Abstract
Angiogenesis and microcirculation play a central role in growth and metastasis of human neoplasms, and, thus, represent a major target for novel treatment strategies. Mechanistic analysis of processes involved in tumor vascularization, however, requires sophisticated in vivo experimental models and techniques. Intravital microscopy allows direct assessment of tumor angiogenesis, microcirculation and overall perfusion. Its application to the study of tumor-induced neovascularization further provides information on molecular transport and delivery, intra- and extravascular cell-to-cell and cell-to-matrix interaction, as well as tumor oxygenation and metabolism. With the recent advances in the field of bioluminescence and fluorescent reporter genes, appropriate for in vivo imaging, the intravital fluorescent microscopic approach has to be considered a powerful tool to study microvascular, cellular and molecular mechanisms of tumor growth.
Keywords: cranial window, dorsal skinfold chamber, in vivo, perfusion, vascularization
Introduction
Neoplastic tissues consist of three compartments, which may be classified as cellular, interstitial and vascular in nature. The majority of earlier experimental studies has focused their research efforts on the understanding of the biology and pathology of the tumor cell, and has paid only minor attention to the in vivo environment. This changed, however, with the increasing awareness of the central role that the microcirculation and the interstitium play for growth, metastasis, detection and treatment of tumors. Angiogenesis and the developing microvasculature are essential for adequate tissue oxygenation and nutritional supply. Without angiogenesis, most tumors will not progress to a clinically relevant size nor will they metastasize to distant organs through the blood stream [1,2]. Moreover, in radiotherapy, chemotherapy or immunotherapy, the effectiveness of the individual treatment strategy critically depends on the delivery of molecules, a process which is governed by tumor blood flow and interstitial transport [3–6]. In the light of the complexity and the dynamics of tumor microcirculation and interstitial transport of molecules, it has also become apparent that the petri dish will never adequately model the full dimensions of local and systemic feedback loops underlying these processes in vivo. This notion prompted the search for in vivo experimental models and techniques, preferentially non- or minimally invasive, that allow a detailed and sophisticated approach to the tumor microcirculation. In the following overview, we will focus on intravital fluorescence videomicroscopy as a versatile experimental tool for the direct in vivo assessment of vascular, cellular and molecular mechanisms of tumor angiogenesis and microcirculation.
Historical Background
The use of intravital microscopy to study the microcirculation dates back to the last century when Waller in 1846 reported on the passage of leukocytes through microvessels of the tongue of frogs [7]. Sandison was then the first to implant transparent observation chambers into the ear of rabbits for noninvasive intravital microscopic studies of living tissues during wound healing [8]. Some years later, Ide et al. used this chamber model to study the vascularization of rabbit epithelioma implants [9]. To facilitate repeat access to the chamber and to allow microscopic investigation in restrained and conscious animals, those chambers were implanted into the dorsal skinfold, allowing microscopic studies over a prolonged period of time. Algire [10] and Algire and Chalkley [11] in 1943 and 1945 were the first to introduce the dorsal skin fold chamber in mice, demonstrating the significance of angiogenesis and microcirculation for tumor growth. Since then, various modifications of this transparent chamber model have been developed and applied to other rodents, such as rats and hamsters [12–15], but also to immunodeficient animals [16,17], which finally allowed the study of tumor xenografts.
Today, advanced optical equipment has significantly improved imaging quality at up to 1000-fold magnification using both trans-and epi-illumination techniques [7]. The combination of classical intravital microscopy with modern video and computer technology allows for sophisticated off-line analysis of complex, dynamic microcirculatory processes [7]. Furthermore, the spectrum of intravital microscopy has been considerably widened during the last years. First, this is due to refined straightforward, atraumatic microsurgical techniques that enabled the increased accessibility of almost all organs and tissues such as brain [18,19], spine [20], heart [21], lung [22], liver [23], pancreas [24], gut [25], kidney [26], lymph nodes [27] and bone marrow [28] in different laboratory animal species. Second, the introduction of a large panel of fluorescent markers that can be applied in vivo without affecting macro- and microhemo-dynamic parameters allows to determine vascular, cellular and molecular function. Fluorescently labeled macromolecules of varying molecular size have been used to enhance the contrast between blood cells, blood plasma and extravascular tissue, thereby visualizing individual microvessels as small as 3 to 5 µm in diameter [13,29–31]. Further, nuclear and cell membrane dyes allow the in vivo identification of implanted tumor mass and analysis of migration of individual tumor cells [32–34]. Finally, cellular tracker dyes like Rhodamine 6G or Acridine Orange have been adopted for in situ staining of circulating blood cells, including leukocytes and platelets [23,35–38]. With these developments and recent progress in the field of bioluminescence and fluorescent reporter genes [39,40], the technique has advanced to an in vivo multifluorescent approach. The strength of the technique is that it provides a direct, continuous and noninvasive approach to visualize the tumor microvasculature. With this, the technique is superior to others, such as histological vessel counts [41], three-dimensional vascular corrosion casts [42,43], microangiography by injection of dyes [44], and blood flow measurements applying [14C]iodoantipyrine autoradiography [45], 133Xe-washout [46], Laser-Doppler flowmetry [47], which are either invasive, limited to a single observation time point, or characterized by a low spatial resolution with little information on distinct vascular development. Moreover, the microscopic technique enables for quantitative analysis of tumor microhemodynamic parameters. This appears mandatory because nutritive tissue perfusion is not reflected by solely morphological parameters (i.e., microangioarchitecture, vessel density, vessel diameter), but involves also microhemodynamics (i.e., microvascular red blood cell velocity and blood flow rate) and rheology (i.e., microvascular hematocrit, viscosity, cell deformability, cell aggregation/adhesion) [6,48].
Experimental Models
Intravital microscopic analysis of tumor angiogenesis and microcirculation can be performed in both acute and chronic animal preparations. The most frequently used organ for the study of tumor microcirculation in an acute setting is the liver, which can be easily exposed for intravital microscopy by transverse laparotomy and exteriorization on a plastic disc held by an adjustable stage [11,37,49] (Figure 1). The advantage of using the liver as site for implantation is the ability to study orthotopic rather than ectopic tumor growth and vascularization (Figure 1). Kan et al. administered three different cell lines (colon adenocarcinoma, mammary carcinoma and Friend erythroleukemia) systemically in rats through the femoral/cecal vein and analyzed the microvasculature of liver metastases by intravital microscopy, placing emphasis on the individual role of the hepatic artery and portal vein for the blood supply to these tumors [50]. This experimental approach was modified by Fukumura et al., who injected the colon adenocarcinoma cell line LS174T into the spleen of nude mice and assessed the effect of the host microenvironment on the microcirculation of liver metastasis when compared with ectopically implanted tumors [51]. They found that hepatic tumors were less vascularized than subcutaneously implanted tumors, and attributed this to differences in the expression of angiogenic growth factors such as vascular endothelial growth factor (VEGF). Apart from studying the microvasculature of hepatic tumors, further interest was focused on the multistep process of metastatic engraftment into the liver, including the initial arrest of tumor cells within the sinusoids, proliferation, angiogenesis and growth to solid tumors. Interestingly, Luzzi et al. demonstrated that only one of 100 metastatic cells progress to a metastatic tumor, and termed this low rate of engraftment “metastatic inefficiency” [52]. Whereas in the past this type of analysis was restricted to melanoma cells, i.e., cells labeled by nature, the use of fluorescent reporter genes (e.g., green fluorescent protein (GFP)) now allows to study metastatic engraftment and growth of any cell line by intravital microscopy [53]. The disadvantage of these acute models is, however, that they do obviously not allow to monitor the time course of angiogenic and microcirculatory events during tumor growth and progression. Consequently, due to the significant biological inter- and intraindividual variability, the use of these models requires the study of a considerable number of animals at different time points of tumor growth before representative conclusions can be drawn with respect to dynamic changes in tumor microcirculation.
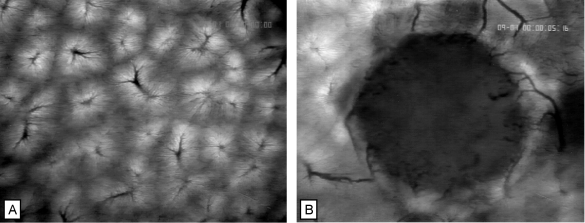
Figure 1.
Microvasculature of normal liver (A) and of hepatic metastasis of a CC531-WAG colon carcinoma (B) as visualized by intravital epi-illumination fluorescence videomicroscopy. Normal liver shows the hexagonal arrangement of acini with dense sinusoidal network, draining blood flow into postsinusoidal venules (A). The vasculature of the hepatic tumor is supplied by and drained into host hepatic vessels. Note the high vascular density in the periphery, and the low vascular density in the centre of the tumor (B). Contrast enhancement by sodium fluorescein i.v. Original magnification, x40.
The most common chronic models to study the dynamics of tumor angiogenesis and microcirculation by repetitive intravital fluorescence microscopy are the dorsal skin fold chamber and the cranial window model. The dorsal skin fold chamber, firstly described by Algire [10], underwent a variety of modifications during the last three decades. The model we presently use consists of two symmetrical titanium frames that are implanted into the dorsal skin fold of the animals so as to sandwich the extended double layer of skin. One layer is completely removed in a circular area of 15 mm in diameter and the remaining layer, consisting of striated muscle, subcutaneous tissue and epidermis, is covered with a glass coverslip incorporated into one of the frames (Figure 2) [17,31]. After a recovery period of 48 hours, the coverslip is temporarily removed, and either tumor cell suspensions, tumor spheroids, or intact tumor specimens are implanted by directly placing them onto the striated muscle [16,32]. The observation window of the chamber allows for repeated intravital microscopic observations of the tumor microvasculature (Figure 2). In this model, background angiogenesis does not alter the analysis of tumor vascularization, because the host tissue consists of striated muscle with little angiogenic potential. In contrast to previously used chamber models, in which the tissue was sandwiched by two cover-slips, thus restricting tumor growth to two dimensions, the backside of the presently used preparation is not covered by a glass slide, facilitating three-dimensional growth of the implanted tumor. Using this modification of the dorsal skin fold chamber, angiogenesis and vascularization of a variety of different tumors have been analyzed, including melanoma, colon adenocarcinoma, rhabdomyosarcoma, prostate cancer, mammary carcinoma and high-grade glioma [13,16,32,54–56].
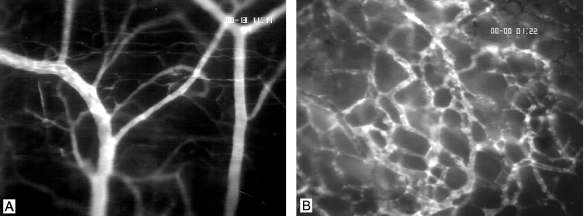
Figure 2.
Microvasculature of striated muscle (A) and C6 glioma on day 18 after implantation into the dorsal skin fold chamber of a nude mouse (B) as visualized by intravital epi-illumination fluorescence videomicroscopy. Note the major feeding arteriolar (right) and draining venular vessels (left) as well as the parallelly arranged capillaries of the muscle tissue (A). In contrast, the glioma microvasculature presents with chaotic angioarchitecture, tortuous and dilated vessel loops, and extravasation of the high-molecular weight fluorescent marker (B). Contrast enhancement by fluorescein isothiocyanate (FITC)-Dextran 150,000 i.v. Original magnification, x 100.
With the aim to develop an orthotopic brain tumor model, the chronic cranial window preparation was used for tumor implantation [47,57]. The model if used as initially described, is restricted to the visualization of the fully developed tumor microvasculature, because the implanted large tumor specimens do not allow to visualize the initial subtle alterations during early tumor angiogenesis. To overcome this drawback, the model has recently been modified by implanting glioma cell suspensions or spheroids orthotopically into the cranial window [34]. For the preparation of the cranial window, a 6x7 mm2 bone flap is created bilaterally over the frontal and parietal regions of the skull, and freed from the underlying dura and sagittal sinus. The dura overlying each hemisphere is removed, avoiding any damage to the sinus and bridging veins (Figure 3). Finally, the glioma cells or spheroids are directly placed onto the pial surface of either hemisphere and the window is sealed with a glass coverslip by adhering to the bone using a histocompatible glue. Although the preparation of the window induces some injury, it is important to note that this injury does not primarily affect the pia and the cortical surface, but the dura at the edges of the window. This injury, in general, induces an angiogenic response, which, however, does not affect the host tissue (pia/cortical surface) within the window for a time period of at least 14 days. Using this cranial window approach, C6 gliomas appear to vascularize quite similar when compared with those implanted ectopically into the skin fold chamber (Figures 2 and 3). Nonetheless, there is detailed analysis of microvascular function, demonstrating that gliomas when implanted into the cranial window, but not when implanted into the dorsal skin fold, develop blood brain barrier characteristics [57]. It should be noted, however, that the ideal orthotopic implantation site for an intraaxial brain tumor such as a glioma would be the parenchymal tissue rather than the pial/cortical surface. Therefore, it remains controversial whether the cranial window as implantation site is more appropriate for the study of glioma vascularization than the dorsal skin fold chamber. In fact, ultrastructural analysis of gliomas implanted into brain and muscle demonstrate that glioma vessels differ significantly from host vessels, but not from each other with respect to vessel morphology and permeability, regardless of the host tissue [58]. In addition, a recent report on vascular corrosion casts of different tumor cells transplanted to the subcutaneous space supports this notion, because the tumor cell could be shown to be the driving force in determining the characteristics of individual types of tumor microvasculature [59].
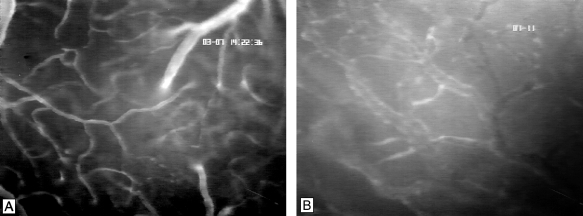
Figure 3.
Microvasculature of cerebral surface (A) and C6 glioma on day 14 after implantation into the chronic cranial window model of a nude mouse (B) as visualized by intravital epi-illumination fluorescence videomicroscopy. Note the feeding arteriole, the cortical capillaries, and the draining venule, representing the cerebral microcirculation (A). The glioma microvasculature presents with chaotic and heterogeneous angioarchitecture, tortuous and dilated vessel loops, and extravasation of the high-molecular weight fluorescent marker (B). Contrast enhancement by FITC-Dextran 150,000 i.v. Original magnification, x200.
The herein presented models, however, do also have distinct limitations. Tumors that are used in the dorsal skin fold and cranial window model are transplantable cell lines or grafted tumor specimens. Thus, the tumor grafts may respond differently to treatment modalities directed toward the vascular compartment when compared with primary tumors [60,61]. So far, tumors which originate spontaneously in situ have not yet been subjected to intravital microscopy. In future, novel transgenic animal models with spontaneous tumor generation may be able to overcome this limitation, and may thus further contribute to elucidate the role of microcirculatory dynamics in the multistep sequelae of tumorigenesis [62]. An additional drawback of the microscopic approach is the limited life-time of the in vivo preparations, which do not allow extended analysis of tumor angiogenesis and microcirculation beyond an observation period of 3 to 4 weeks. Therefore, the studies are restricted to the early stage of tumor growth with a tumor volume rarely exceeding 200 to 400 mm3. In addition, there are also limitations by the microscopic technique itself. The depth which can be imaged by intravital fluorescence microscopy is ∼150 µm in low magnification and becomes less as increasing the magnification. Therefore, to study the microvasculature of deeper tissue areas and, in particular, for its three-dimensional reconstruction, laser confocal fluorescence microscopy may be used [63,64].
Intravital Fluorescence Videomicroscopy to Study Tumor Angiogenesis
Angiogenesis is the continuous formation of new blood vessels from the existing vasculature and represents the result of a dynamic interplay between the progressing tumor mass and microvascular proliferation. The time course and intensity of vascularization may markedly differ between distinct tumor types. In highly vascularized tumors, such as gliomas, intravital microscopy has shown that first signs of angiogenesis (dilation and tortuous elongation of host vessels, microvascular budding and sprouting) can be observed at day 2 to day 4 after implantation [32,33]. By day 6, the newly formed microvessels subsequently branch and interconnect with each other, forming complex micro-vascular networks in the peritumoral area that present with initial red blood cell perfusion. In this early phase, local hyperemia and diapedesis of red blood cells result in multiple intratumoral hemorrhages. By day 10, the glioma mass is completely vascularized and the yet dormant tumor starts to grow steadily [32,33]. With the glioma mass rapidly increasing in size between day 14 and day 22, the microvasculature remodels, finally demonstrating a gradient with high angiogenic activity in the peritumoral area, which decreases toward the centre of the tumor mass [32,33]. Thus, the multistep process of tumor angiogenesis involves three phases: first, an avascular phase (day 0 to day 6) characterized by initial tumor angiogenesis originating from host vessels, but lag of tumor growth; second, an early phase of tumor vascularization (day 6 to day 14) with development of tumor microcirculation, and, consequently, initiation of tumor growth; and third, an advanced phase of tumor vascularization (day 14 to day 22) with maintained high peritumoral angiogenic activity, but microvascular remodeling within the mature tumor [32].
Besides others [34,54,65], the quantitative measure to analyze neovascularization of tumor tissue may be the determination of the total vascular density, which represents the length of all newly formed microvessels per tumor area [16,55]. This measure, however, fails to distinguish between perfused and nonperfused microvessels, an information which may be essential to learn about the dependency of tumor nutritional blood supply, metabolism and growth. With the advantage that intravital microscopy allows to study not only tumor angioarchitecture, but also microcirculation, the measure of tumor functional vascular density, defined as the length of red blood cell perfused microvessels per tumor area, was additionally introduced, now providing information on the function of the angiogenic vessels [32,33]. Moreover, the ratio between the total and functional density of newly formed microvessels represents a microvascular perfusion index, which indicates functional efficacy of tumor angiogenesis [32,33]. Using these measures, intravital microscopic analyses have demonstrated that in contrast to normal tissues with perfusion indices of >95%, in tumor tissue, only 50% to 70% of all newly formed microvessels are perfused, which indeed indicates low functional efficacy of tumor angiogenesis [32].
Intravital Fluorescence Video-microscopy to Study Tumor Microcirculation
The microangioarchitecture, the distribution of vascular volume, vascular density, diameter and surface, as well as functional aspects, such as pressure gradient, microvascular hematocrit and hemorheology, define the microcirculation of a tumor. The tumor microcirculation is highly heterogeneous and does not conform to physiologic microcirculation in well-differentiated tissues. Tumors contain tortuous and maximally dilated vessels, shunts, variable intervascular spaces and large avascular areas [14,16,32,43,66]. In addition, the microcirculation among distinct tumors differs considerably, which is, in part, the result of the individual tumor growth pattern [6,57,59].
As demonstrated by intravital microscopy, the tumor microcirculation can be classified into two distinct compartments: i) peritumoral, i.e., outside the tumor and next to the tumor edge, representing the area of tumor growth and expansion, and ii) intratumoral, i.e., within the tumor mass. These two compartments differ significantly with respect to angiogenic activity [33], expression of angiogenic growth factors and their receptors [67–69], and, thus, microangioarchitecture (vascular density) [32,70]. However, the microcirculation of tumors is not solely dependent on the apparent vascular density. In addition to the vascular length (≈ functional vessel length per area), tissue perfusion Q (in ml/100 g per min) is further determined by mean vessel diameter, mean red blood cell velocity and intervascular spacing [48]. Using intravital microscopy, these parameters can all be measured by computer-assisted image analysis systems, and further information on the tumor microcirculation may be obtained by additionally calculating blood flow rate (nl/s) [16,32], vascular surface area (%) [71,72] and geometrical resistance [73].
The regulation of tumor microvascular perfusion becomes of special interest in the light of evolving novel therapeutic modalities that aim at interfering with tumor angiogenesis and microcirculation, such as anti-angiogenic drug therapy [2,74,75] and vascular targeting [76–78]. In fact, tumors may have several mechanisms to maintain microvascular perfusion despite successful therapeutic reduction of vascular density, including individual vessel dilatation, increase of red blood cell velocity and recruitment of nonperfused microvessels. This view is supported by the results of intravital microscopic studies, demonstrating that i) dilatation of tumor vessels represents one of the major mechanisms to compensate for the natural, progressive intratumoral (central) perfusion failure in growing tumors [16,32,70,79] and ii) targeting of tumor angiogenesis through inhibition of the VEGF receptor Flk-1 is associated with a significant increase of red blood cell velocity and blood flow rate that, in part, compensates for the successful reduction of functional vascular density by 30% to 70% [33].
Besides regulation of individual microvascular blood perfusion, the angioarchitecture and its dynamic changes during tumor growth may also affect overall tumor micro-circulation [48]. So far, however, most knowledge on tumor angioarchitecture is only descriptive and mainly qualitative in nature, including analysis of vascular branching orders and angles, vessel morphology, vessel length and vessel diameter [14,59,80–82]. One of the first more quantitative approaches to elucidate the complex arrangements of the tumor microvasculature used a detailed computer-reconstruction of intravital microscopic images, visualizing small regions of the individual tumors' microvasculature [83]. Alternatively, the influence of tumor angioarchitecture on perfusion and transport was recently assessed by evaluating the fractal characteristics of vascular networks of tumors implanted into the dorsal skin fold chamber preparation of mice [73]. Applying an invasion percolation-based network model, Baish et al. [73] identified the significance of tortuosity, vascular spacing, vessel diameter and viscosity on nutritional and drug transport. Further developments in mathematical modeling of intravital microscopic images may contribute to the understanding of regulatory mechanisms of tumor blood flow, and may thus improve novel strategies counteracting tumor vascularization and growth.
Intravital Fluorescence Videomicroscopy to Study Delivery of Molecules
The delivery of nutrients and drugs to tumor cells depends on both microvascular extravasation and interstitial transport of molecules. First qualitative information on molecule delivery in tumors were derived from ultrastructural analysis and tissue uptake studies [84–88]. In 1982, Nugent and Jain combined photometric techniques and intravital microscopy to monitor transport characteristics of fluorescent molecules, including both the intravascular and extravascular tumor compartments [89]. Since then, numerous fluorescently labeled macromolecules of varying molecular size (albumin, dextran, immunoglobulin fragments) have been used as tracer molecules to quantitatively assess the dynamics of macromolecular plasma clearance and tissue uptake [29,71,90]. Calculation of the microvascular permeability coefficient and the interstitial diffusion coefficient provided data on microvascular permeability and interstitial transport for individual tumor vessel segments or larger tumor regions of interest [5,91]. It should be noted that the use of a fluorescent dye coupled to specific macromolecules, such as dextran (150,000 MW), ideally enables for the simultaneous in vivo microscopic analysis of microvascular perfusion and macromolecular extravasation [32,33].
In fact, a considerable number of studies have confirmed that compared with the microvasculature of normal tissues with closed endothelium, tumor microvessels show an increased permeability for macromolecules up to a molecular weight of 500,000, distinct molecular size-dependent extravasation kinetics and reduced interstitial resistance to macromolecular transport [29,32,71,90]. Future studies with fluorescent microscopic techniques may additionally include analysis of appropriate uptake/receptor binding of specific antibodies and diagnostic tumor markers [92].
Intravital Fluorescence Videomicroscopy to Study Tumor Vessel-Associated Cell Interactions
The various in vivo and ex vivo labeling techniques of tumor cells and blood-born cells allow detailed analysis of the cellular interplay between leukocytes, endothelial cells and tumor cells. Moreover, the multifluorescent microscopic technique with simultaneous in vivo imaging of tumor vessels offers an approach to elucidate the role these cells play in controling angiogenesis and microcirculation, but also metastasis and anti-tumor immune response.
Leukocyte-Endothelial Cell Interaction Several techniques have been proposed so far for the labeling of circulating leukocytes in vivo, including fluorescent DNA markers such as Rhodamine and Acridin Orange [23,36]. In parallel, special care has been taken to optimize and standardize intravital microscopic setups and protocols to prevent artifacts due to phototoxicity or interference of the fluorescent tracers with cellular metabolism [35].
The process of leukocyte-endothelial cell interaction has been shown to be a tightly regulated multistep process [93–95]. Several cellular adhesion molecules are involved in the initial slowing down (“rolling”), firm adhesion to the endothelium (“sticking”) and extravasation of circulating leukocytes. Intravital fluorescence videomicroscopy to a great extent has contributed to identify the biomolecular mechanisms and relevant receptor/ligand systems under-lying these steps under pathological conditions such as inflammation and ischemia/reperfusion. In contrast, the significance of leukocyte-endothelial cell interaction for tumor angiogenesis and microcirculation is still a matter of debate. On the one hand, in vivo fluorescence microscopic studies revealed reduced leukocyte-endothelial cell interaction within tumor vessels when compared with normal control vessels, indicating immuno-incompetence of the host to control tumor growth [36,96,97]. This result has been attributed to a reduced flux of leukocytes in angiogenic vessels or to an immaturity of the new endothelium in upregulating the respective adhesion receptors. However, a large number of immunohistological studies in both human and experimental tumors have clearly shown the presence of leukocytes [45,98,99] as well as the microvascular expression of cell adhesion molecules [97], partly regulated by angiogenic growth factors (fibroblast growth factor-2, VEGF). Thus, the role of leukocyte-endothelial cell interaction in tumor angiogenesis and microcirculation needs further exploration, and intravital microscopy should be the appropriate tool for those future studies.
Tumor Cell-Microvessel Interaction Local tumor cell invasion is a hallmark of the diffuse-infiltrative growth behaviour of many human neoplasms (e.g., gliomas, squamous cell carcinomas, breast tumors). Recently, experimental reports have causally linked tumor cell invasion to tumor angiogenesis and microcirculation [100–102]. Invading tumor cells have been shown to be characterized by a high affinity to the perivascular basal membrane, suggesting that vessels growing from the host tissue toward the tumor might provide a trail for local tumor dissemination through the perivascular space [101]. The detailed mechanisms underlying this relationship, however, are to date incompletely understood, which is in part due to the lack of adequate models that allow for the simultaneous and repetitive assessment of tumor cell invasion and tumor angiogenesis in vivo. To overcome this, fluorescently labeled glioma spheroids instead of tumor cell suspensions may be implanted into both the dorsal skin fold chamber and the cranial window, thereby extending the application of intravital microscopy to a direct and repetitive evaluation of tumor cell migration in vivo (Figure 4). This unique experimental setting offers an approach for concurrent visualization of the dynamics of tumor cell spread and tumor angiogenesis, finally enabling to further elucidate the association between these two processes [34].
Figure 4.
Detachment and spreading (migration) of individual glioma cells 24 hours after C6 spheroid implantation into the dorsal skin fold chamber preparation of a nude mouse. Intravital fluorescence microscopy using epi-illumination techniques. Fluorescent labeling of C6 glioma cells with Oil before implantation. Original magnification, x400.
Intravital Fluorescence Videomicroscopy to Study Tumor Metabolism
The ultimate goal of tumor angiogenesis and microcirculation is the regulation of tissue metabolism and homeostasis. Over the years, major indicators reflecting the metabolic status of a tumor, such as oxygenation, mitochondrial redox state, pH and oxidative stress, have become amenable to intravital fluorescence videomicroscopy by applying advanced signal detection techniques. Accordingly, oxygenation can be assessed noninvasively within intra-and extravascular areas using the phosphorescence decay technique with palladium-porphyrins bound to albumin as an in vivo indicator [103]. The assessment of intracellular NADH fluorescence allows for quantification of the mitochondrial redox state, which represents, in the presence of sufficient substrate and phosphate, an indicator of cellular oxygen [104,105]. Quantitative pH measurements within tumor tissue can be realized by intravital fluorescence ratio imaging microscopy using BCECF as a H+-sensitive fluorochrome [106]. Finally, the formation of reactive oxygen species has been visualized with in vivo microscopic techniques using hydroperoxide-sensitive fluorescent probes that are trapped within viable cells [107].
Future Perspectives
The future challenge for the use of intravital fluorescence videomicroscopy to study tumor angiogenesis and micro-circulation is a further refinement of the technique to enable the in vivo imaging of biological processes taking place beyond the microvascular and cellular level, i.e., at the molecular level. The application of bioluminescence and fluorescent reporter genes opens the door to study in vivo gene activation and regulation as well as functional consequences by noninvasive technologies. This development will be essential to shed more light on the biomolecular mechanisms underlying the regulation of angiogenesis, microcirculation and tumor growth. In fact, first steps toward this future applications have been done by the fluorescent in vivo imaging of VEGF promotor activation through GFP expression during tumor growth and vascularization [69]. Thus, the intravital microscopic technique will remain an essential tool to study tumor biology, bridging over from in vitro observations to in vivo relevance.
Footnotes
This work was supported in part by grants of the Deutsche Forschungsgemeinschaft (VA 151/4-1, UL 60/4-1, and SFB399-A10), SAP and the Forschungsfonds Mannheim (58/96).
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Bedford JS, Mitchell JB. The effect of hypoxia on the growth and radiation response of mammalian cells in culture. Br J Radiol. 1974;47:687–696. doi: 10.1259/0007-1285-47-562-687. [DOI] [PubMed] [Google Scholar]
- 4.Jones DP. Hypoxia and drug metabolism. Biochem Pharmacol. 1981;30:1019–1023. doi: 10.1016/0006-2952(81)90436-6. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 6.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 7.Menger MD, Lehr HA. Scope and perspectives of intravital microscopy — bridge over from in vitro to in vivo. Immunol Today. 1993;14:519–522. doi: 10.1016/0167-5699(93)90179-O. [DOI] [PubMed] [Google Scholar]
- 8.Sandison JC. A new method for the microscopic study of living growing tissue by the introduction of a transparent chamber in the rabbit's ear. Anat Rec. 1924;28:281–287. [Google Scholar]
- 9.Ide AG, Baker NH, Warren SL. Vascularization of the Brown-Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am J Roentgenol. 1939;42:891–899. [Google Scholar]
- 10.Algire GH. An adaption of the transparent chamber technique to the mouse. J Natl Cancer Inst USA. 1943;4:1–11. [Google Scholar]
- 11.Algire GH, Chalkley HW. Vascular reactions of normal and malignant tissues in vivo: I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst USA. 1945;6:73–85. [Google Scholar]
- 12.Papenfuss HD, Gross JF, Intaglietta M, Treese FA. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979;18:311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- 13.Endrich B, Asaishi K, Gotz A, Messmer K. Technical report—a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berlin) 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 14.Dewhirst MW, Tso CY, Oliver R, Gustafson CS, Secomb TW, Gross JF. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int J Radiat Oncol Biol Phys. 1989;17:91–99. doi: 10.1016/0360-3016(89)90375-1. [DOI] [PubMed] [Google Scholar]
- 15.Axelsson H, Bagge U, Lundholm K, Svanberg E. A one-piece plexiglass access chamber for subcutaneous implantation in the dorsal skin fold of the mouse. Int J Microcirc: Clin Exp. 1997;17:328–329. doi: 10.1159/000179248. [DOI] [PubMed] [Google Scholar]
- 16.Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52:6553–6560. [PubMed] [Google Scholar]
- 17.Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- 18.Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab. 1984;4:574–585. doi: 10.1038/jcbfm.1984.82. [DOI] [PubMed] [Google Scholar]
- 19.Rovainen CM, Woolsey TA, Blocher NC, Wang DB, Robinson OF. Blood flow in single surface arterioles and venules on the mouse somatosensory cortex measured with videomicroscopy, fluorescent dextrans, nonoccluding fluorescent beads, and computer-assisted image analysis. J Cereb Blood Flow Metab. 1993;13:359–371. doi: 10.1038/jcbfm.1993.49. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M, Sekizuka E, Sato S, Yamaguchi N, Shimizu K, Kobayashi K, Bertalanffy H, Kawase T. In vivo rat closed spinal window for spinal microcirculation: observation of pial vessels, leukocyte adhesion, and red blood cell velocity. Neurosurgery. 1999;44:156–161. doi: 10.1097/00006123-199901000-00096. [DOI] [PubMed] [Google Scholar]
- 21.Tillmanns H, Ikeda S, Bing RJ. The effects of nicotine on the coronary microcirculation in the cat heart. J Clin Pharmacol. 1974;14:426–433. doi: 10.1002/j.1552-4604.1974.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnle GE, Leipfinger FH, Goetz AE. Measurement of microhemodynamics in the ventilated rabbit lung by intravital fluorescence microscopy. J Appl Physiol. 1993;74:1462–1471. doi: 10.1152/jappl.1993.74.3.1462. [DOI] [PubMed] [Google Scholar]
- 23.Menger MD, Marzi I, Messmer K. In vivo fluorescence microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur Surg Res. 1991;23:158–169. doi: 10.1159/000129148. [DOI] [PubMed] [Google Scholar]
- 24.Klar E, Endrich B, Messmer K. Microcirculation of the pancreas. A quantitative study of physiology and changes in pancreatitis. Int J Microcirc: Clin Exp. 1990;9:85–101. [PubMed] [Google Scholar]
- 25.Bohlen HG, Henrich H, Gore RW, Johnson PC. Intestinal muscle and mucosal blood flow during direct sympathetic stimulation. Am J Physiol. 1978;235:H40–H45. doi: 10.1152/ajpheart.1978.235.1.H40. [DOI] [PubMed] [Google Scholar]
- 26.Steinhausen M, Zimmerhackl B, Thederan H, Dussel R, Parekh N, Esslinger HU, von Hagens G, Komitowski D, Dallenbach FD. Intraglomerular microcirculation: measurements of single glomerular loop flow in rats. Kidney Int. 1981;20:230–239. doi: 10.1038/ki.1981.125. [DOI] [PubMed] [Google Scholar]
- 27.von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3:287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 28.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 30.Menger MD, Pelikan S, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: significance of “reflow paradox.”. Am J Physiol. 1992;263:H1901–H1906. doi: 10.1152/ajpheart.1992.263.6.H1901. [DOI] [PubMed] [Google Scholar]
- 31.Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation. 1995;60:123–127. [PubMed] [Google Scholar]
- 32.Vajkoczy P, Schilling L, Ullrich A, Schmiedek P, Menger MD. Characterization of angiogenesis and microcirculation of high-grade glioma: an intravital multifluorescence microscopic approach in the athymic nude mouse. J Cereb Blood Flow Metab. 1998;18:510–520. doi: 10.1097/00004647-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Vajkoczy P, Menger MD, Vollmar B, Schilling L, Schmiedek P, Hirth KP, Ullrich A, Fong TAT. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia. 1999;1:31–41. doi: 10.1038/sj.neo.7900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vajkoczy P, Goldbrunner R, Farhadi M, Vince G, Schilling L, Tonn JC, Schmiedek P, Menger MD. Glioma cell migration is associated with glioma-induced angiogenesis in vivo. Int J Dev Neurosci. 1999 doi: 10.1016/s0736-5748(99)00021-0. in press. [DOI] [PubMed] [Google Scholar]
- 35.Saetzler RK, Jallo J, Lehr HA, Philips CM, Vasthare U, Arfors KE, Tuma RF. Intravital fluorescence microscopy: impact of light-induced phototoxicity on adhesion of fluorescently labeled leukocytes. J Histochem Cytochem. 1997;45:505–513. doi: 10.1177/002215549704500403. [DOI] [PubMed] [Google Scholar]
- 36.Vajkoczy P, Schilling L, Schmiedek P, Menger MD. Glioma angiogenesis and vascularization: significance of leukocyte/endothelium-interaction. J Cereb Blood Flow Metab. 1997;17(suppl 1):S179. [Google Scholar]
- 37.Lehr HA, Vollmar B, Vajkoczy P, Menger MD. Intravital fluorescence microscopy for the study of leukocyte interaction with platelets and endothelial cells. Methods Enzymol. 1999;300:462–481. doi: 10.1016/s0076-6879(99)00151-2. [DOI] [PubMed] [Google Scholar]
- 38.Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood. 1998;92:507–515. [PubMed] [Google Scholar]
- 39.Heim R, Tsien RY. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 40.Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 41.Wesseling P, van der Laak JA, de Leeuw H, Ruiter DJ, Burger PC. Quantitative immunohistological analysis of the microvasculature in untreated human glioblastoma multiforme. Computer-assisted image analysis of whole-tumor sections. J Neurosurg. 1994;81:902–909. doi: 10.3171/jns.1994.81.6.0902. [DOI] [PubMed] [Google Scholar]
- 42.Orita T, Nishizaki T, Kamiryo T, Aoki H, Harada K, Okamura T. The microvascular architecture of human malignant glioma. A scanning electron microscopic study of a vascular cast. Acta Neuropathol (Berlin) 1988;76:270–274. doi: 10.1007/BF00687774. [DOI] [PubMed] [Google Scholar]
- 43.Konerding MA, Steinberg F, Budach V. The vascular system of xenotransplanted tumors—scanning electron and light microscopic studies. Scanning Microsc. 1989;3:327–335. [PubMed] [Google Scholar]
- 44.Bernsen HJ, Rijken PF, Oostendorp T, van der Kogel AJ. Vascularity and perfusion of human gliomas xenografted in the athymic nude mouse. Br J Cancer. 1995;71:721–726. doi: 10.1038/bjc.1995.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittle IR, Collins F, Kelly PA, Ritchie I, Ironside JW. Nitric oxide synthase is expressed in experimental malignant glioma and influences tumour blood flow. Acta Neurochir (Wien) 1996;138:870–875. doi: 10.1007/BF01411266. [DOI] [PubMed] [Google Scholar]
- 46.Andrade SP, Hart IR, Piper PJ. Inhibitors of nitric oxide synthase selectively reduce flow in tumor—associated neovasculature. Br J Pharmacol. 1992;107:1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foltz RM, McLendon RE, Friedman HS, Dodge RK, Bigner DD, Dewhirst MW. A pial window model for the intracranial study of human glioma microvascular function. Neurosurgery. 1995;36:976–984. doi: 10.1227/00006123-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Intaglietta M, Zweifach BW. Microcirculatory basis of fluid exchange. Adv Biol Med Phys. 1974;15:111–159. doi: 10.1016/b978-0-12-005215-8.50011-5. [DOI] [PubMed] [Google Scholar]
- 49.Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 50.Kan Z, Ivancev K, Lunderquist A, McCuskey PA, Wright KC, Wallace S, McCuskey RS. In vivo microscopy of hepatic tumors in animal models: a dynamic investigation of blood supply to hepatic metastases. Radiology. 1993;187:621–626. doi: 10.1148/radiology.187.3.8497606. [DOI] [PubMed] [Google Scholar]
- 51.Fukumura D, Yuan F, Monsky WL, Chen Y, Jain RK. Effect of host microenvironment on the microcirculation of human colon adenocarcinoma. Am J Pathol. 1997;151:679–688. [PMC free article] [PubMed] [Google Scholar]
- 52.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naumov GN, Wilson SM, MacDonald IC, Schmidt EE, Morris VL, Groom AC, Hoffman RM, Chambers AF. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J Cell Sci. 1999;112:1835–1842. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- 54.Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 55.Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borgstrom P, Bourdon MA, Hillan KJ, Sriramarao P, Ferrara N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 57.Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 58.Coomber BL, Stewart PA, Hayakawa EM, Farrell CL, Del Maestro RF. A quantitative assessment of microvessel ultrastructure in C6 astrocytoma spheroids transplanted to brain and to muscle. J Neuropathol Exp Neurol. 1988;47:29–40. doi: 10.1097/00005072-198801000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Konerding MA, Malkusch W, Klapthor B, van Ackern C, Fait E, Hill SA, Parkins C, Chaplin DJ, Presta M, Denekamp J. Evidence for characteristic vascular patterns in solid tumours: quantitative studies using corrosion casts. Br J Cancer. 1999;80:724–732. doi: 10.1038/sj.bjc.6690416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falk P. Differences in vascular pattern between the spontaneous and the transplanted C3H mouse mammary carcinoma. Eur J Cancer Clin Onco. 1982;18:155–165. doi: 10.1016/0277-5379(82)90059-1. [DOI] [PubMed] [Google Scholar]
- 61.Field SB, Needham S, Burney IA, Maxwell RJ, Coggle JE, Griffiths JR. Differences in vascular response between primary and transplanted tumours. Br J Cancer. 1991;63:723–726. doi: 10.1038/bjc.1991.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergers G, Hanahan D, Coussens LM. Angiogenesis and apoptosis are cellular parameters of neoplastic progression in transgenic mouse models of tumorigenesis. Int J Dev Biol. 1998;42:995–1002. [PubMed] [Google Scholar]
- 63.Dirnagl U, Villringer A, Einhaupl KM. In vivo confocal scanning laser microscopy of the cerebral microcirculation. J Microsc. 1992;165:147–157. doi: 10.1111/j.1365-2818.1992.tb04312.x. [DOI] [PubMed] [Google Scholar]
- 64.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dellian M, Witwer BP, Salehi HA, Yuan F, Jain RK. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol. 1996;149:59–71. [PMC free article] [PubMed] [Google Scholar]
- 66.Baish JW, Jain RK. Cancer, angiogenesis and fractals. Nat Med. 1998;4:984. doi: 10.1038/1952. [DOI] [PubMed] [Google Scholar]
- 67.Hatva E, Kaipainen A, Mentula P, Jaaskelainen J, Paetau A, Haltia M, Alitalo K. Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumors. Am J Pathol. 1995;146:368–378. [PMC free article] [PubMed] [Google Scholar]
- 68.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 69.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 70.Endrich B, Intaglietta M, Reinhold HS, Gross JF. Hemodynamic characteristics in microcirculatory blood channels during early tumor growth. Cancer Res. 1979;39:17–23. [PubMed] [Google Scholar]
- 71.Yuan F, Leunig M, Berk DA, Jain RK. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res. 1993;45:269–289. doi: 10.1006/mvre.1993.1024. [DOI] [PubMed] [Google Scholar]
- 72.Vollmar B, Wolf B, Siegmund S, Katsen AD, Menger MD. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol. 1997;151:169–175. [PMC free article] [PubMed] [Google Scholar]
- 73.Baish JW, Gazit Y, Berk DA, Nozue M, Baxter LT, Jain RK. Role of tumor vascular architecture in nutrient and drug delivery: an invasion percolation-based network model. Microvasc Res. 1996;51:327–346. doi: 10.1006/mvre.1996.0031. [DOI] [PubMed] [Google Scholar]
- 74.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 75.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 76.Huang X, Molema G, King S, Watkins L, Edgington TS, Thorpe PE. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 77.Pasqualini R. Vascular targeting with phage peptide libraries. Q J Nucl Med. 1999;43:159–162. [PubMed] [Google Scholar]
- 78.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, Stratford MR, Dennis MF, Chaplin DJ. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 1999;59:1626–1634. [PubMed] [Google Scholar]
- 79.Asaishi K, Endrich B, Gotz A, Messmer K. Quantitative analysis of microvascular structure and function in the amelanotic melanoma A-Mel-3. Cancer Res. 1981;41:1898–1904. [PubMed] [Google Scholar]
- 80.Skinner SA, Tutton PJ, O'Brien PE. Microvascular architecture of experimental colon tumors in the rat. Cancer Res. 1990;50:2411–2417. [PubMed] [Google Scholar]
- 81.Less JR, Posner MC, Skalak TC, Wolmark N, Jain RK. Geometric resistance and microvascular network architecture of human colorectal carcinoma. Microcirculation. 1997;4:25–33. doi: 10.3109/10739689709148315. [DOI] [PubMed] [Google Scholar]
- 82.Konerding MA, Fait E, Dimitropoulou C, Malkusch W, Ferri C, Giavazzi R, Coltrini D, Presta M. Impact of fibroblast growth factor-2 on tumor microvascular architecture. A tridimensional morphometric study. Am J Pathol. 1998;152:1607–1616. [PMC free article] [PubMed] [Google Scholar]
- 83.Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radial Oncol Biol Phys. 1993;25:481–489. doi: 10.1016/0360-3016(93)90070-c. [DOI] [PubMed] [Google Scholar]
- 84.Hirano A, Matsui T. Vascular structures in brain tumors. Hum Pathol. 1975;6:611–621. doi: 10.1016/s0046-8177(75)80045-1. [DOI] [PubMed] [Google Scholar]
- 85.Waggener JD, Beggs JL. Vasculature of neural neoplasms. Adv Neurol. 1976:27–49. [PubMed] [Google Scholar]
- 86.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 87.Peterson HI, Alpsten M, Skolnik G, Karlsson L. Influence of a prostaglandin synthesis inhibitor and of thrombocytopenia on tumor blood flow and tumor vascular permeability. Experimental studies in the rat. Anticancer Res. 1985;5:253–257. [PubMed] [Google Scholar]
- 88.Sands H, Jones PL, Shah SA, Palme D, Vessella RL, Gallagher BM. Correlation of vascular permeability and blood flow with monoclonal antibody uptake by human Clouser and renal cell xenografts. Cancer Res. 1988;48:188–193. [PubMed] [Google Scholar]
- 89.Nugent LJ, Jain RK. Monitoring transport in the rabbit ear chamber. Microvasc Res. 1982;24:204–209. doi: 10.1016/0026-2862(82)90057-7. [DOI] [PubMed] [Google Scholar]
- 90.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 91.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci USA. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berk DA, Yuan F, Leunig M, Jain RK. Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc Natl Acad Sci USA. 1997;94:1785–1790. doi: 10.1073/pnas.94.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.von Andrian UH, Arfors KE. Neutrophil-endothelial cell interactions in vivo: a chain of events characterized by distinct molecular mechanisms. Agents Actions Suppl. 1993;41:153–164. [PubMed] [Google Scholar]
- 94.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 95.Menger MD, Vollmar B. Adhesion molecules as determinants of disease: from molecular biology to surgical research. Br J Surg. 1996;83:588–601. doi: 10.1002/bjs.1800830506. [DOI] [PubMed] [Google Scholar]
- 96.Fukumura D, Salehi HA, Witwer B, Tuma RF, Melder RJ, Jain RK. Tumor necrosis factor alpha-induced leukocyte adhesion in normal and tumor vessels: effect of tumortype transplantation site, and host strain. Cancer Res. 1995;55:4824–4829. [PubMed] [Google Scholar]
- 97.Jain RK, Koenig GC, Dellian M, Fukumura D, Munn LL, Melder RJ. Leukocyte-endothelial adhesion and angiogenesis in tumors. Cancer Metastasis Rev. 1996;15:195–204. doi: 10.1007/BF00437472. [DOI] [PubMed] [Google Scholar]
- 98.Morioka T, Baba T, Black KL, Streit WJ. Inflammatory cell infiltrates vary in experimental primary and metastatic brain tumors. Neurosurgery. 1992;30:891–896. doi: 10.1227/00006123-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki Y, Ohtani H, Mizoi T, Takeha S, Shiiba K, Matsuno S, Nagura H. Cell adhesion molecule expression by vascular endothelial cells as an immune/inflammatory reaction in human colon carcinoma. Jpn J Cancer Res. 1995;86:585–593. doi: 10.1111/j.1349-7006.1995.tb02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engebraaten O, Bjerkvig R, Pedersen PH, Laerum OD. Effects of EGF, bFGF, NGF and PDGF(bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies in vitro. Int J Cancer. 1993;53:209–214. doi: 10.1002/ijc.2910530206. [DOI] [PubMed] [Google Scholar]
- 101.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 103.Torres Filho IP, Intaglietta M. Microvessel PO2 measurements by phosphorescence decay method. Am J Physiol. 1993;265:H1434–H1438. doi: 10.1152/ajpheart.1993.265.4.H1434. [DOI] [PubMed] [Google Scholar]
- 104.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. The microfluorometry of pyridine nucleotide gives a continuous measurement of the oxidation state. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 105.Vollmar B, Burkhardt M, Minor T, Klauke H, Menger MD. High-resolution microscopic determination of hepatic NADH fluorescence for in vivo monitoring of tissue oxygenation during hemorrhagic shock and resuscitation. Microvasc Res. 1997;54:164–173. doi: 10.1006/mvre.1997.2028. [DOI] [PubMed] [Google Scholar]
- 106.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 107.Suematsu M, Schmid-Schonbein GW, Chavez-Chavez RH, Yee TT, Tamatani T, Miyasaka M, Delano FA, Zweifach BW. In vivo visualization of oxidative changes in microvessels during neutrophil activation. Am J Physiol. 1993;264:H881–H891. doi: 10.1152/ajpheart.1993.264.3.H881. [DOI] [PubMed] [Google Scholar]