Abstract
Mxi2 is a p38α splice isoform that is distinctively activated by mitogenic stimuli. Here we show that Mxi2 immunoprecipitates carry a kinase activity that is persistently activated by epidermal growth factor in a fashion regulated by Ras, Raf, and MEK. We demonstrate that this kinase activity can be attributed not to Mxi2 but rather to extracellular signal-regulated kinases 1 and 2 (ERK1/2), which coimmunoprecipitated with Mxi2 both by ectopic expression and in a physiological environment like the kidney. Furthermore, we provide evidence that Mxi2-ERK interaction has profound effects on ERK function, demonstrating that Mxi2 prolongs the duration of the ERK signal by sustaining its phosphorylation levels. Interestingly, we show that the effects of Mxi2 on ERK are restricted to nuclear events. Mxi2 potently up-regulates ERK-mediated activation of the transcription factors Elk1 and HIF1α but has no effect on the activity of ERK cytoplasmic substrates RSK2 and cPLA2, induced by epidermal growth factor or by MEK. Overall, our findings point to Mxi2 as a unique member of the p38 family that may have an unprecedented role in the regulation of the functions of ERK mitogen-activated protein kinases.
Mitogen-activated protein kinases (MAPKs) are cytoplasmic serine/threonine kinases that are activated in response to a wide array of extracellular stimuli, including those that regulate cell proliferation, differentiation, development, and inflammation. MAPKs are pivotal elements in the transduction of signals from the cell surface, acting as essential mediators in signaling cascades that include, sequentially, MAP kinase kinase kinases, dual-specificity MAP kinase kinases (MAPKKs), and MAPKs. To date, four mammalian MAPKs have been studied in detail: extracellular signal-regulated kinases (ERKs), stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs), p38 MAPKs, and ERK5/BMKs. The ERKs and BMKs are mainly activated by growth factors. By contrast, JNKs and p38 are preferentially induced by stress-inducing agents and proinflammatory cytokines, although activation of various MAPK pathways by the same stimuli often occurs (32, 45).
p38α/RK/CSBP2 MAPK was identified by virtue of its homology to the yeast HOG MAPK (14, 17, 22, 38). p38 is a substrate for the MAPKKs MKK3 and MKK6 (8, 34) and, to a lesser extent, for MKK4 (8, 23). Likewise, p38 is activated by an ever-growing number of upstream MAP kinase kinase kinase kinases and MAP kinase kinase kinases (32). Also, in a fashion similar to that of SAPK/JNKs, the cascade that leads to p38 activation is regulated by Rho family GTPases, unlike ERKs, whose activation is mainly regulated by Ras (36),
A feature common to all MAPKs is the existence of various isoforms in each family. In the case of the p38 family, in addition to p38α/RK/CSBP2, isoforms p38β, p38γ/ERK6/SAPK3, p38δ, and SAPK4 have been described. Also, splicing variants of p38α and p38β, namely, CSBP1, Mxi2, p38-2, and p38β2, have been reported (32). Mxi2 was isolated in a two-hybrid screen for proteins that bind c-Myc partner protein Max. Mxi2 is identical to p38 from amino acid 1 to amino acid 280 and harbors a unique 17-amino-acid C terminus. Its distribution in human tissues resembles that of p38 (49), being most abundant in renal tissues (11). In addition to its ability to bind and phosphorylate Max (49), Mxi2 also interacts with Omi (12), a serine protease with extensive homology to the endoprotease HtrA (18). Omi/HtrA is regulated by kidney ischemia (12) and induces cell death through its interaction with XIAP (41). Other than this, little is known about Mxi2 regulation and functions that could help explain the role of a protein otherwise almost identical to p38. We have recently reported that some Mxi2 biochemical properties make it unique. Thus, Mxi2 is markedly stimulated by mitogens, it is insensible to inhibition by pyridinyl imidazoles, and it is largely unaffected by dual-specificity protein phosphatases. Moreover, Mxi2 phosphotransfer activity on bona fide p38 substrates is remarkably low (39). This divergent behavior may indicate that Mxi2 is involved in as yet unknown regulatory mechanisms that are distinct from those of p38.
In studying the regulation of Mxi2 by mitogenic stimuli, we have found that Mxi2 directly interacts with ERK1/2 and that this interaction has profound effects on the functions of ERKs that take place in the nucleus but not on those that occur in the cytoplasm. Taken together, our results point to Mxi2 as a p38 family member that may play an unprecedented role in the control of the functions of the ERK family MAPKs. In this respect and in combination with the recently reported interaction between p38α and ERKs (50), we identify a direct interaction between isoforms of the p38 and ERK families as a novel mechanism of transregulation between different MAPK signaling modules.
MATERIALS AND METHODS
Materials.
We have previously described the expression vectors encoding Ras-, Rac-, Rho-, and Cdc42-activated (QL) and dominant inhibitory (N17) mutant forms MEK1 A and -E (5), Raf 301 (43), Raf BXB (7), hemagglutinin (HA)-ERK1 and -2, and ERK2 K52R (1). MKK3, MKK4, and MKK6 inhibitory and activated mutant forms (48) were provided by J. L. Woodgett. MEK1 and -2DD (3) were provided by M. J. Weber. HA-Elk1 (46) was provided by M. H. Cobb. HA-RSK2 (30) was provided by T. W. Sturgill. All of the constructs were in the cytomegalovirus background. Mxi2, p38 K52R, MEK1 K97R, and Mxi2 Δ17C were generated by PCR-directed mutagenesis. Mxi2 WI was a product of random mutagenesis resulting from a PCR. All constructs were verified by sequencing. HA- and AU5-tagged p38 and Mxi2 constructs were subcloned in pCDNA3. PD98059 was from Calbiochem, anisomycin was from Sigma, and epidermal growth factor (EGF) was from UBI.
Cell culture and transfection.
HEK 293T and NIH 3T3 cells were grown in Dulbecco modified Eagle medium-10% fetal calf serum. Subconfluent cells were transfected by the calcium phosphate technique (7). The total amount of plasmid DNA was adjusted to 3 to 4 μg per plate with vector DNA when necessary.
Animals.
Three-month-old Wistar rats were housed, fed, and sacrificed in accordance with European Community regulations. Kidneys were extracted and homogenized in radioimmunoprecipitation assay buffer (46). Protein detection was performed as described below.
MAPK kinase assays.
Kinase assays for ERKs, p38, and Mxi2 were performed as described previously (39).
MEK kinase assays.
MEK kinase assays were performed basically as described above, with 10 mM morpholinepropanesulfonic acid (pH 7.5)-12.5 mM β-glycerophosphate-20 mM MgCl2-0.5 mM EGTA-0.5 mM sodium fluoride-0.5 mM vanadate. Reactions were performed in a 100-μl volume containing 5 μCi of [γ-32P]ATP and 50 μM ATP by using glutathione S-transferase (GST) fusions (0.5 μg) as substrates.
RSK kinase assays.
RSK kinase assays were performed as previously described (30). Briefly, HA-RSK2-transfected cells were lysed in 50 mM Tris (pH 8)-1 mM EGTA-1 mM EDTA-150 mM NaCl-1% Nonidet P-40-200 μM vanadate-1 mM phenylmethylsulfonyl fluoride-10 μg of aprotinin per ml-10 μg of leupeptin per ml. Lysates were immunoprecipitated with anti-HA antibody and immunocomplexed with gamma-bind Sepharose. Pellets were washed twice with lysis buffer and once in kinase buffer (25 mM HEPES [pH 7.4], 5 mM β-glycerophosphate [pH 7.4], 4 mM EGTA, 1.5 mM dithiothreitol, 30 mM MgCl2, 150 μM vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml). Kinase reactions were performed for 40 min at 25°C in kinase buffer with 5 μCi of [γ-32P]ATP and 100 μM ATP with 0.5 μg of GST-Myt1 as the substrate.
cPLA2 activation assay.
The cPLA2 activation assay was performed as described in reference 47. Briefly, COS-7 cells were labeled by incubation with [3H]arachidonic acid (ICN) (1μCi per well in a 24-well plate) for 18 h. Cells were then washed twice with phosphate-buffered saline, and new medium (Dulbecco modified Eagle medium, 1% fatty acid-free bovine serum albumin) was added. Where indicated, cells were stimulated with EGF (100 ng/ml) for 30 min. Medium was removed and centrifuged at 2,000 × g, and free, labeled arachidonic acid released into the medium was measured by liquid scintillation counting.
32P metabolic labeling.
32P metabolic labeling was performed as described previously (7).
Recombinant proteins.
GST fusion proteins of kinase-inactive mutant forms of ERK2, p38, Mxi2, and MEK1 and of Elk1 and Myt1 were purified as previously described (39) and used as substrates in kinase reactions as described above.
Immunoblotting and immunoprecipitations.
Lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose filters. Immunocomplexes were visualized by ECL detection (Amersham) with horseradish peroxidase-conjugated secondary antibodies (Cappel). The antibodies used were mouse monoclonal anti-HA and anti-AU5 (Babco), anti-phospho-Elk1 (New England Biolabs), and anti-GST and -phospho-ERK antibodies and rabbit polyclonal anti-MEK, -MKK3, -MKK4, -MKK6, -ERK1/2, -Mxi2, and -p38 N terminus antibodies (Santa Cruz). Immunoprecipitations were performed as described previously (7), with 20 mM HEPES (pH 8)-2 mM MgCl2-2 mM EGTA-150 mM NaCl-2.5 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) for MEK-MAPK interactions or 1% NP-40 for interactions between MAPKs. Low-stringency immunoprecipitations were performed as previously described (50), with a buffer containing 150 mM NaCl, 20 mM Tris (pH 8.0), and 0.5% NP-40.
ERK-Mxi2 in vitro binding assays.
ERK-Mxi2 in vitro binding assays were performed basically as previously described (37). Briefly, histidine-tagged ERK2 was translated in vitro, labeled with [35S]methionine, and purified by standard procedures. Increasing concentrations of radiolabeled ERK2 were incubated with fixed concentrations (40 nmol) of GST, GST-Mxi2, or GST-p38 immobilized in glutathione-Sepharose beads in 50 μl of binding buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 0.1 mg of bovine serum albumin per ml) for 1 h at room temperature. Beads were washed four times with HEPES (pH 7.5)-100 mM NaCl-5 mM MgCl2-0.1 mg of bovine serum albumin per ml-0.1% Triton X-100. Afterwards, loading buffer was added and proteins were fractionated by SDS-PAGE. Gels were dried, and the radiolabeled ERK band was quantified with a phosphorimager.
Reporter gene assays.
Reporter gene assays were performed as described previously (39) with the reporter pGE51-luc and vectors encoding the transactivation domains of Elk-1 (amino acids 307 to 428) (20) and HIF-1α (amino acids 531 to 826) (40) fused to the GAL4 DNA-binding domain. Luciferase activities were determined with a commercial kit (Promega, Madison, Wis.) and normalized to the β-galactosidase activity.
RESULTS
Mxi2 immunoprecipitates harbor a kinase activity activatable by EGF through Ras, Raf, and MEK.
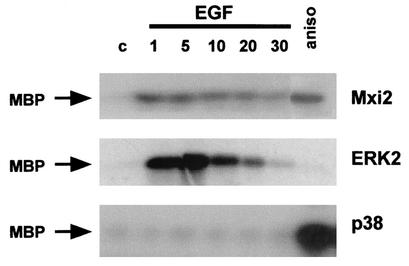
To gain a first insight into Mxi2 regulation by mitogens, its activation kinetics were investigated. It is well established that ERKs respond to mitogens with an acute, almost immediate peak of activity. Conversely, p38 MAPKs are, in most cases, unresponsive to mitogenic stimulation (32). To compare the Mxi2 activation course with those of ERK2 and p38, 293T cells were transfected with HA-tagged Mxi2, ERK2, and p38 and their activities were assayed at different time points after EGF stimulation. The time course assays in cells expressing HA-ERK2 revealed potent ERK2 activation, reaching a maximum after 5 min and decreasing to basal levels after 30 min. On the other hand, EGF failed to stimulate p38 activity (Fig. 1). Under similar conditions, Mxi2 harbored a kinase activity readily activatable by EGF that reached a peak between 1 and 5 min of stimulation. The level of activation detected in HA-Mxi2 immunoprecipitates was, at its maximum, twofold weaker than that exhibited by ERK2 but was more persistent, maintaining >40% of its activity after 30 min (Fig. 1).
FIG. 1.
Time-dependent activation of Mxi2. 293T cells transfected with HA-tagged Mxi2, ERK2, and p38 (1 μg) were starved for 12 h and stimulated with 10 μg of anisomycin (aniso) per ml for 20 min or with EGF (100 ng/ml). At the indicated times (minutes), the phosphotransfer activities in the immunoprecipitated kinases were determined by an in vitro kinase assay with MBP as the substrate. c, control.
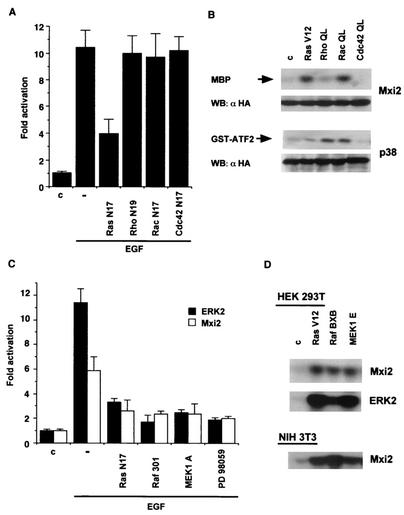
We next focused on identifying the proteins involved in the signaling pathway responsible for the EGF-induced kinase activity detected in Mxi2. It is well established that the MAPK cascades are originally regulated by GTPases of the Ras superfamily (36). Accordingly, we studied which GTPases would mediate Mxi2 mitogenic activation. Initially, we assayed the ability of Ras GTPase dominant inhibitory mutant forms to interfere with the activation of Mxi2. It was found that H-Ras N17 reduced the EGF-induced activation of Mxi2 by 60% (Fig. 2A), while Rac, Rho, and Cdc42 inhibitory mutant forms, otherwise capable of blocking JNK activation in COS-7 and 293T cells (5, 43; data not shown), were ineffective. This indicated that H-Ras was the main mediator in the activation of Mxi2 by EGF. To extend this observation, we analyzed the effects of activated mutant forms of H-Ras (V12), Rac-1, Rho A, and Cdc42 (Q61L) on Mxi2. As shown in Fig. 2B, Mxi2 activity was largely unaffected by Rho and Cdc42, which are capable of potently activating JNK (data not shown), but was significantly stimulated by Ras and Rac. This pattern of activation differed from that exhibited by p38, which was activated by Rho and Rac but was largely unaffected by Ras.
FIG. 2.
Mediation of Mxi2 activation by the components of the ERK pathway. (A) Effects of Ras GTPase dominant inhibitory mutant forms on Mxi2 activation induced by EGF. Kinase activities in 293T cells transfected with HA-Mxi2 and the indicated dominant inhibitory mutant forms (250 ng) are shown. After starvation, the indicated cells were stimulated with EGF (100 ng/ml) for 5 min. The data shown are the average ± the standard error of the mean of three independent experiments, relative to the activity levels in control cells. (B) Effects of activated Ras family GTPases on Mxi2. Kinase activities in cells transfected with HA-Mxi2 or HA-p38 (1 μg) and the indicated GTPase (0.5 μg). (Bottom) Expression of HA-Mxi2 and HA-p38. (C) Effects of the inhibition of the proteins composing the ERK pathway on Mxi2 activation induced by EGF. Cells were cotransfected with HA-tagged Mxi2 or ERK2 and the indicated mutant forms (250 ng). Upon starvation and pretreatment for 30 min with 10 μM PD98059 where indicated, cells were stimulated with EGF and kinase assays were performed. The data shown are the average ± the standard error of the mean of three independent experiments, relative to the activity levels in control cells. (D) Effects of the constitutive activation of the components of the ERK pathway on Mxi2. Kinase activities in 293T or NIH 3T3 cells cotransfected with HA-Mxi2 or HA-ERK2 and expression vectors (0.5 μg) encoding the indicated constructs. WB, Western blot; c, control.
This result prompted us to examine whether other components of the classical ERK pathway would also mediate Mxi2 activation. As before, we tested if the dominant negative mutant forms Raf 301 and MEK1 A could block the effects of EGF on the kinase activity detected in Mxi2 immunoprecipitates. It was found that both Raf and MEK1 inhibitory mutant forms diminished EGF-induced Mxi2 activity by over 50%, just as potently as H-Ras N17. Likewise, MEK1 inhibitor PD98059 was just as efficient (Fig. 2C). In agreement with these observations, constitutively active Raf BXB and MEK1 E were as effective as Ras V12 in stimulating the kinase activity of HA-Mxi2 in both 293T and NIH 3T3 cells (Fig. 2D), indicating that activation of the ERK pathway leads to Mxi2 stimulation irrespective of the cell type. Overall, these results suggested that the kinase activity detected in HA-Mxi2 immunoprecipitates upon stimulation with EGF was regulated through Ras, Raf, and MEK.
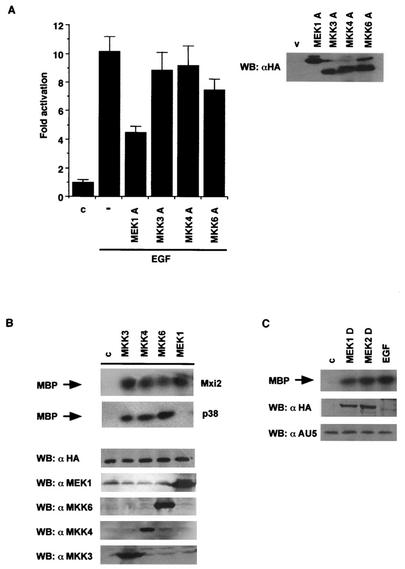
It was important to identify the dual-specificity MAPKKs that regulated Mxi2 stimulation by mitogens. Once again, we followed the dominant inhibitory mutant approach utilized previously. HA-tagged interfering mutant forms of MEK1, MKK3, MKK4, and MKK6 were transfected with AU5-Mxi2, and their effects on the activation of Mxi2 by EGF were analyzed. Even when expressed at similar levels (Fig. 3A, right side), only the MEK1 inhibitory mutant form could significantly reduce the EGF-stimulated kinase activity in Mxi2 precipitates although a 25% drop in Mxi2 activity was also caused by the MKK6 inhibitory mutant form (Fig. 3A, left side). To substantiate this point further, hyperactive forms of MKK3, MKK4, MKK6, and MEK1 were tested for the ability to activate Mxi2. It was found that all four MAPKKs could elicit Mxi2 activation to similar extents. On the other hand, in the same experimental setting, MEK1 could not stimulate p38 (Fig. 3B). Finally, we tested whether the isoforms MEK1 and MEK2 were capable of activating Mxi2 to the same degree. Thus, HA-tagged, activated MEK1 S 218,222 D and MEK2 S 222,226 D were transfected with AU5-Mxi2. Both isoforms induced similar levels of Mxi2 activation, comparable to those elicited by EGF (Fig. 3C). In all, we demonstrated that although all MAPKKs could activate Mxi2, the kinase activity detected in Mxi2 immunoprecipitates upon EGF stimulation was primarily mediated through MEK.
FIG. 3.
MAPKKs as mediators of Mxi2 activation. (A) Effects of MAPKK dominant inhibitory mutant forms on Mxi2 activation induced by EGF. Cells were transfected with AU5-tagged Mxi2 and the indicated HA-tagged inhibitory mutant forms (250 ng). The indicated cells were stimulated with EGF (100 ng/ml) for 5 min. The data shown are the average ± the standard error of the mean of three independent experiments relative to the activity detected in control cells. (Right side) Expression levels of the MAPKKs in total lysates. (B) Activation of Mxi2 by MAPKKs. Kinase activities in cells transfected with HA-tagged Mxi2 or p38, together with the indicated MAPKKs (1 μg). (Bottom) Expression levels of HA-Mxi2 and of the different MAPKKs. (C) Activation of Mxi2 by constitutively active MEK isoforms. Kinase activities detected in cells transfected with AU5-Mxi2 and activated, HA-tagged MEK1 and -2 (1 μg) or stimulated with EGF. (Bottom) Expression levels of AU5-Mxi2 and HA-MEK1 and -2. WB, Western blot; c, control; v, vector.
Mxi2 is not a substrate for MEK1.
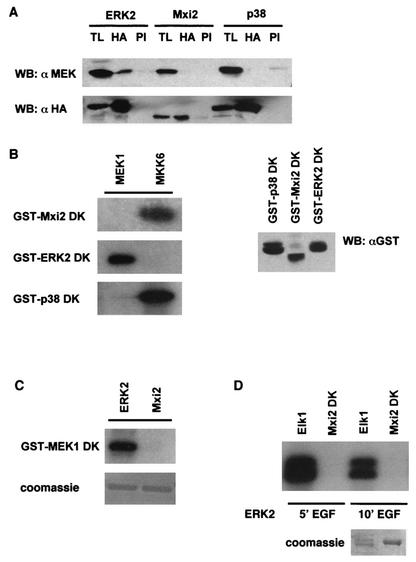
These results pointed to MEK as the kinase responsible for the activation of Mxi2 upon mitogenic stimulation. On the basis of this premise, we investigated the MEK-Mxi2 interaction in more depth. It is known that under quiescent conditions, MEK is bound to ERK1/2 (16). By analogy, if Mxi2 were to be a substrate for MEK, it could be found in association with this MAPKK. To test this hypothesis, HA-tagged Mxi2, ERK2, and p38 were coexpressed with MEK1 and lysates from serum-starved cells were immunoprecipitated with anti-HA antibody to coprecipitate MEK1. Anti-MEK immunoblotting revealed that MEK1 coimmunoprecipitated with ERK2, but it did not associate with Mxi2 or with p38 (Fig. 4A). We then investigated if Mxi2 was a substrate for MEK in vitro. Cells were transfected with HA-tagged, activated MEK1 and MKK6, and their ability to phosphorylate kinase-dead (K52R) Mxi2, ERK2, and p38 was tested. As shown in Fig. 4B, MEK1 potently phosphorylated ERK2 but, in the same experimental setting, it did not recognize Mxi2 as a substrate. On the other hand, Mxi2 was phosphorylated by MKK6 just as efficiently as its bona fide substrate, p38. A known feature of the interaction between MEK1 and ERK1/2 is that ERKs can retrophosphorylate MEK1 (2). To determine if a similar situation occurs with Mxi2, cells were transfected with HA-tagged Mxi2 and ERK2 and stimulated with EGF and their ability to phosphorylate kinase-dead (K97R) MEK1 was tested. It was found that while ERK2 readily phosphorylated MEK1, Mxi2 was incapable of catalyzing any phosphotransfer onto MEK1 (Fig. 4C). These results speak against the notion that Mxi2 is a substrate of MEK and against the idea that a direct interaction between the two kinases takes place.
FIG. 4.
Interactions of Mxi2 with MEK1 and ERK2. (A) Coimmunoprecipitation of MEK with MAPKs. 293T cells were transfected with 1 μg of HA-tagged Mxi2, ERK2, and p38. After 12 h of starvation, anti-HA (HA) and preimmune (PI) immunoprecipitates were immunoblotted with anti-MEK. TL, total lysates. (B) Analysis of MEK substrates. Kinase assays were performed with immunoprecipitated, constitutively active, HA-tagged MEK1 and MKK6 by using as substrates equal amounts (0.5 μg; right side) of fusions of GST with Mxi2, ERK2, and p38 kinase-inactive mutant forms (DK). (C) Analysis of the phosphorylation of MEK1 by Mxi2. Kinase assays of HA-tagged ERK2 and Mxi2 immunoprecipitated from cells treated with EGF for 5 min by using as the substrate 0.5 μg of GST-MEK1 DK. (D) Analysis of the phosphorylation of Mxi2 by ERK2. Kinase assays of HA-tagged ERK2 immunoprecipitated from cells stimulated with EGF for the indicated times by using as substrates fusions of GST with Mxi2 DK and Elk1. WB, Western blot.
The possibility existed that ERKs could phosphorylate Mxi2, thereby facilitating its activation by, for example, MKK6. Indeed, Mxi2 possesses three (S/T)-P motifs, potential ERK phosphorylation sites. To investigate this scenario, cells transfected with HA-ERK2 were stimulated with EGF for 5 and 10 min and the phosphotransfer potential of ERK2 was tested on its physiological substrate Elk1 and on Mxi2 K52R. As shown in Fig. 4D, ERK2 readily phosphorylated Elk1 but not Mxi2. Overall, our data indicate that although the kinase activity in Mxi2 immunoprecipitates is activatable by the ERK pathway, Mxi2 cannot be activated by MEK or phosphorylated by ERK2.
Mxi2 binds to ERK1/2.
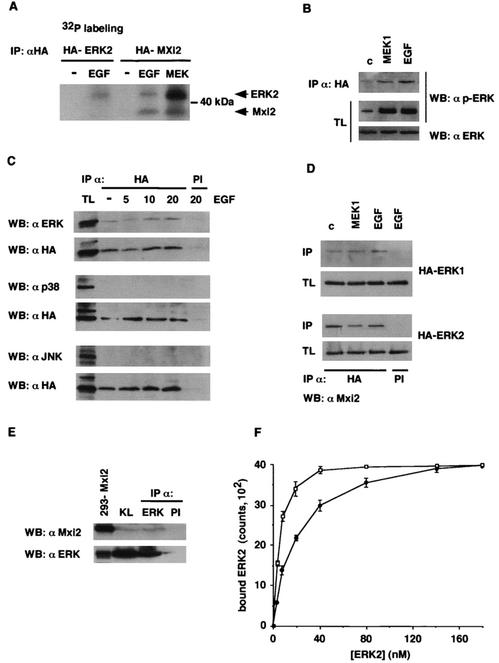
These results opened the possibility that the EGF/ERK pathway-induced kinase activity harbored in anti-Mxi2 precipitates was not due to Mxi2 performance but rather was caused by the function of an unidentified kinase that coimmunoprecipitated with Mxi2. To probe this hypothesis, cells were transfected with HA-Mxi2 and 32P labeled in order to detect proteins, present in anti-HA immunoprecipitates, that incorporated 32P when stimulated with EGF or with MEK1 E. It was found that Mxi2 became phosphorylated in stimulated cells. More interestingly, coprecipitating with Mxi2 was a 42- to 44-kDa protein that was also markedly phosphorylated in cells treated with EGF and, more prominently, in cells cotransfected with MEK1 E. This protein comigrated with a phosphoprotein detected in anti-HA precipitates from cells transfected with HA-ERK2 and treated with EGF (Fig. 5A) that was likely to be ERK2. To ascertain its identity, we tested whether Mxi2 could associate with phosphorylated ERKs. Cells were transfected with HA-Mxi2 in order to coimmunoprecipitate phosphorylated ERKs. Immunoblotting with anti-phospho-ERK revealed the presence of phosphorylated ERKs in anti-HA immunoprecipitates from cells cotransfected with MEK1 E or stimulated with EGF (Fig. 5B). Next, we investigated how the Mxi2-ERKs interaction varied upon mitogenic stimulation. For this purpose, HA-Mxi2-transfected cells were serum starved and stimulated with EGF for different times, after which anti-HA immunoprecipitates were probed for the presence of ERKs. Interestingly, immunoblotting with anti-ERK antibody revealed that ERKs coimmunoprecipitated with Mxi2, even under quiescent conditions. Moreover, the amount of ERKs associated with Mxi2 did not vary significantly upon stimulation with EGF (Fig. 5C). This interaction between Mxi2 and ERKs was specific, as under the same conditions, neither JNK nor p38 was found to coimmunoprecipitate with Mxi2 (Fig. 5C). In the same fashion, no ERKs could be detected in anti-HA immunoprecipitates from cells transfected with HA-p38 and subjected to the same treatment (data not shown). To determine if Mxi2 could interact with both ERK isoforms, Mxi2 was cotransfected with HA-ERK1 and HA-ERK2 and its presence in anti-HA immunoprecipitates was examined by anti-Mxi2 immunoblotting. As shown in Fig. 5D, Mxi2 was found to coprecipitate with ERK1 and ERK2 with similar efficiencies under both stimulated and serum-starved conditions.
FIG. 5.
Association of ERK1 and -2 with Mxi2. (A) Detection of phosphoproteins in Mxi2 immunoprecipitates (IP). 293T cells transfected with HA-ERK2 or HA-Mxi2 and MEK1E (1 μg) were labeled with 32P and stimulated with EGF (100 ng/ml) for 5 min where indicated. After anti-HA immunoprecipitation, phosphoproteins were detected in dried gels. The positions of Mxi2 and ERKs are indicated. (B) Detection of phosphorylated ERKs in association with Mxi2. Cells transfected with HA-Mxi2 and stimulated with cotransfected MEK1E or with EGF for 5 min were probed for phospho-ERK in anti-HA immunoprecipitates and their respective total lysates (TL). (C) Association of MAPKs with Mxi2. Cells transfected with HA-Mxi2 were stimulated with EGF for the indicated times, and anti-HA and preimmune (PI) immunoprecipitates were probed for ERK, JNK, and p38. (Bottom) Expression of immunoprecipitated HA-Mxi2. (D) Association of Mxi2 with ERK1 and -2. Cells transfected with HA-ERK1 and -2, in addition to AU5-Mxi2, were stimulated with cotransfected MEK1E or EGF for 5 min and anti-HA and preimmune immunoprecipitates, and their respective total lysates were probed for Mxi2. (E) Association of Mxi2 with ERKs in rat kidney. Lysates from homogenized kidneys (KL) were immunoprecipitated with anti-ERK or preimmune antibodies and probed for coimmunoprecipitating Mxi2. Total lysate from Mxi2-transfected 293T cells was run alongside as a control. (F) Affinity of ERK2 binding to Mxi2. Increasing concentrations of [35S]methionine-labeled ERK2 were allowed to bind in vitro to GST-Mxi2 (open squares) or to GST-p38 (black circles) bound to glutathione-Sepharose beads. Beads were washed, bound proteins were electrophoresed by SDS-PAGE, and bound ERK2 was counted by phosphorimager. The data shown are the average ± the standard error of the mean of three independent experiments. WB, Western blot; c, control.
It was essential to elucidate whether the interaction between Mxi2 and ERKs occurred in a physiological environment and that it was not an artifactual association due to ectopic overexpression. Mxi2 is mainly expressed in renal distal tubules (11); thus, the interaction between endogenous ERKs and Mxi2 was investigated in rat kidney extracts. Despite its low expression levels, Mxi2 was clearly detected in anti-ERK immunoprecipitates (Fig. 5E). Identical results were obtained with kidney extracts from mice and rabbits (data not shown), thereby demonstrating that the interaction between Mxi2 and ERKs was physiological and widespread among species.
It was important to acquire some notion of the strength of the interaction between Mxi2 and ERK. For this purpose, the association between Mxi2 and ERK2 was studied in vitro. Increasing amounts of ERK2 labeled with [35S]methionine were allowed to bind to either GST-Mxi2 or GST-p38, and the bound ERK2 was recovered by affinity pulldown, resolved by SDS-PAGE, and quantified. As shown in Fig. 5F, the amount of ERK2 binding to Mxi2 saturated at ∼40 nM and half-maximal binding was achieved at ∼20 nM, which gives an approximate estimate of ERK2-Mxi2 binding affinity. In comparison, ERK2 exhibited a reduced affinity for p38. ERK2 binding to p38 saturated at ∼160 nM, with half-maximal binding at ∼80 nM, demonstrating that ERK2 displays an affinity for Mxi2 at least fourfold greater than for p38. These assays were performed at an ionic strength close to physiological levels. No significant binding of ERK2 to GST was detected (data not shown). Overall, our results strongly indicated that Mxi2 directly interacted in vitro, in vivo, and physiologically with ERK1/2.
Mxi2 sustains ERK phosphorylation levels.
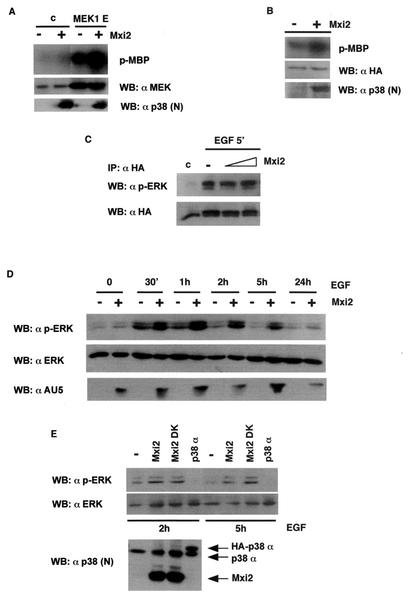
It was of special interest to investigate the existence of changes in ERK1/2 activation and/or functions caused by the interaction with Mxi2. First, we tested whether Mxi2 could have an influence on the activation of ERK brought about by its natural activator, MEK1. For this purpose, ERK2 activity was determined in anti-HA immunoprecipitates from cells transfected with vector and MEK1 E, with or without Mxi2. It was found that in the presence of Mxi2, MEK-induced ERK2 activation was markedly potentiated (Fig. 6A). Furthermore, ERK2 activation also appeared to be slightly higher in those vector-transfected cells that had been cotransfected with Mxi2. Accordingly, we asked if Mxi2 could enhance ERK basal activity. Cells were transfected with HA-ERK2 with or without Mxi2, and after starvation for 18 h, ERK kinase activity was determined in the absence of any further stimulus. As shown in Fig. 6B, after prolonged exposure of the films, it became apparent that in the presence of Mxi2, ERK2 basal activity was significantly augmented. The hallmark of ERK activation is its phosphorylation. Therefore, in light of the above data, we explored whether Mxi2 could alter the phosphorylation status of ERK2 induced by mitogenic stimulation. Cells were transfected with HA-ERK2 and increasing amounts of Mxi2, and the changes in phospho-ERK levels upon EGF stimulation were analyzed in anti-HA immunoprecipitates. Surprisingly, as shown in Fig. 6C, EGF-induced phosphorylation of ERK2 was not significantly altered by Mxi2.
FIG. 6.
Effects of Mxi2 on ERK phosphorylation and activation levels. (A) Effects of Mxi2 on the activation of ERK2 by MEK. 293T cells were transfected with HA-ERK2 cotransfected with vector or with MEK1E (1 μg) in the presence (+) or absence (−) of Mxi2 (1 μg). Phosphorylated MBP (p-MBP) levels indicate ERK activity levels. (Bottom) Expression levels of MEK and Mxi2. (B) Effect of Mxi2 on ERK2 basal activity levels. Cells were transfected with HA-ERK2 (1 μg) in the presence (+) or absence (−) of Mxi2 (1 μg). After 18 h of starvation, ERK kinase activity was determined as described previously. Phospho-MBP levels became apparent after prolonged exposure at −70°C. (Bottom) Expression levels of HA-ERK2 and Mxi2. (C) Effects of Mxi2 on EGF-induced phosphorylation of ERK2. Cells transfected with HA-ERK2 (1 μg) and increasing amounts of Mxi2 (2 and 5 μg) were stimulated with EGF for 5 min and probed for phospho-ERK in anti-HA immunoprecipitates. (Bottom) Levels of immunoprecipitated HA-ERK2. (D) Effects of Mxi2 on the dephosphorylation rate of ERK2. Cells were transfected with (+) or without (−) AU5-Mxi2 and stimulated with EGF (100 ng/ml), and phospho-ERK levels in total lysates were examined at the indicated times. (Bottom) Expression of ERK1/2 and AU5-Mxi2. (E) Effects of Mxi2 dead kinase (DK) on ERK2 dephosphorylation. Cells transfected with the indicated constructs were stimulated with EGF, and phospho-ERK levels were determined after 2 and 5 h. (Bottom) Expression of p38 proteins detected by immunoblotting with anti-p38 N terminus antibody. WB, Western blot; c, control.
It is well established that ERK inactivation occurs through the action of phosphatases, which gradually dephosphorylate the activated ERK pool back to a resting state (21). Thus, we examined if Mxi2 effects on ERK activation could be explained by Mxi2 affecting the ERK inactivation rate. The phosphorylation of ERKs was determined at different time points after an EGF pulse in the presence or absence of AU5-Mxi2. The levels of phosphorylated ERKs dropped gradually from 30 min to 5 h, but surprisingly, the levels of phospho-ERK in cells expressing Mxi2 were significantly higher than those found in vector-transfected cells throughout the complete dephosphorylation course (Fig. 6D), suggesting that Mxi2 somehow impaired ERK deactivation. We then tested if this effect on ERK dephosphorylation was dependent on Mxi2 kinase activity. The amounts of phospho-ERK remaining after 2 and 5 h of EGF treatment were compared in cells transfected with wild-type Mxi2 or with Mxi2 K52R. Interestingly, the levels of phospho-ERK were similar in both cases (Fig. 6E), indicating that the effect of Mxi2 on ERK inactivation was independent of its kinase activity. On the other hand, this protective effect was specific for Mxi2 as, under the same conditions, the overexpression of p38 failed to sustain phospho-ERK levels, which were even lower than those found in control cells (Fig. 6E). These data suggested that the inactivation of ERKs is profoundly influenced by its interaction with Mxi2, resulting in down-regulation of its dephosphorylation rate.
Mxi2 requires its C terminus for binding to ERK and maintenance of ERK phosphorylation levels.
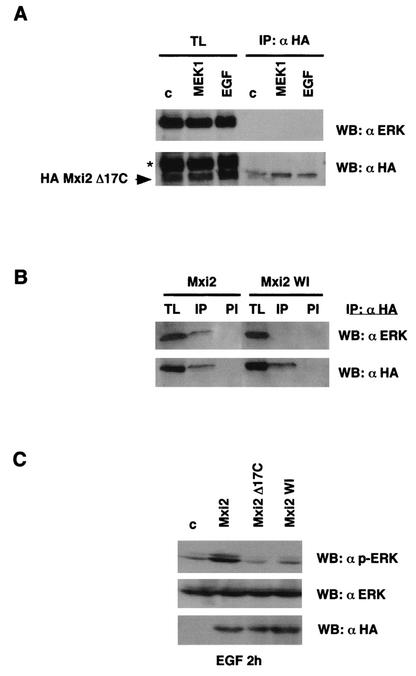
The data indicating that, in our experimental model, p38 could not bind to ERK or sustain ERK phosphorylation levels suggested that, in Mxi2, the determinant(s) responsible for these effects should reside within its unique 17 C-terminal amino acids. To probe this hypothesis, we generated an Mxi2 mutant form in which the unique C terminus was deleted (Mxi2 Δ17C). We then tested whether this mutant form was capable of binding to ERKs. 293T cells were transfected with HA-tagged Mxi2 Δ17C, and the presence of ERKs in anti-HA immunoprecipitates was assayed. As shown in Fig. 7A, no ERKs were found to coimmunoprecipitate with Mxi2 Δ17C, either under basal conditions or upon stimulation with EGF or MEK1. To further prove this point, we made use of another Mxi2 mutant form in which the C terminus was significantly distorted. Mxi2 WI was the result of random mutagenesis, C-terminal M 296 and I 297 were deleted, and in addition, it harbored the substitutions L 294 W and V 295 I. In agreement with our previous result, it was found that Mxi2 WI could not coimmunoprecipitate with ERKs in the same setting in which ERKs could effectively associate with wild-type Mxi2 (Fig. 7B), thereby demonstrating that the C terminus of Mxi2 is directly involved in high-affinity binding to ERKs.
FIG. 7.
Effects of Mxi2 on ERKs require the C terminus of Mxi2. (A) ERKs cannot bind to a deletion mutant form of Mxi2 lacking the C terminus. 293T cells were transfected with HA-Mxi2 Δ17C (1 μg) and stimulated with EGF (100 ng/ml) for 5 min or with cotransfected MEK1 E where indicated. Anti-HA immunoprecipitates (IP) and their respective total lysates (TL) were probed for the presence of ERK. (Bottom) Levels of HA-Mxi2 Δ17C protein. The position of HA-Mxi2 Δ17C is indicated. The asterisk shows the remaining anti-ERK signal from the previous blot. (B) ERKs cannot bind to the Mxi2 WI mutant form. Cells were transfected with HA-Mxi2 or with HA-Mxi2 WI (1 μg). Lysates were immunoprecipitated with an anti-HA antibody or with preimmune (PI) serum and probed for associated ERK. (Bottom) Expression of the HA-tagged proteins. (C) Effect of the mutant form of Mxi2 lacking the C terminus on ERK2 dephosphorylation. Cells transfected with the indicated constructs (1 μg) were stimulated with EGF (100 ng/ml), and phospho-ERK levels were determined after 2 h. (Bottom) Total ERK protein levels and expression of the Mxi2 HA-tagged proteins detected by anti-ERK and anti-HA immunoblotting, respectively. WB, Western blot; c, control.
In light of this finding, we moved on to determine if the binding of Mxi2 to ERKs is an important part of the mechanism by which Mxi2 sustains ERK phosphorylation levels. The amounts of phospho-ERK remaining after 2 h of EGF treatment were compared in cells transfected with wild-type Mxi2, Mxi2 Δ17C, and Mxi2 WI. As shown in Fig. 7C, only wild-type Mxi2 was capable of sustaining the levels of phosphorylated ERKs. Phospho-ERK levels present in wild-type Mxi2-transfected cells were significantly higher than those found in cells transfected with Mxi2 Δ17C or Mxi2 WI, in which the levels of phosphorylated ERKs were very similar to those found in control cells. Therefore, these results clearly indicated that Mxi2 requires its C terminus for binding to ERK and that Mxi2 binding to ERKs is critical to the mechanism by which ERK phosphorylation levels are sustained.
Mxi2 potentiates the functions of ERKs in the nucleus.
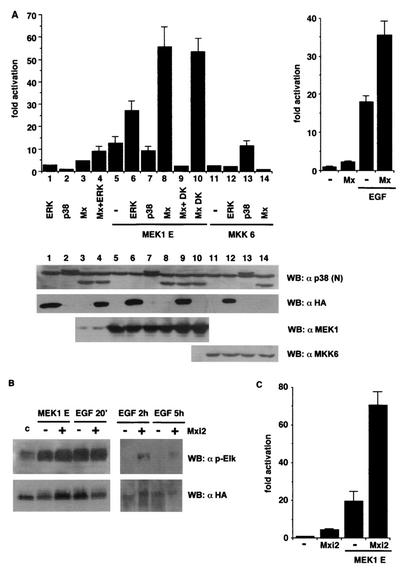
Our final goal was to determine the functional consequences of the sustained activation of ERKs caused by Mxi2. Once activated, ERKs translocate to the nucleus, where they phosphorylate diverse transcription factors, thereby inducing their transactivation potential over their cognate responsive elements (32). Among these, Elk1 sensitivity to stimulation by ERKs is well documented (46). Accordingly, we utilized the response of a GAL4-Elk1-dependent luciferase reporter to study the effects of Mxi2 on an ERK-induced event. Expression of MEK1 E induced an increase of >10-fold in Elk1-dependent luciferase activity (Fig. 8A, left side). Transactivation of Elk1 by MEK1 was significantly enhanced when it was coexpressed with ERK2 but not when it was coexpressed with p38. More interestingly, when MEK1 E was coexpressed with Mxi2, they acted synergistically to induce an up to 55-fold increase in Elk1-dependent gene expression. Mxi2 K52R (DK) had effects identical to those exhibited by wild-type Mxi2. Moreover, this synergistic effect of MEK1 and Mxi2 was entirely dependent on ERKs being functional, as cotransfection with an ERK2 dominant inhibitory mutant form (K52R) completely blocked the Elk1 response. On the other hand, Mxi2 failed to elicit significant stimulation of Elk1 in synergy with MKK6. These results were not due to changes in the protein levels of the different kinases utilized, as variations in their respective expression levels at each experimental point were insignificant (Fig. 8A, bottom).
FIG. 8.
Regulation of ERK-dependent nuclear events by Mxi2. (A, left side) Effects of Mxi2 on Elk1-dependent gene expression. Elk1transactivation was examined in NIH 3T3 cells cotransfected with GAL4-Elk1 (TAD) and the indicated plasmids. ERKs were HA tagged; p38 and Mxi2s were AU5 tagged (Mx + DK = Mxi2 + ERK2 DK). The results shown are the average ± the standard error of the mean of at least five independent experiments. (Bottom) Expression levels of the different kinases. (Right side) Effects of Mxi2 on Elk1transactivation induced by EGF in 293T cells. The results shown are the average ± the standard error of the mean of three independent experiments. In both parts, values are expressed relative to the activity detected in vector-transfected cells. Luciferase activities were normalized to the β-galactosidase activity. (B) Effect of Mxi2 on Elk1 phosphorylation. 293T cells were transfected with HA-Elk1 with (+) or without (−) AU5-Mxi2 and stimulated with cotransfected MEK1E or with EGF (100 ng/ml) for 20 min, 2 h, and 5 h as shown. Phospho-Elk1 levels were determined by immunoblotting. (Bottom) Expression of HA-Elk1. (C) Effects of Mxi2 on HIF1α-dependent expression. HIF1α transactivation was determined in NIH 3T3 cells cotransfected with GAL4-HIF1α (TAD) and the indicated plasmids. Luciferase activities were normalized to the β-galactosidase activity. Values are expressed relative to the activity detected in vector-transfected cells. The results are the average ± the standard error of the mean of three independent experiments. WB, Western blot; c, control.
A similar situation was observed when we tested the effects of Mxi2 on the activation of Elk1-mediated gene expression triggered by a physiological stimulus. As shown in Fig. 8A, right side, treatment of 293T cells with EGF resulted in significant stimulation of Elk1 transcriptional activity. In the presence of Mxi2, EGF-induced Elk1 transactivation was cooperatively enhanced up to 35-fold over basal values. Mxi2-induced dramatic up-regulation of Elk1 did not directly correlate with an increase in Elk1 phosphorylation, as phosphorylation of Elk1 induced by transfection of MEK1 or by stimulation with EGF was only slightly altered by coexpression of Mxi2 (Fig. 8B). On the other hand, Elk1 remained in a phosphorylated state for a longer time. As shown in Fig. 8B, 2 and 5 h after the EGF pulse, phosphorylated Elk1 was still detectable in Mxi2-transfected cells but not in those that lacked Mxi2.
It was of interest to verify whether the observed effect of Mxi2 on ERK-mediated Elk1 transactivation was restricted to this transcription factor or, contrarily, the presence of Mxi2 had the same outcome on other ERK nuclear substrates. To test this point, we looked at the effects of Mxi2 on the activation of another ERK-responsive transcription factor, hypoxia-inducible factor 1α (HIF1α), that has been previously shown to be readily phosphorylated and activated by ERKs (40). As before, we utilized a GAL4-HIF1α chimera that was found to be stably expressed in the nucleus (data not shown). Mxi2 alone caused a small (fourfold), although reproducible, transactivation of HIF1α (Fig. 8C). On the other hand, MEK1 E was capable of inducing a strong response of HIF1α, up to 20-fold over basal values. Upon cotransfection with Mxi2, MEK1 E and Mxi2 synergistically elevated HIF1α transactivation up to 60-fold, thereby demonstrating that Mxi2 had identical effects on two different ERK nuclear substrates. Summarizing, these data indicated that the interaction of ERK1/2 with Mxi2 had direct consequences for ERK nuclear functions.
Mxi2 has no effects on the activation of ERK cytoplasmic substrates.
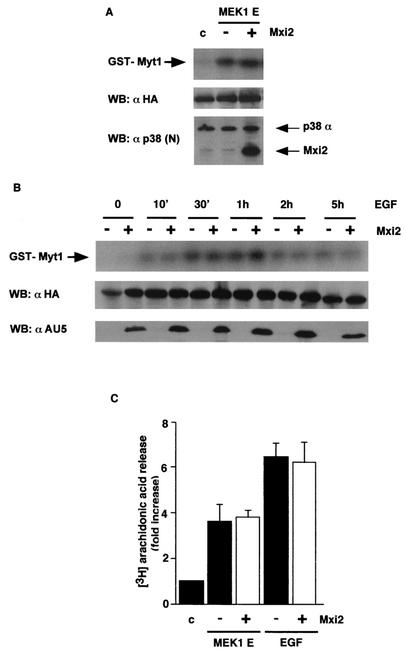
Since ERKs have multiple substrates in different cellular locations (32), we investigated whether the Mxi2 up-regulatory effect on ERK functions would be restricted to nuclear events or, on the other hand, it would be a generalized phenomenon regardless of the cellular site where the activation takes place. Thus, we examined the effects of Mxi2 on the activation of RSK2, a kinase that is predominantly activated in the cytoplasm by ERKs (15). HA-RSK2 was cotransfected with MEK1 E in the presence or absence of Mxi2. It was found that RSK2 activity stimulated by MEK1 was unaffected by Mxi2 (Fig. 9A). The same lack of effect was observed for an acute stimulus like EGF when RSK2 activation was analyzed at different time points after stimulation by comparing cells expressing Mxi2 with control cells (Fig. 9B).
FIG. 9.
Effect of Mxi2 on the activation of ERK cytoplasmic substrates. (A) Effect of Mxi2 on MEK-induced activation of RSK2. RSK2 kinase activities with GST-Myt1 as the substrate in 293T cells transfected with MEK1E with (+) or without (−) AU5-Mxi2 in addition to HA-RSK2. (Bottom) Expression of HA-RSK2 and p38 proteins. (B) Effect of Mxi2 on the long-term activation of RSK2 induced by EGF. Cells were transfected with (+) or without (−) AU5-Mxi2 and stimulated with EGF (100 ng/ml), and RSK2 activation levels were determined after the indicated times. (Middle) Expression of HA-RSK2 in total lysates. (Bottom) AU5-Mxi2 expression levels. (C) Effect of Mxi2 on the activation of cPLA2. Arachidonic acid release was measured in 293T cells labeled with [3H]arachidonic acid previously transfected with MEK1E or stimulated for 30 min with EGF in the presence or absence of transfected Mxi2 as indicated. The data shown are the average ± the standard error of the mean of three independent experiments expressed relative to the values found in unstimulated, vector-transfected cells. WB, Western blot; c, control.
Since ERK-mediated RSK2 activation was unaffected by Mxi2, we then looked at whether this lack of response was a peculiarity of RSK2 or, rather, a characteristic of ERK-Mxi2 interaction in the cytoplasm, regardless of which substrate ERK is acting upon. To verify this point, we tested the effects of Mxi2 on the activation of another ERK cytoplasmic substrate. Cytoplasmic phospholipase A2 (cPLA2) is activated by a wide variety of growth factors, including EGF (24). In most cases, cPLA2 activation is mediated by ERKs, which have been shown to directly phosphorylate and activate cPLA2 (25). Therefore, we tested whether Mxi2 would affect the activation of cPLA2 mediated by ERKs. cPLA2 catalyzes the release of arachidonic acid from cellular phospholipids (19). In the absence of a stimulus, little release of labeled arachidonic acid to the medium was observed. However, in cells that had been transfected with MEK1 E, the release of arachidonic acid was augmented almost fourfold. Stimulation with EGF also induced cPLA2 activation by more than sixfold relative to that of unstimulated cells (Fig. 9C), but in neither case did the cotransfection of Mxi2 result in significant changes, despite the fact that Mxi2 was well expressed (data not shown), thereby demonstrating that Mxi2 does not affect the activation of cPLA2 brought about by ERKs. Overall, our results show that down-regulation of the ERK dephosphorylation rate caused by the interaction with Mxi2 had functional consequences for ERK nuclear events but did not alter the effects of ERKs at other cellular locations, like the cytoplasm.
DISCUSSION
In this study, we have investigated the sensitivity of Mxi2 to mitogens. We report that Mxi2 immunoprecipitates harbor a kinase activity that is stimulated by EGF. Compared to ERK activation, this kinase activity is weaker in magnitude but is more sustained in time, as 40% of its intensity remains 30 min after stimulation. On the other hand, we show that activation of p38 by EGF is minimal, in agreement with previous results (33). p38 can be activated by other mitogenic stimuli, like fibroblast growth factor (26), and plays a relevant role in diverse mitogenic processes, such as myocyte hypertrophy (44) and myeloid (13) and T-cell (6) proliferation. Thus, the existence of a p38 isoform with a marked function under mitogenic stimulation would be in line with the role of p38 in some proliferative situations.
Focusing on the components of the cascade that conveys the mitogenic signal to Mxi2, we show that the EGF-induced activity detected in Mxi2 precipitates is regulated mainly by Ras and not by Rho, Rac, or Cdc42. Although Rac can activate Mxi2, the fact that Mxi2 stimulation by EGF is unaffected by a Rac inhibitory mutant form suggests that Rac may have a role in Mxi2 activation, probably by stress stimuli, by analogy with other p38 members. On the other hand, Ras can mediate the activation of p38 by diverse mitogenic (4, 10, 28, 35) and stress (31) stimuli. The fact that, under some circumstances, p38 can be activated by Ras supports the notion of an isoform specially responsive to Ras-mediated signals. While the mechanisms whereby Ras activates p38 remain elusive, in the case of the kinase activity present in Mxi2 immunoprecipitates, we show that it is activated through the canonical ERK pathway comprising Ras, Raf, and MEK.
It is known that p38 isoforms are mainly activated by MKK3, MKK6, and MKK4 (32). The kinase activity harbored in Mxi2 is responsive to these three MAPKKs, resembling the behavior of p38. In addition, our results indicate that Mxi2 can be activated by MEK1 and -2. However, our data obtained with the inhibitory mutant forms of these MAPKKs indicate that the stimulation of Mxi2 by EGF is essentially conveyed through MEK, although an MKK6-regulated minor component is also apparent. This may indicate that MKK3, -4 and -6 mediate the activation of Mxi2 by the stress-stimulated p38 MAPK module. In light of these results, it could be anticipated that MEK should be the kinase responsible for phosphorylating Mxi2. However, we show that MEK does not coimmunoprecipitate with Mxi2. Even though stable association between MEK and its potential substrates may not be an essential requisite, since ERK mutant forms defective for MEK binding can still be phosphorylated by it (9), this result speaks against the idea that Mxi2 is a good substrate for MEK. This concept is strengthened by our data showing that MEK1 failed to phosphorylate Mxi2 in vitro. On the other hand, in our 32P labeling experiments, phosphorylated Mxi2 appeared in response to EGF and MEK1 stimulation. In the case of EGF, this could be attributed mainly to the MKK6 component, which is capable of phosphorylating Mxi2 in vitro. The fact that MEK1 can also phosphorylate Mxi2 opens the possibility that, in vivo, some unidentified scaffold protein could make low-affinity phosphorylation of Mxi2 possible. This situation would not be unprecedented, as MEK can activate p38 under certain circumstances (4, 29). In any case, the levels of Mxi2 phosphorylation induced by MEK are 10-fold weaker than those detected on the phosphoprotein coimmunoprecipitating with Mxi2, which we have identified as ERK1/2, further demonstrating that Mxi2 is a poor substrate for MEK.
We make evident that ERK1 and -2 can directly associate with Mxi2. Importantly, we have found endogenous Mxi2 and ERKs to coimmunoprecipitate in a physiologically relevant environment like the kidney. This situation is reflected by ectopic expression, as we have detected ERK1/2 in Mxi2 immunoprecipitates and vice versa. Furthermore, ERK-Mxi2 interaction is independent of the activation status of ERKs, since it is equally detected under quiescent and EGF or MEK-stimulated conditions. This association does not imply transphosphorylation between the two kinases, as we demonstrate that, in vitro, Mxi2 is not a substrate for ERK2. Likewise, Mxi2 cannot catalyze phosphotransfer onto ERK2 (our unpublished results). The interaction with Mxi2 appears to be specific for ERKs, as neither JNK nor p38 coimmunoprecipitated with Mxi2. Moreover, our results suggest that Mxi2 binds to ERKs with more efficiency than its splice isoform p38. In our experimental setting, we have not detected any ERKs coimmunoprecipitating with p38. This may seem to contradict a recent report in which an association between p38 and ERK1/2 was shown (50). However, by using the experimental conditions used in that study, a low-stringency lysis buffer, we have been able to reproduce the reported association between p38 and ERKs (unpublished results). This could indicate that the interaction between Mxi2 and ERKs is of a greater affinity than that between p38 and ERKs. Indeed, our in vitro binding experiments demonstrate that Mxi2 binds to ERK2 with fourfold greater affinity than does p38, thus implying that the determinant(s) for high-affinity binding must reside within the unique C terminus of Mxi2. In agreement, we show that deletion or distortion of the C terminus of Mxi2 severely compromises the ability of Mxi2 to bind to ERKs. In this respect, it is noteworthy that the C terminus of Mxi2 exhibits 46% homology to residues 292 to 304 of ERK2 (49). A detailed study of the determinant(s) within the C terminus of Mxi2 responsible for binding to ERKs is under investigation.
Having Mxi2 and ERK in association raises the question of which of the two kinases responds to which stimuli. Since MEK cannot efficiently phosphorylate Mxi2, it is likely that the kinase activity induced by mitogenic stimuli, driven through the Ras-Raf-MEK pathway, is mainly due to ERK function and not to Mxi2. On the other hand, stimuli such as anisomycin and the components of stress-stimulated MAPK modules would preferentially induce Mxi2 activity but not ERKs. Indeed, in vitro, we have shown MKK6 to potently phosphorylate Mxi2. In support of this assumption, our findings from in-gel kinase assays indicate that stimulation with MEK results in myelin basic protein (MBP) phosphorylation at the level of ERK1/2 but none colocalizing with Mxi2. Conversely, upon stimulation with MKK6, phosphorylated MBP is detected comigrating with Mxi2 and not with ERKs (our unpublished results).
We report that interaction of Mxi2 with ERK1/2 has profound consequences for ERK activation. Mxi2 potentiates ERK2 stimulation brought about by activated MEK, but this effect cannot be explained by an augmentation of ERK phosphorylation levels, as Mxi2 does not significantly affect the phosphorylation of ERKs upon stimulation with EGF. On the other hand, it is in agreement with our results indicating that Mxi2 down-regulates the ERK inactivation rate, sustaining the ERK phosphorylated state for >5 h, an effect consistent with the long-lasting kinase activity detected in Mxi2 immunoprecipitates after EGF stimulation that, in light of our results, should be attributed to the function of the associated ERKs. Rather than fixing ERKs in a perpetually phosphorylated state, constitutively active MEK maintains ERK in constant activity by keeping it in a continuous phosphorylation-dephosphorylation cycle. Thus, it is conceivable that a reduction of the rate of the dephosphorylation process, brought about by Mxi2, results in an overall enhancement of ERK activation. Interestingly, we have found that the effect of Mxi2 on ERK dephosphorylation is independent of its kinase activity, as kinase-inactive Mxi2 is identically capable of protecting against ERK deactivation. Moreover, we show that binding of Mxi2 to ERKs is critical to the mechanism by which ERK phosphorylation levels are sustained. Furthermore, this effect is isoform specific, since p38 fails to counteract ERK dephosphorylation. Once again, this may reflect the lower binding affinity of p38 for ERKs.
It could be hypothesized that to exert its effect against ERK dephosphorylation, Mxi2 would interfere with the phosphatase(s) responsible for deactivating ERKs. Binding of Mxi2 to ERKs could pose a steric hindrance, impairing ERK recognition by phosphatases. Since Mxi2 does not affect the phosphorylation of ERKs by MEK or the interaction of ERKs with its substrate Elk1, this would eliminate Mxi2 interference with the binding to a docking domain common to all ERK-interacting proteins, like the CD domain (42). Thus, Mxi2 could impede contacts specific for ERK-phosphatases interactions. In this respect, we demonstrate that Mxi2 enhances ERK2 basal activity, something that speaks in favor of Mxi2 interfering with the dephosphorylation processes that function to maintain ERKs in an inactive state, even under conditions of minimal stimulation. A second possibility is based on our observation that MEK is not phosphorylated by Mxi2 immunoprecipitates, despite the presence of active ERKs. It is conceivable that Mxi2 could somehow interfere with MEK retrophosphorylation by ERKs, something that would hinder the negative feedback control of MEK exerted by ERKs (2), resulting in prolonged MEK activity. These two scenarios are not mutually exclusive and could both contribute to some extent to the maintenance of ERK2 phosphorylation levels, something that is currently under investigation.
Mxi2 binding to ERKs has remarkable consequences for ERK functions. We demonstrate that Mxi2 dramatically up-regulates the transactivation of an Elk1-responsive gene induced by MEK and by EGF. This outcome is independent of Mxi2 kinase activity, as Mxi2 K52R has identical effects, consistent with our results described above. Furthermore, the effect of Mxi2 on Elk1 transactivation is mediated through the ERK pathway and is entirely dependent on ERK being functional. Mxi2 fails to significantly activate Elk1 transcriptional activity when acting alone or in combination with MKK6. In agreement, our previous results demonstrate that Elk1 is a poor substrate for Mxi2 (39). Moreover, an ERK2 inhibitory mutant form completely blocks the synergistic stimulation of Elk1 by MEK-Mxi2. The enhanced Elk1-dependent transcription cannot be primarily due to an incremental increase in the levels of phosphorylated Elk1, as we show that effects of Mxi2 on Elk1 phosphorylation induced by MEK or EGF are, at most, small. Thus, the most likely explanation is that the sustained activity of ERKs caused by Mxi2 results in a more persistent activation of Elk1. In fact, we demonstrate that, upon stimulation with EGF, the time interval in which Elk1 is found in a phosphorylated state is significantly prolonged in the presence of Mxi2. We also demonstrate that a synergistic up-regulation of its transcriptional activity is not a unique response of Elk1 to Mxi2, since another ERK-responsive transcription factor, HIF1α, responds in an identical fashion to the collective action of Mxi2 and MEK. Surprisingly, the potentiating effect of Mxi2 on ERK functions is restricted to nuclear events such as the activation of transcription factors. In line with this, we demonstrate that the presence of Mxi2 has no consequences for ERK-mediated cytoplasmic episodes like the activation of RSK2 or of cPLA2 induced by both MEK and EGF. This is consistent with our observations, to be reported elsewhere, that a considerable proportion of Mxi2 has a nuclear localization.
In summary, our results identify an isoform with unique functions among p38 MAPKs. It is well documented that ERKs and p38 MAPKs have opposing effects in diverse biological (32) and biochemical (50) processes. By contrast, we demonstrate that Mxi2 is capable of potentiating ERK functions. More interestingly, the effects of Mxi2 are site specific, apparently restricted to nuclear events. In light of our findings, it is conceivable that Mxi2, by enhancing ERK functions in the nucleus, could alter the relative contributions of the signals generated by ERK at the different cellular sites. Furthermore, it is well known that sustained versus transient ERK signals have completely different biochemical and biological responses (27). Thus, it can be anticipated that, as a result of its ability to prolong the activation of ERKs in the nucleus, Mxi2 could cause profound changes in ERK-mediated processes.
The biological results of stimuli that switch on multiple MAPK pathways are a consequence of the integration of the signals transduced by each individual route. One way to fine tune such a complex process is through regulatory cross talk between the different MAPK modules. Many such interactions have been described thus far, most of which take place among components upstream of MAPKs (32). Herein, we describe a novel type of regulatory cross talk that occurs through the direct interaction between two MAPKs. A similar interaction has been previously described for p38 (50), albeit with opposing effects. It is intriguing how two almost identical molecules can exert antagonistic effects upon binding to ERKs. One possible explanation is that p38 binds to ERKs in such a way that it sterically blocks ERK phosphorylation by MEK1, as discussed by Zhang et al. (50). On the other hand, binding of the unique high-affinity C terminus of Mxi2 could reposition the bulk of the molecule so that it no longer hinders MEK but instead impedes ERK interaction with phosphatases. Since Mxi2 and p38 are splice variants of the same gene, this opens an attractive scenario in which, by switching the splicing taking place at the p38 locus, the signaling machinery could be programmed to up- or down-regulate ERK activity, with its subsequent alterations of the biological outcomes resulting from ERK activation, an exciting hypothesis that merits some attention.
Acknowledgments
We are indebted to J. L. Woodgett, M. J. Weber, M. H. Cobb, J. S. Gutkind, A. Nebreda, and T. Hunter for providing constructs and L. Bardwell for technical advice.
V.S. is a Universidad de Cantabria predoctoral fellow, and B.C. is a Spanish Ministry of Education predoctoral fellow. This work was supported by grant PM98-0131 from the Spanish Ministry of Education and a grant from the Fundación Marcelino Botín.
REFERENCES
- 1.Ajenjo, N., D. S. Aaronson, E. Ceballos, C. Richard, J. León, and P. Crespo. 2000. Myeloid leukemia cell growth and differentiation are independent of mitogen-activated protein kinase ERK1/2 activation. J. Biol. Chem. 275:7189-7197. [DOI] [PubMed] [Google Scholar]
- 2.Brunet, A., G. Pages, and J. Pouyssegur. 1994. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1). FEBS Lett. 346:299-303. [DOI] [PubMed] [Google Scholar]
- 3.Catling, A. D., H.-J. Schaeffer, C. W. M. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for Raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, G., M. Hitom, J. Han, and D. W. Stacey. 2000. The p38 pathway provides negative feedback for Ras proliferative signaling. J. Biol. Chem. 275:38973-38980. [DOI] [PubMed] [Google Scholar]
- 5.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. Rac-1 and cdc42 control the activity of JNK (SAPK) signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 6.Crawley, J. B., L. Rawlinson, F. V. Lali, T. H. Page, J. Saklatvala, and B. M. Foxwell. 1997. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J. Biol. Chem. 272:15023-15027. [DOI] [PubMed] [Google Scholar]
- 7.Crespo, P., N. Xu, J. L. Daniotti, J. Troppmair, U. R. Rapp, and J. S. Gutkind. 1994. Signaling through transforming G-coupled receptors in NIH 3T3 involves c-Raf: evidence for a protein kinase C independent pathway. J. Biol. Chem. 269:21103-21109. [PubMed] [Google Scholar]
- 8.Dérijard, B., J. Raingeaud, T. Barret, I. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. independent human MAP kinase signal transduction pathway defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 9.Eblen, S. T., A. D. Catling, M. C. Assanah, and M. J. Weber. 2001. Biochemical and biological functions of the N-terminal noncatalytic domain of extracellular-regulated kinase 2. Mol. Cell. Biol. 21:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efimova, T., P. LaCelle, J. F. Welter, and R. L. Eckert. 1998. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem. 273:24387-24395. [DOI] [PubMed] [Google Scholar]
- 11.Faccio, L., A. Chen, C. Fusco, S. Martinotti, J. V. Bonventre, and A. S. Zervos. 2000. Mxi2, a splice variant of p38 stress-activated kinase, is a distal nephron protein regulated with kidney ischemia. Am. J. Physiol. Cell Physiol. 278:781-791. [DOI] [PubMed] [Google Scholar]
- 12.Faccio, L., C. Fusco, A. Chen, S. Martinotti, J. V. Bonventre, and A. S. Zervos. 2000. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J. Biol. Chem. 275:2581-2588. [DOI] [PubMed] [Google Scholar]
- 13.Foltz, I. N., J. C. Lee, P. R. Young, and J. W. Schrader. 1997. Hemopoietic growth factors, with the exception of interleukin-4, activate the p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 272:3296-3301. [DOI] [PubMed] [Google Scholar]
- 14.Freshney, N. W., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in phosphorylation of HSP27. Cell 78:1039-1049. [DOI] [PubMed] [Google Scholar]
- 15.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 25:65-77. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda, M., Y. Gotoh, and E. Nishida. 1997. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, J., J. Lee, L. Bibbs, and R. T. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 18.Hu, S., M. Carozza, M. Klein, P. Nantermet, D. Luk, and R. M. Crowl. 1998. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J. Biol. Chem. 273:34406-34412. [DOI] [PubMed] [Google Scholar]
- 19.Irvine, R. F. 1982. How is the level of free arachidonic acid controlled in mammalian cells? Biochem. J. 204:3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janknecht, R., and T. Hunter. 1997. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 16:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyse, S. M. 2000. Protein Phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr. Opin. Cell Biol. 12:186-192. [DOI] [PubMed] [Google Scholar]
- 22.Lee, J. C., J. C. Laydon, P. C. McDonnel, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, R. J. Heyes, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. Siemens, S. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. 1994. Identification and characterization of a novel protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 23.Lin, A., A. Minden, H. Martinetto, F. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 24.Lin, L., A. Y. Lin, and J. L. Knopf. 1992. Cytosolic phospholipase A2 is coupled to the hormonally regulated release of arachidonic acid. Proc. Natl. Acad. Sci. USA 89:6147-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, L., M. Wartmann, A. Y. Lin, J. L. Knopf, A. Seth, and D. R. J. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72:269-278. [DOI] [PubMed] [Google Scholar]
- 26.Maher, P. 1999. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J. Biol. Chem. 274:17491-17498. [DOI] [PubMed] [Google Scholar]
- 27.Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto, T., K. Yokote, K. Tamura, M. Takemoto, H. Ueno, Y. Saito, and S. Mori. 1999. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J. Biol. Chem. 274:13954-13960. [DOI] [PubMed] [Google Scholar]
- 29.Morooka, T., and E. Nishida. 1998. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J. Biol. Chem. 272:24285-24288. [DOI] [PubMed] [Google Scholar]
- 30.Palmer, A., A. C. Gavin, and A. R. Nebreda. 1998. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17:5037-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palsson, E. M., M. Popoff, M. Thelesman, and L. A. J. O'Neil. 2000. Divergent roles of Ras and Rap in the activation of p38 mitogen-activated protein kinase by interleukin-1. J. Biol. Chem. 275:7818-7825. [DOI] [PubMed] [Google Scholar]
- 32.Pearson, G., F. Robinson, T. G. Gibson, B. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 33.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation in tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 34.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Dérijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rausch, O., and C. J. Marshall. 1999. Cooperation of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways during granulocyte colony-stimulating factor-induced hemopoietic cell proliferation. J. Biol. Chem. 274:4096-4105. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Viciana, P., P. H. Warne, M. D. Waterfield, and J. Downward. 1996. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutations. EMBO J. 15:2242-2251. [PMC free article] [PubMed] [Google Scholar]
- 38.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and M. A. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 39.Sanz, V., I. Arozarena, and P. Crespo. 2000. Distinct carboxy termini confer divergent characteristics to the mitogen-activated protein kinase α and its splice isoform Mxi2. FEBS Lett. 474:169-174. [DOI] [PubMed] [Google Scholar]
- 40.Sohdi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi sarcoma herpes virus G protein-coupled receptor upregulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 41.Suzuki, Y., Y. Imai, H. Nakayama, K. Takahashi, K. Takio, and R. Takahashi. 2001. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8:613-621. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue, T., M. Adachi, T. Moriguchi, and E. Nishida. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2:110-116. [DOI] [PubMed] [Google Scholar]
- 43.Teramoto, H., O. A. Coso, H. Miyata, T. Igishi, T. Miki, and J. S. Gutkind. 1996. Signaling from the small GTP-binding proteins Rac-1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein pathway. J. Biol. Chem. 271:27225-27228. [DOI] [PubMed] [Google Scholar]
- 44.Wang, Y., S. Huang, V. P. Sah, J. J. Ross, J. H. Brown, J. Han, and K. R. Chien. 1999. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273:161-168. [DOI] [PubMed] [Google Scholar]
- 45.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor Ap-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 46.Whitmarsh, A. J., P. Shore, A. D. Sharrocks, and R. J. Davis. 1995. Integration of MAP kinase signal transduction pathways at the serum response element. Science 269:403-407. [DOI] [PubMed]
- 47.Xu, N., L. Bradley, H. Ambdukar, and J. S. Gutkind. 1993. A mutant α subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 90:6741-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanke, B. R., E. A. Rubie, E. Winnet, J. Chan, S. Randall, M. Parsons, K. Boudreau, M. McInns, M. Yan, D. J. Templeton, and J. R. Woodgett. 1996. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J. Biol. Chem. 271:29876-29881. [DOI] [PubMed] [Google Scholar]
- 49.Zervos, A. S., L. Faccio, J. P. Gatto, J. M. Kyriakis, and R. Brent. 1995. Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates max protein. Proc. Natl. Acad. Sci. USA 92:10531-10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, H., X. Shi, M. Hampong, L. Blanis, and S. Pelech. 2001. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J. Biol. Chem. 276:6905-6908. [DOI] [PubMed] [Google Scholar]