Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in a variety of transformed cell lines, but generally spares most normal cells. Transduction by an adenoviral vector expressing human TRAIL cDNA (Ad.TRAIL-GFP) resulted in both direct tumor cell killing as well as a potent bystander effect through presentation of TRAIL by transduced normal cells. Administration of Ad.TRAIL-GFP significantly prolonged survival of mice harboring either intracerebral glioblastomas or breast carcinoma-induced peritoneal carcinomatosis. Additionally, TRAIL induced prolonged transgene expression in normal tissue, presumably as a result of diminished immunemediated destruction of vector-transduced cells. Taken together, these data suggest that vector-mediated transduction of TRAIL may represent an effective strategy for cancer gene therapy.
Keywords: TRAIL, apoptosis, adenoviral vectors, gene therapy, cancer therapeutics
Introduction
Apoptosis is a genetically encoded cell death program involving the interaction between multiple regulators [1]. Tumor cells may acquire a survival advantage by deregulating apoptosis through a number of mechanisms including p53 inactivation and/or induction of the bcl-2 pathway. Multicellular organisms have developed innate mechanisms, some of which involve the induction of apoptosis, for eradicating virally infected and transformed cells. One recently elucidated apoptosis pathway involves tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a transmembrane protein of tumor necrosis factor (TNF) family. Recent evidence suggests that TRAIL has selective ability to induce apoptosis in various tumor cell lines compared to normal cells [2–7]. Systemic injection of a recombinant soluble TRAIL molecule mediated antitumor activity in tumor-bearing mice, whereas little systemic toxicity was seen in mice or monkeys following administration of equivalent doses of the protein [4,5]. Although the tumor selectivity of TRAIL has recently been questioned following demonstration of TRAIL-mediated cytotoxicity toward human hepatocytes and astrocytes [8,9], these observations have been attributed to a nonspecific effect of the recombinant protein preparation rather than the TRAIL molecule itself [10,11]. Despite the promising preliminary data, however, recombinant soluble TRAIL may pose several limitations as a therapeutic agent for routine clinical use secondary to the pharmacologic instability of systemically delivered proteins, problematic distribution kinetics, and the requirement for large amounts of expensive-to-produce recombinant protein.
Replication-deficient, recombinant adenoviral vectors are theoretically attractive gene transfer vehicles due to their ability to transduce a wide variety of cell types and mediate high-level transgene expression. We and others have demonstrated the potential for locally administered adenoviral vectors to produce high local concentrations of biologically active proteins in vivo, thereby overcoming the potential pharmacologic difficulties associated with systemic protein administration [12,13]. The clinical utility of adenovirus as a gene delivery vehicle, however, may be significantly limited by undesirable host immune responses directed toward the virus and vector-transduced cells. We now demonstrate that adenoviral vector-mediated transfer of a TRAIL cDNA results in significant antitumor and bystander effects in vitro and in vivo, without normal tissue toxicity. Furthermore, we demonstrate that vector-encoded TRAIL allows prolongation of adenoviral-mediated transgene expression in normal tissues by delaying death of vectortransduced cells. Our results support a potential role of TRAIL cDNA transfer as an effective local antitumor strategy.
Materials and Methods
Cell lines and animals
The human umbilical vein endothelial cells (HUVECs), U87MG, T98 (human glioblastoma cells), 293 (human embryonic kidney cells), Colo-205 (human colon carcinoma), Ramos (Burkitt lymphoma), and IMR90 (human primary fibroblast cells) were purchased from American Type Culture Collection (ATCC, Rockville, MD). TA3, a mouse mammary ascites tumor cell line was kindly provided by H. F. Dvorak (Beth Israel Hospital, Boston, MA). Human primary astrocytes (NHAs) and human fetal neural progenitor (HNP) cells were purchased from Clonetics (San Diego, CA). NHAs and HNP cells were cultured according to the manufacturer-s protocol. HUVECs were cultured in Medium 199 supplemented with 10% fetal bovine serum (FBS), 100 µg/ml heparin and 30 µg/ml endothelial growth factor (Sigma, St. Louis, MO). Ramos and Colo-205 were maintained in RPMI medium supplemented with 10% FBS. All other cells were maintained in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY) supplemented with 10% FBS. Four-to six-week-old nude mice (Ncr-nufBR) and Swiss Webster mice were obtained from Taconic (Germantown, NY). Animal studies were done in accordance with guidelines of the Animal Care and Use Committee.
Recombinant Adenoviral Vectors
Human TRAIL cDNA was cloned from human fetal brain cDNA library (Clontech, Palo Alto, CA) by PCR using two oligonucleotide primers 5′-CTGAGCGGCCGCAGTCAGACTCTGACAGGAT-3′ and 5′-TGAGGCGGCGGCCGCTTTTTCTTTCCAGGTCAGT-3′. Amplified TRAIL cDNA fragment was inserted into the NotI site of a shuttle plasmid to generate the pTRACK-CMV-TRAIL vector. Transcription of the TRAIL gene was driven by the cytomegalovirus (CMV) enhancer/promoter, terminated with the SV40 poly-A signal. This shuttle vector also contains the green fluorescent protein (GFP) gene, expression of which was useful as a marker for monitoring efficiency of transfection. Control pTRACK-CMV-GFP vector only carries the GFP gene (kindly provided by B. Vogelstein, Johns Hopkins University ). The construction of the recombinant adenoviral vectors Ad.TRAIL-GFP and Ad.GFP followed the protocol described previously [14]. Briefly, a pTRACK-CMV-TRAIL and a backbone plasmid containing the genome of Ad5 with deletions in the E1 and E3 regions (pAdEasy-1) were cotransformed into BJ 5183, a recombination-permissive E. coli strain, for isolation of a pro-adenoviral plasmid. The structure of the resultant recombinant vectors were confirmed by restriction enzyme digestion (BamHI and NotI) and DNA sequencing. Thirty micrograms of proviral DNA was used in transfecting 293 cells by standard calcium phosphate coprecipitation method. Approximately 7 to 10 days later, virus plaques were observed and the recombinant viruses were further propagated in 293 cells as previously described [15]. The virus stocks were purified by two rounds of cesium chloride-gradient ultracentrifugations, dialyzed against 10% glycerol, 10 mM Tris (pH 8.0) and 1 mM MgCl2, and stored at -80°C. Virus titers were determined as plaque-forming units (PFU) assay and GFP-expressing units assay in 293 cells. Typical viral titers obtained were around 5x1010 to 5x1011 PFU/ml.
Western Blot Analysis
Western blot analysis was performed by using anti-TRAIL antibody (C-19, Santa Cruz Biotechnology, Santa Cruz, CA), anti-GFP antibody (clone 11E5, Quantum Biotechnology, Montreal, Canada), anti-TuJ1 antibody (Babco, Richmond, CA), antitubulin and anti-PARP antibody (Pharmingen, Franklin Lakes, NJ), followed by a secondary peroxidase antibody (Santa Cruz Biotechnology).
RT-PCR Analysis
Reverse transcription polymerase chain reaction (RTPCR) was performed using a standard protocol and PCR primer sequences that are described elsewhere [16].
In Situ Apoptotic Cell Detection Assay
Terminal transferase-mediated dUTP nick end labeling (TUNEL) assay was performed on paraffin-embedded and frozen tissue sections, according to the protocol recommended by the supplier (Intergen, Purchase, NY). 3,3′-Diaminobenzidine (DAB) substrate (Vector Laboratories, Burlingame, CA) and rhodamine-conjugated antidigoxygenin antibody were used for visualization.
TRAIL Cytotoxicity Assay
Cells were plated into six-well plates at 105 cells per well in triplicate. Target cells transduced by either Ad.TRAIL-GFP or Ad.GFP (multiplicity of infection, MOI=10 to 200 depending on the cell type) were incubated for 48 to 72 hours and prepared for fluorescence-activated cell sorting (FACS) analysis to determine the apoptotic index. For coculture experiments, effector cells were infected with either Ad.TRAIL-GFP or Ad.GFP and target cells were infected with Ad.CMV-βgal. One day later, both cell populations were replated and mixed. After incubation for 48 to 72 hours, cocultured cells were processed for β-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining in a solution containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 1 mg/ml β-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Survival of target cells was quantified by counting the stained cells per randomly chosen x200 field.
Neural Progenitor Cell Coculture
HNP cells were cultured in B27 media with basic fibroblast growth factor (50 ng/ml) and epidermal growth factor (50 ng/ml) [17]. HNP cells were infected either with Ad.GFP or Ad.TRAIL-GFP (MOI=50) Nearly 100% of cells were infected. Neuronal differentiation was induced by removal of growth factors for 10 days on poly-ornithine-coated slides. For coculture, infected HNP (105) and uninfected U87 MG cells (105) were cocultured for additional 2 days and TUNEL assay was performed.
Intracerebral Tumor Model
U87 MG cells (106) were resuspended in 5 µl of Hank's balanced salt solution (HBSS) and injected into the right caudate nucleus (1 mm posterior to the bregma and 2 mm lateral to the midline) using a small animal stereotactic frame (Kopf Instruments, Tujunga, CA). Five days after tumor implantation, either Ad.TRAIL-GFP or Ad.GFP (5x108 PFU) (in 5 µl HBSS) was injected into the established tumors using the same stereotactic coordinates. Non-tumor bearing mice were treated with 5x108 PFU virus for evaluation of Ad.TRAIL-GFP-mediated neurotoxicity.
Intraperitoneal Ascites Tumor Model
TA3 cells (106) were resuspended in 200 µl of HBSS and injected in the peritoneal cavity of male nude mice. On days 0, 4, and 8, animals received intrapertoneal doses of either Ad.TRAIL-GFP or Ad.GFP (109 PFU). Animals were monitored daily for ascites formation and survival. Autopsies were performed on all mice to determine tumor burden and ascites volume. Statistical significance of survival between different groups was assessed by log-rank analysis of Kaplan-Meier curves.
Duration of Transgene Expression
Either Ad.TRAIL-GFP or Ad.GFP (1010 PFU) was injected into tail veins of immunocompetent Swiss Webster mice (Taconic). Animals were killed 4, 7, 14, and 30 days later. Liver tissue was obtained and processed for cryosection and protein extraction.
Tissue Preparation
Brain and tumor tissues were harvested and fixed in 4% paraformaldehyde/PBS overnight, and then washed in 30% sucrose in PBS. Brains were cut coronally along the needle track and slices were used for paraffin section or embedded in O.C.T. compound (Tissue Tek, Germantown, NY) for cryosection.
Results
Biological Activity of Vector-Transduced TRAIL In Vitro
Previous reports have demonstrated minimal activity of monomeric soluble TRAIL recombinant protein, with significant increases in activity seen following TRAIL oligomerization [16,18]. Because TRAIL exists mainly as a cell-surface protein in vivo, we hypothesized that cell-associated TRAIL might be more active than soluble TRAIL and therefore we constructed an adenovirus vector carrying a transmembrane form of a human TRAIL cDNA under the control of the CMV early promoter (Figure 1A). Western blot analysis from cells transduced by Ad.TRAIL-GFP revealed a strong immunoreactive protein of ∼32 kDa consistent with a transmembrane form of TRAIL (Figure 1B). To assess biologic activity of the vector-expressed TRAIL, several tumor cell lines and human primary cells were directly infected with either Ad.TRAIL-GFP or Ad.GFP, cultured for 2 days and assayed for apoptotic cell death. Using an MOI of 100, >95% of cells were GFP-positive assuring high vector-transduction efficiency. Transduction by Ad.TRAIL-GFP induced apoptosis in 40% of U87 human glioma cells, 20% of T98 human glioma cells, and 22% of TA3 mouse mammary carcinoma-transduced cells (Figure 1C). In contrast, human primary endothelial cells (HUVECs) and human fibroblast (IMR90) and, most importantly, NHA infected with Ad.TRAIL-GFP showed no increase in apoptosis compared to those infected with Ad.GFP (Figure 1C). Western blots of the Ad.TRAIL-transduced U87 tumor cells, but not normal cells, demonstrated the appearance of the characteristic 115-and 85-kDa PARP doublet consistent with a TRAIL-mediated apoptotic mechanism of cell death (Figure 1B). Although we detected TRAIL type 2 (R2) and decoy type 1 (D1) receptor mRNA by semiquantitative RT-PCR in all of our cell lines, we, like other investigators, were unable to correlate receptor type expression with sensitivity to TRAIL-mediated cytotoxicity (Figure 1D).
Figure 1.
Induction of apoptosis in tumor cells by Ad.TRAIL-GFP vector. (A) Structure of Ad.TRAIL-GFP and Ad.GFP. The adenoviral sequences E1 and E3 are deleted. Ad.TRAIL-GFP vector expresses both TRAIL and GFP. (B) Western blot analysis of Ad.GFP-or Ad.TRAIL-GFP-transduced IMR90 cells or U87 glioma cells using an anti-TRAIL antibody (top), an anti-PARP antibody (middle), an antitubulin antibody (bottom), respectively. (C) Apoptotic index of cells (U87, T98, HUVEC, IMR90, NHA [MOI 100], and TA3 [MOI 50]) infected with Ad.TRAIL-GFP or Ad.GFP. Statistical significance between treatment was evaluated by Student's t test (asterisk: P<0.01). (D) Expression of four TRAIL receptors in a panel of cell lines, as determined by semiquantitative RT-PCR.
Adenoviral vectors infect both dividing and nondividing cells. Because our preliminary results suggest that normal primary cells including endothelial cells, astrocytes, and fibroblasts were insensitive to Ad.TRAIL-GFP (Figure 2A), we hypothesized that these transduced normal cells might be capable of “presenting” membrane-associated TRAIL to neighboring tumor cells. Although NHA and endothelial cells effectively expressed TRAIL following vector transduction (data not shown), we chose to use the transduced IMR90 cells for the coculture experiments because these cells, unlike the other normal cell types, grow in a basic defined media, thereby simplifying the conditions for the coculture experiments. Thus, Ad.TRAIL-GFP-transduced IMR90 cells were cocultured with several different lymphocytic cell lines. Unlike IMR90, these cells grow in suspension, which facilitated easy distinction between cell types. Colo-205 and Ramos cells grew normally after 3 days of coculture with Ad.GFP-infected IMR90 cells. In contrast, few cells survived in coculture with Ad.TRAILGFP-infected IMR90 cells. There was no difference in IMR90 cell survival in either group (Figure 2A). To evaluate whether TRAIL-infected IMR90 cells could also mediate cytotoxicity against cocultured adherent tumor cell lines, TA3 and U87 cells were first infected with Ad.βgal as a target cell marker and then cocultured with Ad.TRAIL-GFP -transduced IMR90 cells. As shown in Figure 2;B and C, greater than 70% and 95% of cocultured U87 and TA3 cells were killed, respectively.
Figure 2.
TRAIL-mediated cell killing in cocultures. (A) Human fibroblasts (IMR90) were infected with Ad.GFP or Ad.TRAIL-GFP (MOI 100) and cocultured with target cells for 3 days. Representative photographs (x400) of Colo-205 and Ramos cells after 3 days of coculture. (B) Viability of tumor cells after coculture with different effector cells expressing TRAIL. All target cells were infected with Ad.βgal (MOI 100) 1 day before coculture and then applied to each well. The number of βgal-positive cells in randomly chosen fields (four per group) were counted 3 days later. Relative survival (%) in Ad.TRAIL-GFP coculture was normalized to the control (Ad.GFP) and statistical significance between treatment was evaluated by Student's t test (asterisk: P<0.01). (C) Representative photographs of coculture of TA3 cells and IMR 90 cells were shown.
Because we were unable to kill all of the tumor cells by direct infection with Ad.TRAIL-GFP (Figure 1C), we wondered whether the surviving infected tumor cells could function as “effector” cells and kill uninfected tumor cells. Ad.TRAIL-GFP-transduced U87 cells that survived the initial infection were used as “effector” cells, whereas β-galactosidase-transduced U87 and T98 cells were used as targets in this coculture experiment. Although less effective than IMR90 effector cells, Ad.TRAIL-GFP-transduced U87 cells were capable of eliminating >30% of the uninfected tumor cells (Figure 2B). By contrast, conditioned media from Ad.TRAIL-GFP-transduced normal and tumor cells did not induce apoptosis in target cells arguing against the possibility of a biologically active secreted TRAIL molecule as the mediator of cell death (data not shown).
Vector-Transduced TRAIL-Mediated Cytotoxicity In Vivo
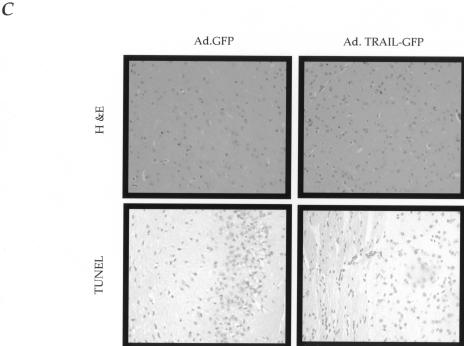
A main concern for exogenous expression of TRAIL in vivo is potential normal tissue toxicity. Indeed, two recent reports suggest that human primary astrocytes and hepatocytes in vitro were susceptible to recombinant TRAIL-mediated death in vitro, although in vivo systemic delivery of recombinant TRAIL did not cause detectable toxicity in the hands of several different investigators [5,8,9]. Recent reports have suggested that such effects were related to nonspecific toxicities of the specific protein preparation that was used for the in vitro experiments [10,11]; however, the potential for neurotoxicity remained a major concern for us. It was, therefore, encouraging to us that transduction of NHA by Ad.TRAIL-GFP did not induce cytotoxic effects in vitro (Figure 1C). Nevertheless, it is still possible that neuronal or non-astrocytic glia might be sensitive to TRAIL, a phenomenon that may not have been seen following systemic injection of recombinant TRAIL secondary to the exclusion of the protein from the central nervous system by the blood-brain barrier. To address the potential for Ad.TRAIL-GFP-mediated neural toxicity, we transduced HNP cells with adenoviral vectors expressing either GFP or TRAIL and then differentiated toward a neuronal lineage. As demonstrated in Figure 3A, TRAIL-infected HNP cells survived and differentiated normally compared to GFP-infected and parental HNP cells, yet were still capable of presenting biologically active TRAIL molecules and mediate a potent bystander effect against U87 glioma cells (Figure 3B). Additionally, we evaluated the acute and chronic affects of TRAIL expression in normal mouse brain by stereotactically injecting Ad.TRAIL-GFP (5x108 PFU) into the caudate nucleus. There were no clinical or histopathologic signs of increased inflammation or apoptosis in the Ad.TRAIL-GFP-injected brains compared to Ad.GFP-injected brains at 2 and 4 days following the injection of the vector (Figure 3C). Animals were monitored for an additional 2 months, with no detectable clinical or histologic abnormality outside of the usual, mostly monocytic infiltration seen following the injection of any adenoviral vector into the brain [19]. In addition, intraperitoneal injection of Ad.TRAIL-GFP vector into non-tumor-bearing immunodeficient mice did not induce gross or histologically evident systemic toxicity compared to that seen with control adenoviral vectors (data not shown).
Figure 3.
Evaluation of neurotoxic effects of Ad.TRAIL-GFP. (A) HNP cells transduced in vitro with Ad.GFP or Ad.TRAIL-GFP and then induced to differentiate along neuronal (TuJ1 +) lines (difference between number of TuJ1 + cells following Ad.GFP or Ad.TRAIL-GFP transduction of HNP not significant; P> 0.4). (B) HNP cells transduced with Ad.GFP or Ad.TRAIL-GFP and coculture with U87 glioma cells. HNP and U87 cells were visualized by DAPI staining and GFP positive cells were HNP cells. White arrows demonstrate apoptotic cells immediately adjacent to Ad.TRAIL-GFP-transduced HNP cells. Difference between number of apoptotic U87 target cells exposed versus Ad.TRAIL-GFP-transduced HNP cells highly significant (P<0.001). (C) Two days following the injection of Ad.TRAIL-GFP (5x108 PFU) into the brains of non-tumor-bearing mice, brains were obtained and prepared for histology and TUNEL assay. Representative photographs (x200) of brain sections stained with H&E (upper panel); representative photographs (x200) of brain frozen section stained for apoptotic cells (lower panel).
To assess the in vivo antitumor activity of Ad.TRAIL-GFP, we used both a human glioblastoma orthotopic xenograft and a mouse mammary peritoneal carcinomatosis model. For the glioblastoma model, U87 cells were stereotactically implanted intracerebrally followed by injection of Ad.TRAIL-GFP or Ad.GFP into the growing tumor 5 days later using the same initial stereotactic coordinates. Animals were then followed for survival. As can be seen in Figure 4A, Ad.TRAIL-GFP-treated animals survived significantly longer than control treated animals (P<0.001, log rank analysis). Likewise, to evaluate the antitumor effect of Ad.TRAIL-GFP on nude mice bearing intraperitoneal, malignant ascites tumors, animals with established TA3 intraperitoneal mammary carcinomas were given three separate intraperitoneal injections of Ad.TRAIL-GFP or Ad.GFP. As shown in Figure 4B, animals treated with Ad.TRAIL-GFP survived significantly longer than those treated with the control virus (P<0.001). TUNEL staining on brain tumor sections from Ad.TRAIL-GFP-treated intracerebral U87 tumor-bearing mice demonstrated a significantly elevated level of tumor cell apoptosis compared to brain tumors from control vector-treated animals (Figure 4C).
Figure 4.
In vivo antitumor activity of Ad.TRAIL-GFP. (A) Kaplan-Meier survival curves of animals treated by stereotactic injection of viral vectors into preestablished U87 gliomas. (B) Kaplan-Meier survival curves of TA3 ascites tumor-bearing mice treated by intraperitoneal injection of Ad.GFP (open square) or Ad.TRAIL-GFP (closed square). Survival is significantly prolonged by log rank analysis (P<0.001). (C) representative photographs of brain frozen section stained for apoptotic cells. Arrows indicate TUNEL-positive cells.
Duration of Ad.TRAIL-GFP Transgene Expression In Vivo
A major limitation to the use of adenoviral vectors for in vivo gene transfer is the loss of transgene expression due to the immune-mediated elimination of virally transduced cells, although prolonged transgene expression is observed in immune privileged sites such as the brain, cornea, and testis [19–21]. FasL, a transmembrane protein belonging to the TNF family, has been shown to be responsible for maintaining immune privileged status in these sites [20,21]. Therefore, we hypothesized that exogenous expression of TRAIL from adenoviral transduced cells might create a situation analogous to the immune-privileged microenvironment, thereby facilitating prolonged transgene expression. To evaluate whether Ad.TRAIL-GFP-transduced cells could prolong the duration of expression from a coexpressed transgene in the liver of immunocompetent mice, Ad.TRAIL-GFP or Ad.GFP was injected through the tail vein. Following a single intravenous injection of Ad.GFP, we found GFP transgene expression in the liver at day 4, with rapid disappearance by day 7. In contrast, GFP expression was seen for >30 days in Ad.TRAIL-GFP-infected liver (Figure 5A). Western blot analysis confirmed significantly prolonged duration of hepatic GFP expression in the Ad.TRAIL-GFP-injected animals (Figure 5B). We observed substantially fewer inflammatory cells in the Ad.TRAIL-GFP-transduced livers compared to the Ad.GFP-transduced livers (data not shown). Additionally, there were significantly fewer numbers of apoptotic hepatocytes seen in the Ad.TRAIL-GFP transduced livers compared to the Ad.GFP control vectortransduced livers at day 7 (Figure 5;C and D). These observations are consistent with a diminished inflammatory cell-mediated anti-adenoviral response directed against the Ad.TRAIL-GFP-transduced hepatocytes compared to the Ad.GFP-transduced hepatocytes.
Figure 5.
Duration of GFP transgene expression in the liver following injection of Ad.TRAIL-GFP. Immunocompetent mice were given Ad.GFP or Ad.TRAIL-GFP (1010 PFU) through the tail vein. (A) On days 4, 7, 14, and 30, the liver was examined for GFP fluorescence. Representative photographs (x400) of unfixed cryosection. (B) Western blot analysis from livers infected with Ad.TRAIL-GFP or Ad.GFP. A single ∼28-kDa band corresponding to the size of GFP protein (arrow) was detected. (C) Increased apoptotic index in Ad.GFP-infected liver compared with Ad.TRAIL-GFP-infected liver 7 days after intravenous injection of virus. (D) Quantitative summary of the apoptotic index from vector-transduced livers (asterisk: P<0.01).
Discussion
TNF family members such as TNF and FasL have significant and potent antitumor activity in vitro and in vivo; however, their clinical utility has been hampered by severe local and systemic toxicity. TRAIL has been recognized as a potentially promising new tool for cancer gene therapy given its ability to induce apoptosis in a variety of transformed cell lines, with few adverse effects on most normal cells in vitro. TRAIL mRNA is widely expressed in most human tissues at significant levels [2]. To date, five TRAIL-binding receptors (TRAIL-R1 to TRAIL-R4, and osteoprotegerin) have been identified [22–24]. TRAIL-R1 and TRAIL-R2 are able to induce apoptosis by engaging TRAIL, whereas, TRAIL-R3 and TRAIL-R4 are incapable of transducing death signal due to deletion or truncation of its cytoplasmic domain, respectively. Curiously, however, expression patterns of different TRAIL receptors in a variety of cell types do not appear to closely correlate with sensitivity to TRAIL. Although the tumor-specific mechanism of TRAIL and its physiological role remain to be elucidated, it is interesting to note that TRAIL-mediated cell killing does not seem to depend on the p53 status of the cell [24]. This makes TRAIL even more attractive as a potential antitumor agent given the frequency with which p53 is mutated in human cancer.
Like other TNF family members, TRAIL exists as a type 2 transmembrane protein, which can be cleaved by specific proteases to a soluble form [25]. Although soluble TNF maintains high biologic activity, both FasL and TRAIL lose significant activity as soluble monomeric proteins. More active forms of soluble TRAIL can be generated, however, by inducing oligomerization of the monomers through genetic, biochemical, or antibody-mediated approaches [26–28]. These large multimeric proteins may, however, present difficult clinical drug delivery challenges particularly for tumors of the central nervous system, where an even partially intact blood-brain barrier is likely to limit efficient systemic delivery of such molecules.
We and others have previously demonstrated the ability of stereotactically injected adenoviral vectors to efficiently transduce relatively large areas of the brain, thereby creating a zone of high transgene protein expression [15,19]. Thus, we hypothesized that TRAIL represented a potentially promising transgene for adenoviral transduction given its tumor-selective cytotoxic profile. A transgene encoding a soluble TRAIL protein, however, would result in a relatively inactive protein. Although we considered constructing adenoviral vectors that encode TRAIL proteins that might spontaneously aggregate (i.e., TRAIL mutants with leucine zippers) [5], it seemed problematic that such aggregation would occur spontaneously and efficiently following transgene delivery in vivo. Thus, given the fact that TRAIL naturally exists as a transmembrane protein, we elected to create vectors that encode a transmembrane form of TRAIL. Our data suggest that transmembrane expression is indeed a highly potent strategy for expressing TRAIL because tumors were more susceptible to TRAIL-induced apoptosis when cocultured with TRAIL-expressing cells than when directly transduced with the vector itself. Why transmembrane expression of TRAIL is so highly efficient for inducing target cell apoptosis is unclear, although possibilities include transmembrane TRAIL undergoing oligomerization at the cell membrane following binding to its receptor on target cells, target cell receptor oligomerization following engagement of membrane bound TRAIL, and/or proper ligand/receptor orientation and engagement. Although these hypotheses need to be formally tested, such mechanisms are consistent with the observed requirement for soluble TRAIL oligomerization for full biologic activity.
We were surprised to find that direct vector-mediated TRAIL transduction of tumor cells appears to be less efficient at inducing apoptosis than by presentation by another cell. Indeed, the surviving TRAIL-transduced tumor cells can themselves act as efficient TRAIL-presenting cells and mediate efficient cell death without dying themselves (Figure 2B). The mechanisms behind this observation are also unclear but may represent intracytoplasmic/intragolgi engagement of newly synthesized monomeric TRAIL molecules with newly synthesized monomeric receptors given that both are transmembrane proteins and are therefore likely to end up in the same golgi/lysosomal transport pathway. Such an intracellular interaction could inhibit proper downstream signaling normally associated with TRAIL receptor engagement. Further studies, however, will be required for final determination of this potentially important agonist/antagonist interaction.
Our data demonstrate that normal human primary cells infected with an adenoviral vector encoding TRAIL were not only viable, but also able to efficiently kill neighboring tumor cells in vitro (Figure 2A). TRAIL-mediated apoptosis may function principally in a paracrine manner in vivo. A paracrine mechanism of TRAIL-mediated cytotoxicity suggests that normal cells infected with TRAIL can function as “TRAIL-effector cells in situ.” Therefore, tumor cells can be killed not only by direct infection, but also by neighboring cells (i.e., bystander effect). This may be particularly important for the treatment of malignant brain tumors because malignant glioma cells infiltrate deep within brain parenchyma, intermixed with normal glial and neuronal cells. It has been previously demonstrated that intracranial injection of adenoviral vectors results in transduction of both normal glial and neuronal cells in vivo, with prolonged transgene expression (>2 months) [19]. Thus, adenoviral transduction of TRAIL to normal brain tissue infiltrated by glioma cells with the subsequent generation of a powerful normal tissue-mediated tumor cell toxic bystander effect, may offer distinct advantages for the treatment of malignant gliomas over systemic delivery of the large recombinant TRAIL protein. Such a approach is supported by our demonstration that Ad.TRAIL-GFP-transduced NHA, human neural progenitor cells and neurons show no signs of toxicity in vitro, yet can mediate an effective antiglioma bystander effect (Figure 3).
Although theoretically attractive for gene therapy, the clinical utility of adenoviral vectors may be limited by potent antiviral immune responses directed against vector-transduced cells. It has been well demonstrated that the initial high-level transgene expression seen following adenoviral vector transduction in vivo, diminishes to virtually nothing within a few days in immunocompetent animals [29–31]. Given the recent demonstration that activated T lymphocytes interacting with cells overexpressing TRAIL undergo apoptosis [31], we hypothesized that cells transduced by adenoviral vectors expressing TRAIL may be less susceptible to T cell-mediated cell death, thus allowing prolonged transgene expression. Indeed, cellular FasL expression is thought to be a major mechanisms responsible for the downregulation of effector immune responses in immunoprivileged areas of the body such as the brain, testes, and eye [32], areas of the body where adenoviral vector-mediated transgene expression is prolonged. Our data demonstrate that adenoviral vectors that coexpress TRAIL, can facilitate prolonged transgene expression in immunocompetent animals by inhibiting the destruction of transduced hepatocytes, thereby, in effect, creating a local area of relative immune privilege (Figure 5). Vectors developed for other gene therapy indications, where prolonged transgene expression is optimal, may benefit from coexpression of TRAIL.
In these studies, we have demonstrated the therapeutic potential of an adenovirus-mediated in vivo transduction strategy that combines the efficiency of adenoviral gene transfer and the tumor-selective cytotoxicity of TRAIL. We have demonstrated both the safety of a TRAIL-expressing adenovirus in mice and its potent antitumor effects in two different human xenograft tumor models. Our data confirm recent studies demonstrating tumor-specific cytotoxicity from various forms of recombinant TRAIL proteins, and suggest gene transfer as a potential alternative strategy for the efficient delivery of TRAIL for localized malignant processes through the generation of a potent, normal and tumor cell-mediated bystander effect.
Abbreviations
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- MOI
multiplicity of infection
- PCR
polymerase chain reaction
- FACS
fluorescence-activated cell sorting
- TUNEL
terminal transferase-mediated dUTP nick end labeling
Footnotes
These authors contributed equally to the work.
References
- 1.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din W S, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Gura T. How TRAIL kills cancer cells, but not normal cells. Science. 1997;277:768. doi: 10.1126/science.277.5327.768. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 6.Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, Parney IF, Roa WH, Petruk KC. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- 7.Kagawa S, He C, Gu J, Koch P, Rha S-J, Roth JA, Curley SA, Stephens C, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 8.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 9.Nitsch R, Bechmann I, Deisz RA, Haas D, Lehmann TN, Wendling U, Zipp F. Human brain-cell death induced by tumour-necrosis factor inducing ligand (TRAIL) Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 11.Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7:1362–1369. [PubMed] [Google Scholar]
- 12.Tanaka T, Cao Y, Folkman J, Fine HA. Viral vector-targeted antiangiogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res. 1998;58:3362–3369. [PubMed] [Google Scholar]
- 13.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 14.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr MJ, Wen PY, Schaub M, Khoury SJ, Sayegh MH, Fine HA. Immune parameters affecting adenoviral vector gene therapy in the brain. J Neurovirol. 1998;4:194–203. doi: 10.3109/13550289809114519. [DOI] [PubMed] [Google Scholar]
- 16.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427:124–128. doi: 10.1016/s0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- 18.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- 19.Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Southgate TD, Klatzmann D, Lassmann H, Castro MG, Lowenstein PR. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 20.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 21.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 22.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 23.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 24.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:55–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 25.Gruss HJ. Molecular, structural, and biological characteristics of the tumor necrosis factor ligand superfamily. Int J Clin Lab Res. 1996;26:143–159. doi: 10.1007/BF02592977. [DOI] [PubMed] [Google Scholar]
- 26.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 27.Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2-or Bcl-xL-overexpressing chemotherapy -resistant tumor cells. Cancer Res. 2000;60:3051–3057. [PubMed] [Google Scholar]
- 28.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Wilson JM. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;155:2560–2564. [PubMed] [Google Scholar]
- 31.Giovarelli M, Musiani P, Garotta G, Ebner R, Di Carlo E, Kim Y, Cappello P, Rigamonti L, Bernabei P, Novelli F, Modesti A, Coletti A, Ferrie AK, Lollini PL, Ruben S, Salcedo T, Forni GA. “Stealth effect”: adenocarcinoma cells engineered to express TRAIL elude tumor-specific and allogeneic T cell reactions. J Immunol. 1999;163:4886–4893. [PubMed] [Google Scholar]
- 32.Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]