 | 0.32 | 0.032 | 2p24 | |
| Translation | Hs.129673 | EIF4A1 | eukaryotic translation initiation factor 4A, isoform 1 | 78 | 0.43 | 0.001 | 17p13 |
| Glycolysis | Hs.2795 | LDHA | lactate dehydrogenase A | 75 | 0.39 | 0.036 | 11p15.4 |
| Cytoskeleton | Hs.821 | BGN | biglycan | 71 | 0.24 | 0.026 | Xq28 |
| Hs.172928 | COL1A1 | collagen, type I, alpha 1 | 74 | 0.46 | 0.007 | 17q21.3-q22 | |
| Hs.90408 | NEO1 | neogenin (chicken) homolog 1 | 78 | 0.48 | 0.006 | 15q22.3-q23 | |
| Immune system | Hs.516 | CCR1 | chemokine (C-C motif) receptor 1 | 71 | 0.28 | 0.039 | 3p21 |
| Hs.198253 | HLA-DQA1 | major histocompatibility complex, class II, DQ alpha 1 | 72.5 | 0.32 | 0.007 | 6p21.3 | |
| Hs.75498 | SCYA20 | small inducible cytokine subfamily A (Cys-Cys), member 20 | 73 | 0.18 | 0.041 | 2q33-q37 | |
| Hs.833 | ISG15 | interferon-stimulated protein, 15 kDa | 78 | 0.25 | 0.042 | 1 | |
| Proteolysis | Hs.61153 | PSMC2 | roteasome (prosome, macropain) 26S subunit, ATPase, 2 | 73 | 0.49 | 0.032 | 7q22.1-q22.3 |
| Apoptosis | Hs.93213 | BAK1 | BCL2-antagonist/killer 1 | 73 | 0.36 | 0.006 | 6p21.3 |
| Hs.278602 | API5 | apoptosis inhibitor 5 | 74 | 0.46 | 0.013 | 11 | |
| Others and ESTs | Hs.75593 | UROS | uroporphyrinogen III synthase | 71 | 0.13 | 0.041 | 10q25.2-q26.3 |
| Hs.108196 | LOC51659 | HSPC037 protein | 71 | 0.37 | 0.027 | 16 | |
| Hs.64595 | AASDHPPT | aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase | 71 | 0.44 | 0.033 | 11q22 | |
| Hs.286049 | PSA | phosphoserine aminotransferase | 72 | 0.47 | 0.009 | 9 | |
| Hs.93659 | ERP70 | protein disulfide isomerase-related protein | 73 | 0.26 | 0.036 | 10 | |
| Hs.2281 | CHGB | chromogranin B (secretogranin 1) | 75 | 0.31 | 0.011 | 20pter-p12 | |
| Hs.110099 | CBFA2T3 | core-binding factor, runt domain, alpha subunit 2; translocated to, 3 | 75 | 0.33 | 0.009 | 16q24 | |
| Hs.4747 | DKC1 | dyskeratosis congenita 1, dyskerin | 75 | 0.40 | 0.003 | Xq28 | |
| Hs.83848 | TPI1 | triosephosphate isomerase 1 | 77 | 0.43 | 0.040 | 12p13 | |
| Hs.75799 | PRSS8 | protease, serine, 8 (prostasin) | 77 | 0.13 | 0.045 | 16p11.2 | |
| Hs.143600 | GPP130 | type II Golgi membrane protein | 77 | 0.35 | 0.024 | 3 | |
| Hs.13565 | T-STAR | Sam68-like phosphotyrosine protein, T-STAR | 78 | 0.44 | 0.013 | 8q24.2 | |
| Hs.169476 | GAPD | glyceraldehyde-3-phosphate dehydrogenase | 80 | 0.47 | 0.042 | 12p13 | |
| Hs.114366 | PYCS | pyrroline-5-carboxylate synthetase | 87 | 0.24 | 0.006 | 10q24.3 | |
| Hs.43445 | PARN | poly(A)-specific ribonuclease (deadenylation nuclease) | 90 | 0.35 | 0.003 | 16p13 | |
| Hs.137556 | H. sapiens mRNA; cDNA DKFZp434A132 | 71 | 0.18 | 0.004 | |||
| Hs.65403 | LOC51323 | hypothetical protein | 71 | 0.50 | 0.003 | 6pter-6q15 | |
| Hs.164285 | ESTs, Weakly similar to Afg1p [S. cerevisiae] | 72 | 0.44 | 0.026 | |||
| Hs.26675 | ESTs | 74 | 0.32 | 0.012 | |||
| Hs.11641 | H. sapiens cDNA: FLJ21432 fis, clone COL04219 | 74 | 0.50 | 0.000 | |||
| Hs.14846 | H. sapiens mRNA; cDNA DKFZp564D016 | 75 | 0.38 | 0.009 | |||
| Hs.283127 | ESTs | 75 | 0.36 | 0.032 | |||
| Hs.63224 | ESTs | 75 | 0.16 | 0.038 | |||
| Hs.227591 | ESTs, Weakly similar to AF1488561 unknown [H. sapiens] | 76 | 0.50 | 0.017 | |||
| Hs.11156 | LOC51255 | hypothetical protein | 76 | 0.21 | 0.005 | 2 | |
| Hs.133207 | H. sapiens mRNA for KIAA1230 protein, partial cds | 77 | 0.42 | 0.011 | |||
| Hs.201925 | H. sapiens cDNA FLJ13446 fis, clone PLACE1002968 | 80 | 0.47 | 0.015 | |||
U, indicates Mann-Whitney statistics. CR/NC, difference ratio between median expression values for each group. P, permutational P value. Genes used for calculating predictive scores are noted in bold type.
Figure 1.
Differential gene expression between the radiosensitive group (CR; 9 samples) and the radioresistant group (NC; 10 samples) with significant difference (P<.05). U indicates the Mann-Whitney test statistic. Expression levels (Ex=Cy5 signal intensity from cancer sample/Cy3 signal intensity from control), of two genes are plotted here. Median Ex values for each group of tumors are denoted by horizontal lines. (A) Retinol-binding protein 1 (RBP1; U=19); (B) X-ray repair cross-complementing 5 (XRCC5; U= 73).
Permutation Test
To evaluate the validity of the 171 genes selected as radiosensitivity-related genes, permutation test was performed as described in the Materials and Methods section. Expression levels of each 19 samples in both groups for each gene were permuted (randomly scrambled) 10,000 times. Pgc values were calculated using µCR, µNC, σCR, and σNC values derived from newly classified CR group and NC group during every permutation. Large absolute values of Pgc indicate a strong correlation between the gene expression and the class distinction, whereas the sign of Pgc being positive or negative corresponds to gene “g” being more highly expressed in the CR or NC group. After the 10,000 times permutation, the probabilities of the genes to be correlated to group distinction, Ps, were estimated under the hypothesis that these 10,000 Pgc values show ideal normal distribution (Table 2, A and B). As a result, all of the selected 62 genes showed P values >.05 without exception. Hence, it was proved that these selected 171 genes were to be radiosensitivity predictive gene under the confidence of P<.05.
Hierarchical Clustering
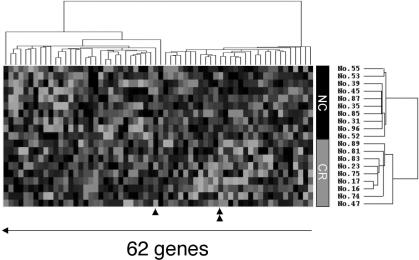
Of these 171 genes were subjected to hierarchical clustering as described in the Materials and Methods section. This procedure clearly separated the two groups from each other, except for tumor No. 47 (data not shown). To achieve complete separation, we selected 62 genes that showed greater than 2.0 standard deviations of expression values among all 19 samples. Cluster analysis using these 62 genes achieved complete separation of the groups (Figure 2).
Figure 2.
Expression patterns of 62 genes across 19 samples of cervical squamous cell carcinoma. Red or green colors indicate higher or lower expression, respectively, relative to the mean signal intensity of a given gene across 19 tumor samples; black, same expression level with mean value; gray, no expression detected (intensities of both Cy3 and Cy5 were below cut-off values). Each row represents each gene and each column a cervical squamous cell carcinoma sample. Single and double triangles indicate the gene-expression profiles of TCF4 and BAK1, respectively.
Cross-Validation Test
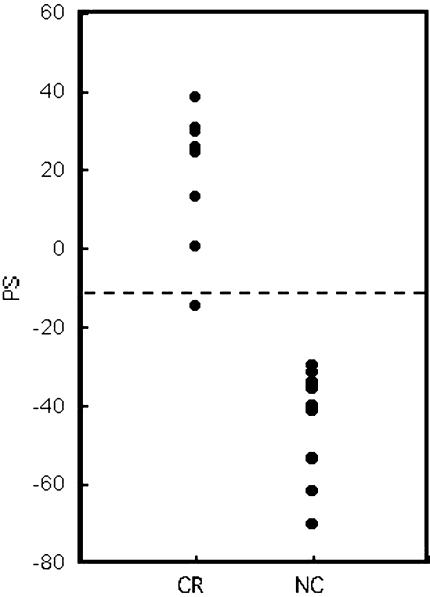
Cross-validation test was performed to examine whether the 62 genes were crucial for classifying CR and NC groups and whether they could predict the group for test samples. Among the 19 samples, 18 samples were used for group predictor and 1 sample was used as the test sample. The sample sets of predictor and test sample were changed 19 times and PS for each 19 samples was calculated as described in the Materials and Methods section (Figure 3 and Table 3). Threshold line for PSs to discriminate CR or NC group were settled at the half point between average PS values of the CR group and that of the NC group: -12. As shown in Figure 3, PSs for samples in CR and NC groups were clearly separated, except for sample No.74 (error ratio was 5.3% (=1 sample/19 samples)).
Figure 3.
Predictive score (PS) for radiosensitive and radioresistant groups by cross-validation test. Details of the calculation method were noted in the Materials and Methods section. A dashed line indicates the threshold.
Table 3.
Predictive Scores to Classify Radiosensitive Group and Radio Resistant Group.
| Sample No. | PS |
| CR | |
| 16 | 36 |
| 17 | 30 |
| 23 | 38 |
| 47 | 0.6 |
| 74 | -15 |
| 75 | 31 |
| 81 | 25 |
| 83 | 26 |
| 89 | 13 |
| NC | |
| 31 | -48 |
| 35 | -40 |
| 39 | -54 |
| 45 | -35 |
| 52 | -62 |
| 53 | -34 |
| 55 | -70 |
| 85 | -32 |
| 87 | -42 |
| 96 | -30 |
PS, predictive score. CR, radiosensitive group. NC, radioresistant group.
Discussion
cDNA Microarray analysis is a powerful tool for obtaining comprehensive information about expression of thousands of genes in cancer cells [12–15]. By combining this technology with statistical analysis, we identified 171 genes that showed different expression patterns between two distinct clinical groups and, therefore, were likely to reflect differences in the response of cervical cancer cells to radiotherapy. To examine the validity of 171 genes selected as radiosensitivity-related genes, random permutation test was performed by calculating Pgcs, and P values for each gene were evaluated. After 10,000 times permutation test, P values for all of the 171 genes were lower than .05, indicating that these genes were significantly correlated to radiosensitivity. Furthermore, in a clustering analysis, the expression profiles of these 171 genes were able to classify each of 19 tumor samples, except for one, to the appropriate group (radiosensitive or radioresistant). However, when the cluster analysis was limited to 62 genes having greater than 2.0 standard deviations of expression level across the 19 samples, all tumors were properly classified into their respective groups. To further evaluate the validity of 62 genes selected as radiosensitivity-related genes, we carried out cross-validation test. After 19 times cross-validation test, each PS value for each sample that belonged to the CR (or NC) group showed higher (or lower) value than threshold line. This study not only supports the feasibility of these 62 genes as radiosensitivity-related genes, but also indicates the possibility of predicting radiosensitivity for discriminating radiation-sensitive from radiation-resistant biopsy samples by PS values calculated from expression values of 62 genes. However, further study with additional tumor samples would be required to apply these genes for predicting radiosensitivity of tumors in patients before therapy begins.
Because all of the cervical cancer samples we used in this study were human papillomavirus (HPV)-positive, p53 as well as RB functions were likely to be eliminated by the viral protein [19]. Hence, the set of genes listed here may be associated with a cell-death pathway independent of p53. Radiation kills tumor cells mainly as a result of double-strand breaks (DSBs) in DNA [20]. If cells are defective in their DNA-repair systems, especially as regards DSB repair, they should be more susceptible to cell death. The gene product of XRCC5, Ku80, a protein that binds double-stranded DNA and a component of DNA-dependent protein kinase holo enzyme, is involved in DSB repair [21]. XRCC5-deficient cells and Ku80-knockout mice are hypersensitive to ionizing radiation [22,23]. Therefore, the higher expression of this gene we observed in radioresistant cancer cells accords with its physiological function. XRCC5 would be one of the most crucial genes for determining the fate of cells under the genotoxic stress caused by irradiation. We also observed a relatively higher level of expression of lactate dehydrogenase (LDHA), which aids glycolysis under hypoxic conditions [24] in radioresistant cells. Cells in fact become radioresistant under hypoxic conditions [25], and a high level of LDHA expression could be an important mechanism conferring radioresistance.
In radiosensitive cells, we found elevated expression of adipogenesis-related genes including ALDH1 and RBP1. The product of ALDH1 is involved in retinoic acid (RA) synthesis [26] and RBP1 is a transporter of retinol. Cervical carcinoma cells treated with RA before irradiation are reported to become radiosensitive [27]; furthermore, RA induces TRAIL expression and causes apoptosis [28]. Therefore, elevated expression of these genes may induce RA synthesis and, thereby, encourage apoptosis after radiation.
Our list of 171 genes should be useful not only as an aid to understanding the mechanism of radiosensitivity, but also as a means to expand the possibilities for effective radiotherapy. For example, if some novel drugs could block gene products that are involved in radioresistance, or if genes that induce apoptotic signals after radiation could be exogenously introduced, the effectiveness of radiotherapy would be increased. In addition, the 62 selected genes might prove of great benefit for diagnosing radiosensitivity of individual cervical cancers, to provide opportunities for selecting appropriate treatment (personalized medicine) for each patient.
Acknowledgements
We appreciate the help of Hiroko Bando, Noriko Nemoto, and Noriko Sudo in fabricating the cDNA microarray.
Abbreviations
- PS
predictive scoring
- aRNA
amplified RNA
- CR
complete response
- NC
no change
- ALDH1
aldehyde dehydrogenase
- XRCC5
X-ray repair cross-complementing 5
- BP1
retinol-binding protein 1
- DSB
double-strand break
Footnotes
This work was supported in part by Research for the Future Program Grant No. 00L01402 from the Japan Society for the Promotion of Science.
References
- 1.Vutuc C, Haidinger G, Waldhoer T, Ahmad F, Breitenecker G. Prevalence of self-reported cervical cancer screening and impact on cervical cancer mortality in Austria. Wien Klin Wochenschr. 1999;111:354–359. [PubMed] [Google Scholar]
- 2.Recio FO, Sahai Srivastava BI, Wong C, Hempling RE, Eltabbakh GH, Piver MS. The clinical value of digene hybrid capture HPV DNA testing in a referral-based population with abnormal pap smears. Eur J Gynaecol Oncol. 1998;19:203–208. [PubMed] [Google Scholar]
- 3.Suzuki K, Watanabe M, Miyoshi J. Differences in effects of oncogenes on resistance of gamma rays, ultraviolet light, and heat shock. Radiat Res. 1992;129:157–162. [PubMed] [Google Scholar]
- 4.FitzGerald TJ, Henault S, Sakakeeny M, Santucci MA, Pierce JH, Anklesaria P, Kase K, Das I, Greenberger JS. Expression of transfected recombinant oncogenes increases radiation resistance of clonal hematopoietic and fibroblast cell lines selectively at clinical low dose rate. Radiat Res. 1990;122:44–52. [PubMed] [Google Scholar]
- 5.Riva C, Lavieille JP, Reyt E, Brambilla E, Lunardi J, Brambilla C. Differential c-myc, c-jun, c-raf and p53 expression in squamous cell carcinoma of the head and neck: implication in drug and radioresistance. Eur J Cancer Part B Oral Oncol. 1995;31B:384–391. doi: 10.1016/0964-1955(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura Y, Yamagishi N, Miyakoshi J, Imamura S, Takebe H. Increase in radiation sensitivity of human malignant melanoma cells by expression of wild-type p16 gene. Cancer Lett. 1997;115:91–96. doi: 10.1016/s0304-3835(97)04714-9. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao M, Tse V, Carmel J, Costanzi E, Strauss B, Haas M, Silverberg GD. Functional expression of human p21(WAF1/CIP1) gene in rat glioma cells suppresses tumor growth in vivo and induces radiosensitivity. Biochem Biophys Res Commun. 1997;233:329–335. doi: 10.1006/bbrc.1997.6450. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert MS, Saad AH, Rupnow BA, Knox SJ. Association of BCL-2 with membrane hyperpolarization and radioresistance. J Cell Physiol. 1996;168:114–122. doi: 10.1002/(SICI)1097-4652(199607)168:1<114::AID-JCP14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Harima Y, Harima K, Shikata N, Oka A, Ohnishi T, Tanaka Y. Bax and Bcl-2 expressions predict response to radiotherapy in human cervical cancer. J Cancer Res Clin Oncol. 1998;124:503–510. doi: 10.1007/s004320050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene. 1996;12:187–192. [PubMed] [Google Scholar]
- 11.Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitahara O, Furukawa Y, Tanaka T, Kihara C, Ono K, Yanagawa R, Nita M E, Takagi T, Nakamura Y, Tsunoda T. Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Res. 2001;61:3544–3549. [PubMed] [Google Scholar]
- 13.Ono K, Tanaka T, Tsunoda T, Kitahara O, Kihara C, Okamoto A, Ochiai K, Takagi T, Nakamura Y. Identification by cDNA microarray of genes involved in ovarian carcinogenesis. Cancer Res. 2000;60:5007–5011. [PubMed] [Google Scholar]
- 14.Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–2137. [PubMed] [Google Scholar]
- 15.Matsushima-Nishiu M, Unoki M, Ono K, Tsunoda T, Minaguchi T, Kuramoto H, Nishida M, Satoh T, Tanaka T, Nakamura Y. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 2001;61:3741–3749. [PubMed] [Google Scholar]
- 16.Luo L, Salunga RC, Guo H, Bittner A, Joy KC, Galindo JE, Xiao H, Rogers KE, Wan JS, Jackson MR, Erlander MG. Gene expression profiles of laser-captured adjacent neuronal subtypes. Nat Med. 1999;5:117–122. doi: 10.1038/4806. [DOI] [PubMed] [Google Scholar]
- 17.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 19.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 20.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 21.Errami A, Smider V, Rathmell WK, He DM, Hendrickson EA, Zdzienicka MZ, Chu G. Ku86 defines the genetic defect and restores X-ray resistance and V(D)J recombination to complementation group 5 hamster cell mutants. Mol Cell Biol. 1996;16:1519–1526. doi: 10.1128/mcb.16.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 23.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig MC, Li GC. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 24.Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci USA. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, Dempsey JF, Perez CA, Purdy JA, Welch MJ. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–1182. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 26.Bhat PV, Labrecque J, Boutin JM, Lacroix A, Yoshida A. Cloning of a cDNA encoding rat aldehyde dehydrogenase with high activity for retinal oxidation. Gene. 1995;166:303–306. doi: 10.1016/0378-1119(96)81752-5. [DOI] [PubMed] [Google Scholar]
- 27.Benbrook DM, Shen-Gunther J, Nunez ER, Dynlacht JR. Differential retinoic acid radiosensitization of cervical carcinoma cell lines. Clin Cancer Res. 1997;3:939–945. [PubMed] [Google Scholar]
- 28.Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7:680–686. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]