Abstract
The adapter protein Grb10 belongs to a superfamily of related proteins, including Grb7, -10, and -14 and Caenorhabditis elegans Mig10. Grb10 is an interacting partner of the insulin-like growth factor I receptor (IGF-IR) and the insulin receptor (IR). Previous work showed an inhibitory effect of mouse Grb10 (mGrb10α) on IGF-I-mediated mitogenesis (A. Morrione et al., J. Biol. Chem. 272:26382-26387, 1997). With mGrb10α as bait in a yeast two-hybrid screen, mouse Nedd4 (mNedd4-1), a ubiquitin protein ligase, was previously isolated as an interacting protein of Grb10 (A. Morrione et al., J. Biol. Chem. 274:24094-24099, 1999). However, Grb10 is not ubiquitinated by Nedd4 in cells. Here we show that in mouse embryo fibroblasts overexpressing Grb10 and the IGF-IR (p6/Grb10), there is a strong ligand-dependent increase in ubiquitination of the IGF-IR compared with that in parental cells (p6). This increased ubiquitination is associated with a shorter half-life and increased internalization of the IGF-IR. The IGF-IR is stabilized following treatment with both MG132 and chloroquine, indicating that both the proteasome and lysosomal pathways mediate degradation of the receptor. Ubiquitination of the IGF-IR likely occurs at the plasma membrane, prior to the formation of endocytic vesicles, as it is insensitive to dansylcadaverine, an inhibitor of early endosome formation in IGF-IR endocytosis. Grb10 coimmunoprecipitates with the IGF-IR and endogenous Nedd4 in p6/Grb10 cells, suggesting the presence of a Grb10/Nedd4/IGF-IR complex. Ubiquitination of the IGF-IR in p6/Grb10 cells is severely impaired by overexpression of a catalytically inactive Nedd4 mutant (Nedd4-CS), which also stabilizes the receptor. Likewise, overexpression of a Grb10 mutant lacking the Src homology 2 (SH2) domain impaired ubiquitination of the IGF-IR in parental p6 and p6/Grb10 cells, indicating that Grb10 binding to Nedd4 is critical for ubiquitination of the receptor. These results suggest a role for the Grb10/Nedd4 complex in regulating ubiquitination and stability of the IGF-IR, and they suggest that Grb10 serves as an adapter to form a bridge between Nedd4 and the IGF-IR. This is the first demonstration of regulation of stability of a tyrosine kinase receptor by the Nedd4 (HECT) family of E3 ligases.
Grb10, originally identified as a binding partner of tyrosine-phosphorylated epidermal growth factor receptor (EGFR) (44), is a member of a family of adapter proteins that includes at least seven isoforms in humans and mice. Grb10 was isolated in our laboratory and others as an interacting partner for the insulin-like growth factor I receptor (IGF-IR) (8, 37), the insulin receptor (IR) (10, 12, 14, 26, 29), or both (43). Grb10 interacts also with the platelet-derived growth factor receptor (62).
All known Grb10 isoforms (41) contain a highly conserved Src homology 2 (SH2) domain at the C terminus, a pleckstrin homology (PH) domain in the central region, and a less conserved N terminus, containing proline-rich sequences which may provide binding sites for SH3 domain-containing proteins (11, 29). Recently, another domain was identified and named BPS (for “between PH and SH2”) (15), and this domain appears to mediate binding to the IGF-IR (15, 56).
The functions of the different Grb10 isoforms have not been fully elucidated, and the available data are partially discordant. We showed an inhibitory effect of mouse Grb 10 (mGrb10α) on IGF-I-mediated cell proliferation (38). An inhibitory effect on IR and IGF-IR signaling has also been reported by other investigators (29, 56). On the other hand, O'Neill et al. and Wang et al. reported stimulatory effects on IGF-I and insulin signaling with human or mGrb10 variants (43, 62). They showed that Grb10-SH2 peptides acted in a dominant negative fashion to inhibit IGF-I and insulin-induced DNA synthesis, and they therefore assumed that Grb10 must have a stimulatory effect on IGF-I and insulin-stimulated cell proliferation. One possible explanation for these apparently contradictory results may be that these authors used DNA synthesis as a parameter of cell proliferation, and there are many examples of cells synthesizing DNA but not dividing (50, 58). Indeed, previous work showed that mGrb10α is able to promote IGF-I-induced DNA synthesis in p6/Grb10 cells to the same level as in parental p6 cells. Fluorescence-activated cell sorting analysis also showed that p6/Grb10 cells are then blocked between the S and G2 phases of the cell cycle, failing to fully respond to IGF-I with cell division (38). Other possible explanations for the discordant results may be related to the different Grb10 isoforms used, which can have different functions and may compete for common substrates.
Recently, the Grb10 gene on mouse chromosome 11 was identified as a maternally expressed imprinted gene (33), and it has been suggested that it may be a candidate gene for the Silver-Russell syndrome in humans, a syndrome that is characterized by pre- and postnatal growth retardation.
The Grb10 SH2 domain was previously shown to interact with MEK1 and Raf1 kinases in a phosphotyrosine-independent manner, and Grb10 mutations within the SH2 domain have been reported to induce apoptosis (41). The Grb10 SH2 domain has also been reported to interact with the growth hormone receptor (39), the Elk receptor (55), the Bcr-Abl tyrosine kinase (3), and the Akt kinase (20).
The yeast two-hybrid system was recently used to screen a mouse embryo library (60) with full-length mGrb10 as bait, and a cDNA (36) encoding the C2 domain of mouse Nedd4 (for “neuronal precursor cell-expressed developmentally down-regulated 4”) was isolated (25).
Mouse Nedd4 (Nedd4-1) is a ubiquitin protein ligase (E3) containing a C2 domain, three WW domains (6), and a HECT (for “homologous to the E6-AP carboxyl terminus”) domain (19). The WW domains of Nedd4 (Nedd4-1 and -2) have been shown to interact with the epithelial sodium channel (ENaC), recognizing proline-rich PY motifs (23, 53), and this interaction promotes ubiquitination and destabilization of the channel (1, 13, 22, 54). Nedd4 has been also shown to bind to and regulate the stability of the guanine nucleotide exchange factor CNrasGEF (46, 47). The C2 domain of rat Nedd4 binds phospholipids and mediates Ca2+-dependent translocation to the plasma membrane (49) through interaction with annexin XIIIb (48).
Deletion mutants of Grb10 have been used to show that it is the SH2 domain of Grb10 that is responsible for binding to the C2 domain of Nedd4. The interaction of mGrb10 and Nedd4 was confirmed in cells, and it was shown that endogenous Nedd4 forms a constitutive complex with Grb10, in a Ca2+- and phosphotyrosine-independent manner (36). Despite this complex formation, Grb10 is not ubiquitinated by Nedd4 in cells. Here we show that the interaction of Grb10 with Nedd4 serves instead to mediate ligand-dependent ubiquitination of the IGF-IR, and we propose that Grb10 acts as an adapter, forming a bridge between Nedd4 and the receptor.
MATERIALS AND METHODS
Cell lines.
p6 and p6/Grb10 cells are mouse embryo fibroblasts overexpressing the IGF-IR (p6) or the IGF-IR and mGrb10α (p6/Grb10) (36, 38).
Ubiquitination assays in cells.
p6 and p6/Grb10 cells were plated in triplicate at a density of 6 × 105 cells/well in six-well plates and transiently transfected with 4 μg of the eight-hemagglutinin (HA)-tagged ubiquitin construct (HA-Ub) (57) (kindly provided by D. Bohmann) per well using 5 μl of DNA/μg of Lipofectamine 2000 (Gibco). After 24 h, cells were shifted to serum-free medium (SFM) for an additional 24 h and then stimulated for 10 min with 20 ng of IGF-I (Gibco) per ml in the presence of 20 μM MG132 (Calbiochem) and 0.4 mM chloroquine (Sigma) to accumulate the ubiquitinated species. Where indicated, 500 μM dansylcadaverine (Sigma) was added with IGF-I. Lysates of p6 or p6/Grb10 cells were pooled, and 800 μg of protein was immunoprecipitated in HNTG buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Triton X-100, 10% glycerol, 0.2 mM sodium orthovanadate, protease inhibitor mix [Roche]) supplemented with MG132 and chloroquine (at the concentrations above) with anti-IGF-IR monoclonal antibodies (Oncogene Sciences). Filters were immunoblotted with anti-HA monoclonal antibodies (Covance) to detect ubiquitinated proteins. Ubiquitinated species appear as a high-molecular-weight smear. The filters were also reprobed with antiphosphotyrosine monoclonal antibodies (Transduction Laboratories) to determine activation of the receptor. The IGF-IR was detected with polyclonal antibodies from Santa Cruz.
To ensure ubiquitination of the IGF-IR and not of associated proteins in the presence of Grb10, we performed ubiquitination assays of the IGF-IR under reducing conditions (47). After transient transfections, pooled lysates of IGF-I-stimulated p6/Grb10 cells were boiled in 1% sodium dodecyl sulfate (SDS) for 5 min. Boiled lysates were diluted 11 times with HNTG buffer to dilute the SDS prior to immunoprecipitation with anti-IGF-IR antibodies, as described above.
To detect ubiquitination of the IGF-IR at endogenous levels of ubiquitin, 2 mg of IGF-I-stimulated (in the presence of MG132 and chloroquine) p6/Grb10 cell lysates was immunoprecipitated with anti-IGF-IR antibodies, and proteins were stained with antiubiquitin antibodies (Covance).
p6/Grb10 cells were also transiently cotransfected with the HA-Ub plasmid in combination with either the empty pRC-CMV vector or the pPRC-CMV-Nedd4(CS) vector, encoding Nedd4(CS). This Nedd4 mutant carries a point mutation of the conserved Cys (Cys to Ser) of the HECT domain and is unable to transfer ubiquitin and hence catalytically inactive (47). The expression of tagged mutant Nedd4 transiently transfected into p6/Grb10 cells was detected with anti-T7 tag monoclonal antibodies (Novagen), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Oncogene Science) or horseradish peroxidase-linked protein A (Amersham Corp. instructions). Blots were then developed with the ECL system according to the manufacturer's instructions (Amersham Corp).
Analysis of IGF-IR levels.
p6 and p6/Grb10 cells were starved in SFM for 24 h and then stimulated with 20 ng of IGF-I (Gibco) per ml for different times (0, 8, 14, 16, 18, and 20 h). This concentration of IGF-I is the one used in previous work (36, 38). Cells were lysed, and the level of the IGF-IR was determined by immunoblotting with polyclonal antibodies against the β subunit of the IGF-IR (Santa Cruz). Normalization of proteins loaded on the gel was done by incubating the same filter with anti-Grb2 monoclonal antibodies (Transduction Laboratories). The inhibitors of the proteasome (MG132) or the lysosomal (chloroquine) pathway or dansylcadaverine was added at the time of stimulation with IGF-I.
Pulse-chase experiments.
p6 and p6/Grb10 cells were plated in triplicate onto six-well plates. Cells were starved overnight in serum-free methionine-cysteine-free medium (Gibco) and then labeled for 1 h in the same medium containing 500 μCi of [35S]Cys-Met (Promix; Amersham)/ml. Cells were extensively washed and placed in fresh SFM plus IGF-I (time zero) and then chased for 2, 4, and 8 h. Pooled lysates (300 μg) from each time point were immunoprecipitated with anti-IGF-IR antibodies (Oncogene Sciences) and loaded on SDS-polyacrylamide gels, and the dried gels were then exposed for autoradiography. p6/Grb10 cells were also transiently transfected as described above with either the empty pRC-CMV vector or pPRC-CMV-Nedd4(CS) before the cells were shifted to serum-free Cys-Met-free medium. Inhibitors were added at time zero. Lysates (50 μg) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted with anti-T7 antibodies (Novagen) to detect expression of Nedd4(CS).
Internalization assay.
Cell surface receptors were assessed by enzyme-linked immunosorbent assay (ELISA), as previously described (45). Briefly, cells were plated onto poly-l-lysine coated 24-well dishes in Dulbecco's modified Eagle medium supplemented with 10% FBS. The following day, they were washed three times with Dulbecco's modified Eagle medium and serum starved for 24 h in SFM. Cells were treated in the absence or presence of 200 ng of IGF-I/ml for 0, 15, 30, and 60 min. Medium was aspirated, and cells were fixed for 10 min at room temperature in 3.7% formaldehyde in Tris-buffered saline (TBS; 50 mM Tris-HCl [pH 7.4], 150 mM NaCl). Cells were washed three times with TBS and then blocked for 45 min with 1% bovine serum albumin-TBS. Cells were incubated for 1 h with a monoclonal antibody against the IGF-IR α subunit (diluted 1:1,000; Oncogene Sciences), washed three times, reblocked for 15 min, and incubated for 1 h with goat anti-mouse alkaline phosphatase-conjugated antibody (diluted 1:1,000; Sigma). They were then washed three times, and antibody binding was visualized by adding 0.25 ml of alkaline phosphatase substrate (Bio-Rad). The reaction was stopped by removing 0.1 ml of the substrate to a 96-well microtiter plate containing 0.1 ml of 0.4 M NaOH. Plates were read at 405 nm in a microplate reader (Bio-Rad) by using Microplate Manager software.
Densitometric analysis.
Densitometric analysis was performed with a Molecular Dynamics densitometer. Data were analyzed with the Image Quant Program at the Kimmel Cancer Center Nucleic Acid Facility.
Coimmunoprecipitations to detect the Grb10/Nedd4/IGF-IR complex.
To investigate the presence of the IGF-IR in complex with Grb10 and Nedd4, serum-starved p6/Grb10 cells were stimulated with 20 ng of IGF-I/ml for 10 min and lysed, and 1 mg of protein was immunoprecipitated with anti-Myc (Oncogene Sciences) antibodies to precipitate the Myc-tagged Grb10 (36, 38). After SDS-PAGE, filters were probed with anti-IGF-IR (Santa Cruz), -Grb10 (UBI), and -Nedd4 (Transduction Laboratories) antibodies, as described previously (36). To test the level of Nedd4 associated with the IGF-IR, 2 mg of IGF-I-stimulated p6 and p6/Grb10 cell lysates was immunoprecipitated with anti-IGF-IR antibodies and stained for Nedd4, Grb10, and IGF-IR, as described above.
Generation and use of the Grb10 SH2 deletion mutant.
The Myc-tagged Grb10 SH2 deletion mutant was generated by standard PCR techniques with the following specific primers: forward, ATG-GAG-CAG-AAG-CTG-ATC-AGC-GAG-GAG-GAC-CTG-AAC-AAC-G; reverse, ATG-CTG-AGT-CCT-GTG-AAT-CAC-TGC-ATT-CAG-GGT-AGA-AGG-AT. The PCR product was cloned first into the PCR Topo II vector (Invitrogen) and then into the pCDNA3.1/Hygro expression vector. In independent experiments, p6 and p6/Grb10 cells were transiently cotransfected with the HA-Ub plasmid in combination with either the empty pCDNA3.1/Hygro vector or the pCDNA3.1/Hygro Grb10ΔSH2 mutant. Serum-starved transfected p6 and p6/Grb10 cells were stimulated with 20 ng of IGF-I/ml for 10 min and lysed, and 1 mg (p6/Grb10) or 2 mg (p6) of proteins was immunoprecipitated with anti-IGF-IR antibodies, as described above. The expression of Myc-tagged Grb10 proteins was detected with anti-Myc polyclonal antibodies (Santa Cruz).
RESULTS
Ligand-dependent ubiquitination of the IGF-IR in cells overexpressing Grb10 (p6/Grb10 cells).
It was recently shown that the SH2 domain of mGrb10α binds to the C2 domain of the E3 ubiquitin ligase Nedd4 and that this binding is phosphotyrosine independent and constitutive (36). Moreover, that work also showed that Grb10 is not a target for ubiquitination by Nedd4 (36) in mouse embryo fibroblasts. We therefore investigated whether the interaction between Grb10 and Nedd4 could instead serve to localize Nedd4 to targets of Grb10 such as the IGF-IR and promote its ubiquitination. We thus performed experiments in which a HA-ubiquitin construct was transiently transfected into parental p6 cells (stably overexpressing the IGF-IR) or p6/Grb10 cells (stably overexpressing the IGF-IR and Myc-tagged Grb10). After transfection with the HA-Ub plasmid, cells were serum starved for 24 h and then stimulated with 20 ng of IGF-I/ml for 10 min (or left unstimulated). Following immunoprecipitation with anti-IGF-IR antibodies, proteins were immunoblotted with anti-HA antibodies to assess their ubiquitination. As seen in Fig. 1A, the appearance of a high-molecular-weight broad band recognized by the HA antibodies in the IGF-IR immunoprecipitates suggests that the receptor is ubiquitinated in vivo. Importantly, following ligand stimulation, there is a strong increase in ubiquitination of the IGF-IR in the p6/Grb10 cells compared with the parental p6 cells (Fig. 1A). In parental p6 cells and in unstimulated p6/Grb10 cells, low levels of ubiquitination of the IGF-IR can be detected, but only after overexposure of the film (not shown). Fig. 1B, C, and D depict controls, demonstrating activation of the IGF-IR following IGF-I stimulation (antiphosphotyrosine blot [Fig. 1B]), equal levels IGF-IR protein were immunoprecipitated in all treatments (Fig. 1C), and equal amounts of HA-Ub expressed in all treatments (Fig. 1D). These data strongly suggest a role of Grb10 in promoting ligand-induced ubiquitination of the IGF-IR.
FIG. 1.
Grb10 increases ligand-dependent ubiquitination of the IGF-IR in cells. p6 and p6/Grb10 cells were plated in triplicate onto six-well plates and transiently transfected with the HA-Ub construct. After 24 h, cells were shifted to SFM for an additional 24 h and then stimulated for 10 min with 20 ng of IGF-I/ml in the presence of 20 μM MG132 and 0.4 mM chloroquine to accumulate the ubiquitinated forms. Cell lysates were pooled, and 800 μg was immunoprecipitated (IP) with anti-IGF-IR antibody and blotted with anti-HA antibodies to detect ubiquitinated species (A). This blot was then stripped and reprobed with antiphosphotyrosine antibodies (B) and anti-IGF-IR antibodies to test for the amount of receptor immunoprecipitated (C). Total lysates served as a positive control for HA-Ub transfection (D). Results in panels A to D are representative of three independent experiments. (E) The IGF-IR, but not coprecipitated protein(s), is ubiquitinated in p6/Grb10 cells. After transient transfections as above, lysates of IGF-I-stimulated p6/Grb10 cells were treated with 1% SDS and boiled for 5 min prior to immunoprecipitation with anti-IGF-IR antibodies. After immunoprecipitation, the filter was probed with anti-HA (left) to detect IGF-IR ubiquitination or anti-IGF-IR (center) antibodies (control). Total lysate served as a positive control for HA-Ub transfection (right). (F) IGF-I-induced ubiquitination of the IGF-IR in p6/Grb10 cells is detectable at endogenous levels of ubiquitin. Lysates (2 mg) of IGF-I-stimulated p6/Grb10 cells were immunoprecipitated with anti-IGF-IR antibodies. After immunoprecipitation the filter was probed with antiubiquitin (UB) antibody (left and right) to detect the ubiquitinated IGF-IR. The blot with anti-IGF-IR antibodies (center) is a control. Results in panels E and F are representative of two independent experiments.
To ensure that the ubiquitination of the IGF-IR in the p6/Grb10 cells shown in Fig. 1A was of the receptor itself and not of associated proteins coprecipitating with the IGF-IR or Grb10, lysates from IGF-I-stimulated p6/Grb10 cells (transiently transfected with the HA-Ub construct) were boiled in 1% SDS for 5 min prior to immunoprecipitation of the IGF-IR. This treatment removes associated proteins while leaving intact ubiquitin moieties, which are covalently linked to their target. As seen in Fig. 1E, the IGF-IR was still ubiquitinated following boiling in SDS, confirming that it is indeed the IGF-IR that is ubiquitinated, and not other putative associated proteins.
To investigate if the ligand-dependent ubiquitination of the IGF-IR in p6/Grb10 cells could be also detected at endogenous levels of ubiquitin, we immunoprecipitated the IGF-IR from IGF-I-stimulated p6/Grb10 cell lysates and immunoblotted the filter with antiubiquitin antibodies. As shown in Fig. 1F, ubiquitination of the IGF-IR in p6/Grb10 cells was detectable with antiubiquitin antibodies.
Grb10 is implicated in the regulation of IGF-IR stability.
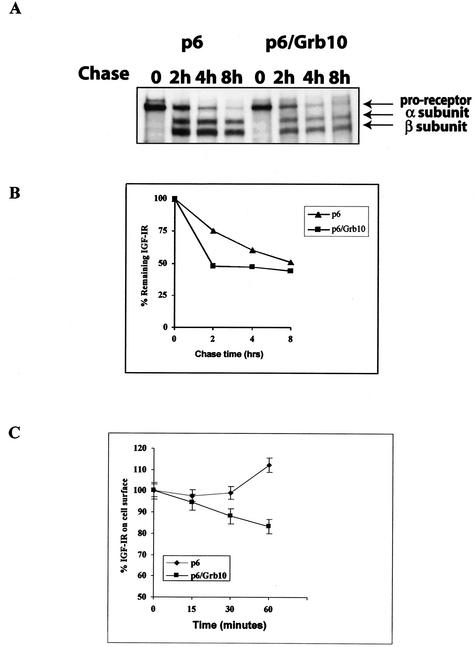
Ubiquitination of proteins regulates their stability and usually targets them for degradation (17). We therefore investigated whether the increased ubiquitination of the IGF-IR in cells overexpressing Grb10 could alter their half-life, using pulse-chase experiments. To this end, p6 and p6/Grb10 were starved in serum-free Met-Cys-free medium, pulsed with [35S]Met-Cys for 1 h, and chased in SFM supplemented with 20 ng of IGF-I/ml for 0 to 8 h. Cells were then lysed, and 300 μg of protein was immunoprecipitated with anti-IGF-IR antibodies. The IGF-IR is newly synthesized as a proreceptor (Fig. 2A) and then processed into α and β subunits. Our results show that in parental p6 cells, after stimulation with IGF-I, the IGF-IR α and β subunits have a half-life of ∼8 h. This half-life is drastically reduced in p6/Grb10 cells, to ∼2 h (Fig. 2A and B). These results suggest that in the presence of Grb10 (p6/Grb10 cells) and IGF-I stimulation, the increased ubiquitination of the IGF-IR is associated with a dramatic decrease in receptor stability.
FIG. 2.
Grb10 is involved in regulating IGF-IR stability and internalization. p6 and p6/Grb10 were serum starved in serum-free Met-Cys-free medium, pulsed with [35S]Met-Cys for 1 h, and chased in SFM supplemented with 20 ng of IGF-I/ml for 0, 2, 4, and 8 h. Cells were then lysed, and 300 μg of protein was immunoprecipitated with anti-IGF-IR antibodies. (A) Immunocomplexes were washed, resolved by SDS-PAGE, dried, and exposed to X-ray film. The IGF-IR is newly synthesized as a proreceptor and then processed into α and β subunits. Results are representative of three independent experiments. (B) Quantitation of IGF-IR using densitometric analysis. The graph was generated with the ImageQuant program. (C) Decreased level of cell surface IGF-IR in p6/Grb10 cells. The level of cell surface IGF-IR in p6 and p6/Grb10 cells was determined by ELISA at different times of IGF-I stimulation, as described in Materials and Methods. IGF-I was used at 200 ng/ml to ensure a saturating concentration of the ligand. The data are the averages of three independent experiments.
Cell surface expression of the IGF-IR is reduced upon ligand stimulation in p6/Grb10 cells.
Ubiquitination of transmembrane receptor proteins has been reported to regulate ligand-mediated internalization (28). We therefore used ELISA (45) to test whether the increased ligand-dependent ubiquitination of the IGF-IR in p6/Grb10 cells was associated with a reduction in the level of cell surface IGF-IR proteins. Figure 2C shows that in p6/Grb10 cells there was a clear decrease in the amount of IGF-IR at the cell surface after 30 min of IGF-I stimulation compared to that in the parental p6 cells, suggesting that the increased ligand-induced ubiquitination of the IGF-IR in p6/Grb10 cells is associated with a faster ligand-mediated internalization of the receptor. The increase in the amount of cell surface IGF-IR in the control p6 cells over time likely represents the accumulation of recycled receptor.
IGF-IR degradation is mediated by both the proteasome and lysosomal pathways.
To further investigate if the increased ubiquitination and internalization of the IGF-IR in cells overexpressing Grb10 could promote a faster degradation of the receptor and to determine the routes of this degradation, we tested by Western blotting the level of the IGF-IR in p6 and p6/Grb10 cells at different time points after IGF-I stimulation. As shown in Fig. 3A, while the level of the IGF-IR protein (β subunit) is stable for up to 20 h of IGF-I stimulation in parental p6 cells, in p6/Grb10 cells the level of the receptor is already decreased by 8 h and severely reduced after 20 h of IGF-I stimulation. The same filters were reprobed with anti-Grb2 antibodies as a control for the amount of loaded proteins (Fig. 3A). Transmembrane receptor tyrosine kinases are targeted for degradation by either the lysosomal or the proteasome pathway (reviewed in references 17, 42, and 59). We investigated, therefore, whether the degradation of the IGF-IR in p6/Grb10 cells is sensitive to proteasome or lysosomal pathway inhibitors. We thus determined the levels of the IGF-IR in p6/Grb10 cells at 20 h after stimulation with IGF-I alone or when supplemented with either a 20 μM concentration of the proteasome inhibitor MG132 or 0.4 mM chloroquine, an inhibitor of the lysosomal pathway. Figure 3B (upper panel) shows that treatment with either MG132 or chloroquine stabilizes the IGF-IR protein, suggesting that the IGF-IR in p6/Grb10 cells is targeted for degradation by both the proteasome and lysosomal pathways.
FIG. 3.
Ligand-stimulated IGF-IR degradation in p6/Grb10 cells is mediated by both the proteasome and lysosomal pathways. (A) p6 or p6/Grb10 cells were serum starved and then stimulated with 20 ng of IGF-I/ml for the indicated times. (B) IGF-IR levels were determined by immunoblotting with anti-IGF-IR polyclonal antibodies. p6/Grb10 cells were stimulated for 20 h with IGF-I alone or supplemented with 20 μM MG132 (MG), 0.4 mM chloroquine (Chl), or both (M+C). The total amount of protein loaded on the gel was monitored with anti-Grb2 monoclonal antibodies (A and B). Results are representative of three independent experiments. Densitometric analysis of the representative experiment was performed with the ImageQuant program, and results are expressed as the percentage of IGF-IR remaining.
Ubiquitination of the IGF-IR occurs prior to entry into endocytotic vesicles.
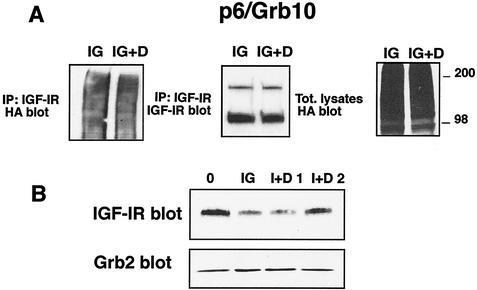
Previous results have suggested that ubiquitination of the EGFR mediated by Cbl occurs in early endosomes (28). We therefore investigated whether the ligand-dependent ubiquitination of the IGF-IR in p6/Grb10 cells requires the formation of early endosomes. We thus repeated the ubiquitination experiments with the IGF-IR in the presence of dansylcadaverine, a specific inhibitor of IGF-IR trafficking in endocytotic vesicles (7). As shown in Fig. 4A, incubation of p6/Grb10 cells with 500 μM dansylcadaverine (7) had no effect on IGF-I-mediated ubiquitination of the IGF-IR, suggesting that the IGF-IR in p6/Grb10 is most likely ubiquitinated at the plasma membrane or at a very early step in vesicle formation, prior to the step inhibited by dansylcadaverine. On the other hand, 200 μM dansylcadaverine was very effective at inhibiting the subsequent degradation of the IGF-IR (Fig. 4B), confirming that degradation of the IGF-IR requires prior endocytosis.
FIG. 4.
Ubiquitination of the IGF-IR occurs prior to the formation of endocytotic vesicles. (A) p6/Grb10 cells were plated in triplicate onto six-well plates and transiently transfected with HA-Ub. After 24 h, cells were shifted to SFM for an additional 24 h and then stimulated for 10 min with 20 ng of IGF-I/ml in the presence of MG132 and chloroquine (IG). Dansylcadaverine (500 μM) was added at the time of stimulation with IGF-I (IG+D). Cell lysates were pooled, and 800 μg was immunoprecipitated with anti-IGF-IR antibody and blotted with anti-HA antibodies to detect ubiquitinated species. This blot was then stripped and reprobed with anti-IGF-IR antibodies to test for the amount of receptor immunoprecipitated. Total lysates served as positive controls for HA-Ub transfection. (B) p6/Grb10 cells were stimulated for 20 h with IGF-I alone or supplemented with 100 (I+D 1) or 200 (I+D 2) μM dansylcadaverine. IGF-IR levels were determined by Western blotting with anti-IGF-IR polyclonal antibodies. The total amount of protein loaded on the gel was monitored with anti-Grb2 monoclonal antibodies. Results are representative of two independent experiments.
Grb10 coimmunoprecipitates with the IGF-IR and endogenous Nedd4 in cells.
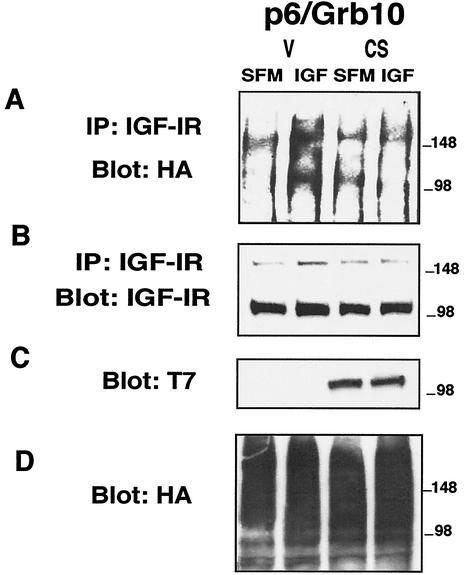
Grb10 binds the IGF-IR in a phosphotyrosine-dependent manner (35), while the binding of Grb10 to Nedd4 is phosphotyrosine independent and constitutive (36). The IGF-IR does not contain PY motifs, which are binding sites for Nedd4 (47, 53), and in fact, using the yeast two-hybrid system, we could not detect any direct interaction between the IGF-IR and Nedd4 (data not shown). We therefore hypothesized that Grb10 may have an adapter function to connect Nedd4 and the IGF-IR. To investigate this hypothesis, we first tested whether the IGF-IR could be recovered in a complex with Grb10 and endogenous Nedd4 in vivo in coimmunoprecipitation experiments. Serum-starved p6/Grb10 cells were stimulated with IGF-I and lysed, and proteins were immunoprecipitated with anti-Myc antibodies to precipitate the Myc-tagged Grb10 (36, 38). As shown in Fig. 5E, Grb10 was immunoprecipitated with anti-Myc antibodies in p6/Grb10 cells. Grb10 was able to coimmunoprecipitate endogenous Nedd4 (Fig. 5C), and this binding is constitutive, as shown previously (36). Grb10 is also able to coimmunoprecipitate the IGF-IR, and the binding is increased after stimulation with IGF-I (Fig. 5A). We also immunoprecipitated the IGF-IR from lysates of IGF-I-stimulated p6 and p6/Grb10 cells, which revealed enhanced Nedd4 recruitment to the IGF-IR in p6/Grb10 cells compared to that in parental p6 cells (Fig. 5D), confirming that Grb10 acts to recruit Nedd4 to the IGF-IR. These results suggest complex formation between Grb10, endogenous Nedd4, and the IGF-IR in p6/Grb10 cells.
FIG. 5.
Grb10 coimmunoprecipitates with the IGF-IR and endogenous Nedd4. Serum-starved p6/Grb10 cells were stimulated with IGF-I for 10 min and lysed, and proteins were immunoprecipitated with anti-Myc antibodies to immunoprecipitate Myc-tagged Grb10 (36, 38). Total lysates were used to show the correct sizes of coprecipitated proteins. In panel E, the third and lower isoform of Grb10 is detected by anti-Grb10 antibodies after longer exposure of the film (not shown). To detect Nedd4 association with the IGF-IR, serum-starved p6 and p6/Grb10 cells were stimulated with IGF-I for 10 min and lysed, and proteins were immunoprecipitated with anti-IGF-IR antibodies.
Overexpression of catalytically inactive Nedd4 abolishes ubiquitination of the IGF-IR in p6/Grb10 cells.
Our results suggest a possible role for Nedd4 in ubiquitination of the IGF-IR in p6/Grb10 cells. To test whether endogenous Nedd4 is the E3 responsible for ubiquitination of the IGF-IR, we transiently cotransfected p6/Grb10 cells with Nedd4(CS) (47) and HA-Ub and assessed the level of ubiquitination of the IGF-IR. As seen in Fig. 6A, there was a marked increase of ubiquitination of the IGF-IR in vector-transfected p6/Grb10 cells after stimulation with IGF-I. In contrast, overexpression of Nedd4(CS) mutant diminished ubiquitination of the IGF-IR (Fig. 6A), suggesting that Nedd4(CS) is acting in a dominant negative fashion to block ubiquitination of the IGF-IR mediated by endogenous Nedd4. These results therefore suggest that Nedd4 is the ubiquitin ligase responsible for ubiquitination of the IGF-IR in p6/Grb10 cells.
FIG. 6.
Catalytically inactive Nedd4 impairs ubiquitination of the IGF-IR in p6/Grb10 cells. p6/Grb10 cells were transiently cotransfected with either an empty vector (V) or Nedd4(CS) and HA-Ub. IGF-IR was then immunoprecipitated, and levels of its ubiquitination were determined with anti-HA antibodies (A), as described for Fig. 1. (B) Level of the IGF-IR immunoprecipitated; (C) same filter reprobed with anti-T7 antibodies to detect the expression of Nedd4(CS); (D) lysates probed with anti-HA antibodies to ensure equal expression of the HA-Ub plasmid. Results are representative of three independent experiments.
Nedd4(CS) increases stability of the IGF-IR in p6/Grb10 cells.
Ubiquitination of proteins targets them for degradation. To confirm the role of Nedd4 in regulating stability of the IGF-IR, we transiently transfected Nedd4(CS) into p6/Grb10 cells. Cells were then starved in serum-free Met-Cys-free medium, pulsed with [35S]Met-Cys for 1 h, and chased in SFM supplemented with 20 ng of IGF-I/ml for 8 h. Cells were then lysed, and 300 μg of protein was immunoprecipitated with anti-IGF-IR antibodies. As seen in Fig. 7, overexpression of Nedd4(CS) partially stabilized the IGF-IR, compared with p6/Grb10 cells transfected with the empty vector. These data suggest that Nedd4 regulates the stability of the IGF-IR in cells expressing Grb10.
FIG. 7.
Catalytically inactive Nedd4(CS) mutant stabilizes the IGF-IR in p6/Grb10 cells. p6/Grb10 cells were transiently transfected with Nedd4(CS). Cells were starved in serum-free Met-Cys-free medium, pulsed with [35S]Met-Cys for 1 h, and chased in SFM supplemented with 20 ng of IGF-I/ml for 8 h. Cells were then lysed, and 300 μg of proteins was immunoprecipitated with anti-IGF-IR antibodies. Immunocomplexes were washed, resolved by SDS-PAGE, dried, and exposed to X-ray film. Densitometric analysis was performed with the ImageQuant program, and results are expressed as the percentage of IGF-IR remaining. (Lower panel) Immunoblot using anti-T7 antibodies to detect the transiently transfected T7-tagged Nedd4(CS) mutant. Results are representative of three independent experiments.
Overexpression of a Grb10 mutant lacking the SH2 domain impairs ubiquitination of the IGF-IR in p6/Grb10 cells.
To confirm that Grb10 acts as a bridge to bring Nedd4 to the IGF-IR, we generated a Grb10 deletion mutant lacking the SH2 domain (ΔSH2); this Grb10 mutant is unable to bind Nedd4 but retains the binding site for the IGF-IR (BPS domain) and is able to bind the receptor (Fig. 8E). This Grb10 mutant was transiently cotransfected into p6/Grb10 cells with HA-Ub, and the level of ubiquitination of the IGF-IR was subsequently assessed. As seen in Fig. 8A, there was strong ubiquitination of the IGF-IR in p6/Grb10 cells after stimulation with IGF-I. In contrast, overexpression of the ΔSH2 Grb10 mutant, which competes with wild-type Grb10 for binding to the receptor (Fig. 8E), severely reduced ubiquitination of the IGF-IR (Fig. 8A), suggesting that Nedd4 binding to Grb10 is critical for ubiquitination of the IGF-IR in p6/Grb10 cells. To confirm that the ubiquitination of the IGF-IR in parental p6 cells was mediated by endogenous Grb10, we transiently cotransfected the ΔSH2 Grb10 mutant into parental p6 cells with the HA-Ub plasmid, and the level of ubiquitination of the IGF-IR was subsequently assessed. As seen in Fig. 8B, there was ubiquitination of the IGF-IR in p6 cells after stimulation with IGF-I. In contrast, overexpression of the ΔSH2 Grb10 mutant almost completely abolished this ubiquitination (Fig. 8B), suggesting that endogenous Grb10 is necessary for the ubiquitination of the IGF-IR in parental p6 cells. These results strongly suggest that endogenous and overexpressed Grb10 acts as a bridge (adapter) between the IGF-IR and endogenous Nedd4, thus promoting ligand-dependent ubiquitination of the IGF-IR.
FIG. 8.
Overexpression of a Grb10 mutant lacking the SH2 domain impairs ubiquitination of the IGF-IR in p6 and p6/Grb10 cells. p6 and p6/Grb10 cells were transiently cotransfected with either an empty vector (V) or the ΔSH2 mutant and HA-Ub. After IGF-I stimulation, cell lysates were pooled, immunoprecipitated with anti-IGF-IR antibody, and blotted with anti-HA antibodies to detect ubiquitinated species (A and B). This blot was then stripped and reprobed with anti-IGF-IR antibodies (C and D) to determine the amount of receptor immunoprecipitated. Panels E and F show the same filter reprobed with anti-Myc antibodies to detect the expression of Myc-tagged wild-type and mutant Grb10 proteins. In panel F, the endogenous Grb10 is not detectable by anti-Myc antibodies. Total lysates blotted with anti-HA antibodies (G and H) serve as positive controls for HA-Ub transfection. Results are representative of three independent experiments.
DISCUSSION
Our results can be summarized as follows. (i) In mouse embryo fibroblasts overexpressing Grb10 and the IGF-IR (p6/Grb10), there is a strong ligand-dependent increase in ubiquitination of the IGF-IR compared with that in p6 cells. (ii) Ubiquitination of the IGF-IR is associated with a shorter half-life and increased internalization of the receptor in p6/Grb10 cells. (iii) The IGF-IR is stabilized following treatment with both MG132 and chloroquine, indicating that both the proteasome and lysosomal pathways mediate degradation of the receptor. (iv) Ubiquitination of the IGF-IR occurs at the plasma membrane or at a very early stage in vesicle formation, as it is insensitive to dansylcadaverine, a specific inhibitor of early endosomes in IGF-IR trafficking. (v) Grb10 coimmunoprecipitates in vivo with the IGF-IR and endogenous Nedd4, suggesting the presence of a complex in p6/Grb10 cells. (vi) Ubiquitination of the IGF-IR in p6/Grb10 cells is impaired by overexpression of Nedd4(CS), which also stabilizes the receptor. (vii) Overexpression of a Grb10 mutant lacking the SH2 domain impairs IGF-I-mediated ubiquitination of the IGF-IR in both p6 and p6/Grb10 cells, suggesting that binding between Grb10 and Nedd4 is critical for ubiquitination of the receptor.
The adapter protein Grb10 belongs to a superfamily of related proteins including Grb7, -10, and -14 and C. elegans Mig10 (reviewed in reference 35). Grb10 was isolated in our and other laboratories as an interacting partner of the IGF-IR or the IR (35). Previous and recent work has shown an inhibitory role for mGrb10α in IGF-I-mediated mitogenesis (38), and mGrb10α was used as bait in a yeast two-hybrid screen to isolate mouse Nedd4, a ubiquitin protein ligase, as an interacting protein of Grb10 (36).
Nedd4 contains a C2 domain, three or four WW domains and a ubiquitin ligase HECT domain. The WW domains mediate binding to target proteins, usually by associating with their PY motifs (PPxY) (23, 53). The IGF-IR does not have PY motifs, suggesting that there is no direct interaction between these two proteins. In fact, when we used the two-hybrid system we did not detect any direct binding between the IGF-IR and Nedd4 (data not shown). The interaction between the C2 domain of Nedd4 and the SH2 domain of Grb10, via a phosphotyrosine-independent association, did not result in ubiquitination of Grb10 (36). It was therefore reasonable to speculate that this interaction may instead serve to localize Nedd4 to targets of Grb10 such as the IGF-IR. Indeed, ubiquitination of the IGF-IR was demonstrated previously (52), and we now present evidence for a strong ligand-dependent in vivo ubiquitination of the IGF-IR in p6/Grb10 cells compared with parental p6 cells. This increased ubiquitination is associated with a shorter half-life and decreased levels of cell surface IGF-IR in p6/Grb10 cells. These results suggest that the ligand-mediated increased ubiquitination of the IGF-IR in p6/Grb10 cells is associated with an increase in the internalization rate for the receptor, as previously reported for the EGFR (28). The IGF-IR in p6/Grb10 cells is likely ubiquitinated at the plasma membrane, as it occurs in the presence of dansylcadaverine, a specific inhibitor of IGF-IR endocytosis (7). Grb10 coprecipitates in vivo with the IGF-IR and endogenous Nedd4, indicating the presence of a complex in p6/Grb10 cells. These data strongly suggest that Grb10 plays an adapter role in Nedd4-mediated ubiquitination of the IGF-IR. Significantly, it was recently shown in Drosophila that DNedd4 binds Commissureless, and Commissureless acts as an adapter protein to target Roundabout for internalization in a DNedd4-dependent manner (40). Moreover, Cbl-induced ubiquitination of the T-cell receptor ζ requires the adapter role of Zap-70 (61), pointing to a possible common alternative molecular mechanism that requires the presence of an adapter protein in E3-mediated ubiquitination of receptor proteins.
p6/Grb10 cells are severely impaired in IGF-I-stimulated cell proliferation compared with parental p6 cells (38), implicating Grb10 in this impairment. Accordingly, our results suggest that Grb10, by recruiting Nedd4, promotes increased ubiquitination, faster internalization, and subsequent enhanced degradation of the IGF-IR in p6/Grb10 cells, thus negatively regulating IGF-IR downstream signaling and mitogenesis. This is reminiscent of the negative role of Cbl, a RING finger E3, in mitogenesis via receptor tyrosine kinases, such as the EGFR, platelet-derived growth factor receptor, colony-stimulating factor 1 receptor, and Met (27, 28, 32, 63). However, we cannot rule out the possibility that Grb10 could also inhibit IGF-IR signaling by additional mechanisms, like direct inhibition of the kinase activity of the receptor by binding of the Grb10 BPS region, as recently reported by other investigators (5, 56).
Our results show that specific inhibitors of both the proteasome and lysosomal pathways stabilize the IGF-IR protein, indicating that both pathways contribute to the degradation of the receptor. There is precedence for degradation of transmembrane proteins by both pathways. For example, in ENaC, the unassembled chains are targeted to the proteasome while the assembled channel is degraded by the lysosome and possibly also by the proteasome (54). Moreover, although many cell surface proteins, particularly in Saccharomyces cerevisiae, are degraded by the lysosome and/or vacuole, proteasome involvement has been demonstrated in several others, such as the growth hormone receptor (59), LMP1 (2), and several tyrosine kinase receptors (21, 28, 34).
Overexpression of the Nedd4(CS) mutant impaired ubiquitination of the IGF-IR in p6/Grb10 cells (Fig. 6) and partially stabilizes the receptor (Fig. 7), indicating that the mutant Nedd4 acts in a dominant negative fashion towards the endogenous Nedd4. The effect of Nedd4(CS) on stability of the IGF-IR is not as pronounced as in inhibiting ubiquitination. This result can be explained given that (i) endogenous Nedd4 is expressed at high levels in p6/Grb10 cells (36, 38) and that (ii) in the ubiquitination experiments Nedd4(CS) competes with the endogenous Nedd4 for ubiquitination of only a fraction of total receptor (the portion that is ubiquitinated), while in pulse-chase experiments the Nedd4(CS) mutant acts on the total pool of newly synthesized IGF-IR. These results therefore suggest that Nedd4 is likely the E3 ligase involved in the regulation of ubiquitination, sorting, and stability of the IGF-IR in p6/Grb10 cells. We cannot rule out the possibility, however, that other ubiquitin ligases could also participate in the ubiquitination of the IGF-IR, utilizing mechanisms independent of Grb10.
Ligand-dependent internalization through clathrin-coated pits and subsequent degradation of tyrosine kinase receptors is a critical step in modulating their biological activity (9, 18). But while the role of the ubiquitin ligase Cbl is well established in tyrosine kinase receptor ubiquitination and regulation (27, 28, 31, 32, 63), our data represent, to our knowledge, the first evidence for the involvement of the Nedd4 (HECT) family of E3 ubiquitin ligases in the regulation of ubiquitination, internalization, and stability of tyrosine kinase receptors. Moreover, unlike the regulation of ENaC by direct binding of Nedd4 family members, regulation of the IGF-IR by Nedd4 employs an adapter protein, Grb10 (Fig. 9). Furthermore, this Nedd4/Grb10 complex formation is not dependent on Nedd4-WW binding to PY motifs on target proteins, as the interaction is mediated by the SH2 domain of Grb10 and the C2 domain of Nedd4 (36). Significantly, the expression in parental p6 and p6/Grb10 cells of the ΔSH2 mutant inhibits IGF-I-mediated ubiquitination of the IGF-IR (Fig. 8), confirming that the binding of endogenous and exogenously expressed Grb10 to Nedd4 is critical for ubiquitination of the receptor.
FIG. 9.
Model for the role of Grb10 as an adapter connecting Nedd4 to the IGF-IR. The Grb10 SH2 domain constitutively binds the C2 domain of Nedd4. Upon ligand stimulation, Grb10 binds the activated IGF-IR through the BPS domain, allowing the formation of a complex that includes the activated IGF-IR, Grb10, and Nedd4. These interactions lead to ligand-mediated and Nedd4-dependent ubiquitination and degradation of the IGF-IR.
Nedd4 and its yeast homologue Rsp5p had been previously shown to regulate the stability of numerous cell surface proteins (reviewed in reference 51), as well as several cytoplasmic and nuclear proteins (4, 46). More recently, Nedd4 members were shown to also regulate ubiquitination involved in vesicular transport and sorting (16) and virus particle budding (18, 24). Our work extends our understanding of Nedd4 function, i.e., regulation of ubiquitination and stability of receptor tyrosine kinases, by use of the adapter protein Grb10 and possibly other family members, such as Grb7 and Grb14 (30), which also bind Nedd4.
Acknowledgments
This work was supported by grant KO1 DK02896 from the National Institutes of Health (to Andrea Morrione) and a grant from the Canadian Institute of Health Research (to Daniela Rotin). Adriano Marchese is supported by a postdoctoral fellowship from the American Heart Association.
We are deeply grateful to Renato Baserga for his mentorship and support and for providing valuable reagents. We acknowledge Pamela Plant for unpublished experiments. We are also grateful to Judy Verdone for her skillful assistance in cell culture.
REFERENCES
- 1.Abriel, H., J. Loffing, J. F. Rebhun, J. H. Pratt, L. Schild, J. D. Horisberger, D. Rotin, and O. Staub. 1999. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome. J. Clin. Investig. 103:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviel, S., G. Winberg, M. Massucci, and A. Ciechanover. 2000. Degradation of the Epstein-Barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J. Biol. Chem. 275:23491-23499. [DOI] [PubMed] [Google Scholar]
- 3.Bai, R. Y., T. Jahn, S. Schrem, G. Munzert, K. M. Weidner, J. Y. Wang, and J. Duyster. 1998. The SH2-containing adapter protein GRB10 interacts with BCR-ABL. Oncogene 17:941-948. [DOI] [PubMed] [Google Scholar]
- 4.Beaudenon, S. L., M. R. Huacani, G. Wang, D. P. McDonnell, and J. M. Huibregtse. 1999. Rsp5 ubiquitin-protein ligase mediates DNA damage-induced degradation of the large subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bereziat, V., A. Kasus-Jacobi, D. Perdereau, B. Cariou, J. Girard, and A. F. Burnol. 2002. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem. 277:4845-4852. [DOI] [PubMed] [Google Scholar]
- 6.Bork, P., and M. Sudol. 1994. The WW domain: a signalling site in dystrophin? Trends Biochem. Sci. 19:531-533. [DOI] [PubMed] [Google Scholar]
- 7.Chow, J. C., G. Condorelli, and R. J. Smith. 1998. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J. Biol. Chem. 273:4672-4680. [DOI] [PubMed] [Google Scholar]
- 8.Dey, B. R., K. Frick, W. Lopaczynski, S. P. Nissley, and R. W. Furlanetto. 1996. Evidence for the direct interaction of the insulin-like growth factor I receptor with IRS-1, Shc, and Grb10. Mol. Endocrinol. 10:631-641. [DOI] [PubMed] [Google Scholar]
- 9.Di Fiore, P. P., and P. De Camilli. 2001. Endocytosis and signaling. an inseparable partnership. Cell 106:1-4. [DOI] [PubMed] [Google Scholar]
- 10.Dong, L. Q., H. Du, S. G. Porter, L. F. Kolakowski, Jr., A. V. Lee, L. J. Mandarino, J. Fan, D. Yee, F. Liu, and J. Mandarino. 1997. Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma. J. Biol. Chem. 272:29104-29112. [DOI] [PubMed] [Google Scholar]
- 11.Dong, L. Q., S. Farris, J. Christal, and F. Liu. 1997. Site-directed mutagenesis and yeast two-hybrid studies of the insulin and insulin-like growth factor-1 receptors: the Src homology-2 domain-containing protein hGrb10 binds to the autophosphorylated tyrosine residues in the kinase domain of the insulin receptor. Mol. Endocrinol. 11:1757-1765. [DOI] [PubMed] [Google Scholar]
- 12.Frantz, J. D., S. Giorgetti-Peraldi, E. A. Ottinger, and S. E. Shoelson. 1997. Human GRB-IRβ/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains. J. Biol. Chem. 272:2659-2667. [DOI] [PubMed] [Google Scholar]
- 13.Goulet, C. C., K. A. Volk, C. M. Adams, L. S. Prince, J. B. Stokes, and P. M. Snyder. 1998. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle's syndrome. J. Biol. Chem. 273:30012-30017. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, H., U. Svensson, J. Zhu, L. Laviola, F. Giorgino, G. Wolf, R. J. Smith, and H. Riedel. 1996. Interaction between the Grb10 SH2 domain and the insulin receptor carboxyl terminus. J. Biol. Chem. 271:8882-8886. [DOI] [PubMed] [Google Scholar]
- 15.He, W., D. W. Rose, J. M. Olefsky, and T. A. Gustafson. 1998. Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains. J. Biol. Chem. 273:6860-6867. [DOI] [PubMed] [Google Scholar]
- 16.Helliwell, S. B., S. Losko, and C. A. Kaiser. 2001. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 153:649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 18.Hicke, L. 2001. A new ticket for entry into budding vesicles—ubiquitin. Cell 106:527-530. [DOI] [PubMed] [Google Scholar]
- 19.Huibregtse, J. M., M. Scheffner, S. Beaudenon, and P. M. Howley. 1995. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA 92:5249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahn, T., P. Seipel, S. Urschel, C. Peschel, and J. Duyster. 2002. Role for the adaptor protein Grb10 in the activation of Akt. Mol. Cell. Biol. 22:979-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffers, M., G. A. Taylor, K. M. Weidner, S. Omura, and G. F. Vande Woude. 1997. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 17:799-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamynina, E., C. Debonneville, M. Bens, A. Vandewalle, and O. Staub. 2001. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 15:204-214. [DOI] [PubMed] [Google Scholar]
- 23.Kanelis, V., D. Rotin, and J. D. Forman-Kay. 2001. Solution structure of a Nedd4 WW domain-ENaC peptide complex. Nat. Struct. Biol. 8:407-412. [DOI] [PubMed] [Google Scholar]
- 24.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, S., Y. Tomooka, and M. Noda. 1992. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem. Biophys. Res. Commun. 185:1155-1161. [DOI] [PubMed] [Google Scholar]
- 26.Laviola, L., F. Giorgino, J. C. Chow, J. A. Baquero, H. Hansen, J. Ooi, J. Zhu, H. Riedel, and R. J. Smith. 1997. The adapter protein Grb10 associates preferentially with the insulin receptor as compared with the IGF-I receptor in mouse fibroblasts. J. Clin. Investig. 99:830-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, P. S., Y. Wang, M. G. Dominguez, Y. G. Yeung, M. A. Murphy, D. D. Bowtell, and E. R. Stanley. 1999. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 18:3616-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levkowitz, G., H. Waterman, E. Zamir, Z. Kam, S. Oved, W. Y. Langdon, L. Beguinot, B. Geiger, and Y. Yarden. 1998. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, F., and R. A. Roth. 1995. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc. Natl. Acad. Sci. USA 92:10287-10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyons, R. J., R. Deane, D. K. Lynch, Z. S. Ye, G. M. Sanderson, H. J. Eyre, G. R. Sutherland, and R. J. Daly. 2001. Identification of a novel human tankyrase through its interaction with the adaptor protein Grb14. J. Biol. Chem. 276:17172-17180. [DOI] [PubMed] [Google Scholar]
- 31.Miyake, S., M. L. Lupher, Jr., B. Druker, and H. Band. 1998. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc. Natl. Acad. Sci. USA 95:7927-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake, S., K. P. Mullane-Robinson, N. L. Lill, P. Douillard, and H. Band. 1999. Cbl-mediated negative regulation of platelet-derived growth factor receptor-dependent cell proliferation. A critical role for Cbl tyrosine kinase-binding domain. J. Biol. Chem. 274:16619-16628. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi, N., Y. Kuroiwa, T. Kohda, H. Shitara, H. Yonekawa, T. Kawabe, H. Hasegawa, S. C. Barton, M. A. Surani, T. Kaneko-Ishino, and F. Ishino. 1998. Identification of the Meg1/Grb10 imprinted gene on mouse proximal chromosome 11, a candidate for the Silver-Russell syndrome gene. Proc. Natl. Acad. Sci. USA 95:1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori, S., K. Tanaka, S. Omura, and Y. Saito. 1995. Degradation process of ligand-stimulated platelet-derived growth factor beta-receptor involves ubiquitin-proteasome proteolytic pathway. J. Biol. Chem. 270:29447-29452. [DOI] [PubMed] [Google Scholar]
- 35.Morrione, A. 2000. Grb10 proteins in insulin-like growth factor and insulin receptor signaling. Int. J. Mol. Med. 5:151-154. [DOI] [PubMed] [Google Scholar]
- 36.Morrione, A., P. Plant, B. Valentinis, O. Staub, S. Kumar, D. Rotin, and R. Baserga. 1999. mGrb10 interacts with Nedd4. J. Biol. Chem. 274:24094-24099. [DOI] [PubMed] [Google Scholar]
- 37.Morrione, A., B. Valentinis, S. Li, J. Y. Ooi, B. Margolis, and R. Baserga. 1996. Grb10: a new substrate of the insulin-like growth factor I receptor. Cancer Res. 56:3165-3167. [PubMed] [Google Scholar]
- 38.Morrione, A., B. Valentinis, M. Resnicoff, S. Xu, and R. Baserga. 1997. The role of mGrb10α in insulin-like growth factor I-mediated growth. J. Biol. Chem. 272:26382-26387. [DOI] [PubMed] [Google Scholar]
- 39.Moutoussamy, S., F. Renaudie, F. Lago, P. A. Kelly, and J. Finidori. 1998. Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins. J. Biol. Chem. 273:15906-15912. [DOI] [PubMed] [Google Scholar]
- 40.Myat, A., P. Henry, V. McCabe, L. Flintoft, D. Rotin, and G. Tear. 2002. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron 35:447-459. [DOI] [PubMed] [Google Scholar]
- 41.Nantel, A., K. Mohammad-Ali, J. Sherk, B. I. Posner, and D. Y. Thomas. 1998. Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases. J. Biol. Chem. 273:10475-10484. [DOI] [PubMed] [Google Scholar]
- 42.Nawaz, Z., D. M. Lonard, A. P. Dennis, C. L. Smith, and B. W. O'Malley. 1999. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA 96:1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill, T. J., D. W. Rose, T. S. Pillay, K. Hotta, J. M. Olefsky, and T. A. Gustafson. 1996. Interaction of a GRB-IR splice variant (a human GRB10 homolog) with the insulin and insulin-like growth factor I receptors. Evidence for a role in mitogenic signaling. J. Biol. Chem. 271:22506-22513. [DOI] [PubMed] [Google Scholar]
- 44.Ooi, J., V. Yajnik, D. Immanuel, M. Gordon, J. J. Moskow, A. M. Buchberg, and B. Margolis. 1995. The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene 10:1621-1630. [PubMed] [Google Scholar]
- 45.Orsini, M. J., J. L. Parent, S. J. Mundell, A. Marchese, and J. L. Benovic. 1999. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J. Biol. Chem. 274:31076-31086. [DOI] [PubMed] [Google Scholar]
- 46.Pham, N., I. Cheglakov, C. A. Koch, C. L. de Hoog, M. F. Moran, and D. Rotin. 2000. The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP. Curr. Biol. 10:555-558. [DOI] [PubMed] [Google Scholar]
- 47.Pham, N., and D. Rotin. 2001. Nedd4 regulates ubiquitination and stability of the guanine-nucleotide exchange factor CNrasGEF. J. Biol. Chem. 276:46995-47003. [DOI] [PubMed] [Google Scholar]
- 48.Plant, P. J., F. Lafont, S. Lecat, P. Verkade, K. Simons, and D. Rotin. 2000. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 149:1473-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plant, P. J., H. Yeger, O. Staub, P. Howard, and D. Rotin. 1997. The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J. Biol. Chem. 272:32329-32336. [DOI] [PubMed] [Google Scholar]
- 50.Reiss, K., B. Valentinis, X. Tu, S. Q. Xu, and R. Baserga. 1998. Molecular markers of IGF-I-mediated mitogenesis. Exp. Cell Res. 242:361-372. [DOI] [PubMed] [Google Scholar]
- 51.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1-17. [DOI] [PubMed] [Google Scholar]
- 52.Sepp-Lorenzino, L., Z. Ma, D. E. Lebwohl, A. Vinitsky, and N. Rosen. 1995. Herbimycin A induces the 20 S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J. Biol. Chem. 270:16580-16587. [DOI] [PubMed] [Google Scholar]
- 53.Staub, O., S. Dho, P. Henry, J. Correa, T. Ishikawa, J. McGlade, and D. Rotin. 1996. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 15:2371-2380. [PMC free article] [PubMed] [Google Scholar]
- 54.Staub, O., I. Gautschi, T. Ishikawa, K. Breitschopf, A. Ciechanover, L. Schild, and D. Rotin. 1997. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 16:6325-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein, E., D. P. Cerretti, and T. O. Daniel. 1996. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J. Biol. Chem. 271:23588-23593. [DOI] [PubMed] [Google Scholar]
- 56.Stein, E. G., T. A. Gustafson, and S. R. Hubbard. 2001. The BPS domain of Grb10 inhibits the catalytic activity of the insulin and IGF1 receptors. FEBS Lett. 493:106-111. [DOI] [PubMed] [Google Scholar]
- 57.Treier, M., L. M. Staszewski, and D. Bohmann. 1994. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78:787-798. [DOI] [PubMed] [Google Scholar]
- 58.Valentinis, B., P. L. Porcu, K. Quinn, and R. Baserga. 1994. The role of the insulin-like growth factor I receptor in the transformation by simian virus 40 T antigen. Oncogene 9:825-831. [PubMed] [Google Scholar]
- 59.van Kerkhof, P., R. Govers, C. M. Alves dos Santos, and G. J. Strous. 2000. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J. Biol. Chem. 275:1575-1580. [DOI] [PubMed] [Google Scholar]
- 60.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 61.Wang, H. Y., Y. Altman, D. Fang, C. Elly, Y. Dai, Y. Shao, and Y. C. Liu. 2001. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J. Biol. Chem. 276:26004-26011. [DOI] [PubMed] [Google Scholar]
- 62.Wang, J., H. Dai, N. Yousaf, M. Moussaif, Y. Deng, A. Boufelliga, O. R. Swamy, M. E. Leone, and H. Riedel. 1999. Grb10, a positive, stimulatory signaling adapter in platelet-derived growth factor BB-, insulin-like growth factor I-, and insulin-mediated mitogenesis. Mol. Cell. Biol. 19:6217-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterman, H., G. Levkowitz, I. Alroy, and Y. Yarden. 1999. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J. Biol. Chem. 274:22151-22154. [DOI] [PubMed] [Google Scholar]