Abstract
Previously, we have demonstrated that deoxycholic acid (DCA)-induced signaling of extracellular signal-regulated kinases 1 and 2 (ERK1/2) in primary hepatocytes is a protective response. In the present study, we examined the roles of the ERK and c-Jun NH2-terminal kinase (JNK) pathways, and downstream transcription factors, in the survival response of hepatocytes. DCA caused activation of the ERK1/2 and JNK1/2 pathways. Inhibition of either DCA-induced ERK1/2 or DCA-induced JNK1/2 signaling enhanced the apoptotic response of hepatocytes. Further analyses demonstrated that DCA-induced JNK2 signaling was cytoprotective whereas DCA-induced JNK1 signaling was cytotoxic. DCA-induced ERK1/2 activation was responsible for increased DNA binding of C/EBPβ, CREB, and c-Jun/AP-1. Inhibition of C/EBPβ, CREB, and c-Jun function promoted apoptosis following DCA treatment, and the level of apoptosis was further increased in the case of CREB and c-Jun, but not C/EBPβ, by inhibition of MEK1/2. The combined loss of CREB and c-Jun function or of C/EBPβ and c-Jun function enhanced DCA-induced apoptosis above the levels resulting from the loss of either factor individually; however, these effects were less than additive. Loss of c-Jun or CREB function correlated with increased expression of FAS death receptor and PUMA and decreased expression of c-FLIP-L and c-FLIP-S, proteins previously implicated in the modulation of the cellular apoptotic response. Collectively, these data demonstrate that multiple DCA-induced signaling pathways and transcription factors control hepatocyte survival.
Bile acids are detergent molecules, synthesized from cholesterol in the liver, and are essential for digestion (2). In the intestine, bile acids function in the solubilization and absorption of fats, certain vitamins, and cholesterol (20). Individually, bile acids are known to have hepatocellular toxicity both in vivo and in vitro (7, 13, 26, 28, 33, 37, 48). Toxic bile acids, when retained within the liver because of impaired secretion into the bile canaliculi, are believed to contribute to liver injury during cholestasis (5, 23, 38). Although bile ducts receive the initial insult from toxic bile salts, the progression of cholestatic liver disease is principally the result of hepatic parenchymal cell damage caused by toxic bile salts (15). In the liver, apoptosis has been implicated as an important form of cell death in various liver diseases, including viral hepatitis, alcoholic liver diseases, cholestasis, toxin-induced liver diseases, allograft rejection reaction following liver transplantation, and hepatocellular carcinoma (3, 22).
Recently, bile acids have been shown to activate multiple signaling pathways within cells that can potentially affect the cells' survival and proliferation (42, 50). This activation may be similar to that due to other toxic stresses, such as chemotherapeutic drugs and ionizing radiation (11, 21). Several groups have shown, for example, that in response to ionizing radiation, the epidermal growth factor receptor (EGFR, also called ErbB1) is activated in a ligand-independent manner in response to irradiation of cells (see, e.g., reference 43). Radiation exposure, via activation of the EGFR, can activate the “classical” mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, which appears to be cytoprotective against ionizing radiation and various cytotoxic drugs (12, 45). Indeed, functional inhibition of EGFR (17), Ras (10), Raf-1 (34), and MEKs 1 and 2 (MEK1/2) (46) has been shown to have radio- and chemosensitizing properties in vitro and in vivo.
Recently, it was reported that the treatment of primary rodent and human hepatocytes with a physiologic concentration of the bile acid deoxycholic acid (DCA) could cause activation of the EGFR, which is responsible for activation of the ERK pathway, and lead to a modest ∼2-fold increase in apoptosis, from ∼3 to ∼6%, in 6 h (39, 40, 41). A blockade of DCA-induced ERK1/2 activation, with inhibitors of Ras, phosphatidylinositol 3-kinase, or MEK1/2 function, increased apoptosis ∼8-fold within 6 h of exposure. Apoptosis was dependent on bile acid-induced, ligand-independent activation of the FAS death receptor. In principle, this apoptosis was mechanistically similar to that previously observed in studies exposing carcinoma cells to ionizing radiation. Other studies by our laboratories have demonstrated that DCA can also strongly activate the c-Jun NH2-terminal kinase (JNK) pathway (16); however, little is currently known about the control of bile acid-induced apoptosis in primary hepatocytes by the JNK and p38 stress pathways. To further characterize the mechanisms controlling bile acid toxicity in hepatocytes, we performed experiments to assess the relative importance of DCA-induced ERK1/2, JNK1/2, and p38 signaling in the control of transcription factor function and the roles these pathways and transcription factors play in the control of hepatocyte cell survival.
MATERIALS AND METHODS
Materials.
Deoxycholic acid was obtained from Sigma Chemical Co. (St. Louis, Mo.). Anti-caspase 3, phospho-ERK1/2, total ERK1/2, phospho-p38α/β, total p38α/β, phospho-JNK1/2, total JNK1/2, anti-BID, anti-FLIP, anti-Bcl-2, anti-Bcl-xl, anti-FAS receptor, anti-Bax, and all the secondary antibodies (anti-rabbit horseradish peroxidase [HRP], anti-mouse HRP, and anti-goat HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-phospho-CREB (1:1,000 dilution; rabbit polyclonal) was obtained from Upstate Biotechnology (Lake Placid, N.Y.), and anti-PARP (1:2,500 dilution; mouse monoclonal) was from Calbiochem (San Diego, Calif.). Radiolabeled [γ-32P]ATP was from NEN Life Science Products (Boston, Mass.). Anti-cytochrome c antibody was from Pharmingen (San Diego, Calif.). An enhanced chemiluminescence kit was purchased from NEN Life Science Products. Pan-caspase inhibitor (Z-VAD-FMK) was purchased from Enzyme System Products (Livermore, Calif.), dissolved in dimethyl sulfoxide, and stored at −20°C. Selective MEK1/2 inhibitors (PD98059 and U0126) were supplied by Calbiochem as powder, dissolved in sterile dimethyl sulfoxide, and stored frozen under light-protected conditions at −80°C. Western immunoblotting was performed with the Amersham Enhanced Chemi-Luminescence system (Bucks, England). Trypsin-EDTA, Williams E medium, and penicillin-streptomycin were purchased from GIBCO BRL Life Technologies (Grand Island, N.Y.). The assay of p38 activity was performed with the Catch and Release system (Upstate Biotechnology) Hoechst 33342 was purchased from Molecular Probes (Eugene, Oreg.). FluroGard Antifade was purchased from Bio-Rad Laboratories (Hercules, Calif.). Other plasmid constructs and reagents were as described in references 1, 39, 40, and 41.
Methods. (i) Primary culture of rodent hepatocytes.
Hepatocytes were isolated either from adult male Sprague-Dawley rats or from adult male wild-type (wt) C57BL/6, wt C57/129, C57BL/6-lpr (FAS receptor null), C57BL/6 JNK2−/− (JNK2 null), or C57/129 JNK1−/− (JNK1 null) mice by the two-step collagenase perfusion technique (39, 40, 41). JNK1 and JNK2 null mice were kindly provided by R. Davis, Howard Hughes Medical Institute, Worcester, Mass. The freshly isolated hepatocytes were plated onto a rat tail collagen (Vitrogen)-coated plate at a density of 2 × 105 cells/well and cultured in Williams E medium supplemented with 50 nM insulin, 0.1 nM dexamethasone, 1 nM thyroxine, and 100 μg of penicillin-streptomycin/ml at 37°C in a humidified atmosphere containing 5% CO2. The initial medium change was performed 3 h after cell seeding to minimize the contamination of dead or mechanically damaged cells.
(ii) Established cell culture.
CHO-K1 cells were cultured in Dulbecco modified Eagle medium containing 10% (vol/vol) fetal calf serum. Cells were plated at a density of 105 cells/cm2 in 15-cm-diameter dishes and cultured for 36 h prior to transfection with C/EBPβ plasmids. C/EBPβ expression vectors were kindly provided by Linda Sealy, Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, Tenn.
(iii) In vitro phosphorylation of C/EBPβ.
(His)6-C/EBPβ wt and (His)6-C/EBPβ P189G (ERK consensus site mutated) were purified from CHO-K1 cells 48 h after transfection by using Ni2+ agarose as described previously for (His)6-MEK1 (35). To remove preexisting phosphorylation of any sites within the C/EBPβ proteins during the final stage of purification, prior to elution, beads (0.25 ml) were incubated in vitro in 0.25 ml of 25 mM Tris-HCl (pH 7.4, 37°C) and 0.5 mM MnCl2 containing 100 mU of PP1 (kindly provided by D. Brautigan, Department of Microbiology, University of Virginia, Charlottesville, Va.). Beads were incubated at 37°C for 30 min, after which the PP1-containing buffer was removed. Beads were washed (three times with 1 ml each time) with 25 mM Tris-HCl (pH 7.4, 37°C) containing 500 mM NaCl, 1 mM sodium pyrophosphate, and 10 μM microcystin-LR. Proteins were eluted from the beads by using 250 mM imidazole-HCl (pH 8.0). Proteins were dialyzed versus 50 mM Tris-HCl (pH 7.4, 37°C) containing 10% (vol/vol) glycerol. Equal protein amounts (2 μg in 25 μl) of (His)6-C/EBPβ wt and (His)6-C/EBPβ P189G were incubated with commercially purchased ERK2 (10 mU) and p38α (10 mU), alone and in combination, together with 0.1 mM [γ-32P]ATP or nonradioactive ATP and 5 mM MgCl2 in a total volume of 50 μl. Phosphorylated C/EBPβ was then either subjected to an electrophoretic mobility shift assay (EMSA) or boiled with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, and proteins were resolved by SDS-PAGE. C/EBP protein bands were visualized by immunoblotting and autoradiography.
Equal amounts of C/EBPβ peptide containing the ERK1/2 consensus sequence PGTP (ATPSGSSGSLSTSSSSSPPGTPSPADAK-RR) (2 μg) were incubated with commercially purchased ERK2 (10 mU) and p38α (10 mU), alone and in combination, as indicated, together with 0.1 mM [γ-32P]ATP or nonradioactive ATP and 5 mM MgCl2 in a total volume of 50 μl. After 20 min, 40-μl aliquots of the reaction mixtures were spotted onto a 2-cm-diameter circle of P81 paper (Whatman, Maidstone, England) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P incorporation into the peptide was quantified by liquid scintillation spectroscopy.
(iv) Recombinant adenoviral vectors: generation and infection in vitro.
Two adenoviral technologies were used. First, replication-defective adenovirus was conjugated to poly-l-lysine and a cDNA plasmid construct as described in references 1 and 34. Second, we generated recombinant adenoviruses (46, 51). Hepatocytes were transfected with these adenoviruses at approximate multiplicities of infection of 250 and 30, respectively. Cells were further incubated for 24 h to ensure adequate expression of transduced gene products.
(v) Transfection of CHO-K1 cells.
CHO-K1 cells (29) were transfected by using Lipofectamine Plus reagent with 5 μg of the indicated C/EBPβ plasmid.
(vi) SDS-PAGE and Western blot analysis.
At various time points after indicated treatment, hepatocytes were lysed in whole-cell lysis buffer (0.5 M Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromophenol blue), and the samples were boiled for 30 min. The boiled samples were loaded onto an SDS-14% PAGE gel, and electrophoresis was run overnight. Proteins were electrophoretically transferred onto 0.22-μm-pore-size nitrocellulose and immunoblotted with various primary antibodies against different proteins. All immunoblots were visualized by enhanced chemiluminescence. For presentation, immunoblots were digitally scanned at 600 dpi by using Adobe PhotoShop 5.0 and their color was removed and figures were generated with Microsoft PowerPoint.
(vii) Morphological detection of apoptosis by Hoechst 33342 assay.
Morphological assessment of apoptosis was performed as follows (39, 40, 41). Hepatocytes were harvested by trypsinization with trypsin-EDTA for ∼10 min at 37°C. As some apoptotic cells detached from the culture substratum into the medium, these cells were also collected by centrifugation of the medium at 1,500 rpm for 5 min. The pooled cell pellets were resuspended, and a fraction of the suspension was centrifuged in a cytospinner (Cytospin 3; Shandon Inc., Pittsburgh, Pa.). The slides were immediately fixed. The slides were then washed. The fixed cells were stained with Hoechst 33342, washed in phosphate-buffered saline to remove excessive dye, air dried, and mounted in FluroGard Antifade. Nuclear morphology was evaluated. Apoptotic cells were identified as those whose nuclei exhibited brightly staining condensed chromatin or nuclear fragmentation or apoptotic bodies. A total of 500 cells from several randomly chosen fields were counted, and the number of apoptotic cells was counted and expressed as a percentage of the total number of cells counted.
(viii) Determination of apoptosis by TUNEL and Wright-Giemsa staining.
After hepatocytes were treated with various regimens, cells were collected by trypsinization followed by cytospinning onto glass slides, as described above. For Wright-Giemsa staining, the slides were fixed and stained in Diff-Quik Stain Set (Dade Diagnostics of P.P. Inc., Aguada, Puerto Rico) according to the manufacturer's instructions and viewed under a light microscope. For terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), cells were fixed in 3% paraformaldehyde for 30 min at 4°C and a TUNEL assay was performed on these cells according to the manufacturer's instructions. The slides were viewed under a fluorescence microscope (39, 40, 41).
(iv) EMSA.
EMSAs and supershift assays for C/EBPβ, CREB, and AP-1 were performed as described previously (4, 49). Briefly, the binding between DNA and transcription factors CREB (5′-AGA GAT TGC CTG ACG TCA GAG AGC TAG-3′), C/EBP (5′-TGC AGA TTG CGC AAT CTG CA-3′), and AP-1/c-Jun (5′-CGC TTG ATG ACT CAG CCG GAA-3′) was examined by EMSA, and the identity of each complex was established with excess unlabeled oligonucleotide (data not shown) and antibody supershifts with specific antibodies. Oligonucleotides and antibodies were purchased from Santa Cruz Biotechnology. Briefly, mixtures containing 5 μg of nuclear protein extract were incubated for 30 min at 4°C in a 20-μl total volume of reaction mixture containing 10 mM HEPES (pH 7.6), 50 mM KCl, 0.2 mM EDTA, 2.5 mM dithiothreitol, 2.5 mM phenylmethylsulfonyl fluoride, 1 μl of aprotinin/ml, 1 μl of leupeptin/ml, and 20% glycerol. The 32P-labeled probe consensus oligonucleotide was added to the mixtures, and the complexes were further incubated for an additional 20 min at room temperature. Complexes were resolved on 5% native polyacrylamide gels for 2 h at 100 V (until the blue dye front reached the bottom). For supershift analysis, 2 μg of antibody against each protein was added to the nuclear extract and the mixtures were incubated for 1 h at 4°C before the addition of 32P-labeled probes. The gels were dried with a vacuum dryer. The complexes were detected by autoradiography.
(x) Data analysis.
Comparison of the effects of various treatments was performed by using one-way analysis of variance and a two-tailed t test. Differences with a P value of <0.05 were considered statistically significant. Experiment results shown are the means of multiple individual points (± standard errors of the means [SEM]).
RESULTS
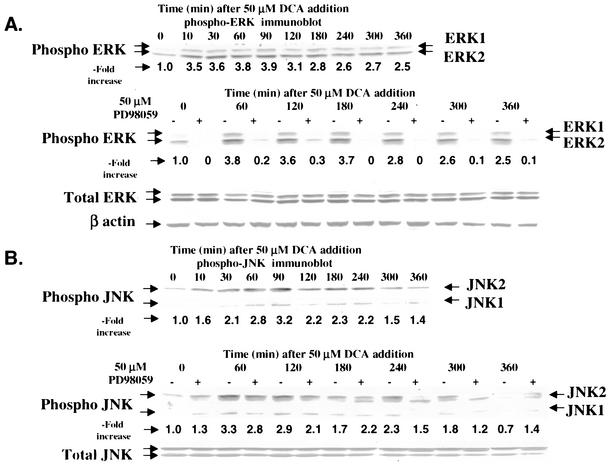
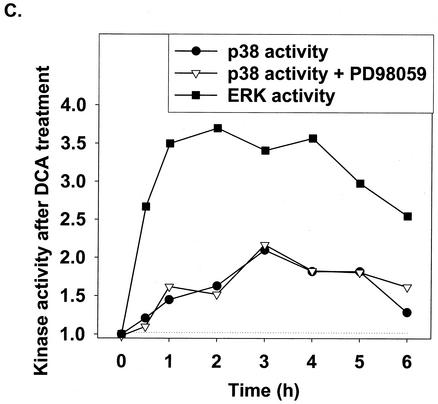
Initial studies characterized the activation of the ERK, JNK, and p38 pathways following treatment of primary rat hepatocytes with a physiologic concentration of DCA (Fig. 1). DCA caused activation of ERK1/2 and JNK1/2 (Fig. 1A and B). The caspase 3 inhibitor DEVD-fmk abolished the appearance of a faster-migrating form of JNK1/2 after DCA and PD98059 treatment (data not shown). p38α/β activation was only weakly detected by the use of immune complex kinase assays (Fig. 1C). Similar data were obtained with primary mouse hepatocytes for the activation of ERK1/2 and JNK1/2 (data are not shown but were in general agreement with those reported in references 16 and 39-41).
FIG. 1.
DCA activates ERK1/2 and JNK1/2 in primary hepatocytes. Hepatocytes were isolated and cultured as described in “Methods.” Twenty-four hours after plating, cells were treated with either vehicle or vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM). Thirty minutes after PD98059 treatment, cells were exposed to bile acid (final concentrations: rat, 50 μM; mouse, 150 μM). At the indicated times after bile acid exposure, cells were lysed in SDS-PAGE sample buffer containing bromophenol blue, boiled, and subjected to SDS-PAGE followed by transfer to nitrocellulose. Immunoblotting was performed as described in “Methods” (39, 40, 41). (A) DCA increases ERK1/2 phosphorylation in rat hepatocytes that is blocked by PD98059. (B) DCA increases JNK1/2 phosphorylation in rat hepatocytes. (C) DCA weakly increases p38 activity in rat hepatocytes that is not altered by PD98059. The JNK1/2/3 inhibitor SP600125 did not alter the activation of either ERK1/2 or p38α (data not shown). Data in all panels are from a representative study (n = 3).
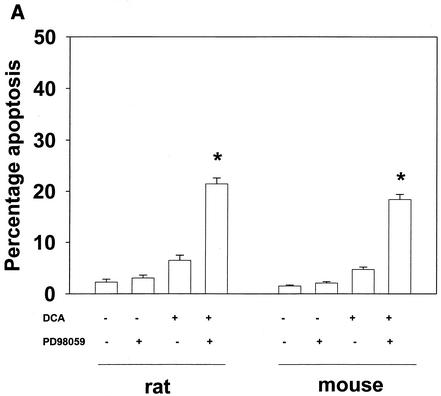
We next investigated the impact on the apoptotic response of DCA-treated rat and mouse hepatocytes of inhibiting each signaling pathway. Inhibition of the p38 pathway by using either a recombinant adenovirus to express dominant-negative p38α or the p38α/β inhibitor SB203580 (1 μM) did not significantly alter the apoptotic response of rat and mouse hepatocytes following DCA treatment (data not shown).
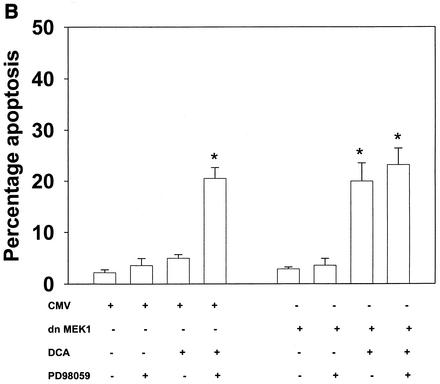
Inhibition of the ERK pathway with the MEK1/2 inhibitor PD98059 enhanced the apoptotic response in rat and mouse hepatocytes (Fig. 2A). Similar data were obtained when either dominant-negative MEK1 (S217A) or dominant-negative Ras N17 was expressed in these cells (Fig. 2B and data not shown [41]). Of note, PD98059 did not enhance apoptosis in cells expressing MEK1 (S217A). These findings are in general agreement with those of published studies with primary hepatocytes and a range of chemically dissimilar MEK1/2 inhibitors (39-41).
FIG. 2.
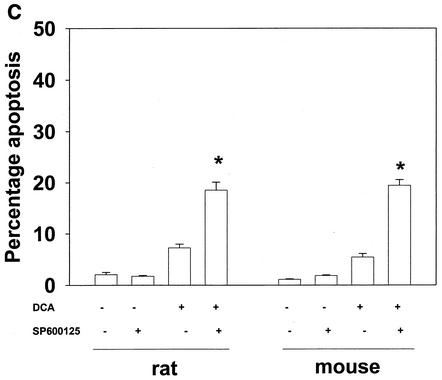
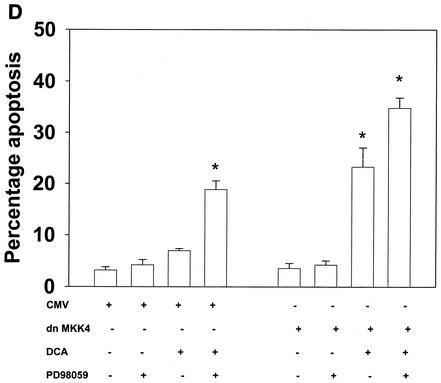
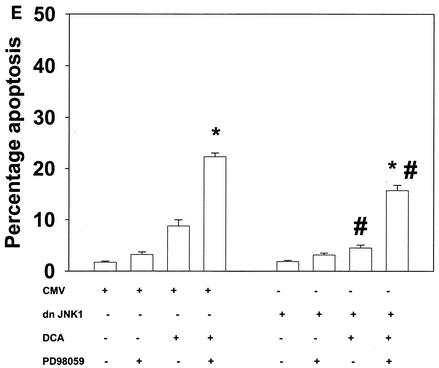
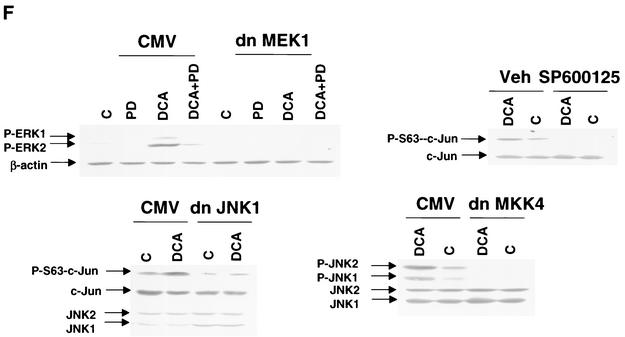
Bile acid-induced apoptosis is enhanced by inhibitors of MEK1/2 or JNK1/2 or by the loss of ERK or JNK pathway function. Hepatocytes were isolated and cultured as described in “Methods.” As indicated in some panels, 4 h after plating, cells were infected either with control poly-l-lysine adenovirus or with dominant-negative MEK1 (dn MEK1) or dominant-negative MKK4 (dn MKK4) poly-l-lysine adenovirus at a multiplicity of infection of 250. As indicated, 24 h after plating, cells were treated with either vehicle, vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM), or vehicle containing the JNK1/2 inhibitor SP600125 (final concentration, 10 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (final concentrations: rat, 50 μM; mouse, 150 μM). Six hours after bile acid exposure, cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). (A) The MEK1/2 inhibitor PD98059 enhances DCA-induced apoptosis in rat and mouse hepatocytes. (B) Dominant-negative MEK1 enhances DCA-induced apoptosis in rat hepatocytes. (C) The JNK1/2/3 inhibitor SP600125 enhances DCA-induced apoptosis in rat and mouse hepatocytes. (D) Dominant-negative MKK4 enhances DCA-induced apoptosis in rat hepatocytes. (E) Expression of dominant-negative JNK1 (dn JNK1) weakly suppresses DCA-induced apoptosis in rat hepatocytes. (F) Dominant-negative MEK1 inhibits ERK1/2 activation. SP600125 and dominant-negative MKK4 inhibit JNK1/2 activation (in agreement with results reported in references 1 and 41). Dominant-negative JNK1 reduces and inhibits c-Jun phosphorylation. Data shown are the means of results from three to four separate determinations (SEM). *, P < 0.05, greater than vehicle-treated control; #, P < 0.05, less than corresponding value for cells not expressing dominant-negative JNK1; %, P < 0.05, greater than corresponding value for wild-type cells; **, P < 0.05, greater than values for cells treated with either PD98059 alone or SP600125 alone. CMV, cytomegalovirus; C, control; PD, PD98059.
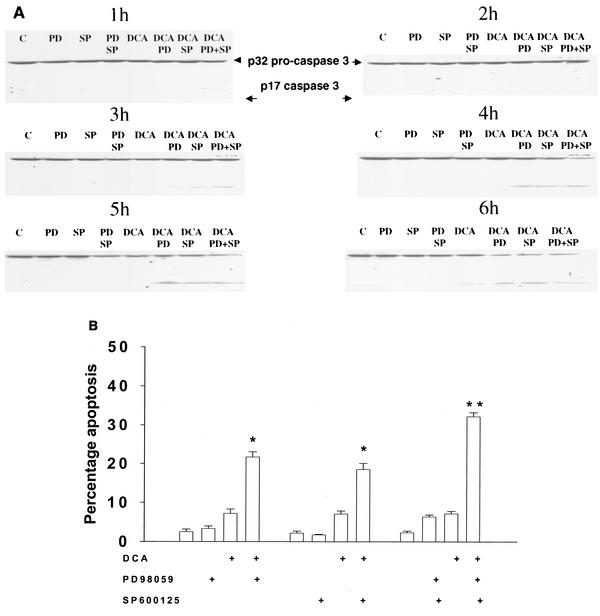
Inhibition of the JNK pathway with the recently described JNK1/2/3 inhibitor SP600125 also increased the apoptotic response in rat and mouse hepatocytes (Fig. 2C to F). SP600125 did not alter the phosphorylation of ERK1/2 or of p38α (data not shown). Apoptosis data similar to those with SP600125 were obtained when dominant-negative MAPK kinase 4 (MKK4) was expressed in rat hepatocytes (Fig. 2D) (1, 16). In contrast, expression of dominant-negative JNK1 weakly suppressed the apoptotic response of hepatocytes (Fig. 2E). Treatment of rat hepatocytes with either DCA plus JNK1/2 inhibitor or DCA plus MEK1/2 inhibitor enhanced caspase 3 cleavage (Fig. 3A) and the apoptotic response (Fig. 3B) (39-41).
FIG. 3.
DCA exposure, together with treatment of rat hepatocytes with either the JNK1/2 inhibitor or the MEK1/2 inhibitor, enhances caspase 3 cleavage and apoptosis. Hepatocytes were isolated and cultured as described in “Methods.” As indicated in some panels, 4 h after plating, cells were infected with either control adenovirus or with dominant-negative JNK1 at a multiplicity of infection of 50. As indicated, 24 h after plating, cells were treated with either vehicle, vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM), or vehicle containing the JNK1/2 inhibitor SP600125 (final concentration, 10 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (rat; final concentration, 50 μM). Six hours after bile acid exposure, cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). (A) DCA exposure, together with treatment of cells with either the JNK1/2 inhibitor or the MEK1/2 inhibitor, enhances caspase 3 cleavage. Twenty-four hours after plating, cells were treated with either vehicle or vehicle containing the MEK1/2 inhibitor PD98059 (PD; final concentration, 50 μM) or the JNK1/2 inhibitor SP600125 (SP; 10 μM). Thirty minutes after kinase inhibitor treatment, cells were exposed to bile acid (final concentration, 50 μM). At the indicated times after bile acid exposure, cells were lysed in SDS-PAGE sample buffer, boiled, and subjected to SDS-PAGE followed by transfer to nitrocellulose. Immunoblotting for caspase 3 was performed as described in “Methods” (39, 40, 41). (B) DCA exposure, together with treatment of cells with either the JNK1/2 inhibitor or the MEK1/2 inhibitor, enhances apoptosis. Data shown in Fig. 3 are the means of results from four separate experiments. *, P < 0.05, greater than vehicle-treated control; **, P < 0.05, greater than values for cells treated with either PD98059 alone or SP600125 alone.
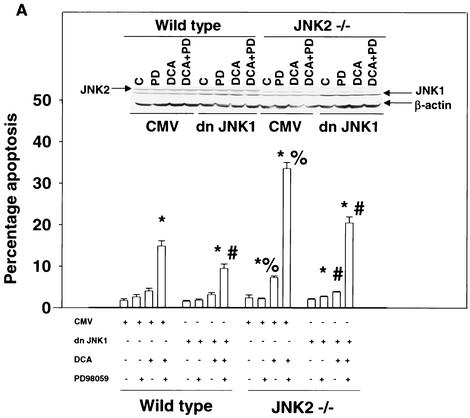
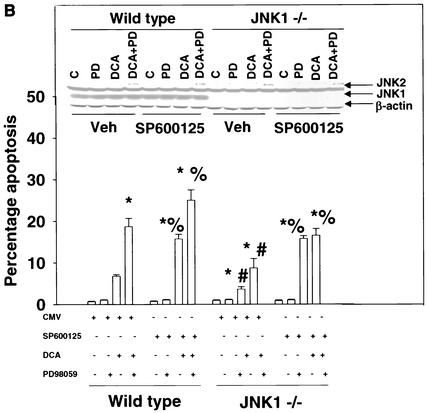
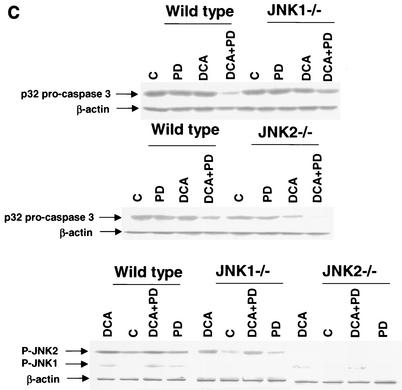
Because of the findings described above, the relative contributions of JNK1 and JNK2 signaling to the apoptotic response in mouse hepatocytes were further investigated. In hepatocytes isolated from JNK2−/− mice, DCA- and DCA-plus-MEK1/2 inhibitor-induced cell killing was enhanced compared to that in wild-type cells and expression of dominant-negative JNK1 reduced the apoptotic response (Fig. 4A). In hepatocytes isolated from JNK1−/− mice, DCA- and DCA-plus-MEK1/2 inhibitor-induced apoptosis was reduced compared to that in wild-type cells (Fig. 4B). In agreement with the fact that JNK2 signaling is protective, inhibition of JNK2 with SP600125 in wild-type and JNK1−/− hepatocytes enhanced cell killing, although the increase in cell killing was weaker in JNK1−/− cells. These findings also correlated with the bile acid-induced reduction of pro-caspase 3 levels in wild-type hepatocytes compared with those in hepatocytes from JNK1−/− and JNK2−/− animals (Fig. 4C).
FIG. 4.
Loss of JNK2 function enhances cell killing by DCA whereas loss of JNK1 function inhibits cell killing by DCA. Hepatocytes were isolated and cultured as described in “Methods.” As indicated in some panels, 4 h after plating, cells were infected with either control adenovirus or with dominant-negative JNK1 (dn JNK1) at a multiplicity of infection of 50. As indicated, 24 h after plating, cells were treated with either vehicle (Veh), vehicle containing the MEK1/2 inhibitor PD98059 (PD; final concentration, 50 μM), or vehicle containing the JNK1/2 inhibitor SP600125 (final concentration, 10 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (mouse; final concentration, 150 μM). Six hours after bile acid exposure, cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). (A) Loss of JNK2 expression enhances the apoptotic response of mouse hepatocytes exposed to DCA, and the response is further enhanced by exposure to DCA and PD98059 and reduced by the expression of dominant-negative JNK1. C, control. (B) Loss of JNK1 expression reduces the apoptotic response of mouse hepatocytes exposed to DCA. The response is enhanced by the JNK1/2/3 inhibitor SP600125. Data shown are the means of results from three separate determinations (SEM). (C) Cleavage of pro-caspase 3 in wild-type C57/BL6, C57/129, JNK1−/−, and JNK2−/− hepatocytes 6 h after bile acid exposure and activation of JNK1 and JNK2 by DCA in JNK1−/− and JNK2−/− hepatocytes 1 h after bile acid addition. *, P < 0.05, greater than vehicle-treated control; #, P < 0.05, less than the corresponding value in cells not expressing dominant-negative JNK1; %, P < 0.05, greater than the corresponding value in wild-type cells. CMV, cytomegalovirus.
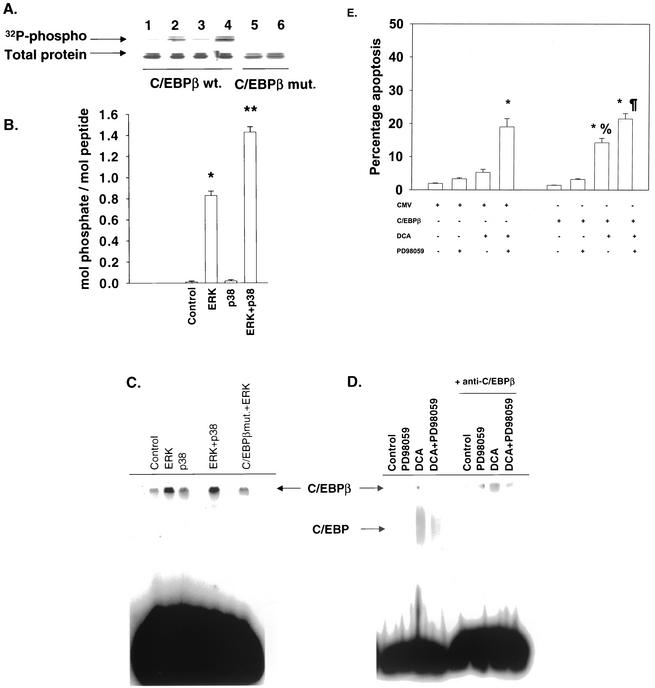
We next determined whether the putative cytoprotective transcription factors C/EBPβ, CREB, and AP-1 (c-Jun) were downstream of ERK1/2 signaling. We first examined the role of C/EBPβ in the protective response. Initial studies investigated the ability of ERK1/2 to phosphorylate C/EBPβ and alter its DNA binding ability in vitro. ERK2 phosphorylated wild-type C/EBPβ but not a mutant C/EBPβ that had its ERK consensus sequence disrupted (Fig. 5A). A peptide containing the ERK consensus sequence in C/EBPβ was phosphorylated by ERK2 but not by p38α or JNK1/2 (Fig. 5B and data not shown). Phosphorylation of the C/EBPβ protein or the peptide by ERK2 permitted subsequent phosphorylation by p38α but not JNK1/2 (Fig. 5B and data not shown). Phosphorylation of purified C/EBPβ in vitro with ERK2 weakly enhanced DNA binding (Fig. 5C). In agreement with a role for ERK signaling in the control of C/EBPβ function in vivo, MEK1/2 inhibition blunted the DCA-induced DNA binding of C/EBPβ in hepatocytes (Fig. 5D). No effect on C/EBPβ DNA binding was observed when the p38α/β inhibitor SB203580 was used (data not shown).
FIG. 5.
ERK1/2 signaling regulates C/EBPβ function, and expression of dominant-negative C/EBPβ T235A enhances DCA-induced apoptosis. (A) Purified ERK2 phosphorylates purified wild-type (wt.) C/EBPβ, but not C/EBPβ P189G (mut.), in vitro. Lane 1, vehicle; lane 2, ERK2; lane 3, p38α; lane 4, ERK2 and p38α; lane 5, vehicle; lane 6, ERK2. (B) Purified ERK2 but not p38 or JNK phosphorylates a peptide containing the ERK site consensus sequence from C/EBPβ. Data include SEM (n = 3). *, P < 0.05, greater than vehicle-treated control; **, P < 0.05, greater than ERK value. (C) Phosphorylation of C/EBPβ in vitro by ERK2 enhances C/EBPβ DNA binding in vitro. Protein was phosphorylated for 30 min. EMSAs were performed as described in “Methods” (4, 49). Results from a representative experiment are shown (n = 2). The identity of each complex was established with excess unlabeled oligonucleotide (data not shown). (D) DCA enhances C/EBPβ DNA binding in vivo, which is blocked by the MEK1/2 inhibitor PD98059. Cells were treated with DCA for 1 h. EMSAs were performed as described in “Methods” (4, 49). Results from a representative experiment are shown (n = 3). The identity of each complex was established with excess unlabeled oligonucleotide (data not shown). (E) Dominant-negative C/EBPβ enhances the apoptotic response of hepatocytes exposed to DCA but does not significantly enhance apoptosis induced by DCA plus the MEK1/2 inhibitor. After plating (4 h), cells were infected with either control plasmid- and poly-l-lysine-conjugated adenovirus or with dominant-negative C/EBPβ plasmid- and poly-l-lysine-conjugated adenovirus at a multiplicity of infection of 250. After plating (24 h), cells were treated with either vehicle or vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM). After kinase inhibitor treatment (30 min), cells were exposed to bile acid (final concentration, 50 μM). After bile acid exposure (6 h), cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). *, P < 0.05, greater than vehicle-treated control; %, P > 0.05, no statistical difference between vector control cell treated with DCA and MEK1/2 inhibitor and dominant-negative C/EBPβ cells treated with DCA alone; ¶, P > 0.05, no statistical significance between vector control cell treated with DCA and MEK1/2 inhibitor and dominant-negative C/EBPβ cells treated with DCA and MEK1/2 inhibitor.
We then investigated whether expression of dominant-negative C/EBPβ altered the apoptotic response of hepatocytes following DCA exposure. C/EBPβ has been linked to a survival response via ERK signaling in UV-irradiated keratinocytes (56). Expression of dominant-negative C/EBPβ in primary rat hepatocytes significantly enhanced the apoptotic response to DCA and caused a weak, nonsignificant enhancement in the response to DCA plus MEK1/2 inhibitor treatment (Fig. 5E).
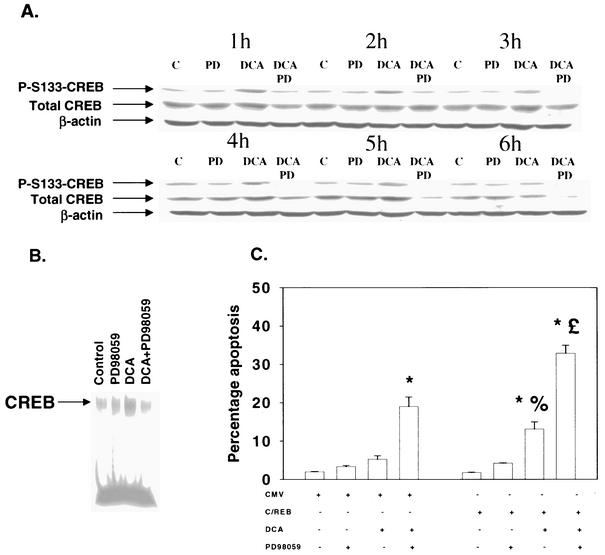
CREB is also a downstream target of the MAPK pathway, and the role of CREB in the protective effect of DCA-induced ERK pathway signaling was examined. Treatment of hepatocytes with DCA increased phosphorylation of CREB S133, which was blocked by a MEK1/2 inhibitor (Fig. 6A) but not by SB203580 (data not shown). These findings correlated with that of a weak increase in CREB DNA binding after DCA treatment that was blocked by an inhibitor of MEK1/2 but not by an inhibitor of p38α/β (Fig. 6B and data not shown). However, inhibition of CREB function by expression of dominant-negative CREB S133A significantly enhanced both DCA-induced and DCA-plus-MEK1/2 inhibitor-induced apoptosis (Fig. 6C).
FIG. 6.
DCA enhances CREB phosphorylation and DNA binding via ERK1/2 signaling: expression of dominant-negative CREB S133A enhances DCA-induced apoptosis. Hepatocytes were isolated and cultured as described in “Methods.” As indicated, 4 h after plating, cells were infected with either control plasmid- and poly-l-lysine-conjugated adenovirus or with dominant-negative CREB S133A plasmid- and poly-l-lysine-conjugated adenovirus at a multiplicity of infection of 250. Twenty-four hours after plating, cells were treated with either vehicle or vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (final concentration, 50 μM). (A) DCA causes an ERK1/2-dependent increase in CREB S133 phosphorylation that is inhibited by PD98059 (PD). At the indicated times after bile acid exposure, cells were lysed in SDS-PAGE sample buffer, boiled, and subjected to SDS-PAGE followed by transfer to nitrocellulose. Immunoblotting for total CREB, phospho-S133-CREB, and β-actin was performed as described in “Methods” (39, 40, 41). (B) DCA increases CREB DNA binding, which is inhibited by PD98059. Cells were treated with DCA for 1 h. EMSAs were performed as described in “Methods” (4, 49). Results from a representative experiment are shown (n = 3). The identity of each complex was established with excess unlabeled oligonucleotide (data not shown). (C) Dominant-negative CREB enhances the apoptotic response following DCA exposure. Six hours after bile acid exposure, cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). *, P < 0.05, greater than vehicle-treated control; %, P < 0.05, greater than corresponding value for cells not expressing dominant-negative CREB; %, P > 0.05, no statistical difference between vector control cell treated with DCA and MEK1/2 inhibitor and dominant-negative CREB cells treated with DCA alone; £, P < 0.05, greater than vector control cell treated with DCA and MEK1/2 inhibitor. CMV, cytomegalovirus.
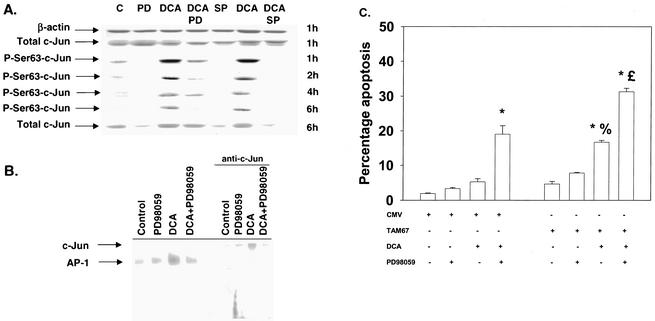
Loss of JNK2 expression and total JNK activity promoted bile acid-induced apoptosis, whereas inhibition of JNK1 reduced the apoptotic response. Studies by several groups have argued that toxic stresses utilize the JNK/c-Jun pathway in the cell death process (36-38). Thus, in parallel with our studies examining C/EBPβ and CREB, we also determined the impact of c-Jun function on the apoptotic response of hepatocytes following DCA treatment. DCA increased the phosphorylation of c-Jun serine 63, which was inhibited by the MEK1/2 inhibitor PD98059 and abolished by SP600125 (Fig. 7A). PD98059 inhibited DCA-induced binding of the AP-1/c-Jun complex (Fig. 7B). Inhibition of c-Jun function by expression of dominant-negative c-Jun (TAM67) enhanced both DCA-induced apoptosis and DCA-plus-MEK1/2 inhibitor-induced apoptosis (Fig. 7C).
FIG. 7.
DCA increases AP-1/c-Jun phosphorylation and DNA binding which is dependent on ERK and JNK signaling. Hepatocytes were isolated and cultured as described in “Methods.” As indicated, 4 h after plating, cells were infected with control plasmid- and poly-l-lysine-conjugated adenovirus or with dominant-negative c-Jun (TAM67) plasmid- and poly-l-lysine-conjugated adenovirus at a multiplicity of infection of 250. Twenty-four hours after plating, cells were treated with either vehicle or vehicle containing either the MEK1/2 inhibitor PD98059 (final concentration, 50 μM) or the JNK1/2 inhibitor SP600125 (final concentration, 10 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (final concentration, 50 μM). (A) DCA increases c-Jun S63 phosphorylation, an effect that is blunted by the MEK1/2 inhibitor PD98059 (PD) and abolished by the JNK1/2 inhibitor SP600125 (SP). C, control. (B) DCA increases AP-1/c-Jun DNA binding, which is inhibited by PD98059. Cells were treated with DCA for 1 h. EMSAs were performed as described in “Methods” (4, 49). Results from a representative experiment are shown (n = 3). The identity of each complex was established with excess unlabeled oligonucleotide (data not shown). (C) Dominant-negative c-Jun (TAM67) enhances the apoptotic response following DCA exposure. Six hours after bile acid exposure, cells were isolated, fixed on glass slides, and stained with Hoechst 33342. Morphological examination of stained cells was performed as described in “Methods” (39, 40, 41). *, P < 0.05, greater than vehicle-treated control; %, P < 0.05, greater than corresponding value for cells not expressing dominant-negative c-Jun (TAM67); %, P > 0.05, no statistical difference between vector control cell treated with DCA and MEK1/2 inhibitor and dominant-negative c-Jun (TAM67) cells treated with DCA alone; £, P < 0.05, greater than vector control cell treated with DCA and MEK1/2 inhibitor. CMV, cytomegalovirus.
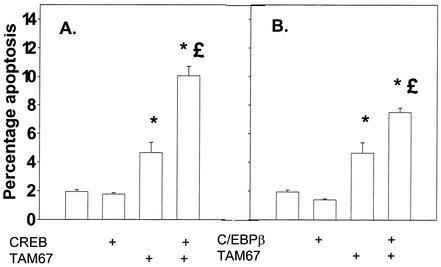
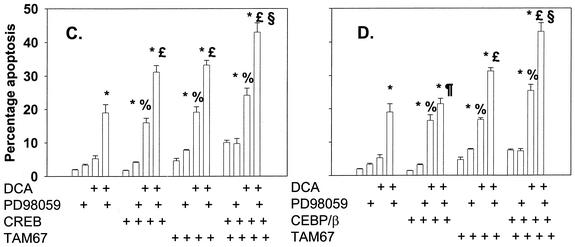
Since c-Jun, CREB, and C/EBPβ all appeared to play important roles in protective signaling following DCA treatment, we also examined the impact on DCA-induced apoptosis of collectively inhibiting C/EBPβ, CREB, and c-Jun function. Combined inhibition of CREB and c-Jun function or of C/EBPβ and c-Jun function, but not of CREB and C/EBPβ function, significantly enhanced basal levels of apoptosis (Fig. 8A and B and data not shown). However, the overall promotion of DCA-induced apoptosis following combined inhibition of transcription factor functions was only marginally greater than that following the expression of each factor alone (Fig. 8C and D). The combination of dominant-negative C/EBPβ and dominant-negative CREB also did not significantly enhance the apoptotic response above that observed for each of the dominant-negative transcription factors alone (data not shown).
FIG. 8.
Dominant-negative c-Jun (TAM67) can interact with either dominant-negative CREB or dominant-negative C/EBPβ to enhance the apoptotic response following DCA exposure. Hepatocytes were isolated and cultured as described in “Methods.” As indicated, 4 h after plating, cells were infected either with control plasmid- and poly-l-lysine-conjugated adenovirus, with dominant-negative c-Jun (TAM67) plasmid- and poly-l-lysine-conjugated adenovirus, with dominant-negative CREB plasmid- and poly-l-lysine-conjugated adenovirus, or with dominant-negative C/EBPβ plasmid- and poly-l-lysine-conjugated adenovirus at a total combined multiplicity of infection of 500. Twenty-four hours after plating, cells were treated with either vehicle or vehicle containing the MEK1/2 inhibitor PD98059 (final concentration, 50 μM). Thirty minutes after kinase inhibitor treatment and 24 h after adenovirus infection, cells were exposed to bile acid (final concentration, 50 μM). (A and B) Expression of dominant-negative c-Jun (TAM67) enhances the basal apoptosis level that is augmented by expression of either dominant-negative CREB or dominant-negative C/EBPβ. (C and D) Expression of dominant-negative c-Jun (TAM67) enhances DCA-induced and DCA-plus-MEK1/2 inhibitor-induced apoptosis levels that are augmented in a less-than-additive fashion by expression of either dominant-negative CREB or dominant-negative C/EBPβ. *, P < 0.05, greater than vehicle-treated control; %, P < 0.05, greater than corresponding value for cells not expressing dominant-negative transcription factor; %, P > 0.05, no statistical difference between vector control cell treated with DCA and MEK1/2 inhibitor and dominant-negative transcription factor-expressing cells treated with DCA alone; £, P < 0.05, greater than vector control cell treated with DCA and MEK1/2 inhibitor; §, P < 0.05, greater than parallel value for treated cells expressing an individual dominant-negative transcription factor.
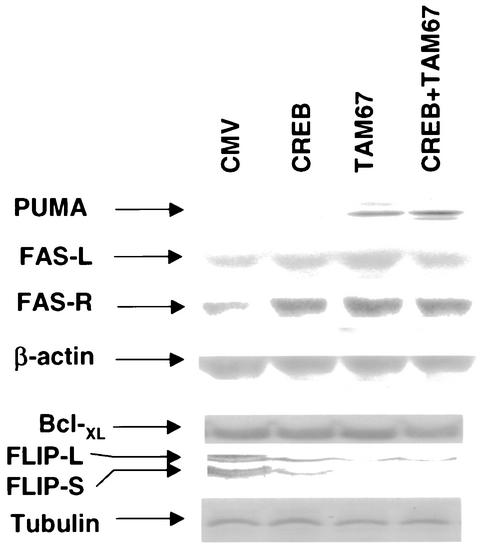
Bile acid-induced apoptosis in hepatocytes has been linked to activation of the FAS, and, more recently, tumor necrosis factor alpha (TNF-α), death receptors (18, 41, 47). Recently, we discovered that DCA-induced ERK signaling could increase the protein levels of the caspase 8/caspase 10 inhibitor proteins c-FLIP-S and c-FLIP-L (41). The FLIP molecules prevent processing of these pro-caspases in the Disk complex. Thus, we next determined whether dominant-negative CREB or dominant-negative c-Jun altered the expression of FLIP molecules as well as other proteins that may be involved in the apoptotic response.
Expression of dominant-negative CREB and dominant-negative c-Jun increased the protein levels of the FAS receptor and PUMA and inhibited basal levels of c-FLIP-L and c-FLIP-S (Fig. 9). Expression of Bcl-XL was weakly reduced in cells expressing both dominant-negative CREB and dominant-negative c-Jun. PUMA is a molecule that can act in a manner similar to that of Bax to cause mitochondrial dysfunction, cytochrome c release into the cytosol, and apoptosis (55). However, no significant alteration was observed in the basal protein levels of other proteins which may alter the apoptotic threshold, such as Bax, BID, Bcl-2, and FAS ligand (data not shown).
FIG. 9.
Expression of dominant-negative transcription factors modulates the expression of pro- and antiapoptotic gene products in hepatocytes. Hepatocytes were isolated and cultured as described in “Methods.” As indicated, 4 h after plating, cells were infected either with control plasmid- and poly-l-lysine-conjugated adenovirus, with dominant-negative c-Jun (TAM67) plasmid- and poly-l-lysine-conjugated adenovirus, or with dominant-negative CREB plasmid- and poly-l-lysine-conjugated adenovirus at a total combined multiplicity of infection of 500. Twenty-four hours after plating, cells were lysed in SDS-PAGE sample buffer, boiled, and subjected to SDS-PAGE followed by transfer to nitrocellulose. Immunoblotting was performed as described in “Methods” (38, 39, 41).
DISCUSSION
A previous study has linked bile acid-induced ERK signaling to a cytoprotective response against bile acid-induced FAS receptor/caspase activation (41). Studies by other groups have linked JNK pathway signaling to increased apoptosis, although more-recent studies have suggested that JNK signaling may have both pro- and anti-apoptosis signaling effects, including those downstream of the TNF-α receptor in hepatocytes (8, 30, 44). The studies in this work were designed to determine whether bile acid-induced JNK signaling enhanced or inhibited the apoptotic response of hepatocytes and whether transcription factors downstream of the cytoprotective ERK pathway altered the apoptotic threshold.
The bile acid DCA activated the ERK and JNK pathways. Inhibition of ERK1/2 activation by PD98059 and dominant-negative MEK1 or JNK1/2 activation by SP600125 and dominant-negative MKK4 enhanced DCA-induced apoptosis. Loss of JNK2 function enhanced DCA-induced apoptosis, and loss of JNK1 function suppressed DCA-induced apoptosis. Collectively, these findings demonstrate that DCA-induced ERK1/2 and JNK2 signaling was cytoprotective whereas DCA-induced JNK1 signaling was cytotoxic. Combined inhibition of both the ERK and JNK pathways caused a greater level of DCA-induced apoptosis than did inhibition of either pathway individually; however, the enhanced apoptotic effect of combined inhibition was less than additive. This finding suggests that, to a certain extent, the protective downstream targets of both the ERK and JNK pathways overlap.
Protective signaling by ERK1/2 has been proposed to be mediated by direct phosphorylation of certain proteins, e.g., BAD (6), as well as by alteration of the transcription of certain genes, e.g., the Bcl-XL gene (31). Thus, further studies were performed to determine the relative importance of these mechanisms for the protective effect of ERK signaling against DCA-induced toxicity. We were unable to detect, under our culture conditions and by immunoblotting, expression of the proapoptotic protein BAD (L. Qiao and P. Dent, unpublished observations). Using freshly cultured hepatocytes, others have detected low levels of BAD expression and shown that total BAD phosphorylation after hepatocyte growth factor treatment was inhibited by the MEK1/2 inhibitor U0126 (54).
Subsequent studies then examined the regulation of putative cytoprotective transcription factors downstream of the ERK pathway. DCA-induced ERK signaling increased C/EBPβ DNA binding. Loss of C/EBPβ function did not alter basal levels of hepatocyte apoptosis but enhanced DCA-induced apoptosis. Loss of C/EBPβ function, however, did not significantly enhance DCA-plus-PD98059-induced apoptosis. This finding implies that C/EBPβ is a key downstream target utilized in a stress-induced protective response following activation of the ERK pathway. Zhu et al. recently presented evidence that C/EBPβ was protective against anthracene treatment in a murine keratinocyte model system and that C/EBPβ was also an essential factor in Ras/ERK-induced tumor formation (56). With our cell system, several groups have previously identified C/EBPβ as an essential component of ERK-induced hepatocyte proliferation, and other studies have linked enhanced proliferation to suppression of apoptosis (9, 52, 56). However, the mechanism(s) by which ERK/C/EBPβ signaling acutely regulates cell survival remains unclear, as dominant-negative C/EBPβ did not alter the expression of proteins known to regulate the apoptotic response in hepatocytes (L. Qiao and P. Dent, unpublished).
In addition to C/EBPβ, the transcription factor CREB has been linked to ERK-dependent protective effects (32). DCA-induced ERK signaling increased CREB DNA binding, in agreement with findings that other stressful stimuli, e.g., ionizing radiation, also enhance compensatory cytoprotective CREB DNA binding via the ERK pathway (4). Prolonged inhibition of CREB function enhanced basal levels of apoptosis as well as the apoptotic response of cells exposed to DCA and DCA plus PD98059. In agreement with this data, dominant-negative CREB increased protein levels of FAS receptor and PUMA and reduced expression of FLIP isoforms. Previously, several groups have linked FLIP expression to protection from toxic stresses in hepatocytes (25, 41). In contrast, elevated expression of PUMA and the FAS receptor has been linked to enhanced apoptosis (41, 55). These findings indicate that while CREB has a basal protective function downstream of ERK signaling, the acute modulation of CREB function by ERK activation is unlikely to play a key role in the compensatory cytoprotective response.
Unexpectedly, we observed that phosphorylation of CREB was abolished under DCA-plus-MEK1/2 inhibitor conditions and that CREB protein levels declined. Another group has shown that CREB is a target for caspase-mediated proteolytic cleavage (14), and we found that the pan-caspase inhibitor Z-VAD-FMK blocked CREB degradation (L. Qiao and P. Dent, unpublished). This finding suggests that under conditions of DCA-plus-MEK1/2 inhibitor treatment, long-term proliferation and survival may be compromised by loss of CREB protein expression.
Enhanced signaling by the JNK/c-Jun pathway has been proposed by many groups to play a positive role in apoptosis (for example, see reference 24). We discovered that JNK2 signaling was protective whereas JNK1 signaling was toxic in the hepatocyte response to DCA. Contemporaneous studies have also suggested that JNK1 and JNK2 may differentially regulate the apoptotic response of cells (19, 27). Hochedlinger et al. (19) discovered that a lack of JNK1 in fibroblasts increased resistance to UV-induced cell death and that JNK2-deficient cells had increased sensitivity to UV irradiation. These investigators also discovered that JNK1−/− and JNK2−/− cells were more sensitive to TNF-α and sorbitol-induced apoptosis. For rat hepatocytes, Liedtke et al. have presented data arguing that JNK pathway signaling is protective against TNF-α-induced apoptosis (30). This is similar to findings of Roulston et al., who discovered that TNF-α-induced apoptosis could be enhanced by inhibition of JNK and p38, but not of ERK, signaling in fibroblasts (44). For our system, it has been paradoxically discovered that DCA-induced JNK1/2 activation is dependent on FAS receptor signaling (S. Gupta, Y. P. Rao, P. B. Hylemon, and P. Dent, submitted for publication). This was surprising because it implies that DCA-induced down-regulation of a gene which regulates cholesterol metabolism, the cholesterol 7α-hydroxylase gene, is dependent on signaling by a death receptor (16). Since DCA-induced apoptosis is also mediated by the FAS receptor (41), it seems reasonable to hypothesize that bile acid-induced activation of the FAS receptor can generate opposing signals, receptor-induced caspase and JNK1 activation, resulting in cell death, and receptor-induced JNK2 activation, resulting in protection and metabolism regulation.
Phosphorylation of c-Jun was dependent on JNK1/2 and MEK1/2 signaling, and loss of AP-1 function by expression of dominant-negative c-Jun (TAM67) enhanced basal and DCA- and DCA-plus-MEK1/2 inhibitor-induced apoptosis. In a manner similar to that of JNK1/2 signaling, the role of c-Jun in apoptotic responses appears to be cell type and stimulus dependent. In many cell types, c-Jun function has been linked to an enhancement of apoptotic responses (23). However, there is also evidence in the literature that AP-1 signaling can provide a protective response to noxious stresses (54). Loss of c-Jun function enhanced basal levels of apoptosis, indicating that AP-1 function plays an important role in hepatocyte survival. c-Jun−/− mouse embryos have been shown to die in utero, in general agreement with this observation. While expression of TAM67 enhanced DCA- and DCA-plus-MEK1/2 inhibitor-induced apoptosis, it also interacted with either dominant-negative CREB or dominant-negative C/EBPβ to further increase the apoptotic response. Collectively, these findings demonstrate that DCA-induced AP-1 signaling interacts with other transcription factors to maintain cell viability.
Loss of AP-1 function also promoted an increase in the expression of the FAS receptor and PUMA and a reduction in the expression of FLIP isoforms. Park et al. have recently argued that FLIP expression is under the control of ERK and JNK signaling, whereas other groups have linked enhanced FLIP expression to either ERK or Akt activation (36, 50). The expression of PUMA is regulated by the tumor suppressor p53, a protein that has previously been implicated in DCA-induced apoptosis (39, 53). In addition, the activity of p53 has been linked to AP-1 function in hepatocytes, and it is possible that our present observations on cell survival may be the result of this mechanism.
Thus, in conclusion, together with the findings of Qiao et al. (39-41), our data strongly suggest that ERK, C/EBPβ, CREB, and c-Jun signaling acts to protect hepatocytes from bile acid toxicity by regulating the expression of multiple apoptosis regulatory proteins.
Acknowledgments
This work was funded by PHS grants (R01-DK52825, P01-CA72955, P01-DK38030, and R01-CA88906), a Department of Defense Career Development Award (BC980148), and a fellowship from the V-Foundation to P.D.; by a PHS grant (P01-DK38030) to P.B.H.; by PHS grants (P01-CA72955, R01-CA63753, and R01-CA77141) and a Leukemia Society of America grant (6405-97) to S.G.; and by a PHS grant (R01-DK51315) to J.F.E.
We thank L. Sealy (Vanderbilt University, Nashville, Tenn.) for C/EBPβ constructs; D. Brautigan (University of Virginia, Charlottesville, Va.) for purified PP1; R. Davis (Howard Hughes Medical Institute, Worcester, Mass.) for JNK1 null and JNK2 null mice; J. C. Reed and S. Krajewski (Burnham Institute, La Jolla, Calif.) for additional anti-FLIP, anti-XIAP, anti-Bcl-XL, and anti-Bcl-2 antibodies; K. Vousden (National Institutes of Health, Bethesda, Md.) for anti-PUMA antibody; and P. Nagarkatti (Virginia Commonwealth University) for FAS receptor null mice.
REFERENCES
- 1.Auer, K., J. Contessa, S. Brenz-Verca, L. Pirola, S. Rusconi, G. Cooper, A. Abo, M. Wymann, R. J. Davis, M. Birrer, and P. Dent. 1998. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol. Biol. Cell 9:561-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benage, D., and K. W. O'Connor. 1990. Cholecystocolonic fistula: malabsorptive consequences of lost bile acids. J. Clin. Gastroenterol. 12:192-194. [PubMed] [Google Scholar]
- 3.Benedetti, A., and L. Marucci. 1999. The significance of apoptosis in the liver. Liver 19:453-463. [DOI] [PubMed] [Google Scholar]
- 4.Berhane, K., and V. Boggaram. 2001. Identification of a novel DNA regulatory element in the rabbit surfactant protein B (SP-B) promoter that is a target for ATF/CREB and AP-1 transcription factors. Gene 268:141-151. [DOI] [PubMed] [Google Scholar]
- 5.Bloomer, J. R., R. M. Allen, and G. Klatskin. 1976. Serum bile acids in primary biliary cirrhosis. Arch. Intern. Med. 136:57-61. [PubMed] [Google Scholar]
- 6.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 7.Botla, R., J. Spivey, H. Aguilar, S. F. Bronk, and G. Gores. 1995. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J. Pharmacol. Exp. Ther. 272:930-938. [PubMed] [Google Scholar]
- 8.Brenner, D. A. 1998. Signal transduction during liver regeneration. J. Gastroenterol. Hepatol. 13:S93-S95. [DOI] [PubMed] [Google Scholar]
- 9.Buck, M., V. Poli, P. van der Geer, M. Chojkier, and T. Hunter. 1999. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBPβ is required for hepatocyte proliferation induced by TGF α. Mol. Cell 4:1087-1092. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Jonathan, E., R. J. Muschel, G. W. McKenna, S. M. Evans, G. Cerniglia, R. Mick, D. Kusewitt, S. M. Sebti, A. D. Hamilton, A. Oliff, N. Kohl, J. B. Gibbs, and E. J. Bernhard. 2000. Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiat. Res. 154:125-132. [DOI] [PubMed] [Google Scholar]
- 11.Dent, P., D. B. Reardon, J. S. Park, G. Bowers, C. Logsdon, K. Valerie, and R. K. Schmidt-Ullrich. 1999. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol. Biol. Cell 10:2493-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent, P., W. D. Jarvis, M. J. Birrer, P. B. Fisher, R. K. Schmidt-Ullrich, and S. Grant. 1998. The roles of signaling by the p42/p44 mitogen-activated protein (MAP) kinase pathway; a potential route to radio- and chemo-sensitization of tumor cells resulting in the induction of apoptosis and loss of clonogenicity. Leukemia 12:400-408. [DOI] [PubMed] [Google Scholar]
- 13.Faubion, W. A., M. E. Guicciardi, H. Miyoshi, S. F. Bronk, P. J. Roberts, P. A. Svingen, S. H. Kaufmann, and G. J. Gores. 1999. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J. Clin. Investig. 103:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francois, F., M. J. Godinho, and M. L. Grimes. 2000. CREB is cleaved by caspases during neural cell apoptosis. FEBS Lett. 486:281-284. [DOI] [PubMed] [Google Scholar]
- 15.Gores, G. J., H. Miyoshi, R. Botla, H. I. Aguilar, and S. F. Bronk. 1998. Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis: a potential role for mitochondrial proteases. Biochim. Biophys. Acta 1366:167-175. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, S., R. T. Stravitz, P. Dent, and P. B. Hylemon. 2001. Down-regulation of cholesterol 7 α-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J. Biol. Chem. 276:15816-15822. [DOI] [PubMed] [Google Scholar]
- 17.Harari, P. M., and S. Huang. 2001. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int. J. Radiat. Oncol. Biol. Phys. 49:427-433. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi, H., S. F. Bronk, Y. Takikawa, N. Werneburg, R. Takimoto, W. El-Deiry, and G. J. Gores. 2001. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J. Biol. Chem. 276:38610-38618. [DOI] [PubMed] [Google Scholar]
- 19.Hochedlinger, K., E. F. Wagner, and K. Sabapathy. 2002. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene 21:2441-2445. [DOI] [PubMed] [Google Scholar]
- 20.Holt, P. R. 1972. The roles of bile acids during the process of normal fat and cholesterol absorption. Arch. Intern. Med. 130:574-583. [PubMed] [Google Scholar]
- 21.Jarvis, W. D., F. A. Fornari, R. M. Tombes, R. K. Erukulla, R. Bittman, G. K. Schwartz, P. Dent, and S. Grant. 1998. Evidence for involvement of mitogen-activated protein kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol. Pharmacol. 54:844-856. [DOI] [PubMed] [Google Scholar]
- 22.Kaplowitz, N. 2000. Mechanisms of liver cell injury. J. Hepatol. 32:39-47. [DOI] [PubMed] [Google Scholar]
- 23.Koeppel, T. A., M. Trauner, J. C. Baas, J. C. Thies, S. F. Schlosser, S. Post, M. M. Gebhard, C. Herfarth, J. L. Boyer, and G. Otto. 1997. Extrahepatic biliary obstruction impairs microvascular perfusion and increases leukocyte adhesion in rat liver. Hepatology 26:1085-1091. [DOI] [PubMed] [Google Scholar]
- 24.Koo, M. S., Y. G. Kwo, J. H. Park, W. J. Choi, T. R. Billiar, and Y. M. Kim. 2002. Signaling and function of caspase and c-jun N-terminal kinase in cisplatin-induced apoptosis. Mol. Cell 13:194-201. [PubMed] [Google Scholar]
- 25.Kovalovich, K., W. Li, R. DeAngelis, L. E. Greenbaum, G. Ciliberto, and R. Taub. 2001. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J. Biol. Chem. 276:26605-26613. [DOI] [PubMed] [Google Scholar]
- 26.Krahenbuhl, S., C. Talos, S. Fischer, and J. Reichen. 1994. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology 19:471-479. [DOI] [PubMed] [Google Scholar]
- 27.Kuan, C. Y., D. D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667-676. [DOI] [PubMed] [Google Scholar]
- 28.Kwo, P., T. Patel, S. Bronk, and G. Gores. 1995. Nuclear serine protease activity contributes to bile acid-induced apoptosis in hepatocytes. Am. J. Physiol. 268:G613-G621. [DOI] [PubMed] [Google Scholar]
- 29.Leach, J. K., S. M. Black, R. K. Schmidt-Ullrich, and P. B. Mikkelsen. 2002. Activation of constitutive nitric-oxide synthase activity is an early signaling event induced by ionizing radiation. J. Biol. Chem. 277:15400-15406. [DOI] [PubMed] [Google Scholar]
- 30.Liedtke, C., J. Plumpe, S. Kubicka, C. A. Bradham, M. P. Manns, D. A. Brenner, and C. Trautwein. 2002. Jun kinase modulates tumor necrosis factor-dependent apoptosis in liver cells. Hepatology 36:315-325. [DOI] [PubMed] [Google Scholar]
- 31.Manna, S. K., V. Haridas, and B. B. Aggarwal. 2000. Bcl-x(L) suppresses TNF-mediated apoptosis and activation of nuclear factor-κB, activation protein-1, and c-Jun N-terminal kinase. J. Interferon Cytokine Res. 20:725-735. [DOI] [PubMed] [Google Scholar]
- 32.McCubrey, J. A., W. S. May, V. Duronio, and A. Mufson. 2000. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia 14:9-21. [DOI] [PubMed] [Google Scholar]
- 33.Noto, H., M. Matsushita, M. Koike, M. Takahashi, H. Matsue, J. Kimura, and S. Todo. 1998. Effect of high concentrations of bile acids on cultured hepatocytes. Artif. Organs 22:300-307. [DOI] [PubMed] [Google Scholar]
- 34.O'Dwyer, P. J., J. P. Stevenson, M. Gallagher, A. Cassella, I. Vasilevskaya, B. P. Monia, J. Holmlund, F. A. Dorr, and K. S. Yao. 1999. c-raf-1 depletion and tumor responses in patients treated with the c-raf-1 antisense oligodeoxynucleotide ISIS 5132 (CGP 69846A). Clin. Cancer Res. 5:3977-3982. [PubMed] [Google Scholar]
- 35.Park, J. S., L. Qiao, G. Gilfor, M. Y. Yang, P. B. Hylemon, C. Benz, G. Darlington, G. Firestone, P. B. Fisher, and P. Dent. 2000. A role for both Ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes. Mol. Biol. Cell 11:2915-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, S. J., Y. Y. Kim, J. W. Ju, B. G. Han, S. I. Park, and B. J. Park. 2001. Alternative splicing variants of c-FLIP transduce the differential signal through the Raf or TRAF2 in TNF-induced cell proliferation. Biochem. Biophys. Res. Commun. 289:1205-1210. [DOI] [PubMed] [Google Scholar]
- 37.Patel, T., S. Bronk, and G. Gores. 1994. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J. Clin. Investig. 94:2183-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poupon, R., O. Chazouilleres, and R. E. Poupon. 2000. Chronic cholestatic diseases. J. Hepatol. 32:129-140. [DOI] [PubMed] [Google Scholar]
- 39.Qiao, L., R. McKinstry, S. Gupta, D. Gilfor, J. J. Windle, P. B. Hylemon, S. Grant, P. B. Fisher, and P. Dent. 2002. Cyclin kinase inhibitor p21 potentiates bile acid-induced apoptosis in hepatocytes that is dependent on p53. Hepatology 36:39-48. [DOI] [PubMed] [Google Scholar]
- 40.Qiao, L., A. Yacoub, E. Studer, S. Gupta, X. Y. Pei, S. Grant, P. B. Hylemon, and P. Dent. 2002. Inhibition of the MAPK and PI3K pathways enhances UDCA-induced apoptosis in primary rodent hepatocytes. Hepatology 35:779-789. [DOI] [PubMed] [Google Scholar]
- 41.Qiao, L., E. Studer, K. Leach, R. McKinstry, S. Gupta, R. Decker, R. Kukreja, K. Valerie, P. Nagarkatti, W. El Deiry, J. Molkentin, R. Schmidt-Ullrich, P. B. Fisher, S. Grant, P. B. Hylemon, and P. Dent. 2001. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol. Biol. Cell 12:2629-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao, Y. P., R. T. Stravitz, Z. R. Vlahcevic, E. C. Gurley, J. J. Sando, and P. B. Hylemon. 1997. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. J. Lipid Res. 38:2446-2454. [PubMed] [Google Scholar]
- 43.Reardon, D. B., J. N. Contessa, R. B. Mikkelsen, K. Valerie, P. Dent, and R. K. Schmidt-Ullrich. 1999. Dominant-negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene 18:4756-4766. [DOI] [PubMed] [Google Scholar]
- 44.Roulston, A., C. Reinhard, P. Amiri, and L. T. Williams. 1998. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J. Biol. Chem. 273:10232-10239. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Ullrich, R. K., P. Dent, S. Grant, R. B. Mikkelsen, and K. Valerie. 2000. Signal transduction and cellular radiation responses. Radiat. Res. 153:245-257. [DOI] [PubMed] [Google Scholar]
- 46.Sebolt-Leopold, J. S., D. T. Dudley, R. Herrera, K. Van Becelaere, A. Wiland, R. C. Gowan, H. Tecle, S. D. Barrett, A. Bridges, S. Przybranowski, W. R. Leopold, and A. R. Saltiel. 1999. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 5:810-816. [DOI] [PubMed] [Google Scholar]
- 47.Sodeman, T., S. F. Bronk, P. J. Roberts, H. Miyoshi, and G. J. Gores. 2000. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G992-G999. [DOI] [PubMed] [Google Scholar]
- 48.Spivey, J. R., S. Bronk, and G. Gores. 1993. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J. Clin. Investig. 92:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strand, P., L. Carlsson, K. Rask, S. Skrtic, S. Ekberg, L. Hedin, J. Oscarsson, and J. O. Jansson. 2000. Growth hormone induces CCAAT/enhancer binding protein α (C/EBPα) in cultured rat hepatocytes. J. Hepatol. 32:618-626. [DOI] [PubMed] [Google Scholar]
- 50.Stravitz, R. T., Y. P. Rao, Z. R. Vlahcevic, E. C. Gurley, W. D. Jarvis, and P. B. Hylemon. 1996. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7 alpha-hydroxylase. Am. J. Physiol. 271:G293-G303. [DOI] [PubMed] [Google Scholar]
- 51.Tombes, R. M., K. L. Auer, R. Mikkelsen, K. Valerie, M. P. Wymann, C. J. Marshall, M. McMahon, and P. Dent. 1998. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem. J. 330:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trautwein, C., T. Rakemann, N. P. Malek, J. Plumpe, G. Tiegs, and M. P. Manns. 1998. Concanavalin A-induced liver injury triggers hepatocyte proliferation. J. Clin. Investig. 101:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueda, T., Y. Takeyama, Y. Hori, M. Shinkai, K. Takase, M. Goshima, M. Yamamoto, and Y. Kuroda. 2000. Hepatocyte growth factor increases in injured organs and functions as an organotrophic factor in rats with experimental acute pancreatitis. Pancreas 20:84-93. [DOI] [PubMed] [Google Scholar]
- 54.Webster, C. R., and M. S. Anwer. 2001. Phosphoinositide 3-kinase, but not mitogen-activated protein kinase, pathway is involved in hepatocyte growth factor-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology 33:608-615. [DOI] [PubMed] [Google Scholar]
- 55.Yu, J., L. Zhang, P. M. Hwang, K. W. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673-682. [DOI] [PubMed] [Google Scholar]
- 56.Zhu, S., K. Yoon, E. Sterneck, P. F. Johnson, and R. C. Smart. 2002. CCAAT/enhancer binding protein-β is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc. Natl. Acad. Sci. USA 99:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]