EDITOR’S KEY POINTS.
Uncomplicated urinary tract infections in women are one of the most frequent presenting complaints seen by family doctors.
A 3-day course of trimethoprim-sulfamethoxazole or trimethoprim alone continues to be recognized as first-line treatment. There are concerns, however, about rare but serious skin reactions to the sulfa component and about growing resistance to these drugs (about 10% in Canada).
Nitrofurantoin has a long history of good efficacy and continues as a first-line choice, even though a 7-day course is required. Despite heavy use, very little resistance to nitrofurantoin has developed.
Fluoroquinolones have more recently been introduced to treat urinary tract infections, and they are very effective in a 3-day course. Their cost and the potential for development of resistance, however, suggest that they should remain a second-line choice for treatment.
POINTS DE REPÈRE DU RÉDACTEUR.
L’infection urinaire non compliquée chez la femme est une des raisons de consulter les plus fréquentes en médecine familiale.
Un traitement de 3 jours à l’aide de triméthoprime-sulfaméthoxazole ou de triméthoprime seul est toujours considéré comme le traitement de premier choix. Il faut toutefois se préoccuper d’éventuelles réactions cutanées à la composante sulfa, qui sont rares mais sévères, et de l’augmentation de la résistance à ces médicaments (environ 10% au Canada).
La nitrofurantoïne a une longue histoire d’efficacité et elle continue d’être une médication de première intention, même s’il faut 7 jours de traitement. Malgré une utilisation abondante, il s’est développé très peu de résistance à cet agent.
Plus récemment, les fluoroquinolones en traitement de 3 jours se sont montrés très efficaces pour traiter l’infection urinaire. Leur coût et le développement éventuel de résistance suggèrent toutefois qu’ils devraient demeurer un traitement de seconde intention.
Acute uncomplicated urinary tract infections (uUTIs) occur in non-pregnant women with normal genitourinary tracts.1 These uUTIs are one of the most common bacterial infections, a frequent presenting complaint for women visiting their family practitioners. Short courses of antibiotic therapy are generally adequate treatment, and beginning empiric therapy without obtaining a urine specimen is recommended.2 The evolution of antimicrobial resistance in community-acquired Escherichia coli, however, requires continuing reevaluation of empiric antimicrobial therapy.3
Widespread empiric use of antibiotics, while convenient, potentially contributes to development of antimicrobial resistance. With concerns about increasing resistance in common community-acquired infections, “antimicrobial stewardship” (using antibiotics in a way that helps limit development of resistance) must also be considered. This review addresses antimicrobial management of uUTIs in the context of evolving antimicrobial susceptibility and family practitioners’ use of guidelines for managing these infections.
Quality of evidence
PubMed was searched using the MeSH terms “uncomplicated urinary infection,” “empiric therapy,” and “antimicrobial resistance.” Additional relevant papers were sought by reviewing references cited in the key papers identified. Canadian guidelines on management of uUTIs were identified through discussions with family physicians. Levels of evidence were assessed. Most evidence is level I from published clinical trials of treatment for uUTIs.
Main message
Uncomplicated urinary tract infection is a common clinical syndrome that occurs in women with otherwise normal genitourinary tracts.1 Reported incidence is 0.5 to 0.7 per person-year in premenopausal women.4 About 3% of all women in the United States visit a physician at least once each year for uUTIs,5 and at least 50% of women report at least one uUTI in a lifetime.6 Some women have frequent infections.7 The burden of disease in Canadian women is likely similar to that in American women. The natural history of uUTIs when antimicrobial therapy is not given is resolution of infection in about 50% of women by 2 to 4 weeks.8,9 Antibiotic therapy shortens the duration of symptoms and will probably cure more than 90% of infections.
Management of uUTIs
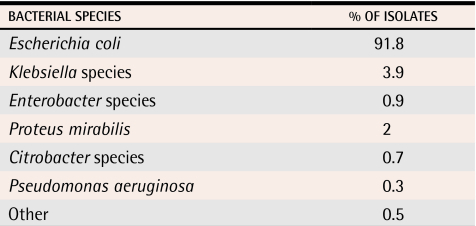
Diagnosis and treatment of uUTIs is usually straightforward. Classic symptoms include burning with urination (dysuria) and increased frequency and urgency.10 Symptoms are characteristic enough that women are highly reliable at self-diagnosis. In addition, the microbiology of infection is consistent, with E coli isolated in 85% to 90% of episodes11 (Table 111). The characteristic symptoms and consistent microbiology support use of empiric antimicrobial therapy initiated as soon as possible after onset of symptoms, without waiting for results of urine culture, and targeted to E coli.2,10,12 Variables to be considered in selecting an antimicrobial drug include efficacy, adverse effects, cost, and potential for future resistance.13,14
Table 1.
Gram-negative organisms isolated from community-acquired uncomplicated urinary tract infections in women in Toronto, Ont
Data from Mazzulli.11
First-line agents for empiric therapy
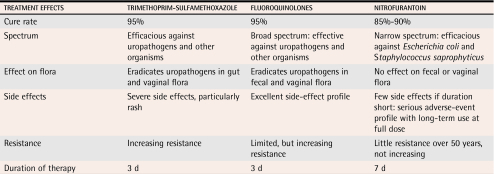
For several decades, trimethoprim-sulfamethoxazole (TMP/SMX), or trimethoprim alone, have been first-line therapy for uUTI.2,10,13 These agents are effective as 3-day therapy, but adverse reactions, particularly allergic reactions to sulfa, sometimes occur and are occasionally serious.2 For women infected with susceptible E coli, cure rates of 90% to 95% are achieved with 3 days’ therapy.15 First-line agents are shown in Table 2.2,13
Table 2.
Current options for first-line antimicrobial treatment of uncomplicated urinary tract infection
Nitrofurantoin is a narrow-spectrum antimicrobial with no systemic activity. It is indicated only for treatment of uUTI caused by E coli and Staphylococcus saprophyticus, the two pathogens isolated from 95% of all uUTIs.13 Nitrofurantoin has been used for treating uUTIs for more than 50 years and has had continuing safety and efficacy.8,13 Early formulations were associated with substantial adverse effects of the gastrointestinal system, but the current macrocrystalline formulation is well tolerated.8 Nitrofurantoin cures 85% to 90% of uUTIs with a 7-day course,16 but only 70% to 80% of uUTIs when given as a 3-day course.8,17
Fluoroquinolones including norfloxacin, ciprofloxacin, ofloxacin, levofloxacin, and gatifloxacin, are effective as 3-day therapy and are well tolerated.2,15,18 For infection with susceptible organisms, outcomes with 3-day fluoroquinolone therapy are similar to outcomes with TMP/SMX: a 90% to 95% cure rate.15 Fluoroquinolones have been evaluated as single-dose therapy, but were shown to have limited efficacy against S saprophyticus with this abbreviated regimen, so single-dose therapy is not recommended.19-22 This class of antimicrobial is also important for treating many other infections, including severe infections of the urinary tract and other sites in the body.
Fosfomycin given as a single dose is also marketed for uUTI in North America. There is limited experience with this agent in Canada, but clinical trials suggest it is slightly less effective than other first-line agents, with a cure rate of about 70%.10 Cephalosporins have a role in treating urinary tract infections, particularly in pregnant women, but are not recommended for empiric therapy because of the relatively high rates of resistance and lower efficacy, especially with short-course therapy.18,23
Antimicrobial resistance
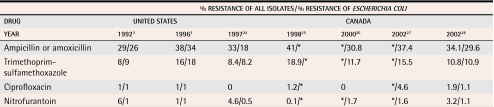
Resistance in community-acquired E coli has evolved with the sequential introduction and widespread use of various antimicrobials over 5 decades of antimicrobial therapy.23 Table 33,24-28 summarizes prevalence surveys of E coli’s resistance to common antimicrobials used to treat uUTIs. The E coli samples were isolated from women outpatients over the last 10 years in Canada and the United States.
Table 3.
Evolution of resistance of uropathogens in North America to first-line antimicrobial agents for uncomplicated urinary tract infection
*Data not available.
Ampicillin or amoxicillin were once standard therapy for uUTI,29 but the resistance of E coli to ampicillin now approaches 50% in most regions of North America.3,11 Trimethoprim-sulfamethoxazole has been considered first-line empiric treatment for more than 30 years—the length of career of most family physicians practising today.2,13 The prevalence of E coli’s resistance to TMP/SMX has increased during the past decade, although resistance varies substantially in different regions.28 Prevalence now exceeds 20% in some regions of North America,29,30 but remains about 10% in Canada.24,28
Resistance to nitrofurantoin among E coli isolates from uUTIs remains low despite more than 50 years’ widespread use of the drug.24,26 Reasons for the lack of emerging resistance are not fully understood, but likely include restricting use to indications for urinary infection, limited systemic absorption, and the need for multiple genetic mutations for the bacteria to develop resistance.
Escherichia coli’s resistance to fluoroquinolones, such as ciprofloxacin, remains relatively low in North America. The prevalence of resistance to ciprofloxacin, however, has increased over the past 5 years in Canada (Table 33,24-28). In some areas of Europe, such as Spain and Portugal, the prevalence of resistance among E coli strains isolated from uUTIs approaches 20%. An increased prevalence of resistance to nalidixic acid, a precursor of resistance to fluoroquinolones, has been reported in several other parts of Europe.26 As a result, increasing antimicrobial resistance to fluoroquinolones is being observed worldwide.
Appropriate antimicrobial use
Antimicrobial resistance is a global issue, and concerns have been raised that some infections for which therapy is now available might become untreatable.31 Appropriate antimicrobial use is defined by the United States’ Centers for Disease Control and Prevention as use that maximizes therapeutic effect while minimizing risk of increased resistance.31 Government and professional organizations, including the Canadian Integrated Action Plan,32 also recommend appropriate antibiotic use, including the major goal of specific therapy that uses the narrowest-spectrum agent possible.32-36 Widespread empiric use of broad-spectrum agents could contravene the principles of antimicrobial stewardship. Drug resistance, a natural response to selective pressure with drug use, is exacerbated by overuse of antimicrobials.31,35 Concurrent with concerns about increased antimicrobial resistance, the pharmaceutical industry is no longer making development of new antibiotics a priority.
Family practitioners are at the front lines of the effort to preserve the effectiveness of antimicrobial drugs. One important strategy for limiting resistance is to avoid unnecessary antibiotic use, but for uUTIs (unlike upper respiratory infections), antibiotics are a consistently appropriate therapeutic choice. Uncomplicated urinary tract infection is one of the most frequent reasons for prescribing antimicrobial therapy in North America.36 Although empiric therapy for uUTI is convenient, effective, and cost-effective, widespread empiric therapy could contribute to development of drug resistance in the community.7
Monitoring antimicrobial resistance
Optimal antibiotic use requires physicians to have timely, accessible information on local prevalence of antimicrobial resistance. It is often difficult to obtain such information for community-acquired E coli.37 As empiric therapy is the standard approach to managing uUTI, urine specimens that are collected and forwarded for culture are more likely to have been obtained from women with recurrent infections, women who have failed therapy, or women with complicated urinary tract infections. Hence, results of laboratory-based prevalence surveys are biased by a preponderance of organisms with a greater likelihood of resistance. Practice-based surveys of urine specimens obtained uniformly from women with uUTIs presenting to practitioners are reported only sporadically.24,28
Antimicrobial susceptibility surveys that summarize resistance in isolates from clinical microbiology laboratories in health care facilities include samples from people with complicated urinary tract infections, even when the isolates included are restricted to samples from outpatients. For example, prevalence surveys of outpatients at health care facilities in Canada25,27 show higher levels of resistance than surveys of women with UTIs presenting to physicians.24,26,28 One example, The Surveillance Network (TSN) Database–Canada, reports susceptibility data on five urinary pathogens from 87 clinical institutions across Canada and both regional and national information.27 Data are timely, as the database is updated 3 times yearly and posted on the www.utizone.ca website for physicians. Isolates from patients with complicated urinary tract infections and with treatment failures would be included in this database, along with data on both upper and lower urinary tract infections. Thus, these reports overestimate the prevalence of antimicrobial resistance in uUTI. The data are collected on a continuing basis, however, so remain useful for monitoring temporal trends.
Evolution of resistance and choice of empiric therapy
The continuing evolution of antimicrobial resistance in community-acquired E coli requires repeated reassessment of recommendations for first-line empiric therapy for uUTI. Practitioners always need to balance antimicrobial selection for optimal patient outcome with the potential for contributing to further antimicrobial resistance through widespread empiric use. The prevalence of resistance at which first-line empiric therapy should be modified is unknown. The Infectious Diseases Society of America’s guidelines suggest that 10% to 20% is an appropriate benchmark, but acknowledge no specific data support this recommendation.2 Prescribing behaviour suggests that, over the past decade, primary care physicians have altered their approach to first-line therapy for uUTI: TMP/SMX prescriptions for uUTI have declined, while fluoroquinolone prescriptions have increased.38
Level I evidence suggests that TMP/SMX, or trimethoprim by itself for women with sulfa allergies, remains optimal first-line empiric therapy where organisms are known or assumed to be susceptible. Thus, where resistance prevalence is lower than 20%, as it appears to be in Canada currently, TMP/SMX should remain the drug of choice for empiric therapy.29 Women with recurrent infections who have received TMP/SMX within 3 months are more likely to have resistant organisms, so alternative empiric therapy is likely appropriate for these patients39 (based on level II evidence).
When antimicrobial resistance or patients’ intolerance to TMP/SMX is of concern, nitrofurantoin, a fluoroquinolone, or fosfomycin are alternative medications. Level I evidence indicates that nitrofurantoin is an effective alternative for empiric therapy when given for 7 days. Use of nitrofurantoin also alleviates concerns about the emergence of resistance. Studies are exploring whether a 5-day course of nitrofurantoin therapy is as effective as a 7-day course. While fluoroquinolones are highly effective (based on level I evidence), widespread empiric use of these agents might promote antimicrobial resistance in organisms that cause severe infections, including organisms that cause infections outside the urinary tract, such as Streptococcus pneumoniae.37,40 It has been suggested that widespread empiric use of fluoroquinolones for uUTI should be avoided as a strategy for limiting resistance and prolonging the efficacy of this class of antibiotics for more serious infections.37 Fosfomycin has not been used much in North America, and the role of this agent remains unclear.
Guidelines for managing uUTI
Recommendations for empiric therapy need to be translated into practice, and guidelines are one way to achieve this. Encouraging guideline-based treatment is an important aspect of changing prescribing behaviour, a goal of antibiotic stewardship. Developing guidelines for treating uUTI in family practice might assist in balancing the dual objectives of providing optimal patient care and limiting antimicrobial resistance.
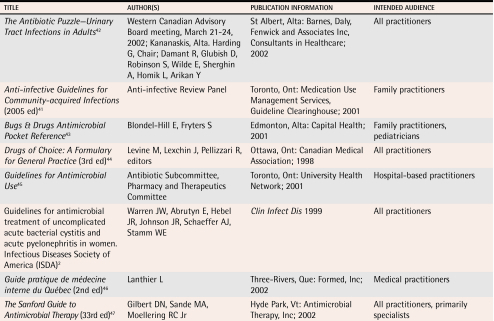
At least 8 sets of uUTI treatment guidelines have been available in Canada for the last 5 years (Table 42,41-47). Despite a consistent spectrum of causative organisms and treatment objectives, antimicrobial regimens for uUTI, including drug selection and duration of therapy, vary widely.14 Various guidelines make conflicting recommendations on alternatives to TMP/SMX. These conflicting recommendations impair optimal decision making and are frustrating for practitioners. In addition, some guidelines were developed for specific settings. The Toronto University Guidelines, for instance, were developed to address resistance issues in a specific hospital system, but were then promoted to general practitioners in the community, despite their lack of relevance in that setting. It is unsurprising, then, that family physicians’ prescribing behaviour varies.38
Table 4.
Guidelines with recommendations for antimicrobial therapy of uncomplicated urinary tract infections
No single set of guidelines applies to every setting. Physicians must identify guidelines relevant to their own practice and use them appropriately. Having family physicians participate in development of guidelines, using evidence-based recommendations, and providing guidelines in an accessible format are desirable attributes. Guidelines also need to be flexible, to allow appropriate modifications for application at local levels, and to address the continuing evolution of organism resistance. Other relevant issues to be considered in development include the intended audience or community for the guidelines, who composes the guidelines (2 or 3 physicians, a board, or a peer-review group), and source of funding for development (corporate sponsorship, independent, or government funded).
Of the guidelines identified and reviewed, the Anti-infective Guidelines for Community-acquired Infections (commonly referred to as the Ontario Guidelines)41 are likely the most relevant for family physicians. These guidelines were developed by an independent panel of physicians led by a family physician and including adequate family physician representation and were specifically targeted at primary care. The publication is in an accessible format and suggests strategies for addressing patient and community expectations and providing specific therapeutic recommendations. Canadian evidence is used wherever possible in these guidelines. For empiric therapy of uUTI, recommended first-line treatments are TMP/SMX, trimethoprim alone, and nitrofurantoin. A quinolone-sparing strategy is recommended because of concerns about resistance.
Beyond development of relevant guidelines, education is key to changing prescribing practice. Physicians need an accurate understanding of the specific clinical problem, access to timely summaries of local resistance, and recommendations for drug regimens. One program that addresses the educational needs of family physicians is the Partners for Appropriate Anti-infective Community Therapy (PAACT) education module.48 This program encourages judicious use of antimicrobials through education rather than restriction.
Conclusion
For treatment of uUTI, narrow-spectrum antimicrobials are appropriate, given the consistent bacteriology, and are preferred, given concerns about antimicrobial resistance. In most parts of Canada, TMP/SMX, trimethoprim alone, or nitrofurantoin are the agents of choice for uUTI. Use of narrow-spectrum antimicrobials is also consistent with the principles of antimicrobial stewardship. Use of broad-spectrum agents, such as the fluoroquinolones, might promote resistance that will negatively affect not only treatment of uUTI, but also treatment of other, more serious, infections. Fluoroquinolones should not be considered first-line therapy. The continuing evolution of antimicrobial resistance requires that timely information describing this resistance is generated and disseminated effectively to practitioners.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Acknowledgments
This paper was based on presentations and discussions at a symposium held in Toronto on May 31, 2003. Funding for this symposium was provided by Procter and Gamble Pharmaceuticals. Participants received honoraria for their time and expertise.
Biography
Dr Nicolle and Dr Zhanel teach at the University of Manitoba in Winnipeg. Dr Anderson teaches at Dalhousie University in Halifax, NS. Dr Conly and Dr Mainprize teach at the University of Calgary in Alberta. Dr Meuser teaches at the University of Toronto in Ontario. Dr Nickel teaches at Queen’s University at Kingston in Ontario. Dr Senikas teaches at McGill University in Montreal, Que.
Footnotes
Competing interests: Dr Nicolle received research funding from Ortho McNeil Inc. Dr Zhanel received research funding from Ortho McNeil Inc and Procter and Gamble Ltd to conduct studies in areas related to the subject matter of this article. He received no funding for preparing this article.
References
- 1.Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(Suppl 1):1–7. [PubMed] [Google Scholar]
- 2.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736–738. doi: 10.1001/jama.281.8.736. [DOI] [PubMed] [Google Scholar]
- 4.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton QE, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. National Ambulatory Medical Care Survey, United States, 1979 summary. Vital and health statistics, 13(66). DHHS Publication no. (PHS) 82-1727. Washington DC: Government Printing Office; 1982. [PubMed] [Google Scholar]
- 6.Kunin CM. Urinary tract infections in females. Clin Infect Dis. 1994;18:1–12. doi: 10.1093/clinids/18.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–333. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomized, controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52:729–734. [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolle LE. Pivmecillinam in the treatment of urinary tract infection. J Antimicrob Chemother. 2000;46(Suppl 1):35–39. [PubMed] [Google Scholar]
- 10.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;1:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 11.Mazzulli T. Antimicrobial resistance trends in common urinary pathogens. Can J Urol. 2001;8(Suppl 1):2–5. [PubMed] [Google Scholar]
- 12.O’Connor PJ, Solberg LI, Christianson J, Amundson G, Mosser G. Mechanism of action and impact of a cystitis clinical practice guideline on outcomes and costs of care in an HMO. Jt Comm J Qual Improv. 1996;22:673–682. doi: 10.1016/s1070-3241(16)30274-7. [DOI] [PubMed] [Google Scholar]
- 13.Nicolle LE. Urinary tract infection: traditional pharmacologic therapies. Am J Med. 2002;113(Suppl 1A):35–44. doi: 10.1016/s0002-9343(02)01058-6. [DOI] [PubMed] [Google Scholar]
- 14.Stamm WE. Scientific and clinical challenges in the management of urinary tract infections. Am J Med. 2002;113(Suppl 1A):1–4. doi: 10.1016/s0002-9343(02)01053-7. [DOI] [PubMed] [Google Scholar]
- 15.McCarty JM, Richard G, Huck W, Tucker RM, Tosiello RL, Shan M, et al. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Am J Med. 1999;106:292–299. doi: 10.1016/s0002-9343(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(Suppl A):67–75. [PubMed] [Google Scholar]
- 17.Hooton TM, Winter C, Tiu F, Stamm WE. Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA. 1995;273:41–45. [PubMed] [Google Scholar]
- 18.Nicolle L. Best pharmacological practice: urinary tract infections. Expert Opin Pharmacother. 2003;4:693–704. doi: 10.1517/14656566.4.5.693. [DOI] [PubMed] [Google Scholar]
- 19.Saginur R, Nicolle LE. Single dose compared with 3-day norfloxacin treatment of uncomplicated urinary tract infection in women. Canadian Infectious Diseases Society Clinical Trials Study Group. Arch Intern Med. 1992;152:1233–1237. [PubMed] [Google Scholar]
- 20.Iravani A. Multicenter study of single-dose and multiple-dose fleroxacin versus ciprofloxacin in the treatment of uncomplicated urinary tract infections. Am J Med. 1993;94(Suppl 3A):89–96. [PubMed] [Google Scholar]
- 21.Ode B, Walder M, Forsgren A. Failure of a single dose of 100 mg ofloxacin in lower urinary tract infections in females. Scand J Infect Dis. 1987;19:677–679. doi: 10.3109/00365548709117203. [DOI] [PubMed] [Google Scholar]
- 22.Garlando F, Rietiker S, Tauber MG, Flepp M, Meier B, Luthy R. Single-dose ciprofloxacin at 100 versus 250 mg for treatment of uncomplicated urinary tract infections in women. Antimicrob Agents Chemother. 1987;31:354–356. doi: 10.1128/aac.31.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta K. Addressing antibiotic resistance. Am J Med. 2002;113(Suppl 1A):29–34. doi: 10.1016/s0002-9343(02)01057-4. [DOI] [PubMed] [Google Scholar]
- 24.Mazzulli T, Skulnick M, Small G, Marshall W, Hoban DJ, Zhanel GG, et al. Susceptibility of community Gram-negative urinary tract isolates to mecillinam and other oral agents. Can J Infect Dis. 2001;12:289–292. doi: 10.1155/2001/601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhanel GG, Karlowsky JA, Harding GK, Carrie A, Mazzulli T, Low DE, et al. A Canadian national surveillance study of urinary tract isolates from outpatients: comparison of the activities of trimethoprim-sulfamethoxazole, ampicillin, mecillinam, nitrofurantoin, and ciprofloxacin. The Canadian Urinary Isolate Study Group. Antimicrob Agents Chemother. 2000;44:1089–1092. doi: 10.1128/aac.44.4.1089-1092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahlmeter G. The ECO. SENS Project: a prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens—interim report. J Antimicrob Chemother. 2000;46(Suppl 1):15–22. [PubMed] [Google Scholar]
- 27.The Surveillance Network (TSN) Database. TSNR Database—USA, 2002. Cypress, Calif and Herndon, Va: Focus Technologies, Inc; 2002. [cited 2006 February 21]. Available at: http://www.utizone.ca. [Google Scholar]
- 28.McIsaac WJ, Mazzulli T, Moineddin R, Raboud J, Ross S. Uropathogen antibiotic resistance in adult women presenting to family physicians with acute uncomplicated cystitis. Can J Infect Dis Med Microbiol. 2004;15:266–270. doi: 10.1155/2004/947026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 30.Zhanel GG, Hisanaga TL, Laing NM, Decorby MR, Nichol KA, Palatnick LP, et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2005;26:380–388. doi: 10.1016/j.ijantimicag.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control. Background on antibiotic resistance. Atlanta, Ga: Centers for Disease Control; 2004. [cited 2006 February 21]. Available at: http://www.cdc.gov/drugresistance/community. [Google Scholar]
- 32.Health Canada. Controlling antimicrobial resistance: an integrated action plan for Canadians. Can Commun Dis Rep. 1997;23(Suppl 7):1–32. [PubMed] [Google Scholar]
- 33.Centers for Disease Control. A public health action plant to combat antimicrobial resistance. Atlanta, Ga: Centers for Disease Control; 2005. [cited 2006 February 21]. Available at: http://www.cdc.gov/drugresistance/actionplan/html/index.htm. [Google Scholar]
- 34.Alliance for Prudent Use of Antibiotics. Antibiotic resistance: synthesis of recommendations by expert policy groups. Geneva, Switz: World Health Organization/CDS/CSR/DRS; 2001. [Google Scholar]
- 35.World Health Organization. WHO global strategy for containment of antimicrobial resistance. Geneva, Switz: WHO/CDS/CSR/DRS; 2001. [cited 2006 February 21]. Available at: http://www.who.int/csr/drugresist/WHO_Global_Strategy_English.pdf. [Google Scholar]
- 36.McCaig LF, Besser RE, Hughes JM. Antimicrobial-drug prescription in ambulatory care settings, United States 1992-2000. Emerg Infect Dis. 2003;9:432–437. doi: 10.3201/eid0904.020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis. 2004;39:75–80. doi: 10.1086/422145. [DOI] [PubMed] [Google Scholar]
- 38.Naber KG. Survey on antibiotic usage in the treatment of urinary tract infections. J Antimicrob Chemother. 2000;46(Suppl 1):49–52. [PubMed] [Google Scholar]
- 39.Brown PD, Freeman A, Foxman B. Prevalence and predictors of trimethoprim/sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis. 2002;34:1061–1066. doi: 10.1086/339491. [DOI] [PubMed] [Google Scholar]
- 40.Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 41.Anti-infective Review Panel. Anti-infective guidelines for community-acquired infections. Toronto, Ont: Medication Use Management Services, Guideline Clearinghouse; 2005. [Google Scholar]
- 42.Harding G, Damant R, Glubish D, Robinson S, Wilde E, Sherghin A, et al. The antibiotic puzzle—urinary tract infections in adults. St Albert, Alta: Barnes, Daly, Fenwick and Associates Inc, Consultants in Healthcare; 1996. Harding G, Chair. [Google Scholar]
- 43.Blondel-Hill E, Fryters S. Bugs & drugs antimicrobial pocket reference. Edmonton, Alta: Capital Health; 2001. pp. 212–213. [Google Scholar]
- 44.Levine M, Lexchin J, Pellizzari R. Drugs of choice: a formulary for general practice. 3rd ed. Ottawa, Ont: Canadian Medical Association; 1998. pp. 152–153. [Google Scholar]
- 45.Antibiotic Subcommittee, Pharmacy and Therapeutics Committee. Guidelines for antimicrobial use. Toronto, Ont: University Health Network; 2001. p. 91. [Google Scholar]
- 46.Lanthier L. Guide pratique de médecine interne du Québec. 2nd ed. Three-Rivers, Que: Formed, Inc; 2002. pp. 138–139. [Google Scholar]
- 47.Gilbert DN, Sande MA, Moellering RC., Jr The Sanford guide to antimicrobial therapy. 33rd ed. Hyde Park, Vt: Antimicrobial Therapy, Inc; 2002. p. 23. [Google Scholar]
- 48.Stewart J, Pilla J, Dunn L. Pilot study for appropriate anti-infective community therapy. Effect of a guideline-based strategy to optimize use of antibiotics. Can Fam Physician. 2000;46:851–859. [PMC free article] [PubMed] [Google Scholar]