Abstract
Phospholipase D (PLD) has been implicated in the signal transduction pathways initiated by several mitogenic protein tyrosine kinases. We demonstrate for the first time that most notably PLD2 and to a lesser extent the PLD1 isoform are tyrosine phosphorylated by c-Src tyrosine kinase via direct association. Moreover, epidermal growth factor induced tyrosine phosphorylation of PLD2 and its interaction with c-Src in A431 cells. Interaction between these proteins is via the pleckstrin homology domain of PLD2 and the catalytic domain of c-Src. Coexpression of PLD1 or PLD2 with c-Src synergistically enhances cellular proliferation compared with expression of either molecule. While PLD activity as a lipid-hydrolyzing enzyme is not affected by c-Src, wild-type PLDs but not catalytically inactive PLD mutants significantly increase c-Src kinase activity, up-regulating c-Src-mediated paxillin phosphorylation and extracellular signal-regulated kinase activity. These results demonstrate the critical role of PLD catalytic activity in the stimulation of Src signaling. In conclusion, we provide the first evidence that c-Src acts as a kinase of PLD and PLD acts as an activator of c-Src. This transmodulation between c-Src and PLD may contribute to the promotion of cellular proliferation via amplification of mitogenic signaling pathways.
Phospholipase D (PLD) catalyzes the hydrolysis of phosphatidylcholine, the major membrane phospholipid, to form phosphatidic acid (PA) and choline. PA is generally recognized as the signaling product of PLD and functions as an effector in multiple physiological processes. The PLD pathway is thought to play a critical role in regulating cell responses that contribute to mitotic signaling and transformation (14, 17, 23, 28, 36). To date, two PLD isoforms have been cloned and characterized. PLD1 has a low basal activity and is up-regulated by small G proteins (ARF, Rho, and Ral), protein kinase C (PKC), and phosphatidylinositol 4, 5-bisphosphate (PIP2) in vitro. In contrast, PLD2 has a high basal activity, requires PIP2, and is up-regulated by ADP-ribosylation factor and protein kinase C (6, 16). Although many studies focusing on the regulation of PLD have been reported, the cellular role of PLD remains still unclear.
Protein tyrosine phosphorylation is important in regulating signaling pathways through its effects on protein-protein interactions. Tyrosine kinase receptors such as epidermal growth factor (EGF) and platelet-derived growth factor receptors stimulate PLD activity in some cell types, suggesting that PLD activity is modulated by tyrosine phosphorylation (2, 13, 18). Recently, Min et al. (33) and Marcil et al. (31) reported that pervanadate induced tyrosine phosphorylation on PLD1 in Swiss 3T3 fibroblasts and HL60 cells, respectively. Expression of PLD1 or PLD2 in HEK 293 cells revealed that PLD2, but not PLD1, is constitutively associated with the EGF receptor and becomes phosphorylated on tyrosine-11 upon stimulation with EGF (49). Mutation of tyrosine-11 to phenylalanine did not alter the magnitude of EGF stimulation. Thus, it was suggested that tyrosine phosphorylation of PLD2 is important for interaction with SH2-containing proteins but not for its intrinsic activity as a lipid-hydrolyzing enzyme. Not only the identity of the kinase responsible for phosphorylation of PLD but also the role of phosphorylated PLD in cellular responses needs to be clarified. Furthermore, in neutrophils stimulated by fMLP (N-formyl-methionyl-leucyl-phenylalanine) and monosodium urate crystals, PLD1 was activated but not tyrosine phosphorylated (32). Therefore, the physiological relevance of tyrosine phosphorylation as an activation mechanism of PLD remains to be established.
PLD has been reported to be overexpressed and hyperactivated in some human cancers (40, 54, 55, 59). Consistent with these findings, PLD can facilitate mitogenesis and oncogenic transformation (17, 28, 36), and protein tyrosine kinase (PTK) activity occurs widely in mitogenic signaling (12). Jiang et al. (20-22) demonstrated that v-Src (pp60v-src), the viral counterpart of the nonreceptor tyrosine kinase c-Src (pp60c-src), stimulated PLD activity in fibroblasts and that small G proteins Ras and GTPase Ral were involved in PLD activation. The src oncogene encodes a membrane-localized tyrosine-specific protein kinase whose enzymatic activity is necessary to induce oncogenic transformation (7, 24, 42). Because the oncogenicity of c-Src correlates with its kinase activity, the Src oncoprotein probably induces transformation by phosphorylating critical cellular proteins that control cell proliferation. Src kinase activity and sometimes Src protein levels were found to be elevated in a wide variety of human cancers, with a frequent correlation between Src kinase activity and the degree of malignancy and/or invasiveness (3, 19). Recently, it was reported that elevated expression of PLD1 or PLD2 resulted in the transformation of rat fibroblasts overexpressing c-Src (23). However, little is known about how PLD cooperates with c-Src to transform cells.
In the present study, we demonstrate that PLD associates with c-Src. For the first time, we provide the evidence that PLD is an effector for c-Src activation as well as a physiologically relevant substrate by c-Src kinase. Therefore, we hypothesize that this intimate cross talk between PLD and c-Src contributes to cell proliferation by amplifying Src signaling pathways.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, and Lipofectamine were purchased from Invitrogen. Protein A-Sepharose and glutathione-Sepharose 4B were from Amersham Bioscience Biotech. Hydrogen peroxide and phorbol myristate acetate were from Sigma. EGF and anti-phosphotyrosine antibody (P-Tyr) (4G10) were from Upstate Biotechnology, and PP2 was from Calbiochem. Antipaxillin antibody was from Transduction Laboratories (Lexington, Ky.). The antibody to c-Src was from Santa Cruz. Phospho-Tyr416 c-Src, the activated form of the kinase (8, 10), phospho-paxillin, phospho-ERK, and ERK were assessed by using an antibody from Cell Signaling. A polyclonal antibody that recognizes both PLD1 and PLD2 was generated as previously described (34). Phosphatidylbutanol (PtdBut) standard was from Avanti Polar Lipid. [9, 10-3H]myristate was purchased from Perkin-Elmer Life Sciences. Silica gel 60 A thin-layer chromatography plates were from Whatman. Horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG were from Kirkegaard & Perry Lab (Gaithersburg, Md.). The ECL Western blotting detection kit was from Amersham Bioscience.
Cell culture and transfections.
Human A431 epidermoid carcinoma cells were purchased from the American Type Culture Collection. The establishment of mouse fibroblast cells overexpressing wild-type PLD2 was described previously (36). FaO rat hepatoma cell lines overexpressing wild-type, dominant negative mutant, and dominant active mutant of c-Src were obtained by transfection by using Lipofectamine according to the manufacturer's instructions. Transfected cells were selected with G418 (400 μg/ml) for 21 days at 37°C. At that time antibiotic-resistant colonies were pooled and expanded for further analysis under selective conditions. COS-7 cells were transfected with human PLD1, PLD2, catalytically inactive mutants PLD1-K898R, PLD2-K758R, N-terminally deleted mutants of PLD2 Δ183(184-933) and PLD2 Δ313(314-933), Lck, Fyn, and wild-type and mutant c-Src (K295M) expression vectors by using Lipofectamine. All cells were maintained in DMEM supplemented with 10% fetal bovine serum.
Plasmid construction and preparation of GST fusion proteins.
The c-Src truncation mutants (SH3, SH2, or kinase domains) with coding regions corresponding to amino acids 88 to 137, 144 to 245, and 265 to 523, respectively, were created by PCR amplification. The forward primers used to amplify the truncation mutants of c-Src with coding region starting at amino acid 88, 144, or 265 were 5′-CCGGAATTCATGGCTCTCTACGACTACGAGTCC-3′, 5′-CGGAATTCATGCAGGCTGAAGAGTGGTAC-3′, and 5′-CCGGAATTCATGGAGTCGCTGCGGCTGGAGGTGAA-3′, respectively. The reverse primers that were used to amplify the truncation mutants of c-Src constructs that end at amino acid 137, 245, or 523 were 5′-CCGCTCGAGCTAGACATAGTTACTGGGGATGTA-3′ , 5′-CGCTCGAGTCAGCAGACGTTGGTCAGGCG-3′, and 5′-CCGCTCGAGCTATGTCGAGGTGAAGTAGTCCTC-3′, respectively. The PCR fragments were then subcloned in frame with the N-terminal fusion glutathione S-transferase (GST) at the EcoRI/XhoI site in the pGEX4T-1 vector (Amersham Bioscience Biotech). All the constructs were sequenced to verify the coding regions of c-Src. The full-length PLD2 and its two deletion mutants with coding regions corresponding to amino acids 184 to 933 or 314 to 933 were created by PCR. A cDNA fragment encoding PLD2 Δ183(184-933) was amplified from pcDNA3.1-PLD2 by PCR using sense (5′-GAATTCATGACAGAGTTCCTGGAAGTCAGTGC-3′) and antisense (5′-GCGGCCGCTATGTCCACACTTCTAGGGGGATC-3′) oligonucleotides. It was then digested with EcoRI and NotI and ligated together with the EcoRI/NotI fragment encoding PLD2 Δ183(184-933) into pCMV-Flag5b (Stratagene) vector. A cDNA fragment encoding hPLD2 Δ313(314-933) was amplified from pcDNA3.1-PLD2 by PCR using sense (5′-GAATTCATGGGCAGAGACTTCCTACAGCTGCAC-3′) and antisense (5′-CTCGAGTGTCCACACTTCTAGGGGGATC-3′) oligonucleotides. The PCR fragments were digested by EcoRI and XhoI and ligated together with the same digestion fragment into pCMV-Flag5b expression vector. Each deletion construct was confirmed by DNA sequencing.
PLD activity assay.
PLD activity was assessed by measuring the formation of [3H]PtdBut, the product of PLD-mediated transphosphatidylation, in the presence of 1-butanol. After 20 h of transfection with Lipofectamine, COS-7 cells in six-well plates were serum starved in the presence of 1 μCi of [3H]myristic acid/ml. After overnight starvation, the cells were washed three times with 5 ml of phosphate-buffered saline (PBS) and preequilibrated in serum-free DMEM for 1 h. For the final 10 min of preincubation, 0.3% butan-1-ol was included. At the end of the preincubation, cells were treated with agonists for the indicated times. The extraction and characterization of lipids by thin-layer chromatography were performed as previously described (34).
Immunoprecipitation.
Cells or tissues were washed twice with ice-cold PBS and then lysed in the extraction buffer (20 mM HEPES [pH 7.2], 1% Triton X-100, 1% sodium deoxycholate, 0.2% sodium dodecyl sulfate [SDS], 200 mM NaCl, 1 mM Na3VO4, 1 mM NaF, 10% glycerol, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride). The resulting cell lysates were spun at 15,000 × g in an Eppendorf microcentrifuge for 10 min at 4°C to pellet the unbroken cells. The supernatant was then precleared for 30 min with preimmune IgG and protein A Sepharose at 4°C with rocking. Protein concentrations were determined by using the Bradford method with bovine serum albumin as a standard (4). Equal protein aliquots of precleared cell lysates (1 mg) were incubated with the indicated antibodies and 40 μl of a 1:1 slurry of protein A-Sepharose beads for 4 h at 4°C. The immune complexes were collected by centrifugation, washed five times with a buffer (20 mM Tris [pH 7.5], 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 2 mM Na3VO4, 10% glycerol, and 1% Nonidet P-40), and resuspended in sample buffer. Immune complexes were boiled in SDS sample buffer.
Western blotting.
Protein samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) on 8% gels and were transferred to a nitrocellulose membrane. The blots were then blocked with 5% nonfat milk and incubated with the appropriate primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were detected by enhanced chemiluminescence.
In vitro binding assay.
Wild-type human PLD2, N-terminal 184-amino-acid-deleted mutant PLD2 Δ183(184-933), and the N-terminal 313-amino-acid-deleted mutant PLD2 Δ313(314-933) were transfected in COS-7 cells as described previously (1). Transfected cells were lysed in lysis buffer. After centrifugation, aliquots of the soluble extract were coimmunoprecipitated and subjected to immunoblot analysis by using an antibody against PLD or c-Src as described above. After cotransfecting COS-7 cells with PLD2 and c-Src, a GST pull-down assay was performed by using GST fusion proteins attached to glutathione-Sepharose beads. Clarified lysates (1 mg) were incubated with 2 μg of GST fusion proteins immobilized on glutathione-Sepharose beads in a final volume of 500 μl of the extraction buffer for 1 h at 4°C. Protein complexes were collected by centrifugation and washed four times with washing buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris-HCl [pH 8.0], 20 mM NaF, 200 μM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml). Associated protein complexes were resolved by SDS-PAGE and transferred to a nitrocellulose membrane, followed by immunoblot analysis with anti-PLD antibody.
Generation of mouse monoclonal antibody specific for PLD2.
The fragment of PLD2 (724 to 825 amino acids) was fused to GST. For the production of monoclonal antibody, a female BALB/c mouse (7 to 8 weeks old) was immunized with 50 μg of the purified GST fusion protein in complete Freund's adjuvant. At intervals of 2 weeks, three booster injections were given. Three days before cell fusion, the mouse was further boosted by an injection of 50 μg of the protein in PBS. The spleen cells from the immunized mouse were fused with SP2 myeloma cells. Standard fusion, screening, and cloning procedures were followed. Culture supernatants were screened for the immunoblotting. The cells from the positive wells were cloned twice to ensure monoclonality.
Cell viability assays.
The trypan blue exclusion method was used to quantify cell proliferation. After transfection, cells were harvested by trypsin and washed in PBS. Trypan blue (Sigma) was added to suspended cells at a concentration of 0.4% (wt/vol). Live cell numbers were determined by counting with a hemocytometer.
Measurement of c-Src activation.
To detect activated c-Src, clarified whole-cell lysates of transfected cells were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Tyr416-phosphorylated c-Src was detected by using a 1:1,000 dilution of anti-phospho Tyr416 c-Src antibody, with horseradish peroxidase-conjugated goat anti-rabbit IgG as secondary antibody. Identical samples were immunoblotted with c-Src antibody as controls. Immune complexes on nitrocellulose were visualized by enzyme-linked chemiluminescence.
RESULTS
Src family PTKs induce tyrosine phosphorylation of PLD isozymes.
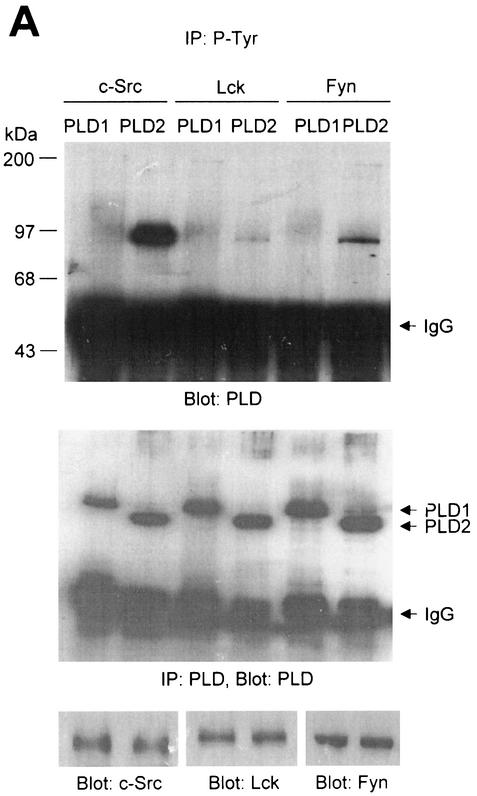
We investigated whether the Src family PTKs were capable of phosphorylating PLD isozymes. Cotransfection experiments were performed with COS-7 cells utilizing Src family PTKs and PLD expression vectors as depicted in Fig. 1A. After transfection, cell lysates were prepared and subjected to immunoprecipitation by using an antibody directed against P-Tyr, and tyrosine phosphorylation of PLD isozymes was monitored by successive immunoblotting for PLD. The anti-PLD antibody generated by us recognized both PLD1 and PLD2. The data presented in Fig. 1A show that transfection of vectors containing c-Src, Lck, and Fyn resulted in tyrosine phosphorylation of PLD2, while faint phosphorylation of PLD1 was observed only after a long exposure of the membrane to film (data not shown).
FIG. 1.
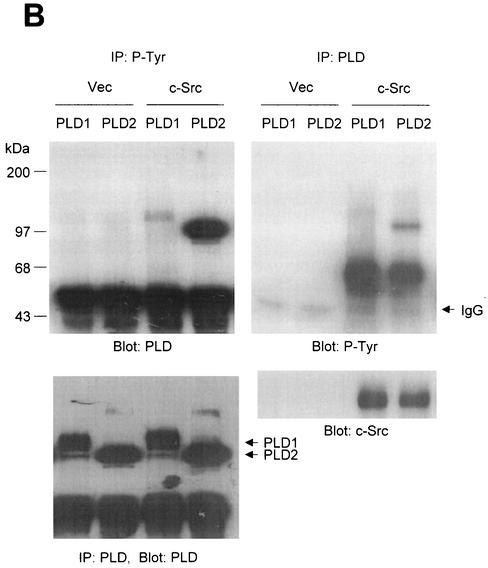
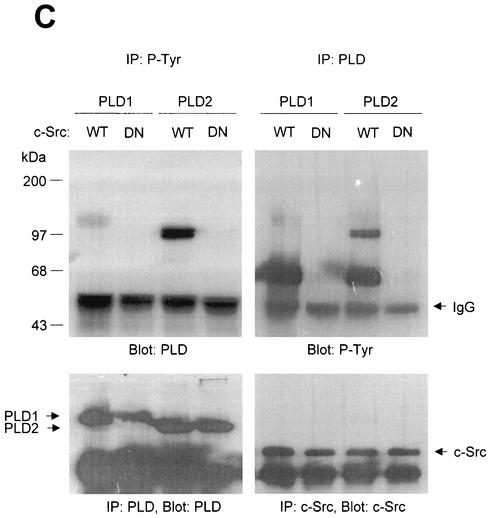
PLD2 and, to a lesser extent, PLD1 are tyrosine phosphorylated by c-Src tyrosine kinase. (A) COS-7 cells were transiently cotransfected for 40 h with various combinations of plasmids encoding PLD1, PLD2, c-Src, Lck, and Fyn. Cell lysates were immunoprecipitated with anti-P-Tyr antibody, and precipitated proteins were subjected to immunoblot analysis with an anti-PLD antibody. Western blot analysis using PLDs and c-Src family tyrosine kinase demonstrated that the respective proteins were expressed to equal extents. (B) COS-7 cells were transiently transfected with the indicated expression plasmids, and cell lysates were subjected to immunoprecipitation with anti-P-Tyr antibody or anti-PLD antibodies. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-PLD or anti-P-Tyr antibody. Expression of PLD and c-Src was determined by using anti-PLD or anti-c-Src antibodies. (C) COS-7 cells were transiently transfected with various combinations of plasmids encoding PLD1, PLD2, wild-type (WT) c-Src, and dominant negative (DN) c-Src. Cell lysates were immunoprecipitated by using anti-P-Tyr antibody or anti-PLD antibody, and immune complexes were analyzed by immunoblotting. Immunoreactive proteins were visualized by use of horseradish peroxidase-coupled secondary antibody and chemiluminescence. Data are representative of three experiments.
The studies that are described next were focused on c-Src, since c-Src of the tested Src family PTKs induced the strongest tyrosine phosphorylation of PLD. In cells cotransfected with PLD1 or PLD2 plus c-Src, both PLD1 and PLD2 (approximately 125 and 105 kDa, respectively) were tyrosine phosphorylated, whereas no tyrosine-phosphorylated proteins (except for IgG chains) were detected in cells transfected with PLD1 or 2 plus empty vector (Fig. 1B). Similar results were obtained by immunoblotting analysis with the antibody directed against P-Tyr after immunoprecipitation with anti-PLD antibody. Since equal amounts of PLD1 and PLD2 were immunoprecipitated from all samples (Fig. 1B), it appears that PLD2 is notably more strongly tyrosine phosphorylated than PLD1. Western blot analysis showed that the 60-kDa tyrosine-phosphorylated protein was c-Src.
The c-Src mutant K295M is kinase inactive due to disrupted phosphotransfer activity (9). Cotransfection of the kinase-inactive mutant c-Src with PLDs did not lead to tyrosine phosphorylation of PLD1 and PLD2, whereas wild-type c-Src did (Fig. 1C). These results indicate that both PLD1 and PLD2 are tyrosine phosphorylated by the expression of c-Src tyrosine kinase and that PLD2 is its preferred substrate.
PLD1 and PLD2 bind to wild-type c-Src but not to the kinase-inactive c-Src mutant.
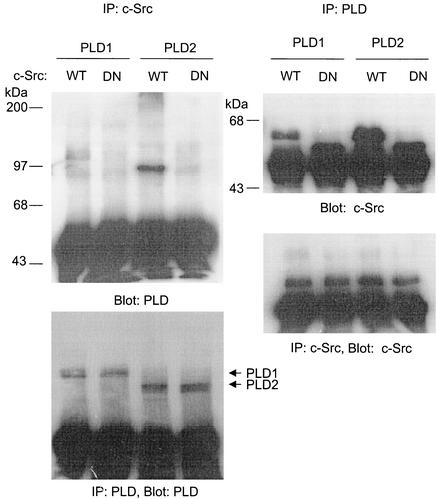
Next, we examined whether tyrosine phosphorylation of PLD occurs through its direct interaction with c-Src having kinase activity. Cells were cotransfected with PLD1 or PLD2 plus wild-type or mutant c-Src. Coimmunoprecipitation assays indicated that both PLD1 and PLD2 associated with wild-type c-Src, but not with the kinase-inactive mutant (Fig. 2). PLD2, which was more strongly tyrosine phosphorylated by c-Src than PLD1, revealed a stronger interaction with c-Src. These results demonstrate that the extent of phosphorylation in PLD isozymes by c-Src kinase is closely correlated with the binding affinity between PLD isozymes and c-Src protein.
FIG. 2.
PLD isozymes, most notably PLD2, bind to wild-type c-Src but not to the kinase-inactive c-Src mutant. COS-7 cells were transiently transfected for 40 h with various combinations of plasmids encoding PLD1, PLD2, wild-type (WT) c-Src, and dominant negative (DN) c-Src. Cell lysates were immunoprecipitated with anti-PLD or anti-c-Src antibodies, and immune complexes were subjected to immunoblotting analysis using anti-c-Src or anti-PLD antibodies, respectively. Expressions of PLDs and c-Src were determined by immunoprecipitation and immunoblotting. Data are representative of three experiments.
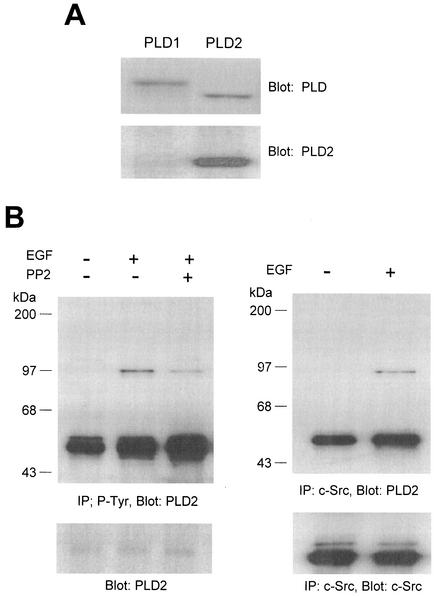
EGF induces tyrosine phosphorylation of PLD2 and its association with c-Src in A431 cells.
Next, we investigated whether the interaction between Src and PLD2 could be induced by extracellular stimuli without the enforced overexpression of these proteins. We used A431 cells which express PLD2 isozyme and EGF receptors as a model system (35). For this study we generated mouse monoclonal antibody specific for PLD2 but not PLD1, and this PLD2 antibody was used for immunoblotting due to its inability to immunoprecipitate PLD protein (Fig. 3A). Serum-starved cultures of the cells were exposed to EGF in the presence or absence of a selective inhibitor of Src family tyrosine kinase, PP2, and cell lysates were prepared and subjected to immunoprecipitation with antibody against either P-Tyr or c-Src. Tyrosine phosphorylation of PLD2 isozyme and its association with c-Src were monitored by successive immunoblotting for mouse monoclonal anti-PLD2 antibody. As shown in Fig. 3B, EGF induced tyrosine phosphorylation of PLD2 and pretreatment of c-Src kinase inhibitor reduced EGF-induced tyrosine phosphorylation of PLD2. PLD2 was associated with c-Src in response to EGF (Fig. 3B), suggesting that interaction of PLD2 and c-Src is regulated by a physiological ligand such as EGF.
FIG. 3.
EGF induces tyrosine phosphorylation of PLD2 and its association with c-Src in A431 cells. (A) Mouse monoclonal antibody specific for PLD2 was generated as described in Materials and Methods. To analyze its specificity, PLD1 or PLD2 was transfected into COS-7 cells and the lysates were immunoblotted with anti-PLD antibody which recognizes both PLD1 and PLD2 or anti-PLD2 antibody. (B) Quiescent A431 cells were pretreated with or without a selective inhibitor of Src family tyrosine kinase, PP2 (2 μM), for 30 min and stimulated with 200 ng of EGF/ml for 20 min. Cell lysates were prepared, and 1 mg of the lysates were immunoprecipitated with anti-P-Tyr antibody or anti-c-Src antibody. Immunoprecipitated proteins were separated on SDS-PAGE gel and probed with mouse monoclonal antibody specific for PLD2. The result shown is representative of three experiments.
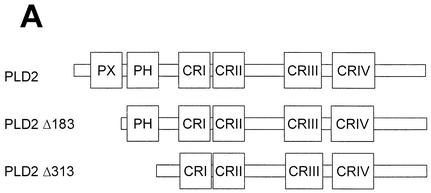
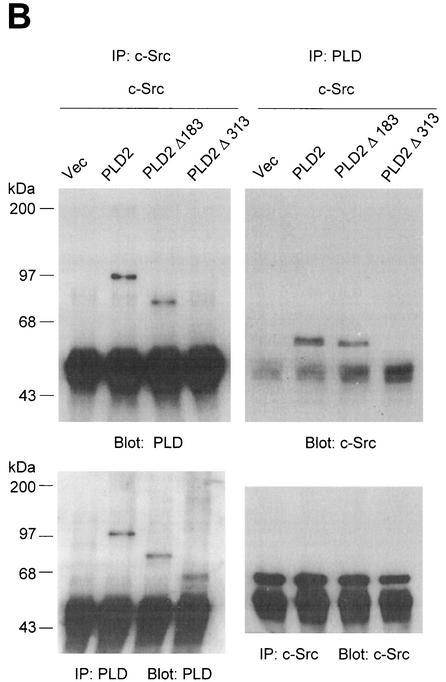
The N-terminal PH domain of PLD2 is required for its interaction with c-Src.
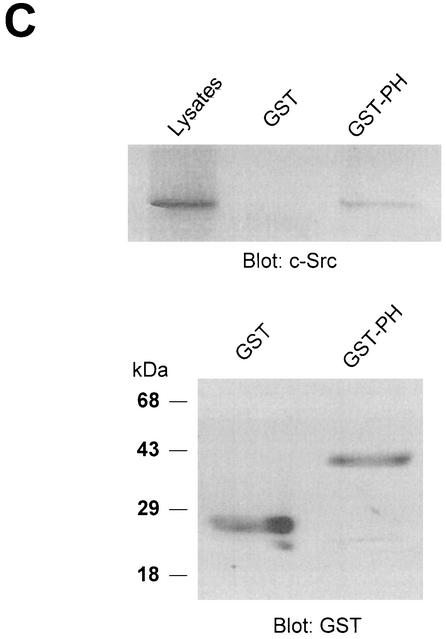
PLD2 contains a number of functional domains, including conserved catalytic regions (I, II, III, and IV), a PIP2-binding site, a pleckstrin homology (PH) domain, and an N-terminal phox (PX) domain (Fig. 4A) that has been proposed to mediate a wide variety of protein-protein interactions as well as binding of phospholipids (15, 18, 56). To determine the domain(s) of PLD2 required for c-Src interaction, cells were cotransfected with an N-terminally deleted mutant construct of PLD2, namely Δ183(184-933) or Δ313(314-933) plus c-Src. Using an antibody to PLD that recognizes the C-terminal region of PLD, we found that truncation of the N-terminal 313 amino acids from PLD2 (which removes both the PX domain and the PH domain) resulted in the loss of c-Src association, while truncation of the N-terminal 183 amino acids (which removes the PX domain) did not affect its association (Fig. 4B). These data indicate that the PH domain of PLD2 is critical for the interaction with c-Src. Moreover, using a GST pull-down assay, we confirmed that the GST PH domain interacted with c-Src (Fig. 4C).
FIG. 4.
The N-terminal PH domain of PLD2 is required for interaction with c-Src. (A) Schematic representation of PLD2. The boxes indicate highly conserved sequences of PLD; their possible functions have been proposed or demonstrated in reference 52. CR, conserved region. (B) COS-7 cells were transiently cotransfected with plasmids encoding the empty vector (Vec) and c-Src, or PLD2 and c-Src, or N-terminal 183-amino-acid-truncated PLD2 Δ183(184-933) and c-Src, or N-terminal 313-amino-acid-truncated PLD2 Δ313(314-933) and c-Src. Cell lysates were subjected to immunoprecipitation by using anti-PLD or anti-c-Src antibodies, and the amount of coimmunoprecipitated c-Src or PLD was determined by immunoblotting with anti-c-Src or anti-PLD antibodies. Expression of PLD2 and c-Src was determined by using anti-PLD or anti-c-Src antibodies. (C) For the GST pull-down assay, GST-PH fusion protein was used as described in reference 29. The lysates transfected with c-Src were incubated with 2 μg of GST or GST-PH fragment and immunoblotted with antibody to c-Src. The amount of the GST fusion proteins was visualized by immunoblotting with an anti-GST antibody. Data are representative of three experiments.
Association of PLD2 with the kinase domain of c-Src.
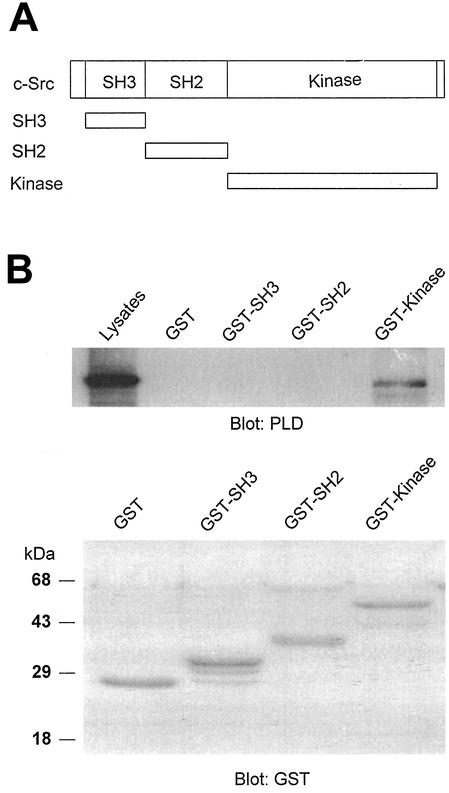
Having identified the PLD2 region required for association with c-Src, we next sought to identify the c-Src region involved in binding to PLD2. GST fusion proteins that contained the specific domains of c-Src, namely, GST-SH3, GST-SH2, and GST-kinase, were prepared (Fig. 5A). c-Src and PLD2 were coexpressed in COS-7 cells, cell lysates were prepared, incubated with fusion proteins, and subjected to a GST pull-down assay, and proteins were analyzed by immunoblotting with an anti-PLD antibody. We found that the catalytic domain, but not SH3 or SH2, was required for binding to PLD2 (Fig. 5B).
FIG. 5.
The catalytic domain of c-Src interacts with PLD2. (A) Schematic representation of GST-fusion protein constructs. (B) c-Src was fragmented into SH3 (88-137), SH2 (144-245), and kinase (265-523) domains. The fragments were cloned as GST fusion proteins, expressed in Escherichia coli, and purified by use of glutathione-conjugated Sepharose beads. An equal amount (2 μg) of GST or GST-Src fragment was incubated with the lysates of COS-7 cells transfected with PLD2 and c-Src. The pull-down proteins were subjected to immunoblot analysis with an antibody against PLD (upper panel). The amount of the GST fusion proteins was visualized by immunoblotting with an anti-GST antibody (lower panel). Data are representative of three experiments.
Modulation between PLD isozymes and c-Src significantly enhances cellular proliferation.
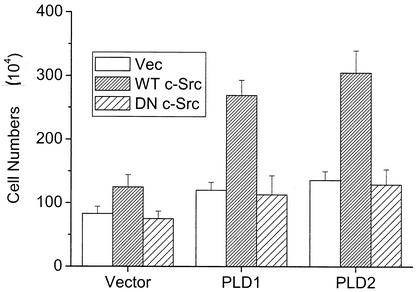
Next, we investigated the roles of PLD and c-Src in the cellular growth. Mouse fibroblasts overexpressing PLD1 or PLD2 (36) were plated at a density of 5 × 104 cells in 12-well plates and transfected with control vector or wild-type or a kinase-inactive mutant c-Src. After incubation for 40 h, the live cells were counted by using the trypan blue exclusion assay. As we reported recently (36), overexpression of PLD in the fibroblast increased growth rates compared to those of vector-transfected cells. Cells transfected with wild-type c-Src displayed increased cell proliferation compared to cells transfected with the control vector or the kinase-inactive mutant (Fig. 6). Coexpression of c-Src and PLD1 or 2 synergistically increased the cellular proliferation over that observed with expression of either molecule. Cells proliferated even at high density, suggesting that these cells overcame density arrest. These results based on the number of cells were similar to those obtained from the proliferation assay based on metabolic conversion of a tetrazolium compound, MTS (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxylmethoxylphenyl]-2-[4-sulfophenyl]-2H-tetrazolium), to a colored product by living cells (data not shown). These results demonstrate that modulation between PLD2 and c-Src might contribute to enhanced cellular proliferation.
FIG. 6.
Modulation between PLD2 and c-Src significantly enhances cellular proliferation. Mouse fibroblasts overexpressing PLD isozymes were plated at a density of 5 × 104 cells in 12-well plates and transfected with empty vector (Vec), wild-type (WT) c-Src, or dominant negative (DN) c-Src mutant. After 40 h of incubation, viable cells were counted by a trypan blue exclusion method. Results show means ± standard deviations of three independent experiments.
Tyrosine phosphorylation of PLD by c-Src does not affect its phospholipase activity.
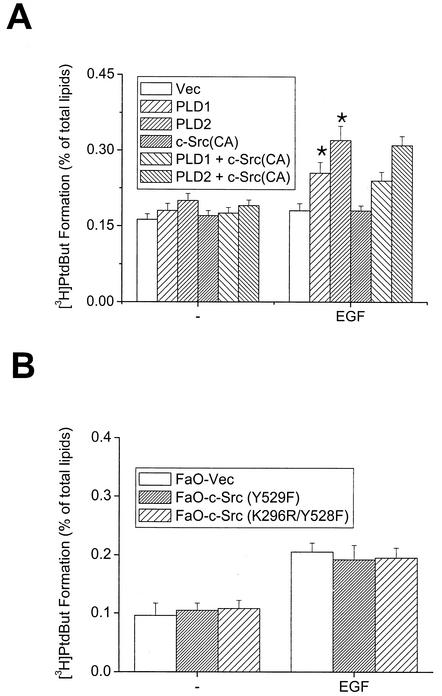
Next, we attempted to examine the underlying mechanisms of the modulation between PLD and c-Src, possibly leading to enhancement of cellular proliferation. We first investigated whether PLD phosphorylation by c-Src has any effect on its phospholipase activity. Constitutively active c-Src mutant was coexpressed with PLD1 or 2 in COS-7 cells, and in vivo PLD activity was measured following EGF stimulation. The results of these experiments showed that EGF-induced PLD activity in cells cotransfected with PLD1 or 2 plus constitutively active c-Src was similar to that in cells cotransfected with PLDs plus control vector (Fig. 7A). Transient expression of the dominant negative mutant c-Src also did not affect PLD activity (data not shown). Similar data were obtained by using FaO hepatoma cells stably overexpressing the constitutively active mutant c-Src, in which EGF-induced PLD activity was not different from that in cells expressing empty vector or inactive c-Src (Fig. 7B). These results suggest that tyrosine phosphorylation of PLD by c-Src does not increase its phospholipase activity. Therefore, other regulatory mechanism(s) contributing to the promotion of cellular proliferation by interaction between PLD and c-Src may exist.
FIG. 7.
c-Src does not affect EGF-induced PLD activation in COS-7 and FaO cells. COS-7 cells transiently transfected with various constructs (A) and FaO cells expressing constitutive active c-Src (Y529F) or dominant inactive c-Src mutant (K296R/Y528F) (B) were labeled with [3H]myristic acid for 18 h. The cells were untreated or treated with 200 ng of EGF/ml for 30 min in the presence of 0.3% 1-butanol. Radioactivity incorporated into PtdBut was measured as described in Materials and Methods. *, P < 0.05 compared to cells transfected with vector and treated with EGF. Results show means ± standard deviations of three independent experiments.
Stimulation of c-Src kinase activity by PLD.
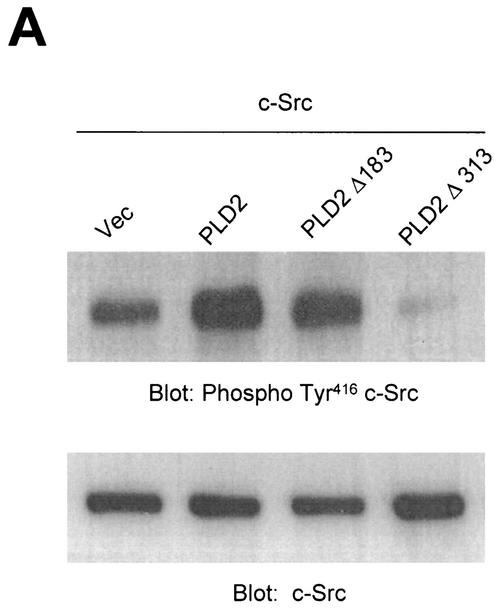
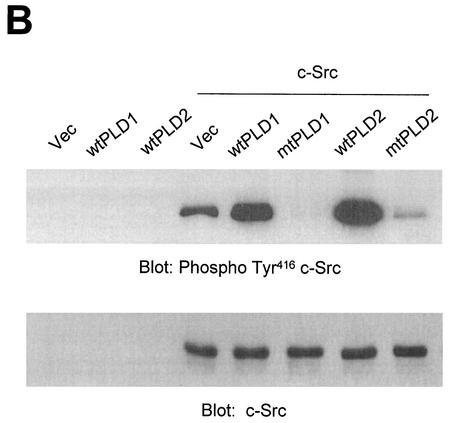
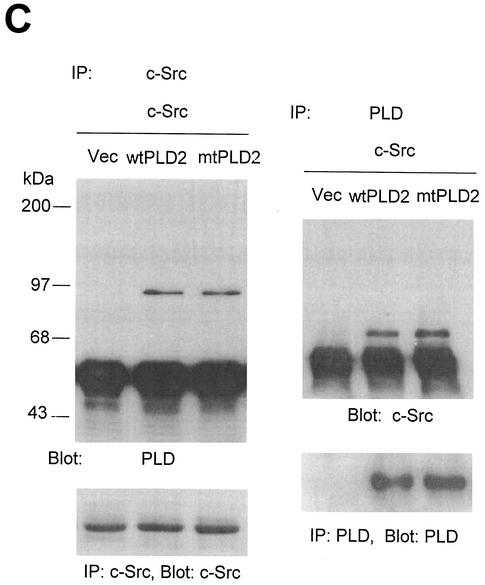
Next, we investigated whether PLD might lead to an increase in Src kinase activity. It has become clear that autophosphorylation of Tyr416 in the activation loop is critical for full activation of Src-family tyrosine kinases (26, 53, 57). First, COS-7 cells were transiently cotransfected with c-Src and various PLD2 constructs, and the lysates were assayed for c-Src activity by immunoblotting with an antibody specific for c-Src autophosphorylated at Tyr416 (anti-phospho-Y416 c-Src [37]). The data presented in Fig. 9A show that cells coexpressing wild-type PLD2 and c-Src contained high levels of autophosphorylated kinase. Truncation of the N-terminal region containing the PX and PH domains of PLD2 (which eliminates 85% of the basal activity) resulted in decreased c-Src activation, whereas truncation of the N-terminal 183 amino acids from PLD2 (which removes the PX domains but still yields a catalytically active enzymes) induced an activation of c-Src similar to that in cells transfected with wild-type PLD2 and c-Src (Fig. 8A). Since the N-terminal 313-amino-acid-truncated PLD2 mutant that does not bind to c-Src (PLD2 Δ313(314-933) failed to activate c-Src (Fig. 4B), we further examined whether PLD catalytic activity or the binding of PLD isozyme with c-Src is more critical for c-Src activation by PLD isozyme. Vector or PLD alone did not induce autophosphorylation of Tyr416 in transfected cells. Interestingly, not only wild-type PLD2 but also wild-type PLD1 significantly stimulated c-Src kinase activity compared to cells transfected with c-Src alone. In contrast, both catalytically inactive mutants of PLD1 and PLD2 reduced c-Src kinase activity (Fig. 8B). As shown in Fig. 2, PLD1 has a much lower binding affinity with c-Src than does PLD2. Furthermore, a catalytically inactive PLD2 mutant also interacted with c-Src to the same extent as wild-type PLD2 (Fig. 8C). These results demonstrate that the catalytic activity of PLD isozyme rather than its binding with c-Src is required for c-Src activation by PLD.
FIG. 9.
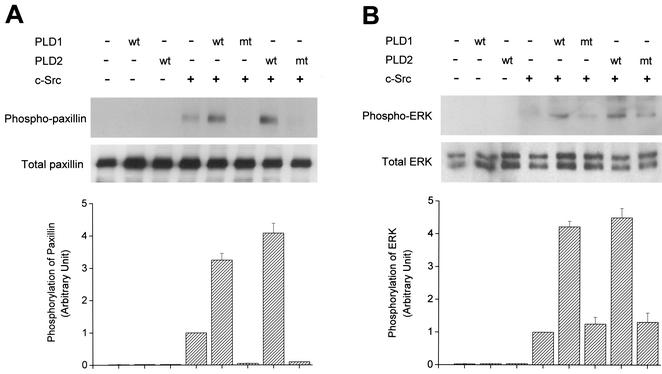
PLD enhances not only c-Src-induced tyrosine phosphorylation of paxillin but also c-Src-mediated ERK activation. COS-7 cells were transiently cotransfected for 40 h with various combinations of plasmids encoding an empty vector, PLD1, PLD2, c-Src, and c-Src plus either wild-type (wt) or catalytically inactive mutant (mt) PLD1 or PLD2. Cell lysates were immunoblotted with an antibody directed against tyrosine-phosphorylated paxillin (A) or an anti-phospho-ERK antibody (B). Expression of total paxillin and ERK was visualized by immunoblotting with antipaxillin antibody or anti-ERK antibody. Data are representative of three experiments. The intensity of phosphorylated paxillin or phospho-ERK immunoreactive bands quantified by densitometry of immunoblot was expressed as relative intensity of the bands. Results show means ± standard deviations of three independent experiments.
FIG. 8.
Overexpression of PLD stimulates c-Src kinase activity. (A) COS-7 cells were transiently cotransfected with various combinations of plasmids encoding empty vector (Vec) and c-Src, or PLD2 and c-Src, or N-terminal 183-amino-acid-truncated PLD2 Δ183(184-933) and c-Src, or N-terminal 313-amino-acid-truncated PLD2 Δ313(314-933) and c-Src. Cell lysates were subjected to immunoblot analysis using a phospho Tyr416 c-Src antibody. Expression of c-Src was determined by using an anti-c-Src antibody. (B) COS-7 cells were transiently transfected for 40 h with various combinations of plasmids encoding an empty vector, PLD1, PLD2, c-Src, and c-Src plus either wild-type (wt) or catalytically inactive mutant (mt) PLD1 or PLD2. Data are representative of three experiments. (C) COS-7 cells were transiently cotransfected with a catalytically inactive PLD2 mutant and wild-type c-Src, and cell lysates were subjected to immunoprecipitation with anti-c-Src antibody or anti-PLD antibodies. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-PLD or anti-c-Src antibody. Expression of PLD and c-Src was determined by using anti-PLD or anti-c-Src antibodies. Data are representative of three experiments.
Not only c-Src-induced tyrosine phosphorylation of paxillin but also ERK activation is potentiated by expression of PLD isozymes.
c-Src has been reported to phosphorylate paxillin in integrin signaling or various mitogenic signaling pathways (58). Therefore, we investigated whether PLD affects c-Src-induced phosphorylation of paxillin. Transient cotransfection of c-Src and PLD into COS-7 cells showed that overexpression of wild-type PLDs, but not catalytically inactive mutant PLDs, increased tyrosine phosphorylation of paxillin compared to cells expressing c-Src and empty vector (Fig. 9). Tyrosine phosphorylation of paxillin was not detected in cells transfected with vector or PLD only. This result suggests that PLD with a catalytic activity might be involved in the potentiation of tyrosine phosphorylation of paxillin, possibly through activation of c-Src. In addition, it has been reported that overexpression or activation of c-Src is involved in the activation of the Ras/ERK pathway in COS-7 cells (29). Using phospho-specific antibodies that bind only activated forms of ERK, we examined whether PLD enhanced c-Src-stimulated ERK activity. Transient expression of wild-type PLD1 or PLD2 plus c-Src resulted in increased ERK activity compared to that observed with cells transfected with c-Src and empty vector or catalytically inactive PLD mutant plus c-Src (Fig. 9). Activation of ERK was not detected in cells transfected with vector or PLD only. Taken together, these results suggest that activation of the Src signaling cascade is also dependent upon PLD activity.
DISCUSSION
It has recently become apparent that PLD activity plays a significant role in transduction of intracellular signals (11, 27, 51). Since tyrosine kinases have been shown to form networks with a diverse array of cellular signal transduction pathways, a PLD-tyrosine kinase link provides novel mechanisms for PLD signaling to modulate a broad range of physiological responses. Therefore, it is crucial to understand how these two signaling components communicate with each other.
Several studies indicate that PTKs participate in the PLD activation via an unidentified mechanism (38). However, the biological significance of phosphorylation as one of the activation mechanisms of PLD still remains to be determined, since PLD activity has not been correlated with its phosphorylation level in many studies (18, 32). In addition, the nature of the tyrosine kinase has not yet been defined. Recent studies demonstrated that PLD1 and PLD2 were phosphorylated in cell-free preparations by p38MAP kinase (39) but such phosphorylation did not enhance PLD activity. It was suggested that an ERK-dependent tyrosine kinase might mediate the effect of norepinephrine on tyrosine phosphorylation of PLD2 (41). Although PLD is phosphorylated on serine and threonine residues in intact cells in response to phorbol myristate acetate (25) or on tyrosine residues by pervanadate (31, 33), it is still unclear whether this phosphorylation of PLD is of physiological relevance to enhanced hydrolysis of phosphatidylcholine or other phospholipid substrates.
Although Src tyrosine kinase has been shown to play a role in PLD-mediated signaling (23, 43), the link between c-Src and PLD was not clear. In this study, we demonstrate for the first time that Src family protein kinases induced tyrosine phosphorylation of PLD isozymes, most notably PLD2, by transient transfection. Moreover, EGF induced tyrosine phosphorylation of PLD2 in A431 cells. However, EGF-induced PLD activity was not altered by coexpression of c-Src in COS-7 cells. In addition, EGF-induced PLD activity in hepatoma cells overexpressing constitutive active mutant c-Src was not different from the activity shown in cells transfected with empty vector or dominant inactive c-Src. These results suggest that tyrosine phosphorylation of PLD by c-Src does not increase PLD phospholipase activity. This correlates with a previous report that overexpression of active c-Src or kinase-negative mutant c-Src did not affect PLD activity in rat mesangial cells (44) but is in contrast to observations with fibroblasts transformed by the viral counterpart and oncogenic PTK v-Src (20). A comparison of the effects of pp60c-src and pp60v-src would suggest that only the oncogenic tyrosine kinase is able to interfere with the Ras pathway. The significance of PLD activation by v-src tyrosine kinase is unknown. Moreover, the molecular basis by which PLD activity is differentially regulated by v-Src and c-Src remains to be clarified. In some pathways, the enzymatic activity of PLD as a lipid hydrolytic enzyme might not be strictly required for PLD function, pointing to the multifaceted nature of the PLD protein. Consistent with this hypothesis, it was reported that the mitogenic activity of phospholipase C-γ1, which is known to play an important role in cell proliferation, does not exclusively result from the enzymatic activity of the lipase and that another activity inherent to the phospholipase C-γ1 molecule can also induce DNA synthesis in quiescent cells (50). Although it is possible that nonphospholipase activities of PLD through tyrosine phosphorylation play a role in PLD-mediated cellular signaling, at present it is still not clear how tyrosine phosphorylation of PLD contributes to PLD-mediated signal transduction in cells. Tyrosine phosphorylation of PLD may not be critical for proliferation because PLD1 and PLD2 show enhancement in a similar potency proliferation by coexpression of c-Src synergistically, even though the extent of tyrosine phosphorylation of PLD1 is very different from that of PLD2. We cannot exclude the possibility that tyrosine-phosphorylated residues in PLD2 by c-Src may provide the docking sites to recruit the proteins with the SH2 domain, leading to amplification of Src signaling cascades. The biological significance of PLD tyrosine phosphorylation remains to be determined in detail.
PLD associated with c-Src, and it was the catalytic domain, not the SH3 and SH2 domains, of c-Src that was required for binding to PLD2. This is reminiscent of the interaction of Gαs and Gαi with the catalytic domain of c-Src (30). c-Src binds to a region of PLD2 containing PH, which is known to be involved in protein-protein interactions as well as binding of phospholipids (56). Highly tyrosine-phosphorylated PLD2 was strongly associated with c-Src in cotransfection experiments. This was comparable to EGF-induced association of phosphorylated PLD2 with c-Src in A431 cells.
To understand PLD signaling and its biological functions, it is essential to identify the direct targets of PLD. The effectors of PLD might be proteins interacting with it or the signaling molecules that are activated by PA. Therefore, we investigated the possibility that PLD has a role not only as a substrate of c-Src but also as a regulator of c-Src. Coexpression of wild-type PLD1 or PLD2, but not catalytically inactive PLD mutants, along with c-Src, stimulated c-Src kinase activity. There seems to be little difference between PLD1 and PLD2 in c-Src activation, even though the extent of their interaction with c-Src is very different. Although catalytically inactive PLD mutant associated with c-Src, overexpression of the PLD mutant rather decreased c-Src activation. Therefore, c-Src activation via PLD appeared to be dependent upon the catalytic activity of PLD rather than interaction of PLD with c-Src. The present work identifies PLD as a regulator of Src family tyrosine kinases and provides substantial new insights for future investigations in the two fields of PLD function and Src regulators.
Until now, PLDs have not been studied for their ability to increase c-Src kinase activity. In the present study, we demonstrate for the first time that there is an intimate cross-regulation between PLD and c-Src. Critical downstream targets of the Src kinase remain controversial and probably depend on the particular signaling pathway that activated the kinase. Many proposed substrates of c-Src are derived from studies of proteins phosphorylated by the activated viral Src protein, such as phosphatidylinositol 3 kinase, Shc, phospholipase C-γ, paxillin, vinculin, and ERK (5, 29, 48). In the present study, we demonstrated that PLD-induced activation of c-Src increased tyrosine phosphorylation of paxillin and activation of ERK, downstream target molecules of Src. Activation of these molecules was also dependent on the catalytic activity of PLD. Our observations are consistent with the recent report demonstrating that EGF-induced ERK activation is dependent upon PLD activity in A431 cells (46). This may occur by stimulation of c-Src signaling via PLD activity-dependent mechanism. Therefore, our results suggested that PA generated by PLD activity might be involved in the c-Src/ERK pathway. A number of studies have demonstrated that PA induces tyrosine phosphorylation in neutrophils and other cell types (45, 47). While it is tempting to speculate that PA-dependent tyrosine phosphorylation may be involved in activation of c-Src and stimulation of the ERK, additional experiments are required to prove this hypothesis.
Both PLD and c-Src are overexpressed and highly activated in a wide variety of human cancers (19, 40, 54, 55, 59). A connection between activation of Src via PLD and cancer progression would be a significant finding. Consistent with such a connection, Joseph et al. (23) reported that elevated expression of either PLD1 or PLD2 transformed cells overexpressing c-Src. Therefore, it is reasonable to propose that these two proteins have coordinated functions.
The present studies significantly advance our understanding of the cross talk between PLDs and c-Src tyrosine kinases. In summary, our novel findings show that there is an interaction between Src and PLD protein leading to tyrosine phosphorylation of both PLD1 and PLD2 and that this interaction and PLD activity activate c-Src, leading to autophosphorylation, paxillin phosphorylation, and ERK activation. PLD is tyrosine phosphorylated as a novel substrate of c-Src and stimulates Src kinase signaling in a PLD activity-dependent mechanism, inducing cell proliferation (Fig. 10). These characteristics suggest that PLD lies at an interesting nexus of Src-dependent signaling. Further studies aimed at the elucidation of cell growth signal → c-Src → signaling event of PLD phosphorylation and PLD-stimulated c-Src downstream signaling events leading to the gene expression are necessary to gain a better understanding of the functional role of PLD in mediating signals from c-Src tyrosine kinase.
FIG. 10.
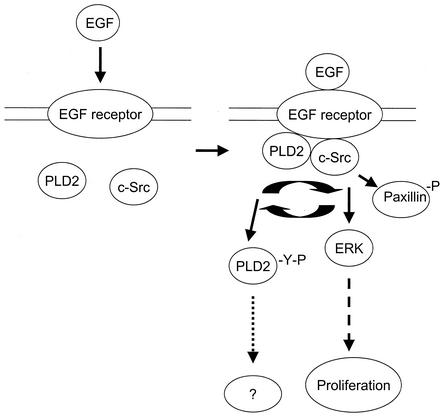
Proposed model of PLD-stimulated c-Src downstream signaling. EGF binds to its receptor, thereby stimulating both PLD and c-Src activation. It is likely that the resulting PLD activity is involved in the stimulation of Src-mediated signaling pathways such as ERK and then in the stimulation of cell proliferation. EGF induces tyrosine phosphorylation of PLD2 via c-Src and its interaction with c-Src. At present, it is not clear, however, how tyrosine phosphorylation of PLD is involved in PLD-mediated signaling mechanism.
Acknowledgments
We thank Sung Ho Ryu (POSTECH, Pohang, Korea) for providing cDNA encoding PLD constructs (wild-type and mutant GST-PH) and helpful comments in the preparation of the manuscript and Pamela L. Schwartzberg for providing cDNA encoding wild-type and mutant c-Src (K295M) expression vectors.
This work was supported by a Korea Research Foundation grant (KRF-2002-070-C00074).
REFERENCES
- 1.Ahn, B. H., H. Rhim, S. Y. Kim, Y. M. Sung, M. Y. Lee, J. Y. Choi, B. Wolozin, J. S. Chang, Y. H. Lee, T. K. Kwon, K. C. Chung, S. H. Yoon, S. J. Hahn, M. S. Kim, Y. H. Jo, and D. S. Min. 2002. α-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J. Biol. Chem. 277:12334-12442. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Av, P., and M. Liscovitch. 1989. Phospholipase D activation by the mitogens platelet-derived growth factor and 12-O-tetradecanoylphorbol 13-acetate in NIH-3T3 cells. FEBS Lett. 259:64-66. [DOI] [PubMed] [Google Scholar]
- 3.Bjorge, J. D., A. Jakymiw, and D. J. Fujita. 2000. Selected glimpses into the activation and function of Src kinase. Oncogene 19:5620-5635. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. T., and J. A. Cooper. 1996. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287:121-149. [DOI] [PubMed] [Google Scholar]
- 6.Colley, W. C., R. Sung, R. L. Jenco, S. M. Hammond, Y. Altshuller, D. Bar-Sagi, A. J. Morris, and M. A. Frohman. 1997. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7:191-201. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, J. A., and B. Howell. 1993. The when and how of Src regulation. Cell 73:1051-1054. [DOI] [PubMed] [Google Scholar]
- 8.David-Pfeuty, T., and Y. Nouvian-Dooghe. 1990. Immunolocalization of the cellular src protein in interphase and mitotic NIH c-src overexpresser cells. J. Cell Biol. 111:3097-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debnath, J., M. Chamorro, M. J. Czar, E. M. Schaeffer, M. J. Lenardo, H. E. Varmus, and P. L. Schwartzberg. 1999. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol. Cell. Biol. 19:1498-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunant, N., and K. Ballmer-Hofer. 1997. Signalling by Src family kinases: lessons learnt from DNA tumour viruses. Cell. Signal. 9:385-393. [DOI] [PubMed] [Google Scholar]
- 11.Exton, J. H. 1999. Regulation of phospholipase D. Biochim. Biophys. Acta 1439:121-133. [DOI] [PubMed] [Google Scholar]
- 12.Fantl, W. J., D. E. Johnson, and L. T. Williams. 1993. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 62:453-481. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, G. J., P. A. Henderson, J. J. Voorhees, and J. J. Baldassare. 1991. Epidermal growth factor-induced hydrolysis of phosphatidylcholine by phospholipase D and phospholipase C in human dermal fibroblasts. J. Cell. Physiol. 146:309-317. [DOI] [PubMed] [Google Scholar]
- 14.Frankel, P. A., M. Ramos, J. Flom, S. Bychenok, E. Kerkhoff, U. R. Rapp, L. A. Feig, and D. A. Foster. 1999. Ral and Rho dependent activation of phospholipase D in v-Raf transformed cells. Biochem. Biophys. Res. Commun. 255:502-507. [DOI] [PubMed] [Google Scholar]
- 15.Frohman, M. A., and A. J. Morris. 1999. Phospholipase D structure and regulation. Chem. Phys. Lipids 98:127-140. [DOI] [PubMed] [Google Scholar]
- 16.Hammond, S. M., J. M. Jenco, S. Nakashima, K. Cadwallader, Q.-M. Gu, S. Cook, Y. Nozawa, G. D. Prestwich, M. A. Frohman, and A. J. Morris. 1997. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J. Biol. Chem. 272:3860-3868. [DOI] [PubMed] [Google Scholar]
- 17.Hornia, A., Z. Lu, T. Sukezane, M. Zhong, T. Joseph, P. Frankel, and D. A. Foster. 1999. Antagonistic effects of protein kinase C α and δ on both transformation and phospholipase D activity mediated by the EGF receptor. Mol. Cell. Biol. 19:7672-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houle, M. G., and S. Bourgoin. 1999. Regulation of phospholipase D by phosphorylation-dependent mechanisms. Biochim. Biophys. Acta 1439:135-150. [DOI] [PubMed] [Google Scholar]
- 19.Irby, R. B., and T. J. Yeatman. 2000. Role of Src expression and activation in human cancer. Oncogene 19:5636-5642. [DOI] [PubMed] [Google Scholar]
- 20.Jiang, H., K. Alexandropoulos, J. Song, and D. A. Foster. 1994. Evidence that v-Src-induced phospholipase D activity is mediated by a G protein. Mol. Cell. Biol. 14:3676-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, H., Z. Lu, J. Q. Luo, A. Wolfman, and D. A. Foster. 1995. Ras mediates the activation of phospholipase D by v-Src. J. Biol. Chem. 270:6006-6009. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, H., Z. Lu, J. Q. Luo, T. Urano, P. Frankel, Z. Lu, D. A. Foster, and L. A. Feig. 1995. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature 378:409-412. [DOI] [PubMed] [Google Scholar]
- 23.Joseph, T., R. Wooden, A. Bryant, M. Zhong, Z. Lu, and D. A. Foster. 2001. Transformation of cells overexpressing a tyrosine kinase by phospholipase D1 and D2. Biochem. Biophys. Res. Commun. 289:1019-1024. [DOI] [PubMed] [Google Scholar]
- 24.Jove, R., and H. Hanafusa. 1987. Cell transformation by the viral src oncogene. Annu. Rev. Cell Biol. 3:31-56. [DOI] [PubMed] [Google Scholar]
- 25.Kim, Y., J. M. Han, B. R. Han, A. Lee, J. H. Kim, B. D. Lee, I. H. Jang, P. G. Suh, and S. H. Ryu. 2000. Phospholipase D1 is phosphorylated and activated by protein kinase C in caveolin-enriched microdomains within the plasma membrane. J. Biol. Chem. 275:13621-13627. [DOI] [PubMed] [Google Scholar]
- 26.Kmiecik, T. E., and D. Shalloway. 1987. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49:65-73. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S., J. W. Kim, C. S. Lee, J. H. Kim, Y. Kim, K. Heo, Y. Ihara, Y. Goshima, P.-G. Suh, and S. H. Ryu. 2002. Collapsin response mediator protein-2 inhibit neuronal phospholipase D2 activity by direct interaction. J. Biol. Chem. 277:6542-6549. [DOI] [PubMed] [Google Scholar]
- 28.Lu, Z., A. Hornia, T. Joseph, T. Sukezane, P. Frankel, M. Zhong, S. Bychenok, L. Xu, L. A. Feig, and D. A. Foster. 2000. Phospholipase D and RalA cooperate with the epidermal growth factor receptor to transform 3Y1 rat fibroblasts. Mol. Cell. Biol. 20:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luttrell, L. M., B. E. Hawes, T. V. Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 30.Ma, Y.-C., J. Huang, S. Ali, W. Lowry, and X.-Y. Huang. 2000. Src tyrosine kinase is a novel direct effector of G proteins. Cell 102:635-646. [DOI] [PubMed] [Google Scholar]
- 31.Marcil, J., D. Harbour, P. H. Naccache, and S. Bourgoin. 1997. Phospholipase D1 can be tyrosine-phosphorylated in HL-60 granulocytes. J. Biol. Chem. 272:20660-20664. [DOI] [PubMed] [Google Scholar]
- 32.Marcil, J., D. Harbour, M. G. Houle, P. H. Naccache, and S. Bourgoin. 1999. Monosodium urate-crystal-stimulated phospholipase D in human neutrophils. Biochem. J. 337:185-192. [PMC free article] [PubMed] [Google Scholar]
- 33.Min, D. S., E.-G. Kim, and J. H. Exton. 1998. Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J. Biol. Chem. 273:29986-29994. [DOI] [PubMed] [Google Scholar]
- 34.Min, D. S., B.-H. Ahn, D.-J. Rhie, S.-H. Yoon,. S. J. Hahn, M.-S. Kim, and Y. H. Jo. 2001. Expression and regulation of phospholipase D during neuronal differentiation of PC12 cells. Neuropharmacology 41:384-391. [DOI] [PubMed] [Google Scholar]
- 35.Min, D. S., B.-H. Ahn, and Y. H. Jo. 2001. Differential tyrosine phosphorylation of phospholipase D isozymes by hydrogen peroxide and the epidermal growth factor in A431 epidermoid carcinoma cells. Mol. Cells 11:369-378. [PubMed] [Google Scholar]
- 36.Min, D. S., T. K. Kwon, W.-S. Park, J.-S. Chang, S.-K. Park, B.-H. Ahn, Z.-H. Ryu, Y. H. Lee, Y. S. Lee, D.-J. Rhie, S.-H. Yoon, S. J. Hahn, M.-S. Kim, and Y.-H. Jo,. 2001. Neoplastic transformation and tumorigenesis associated with overexpression of phospholipase D isozymes in cultured murine fibroblasts. Carcinogenesis 22:1641-1647. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay, D., L. Tsiokas, X. M. Zhou, D. Foster, J. S. Brugge, and V. P. Sukhatme. 1995. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375:577-581. [DOI] [PubMed] [Google Scholar]
- 38.Natarajan, V., W. M. Scribner, and S. Vepa. 1996. Regulation of phospholipase D by tyrosine kinases. Chem. Phys. Lipids 80:103-116. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan, V., W. M. Scribner, A. J. Morris, S. Roys, S. Vepa, J. Yang, R. Wadgaonkar, S. P. M. Reddy, J. G. Garcia, and N. L. Parinandi. 2001. Role of p38 MAP kinase in diperoxovanadate-induced phospholipase D activation in endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 281:L435-L449. [DOI] [PubMed] [Google Scholar]
- 40.Noh, D., S. Ahn, R. Lee, I. Park, J. Kim, P. G. Suh, S. H. Ryu, K. Lee, and J. Han. 2000. Overexpression of phospholipase D1 in human breast cancer tissues. Cancer Lett. 161:207-214. [DOI] [PubMed] [Google Scholar]
- 41.Parmentier, J.-H., M. M. Muthalif, A. E. Saeed, and K. U. Malik. 2001. Phospholipase D activation by norepinephrine is mediated by 12(s)-, 15(s)-, and 20-hydroxyeicosatetraenoic acids generated by stimulation of cytosolic phospholipase a2. tyrosine phosphorylation of phospholipase D2 in response to norepinephrine. J. Biol. Chem. 276:15704-15711. [DOI] [PubMed] [Google Scholar]
- 42.Parsons, J. T., and M. J. Weber. 1989. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr. Top. Microbiol. Immunol. 147:79-127. [DOI] [PubMed] [Google Scholar]
- 43.Reiser, C. O. A., and M. Goppelt-Struebe. 1996. Modulation of phospholipase D stimulation in c-Src transfected mesangial cells. J. Lipid Mediat. Cell Signal. 15:193-202. [DOI] [PubMed] [Google Scholar]
- 44.Rizzo, M. A., K. Shome, C. Vasudevant, D. B. Stolz, T.-C. Sung, M. A. Frohman, S. C. Watkins, and G. Romero. 1999. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274:1131-1139. [DOI] [PubMed] [Google Scholar]
- 45.Sergeant, S., K. A. Waite, J. Heravi, and L. C. McPhail. 2001. Phosphatidic acid regulates tyrosine phosphorylating activity in human neutrophils: enhancement of Fgr activity. J. Biol. Chem. 76:4737-4746. [DOI] [PubMed] [Google Scholar]
- 46.Shen, Y., L. Xu, and D. A. Foster. 2001. Role of phospholipase D in receptor-mediated endocytosis. Mol. Cell. Biol. 21:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui, R. A., and D. English. 1997. Phosphatidic acid elicits calcium mobilization and actin polymerization through a tyrosine kinase-dependent process in human neutrophils: a mechanism for induction of chemotaxis. Biochim. Biophys. Acta 1349:81-95. [DOI] [PubMed] [Google Scholar]
- 48.Simeonova, P. P., S. Wang, T. Hulderman, and M. I. Luster. 2002. c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic. Role in carcinogenesis. J. Biol. Chem. 277:2945-2950. [DOI] [PubMed] [Google Scholar]
- 49.Slaaby, R., T. Jensen, H. S. Hansen, M. A. Frohman, and K. Seedorf. 1998. PLD2 complexes with the EGF receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J. Biol. Chem. 273:33722-33727. [DOI] [PubMed] [Google Scholar]
- 50.Smith, M. R., Y.-L. Liu, N. T. Matthews, S. G. Rhee, W. K. Sung, and H.-F. Kung. 1994. Phospholipase C-γ1 can induce DNA synthesis by a mechanism independent of its lipase activity. Proc. Natl. Acad. Sci. USA 91:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stted, P. M., and A. H. M. Chow. 2001. Intracellular signaling by phospholipase D as a therapeutic target. Curr. Pharm. Biotechnol. 2:241-256. [DOI] [PubMed] [Google Scholar]
- 52.Sung, T. C., Y. M. Altshuller, A. J. Morris, and M. A. Frohman. 1999. Molecular analysis of mammalian phospholipase D2. J. Biol. Chem. 274:494-502. [DOI] [PubMed] [Google Scholar]
- 53.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 54.Uchida, N., S. Okamura, and Y. Nagamachi. 1997. Increased phospholipase D activity in human breast cancer. J. Cancer Res. Clin. Oncol. 123:280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchida, N., S. Okamura, and H. Kuwano. 1999. Phospholipase D activity in human gastric carcinoma. Anticancer Res. 19:671-676. [PubMed] [Google Scholar]
- 56.Xu, Y., L.-F. Seet, B. Hanson, and W. Hong. 2001. The phox homology (PX) domain, a new player in phosphoinositide signaling. Biochem. J. 360:513-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu, W., A. Doshi, M. Lei, M. J. Eck, and S. C. Harrison. 1999. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell 3:629-638. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Z., R. Baron, and W. C. Horne. 2000. Integrin engagement, the actin cytoskeleton, and c-Src are required for the calcitonin-induced tyrosine phosphorylation of paxillin and HEF1, but not for calcitonin-induced Erk1/2 phosphorylation. J. Biol. Chem. 275:37219-37223. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, Y., H. Ehara, Y. Akao, M. Shamoto, Y. Nakagawa, Y. Banno, T. Deguchi, N. Ohishi, K. Yagi, and Y. Nozawa. 2000. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem. Biophys. Res. Commun. 278:140-143. [DOI] [PubMed] [Google Scholar]