Abstract
The degree of cooperation and redundancy between different chaperones is an important problem in understanding how proteins fold in the cell. Here we use the yeast Saccharomyces cerevisiae as a model system to examine in vivo the chaperone requirements for assembly of the von Hippel-Lindau protein (VHL)-elongin BC (VBC) tumor suppressor complex. VHL and elongin BC expressed in yeast assembled into a correctly folded VBC complex that resembles the complex from mammalian cells. Unassembled VHL did not fold and remained associated with the cytosolic chaperones Hsp70 and TRiC/CCT, in agreement with results from mammalian cells. Analysis of the folding reaction in yeast strains carrying conditional chaperone mutants indicates that incorporation of VHL into VBC requires both functional TRiC and Hsp70. VBC assembly was defective in cells carrying either a temperature-sensitive ssa1 gene as their sole source of cytosolic Hsp70/SSA function or a temperature-sensitive mutation in CCT4, a subunit of the TRiC/CCT complex. Analysis of the VHL-chaperone interactions in these strains revealed that the cct4ts mutation decreased binding to TRiC but did not affect the interaction with Hsp70. In contrast, loss of Hsp70 function disrupted the interaction of VHL with both Hsp70 and TRiC. We conclude that, in vivo, folding of some polypeptides requires the cooperation of Hsp70 and TRiC and that Hsp70 acts to promote substrate binding to TRiC.
Protein folding is an essential step in the expression of genetic information, and defects in this process lead to a variety of disease states (11, 19). It is clear that molecular chaperones are key mediators of protein folding in the cell (7, 14, 22). Most studies of cellular folding focus on monomeric protein substrates, but chaperones are also of critical importance in the assembly of oligomeric complexes. There are several functionally and structurally distinct chaperone systems in the eukaryotic cytosol, including Hsp70 and the chaperonin TRiC (TCP1-ring complex; also called CCT) (reviewed in references 8, 12, and 14). The monomeric Hsp70s, in conjunction with cofactors of the Hsp40 family, prevent aggregation by binding to extended polypeptide sequences enriched in hydrophobic amino acids. In contrast, the chaperonin TRiC/CCT is a 900-kDa hetero-oligomeric, toroid-shaped complex that binds nonnative polypeptides within its central cavity and promotes their folding in an ATP-dependent reaction (8, 14). Both Hsp70 and the chaperonin TRiC have been implicated in the folding of a substantial fraction of cellular proteins (45).
As the substrate repertoires observed for these chaperones are not identical, it appears that different cytosolic proteins have different chaperone requirements for folding in the cell. It has been controversial whether newly translated polypeptides normally interact with several different chaperones during folding and, if so, what the functional significance is of these interactions. In some cases, functional overlap between chaperone systems may serve to provide parallel pathways of assisted folding. For instance, DnaK, the Hsp70 system in Escherichia coli, is redundant with the prolyl isomerase trigger factor in the folding of newly translated polypeptides (10, 44). On the other hand, it has been proposed that different chaperone systems cooperate in the folding of some polypeptides. For instance, in vitro translation experiments indicated that folding of firefly luciferase requires the cooperation of Hsp70 and TRiC (17). TRiC has also been proposed to cooperate with the prefoldin/GimC complex in the folding of actin and tubulin (42, 46). However, the degree of cooperation and redundancy between TRiC, Hsp70, and GimC remains poorly defined and controversial. In principle, it is possible that the interaction with multiple chaperone systems is not relevant to the folding reaction and reflects a kinetic partitioning process that facilitates degradation. To gain insight into this question, we here examine the role of these chaperones in the formation of the von Hippel-Lindau protein (VHL)-elongin BC (VBC) tumor suppressor complex, which interacts with both Hsp70 and TRiC upon expression in mammalian cells (13).
Mutations in the VHL gene are associated with both benign and malignant tumors (reviewed in references 9 and 32). Inherited mutations lead to von Hippel-Lindau disease, a disorder that affects approximately 1 in 32,000 people worldwide (9, 32). VHL is also inactivated in ∼80% of sporadic renal clear cell carcinomas, the most common form of kidney cancer. The tumor suppressor function of the 213-amino-acid VHL is dependent on its association with a ubiquitous complex of two small proteins called elongin B and elongin C (herein elongin BC). VBC assembly is essential for VHL function, and the elongin BC-binding site (corresponding to amino acids 157 to 172) is a hot spot for disease-causing mutations (9, 32). VBC associates with a cullin (Cul2) and a ring-finger protein (Rbx1) to form a larger SCF (Skp-Cullin-F box protein)-like ubiquitin-ligase complex (26, 30, 34, 39) that promotes the oxygen-dependent degradation of a subset of proteins required for tumor growth and vascularization, most notably the hypoxia-inducible transcription factor, Hif-1α (25, 27, 36, 38).
We recently proposed that formation of the VBC complex requires the assistance of molecular chaperones (13) which fold the VHL subunit to an assembly-competent conformation that can bind to elongin BC (13, 21). Importantly, VHL folds correctly only in the presence of elongin BC, suggesting that VHL folding is coupled with its assembly into VBC (13). Accordingly, unassembled VHL is unable to fold and adopts an unstructured, protease-sensitive conformation (13). In mammalian cells, the unassembled form of VHL is associated with two different molecular chaperones, Hsp70 (13) and the chaperonin TRiC (13, 21). In vitro translation experiments in TRiC-immunodepleted extracts indicate that this chaperonin is required for VHL incorporation into VBC (13).
The association of VHL with two different chaperone systems, Hsp70 and TRiC, provides an opportunity to explore the interplay between these chaperones. We envision several possibilities to explain the observed association of VHL with both Hsp70 and TRiC. First, TRiC may participate in VHL folding, as suggested by in vitro translation experiments (13), but the interaction with Hsp70 may not be required for folding. Alternatively, these different chaperones may fold VHL by two independent, and perhaps redundant, pathways. Finally, VHL may require that both chaperones cooperate in the folding reaction. To distinguish between these possibilities, we reconstituted VBC assembly in Saccharomyces cerevisiae to exploit conditional chaperone mutants available in this system. We found that VBC is correctly assembled upon the expression of VHL and elongin BC in S. cerevisiae. Using cells bearing temperature-sensitive mutations in a TRiC subunit, we demonstrate the requirement of TRiC for VHL folding and VBC assembly in intact cells. Furthermore, analyses of a conditional mutation in the cytosolic Hsp70 Ssa1p indicate that Hsp70 is also required for this process. As both chaperone systems must be functional for folding, our results indicate that formation of the VHL tumor suppressor complex requires the cooperation of both chaperone systems. Furthermore, since the VHL-TRiC interaction is lost in the absence of functional Hsp70, it appears that Hsp70 functions to promote or stabilize substrate binding to the chaperonin TRiC/CCT.
MATERIALS AND METHODS
Strains and plasmids.
The temperature-sensitive TRiC/CCT mutant cct4ts (DDY0299; also known as anc2-1) and its isogenic wild type (DDY0186) were the gift of David Drubin (47). The temperature-sensitive ssa1-45 (herein ssa1ts) and isogenic wild-type SSA1 (herein SSA1wt) yeast strains were the gift of Elizabeth Craig (31). The His6-VHL coding region from pET24a His6-VHL (13) was cloned into pESC (Ura3) under the control of a Gal1 promoter (Stratagene). The pCup His6-VHL (Ura3) plasmid was then generated by inserting His6-VHL from pESC-His6-VHL (Ura3) downstream of the copper-inducible promoter of pCu426 (33). myc-tagged elongin B from pCITE-myc-elongin B (13) was cloned into pESC (Leu2) downstream of the Gal10 promoter. pESC-mycBC (Leu2) was generated by inserting myc-tagged elongin C downstream of the Gal1 promoter in the pESC-myc-elongin B (Leu2) construct. To make the pESC-mycBC (His3) plasmid, the entire myc-elongin B-Gal1/10-myc-elongin C cassette from pESC-mycBC (Leu2) was replaced in the corresponding region in pESC (His3).
Protein expression and lysate preparation.
Yeast cells were transformed by the lithium acetate method, as previously described (2). Transformants were plated on synthetic glucose medium (SD medium; Qbiogene) lacking the appropriate amino acid(s) for selection. To prepare cell extracts, transformants were inoculated in 100- to 200-ml SD medium and grown overnight at 30°C (or 23°C when indicated). For galactose-inducible proteins, cells were collected by centrifugation, resuspended in synthetic galactose medium (SGal; Qbiogene) at a final optical density at 600 nm (OD600) of 0.4 in a 1-liter culture, and incubated for 16 to 20 h with shaking. For analysis of the ssa1ts strain, VHL was also expressed from a copper-inducible promoter to exploit its rapid induction kinetics. Cultures of wild-type and mutant cells were incubated as described above to generate a pool of galactose-induced elongin BC and were then shifted to 37°C. Following a 15-min incubation to achieve the temperature-sensitive phenotype of ssa1ts, VHL expression was induced for 45 min by the addition of 0.2 mM CuSO4.
For the analysis of radiolabeled cells, overnight cultures of yeast transformants grown in SD medium were harvested by centrifugation, resuspended in SGal lacking amino acid selection markers to an OD600 of 0.4, and grown overnight with shaking. Cells were then labeled at an OD600 of 8 for 2 h after transfer to SGal lacking methionine as well as the selective amino acids and containing 0.1 mCi of [35S]Met-Cys per ml of culture.
Yeast lysates were prepared essentially as described previously (2). Briefly, cells were harvested by centrifugation, weighed, and resuspended in 3 volumes (wt/vol) of ice-cold water. The cell slurry was mixed with 3 volumes of ice-cold buffer A (50 mM Tris-HCl [pH 8.0], 150 mM ammonium sulfate, 10% glycerol) supplemented with 50 μl of mammalian protease inhibitor cocktail (Sigma)/ml and 4 volumes of acid-washed glass beads. Cells were then lysed by four cycles of vigorous vortex mixing for 1 min, followed by a 2-min incubation on ice. The supernatant was removed, and the beads were washed with 2 volumes of buffer A-50 μl of mammalian protease inhibitor cocktail/ml. The combined supernatants and washes were then clarified by centrifugation at 30,000 × g for 1 h. Cell extracts were aliquoted and stored at −80°C. Protein concentrations, typically between 2 and 5 mg/ml, were measured using the BCA assay (Pierce), and [35S]methionine incorporation was measured by trichloroacetic acid precipitation.
To generate recombinant VBC, His6-VHL, His6-elongin B, and His6-elongin C were expressed and purified from E. coli BL-21 inclusion bodies. The purified inclusion bodies were denatured in buffer B (50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 0.5% Triton X-100, 10% glycerol)-6 M guanidine-HCl, bound to TALON cobalt metal affinity resin (Clontech), and purified per the protocols of the manufacturers. The purified, denatured proteins were mixed in a 1:4:1 V:B:C ratio and renatured by stepwise dialysis from 6 to 0 M guanidine in buffer B. Mammalian cell extracts expressing VBC were generated by transfection as described previously (13).
Antibodies and immunoprecipitation.
Mouse monoclonal anti-myc 9E10 antibody was purchased from Convance-Babco. The elongin-specific antibodies were the kind gift of Joan Weliky-Conaway. Guinea pig antiactin was the kind gift of David Botstein. Rabbit anti-Ssa1/2p polyclonal serum was a generous gift from Doug Cyr. Rabbit anti-TRiC polyclonal serum was prepared against subunit CCT1 of yeast. To facilitate VHL detection, we raised a mouse monoclonal anti-VHL antibody (6D10), which was prepared as described previously (3). This anti-VHL antibody can specifically recognize VHL in immunoprecipitation and immunoblot analyses.
Immunoprecipitations for the myc-tagged elongin proteins were performed as follows. Anti-myc 9E10 mouse monoclonal antibody (between 2 and 5 μl) was incubated with lysates for 1 h at 4°C, followed by additional incubation with 25 μl of protein G-agarose (Amersham-Pharmacia) (packed volume) for 30 min at 4°C. The immune complexes were washed five times with buffer B. Correctly folded VBC is resistant to stringent detergent washes, and thus, 0.1% Sarkosyl was included in the wash to evaluate the strength of the VHL-elongin BC interaction in the wild-type and mutant strains. The anti-VHL immunoprecipitations were performed in a similar manner, except that the anti-VHL monoclonal antibody 6D10 was prebound to the protein G-agarose beads for 30 min before addition of the lysate. The immunoprecipitation reaction was incubated for 1 h at 4°C and washed as above. Typically, immunoprecipitation reactions of total lysates contained between 0.5 and 5 mg of total protein.
Other biochemical assays.
Size exclusion chromatography was carried out using a Superose 6 column (Pharmacia) equilibrated in buffer B according to the manufacturer's instructions. Nondenaturing gel electrophoresis, protease sensitivity assays, and actin folding assays were performed essentially as described previously (15, 17). Using a NAP-5 column (Amersham-Pharmacia) to remove protease inhibitors, yeast lysates were prepared for protease sensitivity assays by buffer exchange. To test the protease sensitivity of VHL, lysates in buffer C (50 mM Tris-HCl [pH 8.0], 50 mM KCl, 10 mM CaCl2, 10% glycerol) were incubated at 4°C with 200 μg of thermolysin/ml for the times indicated. The amount of lysate subjected to digestion was normalized to contain the same total amount of VHL, and the reaction was stopped by the addition of EDTA to achieve a 10 mM final concentration. To evaluate actin folding, lysates were exchanged into buffer D (10 mM Tris [pH 7.5], 1 mM CaCl2, 1 mM dithiothreitol, 10% formamide, 10% glycerol, 0.2 mM ATP) and adjusted to equal protein concentrations. Binding to DNase I beads was performed exactly as described previously (15), and protease sensitivity was tested by incubation with 10 μg of proteinase K/ml at 4°C. Samples were removed at the times indicated, and reactions were stopped with the addition of phenylmethylsulfonyl fluoride to achieve a 2 mM final concentration. In all cases, the reactions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblot analysis using VHL-, myc-, or actin-specific antiserum.
RESULTS
Assembly of correctly folded VBC upon expression of VHL and elongin BC in S. cerevisiae.
To assess the chaperone requirements for VHL folding and assembly in vivo, we established a system to study VBC biogenesis in yeast. There is a high level of conservation between the types of cytosolic chaperone machinery seen in mammalian and yeast cells. The yeast chaperonin TRiC/CCT is encoded by eight genes (named CCT1-8) (43). The yeast cytosol also contains multiple Hsp70 homologues, including the four soluble cytosolic Hsp70 genes (named Ssa1 to -4) and three ribosome-associated Hsp70s (named Ssb1/2 and Ssz/Pdr13) (5, 14). Whereas the ribosome-associated Hsp70s are dispensable, Ssa function is essential for viability (5, 29). S. cerevisiae appears to lack homologues of VHL and elongin B, although it contains a homologue of elongin C (1, 28).
VHL and the elongin BC proteins were expressed in yeast under the control of inducible promoters (Fig. 1A). To facilitate detection, VHL contained an N-terminal polyhistidine tag and the elongin proteins contained N-terminal myc epitope tags. Importantly, previous experiments indicated that the tags do not interfere either with VHL folding and chaperone interactions or with VBC assembly (13). Extracts from cells transformed with plasmids encoding the components of VBC were subjected to SDS-PAGE followed by immunoblot analysis with antibodies specific for VHL, elongin B, and elongin C (Fig. 1B). Since both elongin proteins contained a myc epitope tag, they were also detected using myc-specific antibodies (Fig. 1B; note that under these PAGE conditions, elongin B and elongin C comigrate). Our analysis indicated that all three proteins were expressed as expected. The highest level of expression was reproducibly obtained in cells expressing all three components of VBC, suggesting that assembly of the complex stabilizes both VHL and the elongin proteins against degradation.
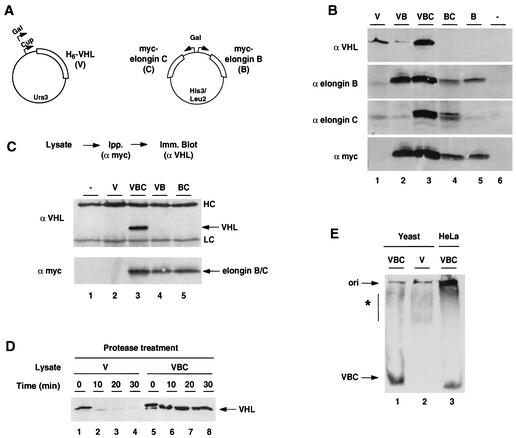
FIG. 1.
Expression and assembly of correctly folded VBC complex in S. cerevisiae. (A) The scheme shows the expression strategy for VHL and the elongin BC proteins. Polyhistidine-tagged VHL (V) was expressed under control of either galactose or copper-inducible promoters. The myc-tagged elongin BC proteins (in a separate plasmid) were under galactose-inducible control; one version of the plasmid contained myc-elongin B alone (designated B), and the other contained both myc-elongin B and myc-elongin C (designated C). The selection markers are also indicated. (B) VHL and the elongin BC proteins are expressed in yeast. Lysates (50 μg) were prepared from yeast expressing His6-VHL (V), His6-VHL plus myc-elongin B (VB), His6-VHL plus myc-elongins B and C (VBC), myc-elongins B and C (BC), myc-elongin B (B), or empty vectors (−); separated by SDS-PAGE; and immunoblotted with antibodies against VHL, elongin B, elongin C, or the myc epitope tag. (C) Formation of a stable VHL-elongin B-elongin C complex. Using antibodies against the myc epitope tag followed by immunoblot detection (Imm. Blot) of VHL, the association between different components of VBC in yeast was monitored by immunoprecipitation (Ipp.) of the elongin proteins. Lysates from yeast transformed with empty vectors (−), V, VBC, VB, or BC (as indicated) were used in this analysis. VHL was associated with elongins only in lysates containing all three components of VBC. The heavy and light chains from the anti-myc antibodies are indicated (HC and LC, respectively). (D) VHL in VBC is in a protease-resistant conformation. Lysates prepared from yeast transformed with VHL alone (V) or with VBC were treated with thermolysin and analyzed by SDS-PAGE and immunoblotting with VHL-specific antibodies. (E) Nondenaturing gel analysis of VHL-containing complexes. The complexes formed by VHL in yeast expressing either VHL with elongin BC (VBC; lane 1) or VHL alone (V; lane 2) were compared with the VBC complexes expressed in mammalian cells (lane 3). Lysates containing approximately equivalent levels of VHL were separated by nondenaturing PAGE, and VHL was detected by immunoblot analysis with specific antibodies. The slight differences in migration of the VBC complexes from different sources are caused by differences in the epitope tags of the components of the complexes (N-terminal polyhistidine-tagged VHL in yeast and FLAG-tagged VHL in mammalian cells). The origin of the gel is indicated (ori). The asterisk indicates slowly migrating putative complexes between VHL and endogenous yeast components.
We next examined whether VBC was assembled in yeast. The association of VHL with elongin BC was assessed by immunoprecipitation of the yeast lysates with anti-myc antibodies, followed by immunoblot detection of VHL (Fig. 1C). VHL was only found in a complex with elongins when both elongin B and elongin C were expressed (Fig. 1C; compare lanes 3 and 4). Similar results were obtained when VBC assembly was examined by immunoprecipitation of VHL followed by immunoblot detection of the elongin proteins (data not shown). The observed requirement for all three components of VBC for assembly is consistent with previous results that examined these interactions using two-hybrid analysis (40). Importantly, VBC assembled in yeast was resistant to treatment with 0.1% Sarkosyl, as observed for correctly folded mammalian VBCs (13).
Sensitivity to mild protease treatment was employed as an independent assay for VHL folding. Resistance to low concentrations of protease is a characteristic of correctly folded, compact proteins (17). Indeed, experiments in mammalian systems indicated that correctly assembled VHL in VBC is in a folded, protease-resistant conformation whereas mutant or unassembled VHL is susceptible to protease digestion (13). Treatment of yeast extracts with thermolysin indicated that VHL expressed in the absence of elongin BC was in a protease-sensitive conformation (Fig. 1D). In contrast, VHL coexpressed with elongin BC was protease resistant (Fig. 1D), supporting the idea that yeast cells contain the machinery to incorporate VHL into a correctly folded VBC complex, as observed in mammalian cells.
The VHL-containing complexes formed in S. cerevisiae were also examined by nondenaturing gel electrophoresis followed by immunoblot detection of VHL (Fig. 1E). The yeast VBC lysate (Fig. 1E, lane 1) contained a VHL-reactive complex with similar mobility to that of VBC expressed in mammalian cells (Fig. 1E, lane 3). Most of the elongin BC in the lysate comigrated with this VHL-reactive band (data not shown), arguing that it corresponds to VBC. Importantly, the majority of VHL in the VBC lysate was present in this complex, indicating that most of the VHL was incorporated into VBC. Furthermore, this band was not observed when VHL was expressed in the absence of elongin BC (Fig. 1E, lane 2). Instead, VHL was distributed in several slowly migrating broad bands (Fig. 1E; note that some of these bands are also present in lane 3). Since immunoblot analysis indicates that SSA and TRiC migrate in this region of the gel (data not shown), these bands may correspond to complexes between VHL and these endogenous chaperones or other cellular components involved in either assembly or degradation (see below and Fig. 2). Notably, we obtained identical VHL migration patterns in wild-type cells and in cells lacking the yeast elongin C homologue (data not shown), suggesting that the endogenous yeast elongin C cannot substitute for mammalian elongin BC in VHL folding.
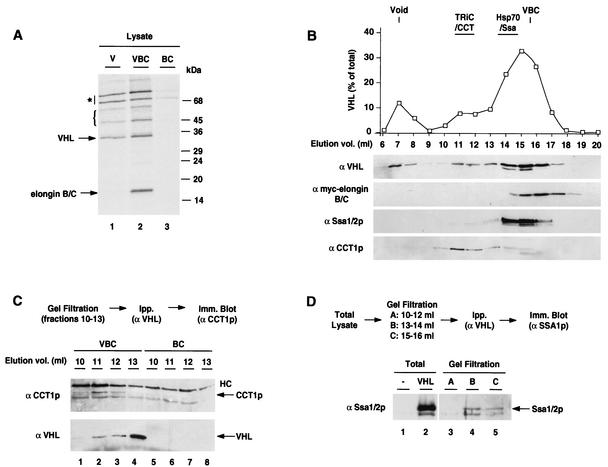
FIG. 2.
Association of VHL with molecular chaperones in S. cerevisiae. (A) VHL-containing complexes immunoisolated from 35S-labeled lysates. Yeast cells expressing VHL alone (V; lane 1), VHL and elongin BC (VBC; lane 2), or elongin BC (BC; lane 3) were metabolically labeled with [35S]methionine. Following lysis, equal amounts of total counts (106 cpm) were immunoprecipitated using VHL-specific antibodies and analyzed by SDS-PAGE and autoradiography. The positions of VHL and the elongin BC bands are indicated. The position of endogenous yeast proteins specifically associated with VHL are also indicated; the bracket indicates a set of bands with molecular masses similar to those of the components of the TRiC/CCT complex, whereas the asterisk indicates two bands migrating around 70 to 80 kDa. (B to D) Size exclusion chromatography analysis of VHL complexes in VBC lysates. (B) Elution profile of VHL, elongin BC, the Hsp70 Ssa1/2p, and TRiC/CCT following fractionation on a Superose 6 column. The graph shows the elution profile of VHL quantitated by densitometric analysis of immunoblots. (C) Association of VHL with the chaperonin TRiC/CCT. High-molecular-weight fractions (10 to 13 ml) of a Superose 6 fractionation of VBC lysates (as described for panel B) or BC lysates (as negative controls) were subjected to immunoprecipitation with VHL-specific antibodies followed by immunodetection (using antibodies directed against subunit CCT1) of TRiC. TRiC was only detected in the high-molecular-weight fractions of the VHL-containing lysates. The IgG heavy chain (HC) is indicated. (D) Association of VHL with the Hsp70 Ssa1/2p. Lysates and Superose 6 column fractions (pooled as indicated) were immunoprecipitated using a VHL-directed antibody followed by immunoblot detection of Ssa1/2p. A lysate from cells transformed with vector alone was used as a control.
Taken together, these independent assays of folding and assembly indicate that yeast contains the machinery to incorporate VHL into a correctly folded VBC complex similar to that generated in mammalian cells. We next employed these assays to assess the chaperone interactions of VHL and their requirement for VBC assembly.
Association of VHL with yeast chaperones.
The possible association of VHL with endogenous yeast chaperones was investigated by two independent approaches. First, VHL complexes were immunoisolated from 35S-labeled lysates from yeast expressing VHL, VBC, or BC (Fig. 2A). In addition to elongin BC, several endogenous 35S-labeled proteins were associated with VHL, including a set of bands ranging in size from 50 to 60 kDa and two prominent bands at ∼70 to 80 kDa. Notably, the size range of these bands is consistent with the molecular mass of the chaperones TRiC and Hsp70, suggesting that these chaperones may also participate in VHL folding in yeast. Indeed, immunoblot analysis of VHL immunoprecipitations confirmed the association with TRiC and the Hsp70 Ssa1/2p (see below and Fig. 2C and D).
We next examined the distribution of VHL in cellular complexes using size exclusion chromatography. To analyze the interactions of VHL under conditions in which it forms VBC, lysates from cells expressing VHL and elongin BC were separated on a Superose 6 column and the elution profiles of different proteins were assessed by immunoblotting (Fig. 2B). The majority of VHL was present in a broad peak that overlapped with the elongin BC peak (15 to 16 ml; Fig. 2B); importantly, native gel analysis confirmed that most of the VHL in these fractions was assembled in VBC (data not shown). VHL was also present in higher-molecular-weight fractions containing the yeast Hsp70 Ssa1/2p (14 ml; Fig. 2B). In addition, VHL also eluted in fractions containing the chaperonin TRiC/CCT (fractions 11 to 13; Fig. 2B). The elution profile resembled that previously observed for VHL expressed in mammalian cells (13) and was consistent with the idea that VHL interacts with both Hsp70/Ssa and the chaperonin TRiC/CCT. Indeed, immunoprecipitation of individual column fractions with a VHL-specific antibody followed by immunoblot detection of the CCT1 subunit of the TRiC complex confirmed the VHL-TRiC interaction in the high-molecular-weight complex (Fig. 2C). In contrast, TRiC was not detected in control immunoprecipitations using the equivalent column fractions from lysates lacking VHL but expressing elongin BC (Fig. 2C; compare lanes 2 and 6). Notably, binding to the chaperonin appeared to render VHL less accessible to recognition by the antibody, since the efficiency of the VHL immunoprecipitation was reduced in the VHL-TRiC column fractions (compare immunoprecipitation results in Fig. 2C with totals in panel B). Presumably, VHL binding within the central cavity of the chaperonin decreases the availability of some VHL regions to the solution.
Similar immunoprecipitation experiments confirmed the VHL-Ssa1/2p interaction in the lower-molecular-weight fractions (Fig. 2D). Ssa1/2p eluted in a broad peak that partially overlapped with the VBC-containing fractions. However, most of the VHL-associated Ssa1/2p was detected in the early-eluting fractions, where VBC was absent (Fig. 2D; compare lanes 4 and 5). Although VHL-Hsp70 and VBC had overlapping elution profiles when analyzed by gel filtration, the Hsp70-containing complexes displayed very different mobilities from VBC upon native gel analysis (Fig. 1E and data not shown). Furthermore, immunoblot analysis of native gels indicated that VBC does not contain Hsp70 (data not shown). Since elongin BC is also absent from the TRiC-VHL high-molecular-weight fractions, it appears that only unassembled VHL interacts with Hsp70 or TRiC. From these experiments, we conclude that VHL associates with TRiC and Hsp70 in yeast, indicating that VBC assembly in S. cerevisiae is assisted by the same chaperone machinery as in mammalian cells.
The cytosolic Hsp70 Ssa is required for VHL folding.
We next examined the role of Hsp70 in VHL biogenesis using a genetic approach. All components of VBC were expressed in a strain lacking SSA2-4 and carrying as its only source of cytosolic Hsp70 a temperature-sensitive allele of SSA1 (ssa1ts) (4). Cells carrying this mutation grow well at 30°C but stop growing upon exposure to 37°C (reference 4 and data not shown). This strain has been previously used to demonstrate that Ssa1p function is required to fold the cytosolic enzyme ornithine transcarbamylase (31). To specifically assess the requirement of Ssa1p in VHL folding, we separated the induction of VHL from synthesis of elongin BC. The elongin BC complex (under the control of a galactose-inducible promoter) was expressed overnight at the permissive temperature. The cells were then shifted to the nonpermissive temperature, and VHL (under the control of a copper-inducible promoter) was induced for 45 min. This experimental design allowed us to express VHL in the absence of functional Ssa1p but in the presence of preformed elongin BC.
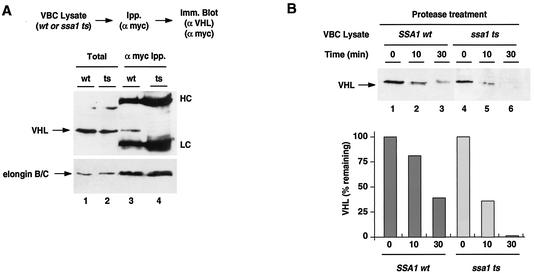
The incorporation of VHL into VBC was examined by coimmunoprecipitation using stringent conditions as described for Fig. 1C. Briefly, VHL was induced in either ssa1ts or SSA1wt at 37°C, and its binding to elongin BC was assessed by immunoprecipitation using myc-specific antibodies, followed by immunoblot analysis (Fig. 3A). Although the elongin BC complex was efficiently immunoprecipitated from these extracts, loss of SSA function resulted in a dramatic reduction in the amount of VHL associated with elongin BC (Fig. 3A, compare lanes 3 and 4), indicating that functional Hsp70 is required for VBC assembly. Similar results were obtained when VBC assembly was examined by immunoprecipitation using VHL-specific antibodies followed by immunoblot analysis of elongin BC (data not shown). As an independent test of VHL folding in these strains, the protease sensitivity of VHL was examined. Properly folded VHL (assembled into VBC) is more resistant to protease digestion than incorrectly folded VHL (13) (Fig. 1D). The folded state of VHL expressed in the absence of functional Ssa1p was thus examined by mild thermolysin treatment (Fig. 3B). VHL expressed at the nonpermissive temperature in the ssa1ts strain was substantially more protease sensitive than VHL expressed in the SSA1wt strain at the same temperature (Fig. 3B). These separate assays indicate that functional Hsp70 is required for correct VHL folding and assembly into VBC.
FIG. 3.
The cytosolic Hsp70 Ssa1/2p is required for VHL folding and VBC assembly. (A) VHL is not incorporated into VBC in cells lacking Hsp70/Ssa1/2p function. VHL and elongin BC were expressed in SSA1wt and ssa1ts cells. Formation of VBC in SSA1wt (lanes 1 and 3) and ssa1ts (lanes 2 and 4) was monitored by immunoprecipitation (Ipp.) of myc-elongin BC-associated proteins (lanes 3 and 4). The top panel shows the results of immunoblot detection (Imm. Blot) of VHL; the bottom panel shows the results of immunoblot detection of elongin BC. Lanes 1 and 2 (Total) represent 10% of the amount of input lysate used in the immunoprecipitations. The heavy and light IgG chains are indicated (HC and LC, respectively). (B) VHL is in a protease-sensitive conformation in the ssa1ts cells. Lysates from VBC-expressing cells (SSA1wt, lanes 1 to 3; ssa1ts, lanes 4 to 6) were treated with thermolysin for the indicated times, and VHL was detected by immunoblot analysis. The graph shows the fraction of VHL remaining at each time point (expressed as percentage of initial amount) as quantified by densitometry. Results are representative of three similar experiments.
The cytosolic chaperonin TRiC/CCT is required for VHL folding.
We next examined whether TRiC is required for VBC assembly. To this end, we employed a mutant strain carrying a temperature-sensitive mutation in subunit 4 of the TRiC/CCT complex (herein cct4ts; also called anc2-1) (47). This mutant is defective in actin filament assembly and is hypersensitive to the anti-microtubule drug benomyl, suggesting that tubulin assembly is also impaired (47). To provide direct evidence that this mutation impairs chaperonin-mediated folding, we first examined its effect on the biogenesis of correctly folded actin. Two established folding assays, binding to DNase I and sensitivity to mild protease treatment, were used to assess actin folding in the cct4ts strain and its isogenic wild-type strain (Fig. 4A and B). Since tight binding to DNase I is a hallmark of correctly folded actin (15), lysates of both strains containing equal amounts of actin were incubated with DNase I coupled to Sepharose beads and the levels of bound actin were determined using actin-specific antibodies (Fig. 4A). The results of this experiment indicated that the levels of folded actin were greatly reduced in the cct4ts strain (Fig. 4A, lanes 3 and 4). This conclusion was further supported by the results of the protease sensitivity assay, which indicated that actin expressed in the cct4ts strain was substantially more susceptible to proteinase K treatment than actin from the wild-type strain (Fig. 4B). Thus, this mutation in CCT4 results in a biochemical defect in the ability of the chaperonin to fold actin.
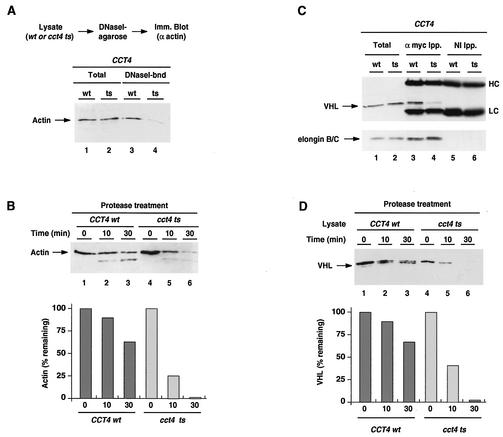
FIG. 4.
The cytosolic chaperonin TRiC/CCT is required for VHL folding and VBC assembly. (A and B) TRiC/CCT-mediated folding is impaired in the anc2-1/cct4ts cells. TRiC/CCT function in mediation of folding of endogenous actin was monitored according to binding to DNase I beads (A) and formation of protease-resistant actin (B). (A) Actin binding to DNase I-Sepharose beads examined in lysates of the cct4ts strain (lanes 1 and 3) and its isogenic wild-type strain (lanes 2 and 4). The binding assay was performed as described previously (15), and actin was detected by immunoblot analysis (Imm. Blot). Lanes 1 and 2 show the results for 10% input lysate; lanes 3 and 4 show the results for actin bound to DNase I-Sepharose beads. (B) Actin from cct4ts cells is in a protease-sensitive conformation. CCT4wt (lanes 1 to 3) and cct4ts (lanes 4 to 6) lysates were treated with proteinase K for the indicated times, and actin protein was detected by immunoblot analysis. The graph shows the fraction of actin remaining at each time point (expressed as percentage of initial amount) as quantified by densitometry. Results are representative of four similar experiments. (C and D) VHL folding and assembly are impaired in cct4ts cells. (C) VBC formation in the CCT4wt (lanes 1, 3, and 5) and cct4ts (lanes 2, 4, and 6) strains was examined by coimmunoprecipitation (Ipp.) of VHL with the elongin BC proteins (lanes 3 and 4). Lanes 1 and 2 (Total) represent 10% of the amount of input lysate used in the immunoprecipitations. Nonimmune mouse antibodies were used as negative controls (lanes 5 and 6). The top panel shows results for immunoblot detection of VHL; the bottom panel shows results for immunoblot detection of elongin BC. IgG heavy and light chains from the immunoprecipitations are indicated (HC and LC, respectively). (D) VHL is in a protease-sensitive conformation in the cct4ts cells. Lysates from VBC-expressing cells (CCT4wt, lanes 1 to 3; cct4ts, lanes 4 to 6) were treated with thermolysin for the indicated times, and VHL was detected by immunoblot analysis. The graph shows the fraction of VHL remaining at each time point (expressed as percentage of initial amount) as quantified by densitometry. Results are representative of six similar experiments.
We next employed the cct4ts mutant to examine the chaperonin requirement in VHL folding and assembly into VBC. VHL and elongin BC were expressed in wild-type and mutant CCT4 strains, and the formation of VBC was analyzed by immunoprecipitation using myc-specific antibodies, as described above (Fig. 1C and 3A). As observed for actin folding, VBC formation was substantially impaired in the cct4ts mutant strain at the nonpermissive temperature (Fig. 4C; compare lanes 3 and 4). Furthermore, VHL expressed in the cct4ts strain was also more susceptible to proteolytic treatment than VHL from the wild-type strain (Fig. 4D). Notably, the extent of the folding defect was comparable to that observed for actin folding (Fig. 4B). We conclude from these experiments that TRiC/CCT function is required for VHL folding and the correct assembly of VBC.
Hsp70 function is required for interaction of VHL with TRiC.
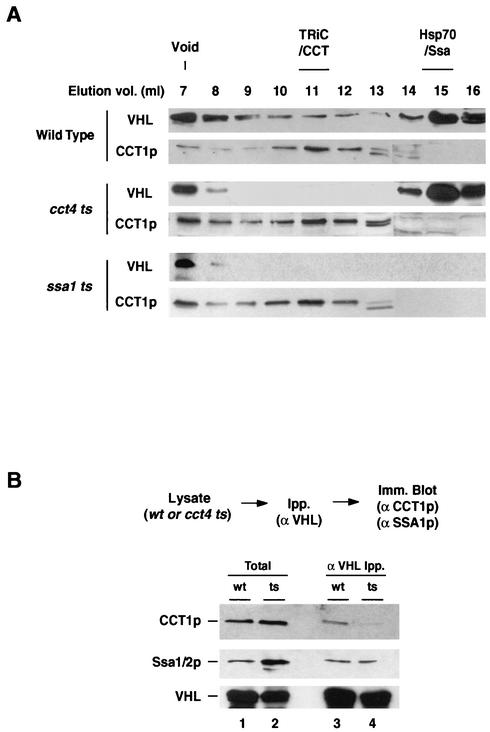
Several models may account for the observed association of VHL with Hsp70 and TRiC. Hsp70 and TRiC may associate with the folding polypeptide independently of one another. If this were the case, inactivation of one chaperone system would not affect interaction of VHL with the other chaperone. Alternatively, VHL may interact with these chaperones along a sequential pathway of assisted protein folding, in which case loss of function of one chaperone protein would affect the association of VHL with the remaining one. To distinguish between these possibilities, we used gel filtration chromatography to examine the VHL-chaperone interactions in the ssa1ts and cct4ts mutant cells at the nonpermissive temperature (Fig. 5A). The elution profiles of VHL in both CCT4 and SSA1 wild-type VBC extracts were comparable to those obtained at the permissive temperature (shown for CCT4wt in Fig. 5A; compare with Fig. 2B). In contrast, the elution profile of VHL in the cct4ts strain showed a clear reduction in the levels of VHL-TRiC compared to that in the wild type but no reduction in the VHL-Hsp70 peak (Fig. 5A; compare VHL elution profiles for wild-type and cct4ts extracts). Strikingly, both the Hsp70-VHL complex and the TRiC-VHL complex were absent in the ssa1ts cells (Fig. 5A). Instead, all of the VHL expressed in the Hsp70 mutant cells was present in high-molecular-weight species that eluted in the void volume of the Superose 6 column (Fig. 5A). These results suggest that in the absence of functional Hsp70, VHL is unable to bind to the chaperonin and probably forms soluble high-molecular-weight aggregates. Since the loss of TRiC-VHL interaction may arise from a reduction in the chaperonin levels in the mutant cells, we examined the elution profiles of TRiC in these extracts. All mutant strains contained a prominent chaperonin peak eluting at its expected volume on gel filtration chromatography (Fig. 5A), indicating that the TRiC complex is still present in these cells. Furthermore, TRiC in the ssa1ts lysates can bind exogenously added, chemically denatured 35S-labeled actin, indicating that it retains its intrinsic substrate-binding ability (A. S. Meyer, A. J. McClellan, and J. Frydman, unpublished observations). The effect of the cct4ts mutation on VHL-chaperone interactions was also examined by immunoprecipitation of VHL followed by immunoblot analysis (Fig. 5B). Consistent with the gel filtration chromatography results, we observed decreased VHL-TRiC binding in the cct4ts strain compared to that observed with the wild type (Fig. 5B, top panel; compare lanes 3 and 4). In contrast, the interaction of VHL with Hsp70 appeared largely unaffected (Fig. 5B, middle panel, lanes 3 and 4). It thus appears that binding to Hsp70 is independent of TRiC function, whereas Hsp70 is required to load VHL onto the chaperonin complex. These results are consistent with previous findings indicating that Hsp70 and TRiC cooperate in an organized and sequential pathway of chaperone-assisted protein folding (17).
FIG. 5.
Association of VHL with TRiC requires Hsp70 function. (A) Size exclusion chromatography analysis of VHL complexes in cells defective for TRiC and Hsp70 function. VBC lysates were prepared following expression in wild-type, cct4ts, and ssa1ts cells, fractionated on a Superose 6 column, and analyzed by immunoblot analysis as described for Fig. 2 except that expression was carried out at 37°C for 4 h. The elution profiles for VHL and TRiC/CCT are presented. Similar elution profiles (shown for CCT4 wt) were obtained for the CCT4wt and the SSA1wt strains. Void, void volume of the Superose 6 column. (B) Effect of the cct4ts mutation on VHL-chaperone interactions. Lysates from cells expressing VBC at 37°C (CCT4wt, lanes 1 and 3; cct4ts, lanes 2 and 4) were analyzed by immunoprecipitation (Ipp.) with anti-VHL antibodies. Associated chaperones were detected by immunoblot analysis (Imm. Blot). Lanes 1 and 2 show results for the 10% input lysate used in the immunoprecipitations; lanes 3 and 4 show immunoprecipitated material. The top panel shows results for immunoblot detection of CCT1, and the middle panel shows results for immunoblot detection of Ssa1/2p. An immunoblot for VHL is included as an immunoprecipitation control (bottom panel).
DISCUSSION
To gain insight into the interplay between different chaperone systems in the eukaryotic cytosol, we have examined the role of Hsp70 and TRiC in the folding and assembly of the VHL tumor suppressor protein in intact cells. To this end, we employed S. cerevisiae as a model system that can successfully reproduce the VBC assembly process observed in mammalian cells. According to various criteria, including resistance to stringent detergent washes, migration on nondenaturing gels, and resistance to mild proteolytic treatment, VHL and the elongin BC proteins expressed in S. cerevisiae assemble into a complex that behaves like VBC from mammalian cells (Fig. 1). Native gel analysis and gel filtration chromatography indicate that in the presence of elongin BC, the majority of VHL expressed in yeast is assembled into VBC (Fig. 1E and 2B). VHL folding in vivo requires expression of all three components of VBC, even though yeast contains an elongin C homologue that can bind to VHL in vitro (1, 6). These results suggest that yeast elongin C cannot replace the mammalian elongin C in VHL folding. Notably, VHL appeared to be unstable in the absence of elongin BC coexpression, consistent with reports that tumor-causing mutants of VHL that impair VBC formation are degraded by the ubiquitin-proteasome system (41).
The interaction of VHL with cellular chaperones was also conserved in the yeast system. Previous experiments in mammalian cells indicated that VHL associates with both TRiC and Hsp70 (13). These chaperones also associate with VHL expressed in yeast (Fig. 2). This result led us to examine whether these different chaperone systems are indeed required for formation of VBC in intact cells. VHL folding and assembly into VBC was severely impaired in a strain carrying a temperature-sensitive mutation of SSA1 as its only source of cytosolic Hsp70 (Fig. 3). First, there was a dramatic reduction in the amount of VHL associated with elongin BC at the nonpermissive temperature (Fig. 3A). Second, VHL expressed under these conditions was in a protease-sensitive conformation (Fig. 3B). The severity with which VBC formation is compromised in the ssa1ts strain suggests that Hsp70 plays a major role in the incorporation of VHL into the VBC (see below).
The requirement of the chaperonin TRiC/CCT for VHL folding in vivo was examined using a temperature-sensitive mutation in subunit CCT4. This strain exhibits defects in both actin and tubulin filament networks (47). Analysis of the effect of this mutation on the conformation of actin demonstrated that folding of this chaperonin substrate was indeed impaired in the cct4ts strain (Fig. 4A and B), indicating that TRiC-mediated folding is defective in these cells. Thus, the cytoskeletal phenotypes correlate with defects in chaperonin-mediated folding. Importantly, assembly of correctly folded VBC was also dramatically reduced (Fig. 4C and D). Our results demonstrate that TRiC is required for VBC assembly in vivo, consistent with our previous findings in cell extracts. Notably, a recent survey of protein interactions in S. cerevisiae indicated that TRiC/CCT interacts with a significant number of proteins that are subunits of oligomeric complexes (18, 24), suggesting that our analysis of VBC biogenesis may apply to other chaperonin-mediated assembly processes.
Our results provide novel insight into the cooperation between different chaperone systems in vivo. Although some proteins, such as ornithine transcarbamylase, which requires Hsp70 (31), require the assistance of only one chaperone system to fold, there are several examples of polypeptides that interact with multiple chaperone systems during folding (14). However, it is not clear whether these observations reflect a requirement for cooperation of different chaperones in polypeptide folding. In some cases, functional overlap between chaperone systems may serve to provide parallel pathways of assisted folding, as previously shown for the Hsp70 DnaK and the prolyl isomerase trigger factor in E. coli (10, 44). On the other hand, in vitro and in vivo studies have indicated that some newly translated polypeptides interact sequentially with multiple chaperones, both in the cytosol and upon translocation into organelles such as mitochondria and the endoplasmic reticulum (ER). Interestingly, these studies observed that the folding polypeptide was initially bound by an Hsp70 homologue and was subsequently associated with another chaperone system. For instance, in vitro translation experiments indicated that folding of firefly luciferase requires the sequential cooperation of Hsp70 and TRiC (17). A sequential action of Hsp70 and Hsp90 also appears to mediate the maturation of steroid hormone receptors and tyrosine kinases (16). In a similar manner, when Mas2p and luciferase are translocated into mitochondria, binding to Hsp70 precedes the interaction with the chaperonin Hsp60 (23, 35). Likewise, binding to the ER Hsp70 homologue BiP also precedes binding to the ER chaperones Grp94 (in the folding of newly translocated immunoglobulin G (IgG) light chain [37]) and calnexin (in the folding of the viral protein VSV-G [20]). These studies led to the proposal that different chaperone systems cooperate in the folding of some polypeptides along a sequential pathway of interactions; in these scenarios, Hsp70 acts to stabilize early-folding intermediates for subsequent transfer to another chaperone system.
While the above-described studies established that some polypeptides interact with multiple chaperone systems, the physiological significance of these observations has been unclear. By analyzing VHL formation in cells carrying conditional chaperone mutants, we demonstrate that folding of some cellular proteins indeed requires the cooperation of different chaperone systems. Our data show that both Hsp70 and TRiC are required for VHL folding. It is possible in principle that these chaperones mediate VHL folding through two parallel pathways involving either chaperone. However, this model predicts that mutations that affect only Hsp70 or TRiC would still permit VHL folding through the alternate chaperone pathway. The dramatic effect of mutations in either chaperone on VBC assembly suggests an obligate cooperation between Hsp70 and TRiC. Interestingly, TRiC has also been suggested to cooperate with another chaperone, the prefoldin/GimC complex, in the folding of actin and tubulin (42, 46). However, deletion of the prefoldin/GimC complex was without effect on VBC assembly (data not shown). Thus, cooperation of TRiC with different chaperone systems, such as Hsp70 or GimC, might be determined by the properties of the substrate.
Our study also addresses the nature of the cooperation between Hsp70 and TRiC in substrate binding. In principle, VHL folding might result from the simultaneous action of both chaperones binding independently to the folding polypeptide. Alternatively, association of Hsp70 and TRiC with VHL might require a defined order of chaperone interactions. To explore these possibilities, we examined the fate of VHL in the chaperone mutant cells. The cct4ts defect resulted in reduced association of VHL with TRiC but had little effect on the VHL-Hsp70 interaction (Fig. 5A and B). In contrast, loss of Hsp70 function abolished VHL binding to both Hsp70 and TRiC (Fig. 5A). The loss of VHL interaction with TRiC is striking, since the chaperonin complex was present in the ssa1ts lysates. Thus, it appears that Hsp70 function is required for VHL binding to TRiC in vivo. These experiments highlight the hierarchical nature of chaperone interactions in the cell and support the idea that Hsp70 and TRiC function sequentially along the VHL folding pathway. Since purified TRiC can mediate incorporation of chemically denatured VHL into folded VBC in the absence of Hsp70 (A. S. Meyer and J. Frydman, unpublished observations), it is tempting to speculate that, in vivo, Hsp70 functions primarily to stabilize nonnative forms of VHL for subsequent transfer to TRiC. On the other hand, folding in vivo differs in significant aspects from refolding of chemically denatured proteins (15). Future experiments elucidating the precise mechanism of chaperone cooperation should clarify the contributions of Hsp70 and TRiC to VHL folding.
Acknowledgments
We thank Daniel E. Howard for useful discussions and help in some of the experiments, Joan Conaway and Doug Cyr for their generous gift of antibodies, and David Drubin and Elizabeth Craig for providing the strains used in this study. We also thank members of the Frydman lab for discussions and comments on the manuscript.
J.F. is a recipient of a Terman Award and is a Distinguished Young Scholar of the W. M. Keck Foundation. This work was supported by NIH grant GM56433. M.W.M. and A.J.M. were partially supported by PHS training grant CA09302 and by postdoctoral fellowships from the California Division of the ACS (to M.W.M.) and NIH (NRSA GM64992 to A.J.M.). A.S.M. is the recipient of a predoctoral fellowship from NSF and a Stanford Graduate Fellowship.
REFERENCES
- 1.Aso, T., and M. N. Conrad. 1997. Molecular cloning of DNAs encoding the regulatory subunits of elongin from Saccharomyces cerevisiae and Drosophila melanogaster. Biochem. Biophys. Res. Commun. 241:334-340. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1992. Current protocols in molecular biology, vol. 1. Wiley-Interscience, New York, N.Y.
- 3.Bazin, R., and R. Lemieux. 1989. Increased proportion of B-cell hybridomas secreting monoclonal antibodies of desired specificity in cultures containing macrophage-derived hybridoma growth factor (IL-6). J. Immunol. Methods 116:245-249. [DOI] [PubMed] [Google Scholar]
- 4.Becker, J., W. Walter, W. Yan, and E. A. Craig. 1996. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol. Cell. Biol. 16:4378-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boorstein, W. R., T. Ziegelhoffer, and E. A. Craig. 1994. Molecular evolution of the Hsp70 multigene family. J. Mol. Evol. 38:1-17. [DOI] [PubMed] [Google Scholar]
- 6.Botuyan, M. V., G. Mer, G. S. Yi, C. M. Koth, D. A. Case, A. M. Edwards, W. J. Chazin, and C. H. Arrowsmith. 2001. Solution structure and dynamics of yeast elongin C in complex with a von Hippel-Lindau peptide. J. Mol. Biol. 312:177-186. [DOI] [PubMed] [Google Scholar]
- 7.Bukau, B., E. Deuerling, C. Pfund, and E. A. Craig. 2000. Getting newly synthesized proteins into shape. Cell 101:119-122. [DOI] [PubMed] [Google Scholar]
- 8.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 9.Clifford, S. C., and E. R. Maher. 2001. von Hippel-Lindau disease: clinical and molecular perspectives. Adv. Cancer Res. 82:85-105. [DOI] [PubMed] [Google Scholar]
- 10.Deuerling, E., A. Schulze-Specking, T. Tomoyasu, A. Mogk, and B. Bukau. 1999. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400:693-696. [DOI] [PubMed] [Google Scholar]
- 11.Dobson, C. M. 1999. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24:329-332. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, A. Y., M. W. Melville, and J. Frydman. 2001. Review: cellular substrates of the eukaryotic chaperonin TRiC/CCT. J. Struct. Biol. 135:176-184. [DOI] [PubMed] [Google Scholar]
- 13.Feldman, D. E., V. Thulasiraman, R. G. Ferreyra, and J. Frydman. 1999. Formation of the VHL-elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell 4:1051-1061. [DOI] [PubMed] [Google Scholar]
- 14.Frydman, J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70:603-647. [DOI] [PubMed] [Google Scholar]
- 15.Frydman, J., and F. U. Hartl. 1996. Principles of chaperone-assisted protein folding: differences between in vitro and in vivo mechanisms. Science 272:1497-1502. [DOI] [PubMed] [Google Scholar]
- 16.Frydman, J., and J. Hohfeld. 1997. Chaperones get in touch: the Hip-Hop connection. Trends Biochem. Sci. 22:87-92. [DOI] [PubMed] [Google Scholar]
- 17.Frydman, J., E. Nimmesgern, K. Ohtsuka, and F. U. Hartl. 1994. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370:111-117. [DOI] [PubMed] [Google Scholar]
- 18.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 19.Gregersen, N., P. Bross, M. M. Jorgensen, T. J. Corydon, and B. S. Andresen. 2000. Defective folding and rapid degradation of mutant proteins is a common disease mechanism in genetic disorders. J. Inherit. Metab. Dis. 23:441-447. [DOI] [PubMed] [Google Scholar]
- 20.Hammond, C., and A. Helenius. 1994. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science 266:456-458. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, W. J., M. Ohh, J. Moslehi, K. Kondo, W. G. Kaelin, and W. J. Welch. 2002. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol. Cell. Biol. 22:1947-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 23.Heyrovska, N., J. Frydman, J. Hohfeld, and F. U. Hartl. 1998. Directionality of polypeptide transfer in the mitochondrial pathway of chaperone-mediated protein folding. Biol. Chem. 379:301-309. [DOI] [PubMed] [Google Scholar]
- 24.Ho, Y., A. Gruhler, A. Heilbut, G. D. Bader, L. Moore, S. L. Adams, A. Millar, P. Taylor, K. Bennett, K. Boutilier, L. Yang, C. Wolting, I. Donaldson, S. Schandorff, J. Shewnarane, M. Vo, J. Taggart, M. Goudreault, B. Muskat, C. Alfarano, D. Dewar, Z. Lin, K. Michalickova, A. R. Willems, H. Sassi, P. A. Nielsen, K. J. Rasmussen, J. R. Andersen, L. E. Johansen, L. H. Hansen, H. Jespersen, A. Podtelejnikov, E. Nielsen, J. Crawford, V. Poulsen, B. D. Sorensen, J. Matthiesen, R. C. Hendrickson, F. Gleeson, T. Pawson, M. F. Moran, D. Durocher, M. Mann, C. W. Hogue, D. Figeys, and M. Tyers. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180-183. [DOI] [PubMed] [Google Scholar]
- 25.Ivan, M., K. Kondo, H. F. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin. 2001. HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O-2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 26.Iwai, K., K. Yamanaka, T. Kamura, N. Minato, R. C. Conaway, J. W. Canaway, R. D. Klausner, and A. Pause. 1999. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 96:12436-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. von Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O-2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 28.Jackson, T., E. Kwon, A. M. Chachulska, and L. E. Hyman. 2000. Novel roles for elongin C in yeast. Biochim. Biophys. Acta 1491:161-176. [DOI] [PubMed] [Google Scholar]
- 29.James, P., T. Ziegelhoffer, C. Pfund, and E. Craig. 1995. Functionally distinct cytosolic Hsp70s of yeast; the Ssa and Ssb proteins. Mol. Biol. Cell 6:33.
- 30.Kamura, T., D. M. Koepp, M. N. Conrad, D. Skowyra, R. J. Moreland, O. Iliopoulos, W. S. Lane, W. G. Kaelin, Jr., S. J. Elledge, R. C. Conaway, J. W. Harper, and J. W. Conaway. 1999. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284:657-661. [DOI] [PubMed] [Google Scholar]
- 31.Kim, S., B. Schilke, E. A. Craig, and A. L. Horwich. 1998. Folding in vivo of a newly translated yeast cytosolic enzyme is mediated by the SSA class of cytosolic yeast Hsp70 proteins. Proc. Natl. Acad. Sci. USA 95:12860-12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo, K., and W. G. Kaelin. 2001. The von Hippel-Lindau tumor suppressor gene. Exp. Cell Res. 264:117-125. [DOI] [PubMed] [Google Scholar]
- 33.Labbe, S., and D. J. Thiele. 1999. Copper ion inducible and repressible promoter systems in yeast. Methods Enzymol. 306:145-153. [DOI] [PubMed] [Google Scholar]
- 34.Lisztwan, J., G. Imbert, C. Wirbelauer, M. Gstaiger, and W. Krek. 1999. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 13:1822-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning, K. U., P. E. Scherer, and G. Schatz. 1991. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 10:3273-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 37.Melnick, J., J. L. Dul, and Y. Argon. 1994. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature 370:373-375. [DOI] [PubMed] [Google Scholar]
- 38.Ohh, M., C. W. Park, N. Ivan, M. A. Hoffman, T. Y. Kim, L. E. Huang, N. Pavletich, V. Chau, and W. G. Kaelin. 2000. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2:423-427. [DOI] [PubMed] [Google Scholar]
- 39.Pause, A., S. Lee, R. A. Worrell, D. Y. Chen, W. H. Burgess, W. M. Linehan, and R. D. Klausner. 1997. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl. Acad. Sci. USA 94:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pause, A., B. Peterson, G. Schaffar, R. Stearman, and R. D. Klausner. 1999. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc. Natl. Acad. Sci. USA 96:9533-9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenfeld, A. R., E. J. Davidowitz, and R. D. Burk. 2000. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc. Natl. Acad. Sci. USA 97:8507-8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegers, K., T. Waldmann, M. R. Leroux, K. Grein, A. Shevchenko, E. Schiebel, and F. U. Hartl. 1999. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 18:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoldt, V., F. Rademacher, V. Kehren, J. F. Ernst, D. A. Pearce, and F. Sherman. 1996. Review: the Cct eukaryotic chaperonin subunits of Saccharomyces cerevisiae and other yeasts. Yeast 12:523-529. [DOI] [PubMed] [Google Scholar]
- 44.Teter, S. A., W. A. Houry, D. Ang, T. Tradler, D. Rockabrand, G. Fischer, P. Blum, C. Georgopoulos, and F. U. Hartl. 1999. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell 97:755-765. [DOI] [PubMed] [Google Scholar]
- 45.Thulasiraman, V., C. F. Yang, and J. Frydman. 1999. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 18:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vainberg, I. E., S. A. Lewis, H. Rommelaere, C. Ampe, J. Vandekerckhove, H. L. Klein, and N. J. Cowan. 1998. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 93:863-873. [DOI] [PubMed] [Google Scholar]
- 47.Vinh, D. B., and D. G. Drubin. 1994. A yeast TCP-1-like protein is required for actin function in vivo. Proc. Natl. Acad. Sci. USA 91:9116-9120. [DOI] [PMC free article] [PubMed] [Google Scholar]