Abstract
The high-mobility-group (HMG) box is a conserved DNA-binding domain found in a family of transcription factors that regulate growth and development. One family member, Ste11p, directs sexual differentiation of Schizosaccharomyces pombe by binding specific DNA sequences upstream of genes required for mating and meiosis. Here, we show that Ste11p is a shuttling protein. In growing cells, Ste11p is present in low levels and is pancellular. Mating pheromones and nutrient limitation trigger nuclear accumulation and increased expression of the transcription factor. Several mechanisms likely control Ste11p localization. First, the 14-3-3 protein, Rad24p, binds phosphorylated Ste11p and inhibits its nuclear accumulation. Second, the HMG domain of Ste11p contains a basic cluster nuclear localization signal. Finally, treatment of cells with leptomycin B, an exportin inhibitor, results in the nuclear accumulation of Ste11p. A Ste11p deletion mutation, ΔC54, mimics the effects of leptomycin B. The C54 region contains no identifiable nuclear export signal but instead is required for biological activity and to stimulate Ste11p target gene expression. These results provide evidence that both nuclear import and export mechanisms operate to regulate cellular localization of an HMG box protein. In addition, they establish a paradigm for the potential role of pheromone/hormone-like polypeptides in cellular localization of this important class of developmental regulators.
Transcription factors belonging to the high-mobility-group (HMG) box superfamily share a conserved DNA-binding domain. Members include Sry, the developmental regulator of sex determination in humans; the related Sox (Sry box) proteins; and the TCF and LEF transcription factors (28, 39). Sequences within the HMG box bend DNA by interacting with the minor groove (43). This property allows HMG box proteins to act as architectural elements specifying the assembly of higher-order nucleoprotein complexes (12, 25). HMG proteins are important for differentiation of diverse cell types, such as those specifying cardiac, neural, and lymphoid tissues (49). Dysfunctional HMG proteins are linked to several disease states, including malignancy (35).
Ste11p is a 52-kDa HMG box protein that regulates expression of cell mating type genes required for conjugation and sporulation of Schizosaccharomyces pombe (41). Since Ste11p is structurally related to members of the HMG domain superfamily (28), the molecular mechanisms used to regulate its activity are likely to be of general significance.
Yeast cells reproduce vegetatively, but specific conditions can cause cells of opposite mating types to conjugate and form a diploid zygote that is capable of meiosis (5, 8). Conjugation is a complex process initiated by deprivation of nutrients from the growth medium and by pheromone signals. These cause G1 cell cycle delay, cell-cell agglutination and fusion, and karyogamy. The newly formed diploid zygote is capable of meiosis if pheromone signaling and nutrient limitation conditions continue. Pat1p/Ran1p protein kinase (referred to as Pat1p hereafter) has a pivotal function in establishing both competence to differentiate and commitment to the process.
During vegetative growth, Pat1p kinase inhibits conjugation and sporulation (26). Nutrient limitation and pheromone signaling cause inactivation of Pat1p (2). Inhibition of the kinase is both necessary and sufficient for differentiation (2, 14, 30), though not all means by which inactivation is accomplished have been described at the molecular level. Genetic and biochemical studies identified Ste11p and Mei2p as substrates for Pat1p (16, 22, 47). Mei2p is an RNA-binding protein (48). Phosphorylation by Pat1p is inhibitory for Mei2p. It converts Mei2p into a substrate for ubiquitin-dependent proteolysis (16) and inhibits its RNA-binding ability (36). In the absence of Mei2p, cells conjugate, and the resulting diploid zygote arrests prior to premeiotic DNA synthesis (3, 37, 48). Thus, Mei2p is not essential for the early steps of sexual differentiation. In contrast, Ste11p is essential for both conjugation and sporulation (38, 41) and is the most immediate downstream target of Pat1p (22).
As it does for Mei2p, phosphorylation of Ste11p inhibits its activity. Inactivation of Pat1p is sufficient for robust expression of ste11 (31), since Ste11p is autoregulatory (20, 41). Ste11p binds a specific DNA sequence, the TR box, found upstream of genes required for mating and sporulation. This includes the mating type genes, mei2, and ste11 itself (18, 41). The mating type genes regulate production of cell type-specific pheromones and pheromone receptors. Pheromone communication allows cells of opposite mating types to conjugate and to undergo meiosis (5, 8). Meiosis is caused by activation of mei3 expression. Two mating type proteins, MatPm and MatMc, directly regulate mei3 transcription (44). Mei3p is an inhibitor of Pat1p that is essential for full inactivation of the kinase and commitment to meiosis (26, 27, 45, 50). Thus, Pat1p and Ste11p are elements of a positive feedback loop.
The requirement for nutrient limitation in sexual differentiation and its role in expression of ste11 are well established. However, mating pheromone communication is only indirectly implicated in regulation of Ste11p activity. We have proposed that Ste11p activity is directly repressed by Pat1p phosphorylation (22). In this model, inactivation of Pat1p by nutrient limitation leads to accumulation of a nonphosphorylated, activated form of Ste11p. Ste11p autoregulation amplifies the initial starvation signal and causes expression of mating pheromones and receptors. Pheromone signaling further inactivates Pat1p kinase, consequently activating Ste11p and amplifying expression of Ste11p and its downstream targets. Molecular details to support this model are lacking. For instance, it is not known if Ste11p is an in vivo substrate for Pat1p or if Pat1p-specific phosphorylation alters the activity of the transcription factor.
Some regulators of Ste11p transcription factor activity are known. For example, cell type-specific expression of M factor, encoded by mfm1, requires an interaction between Ste11p and another HMG box protein, Mat1-Mc. The interaction strengthens Ste11p binding to weak TR boxes (18). Additionally, Rad24p physically interacts with Ste11p and inhibits its activity by an as yet unknown mechanism (16). Rad24p is a 14-3-3 protein (9). Members of the 14-3-3 family coordinate multiple signal transduction pathways. In the fission yeast Schizosaccharomyces pombe, Rad24p functions in the DNA synthesis checkpoint as a cytoplasmic tether for the Cdc25p mitotic activator (9, 23, 24). Rad24p also has a role in conjugation. In its absence, cells conjugate and ste11 is expressed without the need for starvation (9). On the other hand, high-level expression of Rad24p causes a reduction in the steady-state level of both the ste11 transcript and its protein product. Rad24p binds preferentially to Ste11p that has been phosphorylated by Pat1p (16). In support of this observation, the amino acid sequences around both phosphorylation sites resemble the consensus recognition sequence for members of the 14-3-3 protein family. Although Rad24p functions to regulate the localization of some regulatory proteins (23), Rad24p did not appear to alter the localization of Ste11p. Thus, it has been proposed that Rad24p may be important in the transcriptional activation or DNA binding of Ste11p (16).
In the present study, evidence is presented that localization of Ste11p is one means of regulating its activity. Ste11p is not constitutively nuclear but accumulates in that compartment during specific stages of the cell's life cycle. Systematic deletion analysis showed that the HMG box of Ste11p contains a functional basic cluster nuclear import signal. Pheromone signaling and nutrient limitation likely control accumulation of Ste11p in the nucleus by inhibiting its crm1 (exportin)-dependent export. An ste11 allele, ste11ΔC54, produces a truncated polypeptide found constitutively localized to the nucleus. The Ste11ΔC54p polypeptide is inactive, indicating that nuclear accumulation of Ste11p is not sufficient for its function. In addition to import and export, the subcellular distribution of Ste11p is regulated by other mechanisms. Contrary to other reports, we found that Rad24p alters the subcellular distribution of Ste11p. Rad24p sequesters Ste11p in the cytoplasm so that it is not available for import into the nucleus.
MATERIALS AND METHODS
Yeast strains and media.
The S. pombe strains used are listed in Table 1. Strains listed as laboratory stocks were derived by crosses from fission yeast strains generously supplied by David Beach (London, England). Media, supplements, and other yeast techniques have been described previously (1).
TABLE 1.
Strain list
| Strain | Genotype | Reference |
|---|---|---|
| SPB6 | h90 ade6-M216 leu1-32 ura4-D18 | Lab stock |
| SPB59 | h+N ade6-M216 mei2::lacZ | Lab stock |
| SPB61 | h−s ade6-M216 leu1-32 ura4-D18 | Lab stock |
| SPB162 | h90 ade6-M216 leu1-32 ura4-D18 ste11::ura4 mei2::lacZ | Lab stock |
| SPB361 | h90 ade6-M216 ste11::ste11T173A,S218A | 22; this study |
| SPB363 | h90 ade-M216 | Lab stock |
| SPB366 | h90 ade6-M216 ste11::ste11T173A,S218Amei2::lacZ | 22; this study |
| SPB367 | h+N ade6-M216 ste11::ste11T173A,S218Amei2::lacZ | 22; this study |
| SPB368 | h90 ade6-M216 mei2::lacZ | Lab stock |
| SPB371 | h90 leu1-32 ura4-D18 ade6-M216 ste11::pste11-gfp | This study |
| SPB373 | h+N leu1-32 ura4-D18 ade6-M216 ste11::pste11-gfp | This study |
| SPB375 | h−s leu1-32 ura4-D18 ade6-M216 ste11::pste11-gfp | This study |
| SPB380 | h90 leu1-32 ura4-D18 ade6-M216 fus1-B20 ste11:: pste11-gfp | This study |
| SPB394 | h−s leu1-32 ura4-D18 ade6-M216 sxa2-563 ste11:: pste11-gfp | This study |
| SPB399 | h90 leu1-32 ura4-D18 ade6-M216 mei3::lacZ ste11:: pste11-gfp | This study |
| SPB408 | h−s leu1-32 ura4-D18 mei2ts pat1::ura4 ade6-M210 | Lab stock |
| SPB410 | h−s leu1-32 ade6-M210 mei2ts | Lab stock |
Nitrogen starvation.
Cells were cultured in YEA to the stationary phase and then diluted into EMM containing the desired amino acid supplements (1). When the density of the culture attained 5 × 106 to 1 × 107 cells/ml, cells were washed twice in nitrogen-free EMM and then resuspended at 107 cells/ml in nitrogen-free EMM.
Plasmid constructions and DNA manipulations.
Relevant features of the plasmids used for this study are described in Table 2. Exact details are available upon request. Expression of Ste11-GFP and its variants was done under the control of either the thiamine-inducible nmt1 promoter or the constitutive adh promoter. The cellular distribution of the fusion proteins was promoter independent in all cases.
TABLE 2.
Plasmid list
| Plasmid | Relevant features | Source |
|---|---|---|
| pSte11.102 | ars1 LEU2 adh-ste11(aa1-468)-GFP | This study |
| pSte11.108 | ars1 LEU2 adh-ste11(aa1-456)-GFP | This study |
| pSte11.60 | ars1 LEU2 adh-ste11(aa1-414)-GFP | This study |
| pSte11.69 | ars1 LEU2 adh-ste11(aa1-235)-GFP | This study |
| pSte11.91 | ars1 LEU2 nmt1-ste11(aa1-192)-GFP | This study |
| pSte11.90 | ars1 LEU2 nmt1-ste11(aa1-139)-GFP | This study |
| pSte11.89 | ars1 LEU2 nmt1-ste11(aa1-99)-GFP | This study |
| pSte11.73 | ars1 LEU2 nmt1-ste11(aa1-64)-GFP | This study |
| pSte11.114 | ars1 LEU2 adh-ste11(aa1-456)-GFP | This study |
| pSte11.118L | ars1 LEU2 adh-ste11(aa65-99)-LGFP | This study |
| pSte11.120 | ars1 LEU2 adh-ste11(aa1-414Δ64-110)-GFP | This study |
| pSte11.121 | ars1 LEU2 adh-ste11(aa65-414)-GFP | This study |
| pSte11.97 | ars1 LEU2 nmt1-ste11(aa92-414)-GFP | This study |
| pRad24.1 | ars1 LEU2 adh-rad24 | This study |
Site-directed mutagenesis.
ste11 deletions were constructed by inserting a NotI restriction enzyme site in the ste11 coding region at specified locations (see Fig. 4). The NotI site was used to fuse ste11 sequences in-frame with green fluorescent protein (GFP) coding sequences. Site-directed mutagenesis was used to create NotI sites in ste11, and this was accomplished with a plasmid-based (BH95NN) mutagenesis system (33a). In this technique, two mutagenic oligonucleotides are used. One carries the desired mutation, and the other, referred to as the selection primer, repairs a small deletion in the β-lactamase gene. ste11 was inserted into BH95NN as a cassette contained on an NheI-BamHI DNA fragment. Mutagenic oligonucleotides (containing NotI sites) were annealed to denatured plasmid DNA, elongated in vitro, and used to transform a repair-defective strain of Escherichia coli. Ampicillin-resistant colonies, resulting from transformation by DNA to which the ampicillin selection primer had annealed, were tested for the presence of the NotI site. The sequences of oligonucleotides used for mutagenesis are available on request.
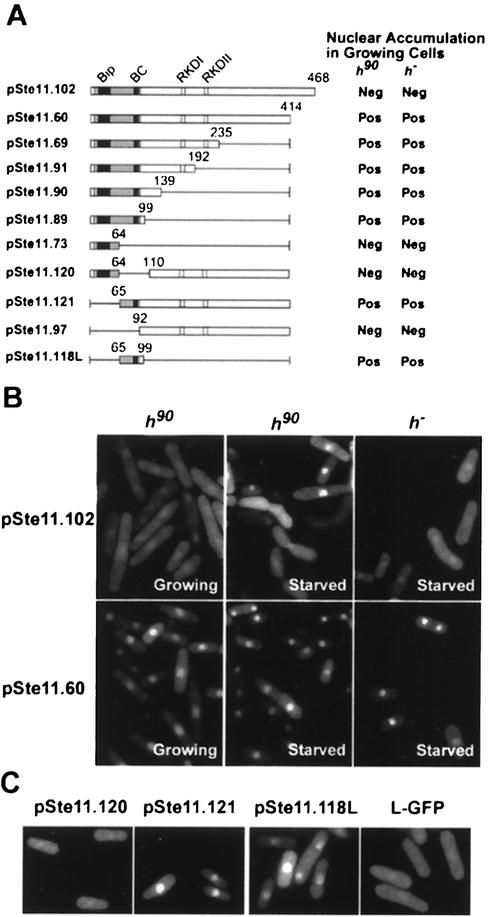
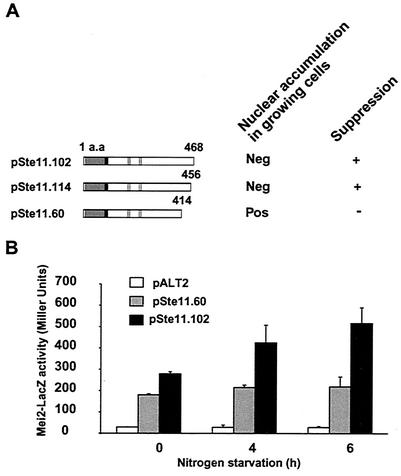
FIG. 4.
Ste11p contains specific regions required for nuclear accumulation. (A) Full-length Ste11p (represented by a box) and Ste11p deletion derivatives were fused to GFP. The gray bar represents the HMG domain, with potential bipartite (Bip) and basic cluster (BC) NLS sequences in black. The RKDI and RKDII regions contain Pat1p substrate specificity determinants (22). The fusion proteins were produced from plasmids in h90 (SPB6) and h− (SPB61) cells, and nuclear accumulation was scored in actively growing cells. A positive score indicates that ≥1.0% of the cells accumulated the fusion protein in the nucleus; a negative score indicates that ≤1.0% cells had nucleus-accumulated GFP. (B) A montage illustrating the distribution of Ste11p-GFP (pSte11.102) and C54-GFP (pSte11.60) in growing and starved cells. Cells transformed with plasmids expressing either complete Ste11p (pSte11.102) or a version deleted of 54 carboxyl-terminal residues (pSte11.60) were examined with a microscope. Nuclear accumulation was scored in growing and starved h90 (SPB6) and h− (SPB61) cells. Note that in the starved h90 culture, the subcellular distribution of Ste11p-GFP is regulated so that only a subset of the cells display nuclear accumulation of the protein (see Fig. 1B). In contrast, cells containing the deletion derivative (pSte11.60) accumulate nuclear GFP at all stages of the life cycle. (C) The basic cluster is necessary and sufficient to target Ste11p-GFP to the nucleus. h− cells (SPB61) were transformed with the indicated plasmids. pSte11.118L was fused to GFP via a 75-amino-acid linker sequence (L-GFP).
Construction of ste11T173A,S218A.
A gene replacement experiment (1, 11) was used so that ste11 was expressed under control of its endogenous promoter. Two-step sequential gene replacement was designed to obtain a strain of cells congenic with wild-type cells except for the two amino acid changes in Ste11p. First, the endogenous ste11 allele was replaced with a PCR fragment containing ste11 interrupted by ura4 sequences. The disrupted cells were selected as Ura+ cells with a sterile phenotype. The resulting strain was used for a second gene replacement with a PCR fragment containing the ste11T173A,S218A gene. ste11T173A,S218A cells were selected as Ura− cells on plates containing 5-fluoroorotic acid (11). Genomic PCR combined with restriction enzyme digestion was used at each step to confirm the correct chromosomal gene replacement (not shown). ste11T173A,S218A is referred to here as ste11-AA.
Construction of Ste11p-GFP.
The enhanced version of GFP (kindly supplied by Brehon Laurent, SUNY Downstate) was used to tag Ste11p. In some cases, linker DNA was fused to GFP sequences prior to fusion to Ste11p. The linker encodes a 75-amino-acid polypeptide with no nuclear localization signal (NLS) activity. ste11-gfp was integrated into the chromosome as previously described (11). The commercial vector Litmus 28-7 (New England Biolabs, Inc, Beverly, Mass.), containing the ura4 gene (as a blunt-end fragment cloned into the EcoRV site of Litmus) and ste11-gfp (as an NheI-BamHI fragment), was linearized with pst1 prior to transformation. Stable Ura+ transformants were identified. Integration of the fusion gene was confirmed by PCR. Measurement of conjugation and sporulation rates showed that the fusion allele is indistinguishable from endogenous ste11 (data not shown). Western blots of proteins encoded by ste11-gfp and its mutant alleles revealed that the full-length protein was produced in all cases. When expressed from the adh promoter, approximately equivalent amounts of protein of the expected molecular weight were produced by each allele.
Microscopy.
For microscopy, cells were freshly grown and transferred to coverslips for observation within 5 min. The GFP signal became weak and diffuse if the slides were not freshly observed. All micrographs were taken with the same exposure parameters. Cells were observed with a Nikon Axiphot fluorescence microscope equipped with a BA 520-560 filter. Digital images were exported to Adobe Photoshop and quantified blindly and independently by two persons. Cells (at least 200 per sample) were scored as having either nucleus-accumulated fluorescence (N) or fluorescence equally distributed in both the nucleus and cytoplasm (N+C). We never observed exclusion of Ste11p-GFP from the nucleus or an exclusively nuclear localization of the fusion protein. A cell was scored as N when the GFP-tagged protein accumulated in the nucleus to the extent that the nuclear compartment was clearly demarcated from the cytoplasm by fluorescence. A cell was scored as N+C when nuclear accumulation of the fluorescent protein was not strong enough to clearly distinguish between the nuclear compartment and the cytoplasm by fluorescence. Heterothallic cells containing a replacement of endogenous ste11 with ste11::gfp served as a negative control for nuclear accumulation (N < 1.0%).
For some experiments, confocal images were obtained with a Radiance 2000 confocal microscope (Bio-Rad, Hercules, Calif.). Images were obtained with a 40× objective lens as 1,024 by 1,024 pixels. Images were processed with Adobe Photoshop 5.5 (Adobe Systems, Mountain View, Calif.) and printed on a Tektronix Phaser 780 laser printer. To stain nuclei, cells were incubated with Hoechst 33324 (Sigma) at 1 μg/ml for 10 min.
Measurement of β-galactosidase activity.
A permeabilized cell assay (1) was used to measure β-galactosidase activity. Units were calculated as follows: 1 Miller unit = 1,000 × [optical density at 420 nm/(reaction time in minutes × culture volume in milliliters × optical density at 600 nm)].
P factor treatment.
Synthetic P factor (15) was synthesized by Genemed Synthesis, Inc. (South San Francisco, Calif.). Pheromone was dissolved in methanol and added to the medium at a final concentration of 50 μg/ml.
RESULTS
Cellular distribution of Ste11p in living cells is dynamic.
When proliferating fission yeast cells are starved of nitrogen, they exit the mitotic cell cycle, conjugate, and differentiate into ascospores. Ste11p is essential for this process. To examine the subcellular distribution of Ste11p during the yeast life cycle (Fig. 1A), we constructed an allele encoding a fusion between the coding sequences of GFP and the carboxyl-terminal region of Ste11p. The allele (ste11-gfp) was integrated into the chromosome of an h90 strain. h90 cells interconvert genes at the mating type locus so that a colony contains a mixture of plus and minus cells capable of conjugation when starved of nutrients (usually nitrogen). h90 ste11-gfp cells displayed a weak, pancellular GFP signal in growing cells (Fig. 1B, 0 h), consistent with the observation that elevated expression of ste11 requires nitrogen starvation (41).
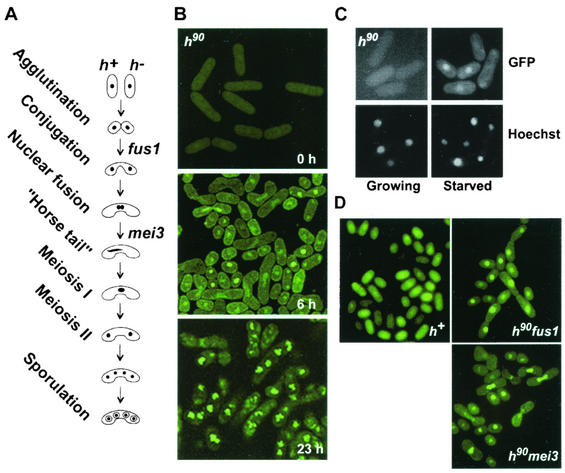
FIG. 1.
Ste11p accumulates in the nucleus of signaling cells prior to and during conjugation. (A) Schematic drawing of cells at visually distinguishable stages of sexual development. Some genes required at several defined stages are indicated on the right. (B) Images of h90 ste11-gfp cells (SPB371) were acquired by confocal microscopy at the indicated times following a shift to nitrogen-free medium. (C) Growing and starved SPB371 cells containing Ste11p-GFP (top panels) were stained with Hoechst 33324 (bottom panels) to visualize the nucleus. (D) Images of the designated nitrogen-starved cells (h+, SPB373; h90 fus1, SPB380; h90 mei3, SPB399) were visualized by fluorescence microscopy.
To induce sexual differentiation, actively dividing cells were shifted to nitrogen-free medium. Microscopic examination 6 h following the shift revealed that the culture contained a mixture of haploid cells, conjugating pairs, and zygotes. In contrast to the location of Ste11p-GFP in growing cells, distinct nuclear accumulation of the fusion protein was observed in individual cells but not paired cells. Some cells displayed strong nuclear fluorescence extending across the fusion tube formed by mating cells. This staining pattern is indicative of meiotic prophase, which is defined cytologically by the presence of horse-tail nuclei that result from rapid nuclear oscillations (Fig. 1A and B). Continued starvation produced cells containing immature spores (Fig. 1B, 23 h). These contained several bright punctate fluorescent spots in the four nuclei resulting from meiosis. To confirm that Ste11p-GFP accumulated in the nucleus of starved h90 cells, we used Hoechst 33324. Staining of Ste11p-GFP in the nuclear region colocalized with the DNA-specific Hoechst stain (Fig. 1C).
h90 cells do not conjugate synchronously.
To examine localization of Ste11p-GFP at defined stages of sexual differentiation, we used several characterized mutant strains (Fig. 1D). The requirement for conjugation was examined in heterothallic h+ cells. Nitrogen-starved h+ cells arrest in G1 but fail to mount a pheromone response or to conjugate unless they are experimentally mixed with h− cells. Surprisingly, and in contrast to observations made with h90 cells, nuclear accumulation of Ste11p-GFP was not observed even after prolonged incubation in nitrogen-free medium. Similar results were obtained when Ste11p-GFP was examined in starved h− cells (Fig. 2).
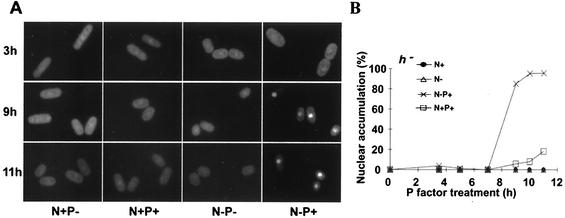
FIG. 2.
Nuclear accumulation of Ste11p-GFP requires nitrogen starvation and pheromone signaling. (A) A montage of h− ste11-gfp cells (SPB394) incubated in the presence (N+) or absence (N−) of a nitrogen source. At the time of the nutritional shift, either synthetic pheromone (P+) or vehicle (P−) was added to the indicated samples. (B) The number of cells displaying nuclear accumulation of Ste11p-GFP was determined as described in Materials and Methods.
To determine if cell-cell fusion was required for nuclear accumulation of Ste11p, we used fus1 cells (see Fig. 10). fus1 encodes a formin-like protein required for cell wall breakdown during conjugation (32, 33). fus1 was not required for Ste11p-GFP nuclear accumulation; in its absence, nuclear accumulation of Ste11p-GFP was stable.
The subcellular distribution of Ste11p-GFP was examined in cells arrested during meiotic prophase with a mei3 mutant. Microscopic examination of the cells showed that Ste11p-GFP accumulated in the nucleus prior to nuclear fusion and during meiotic prophase.
Nuclear accumulation of Ste11p-GFP requires nitrogen starvation and mating pheromone.
The above results provide a strong correlation between mating competency and nuclear accumulation of Ste11p-GFP. This, and the striking observation that nonconjugating, heterothallic cells failed to accumulate Ste11p-GFP in the nucleus, suggested an important role for pheromone signaling in localization of the transcription factor. To investigate this, h− cells containing the ste11-gfp allele were shifted to medium with various amounts of nitrogen. Artificially synthesized pheromone (P factor) was added to some cultures, as indicated in Fig. 2. Cells were photographed during the course of the treatments (Fig. 2A), and the fraction containing distinct nuclear fluorescence was determined (Fig. 2B). Microscopic examination of the cells revealed weak pancellular fluorescence in growing cells (data not shown), consistent with previous observations (Fig. 1B). Nuclear accumulation of Ste11p-GFP became evident 9 h following the nutritional shift, but only in cultures to which P factor had been added. Eventually, this culture amassed a large number of cells (94%) with nuclear Ste11p-GFP (N-P+; 23 h, Fig. 2). Twenty-three hours after initiation of the experiment, nitrogen-starved cells treated with P factor contained extended conjugation tubes, a well-documented response to pheromone signaling (data not shown). For all other conditions, Ste11p-GFP was pancellular in most cells examined. A small fraction of cells (15%) treated with P factor in the presence of nitrogen (N+P+, Fig. 2B) accumulated Ste11p-GFP in the nucleus. However, the absence of mating projections indicates that these cells responded poorly to the presence of the pheromone (data not shown). This may reflect a requirement for nitrogen starvation to obtain maximum transcription of the P factor receptor (17). These experiments provide strong evidence that nuclear accumulation of Ste11p-GFP is mediated by diffusible mating pheromones and nitrogen starvation.
Inactivation of Pat1p causes nuclear accumulation of Ste11p in the absence of nitrogen limitation and pheromone signaling.
Phosphorylation is a well-documented means for regulating the cellular distribution of proteins. Ste11p is phosphorylated by Pat1p kinase in vitro on two amino acid residues, Thr173 and Ser218, located in two homologous domains, referred to as RKDI and RKDII (22). Much indirect evidence indicates that phosphorylation inhibits Ste11p activity. The finding that the subcellular distribution of Ste11p is dynamic raised the possibility that Pat1p inactivates Ste11p by hindering its nuclear accumulation. To assess the role of Pat1p in Ste11p localization, we expressed Ste11p-GFP in cells containing a pat1::ura4 null allele (SPB408). Microscopic examination revealed that approximately 95% of growing pat1::ura4 cells displayed nucleus-accumulated Ste11p-GFP. In contrast, Ste11p-GFP was pancellular in greater than 90% of the growing pat1+ cells (Fig. 3A). The functional consequences of inactivating Pat1p are well documented. Loss of pat1 induces cells to conjugate and sporulate (2, 14, 26, 27, 30) and causes activation of ste11 target genes (6, 16). These findings provide a correlation between Ste11p nuclear accumulation and its biological activity and are consistent with the hypothesis that Pat1p inhibits Ste11p and prevents its accumulation in the nucleus.
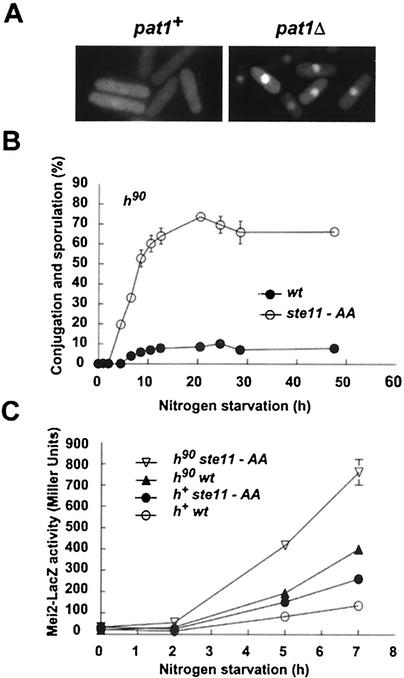
FIG. 3.
Role of Pat1p in activation of Ste11p. (A) Inactivation of Pat1p kinase bypasses the starvation and pheromone signals for nuclear accumulation of Ste11p. Shown is a montage of h− pat1::ura4 mei2ts cells (right panel; SPB408)) and h− mei2ts cells (left panel; SPB410) expressing Ste11p-GFP. Cells were incubated at 34°C (to inactivate mei2ts) in complete medium. (B) Conjugation and sporulation rates indicate that ste11-AA is an activated allele. The percentage of h90 cells containing either ste11-AA (○; SPB361) or ste11 (•; SPB363) that were able to undergo conjugation and meiosis is indicated. Cells were shifted to nitrogen-free medium. At various times, a portion of the culture was examined to determine the number of single cells, conjugated cells and asci. wt, wild type. (C) Expression of the mei2-lacZ reporter gene in h90 and h+ cells combined with either ste11 or ste11-AA following nitrogen starvation. Cells with the indicated genotypes were shifted to nitrogen-free medium. At various times, a portion of each culture was removed, and β-galactosidase activity was measured with a permeabilized cell assay. Activity is expressed in Miller units (see Materials and Methods). Mean values from three independent samples are shown. Genotypes of the strains used are listed in Table 1. They were SPB368, SPB366, SPB59, and SPB367.
To further investigate the effects of phosphorylation on Ste11p activity, we examined the cellular localization of a nonphosphorylatable ste11 allele, ste11-AA. This allele contains alanine substitutions for Thr173 and Ser218, the two amino acid residues phosphorylated by Pat1p in vitro (22). A cell strain in which the endogenous ste11 allele was replaced with ste11-AA fused to GFP sequences was constructed. Localization of the fusion protein was examined in h90 and h− cells in the presence and absence of nitrogen. This experiment revealed that ste11-AA-GFP did not bypass the nitrogen starvation and pheromone signaling requirements for Ste11p nuclear accumulation (data not shown; see Fig. 1 and 2). Like Ste11p-GFP, Ste11p-AA-GFP required starvation and pheromone signals to accumulate in the nucleus prior to and during conjugation.
The inability of Ste11p-AA to mimic inactivation of Pat1p with respect to its subcellular distribution prompted us to examine ste11-AA for other phenotypes caused by inactivation of pat1. The effects of expressing ste11-AA on conjugation and sporulation rates were measured in nitrogen-starved liquid cultures. This experiment revealed that ste11-AA enhanced both the onset and the extent of sexual differentiation (Fig. 3B). Thus, the T173A and S218A substitutions mimic the effects of a dephosphorylated and activated protein.
Next, we examined the effects of pheromone communication and the T173A and S218A substitutions on Ste11p target gene expression. Growing cells were shifted to nitrogen-free medium and collected for measurement of β-galactosidase activity at various times thereafter (Fig. 3C). Reporter gene expression was first observed 5 h following the nutritional shift. This experiment revealed that both conditions, pheromone communication and the substitutions of Thr173 and Ser218 with alanine residues, individually and additively contributed to Ste11p activity. The most robust activation (sixfold) caused by ste11-AA was in pheromone signaling cells (compare h90 ste11-AA with h+wt in Fig. 3C). Pheromone signaling was responsible for a threefold increase in β-galactosidase expression (compare the h90 cells with their h+ counterparts, Fig. 3C), while the ste11-AA allele caused a 1.8-fold increase (compare ste11-AA cells with their ste11 counterparts, Fig. 3C). These results provide strong support that ste11-AA is an activated allele and that phosphorylation by Pat1p functions to inactivate the transcription factor. The additive nature of the two conditions (replacement of Thr173 and Ser218 with alanine residues and pheromone communication) indicates that each activates Ste11p by a different mechanism. However, the observation that inactivation of pat1 is sufficient for nuclear accumulation of Ste11p but substitution of Thr173 and Ser218 with alanine residues is not predicts that Pat1p has an additional function in regulating subcellular distribution of Ste11p besides substrate phosphorylation.
Deletion analysis defines regions of Ste11p that function to regulate its subcellular distribution.
To define sequences required for nuclear accumulation of Ste11p-GFP, systematic deletion analysis was employed (Fig. 4A). Since mutations that inactivate Ste11p prevent its expression, each allele was expressed under the control of an ectopic promoter, either the constitutive adh promoter or the thiamine-repressible nmt1 promoter. First, we determined if these expression methods interfered with regulated nuclear accumulation of Ste11p. Ste11p-GFP was produced in high levels from a plasmid by expressing the fusion gene under control of the adh promoter. Transformants were examined to determine the location of the fusion protein in growing and starved h90 cells and in h− cells (Fig. 4B). This analysis revealed that nuclear accumulation of the highly expressed protein was not observed in growing h90 cells or in starved h− cells. On the other hand, nuclear accumulation was observed in a subset of cells from the starved h90 culture, which contains cells capable of pheromone signaling (see Fig. 1B). Thus, ectopic production of Ste11p-GFP does not alter the cell's ability to regulate nuclear accumulation of the protein.
Next, plasmids containing the different fusion constructs were transformed into both h90 and h− cells. Surprisingly, deletion of 54 amino acids from the carboxyl-terminal region of Ste11p (pSte11.60; Fig. 4A) produced a protein that accumulated in the nucleus of growing h90 cells and even in h− cells (Fig. 4B). This result suggests two important conclusions. One is that the carboxyl-terminal 54-amino-acid region (referred to as C54) contains sequences required for the pancellular distribution of Ste11p observed in growing cells. The other is that deletion of C54 allows Ste11p to bypass the requirements of pheromone signaling and nutrient limitation for its nuclear accumulation. Studies examining the exact function of the C54 region are presented in a later section.
Progressive deletion of the carboxyl terminus revealed that the HMG box is sufficient for nuclear accumulation of Ste11p in growing cells (pSte11.89). The HMG box contains two potential NLS sequences, a bipartite NLS (17KRPLNSFMLYRRDRQAE) and a classical, basic cluster motif (84KKRSTVRRRHKK). The requirement for either the bipartite or the basic cluster sequence was investigated by deletion of each. pSte11.120 was derived from pSte11.60 by deletion of the basic cluster region. pSte11.121 was derived from pSte11.60 by deletion of the bipartite sequences. Each plasmid was transformed into S. pombe, and the cells were examined to determine the subcellular distribution of the GFP-tagged polypeptide. We observed that the basic cluster was both necessary and sufficient for nuclear accumulation of Ste11p. In contrast, the bipartite region was dispensable for nuclear accumulation of the transcription factor (Fig. 4C). This result suggests that the basic cluster may define an NLS.
To determine if the basic cluster is sufficient to function as a transferable NLS, amino acids 65 to 99 were fused to GFP. This construct (pSte11.118L) produced a protein that accumulated in the nucleus (Fig. 4C). Thus, nuclear import capability can be acquired by transferring the basic cluster polypeptide to heterologous sequences. Additionally, although fusion of the basic cluster region to GFP was sufficient for nuclear accumulation of the reporter polypeptide, those sequences did not impart nutrition- and pheromone-regulated nuclear accumulation of the protein.
Inhibition of nuclear export is a major mechanism allowing Ste11p nuclear accumulation.
Some proteins contain a hydrophobic nuclear export signal (NES) that binds Crm1p/exportin. Since deletion of 54 amino acids from the carboxyl terminus of Ste11p produced a protein that accumulated in the nucleus of growing cells (pSte11.60; Fig. 4A), we inspected the C54 region for NES-like sequences. This analysis revealed the presence of a short hydrophobic region (457LLEPWLPNSNLF) with the potential to act as a Crm1p-dependent NES.
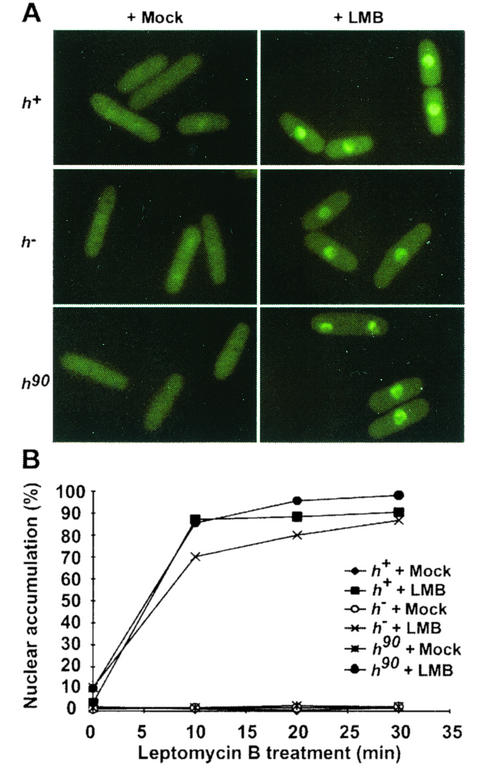
To determine if regulated nuclear accumulation of Ste11p depends on Crm1p activity, we investigated Ste11p-GFP localization in cells treated with leptomycin B (LMB). LMB binds Crm1p and inhibits export in S. pombe (19). Homothallic (h90) and heterothallic (h+ or h−) cells carrying ste11-gfp were treated with 100 ng of LMB per ml or with carrier. At various times following addition of the drug, cells were photographed (Fig. 5A) and counted to determine the number of cells with nuclear distribution of Ste11p (Fig. 5B). The kinetics of Ste11p-GFP nuclear accumulation was nearly identical for all three strains. In the absence of LMB, Ste11p-GFP was pancellular in growing homothallic or heterothallic cells, as shown previously. Following LMB treatment for 10 min, Ste11p-GFP accumulated in the nucleus of most cells (70 to 90%). Within 30 min, 80% to 95% of the cells displayed nuclear accumulation of Ste11p-GFP.
FIG. 5.
LMB treatment causes nuclear accumulation of Ste11p in the absence of nutritional and mating pheromone signals. (A) h90 (SPB371), h+ (SPB373), and h− (SPB375) cells growing in nutrient-rich medium were mock treated or treated with LMB. At the indicated times, a portion of each culture was removed for photography. (B) A graphic representation of the number of cells containing nucleus-accumulated Ste11p-GFP.
Next, mutations were constructed in the hydrophobic residue-rich region of C54 (amino acids 457 to 468) to determine if NES activity could be disrupted. Deletion of the hydrophobic region (pSte11.114; Fig. 6A and data not shown) produced a polypeptide indistinguishable from wild-type Ste11p in that it displayed a pancellular distribution in growing cells. Substitution of the hydrophobic residues with alanine residues produced a similar result. Additionally, fusion of C54 to an NLS-GFP reporter gene did not diminish nuclear accumulation of the reporter protein (data not shown). These results argue that the C54 region does not contain a classic Crm1p-dependent NES.
FIG. 6.
ΔC54 is required for biological activity. (A) A schematic representation of several ste11 alleles expressed from plasmids with the adh promoter. The HMG box is shaded in gray. The basic cluster is illustrated in black. Also indicated are the RKDI and RKDII regions (gray lines). Plasmids containing the deletions fused to GFP were transformed into h90 cells, and the subcellular distribution of the chimeric proteins was determined in growing cells. The cells were scored as indicated in the legend to Fig. 4. Suppression is the ability to reverse the conjugation and sporulation defect of h90 ste11::ura4 cells. a.a., amino acid. (B) Expression of the mei2-lacZ reporter gene in h90 ste11::ura4 cells (SPB162) transformed with the indicated plasmids. pALT2 is the empty vector. pSte11.60 and pSte11.102 are described in Fig. 4. Cells were shifted to nitrogen-free medium. At various times, a portion of each culture was removed, and β-galactosidase activity was measured. Activity is expressed in Miller units (see Materials and Methods). Mean values from three independent samples are shown.
C54 region of Ste11p is required to activate target gene expression.
Although an NES was not identified, our results strongly suggest that Crm1p-mediated nuclear export is a major means of regulating the cellular distribution of Ste11p in response to nutrient and pheromone signaling. Export signals sometimes are not found as a linear array of amino acid sequences. Moreover, the interaction between an export protein and its target need not be direct. To gain insight into the function of the C54 region, we examined the ability of various carboxyl-terminal deletion mutations to complement the conjugation defect of an ste11Δ strain. Deletion of C54 (pSte11.60) produced a protein that was unable to substitute for endogenous Ste11p. In contrast, removal of only the hydrophobic region (amino acids 457 to 468; pSte11.114) resulted in an active protein (Fig. 6).
To further characterize the carboxy-terminal region of Ste11p, reporter gene expression was examined. h90 ste11::ura4 mei2-lacZ cells were transformed with pSte11.114 or pSte11.60 to examine the ability of each to activate expression of mei2-lacZ. Prior to nitrogen starvation, both polypeptides activated expression of the mei2-lacZ reporter gene to nearly the same extent (Fig. 6B, 0 h), even though Ste11p-GFP is primarily pancellular in growing cells, while Ste11ΔC54p-GFP appears to be exclusively nuclear. In response to nitrogen starvation, Ste11p-GFP accumulated in the nucleus, and this was accompanied by a time-dependent increase in β-galactosidase activity. By contrast, β-galactosidase activity did not increase during nitrogen starvation of cells carrying the plasmid producing Ste11ΔC54p-GFP. This result indicates that nuclear accumulation of Ste11p is not sufficient for its activity. One other function defined by the ste11ΔC54 allele is required. It is noteworthy that this allele defines two independent and antagonistic functions; one mediates signal-directed nuclear export of Ste11p, and the other is required to activate target gene expression.
Rad24p promotes cytoplasmic distribution of Ste11p.
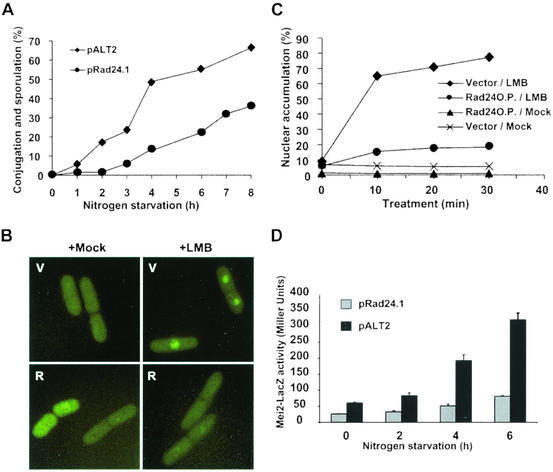
Generally, inactivation of an NES or of a cytoplasmic retention factor and activation of an NLS or a nuclear retention factor are conditions that favor nuclear accumulation of a protein. The Rad24p differentiation inhibitor is a mostly cytoplasmic 14-3-3 protein (23) with the potential to function as a cytoplasmic tether for Ste11p. Recent studies indicate that Rad24p preferentially binds the phosphorylated RKD regions of Ste11p, but its mechanism of inhibition is not known (16). To investigate a potential function for Rad24p in nuclear accumulation of Ste11p, we examined the biological consequences of high-level production of the protein on sexual differentiation. Compared with cells transformed with empty vector, cells expressing rad24 from a plasmid were significantly delayed in conjugation (Fig. 7). Fluorescent microscopic examination of the cells revealed that nuclear accumulation of Ste11p-GFP was also delayed in cells containing Rad24p, indicating that one effect of Rad24p could be to interfere with nuclear accumulation of Ste11p (not shown). However, this experiment did not reveal at which step Rad24p performs its function. Rad24p could interfere directly with nuclear accumulation of Ste11p by, for example, tethering the protein in the cytoplasm. Alternatively, Rad24p could indirectly inhibit nuclear accumulation of Ste11p by hampering expression of mating pheromones or receptors.
FIG. 7.
Expression of Rad24p delays sexual differentiation and causes a defect in nuclear accumulation of Ste11p. (A) Cells transformed with empty plasmid (pALT2) or a plasmid expressing rad24 from the adh promoter (pRad24.1) were shifted to nitrogen-free medium. At various times thereafter, the number of individual cells, conjugating cells, and spore-containing cells was determined. (B) Expression of Rad24p alters accumulation of Ste11p-GFP in the nucleus. h− cells containing empty vector (V) or pRad24.1 (R) were mock treated or treated with LMB for 10 min prior to photography. (C) Quantitation of nucleus-localized Ste11p-GFP from the above experiment. Cells treated with LMB (⧫ and •) or mock treated (▴ and ×) were photographed at the indicated times following the addition of drugs. Cells were counted to determine the percentage containing nuclear fluorescence. (D) Expression of Rad24p inhibits activation of the mei2-lacZ reporter gene in h90 mei2::lacZ cells. SPB162 cells were transformed with empty vector (pALT2) or a plasmid expressing Rad24p (pRAD24.1). Growing transformants were shifted to nitrogen-free medium. At various times, a portion of each culture was removed, and β-galactosidase activity was measured with a permeabilized cell assay. Activity is expressed in Miller units (see Materials and Methods). Mean values from three independent samples are shown.
To distinguish between these possibilities, Rad24p was produced from a plasmid in h− cells treated with LMB. As shown previously (Fig. 5), LMB bypasses the nutritional and mating pheromone-signaling requirements for nuclear accumulation of Ste11p by preventing export of the transcription factor from the nucleus. Therefore, it follows that in the presence of LMB, nuclear accumulation of Ste11p-GFP ought to reflect the amount of protein in the cell that is free to translocate to the nucleus. Growing cells containing either rad24 or empty vector were treated with LMB and examined at various times thereafter. Following LMB treatment for 10 min, clear nuclear accumulation was observed in 85% of the cells carrying a control vector. By contrast, the subcellular distribution of Ste11p-GFP in cells containing Rad24p varied both quantitatively and qualitatively from the control. Only approximately 15% of those cells contained a clear nuclear concentration of Ste11p-GFP. In addition, the nuclear fluorescence in these few cells was rarely as intense as that observed in the control cells (Fig. 7B and 7C). During the 30-min time course of the experiment, these patterns did not change.
To examine the functional consequences of Rad24p production, cells containing a mei2::lacZ reporter gene were transformed with a plasmid producing Rad24p. Nitrogen starvation was used to induce expression of the reporter gene. β-Galactosidase activity in cells transformed with a control plasmid increased sixfold during 6 h of nitrogen starvation. In contrast, Rad24p production increased β-galactosidase activity only twofold (Fig. 7D). These results are consistent with the idea that Rad24p interferes with Ste11p activity by hindering its accumulation in the nucleus.
DISCUSSION
Here, we show that Ste11p is a shuttling protein. In growing cells, Ste11p is pancellular and maintained at low levels. Starvation and pheromone signaling trigger its accumulation in the nucleus. A major mechanism allowing Ste11p nuclear accumulation is inactivation of Crm1p-dependent nuclear export. Besides export, other mechanisms regulate the dynamic nuclear-cytoplasmic distribution of Ste11p. Ste11p contains a functional basic cluster NLS that is both necessary and sufficient for nuclear import of a chimeric protein. Also, Ste11p is a binding partner for Rad24p, a 14-3-3 protein. Rad24p may interfere with Ste11p activity, at least in part, by sequestering it in the cytoplasm.
Nuclear export is a major mechanism regulating nuclear accumulation of Ste11p.
In general, nuclear accumulation represents either an increase in the rate of import over that of export or a decrease in the rate of export so that import into the nucleus is preferred. Ste11p is pancellular in growing cells, but treatment with the Crm1p inhibitor leptomycin B causes very rapid (within 5 min) nuclear accumulation of Ste11p. This suggests that nuclear accumulation of Ste11p depends on inactivation of a nuclear export signal. These data do not indicate if the interaction between Ste11p and a Crm1p-dependent exportin is direct. Our studies failed to identify Ste11p regions that could function as transferable NES sequences (see below). Export of Ste11p could be regulated by several mechanisms. For instance, extracellular signaling may trigger an alteration in Ste11p folding that masks an otherwise exposed nuclear export signal from the cell's transport machinery. This model has been proposed as a mechanism that functions in stressed cells to regulate nuclear export of p53 (40).
Two other conditions lead to nuclear accumulation of Ste11p in the absence of signaling: deletion of 54 amino acids from the polypeptide's carboxy terminus (C54) and inactivation of Pat1p kinase. The C54 region contains a hydrophobic motif similar to that of other defined NES regions that utilize Crm1p/exportin (29). Unexpectedly, C54 does not function as an NES when fused to NLS-GFP reporter constructs, and mutation of its hydrophobic residues does not inactivate the Ste11p export function (data not shown). Deletion of C54 may inactivate or mask an NES located elsewhere on the protein. While the studies presented here were in progress, investigations of SOX9 and SOX10 reported that those HMG box proteins contain functional nuclear export signals within their HMG boxes (10, 34) and raised the intriguing possibility that as yet undefined sequences in the HMG domain of Ste11p could function as an NES.
The C54 region is required for Ste11p activity and, based on measurements of reporter gene expression, may contain a transactivation domain. Formally, ste11ΔC54 defines two functions, one for nuclear export of Ste11p and the other to activate target gene expression. Functional overlap between nuclear export signals and transactivation domains has been reported for other proteins. For instance, it is thought that Smad4 accumulates in the nucleus by masking of its NES through complex formation with R-Smad proteins (46). One potential candidate for an Ste11p interaction partner is Rst2p. Rst2p directly activates ste11 expression and is responsive to nutritional conditions. In cells containing high cyclic AMP-dependent protein kinase activity, Rst2p is mainly cytoplasmic but accumulates in the nucleus when protein kinase A activity is low (13, 20). Future studies are required to identify protein partners for Ste11p and to determine the role of the C54 region in those interactions.
Regulation of Ste11p activity by Pat1p phosphorylation.
Ste11p is an in vitro substrate for Pat1p, and two potential phosphorylation sites have been identified (22). Genetic studies indicated that phosphorylation of Ste11p at those sites would inactivate the transcription factor. In support of this evidence, mutagenesis of the in vitro phosphoacceptor residues Thr173 and Ser218 to alanine resulted in an activated protein. This experiment establishes the physiological significance of Pat1p phosphorylation. The activated protein, Ste11p-AA, causes advanced sexual differentiation and increased expression of mei2, a gene regulated directly by Ste11p.
How does Pat1p phosphorylation alter the activity of Ste11p? A recent study demonstrated that Pat1p phosphorylation of Ste11p creates binding sites for the 14-3-3 protein Rad24p. The interaction between Rad24p and Ste11p weakens the activity of the transcription factor, although the mechanism by which this is accomplished was not determined (16). Those studies reported that Rad24p did not alter the cellular distribution of Ste11p. However, our results suggest that Rad24p functions as a cytoplasmic tether for Ste11p. We used LMB to inhibit nuclear export. In the absence of export, nuclear accumulation of Ste11p would theoretically reflect its availability to the nuclear import system. Under these conditions, Ste11p was found in the cytoplasm and did not accumulate in the nucleus. Thus, Rad24p substantially reduced the availability of Ste11p for nuclear import. Rad24p is primarily cytoplasmic (23), a subcellular distribution which would be expected in our hypothesis. This result provides a mechanistic explanation for the function of Pat1p phosphorylation in regulation of Ste11p, though additional mechanisms may be operative. Rad24p may inhibit another Ste11p function, such as DNA binding or transactivation. Mei2p is a Pat1p substrate, and like Ste11p, phosphorylated Mei2p binds Rad24p. Interestingly, this interaction interferes with the ability of Mei2p to bind meiRNA, which is required for meiosis (36).
The experiments reported here indicate that Pat1p inhibits nuclear accumulation through at least one other target. Ste11p-AA, which cannot be phosphorylated by Pat1p, is an activated protein, but it requires starvation and pheromone signaling before it accumulates in the nucleus. Both conditions are bypassed for nuclear accumulation of Ste11p-GFP in cells containing a loss-of-function pat1 allele. This indicates that Pat1p regulates at least one other factor that is required for nuclear accumulation of Ste11p. Besides Ste11p and Mei2p, no other direct Pat1p targets have been identified.
Ste11 contains a nuclear import signal.
Systematic deletion analysis revealed that the HMG box DNA-binding domain contains sequences that target Ste11p to the nucleus. The HMG box contains both bipartite and basic cluster sequences, each with the potential to function independently as an NLS (4). The basic cluster but not the bipartite motif is both necessary and sufficient to target GFP sequences to the nucleus. This indicates that DNA binding has no major role in the nuclear accumulation of Ste11p, since amino acids important for binding DNA overlap the bipartite cluster (35).
Is nuclear accumulation of Ste11p a major regulatory mechanism for its function? Sexual differentiation of fission yeast requires coordination of nutritional starvation and pheromone signaling and is initiated by inactivation of Pat1p kinase. The results presented here, in context with the results of others, suggest a model for the function of Pat1p and Ste11p during conjugation. We propose that, during vegetative growth, active Pat1p phosphorylates Ste11p, making it an efficient binding partner for Rad24p. This complex is primarily cytoplasmic, but some Ste11p is available to shuttle to the nucleus. Nuclear accumulation of the transcription factor is prevented by efficient export. This mechanism ensures that only a limited amount of Ste11p is available in the nucleus for its own low-level expression and prevents strong nuclear accumulation of the transcription factor and subsequent activation of the developmental pathway in growing cells. As cells starve, Pat1p is inactivated, and dephosphorylated Ste11p becomes available for nuclear import. This leads to increased expression of genes required for mating pheromone signaling. Pheromone signaling leads to a robust activation of Ste11p by inhibiting export of the transcription factor from the nucleus.
Our results may provide insight into the regulation of other developmental programs. Human sexual development is regulated by a cascade of transcription factors, one being the HMG box protein SOX9. SOX9 is required to commit cells to the male lineage. During testis differentiation, SOX9 shifts from a primarily cytoplasmic location to one that is nuclear. Nuclear accumulation of the HMG box protein correlates with expression of anti-Müllerian hormone, which causes regression of Müllerian ducts that would otherwise develop into female gonadal tissue. This suggests that regulation of subcellular distribution may be important for the function of SOX9 and, perhaps, for other SOX developmental regulators (7). Notably, a mutation in the nuclear localization signal of SRY that causes human sex reversal was recently described (21). Our present study provides evidence that the subcellular distribution of an HMG box protein is a regulatory mechanism for its activity and serves as a model for HMG proteins that are under hormonal control of cellular localization.
Acknowledgments
Curt Horvath, Christopher Roman, and Brehon Laurent are thanked for many helpful discussions. Annette Kirchgessner is gratefully acknowledged for assistance with the confocal microscope. Olaf Nielsen is thanked for fission yeast strains.
This work was supported by grants from the American Heart Association (9850102T) and the National Institutes of Health (5RO1GM56875).
REFERENCES
- 1.Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Beach, D., L. Rodgers, and J. Gould. 1985. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr. Genet. 10:297-311. [DOI] [PubMed] [Google Scholar]
- 3.Bresch, C., G. Muller, and R. Egel. 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102:301-306. [DOI] [PubMed] [Google Scholar]
- 4.Christophe, D., C. Christophe-Hobertus, and B. Pichon. 2000. Nuclear targeting of proteins: how many different signals? Cell Signal. 12:337-341. [DOI] [PubMed] [Google Scholar]
- 5.Davey, J. 1998. Fusion of a fission yeast. Yeast 14:1529-1566. [DOI] [PubMed] [Google Scholar]
- 6.Davey, J., and O. Nielsen. 1994. Mutations in cyr1 and pat1 reveal pheromone-induced G1 arrest in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 26:105-112. [DOI] [PubMed] [Google Scholar]
- 7.de Santa Barbara, P., B. Moniot, F. Poulat, and P. Berta. 2000. Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev. Dyn. 217:293-298. [DOI] [PubMed] [Google Scholar]
- 8.Egel, R. 2000. Fission yeast on the brink of meiosis. Bioessays 22:854-860. [DOI] [PubMed] [Google Scholar]
- 9.Ford, J. C., F. al-Khodairy, E. Fotou, K. S. Sheldrick, D. J. Griffiths, and A. M. Carr. 1994. 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science 265:533-535. [DOI] [PubMed] [Google Scholar]
- 10.Gasca, S., J. Canizares, P. De Santa Barbara, C. Mejean, F. Poulat, P. Berta, and B. Boizet-Bonhoure. 2002. A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc. Natl. Acad. Sci. USA 99:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm, C., J. Kohli, J. Murray, and K. Maundrell. 1988. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement with the ura4 gene as a selectable marker. Mol. Gen. Genet. 215:81-86. [DOI] [PubMed] [Google Scholar]
- 12.Grosschedl, R., K. Giese, and J. Pagel. 1994. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 10:94-100. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi, T., Y. Watanabe, and M. Yamamoto. 2002. Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol. Cell. Biol. 22:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iino, Y., and M. Yamamoto. 1985. Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol. Gen. Genet. 198:416-421. [DOI] [PubMed] [Google Scholar]
- 15.Imai, Y., and M. Yamamoto. 1994. The fission yeast mating pheromone P-factor: its molecular structure, gene structure, and ability to induce gene expression and G1 arrest in the mating partner. Genes Dev. 8:328-338. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura, K., S. Katayama, S. Dhut, M. Sato, Y. Watanabe, M. Yamamoto, and T. Toda. 2001. Phosphorylation of Mei2 and Ste11 by Pat1 kinase inhibits sexual differentiation via ubiquitin proteolysis and 14-3-3 protein in fission yeast. Dev. Cell 1:389-399. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura, K., and C. Shimoda. 1991. The Schizosaccharomyces pombe mam2 gene encodes a putative pheromone receptor which has a significant homology with the Saccharomyces cerevisiae Ste2 protein. EMBO J. 10:3743-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaerulff, S., D. Dooijes, H. Clevers, and O. Nielsen. 1997. Cell differentiation by interaction of two HMG-box proteins: Mat1-Mc activates M cell-specific genes in S. pombe by recruiting the ubiquitous transcription factor Ste11 to weak binding sites. EMBO J. 16:4021-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo, N., B. Wolff, T. Sekimoto, E. P. Schreiner, Y. Yoneda, M. Yanagida, S. Horinouchi, and M. Yoshida. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540-547. [DOI] [PubMed] [Google Scholar]
- 20.Kunitomo, H., T. Higuchi, Y. Iino, and M. Yamamoto. 2000. A zinc-finger protein, Rst2p, regulates transcription of the fission yeast STE11+ gene, which encodes a pivotal transcription factor for sexual development. Mol. Biol. Cell 11:3205-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, B., W. Zhang, G. Chan, A. Jancso-Radek, S. Liu, and M. A. Weiss. 2001. Human sex reversal due to impaired nuclear localization of SRY. A clinical correlation. J. Biol. Chem. 276:46480-46484. [DOI] [PubMed] [Google Scholar]
- 22.Li, P., and M. McLeod. 1996. Molecular mimicry in development: identification of ste11+ as a substrate and mei3+ as a pseudosubstrate inhibitor of ran1+ kinase. Cell 87:869-880. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Girona, A., B. Furnari, O. Mondesert, and P. Russell. 1999. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397:172-175. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Girona, A., J. Kanoh, and P. Russell. 2001. Nuclear exclusion of Cdc25 is not required for the DNA damage checkpoint in fission yeast. Curr. Biol. 11:50-54. [DOI] [PubMed] [Google Scholar]
- 25.Love, J. J., X. Li, D. A. Case, K. Giese, R. Grosschedl, and P. E. Wright. 1995. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376:791-795. [DOI] [PubMed] [Google Scholar]
- 26.McLeod, M., and D. Beach. 1988. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature 332:509-514. [DOI] [PubMed] [Google Scholar]
- 27.McLeod, M., M. Stein, and D. Beach. 1987. The product of the mei3+ gene, expressed under control of the mating type locus, induces meiosis and sporulation in fission yeast. EMBO J. 6:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai, K. 2001. Molecular evolution of Sry and Sox gene. Gene 270:161-169. [DOI] [PubMed] [Google Scholar]
- 29.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell 99:677-690. [DOI] [PubMed] [Google Scholar]
- 30.Nurse, P. 1985. Mutations of the fission yeast Schizosaccharomyces pombe which alter the shift between cell proliferation and sporulation. Mol. Gen. Genet. 198:497-502. [Google Scholar]
- 31.Okazaki, N., K. Okazaki, Y. Watanabe, M. Kato-Hayashi, M. Yamamoto, and H. Okayama. 1998. Novel factor highly conserved among eukaryotes controls sexual development in fission yeast. Mol. Cell. Biol. 18:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen, J., O. Nielsen, R. Egel, and I. M. Hagan. 1998. FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J. Cell Biol. 141:1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen, J., D. Weilguny, R. Egel, and O. Nielsen. 1995. Characterization of fus1 of Schizosaccharomyces pombe: a developmentally controlled function needed for conjugation. Mol. Cell. Biol. 15:3697-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Qin, J., Z. Peng, and M. McLeod. In vitro mutagenesis to define functional domains. Methods Mol. Biol., in press. [DOI] [PubMed]
- 34.Rehberg, S., P. Lischka, G. Glaser, T. Stamminger, M. Wegner, and O. Rosorius. 2002. Sox10 is an active nucleocytoplasmic shuttle protein, and shuttling is crucial for Sox10-mediated transactivation. Mol. Cell. Biol. 22:5826-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roose, J., and H. Clevers. 1999. TCF transcription factors: molecular switches in carcinogenesis. Biochim. Biophys. Acta 1424:M23-37. [DOI] [PubMed] [Google Scholar]
- 36.Sato, M., Y. Watanabe, Y. Akiyoshi, and M. Yamamoto. 2002. 14-3-3 protein interferes with the binding of RNA to the phosphorylated form of fission yeast meiotic regulator mei2p. Curr. Biol. 12:141-145. [DOI] [PubMed] [Google Scholar]
- 37.Shimoda, C., A. Hirata, M. Kishida, T. Hashida, and K. Tanaka. 1985. Characterization of meiosis-deficient mutants by electron microscopy and mapping of four essential genes in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 200:252-257. [DOI] [PubMed] [Google Scholar]
- 38.Sipiczki, M. 1988. The role of sterility genes (ste and aff) in the initiation of sexual development in Schizosaccharomyces pombe. Mol. Gen. Genet. 213:529-534. [DOI] [PubMed] [Google Scholar]
- 39.Soullier, S., P. Jay, F. Poulat, J. M. Vanacker, P. Berta, and V. Laudet. 1999. Diversification pattern of the HMG and SOX family members during evolution. J. Mol. Evol. 48:517-527. [DOI] [PubMed] [Google Scholar]
- 40.Stommel, J. M., N. D. Marchenko, G. S. Jimenez, U. M. Moll, T. J. Hope, and G. M. Wahl. 1999. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18:1660-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto, A., Y. Iino, T. Maeda, Y. Watanabe, and M. Yamamoto. 1991. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 5:1990-1999. [DOI] [PubMed] [Google Scholar]
- 42.Travers, A. 2000. Recognition of distorted DNA structures by HMG domains. Curr. Opin. Struct. Biol. 10:102-109. [DOI] [PubMed] [Google Scholar]
- 43.van de Wetering, M., and H. Clevers. 1992. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 11:3039-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Heeckeren, W. J., D. R. Dorris, and K. Struhl. 1998. The mating type proteins of fission yeast induce meiosis by directly activating mei3 transcription. Mol. Cell. Biol. 18:7317-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, W., P. Li, A. Schettino, Z. Peng, and M. McLeod. 1998. Characterization of functional regions in the Schizosaccharomyces pombe mei3 developmental activator. Genetics 150:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe, M., N. Masuyama, M. Fukuda, and E. Nishida. 2000. Regulationof intracellular dynamics of Smad4 by its leucine-rich nuclear export signal. EMBO Rep. 1:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe, Y., S. Shinozaki-Yabana, Y. Chikashige, Y. Hiraoka, and M. Yamamoto. 1997. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 386:187-190. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe, Y., and M. Yamamoto. 1994. S. pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species, meiRNA. Cell 78:487-498. [DOI] [PubMed] [Google Scholar]
- 49.Wegner, M. 1999. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27:1409-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willer, M., L. Hoffmann, U. Styrkarsdottir, R. Egel, J. Davey, and O. Nielsen. 1995. Two-step activation of meiosis by the mat1 locus in Schizosaccharomyces pombe. Mol. Cell. Biol. 15:4964-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]