Abstract
A 16-aa insertion loop present in eubacterial methionyl-tRNA formyltransferases (MTF) is critical for specific recognition of the initiator tRNA in Escherichia coli. We have studied the interactions between this region of the E. coli enzyme and initiator methionyl-tRNA (Met-tRNA) by using two complementary protection experiments: protection of MTF against proteolytic cleavage by tRNA and protection of tRNA against nucleolytic cleavage by MTF. The insertion loop in MTF is uniquely sensitive to cleavage by trypsin. We show that the substrate initiator Met-tRNA protects MTF against trypsin cleavage, whereas a formylation-defective mutant initiator Met-tRNA, which binds to MTF with approximately the same affinity, does not. Also, mutants of MTF within the insertion loop (which are defective in formylation) are not protected by the initiator Met-tRNA. Thus, a functional enzyme–substrate complex is necessary for protection of MTF against trypsin cleavage. Along with other data, these results strongly suggest that a segment of the insertion loop, which is exposed and unstructured in MTF, undergoes an induced fit in the functional MTF⋅Met-tRNA complex but not in the nonfunctional one. Footprinting experiments show that MTF specifically protects the acceptor stem and the 3′-end region of the initiator Met-tRNA against cleavage by double and single strand-specific nucleases. This protection also depends on formation of a functional MTF⋅Met-tRNA complex. Thus, the insertion loop interacts mostly with the acceptor stem of the initiator Met-tRNA, which contains the critical determinants for formylation.
Keywords: RNA–protein interactions, initiator tRNA formylation, RNA footprinting, protein conformational change
Formylation of the initiator Met-tRNA (Met-tRNAfMet) by the enzyme methionyl-tRNA formyltransferase (MTF) is important for the initiation of protein synthesis in eubacteria, mitochondria, and chloroplasts (1–3). Escherichia coli initiator tRNA mutants defective in formylation are essentially inactive in initiation (4). Similarly, a strain of E. coli having a disruption in the formylase gene grows extremely slowly (5). The formyl group on the initiator methionyl-tRNA facilitates its binding to the initiation factor IF2, which is necessary for the binding of the tRNA to the ribosomal P site during initiation of protein synthesis (6). MTF is highly specific in that it formylates only the initiator Met-tRNA and no other aminoacyl-tRNA including the elongator methionyl-tRNA (7, 8). The most important sequence and structural elements for formylation are clustered in the acceptor stem of E. coli initiator tRNA (9–12). The absence of a Watson-Crick base pair between positions 1 and 72, a unique feature of all eubacterial initiator tRNAs, is one of the critical determinants.
The crystal structure of E. coli MTF shows that the enzyme consists of two domains connected by a linker region (refs. 13–16; Fig. 1). Cross-linking studies, mutational analysis, and binding studies support the notion that the C-terminal domain, although essential, is involved mostly in nonspecific interactions with the tRNA (refs. 13 and 17; S.G., Y. Li, V.R., and U.L.R., unpublished results). The N-terminal domain is strikingly homologous in structure to E. coli glycinamide ribonucleotide formyltransferase (GARF), which, like MTF, also uses N10-formyltetrahydrofolate as a formyl donor in formylation (13–15). A notable difference, however, is that the N-terminal domain of MTF contains a highly conserved 16-aa insertion sequence (residues 34–49 in the E. coli enzyme) in the loop region between the second β-strand and second α-helix (Fig. 1). Suppressor mutants (18) in the E. coli MTF, which compensate for a formylation-defective mutant initiator tRNA fall within this insertion loop. Mutational analysis of the conserved amino acid residues within the insertion sequence indicates an important role for an invariant arginine, Arg-42, in both substrate binding and catalysis (19). Further characterization of the arginine mutants suggested interactions between the invariant arginine and the determinants for formylation in the acceptor stem of initiator tRNA (19). These results of suppressor and mutational analysis suggest strongly that the 16-aa insertion sequence in MTF acts as a recognition module for specific interactions with the determinants for formylation in the acceptor stem of the initiator tRNA.
Figure 1.
Schematic alignment of GARF and MTF sequences from various sources. The amino acid numbering of MTF begins with serine found at the N terminus of the E. coli protein. The amino acids Asn, His, Ser/Thr, and Asp [thought to be involved in catalysis in GARF (14–16) and also found in MTF] are indicated. Arrows indicate sites of insertion in MTF compared with GARF.
A segment of the insertion loop, 40AGRGKK45, spanning the critical arginine (13), is unstructured in the crystal structure of the native enzyme and is uniquely susceptible to proteolysis in MTF. Here we have used two types of experiments—protection of MTF against proteases and protection of tRNA against nucleases—to study the specific interactions between MTF and the initiator Met-tRNA.‡ The initiator Met-tRNA specifically protects the enzyme against proteolytic cleavage within the insertion loop. By using Arg-42 mutant enzymes and mutant initiator tRNAs in protection experiments we show that the formation of a functional MTF⋅Met-tRNA (enzyme–substrate) complex is necessary for this protection. These results support the idea that a segment of the recognition module in MTF, which is unstructured and accessible to proteases in the free enzyme, adopts a defined conformation in the enzyme–substrate complex. Footprint experiments (20) using RNase V1, T2, and T1 also suggest that the recognition module protects mostly the acceptor stem of the initiator Met-tRNA, which contains the critical determinants for formylation, in the functional, but not in the nonfunctional, complex.
MATERIALS AND METHODS
Strains, Plasmids, and Enzymes.
The cloning and expression of wild-type and mutant MTFs and MetRS as C-terminal 6×His fusion proteins has been described (18, 19). The purified enzymes were stored in 20 mM Imidazole⋅HCl (pH 7.6), 150 mM NaCl, 10 mM 2-mercaptoethanol, and 50% glycerol. E. coli UT481 containing the plasmid pEC7 and overexpressing tRNA nucleotidyl transferase was provided by M. P. Deutscher (University of Miami School of Medicine). The enzyme was purified as described in ref. 21. RNase V1 (Cobra venom, 0.72 units/μl) was from Pharmacia Biotech and was stored in 50% glycerol at −20°C. RNases T2 and T1 (Sankyo, 10 units/μl) were from Calbiochem and were stored at 4°C.
Preparation of Initiator tRNA Containing 3′-Amino-3′-DeoxyA (tRNAN) at the 3′ End.
The cloned wild-type and G72 mutant initiator tRNAs were purified from E. coli B by electrophoresis of total tRNA on a 15% native polyacrylamide gel (22). The purity of tRNAs, assessed by aminoacylation assays, was >95%. The 3′-terminal A of tRNA was exchanged for 3′-amino-3′-deoxyA by using tRNA nucleotidyl transferase. The incubation mixture (20 μl) for this contained 50 mM Tris⋅HCl (pH 8.0), 10 mM MgCl2, 10 mM reduced glutathione, 0.5 mM CTP, 4.8 mM 3′-amino-3′-deoxyATP (Fluka), 1 mM sodium pyrophosphate, 1 A260 unit of initiator tRNA, and 2 units of tRNA nucleotidyl transferase. Incubation was at 37°C for 2 hr. After the incubation, the tRNA was separated from the excess of 3′-amino-3′-deoxyATP by DEAE-cellulose chromatography and recovered by ethanol precipitation. The purified initiator tRNA analog (tRNAN) could be quantitatively aminoacylated to yield [35S]Met-tRNAN in which the [35S]methionine is linked to the tRNA by a base-stable peptide linkage. When using nonradioactive methionine, the extent of aminoacylation of the initiator tRNAN was monitored by silver staining of 15% polyacrylamide gels. The aminoacyl-tRNAN migrated slower than tRNAN, and aminoacylation was essentially complete.
Preparative Aminoacylation of Initiator tRNAN with [35S]l-Methionine.
tRNAN (1.2 μM) was incubated in the aminoacylation buffer containing 250 ng of MetRS and 10 μM [35S]l-methionine (1 × 106 cpm per pmol) at 37°C for 25 min. The [35S]Met-tRNAN was purified by electrophoresis on a 12% polyacrylamide–8M urea gel, located by autoradiography, and eluted into a buffer containing 0.3 M sodium acetate (pH 5.5) and 1 mM EDTA for 2–5 hr at 20°C. The tRNA was recovered by ethanol precipitation and dissolved in distilled water to approximately 30,000 cpm per microliter.
Expression and Purification of [35S]Methionine-Labeled MTFs.
Cultures of E. coli JM109/pQE16FMTp (or the appropriate MTF mutant) were grown to mid-logarithmic phase in 100 ml of 2×YT (1% yeast extract/1.6% tryptone/0.5% NaCl) medium. The cells were collected, washed with 0.1 M KH2PO4 (pH 7.4), and suspended in 50 ml of Dulbecco’s Modified Eagle Medium without glutamine, methionine, and cystine (ICN). The culture was then supplemented with glutamine (1 mM), ampicillin (100 μg/ml), [35S]l-methionine [1 mCi (1 Ci = 37 GBq); 1,175 Ci/mmole, NEN] and isopropyl β-d-thiogalactoside (IPTG) (1 mM) and grown for 3 hr at 37°C (23). The [35S]methionine-labeled MTF was purified by using Talon Sepharose affinity chromatography and was diluted with the corresponding nonradioactive MTF or mutant MTF to a specific activity of 86 × 103 cpm per microgram.
Proteolysis of MTF.
Protease digestions were performed in the standard buffer used for aminoacylation and formylation containing 20 mM imidazole⋅HCl (pH 7.6), 150 mM NH4Cl, 2 mM ATP, 0.1 mM Na2EDTA, and 10 mM MgCl2 at 37°C with trypsin (Promega; modified trypsin, 21, 500 units/mg). The reaction (10 μl) consisting of [35S]methionine-labeled MTF (0.5 μM), wild-type or the G72 mutant initiator tRNAN (1.25–80 μM), and MetRS (200 ng) was incubated in the presence or absence of 1 mM methionine in the aminoacylation buffer for 15 min at 37°C. Trypsin (1:3.3 wt/wt ratio of trypsin to protein) was added and the mixture was incubated for 10 min at 37°C. The reaction was stopped by the addition of SDS buffer (24) and freezing in dry ice. The samples were analyzed on 12% SDS/PAGE gels, and the gels were dried and subjected to autoradiography. The radioactivity was quantitated by phosphorimaging (Molecular Dynamics).
The G34C36 mutant initiator tRNA was purified and aminoacylated with valine by using a ValRS-enriched extract (25). The Val-tRNA (G34C36) was recovered by using phenol extraction and ethanol precipitation and was used for protease digestion as described above.
Gel Mobility Shift Analysis of MTF⋅Met-tRNAN Complexes.
Binding reactions (10 μl) were performed in 20 mM imidazole⋅HCl (pH 7.6), 5 mM MgCl2, 150 mM NaCl, and 5% glycerol. The mixture was incubated at room temperature for 15 min, and the complexes were resolved on a 6% native polyacrylamide gel at 150 V for 2 hr. The gels were fixed and dried, and the radioactivity was quantitated by phosphorimaging.
RNase V1, T2, and T1 Footprinting.
The initiator Met-tRNAN, labeled either at the 5′ end with [32P] or at the 3′ end with [35S]Met, (0.02 μM, ≈40,000 cpm) was preincubated in a buffer containing 20 mM imidazole⋅HCl, (pH 7.6), 150 mM NH4Cl, and 5 mM MgCl2 for 10 min at 55°C and slowly cooled to 20°C. MTF (0.09–18 or 27 μM) was added and the mixture (10 μl) was left at 20°C for 10 min; 0.1 μg of yeast tRNAPhe and either RNase V1 (0.005 units), RNase T2 (0.002 units), or RNase T1 (0.01 units) was added, and the mixture was incubated at 20°C. After 10 min, 0.3 M sodium acetate (pH 5.5; 90 μl) and total E. coli tRNA (2 μg) was added. The mixture was extracted with an equal volume of phenol/chloroform, an additional 2 μg of carrier tRNA was added to the aqueous layer, and tRNA fragments were recovered by ethanol precipitation on dry ice. The pellet was washed once in 80% ethanol and dissolved in 6 μl of 8 M urea containing 0.03% (wt/vol) bromophenol blue and xylene cyanole and analyzed on 18% polyacrylamide/7 M urea sequencing gels. Products of partial cleavage with alkali and RNase T1 were used as size markers to identify the tRNA bands (26). The gel was fixed, dried on a DEAE-cellulose paper, and subjected to autoradiography. Radioactivity in the bands on each lane was quantitated by phosphorimaging (Molecular Dynamics).
RESULTS
Susceptibility of the Insertion Loop in MTF to Proteolytic Cleavage.
Several lines of evidence indicate that the sequence 40AGRGKK45 within the insertion loop of MTF is uniquely sensitive to proteases. First, attempts to purify MTF overproduced in E. coli by factor Xa cleavage of a maltose-binding protein fused to MTF yielded a truncated MTF protein that had been cleaved after Arg-42 within the insertion loop (D. Mangroo, S.G., and U.L.R., unpublished data). This truncated MTF is inactive in formylation. Second, suppressor mutants of MTF with Gly-41 changed to Arg-41 or Lys-41 were found to be partially cleaved in E. coli after Arg-41 or Lys-41 (18). Third, the crystal structure of MTF (13) shows that amino acids 40–45 are disordered. Fourth, limited treatment of MTF with trypsin (13) or with clostripain, an arginine-specific protease (data not shown), resulted in a single cleavage after Arg-42. Interestingly, the R42L mutant is not cleaved by clostripain (data not shown), suggesting that Arg-38, the other arginine in the insertion loop, is structured (13) and is not accessible to cleavage. The R42L mutant is, however, susceptible to cleavage with trypsin after Lys-44 and Lys-45 (data not shown), suggesting that these residues are, as indicated in the crystal structure (13), also unstructured and therefore accessible to cleavage by trypsin.
Protection of MTF Against Trypsin Cleavage by tRNA.
We showed previously that the R42L mutant of MTF was much less active in formylation. Interestingly, the G41R/R42L double mutant also was equally inactive, suggesting that the requirements for arginine at position 42 cannot be fulfilled by an arginine at position 41 (19). This result suggested that amino acids 40–45 in the insertion loop, normally unstructured and flexible, adopt a defined conformation upon binding to the tRNA. Here, we have used protection against cleavage with trypsin to probe for conformational changes in the insertion loop on binding of the enzyme to the initiator Met-tRNA. Because of the chemical instability of the ester linkage between methionine and tRNA in Met-tRNA, an initiator tRNA analog, tRNAN, in which the 3′-terminal A residue is replaced by 3′-amino-3′-deoxyA, was used (27). Upon aminoacylation of tRNAN with MetRS, the methionine, which is initially attached to the 2′-OH group through an ester linkage, migrates to the 3′ position and forms a base stable peptide linkage (28). MTF labeled in vivo with [35S]Met was used to follow the extent of cleavage of MTF with trypsin.
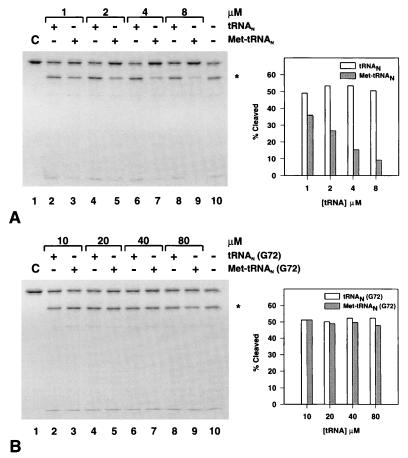
The extent of cleavage of MTF was monitored at a fixed concentration of MTF (0.5 μM) and increasing concentrations of tRNAN or Met-tRNAN (Fig. 2A). Increasing amounts of tRNAN up to a 16-fold molar excess over MTF had little effect on the extent of cleavage (Fig. 2A, lanes 2, 4, 6, and 8) over that, 48.7%, seen in the absence of any added tRNA (Fig. 2A, lane 10 and Right). In contrast, addition of Met-tRNAN protected MTF against cleavage by trypsin in a concentration-dependent manner (Fig. 2A, lanes 3, 5, 7, and 9 and Right). At the highest concentration of Met-tRNAN used here (8 μM), there was >80% protection of MTF against trypsin cleavage compared with the cleavage seen in presence of the uncharged tRNAN (Fig. 2A, lane 9 and Right).
Figure 2.
Effect of tRNA, uncharged (□) or aminoacylated (■), on partial trypsin digestion of 35S-labeled MTF. Left panels show SDS-gel electrophoretic analysis of the incubation mixture in the presence of increasing amounts of initiator tRNAN or Met-tRNAN. (A) Wild-type initiator tRNAN. (B) The G72 mutant initiator tRNAN. ∗ indicates the position of the long C-terminal MTF fragment, the radioactive band at the bottom of the gel is the corresponding N-terminal fragment. Percent cleavage in each lane was determined by adding the radioactivity in the fragments divided by the total radioactivity in that lane based on phosphorimager analysis. (Right) Results of quantitation.
In contrast to the protection against trypsin cleavage of MTF by Met-tRNAN, there was virtually no protection (Fig. 2B) by the G72 mutant initiator tRNAN (G72) or Met-tRNAN (G72). The G72 mutant initiator tRNA, which contains a C1:G72 base pair at the end of the acceptor stem, is a very poor substrate for MTF both in vitro (9–11) and in vivo (12). Protection against cleavage was marginal at best even at the highest concentration (80 μM) of Met-tRNAN (G72), corresponding to a 160-fold molar excess of tRNA over MTF (Fig. 2B, Right). The G72 mutant Met-tRNAN, however, forms a complex with MTF with about the same affinity as wild-type Met-tRNAN (Table 1). These results suggest that formation of a complex alone between MTF and the G72 mutant Met-tRNAN is not sufficient for tRNA-dependent protection of MTF against cleavage within the insertion loop by trypsin. A functional MTF⋅Met-tRNAN complex is necessary for the protection.
Table 1.
Apparent Kds of wild-type and mutant MTF⋅Met-tRNAN complexes
Data are based on results of gel mobility shift analyses (29). The binding reactions contained either the wild-type MTF (2.5 μM), the R42K mutant (2.5 μM), or the R42L mutant (5 μM) and increasing amounts of Met-tRNAN, wild-type, or the G72 mutant (0.25 μM to 1.5 μM). The apparent Kd values were calculated from the slope of a double reciprocal plot of 1/r, where r is the fraction of MTF bound to Met-tRNAN against 1/Met-tRNAN free using the equation 1/r = Kd (1/Met-tRNAN free) + 1 (30, 31). The apparent Kd measured here for the His-tagged wild-type MTF⋅Met-tRNAN complex using gel mobility shift experiments is about the same as the Kd reported for MTF⋅Met-tRNA complex based on quenching of tryptophan fluorescence of MTF (32).
Results of separate set of experiments.
Effect of tRNAN and Met-tRNAN on Trypsin Cleavage of Mutant MTFs.
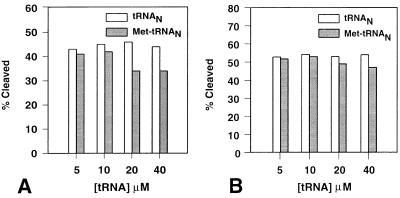
In view of the results above with the formylation-defective G72 mutant initiator tRNA, we examined whether MTF mutants that are defective in enzyme function would be protected against trypsin cleavage by wild-type initiator Met-tRNA. The results summarized in Fig. 3 show that the R42K mutant is protected to a small extent by Met-tRNAN but only at very high concentrations (20 μM and 40 μM), whereas there was virtually no protection of the G41R/R42L mutant even at the highest concentration (40 μM) of Met-tRNAN. The R42K mutant of MTF forms a complex with Met-tRNAN with about the same affinity as wild-type MTF (Table 1). These results further support the conclusion above that the formation of a functional MTF⋅Met-tRNAN complex is necessary for protection of MTF against trypsin cleavage.
Figure 3.
Effect of tRNA, uncharged (□) or aminoacylated (■), on partial trypsin digestion of [35S]Met-labeled mutant MTFs. (A) The R42K mutant MTF. (B) The G41R/R42L mutant MTF. Percent cleavage was calculated as described in the Legend of Fig. 2.
Effect of Mutant Initiator tRNA Aminoacylated with Valine on Protection Against Trypsin Cleavage.
The amino acid attached to the initiator tRNA is also important for formylation of tRNA by MTF (25, 33–35). Previous studies have shown that E. coli initiator tRNA that had been “misaminoacylated” with valine is a poor substrate for formylation, Vmax down 57-fold and Km up 2.2-fold (33). Therefore, we have investigated whether the G34C36 anticodon mutant of E. coli initiator tRNA, which is aminoacylated with valine (25), protects MTF against trypsin cleavage. Because the ester linkage in Val-tRNA is stable under the conditions of incubation (36, 37), the G34C36 mutant initiator Val-tRNA was used as such without exchange of the 3′-terminal A for 3′-deoxy-3′-amino A. There was no protection of MTF against trypsin cleavage even at the highest concentration (40 μM) of the G34C36 mutant Val-tRNA (data not shown).
Protection of Met-tRNAN by MTF Against RNases V1, T2, and T1 Cleavages.
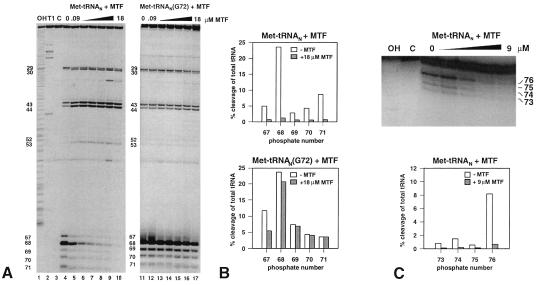
The most important determinants for formylation in the initiator tRNA are clustered toward the end of the acceptor stem (10–12). A minor determinant is the A11:U24 base pair in the D stem (10, 38). It was, therefore, of interest to see whether the interaction of MTF with the initiator tRNA is localized to these regions or extends to the rest of the tRNA. This was studied by analyzing regions of the tRNA protected by MTF against cleavages by the double strand-specific RNase V1 and the single strand-specific RNases T2 and T1 (20). Besides Met-tRNAN and the nuclease, the incubation mixture contained either no MTF or increasing amounts of MTF from 0.09 μM to 18 or 27 μM. To ensure that the cleavages being monitored were caused by primary hits, the Met-tRNAN was labeled either at the 3′ end with [35S]Met or at the 5′ end with 32P. The results obtained with either labeled tRNA were essentially the same. Fig. 4A (Left) shows the results of a gel electrophoretic analysis of limited digests of [35S]Met-tRNAN with RNase V1. The cleavage sites are numbered according to the phosphate, with the 5′-terminal phosphate being number 1. It can be seen that MTF protects the 3′ end of the acceptor stem at phosphates 67–71 in a concentration-dependent manner. At 0.09 μM MTF, there was already 50% protection of cleavage at phosphate 68 (Fig. 4A Left), whereas at 18 μM MTF, there was >90% protection of cleavage at phosphates 67–71 (Fig. 4B Upper). Some other sites showed enhanced cleavage at phosphates 30, 43, and 44 in the anticodon stem and at phosphate 53 in the TψC stem whereas others showed slightly reduced cleavage at phosphates 29 in the anticodon stem and phosphate 52 in the TψC stem. These are most likely the result of structural changes in the tRNA induced by MTF. Similar enhancements of RNase V1 cleavage in the anticodon stem have been noted before in complexes of EF-Tu with aminoacyl-tRNA and IF2 with fMet-tRNA (39, 40).
Figure 4.
(A) Electrophoretic analysis of partial RNase V1 cleavage of 3′-end [35S]Met-labeled initiator Met-tRNAN and Met-tRNAN (G72) on polyacrylamide gels. Met-tRNAN (lanes 4–10) and the G72 mutant Met-tRNAN (lanes 11–17) were incubated with increasing amounts of MTF (0, 0.09, 0.3, 0.9, 3, 9, and 18 μM) and subjected to partial digestion with RNase V1. Lane 1, OH, alkaline ladder; lane 2, T1, RNase T1 digest under denaturing conditions; lane 3, C, control without RNase VI treatment. (B) Quantitation of RNase VI cleavages in A by phosphorimager analysis. The amount of tRNA cut at a given position (67–71) is plotted as a percentage of total tRNA loaded per lane. Open bars, cleavage of Met-tRNAN, hatched bars, cleavage in the presence of 18 μM MTF. (C) Upper, electrophoretic analysis (on a 15% polyacrylamide gel) of a partial RNase T2 digest of 5′-end 32P-labeled Met-tRNAN. The tRNA was bound to increasing amounts of MTF (0.09–9 μM), and the complexes were analyzed by RNase T2 digestion; Lower, quantitation of RNase T2 footprinting by phosphorimager analysis.
In contrast to protection against RNase V1 cleavage of Met-tRNAN by MTF, there was only very weak protection of the G72 mutant initiator tRNA, Met-tRNAN (G72), at phosphates 67 and 68 and none at phosphates 69, 70, and 71 in the acceptor stem. (Fig. 4A Right and Fig. 4B Lower). Because the G72 mutant Met-tRNAN forms a complex with MTF with about the same affinity as wild-type Met-tRNAN (Table 1), this result suggests that MTF dependent protection of the initiator Met-tRNAN against RNase V1 cleavage also requires the formation of a functional MTF⋅Met-tRNAN complex.
RNase T2 cleaved Met-tRNAN predominantly at the end of the acceptor stem at phosphates 73–76 (Fig. 4C) and to some extent in the D loop, the anticodon loop, and the variable loop (data not shown). Because of the very short size of the 3′-terminal fragment(s) resulting from cleavages at phosphates 73–76, only 5′ 32P-labeled Met-tRNAN was used in the RNase T2 cleavage experiments. MTF protected the cleavage of tRNA by RNase T2 in the acceptor stem (Fig. 4C) but not in the D loop, the anticodon loop, or the variable loop (data not shown).
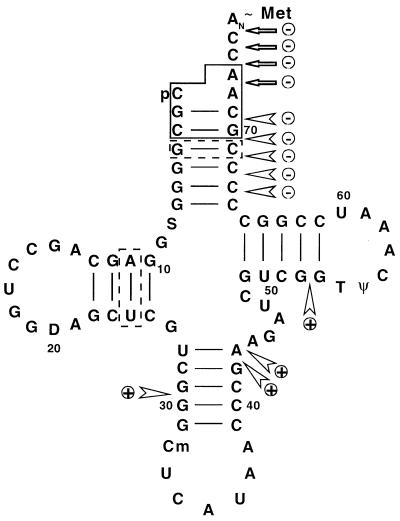
RNase T1 cleaved Met-tRNAN in the D loop and in the variable loop. Neither of these regions were protected against RNase T1 cleavage by MTF (data not shown). Thus, the combined results on the effect of MTF on cleavage of Met-tRNAN by RNases V1, T2, and T1 suggest that MTF binds primarily to the acceptor stem of Met-tRNAN (Fig. 5). There are no major contacts between MTF and other regions of the tRNA. We cannot exclude contacts between MTF and the tRNA in the D stem, because the enzyme probes that we have used do not cleave within the D stem.
Figure 5.
Cloverleaf structure of E. coli initiator Met-tRNAN. Nucleotides playing a major role in formylation are boxed by solid lines, whereas those playing a relatively minor role are boxed by dotted lines. RNase cleavages that are decreased in the presence of MTF are indicated with ⊝ whereas those that are increased are indicated with ⊕.  , RNase V1 cleavage;
, RNase V1 cleavage;  , RNase T2 cleavage.
, RNase T2 cleavage.
Use of Mutant MTF Proteins in RNase V1 Footprinting of Met-tRNAN.
Three mutant proteins all carrying alterations in Arg-42, MTF (R42K), MTF (R42L), and MTF (G41R/R42L) were also used for RNase V1 footprint analysis of the MTF⋅Met-tRNA complex. The difference among the mutants was marginal, although MTF (R42K) was somewhat better than either MTF (R42L) or MTF (G41R/R42L) mutants. Both the MTF (R42K) and MTF (R42L) mutants required at least 100 times more protein than wild-type MTF (>27 μM for the mutants as compared with 0.09 μM for the wild-type MTF) to provide 50% protection of cleavage at phosphate 68 (data not shown). Although the binding affinity of the R42L mutant MTF for Met-tRNAN is about 13-fold lower than that of the wild-type MTF, the R42K enzyme forms a complex with Met-tRNAN with about the same affinity as wild-type MTF (Table 1). These data, therefore, support the conclusion that the interaction of MTF with Met-tRNAN is strongly affected by the mutation of Arg-42 and that a specific interaction between the MTF and the acceptor stem of the tRNA is lost in the mutants (19).
Use of Mutant Initiator tRNA Aminoacylated with Valine in RNase V1 Footprinting.
As mentioned above, initiator tRNA aminoacylated with valine is a poor substrate for MTF (33). Therefore, we have performed RNase V1 footprinting of Val-tRNA (G34C36) in the presence of increasing amounts of MTF. There was no protection against RNase V1 cleavages in the acceptor stem except perhaps at the highest concentration (9 μM) of MTF (data not shown).
DISCUSSION
Our results on protection of MTF against trypsin cleavage in the insertion loop by the substrate Met-tRNAN strongly suggest that amino acids 40–45 in this region of MTF (which are unstructured and flexible in the free enzyme) adopt a defined structure in a functional (but not in an inactive) MTF⋅Met-tRNAN complex. The protection requires the formation of a functional MTF⋅Met-tRNAN complex because the G72 mutant initiator tRNA, which is a poor substrate for formylation, and the G34C36 mutant initiator tRNA, which is aminoacylated with valine, are both ineffective in protection of MTF against trypsin cleavage (Figs. 2 and 4). Also, MTF with mutations in the critical arginine in the insertion loop are not protected against trypsin cleavage by the initiator Met-tRNAN (Fig. 3). Other studies have shown that the insertion loop is important for formylation and that amino acids in the insertion loop come close to and interact with the determinants for formylation in the acceptor stem of the initiator tRNA. For example, (i) a single trypsin cleavage within the insertion loop results in an enzyme that is inactive in formylation (ref. 13, D. Mangroo, S.G., and U.L.R., unpublished results). (ii) Suppressor mutants of MTF that formylate partially the G72 mutant initiator tRNA carry a mutation at amino acid 41 within the insertion sequence (18, 19). (iii) Mutagenesis experiments show that the basic amino acids Arg-38 and Arg-42 play important roles in MTF function and that Arg-42 functionally interacts with base pairs 3:70 and possibly 2:71 in the acceptor stem of the tRNA (ref. 19; E. Schmitt, M. Panvert, S. Blanquet, and Y. Mechulam, personal communication). Also, comparison of the kinetic parameters of the double mutant G41R/R42L with those of the single mutant R42L show that the requirement for an arginine at position 42 cannot be fulfilled by having an arginine at position 41 (19). Therefore, although the protection of MTF by the initiator Met-tRNAN against trypsin cleavage, could, on its own, be ascribed to the tRNA shielding an otherwise unstructured and exposed region of MTF in the MTF⋅Met-tRNAN complex, the combined evidence suggests very strongly that amino acids 40–45 of MTF undergo a local conformational change in the MTF⋅Met-tRNAN complex in an induced-fit mechanism (41–44). Such an induced conformational change would be akin to what happens in the HIV-1 Rev peptide–RRE (Rev response element) interactions (45) or bovine immunodeficiency virus Tat peptide–TAR RNA interactions (46, 47). In the latter case, a small arginine-rich peptide segment, normally unstructured and flexible, undergoes a conformational change to a β-hairpin on binding to the corresponding RNA (48, 49).
As mentioned above, protection of MTF against trypsin cleavage requires the formation of a functional MTF⋅Met-tRNAN complex. The G72 mutant Met-tRNAN also forms a complex with MTF (Table 1), however, there is no protection of MTF against trypsin cleavage, presumably because the complex is “inactive.” It is also known that MTF can bind nonspecifically to other tRNAs with comparable affinities (ref. 32; L. Hancox, D. Mangroo, and U.L.R., unpublished results), although only the initiator Met-tRNA (or Met-tRNAN) is a substrate for formylation. The notion of a conformational change within the insertion loop of MTF, subsequent to binding, only in a functional MTF⋅Met-tRNAN complex is reminiscent of the situation with aminoacyl-tRNA synthetase⋅tRNA complexes, in which a conformational change is triggered by cognate tRNAs but not by noncognate tRNAs (ref. 50, D. Moras, personal communication).
The results of RNase footprinting show that MTF protects the acceptor stem and the 3′-terminal nucleotides, but not other regions of the tRNA. We cannot comment on contacts between MTF and tRNA in the D stem, because the enzyme probes we have used do not cleave within the D stem. Thus, the interaction of MTF to the initiator Met-tRNA is localized to the region that contains the most important determinants for formylation. Here, too, the formation of a functional complex is necessary for protection of the tRNA against nucleases. The G72 mutant initiator Met-tRNAN has approximately the same affinity for MTF as wild-type initiator Met-tRNAN (Table 1) but is not protected against RNase V1 cleavage by MTF. The finding that MTF mutants with a single amino acid mutation (R42 to K or L) in the insertion loop do not protect the Met-tRNAN against RNase cleavage highlights the critical role of the insertion loop in this process. A likely explanation is that during the formation of a functional MTF⋅Met-tRNA complex, the tRNA is initially bound to MTF mostly through nonspecific electrostatic interactions involving the basic amino acids in the C-terminal region (13) that form a positively charged channel on the surface of the enzyme. This is followed by interactions between the amino acids in the insertion loop and the acceptor stem of the initiator tRNA, accompanied by changes in the local structure of the insertion loop and possibly of the tRNA in an induced-fit mechanism.
Acknowledgments
We thank Dr. Paul Schimmel for comments and suggestions on the manuscript and Annmarie McInnis for her enthusiasm and care in the preparation of this manuscript. This work was supported by Grant R37GM17151 from the National Institutes of Health.
ABBREVIATIONS
- MTF
methionyl-tRNA formyltransferases
- GARF
glycinamide ribonucleotide formyltransferase
Note Added in Proof
After the submission of this manuscript, a paper describing the three-dimensional structure of E. coli MTF complexed with fMet-tRNA, the product of MTF reaction, was published (51). Our conclusion that amino acids 40–45 undergo an induced fit in the presence of the initiator tRNA substrate, based on biochemical analyses described in here and in previous studies (19), is now also supported by the crystal structure data.
Footnotes
The initiator tRNA2fMet species was used throughout this work.
References
- 1.Gualerzi C O, Pon C L. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 2.Kozak M. Microbiol Rev. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RajBhandary U L. J Bacteriol. 1994;176:547–552. doi: 10.1128/jb.176.3.547-552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varshney U, Lee C P, Seong B L, RajBhandary U L. J Biol Chem. 1991;266:180018–18024. [PubMed] [Google Scholar]
- 5.Guillon J M, Mechulam Y, Schmitter J M, Blanquet S, Fayat G. J Bacteriol. 1992;174:4294–4301. doi: 10.1128/jb.174.13.4294-4301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundari R M, Stringer E A, Schulman L H, Maitra U. J Biol Chem. 1976;251:3338–3345. [PubMed] [Google Scholar]
- 7.Dickerman H W, Steers E, Jr, Redfield B G, Weissbach H. J Biol Chem. 1967;242:1522–1525. [PubMed] [Google Scholar]
- 8.Marcker K, Sanger F. J Mol Biol. 1964;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- 9.Seong B L, RajBhandary U L. Proc Natl Acad Sci USA. 1987;84:8859–8863. doi: 10.1073/pnas.84.24.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C P, Seong B L, RajBhandary U L. J Biol Chem. 1991;266:18012–18017. [PubMed] [Google Scholar]
- 11.Guillon J M, Meinnel T, Mechulam Y, Lazennec C, Blanquet S, Fayat G. J Mol Biol. 1992;224:359–367. doi: 10.1016/0022-2836(92)91000-f. [DOI] [PubMed] [Google Scholar]
- 12.Lee C P, Dyson M R, Mandal N, Varshney U, Bahramian B, RajBhandary U L. Proc Natl Acad Sci USA. 1992;89:9262–9266. doi: 10.1073/pnas.89.19.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt E, Blanquet S, Mechulam Y. EMBO J. 1996;15:4749–4758. [PMC free article] [PubMed] [Google Scholar]
- 14.Almassy R J, Janson C A, Kan C-C, Hostomska Z. Proc Natl Acad Sci USA. 1992;89:6114–6118. doi: 10.1073/pnas.89.13.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein C, Chen P, Arevalo J J, Stura E A, Marolewski A, Warren M S, Benkovic S J, Wilson I. J Mol Biol. 1995;249:153–175. doi: 10.1006/jmbi.1995.0286. [DOI] [PubMed] [Google Scholar]
- 16.Warren M S, Marolewski A E, Benkovic S J. Biochemistry. 1996;35:8855–8862. doi: 10.1021/bi9528715. [DOI] [PubMed] [Google Scholar]
- 17.Gite S, RajBhandary U L. J Biol Chem. 1997;272:5305–5312. doi: 10.1074/jbc.272.8.5305. [DOI] [PubMed] [Google Scholar]
- 18.Ramesh V, Gite S, Li Y, RajBhandary U L. Proc Natl Acad Sci USA. 1997;94:13524–13529. doi: 10.1073/pnas.94.25.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramesh V, Gite S, RajBhandary U L. Biochemistry. 1998;37:15925–15932. doi: 10.1021/bi981873x. [DOI] [PubMed] [Google Scholar]
- 20.Ehresmann C, Baudin F, Mougel M, Romby P, Ebel J-P, Ehresmann B. Nucleic Acids Res. 1987;15:9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cudny H, Deutscher M P. J Biol Chem. 1986;260:6450–6453. [PubMed] [Google Scholar]
- 22.Mandal N, RajBhandary U L. J Bacteriol. 1992;174:7828–7830. doi: 10.1128/jb.174.23.7827-7830.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovane C, Schwalbach G, Weiss E. Biotechniques. 1977;22:796–798. doi: 10.2144/97225bm01. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Wu X-Q, Iyengar P, RajBhandary U L. EMBO J. 1996;15:4734–4739. [PMC free article] [PubMed] [Google Scholar]
- 26.Donis-Keller H, Maxam A, Gilbert W. Nucleic Acids Res. 1977;4:2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser T H, Rich A. Proc Natl Acad Sci USA. 1973;70:2671–2675. doi: 10.1073/pnas.70.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprinzl M. In: Structure, Function and Genetics of Ribosomes. Hardesty B, Kramer G, editors. Berlin: Springer; 1986. pp. 509–522. [Google Scholar]
- 29.Lane D, Prentki P, Chandler M. Microbiol Rev. 1992;56:509–528. doi: 10.1128/mr.56.4.509-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner T, Gross M, Sigler P B. J Biol Chem. 1984;259:4706–4709. [PubMed] [Google Scholar]
- 31.Wu X-Q, RajBhandary U L. J Biol Chem. 1997;272:1891–1895. doi: 10.1074/jbc.272.3.1891. [DOI] [PubMed] [Google Scholar]
- 32.Kahn D, Fromant M, Fayat G, Dessen P, Blanquet S. Eur J Biochem. 1980;105:489–497. doi: 10.1111/j.1432-1033.1980.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 33.Giege R, Ebel J P, Clark B F C. FEBS Lett. 1973;30:291–295. doi: 10.1016/0014-5793(73)80672-6. [DOI] [PubMed] [Google Scholar]
- 34.Varshney U, Lee C P, RajBhandary U L. Proc Natl Acad Sci USA. 1993;90:2305–2309. doi: 10.1073/pnas.90.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Kumar N V, Varshney U, RajBhandary U L. J Biol Chem. 1996;271:1022–1028. doi: 10.1074/jbc.271.2.1022. [DOI] [PubMed] [Google Scholar]
- 36.Drabkin H, RajBhandary U L. Mol Cell Biol. 1998;18:5140–5147. doi: 10.1128/mcb.18.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthaei J H, Voigt H P, Heller G, Neth R, Schöch G, Kübler H, Amaelunxeu F, Sander G, Parmeggiani A. Cold Spring Harbor Symp Quant Biol. 1966;33:25–38. doi: 10.1101/sqb.1966.031.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh V, Varshney U, RajBhandary U L. RNA. 1997;3:1220–1232. [PMC free article] [PubMed] [Google Scholar]
- 39.Boutorin A S, Clark B F C, Ebel J-P, Kruse T A, Petersen H U, Remy P, Vassilenko S. J Mol Biol. 1981;152:593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- 40.Wakao H, Romby P, Westhof E, Laalami S, Grunberg-Manago M, Ebel J-P, Ehresmann C, Ehresmann B. J Biol Chem. 1989;264:20363–20371. [PubMed] [Google Scholar]
- 41.Koshland D E. Proc Natl Acad Sci USA. 1958;44:98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fersht A R, Knill-Jones J W, Bedouelle H, Winter G. Biochemistry. 1988;27:1581–1587. doi: 10.1021/bi00405a028. [DOI] [PubMed] [Google Scholar]
- 43.Spolar R S, Record T M., Jr Science. 1994;263:777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- 44.Ribas De Pouplana L, Auld D S, Kim S, Schimmel P. Biochemistry. 1996;35:8095–8102. doi: 10.1021/bi960256a. [DOI] [PubMed] [Google Scholar]
- 45.Tan R, Frankel A D. Biochemistry. 1994;33:14579–14585. doi: 10.1021/bi00252a025. [DOI] [PubMed] [Google Scholar]
- 46.Ye X, Kumar R A, Patel D J. Chem Biol. 1995;2:827–840. doi: 10.1016/1074-5521(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 47.Puglisi J D, Chen L, Blanchard S, Frankel A D. Science. 1995;270:1200–1203. doi: 10.1126/science.270.5239.1200. [DOI] [PubMed] [Google Scholar]
- 48.Sundquist W I. Nat Struct Biol. 1996;3:8–11. doi: 10.1038/nsb0196-8. [DOI] [PubMed] [Google Scholar]
- 49.Frankel A D, Smith C A. Cell. 1998;92:149–151. doi: 10.1016/s0092-8674(00)80908-3. [DOI] [PubMed] [Google Scholar]
- 50.Schimmel P R, Söll D. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt E, Panvert M, Blanquet S, Mechulam Y. EMBO J. 1998;17:6819–6826. doi: 10.1093/emboj/17.23.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]