Abstract
DNA mismatch repair maintains genomic stability by detecting and correcting mispaired DNA sequences and by signaling cell death when DNA repair fails. The mechanism by which mismatch repair coordinates DNA damage and repair with cell survival or death is not understood, but it suggests the need for regulation. Since the functions of mismatch repair are initiated in the nucleus, we asked whether nuclear transport of MLH1 and PMS2 is limiting for the nuclear localization of MutLα (the MLH1-PMS2 dimer). We found that MLH1 and PMS2 have functional nuclear localization signals (NLS) and nuclear export sequences, yet nuclear import depended on their C-terminal dimerization to form MutLα. Our studies are consistent with the idea that dimerization of MLH1 and PMS2 regulates nuclear import by unmasking the NLS. Limited nuclear localization of MutLα may thus represent a novel mechanism by which cells fine-tune mismatch repair functions. This mechanism may have implications in the pathogenesis of hereditary non-polyposis colon cancer.
DNA mismatch repair maintains genomic stability by correcting mismatches generated during DNA replication and recombination (3). As a consequence of mismatch repair, the fidelity of DNA synthesis is increased by two to three orders of magnitude, and the extent of genetic diversity generated in meiosis is limited (24, 31). The mismatch repair system is also a molecular sensor of DNA sequence errors establishing the threshold for cell survival in the presence of excess DNA damage (8, 11, 13, 15, 18) and may control somatic hypermutation (5).
Mismatch repair family proteins are conserved in evolution, and the study of DNA repair in Escherichia coli provides a useful paradigm. In E. coli, a MutS homodimer detects DNA mismatches and recruits a MutL homodimer to the repair sites. The mobilized MutL then activates an endonuclease, MutH, that cleaves the newly synthesized strand and initiates repair (31). In eukaryotic cells, at least six MutS homologues (MSH1 to -6) and four MutL homologues (MLH1, PMS2, PMS1, and MLH3) have been identified. MSH2 pairs with MSH6 or MSH3 to form distinct heterodimers, MutSα (MSH2/MSH3) and MutSβ (MSH2/MSH6), that recognize single-base-pair mismatches and insertion-deletion loops, respectively. Similarly, MLH1 dimerizes with PMS2, PMS1, or MLH3 to form MutL heterodimers with discrete repairing specificities, including MutLα (MLH1/PMS2), the major MutL complex for mismatch repair (24).
Deficiency in mismatch repair causes a mutator phenotype in most cells because of decreased fidelity of DNA replication (24, 31) and because of increased cell survival in the presence of DNA damage (11, 13). Therefore, it is not surprising that mutations in the mismatch repair genes are associated with cancer (17) and, in particular, with hereditary nonpolyposis colon cancer (HNPCC) (35, 36). On the other hand, mice deficient in mismatch repair paradoxically have impaired somatic hypermutation of the immunoglobulin genes in B lymphocytes (5, 12, 22, 23, 37, 41, 43, 44). To explain this paradox, we proposed that during hypermutation of the immunoglobulin genes, mismatch repair is co-opted (5). Alternatively, mismatch repair may be necessary for survival of hypermutated B cells rather than directly participating in the mutation process (41). How mismatch repair articulates between DNA repair functions and being a promoter of mutations is not known, but it suggests that mismatch repair functions are adaptable.
In this work we asked whether nuclear import of MutLα components is a limiting step for the nuclear functions of mismatch repair. The finding that primary deficiency in MLH1 is associated with PMS2 deficiency (6, 26) is consistent with a regulatory interplay between MLH1 and PMS2, as suggested by the work of several laboratories (1, 9, 36). We therefore tested whether MLH1 and PMS2 nuclear import were interdependent. Our results suggested that C-terminal dimerization of MLH1 and PMS2 limits nuclear localization of MutLα. One implication of the findings here described is that, perhaps, nuclear import adaptively regulates mismatch repair functions.
MATERIALS AND METHODS
Cloning of mouse full-length MLH1 and PMS2 cDNAs.
MLH1 and PMS2 cDNAs were obtained from total mouse spleen RNA by reverse transcription-PCR, with the thermoscript system II (Invitrogen) and oligo(dT) primer followed by amplification with Turbo Pfu polymerase (Stratagene). Full-length PMS2 cDNA was generated by using overlap extension PCR and primer sets wu7/wu4 and wu3/wu8. The resulting two overlapping fragments served as templates for a second PCR with primers wu7 and wu8. Full-length MLH1 cDNA was generated by one-step PCR with primers wu20 and wu21. Both MLH1 and PMS2 cDNAs were verified by DNA sequencing.
Site-directed mutagenesis.
Mutant MLH1 and PMS2 constructs were generated by PCR-based site-directed mutagenesis with Turbo Pfu polymerase from their full-length cDNAs. PCR fragments were flanked by a 5′ NheI site and a 3′ XbaI site to allow subsequent cloning into the pCI vector (Promega) at the same sites. MLH1 constructs were fused in frame with two copies of Flag tags at their N termini. PMS2 constructs were fused in frame with two copies of hemagglutinin (HA) tags at their N termini.
The MLH1ΔC, MLH1ΔC-ΔNLS, and MLH1ΔN fragments were obtained by PCR amplification with primers wu20/wu38, primers wu20/wu101, and primers wu95/wu21, respectively. MLH1-ΔNLS was generated by two PCR rounds. The first rounds of amplification were with primers wu20/wu102 and primers wu103/wu21. The products of the first rounds were used as templates in a second PCR round with primers wu20/wu21.
PMS2ΔC and PMS2ΔC-ΔNLS were generated by PCR with primers wu7/wu39 and wu7/wu111, respectively. PMS2-ΔNLS was produced by two rounds of PCR. The first rounds were with primers wu7/wu112 and wu113/wu8 and generated the substrates for the second round of amplification with primers wu7/wu8. The MLH1-PMS2 chimeras were also generated by two rounds of PCR. The MLH1/PMS2 chimera was produced with primers wu20/wu93 and wu92/wu8 in the first rounds that generated the substrates for the second round of amplification with wu20/wu8. The PMS2/MLH1 chimera was generated with primers wu7/wu91 and wu90/wu21 in the first rounds that generated the substrates for the second round with wu7/wu21.
Nuclear export reporter constructs.
The nuclear export reporter constructs were generated by assembling three fragments, the nuclear export sequences (NES) obtained from MLH1 (MNES) or from PMS2 (PNES), the steroid-responsive element of the rat glucocorticoid receptor (Gr element), and enhanced green fluorescent protein (EGFP; G element) in the pUHD10S vector (21). The MNES fragment (positions 561 to 667) was generated by PCR from MLH1 cDNA with wu106/wu107 and was engineered to contain 5′ NheI and 3′ SpeI sites. The PNES fragment (positions 707 to 779) was amplified by PCR from PMS2 cDNA with wu116/wu117. The Gr element was amplified by PCR from pGXXG (29) with wu118/wu120 and was engineered to contain 5′ SpeI and 3′ AvrII sites. The EGFP fragment (including the stop codon) was produced by PCR from pEGFP-N1 (Clontech) with wu109/wu105 and was flanked by 5′ AvrII and 3′ XbaI sites. The three fragments of MNES or, alternatively, PNES, Gr, and G, were cloned into the pUHD10S vector to generate MNES-GrG or PNES-GrG.
Oligonucleotides.
The primers (and sequences) used in the PCRs were as follows: wu3 (5′-AAGAGAAGCTATTGCTGGCCG), wu4 (5′-TCAAACATTCCTATCAAGGAGG), wu7 (5′-CTATGCTAGCATGGAGCAAACCGAAGGCGTGAG), wu8 (5′-CTAGTCTAGATCAGTTCTGAGAGATGACATCC), wu20 (5′-CCGTGCTAGCATGGCGTTTGTAGCAGGAGTTATTCG), wu21 (5′-TACCTCTAGATTAACACCGCTCAAAGACTTTGTATAG), wu38 (5′-TTCTAGTCTAGATTATGCAAACATCGATTTACTAATCTC), wu39 (5′-TTCTAGTCTAGATTAGCTGGTGAGGTTAATGATCTTCC), wu90 (5′-GAGATTAGTAAATCGATGTTTGCAGTCTTGAGTCTCCAGGAAGAGATT),wu91 (5′-AATCTCTTCCTGGAGACTCAAGACTGCAAACATCGATTTACTAATCTC), wu92 (5′-AGGAGGATCATTAACCTCACCAGCGAGATGGAGATCTTGGGTCAGTTT), wu93 (5′-AAACTGACCCAAGATCTCCATCTCGCTGGTGAGGTTAATGATCCTCCT), wu95(5′-GAATCAGCTAGCGTCTTGAGTCTCCAGGAAGAGATT), wu101 (5′-GAATCATCTAGATTAGGAGCTTCCTGGACTGGAAGTGG), wu102 (5′-CACATCAGAGTCCTCGGAGCTTCCTGGACTGGAAGTGG), wu103 (5′-AGTCCAGGAAGCTCCGAGGACTCTGATGTGGAAATGGTG), wu105 (5′-GAATCATCTAGATTACTTGTACAGCTCGTCCATGCC), wu106 (5′-GAATCAGCTAGCGAAGAGCTGTTCTACCAGATACTC), wu107 (5′-GAATCAACTAGTCTCAGTGGCCAGTCGAAGAATGAAG), wu109 (5′-GAATCACCTAGGGTGAGCAAGGGCGAGGAGCTGTCC), wu111 (5′-GAATCATCTAGATTAGGCATTTGTGGGTGACAGACGAGC), wu112 (5′-CAAGATCTCCATCTCGGCATTTGTGGGTGACAGACGAGC), wu113 (5′-TCACCCACAAATGCCGAGATGGAGATCTTGGGTCAGTTTAACC), wu116 (5′-GAATCAGCTAGCGAGATGGAGATCTTGGGTCAGTTTAAC), wu117 (5′-GGATCAACTAGTTCTGAATATTTCCAGATTTTCTATCAG), wu118 (5′-GGATCAACTAGTCGAAAAACAAAGAAAAAAATCAAAGGG), and wu120 (5′-GAACAGCTCCTCGCCCTTGCTCACCCTAGGTTTTTGATGAAACAGAAGCTTTTTGAT).
Cell culture and transfection.
Mismatch repair-proficient HeLa-tTA (HtTA) cells (14) were cultured in Dulbecco's modified Eagle's medium and were supplemented with 10% fetal calf serum. Mismatch repair-deficient HCT 116 cells (ATCC CCL-247) (27, 34) were maintained in McCoy's 5A medium with 10% fetal calf serum. HeLa or HCT 116 cells were seeded on 12-well microscope slides (Erie Scientific) 24 h prior to transfection. DNA transfections were performed by using Lipofectamine (Invitrogen) with 0.5 μg of total construct DNA per slide well, according to the manufacturer's instructions. For the immunoprecipitation experiments, HCT 116 cells in 100-mm-diameter dishes were transfected with 10 μg of DNA and were analyzed at 24 h. For the nuclear export inhibition experiments, cells were incubated for 2 h at 37°C with 20 ng of leptomycin B (Sigma)/ml in culture medium 22 h after transfection and prior to immunofluorescence staining.
Immunofluorescence staining and microscopy.
Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature, followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. The cells were then blocked with 3% nonfat milk in PBS for at least 1 h at room temperature. Incubation with primary antibodies was done overnight at room temperature, and antibodies were diluted in 3% nonfat milk in PBS. HA and Flag tags were detected by mouse monoclonal anti-HA (12CA5 [Roche], or 16B12 [Convance]) and anti-Flag (M2; Sigma) diluted to concentrations of 2 μg/ml. MLH1 and PMS2 were identified by affinity-purified rabbit anti-mouse MLH1 (sc-582) and rabbit anti-mouse PMS2 (sc-618) polyclonal antibodies (Santa Cruz Biotechnologies) recognizing the C-terminal region of MLH1 or PMS2 of either human or mouse origin and were diluted to 1:10 and 1:100, respectively. Primary antibodies were revealed by goat anti-rabbit fluorescein isothiocyanate-conjugated antibodies (Jackson Immuno-Research Laboratories) diluted to 4 μg/ml. In the anti-HA and anti-Flag double stainings, cells were first stained with anti-HA Texas Red-conjugated (Roche) antibodies followed by anti-Flag fluorescein isothiocyanate-conjugated (Sigma) antibodies. Slides were mounted with Vectoshield containing 4′,6′-diamidino-2-phenylindole (Vector Laboratories) to stain the nuclei and were examined with a LSM510 laser-scanning microscope (Carl Zeiss) with 100× objectives, as previously described (45). Typically, 500 transfected cells were examined in each experiment. Representative images were then collected and processed by using Adobe Photoshop 6.0 software (Adobe Systems).
Immunoprecipitation and Western blotting.
Twenty four hours after transfection, the cells (about 107 cells/dish) were washed three times with ice-cold PBS, scraped off the dishes, and lysed in 1.4 ml of NP-40 lysis buffer (1.0% Nonidet P-40, 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5.0 mM EGTA, 5.0 mM EDTA, 15 mM MgCl2, 60 mM glycerolphosphate, 1.0 mM dithiothreitol, 0.1 mM Na3VO4, 0.1 mM NaF, 15 mM p-nitrophenylphosphate, and 1 tablet of a protease inhibitor cocktail [Roche] per 40 ml). Immunoprecipitation was performed essentially as described previously (21). About one-fifth of precipitated proteins in 20 μl of sample buffer were separated on a sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE) followed by electroblotting onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). After a brief wash with 0.05% Tween 20-PBS (PBST), the membranes were blocked for 1 h with 5% nonfat milk in PBST and were probed with respective primary antibodies, as indicated. Blots were developed with an ECL Western detection kit (Amersham Biotech.).
RESULTS
MLH1 and PMS2 are localized in the nucleus.
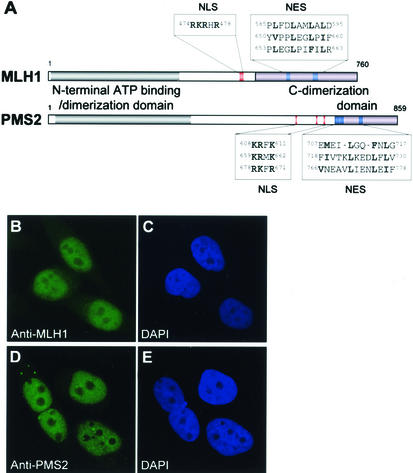
Because DNA mismatch repair takes place in the nucleus, mismatch repair proteins need to be imported to and retained in the nucleus to function. Nuclear import of proteins is determined by nuclear localization signals (NLS), and nuclear export is directed by NES (30) contained in the sequences of the protein itself or in the sequence of an associated partner. Homology search revealed one or more conserved monopartite NLS and several leucine-rich NES in the amino acid sequences of mouse MutLα components MLH1 and PMS2, as indicated in Fig. 1A. Because both NLS and NES are present in MLH1 and PMS2, we examined the protein intracellular localization. Figures 1B to E show that MLH1 and PMS2 localized predominantly in the nucleus of mismatch repair-proficient HeLa cells, corroborating the results obtained by others (10).
FIG. 1.
MLH1 and PMS2 have putative NLS and NES and localize in the nucleus. (A) Domain structure of mouse MLH1 and PMS2. C-terminal dimerization domains were identified as described by Guerrette et al. (16), and ATP binding domains were identified as described by Ban and Yang (2). Putative NLS and NES were identified by homology with known functional sequences from simian virus 40 large T antigen (32), c-myc (20), human ribosomal protein S6 (39), human immunodeficiency virus rev protein (42), human cyclic AMP-dependent protein kinase inhibitor (42), and p53 (46). The alignment identified three putative NLS in the PMS2 and one in the MLH1. In addition, three putative NES sequences were identified in PMS2 and MLH1. Mouse and human MLH1 and PMS2 NLS and NES were identical. (B to E) Confocal microscopy showing the subcellular localization of endogenous MLH1 (B) and PMS2 (D) in HeLa cells. 4′,6′-Diamidino-2-phenylindole (DAPI) nuclear staining is shown in panels C and E. Images are representative of 99% of the cells examined (496 out of 500).
MLH1 and PMS2 possess functional NLS and functional NES.
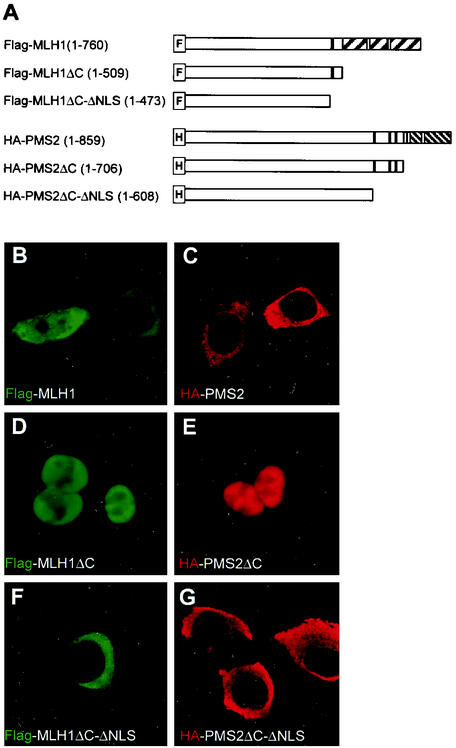
Since PMS2 and MLH1 possess both putative NLS and NES, we investigated how the proteins preferentially localized in the nucleus. We first examined the intracellular localization of mouse PMS2 and mouse MLH1, individually expressed in HCT 116 cells. The HCT 116 cells are deficient in mismatch repair due to lack of endogenous MLH1 and PMS2 (6). Figures 2B and C show that Flag-MLH1 and HA-PMS2, expressed alone in HCT 116 cells, are detected mainly in the cytoplasm, unlike the endogenous proteins in HeLa cells (Fig. 1B to E). While PMS2 was exclusively detected in the cytoplasm in 95% of the HA-PMS2 transfectants, MLH1 localized only in the cytoplasm in 51% of the Flag-MLH1 transfectants and fractioned between cytoplasm and the nucleus in the remaining 49% of cells examined (Fig. 2B). Predominant cytoplasmic localization of PMS2 or MLH1 was surprising and suggested either a compromised nuclear import or a very active nuclear export precluding nuclear accumulation, when the proteins are individually expressed. Since nuclear trafficking is determined by NLS and NES, we tested the functionality of these sequences in MLH1 and PMS2 by analyzing the fate of the deletion mutants shown in Fig. 2A. MLH1 and PMS2 C-terminal deletion mutants (positions 1 to 509 and positions 1 to 706, respectively) that retained the NLS each localized in the nucleus (Fig. 2D and E). On the other hand, C-terminal deletion mutants that additionally lacked the NLS domain of MLH1 and PMS2 (positions 1 to 473 and positions 1 to 608, respectively) localized in the cytoplasm (Fig. 2F and G). These results suggest that the NLS-containing domains of MLH1 and PMS2 are functional and necessary to direct nuclear localization of each protein but fail to do so in the presence of the C-terminal region.
FIG. 2.
MLH1 and PMS2 possess functional NLS. (A) MLH1 and PMS2 full-length and mutant constructs. An F- or H-marked box identifies the Flag or the HA tag cloned in frame 5′ to the coding sequence of MLH1 or PMS2, respectively. The NLS are identified by black-filled rectangles, and the NES are identified by gray-filled rectangles. The hatched areas represent the C-dimerization domain of MLH1 or PMS2, respectively. (B to G) Intracellular localization of MLH1 (green) or PMS2 (red) in HCT 116 cells transfected with Flag-MLH1 (B), Flag-MLH1ΔC (D), Flag-MLH1ΔC-ΔNLS (F), HA-PMS2 (C), HA-PMS2ΔC (E), or HA-PMS2ΔC-ΔNLS (G). MLH1 or PMS2 was detected with anti-Flag (B, D, and F) or anti-HA (C, E, and G) antibodies, respectively, as indicated. Images C to G are representative of 487 (97%), 382 (76%), 474 (95%), 481 (96%), and 477 (95%) cells out of 500 transfected cells examined. (B) Flag-MLH1 was exclusively localized to the cytoplasm in 255 (51%) cells and is partitioned between the cytoplasm and the nucleus in 245 (49%) cells out of 500 transfectants examined. Confocal images were collected and processed by using the same settings, as explained in Materials and Methods.
Next we tested whether the NES-containing domains of PMS2 or MLH1 were capable of directing the nuclear export of a heterologous GFP reporter. In these constructs, the putative NES-containing domains of MLH1 (positions 561 to 667) or PMS2 (positions 707 to 779) were fused 5′ to the rat glucocorticoid receptor and to GFP. Nuclear import of the fusion protein was induced by dexamethasone, and nuclear export depended on functional NES upon steroid removal, as described by Love et al. (29). GFP fusion proteins containing the NES domains of MLH1 or PMS2 directed cytoplasmic localization of GFP upon dexamethasone withdrawal (Fig. 3H to I), while a control GFP fusion protein remained in the nucleus (Fig. 3J). These results are consistent with the idea that the C-terminal fragments of MLH1 and PMS2 contain functional NES.
FIG. 3.
MLH1 and PMS2 possess functional NES. (A) MLH1-NES (MNES-GrG), PMS2-NES (PNES-GrG), and control (GrG) reporter constructs. GrG is an abbreviation for glucocorticoid receptor (Gr)-GFP (G) fusion, M refers to MLH1, and P refers to PMS2. All constructs were cloned into the pUHD10S vector. (B to J) Intracellular localization of GFP in HtTA-expressing cells transfected with MNES-GrG (B, E, and H), PNES-GrG (C, F, and I), or control construct GrG (D, G, and J). Panels B, C, and D show transfectants not treated with dexamethasone (DEX); panels E, F, and G represent transfectants treated with 1 μM dexamethasone for 30 min; panels H, I, and J represent cells treated with dexamethasone, followed by removal for two hours, in the presence of 30 μg of cycloheximide/ml. Confocal images were representative of at least 85% of 500 transfected cells examined and were collected and processed by using the settings explained in Materials and Methods.
Nuclear import governs nuclear localization of MLH1 and PMS2.
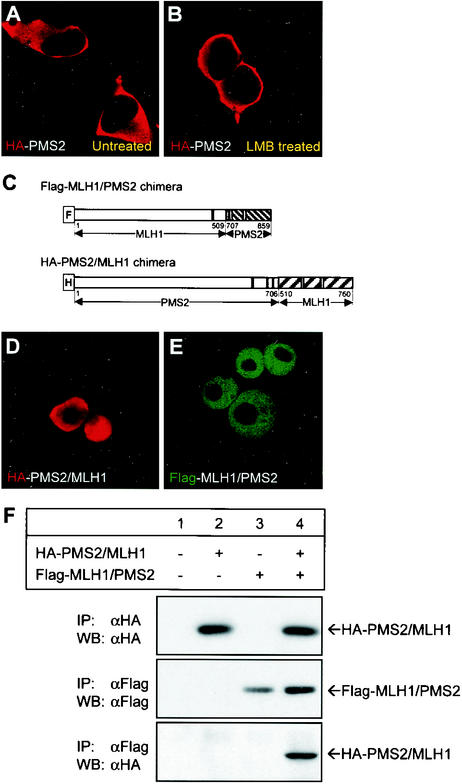
Our results suggest that MLH1 and PMS2 possess functional NLS, yet when expressed alone they fail to localize into the nucleus. We asked whether the exclusive cytoplasmic localization of full-length PMS2 expressed alone is owed to impaired nuclear import or to efficient nuclear export. To this effect, we analyzed the intracellular localization of PMS2 in the presence of leptomycin B. Leptomycin B inhibits nuclear export mediated by leucine-rich NES. We reasoned that if nuclear export governs localization of PMS2, then the protein should accumulate in the nucleus of treated cells, whereas if nuclear import governs the localization, the protein should remain in the cytoplasm. Figures 4A and B show that when nuclear export is inhibited by leptomycin B, PMS2 remained in the cytoplasm. These results suggest that PMS2 expressed alone does not reside in the nucleus because of impaired nuclear import.
FIG. 4.
MLH1 and PMS2 nuclear import is impaired by their C-terminal domains. (A and B) Intracellular localization of HA-PMS2 expressed in HCT 116 cells with (B) or without (A) 20.0 ng of leptomycin B (LMB)/ml for 2 h prior to immunostaining. PMS2 was detected with an anti-HA antibody. (C) MLH1/PMS2 and PMS2/MLH1 chimeric constructs were obtained by replacing the C-terminal domains of MLH1 and PMS2 with each other. (D and E) Intracellular localization of HA-PMS2/MLH1 (D) or Flag-MLH1/PMS2 (E) fusion proteins in transfected HCT 116 cells. The HA or Flag tags were detected with anti-HA (D) or anti-Flag (E) antibodies, as indicated in the photographs. The confocal images were representative of at least 95% of 500 transfected cells analyzed. (F) Western blot (WB) analysis of immunoprecipitation (IP) of MLH1/PMS2 and PMS2/MLH1 chimeric proteins in total cell lysates of HCT 116 cells (lane 1) or HCT 116 cells transfected with HA-PMS2/MLH1 (lanes 2 and 4) or Flag-MLH1/PMS2 (lane 3 and 4). Whole-cell lysates were immunoprecipitated with anti-Flag or anti-HA antibodies, as indicated. Precipitated proteins were resolved on SDS-8% PAGE and were blotted onto PVDF membranes, followed by probing with anti-Flag or anti-HA antibodies, as indicated. Bands were visualized with the ECL detection kit.
Since C-terminal deletion promoted nuclear localization of MLH1 and PMS2 (Fig. 2D and E), impaired nuclear import could be owed to a C-terminal cytoplasmic retention property or to NLS masking. To distinguish between these two possibilities, we generated chimeric mutants by swapping the C-terminal domains of MLH1 and PMS2 (Fig. 4C). We reasoned that while cytoplasmic retention is likely to reflect an intrinsic property of the C terminus, masking of the NLS probably requires intramolecular interactions more likely to be disrupted in the chimeras. Figures 4D and E show that Flag-MLH1/PMS2 and HA-PMS2/MLH1 chimeras localized in the nucleus, indicating that a heterologous C terminus is compatible with nuclear localization. Because the heterologous C termini in the chimeric proteins likely retain their native conformation as shown by coimmunoprecipitation (Fig. 4F), our results are consistent with the idea that the C-terminal domains of MLH1 and PMS2 impaired nuclear import by masking the function of NLS in the wild-type proteins.
MLH1 and PMS2 are coimported into the nucleus.
Our results indicated, however, that in mismatch repair-proficient cells expressing endogenous MLH1 and PMS2, the proteins localize in the nucleus (Fig. 1B to E), and that full-length PMS2 and perhaps also MLH1 expressed alone are not imported into the nucleus. Because mouse PMS2 associates with mouse MLH1 (Fig. 5H, lane 2) like their human counterparts (24), we questioned whether MLH1 and PMS2 mutually facilitate their nuclear localization, perhaps by dimerization. To this end, we cotransfected Flag-MLH1 and HA-PMS2 into HCT 116 cells and double stained them with anti-Flag and anti-HA antibodies. Figures 5B and C show that, indeed, coexpression of MLH1 and PMS2 resulted in their nuclear localization, suggesting that MLH1 and PMS2 are coimported into the nucleus.
FIG. 5.
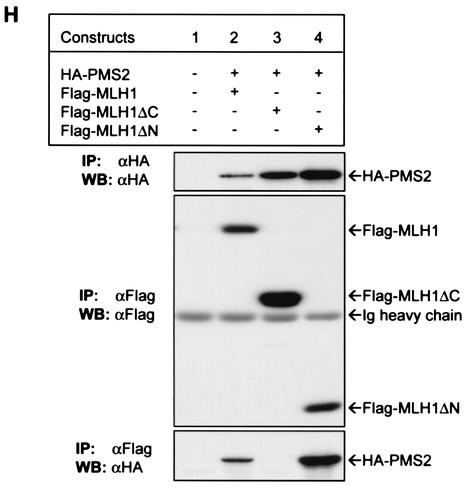
MLH1 and PMS2 are imported into the nucleus as a C-terminal MutLα heterodimer. (A) MLH1 and PMS2 full-length or deletion mutants were marked as described in the legend to Fig. 2. (B to G) Intracellular localization of MLH1 (green) or PMS2 (red) in HCT 116 cells cotransfected with HA-PMS2 and Flag-MLH1 (B and C), Flag-MLH1ΔC (D and E), or Flag-MLH1ΔN (F and G). MLH1 or PMS2 was detected with anti-Flag (B, D, and F) or anti-HA (C, E, and G) antibodies. Images reflected 476 (95%) cells in panels B and C, 479 (96%) cells in panels D and E, and 485 (97%) cells in panels F and G, out of 500 transfected cells examined. Images were collected and processed by using the same settings, as explained in Materials and Methods. (H) Western blot (WB) analysis of immunoprecipitation (IP) of MLH1 and PMS2 in total cell lysates of HCT 116 cells (lane 1) or HCT 116 cells transfected with full-length HA-PMS2 (lanes 2 to 4), Flag-MLH1 (lane 2), Flag-MLH1ΔC (lane 3), or Flag-MLH1ΔN (lane 4). Whole-cell lysates were immunoprecipitated with anti-Flag or anti-HA antibodies, as indicated. Precipitated proteins were resolved on SDS-8% PAGE and were blotted onto PVDF membranes, followed by probing with anti-Flag or anti-HA antibodies, as indicated. Bands were visualized with the ECL detection kit. Ig, immunoglobulin.
Since expression of MLH1 in HCT 116 cells restores endogenous PMS2 (6) and mouse-human transspecies MutLα can be formed (Fig. 6A, lane 3), it is possible that the partial nuclear localization of MLH1, shown in Fig. 2B, reflects the association of mouse Flag-MLH1 and the restored endogenous human PMS2. In agreement with this idea is the nuclear localization of mouse Flag-MLH1 in 82% of transfected HeLa cells that constitutively express endogenous PMS2 (Fig. 6B).
FIG. 6.
Formation and nuclear import of transspecies MutLα. (A) Western blot analysis of immunoprecipitation of Flag-MLH1 and HA-PMS2 in whole-cell lysates of HtTA cells nontransfected (lane 1), transfected with HA-PMS2 alone (lane 2), or transfected with HA-PMS2 and Flag-MLH1 (lane 3). Immunoprecipitation (IP) was with anti-Flag or anti-HA antibodies, as indicated. Precipitated proteins were resolved on SDS-6% PAGE and blotted onto PVDF membranes (WB), followed by probing with anti-Flag, anti-HA, or anti-MLH1 antibodies as indicated. Bands were visualized with the ECL detection kit. (B) Intracellular localization of mouse MLH1 in HeLa cells transfected with Flag-MLH1. Mouse MLH1 protein was detected with anti-Flag antibody. Image represents more than 95% of 500 transfectants examined.
Since MLH1 and PMS2 contain a carboxyl-terminal dimerization domain (7, 16, 33), we asked whether C-terminal dimerization of the proteins is necessary for their nuclear import. Deletion of the C terminus of MLH1 (MLH1ΔC) abolished nuclear localization of PMS2 (Fig. 5D and E). On the other hand, coexpression of the MLH1 C terminus (MLH1ΔN) facilitated nuclear localization of PMS2 (Fig. 5F and G). Since MLH1ΔC does not dimerize with PMS2 and MLH1ΔN does dimerize with PMS2 as demonstrated by coimmunoprecipitation (Fig. 5H, lanes 3 and 4, respectively), our results suggest that C-terminal dimerization of MLH1 and PMS2 is necessary for nuclear import.
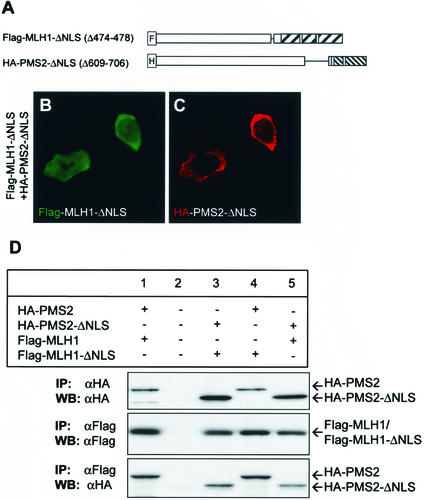
To test whether C-terminal dimerization of MLH1 and PMS2 mediated nuclear import of MutLα by unmasking the NLS, we generated mutants MLH1 (deletion of positions 474 to 478) and PMS2 (deletion of positions 609 to 706) lacking the NLS-containing domains (Fig. 7A) but retaining the dimerization domains. Figures 7B and C show that the NLS-deficient MLH1 and PMS2 mutants failed to localize in the nucleus despite the fact that they were able to dimerize, as shown by coimmunoprecipitation (Fig. 7D, lanes 3, 4, and 5). Since deleting the NLS from either MLH1 or PMS2 is sufficient to abolish the nuclear import of MutLα, our results are consistent with the idea that the C-terminal dimerization of MLH1 and PMS2 promote nuclear import by unmasking the NLS.
FIG. 7.
NLS of MLH1 and PMS2 are required for nuclear localization. (A) Schematic representation of NLS MLH1 and PMS2 deletion mutant constructs Flag-MLH1-ΔNLS and HA-PMS2-ΔNLS, respectively. (B and C) Intracellular localization of MLH1 (green) or PMS2 (red) mutants in HCT 116 cells cotransfected with Flag-MLH1-ΔNLS and HA-PMS2-ΔNLS. MLH1 or PMS2 was detected with anti-Flag (B) or anti-HA (C) antibodies. Images B and C represent 439 (88%) of 500 transfected cells examined and were collected and processed by using the same settings, as explained in Materials and Methods. (D) Western blot analysis of MLH1 and PMS2 immunoprecipitated from nontransfected HCT 116 cells (lane 2), cells transfected with full-length HA-PMS2 (lanes 1 and 4) and Flag-MLH1 (lane 1) or Flag-MLH1-ΔNLS (lane 4), and cells transfected with HA-PMS2-ΔNLS (lanes 3 and 5) and Flag-MLH1-ΔNLS (lane 3) or Flag-MLH1 (lane 5). Whole-cell lysates were immunoprecipitated (IP) with anti-Flag or anti-HA antibodies, as indicated. Precipitated proteins were resolved on SDS-8% PAGE and were blotted onto PVDF membranes (WB), followed by probing with anti-Flag or anti-HA antibodies, as indicated. Bands were visualized with the ECL detection kit.
DISCUSSION
In eukaryotic cells, nuclear import and export of proteins provides cells with the means to respond rapidly but transiently to internal and environmental challenges. For example, activation of p53 in response to DNA damage is regulated through nuclear import and export (28, 46). How the functions of mismatch repair are activated to repair DNA errors or to signal how much DNA damage is incompatible with cell survival is not known. Here we examined whether nuclear localization of MutLα is regulated by limiting nuclear transport. We propose that by limiting the nuclear transport of MutLα, dimerization of MLH1 and PMS2 may regulate mismatch repair responses to DNA damage by promoting repair or by signaling apoptosis.
In this report we demonstrated that nuclear localization of MLH1 and PMS2 requires C-terminal dimerization of the two proteins. Our results suggest that the unbound C terminus masks the NLS, perhaps by forming a hairpin structure, and that MLH1 and PMS2 C-terminal dimerization may promote nuclear import by rendering the NLS accessible. Since the NES are contained within the C-terminal domains, nuclear export may also be limited by protein dimerization. If C-terminal dimerization precludes nuclear export, then dissociation would be required before the proteins can be exported from the nucleus. Work from several quarters indicates that MutLα undergoes a conformational transformation upon ATP binding that includes N-terminal dimerization (2, 38, 40). On the other hand, work by Jager et al. suggests that C-terminal dimerization of MLH1 and PMS2 may not be sustained in the nucleus, because an N-terminal dimerization-defective mutant MLH1 (T117 M) protein binds to PMS2 in vitro but not in a yeast two-hybrid assay that only detects protein interactions in the nucleus in vivo (19). While current evidence from in vitro binding assays suggests that MLH1-PMS2 C-terminal dimerization appears to be obligatory, the question remains of whether C-terminal dimerization is conditioned differently in the cytoplasm and in the nucleus. Conditional C-terminal dimerization could potentially be a way to regulate MutLα intracellular localization.
How may dimerization of MLH1 and PMS2 regulate mismatch repair functions? While MutLα is the major MutL complex in mammalian cells, MLH1 pairs with two other partners, PMS1 and MLH3, that have conserved NLS and NES (unpublished observations) and dimerize with MLH1 at their C termini (25). It is possible that competition for binding to MLH1 may alter the amount of nuclear MutLα vis a vis other MutL complexes. Therefore, altering the relative amount of the different MutL complexes in the nucleus may be a mechanism by which adaptive responses are generated to repair DNA and promote cell survival or cell death.
Our findings may have implications for the pathogenesis of colon cancer and perhaps other cancers. It is generally accepted that germ line missense mutations in the Mlh1 gene cause HNPCC by inactivating the protein activity. However, a search of the HNPCC Mlh1 mutation database (http://www.nfdht.nl/) revealed substitution mutation hot spots (ΔR616 and R618A), located within the C-terminal domain. Studies by Guerrette and collaborators showed that the ΔR616 and R618A mutants are deficient in C-terminal dimerization with PMS2 (16). Because our findings indicate that MLH1 and PMS2 C-terminal dimerization is required for MutLα nuclear localization, we propose that the ΔR616 and R618A mutants may contribute to HNPCC, owing to a defective nuclear localization. In addition, compromised dimerization of MLH1 and PMS2 may also shift the equilibrium towards alternative MutL complexes as well as towards unbound MLH1 in the cytoplasm. Alternative forms of MutL and nuclear absence of MLH1 may contribute to tumorigenesis by altering the specificity and efficiency of DNA repair and/or by conferring resistance to cell death in the presence of DNA damage.
We propose that mismatch repair functions may be regulated, at least in part, at the level of nuclear transport, endowing cells with a fast response mechanism to internal or environmental stimuli, manifested by altering nuclear concentrations, the composition, and/or the intracellular distribution of mismatch repair complexes. Based on our findings, mismatch repair components add to the list of nuclear factors, such as tumor suppressors p53, APC, and p21Cip1/WAF1, and cell cycle regulators, such as cyclin D1, cdc25, and p27kip1, that are regulated by nuclear import and/or export (4).
Acknowledgments
We thank Jan van Deursen for reagents and Amy Tang for critically reading the manuscript. We also thank members of the laboratory for assistance in many steps of this work, namely, Michelle Rebrovich, Cecilia Rietz, and Peter Ouillette.
Work in the laboratories of the authors is supported by grants from the National Institutes of Health (AI48602 to M.C. and HL46810 and HL52297 to J.P.) and by a grant (BPD Praxis XXI/BPD 18835/98) from the Fundação para a Ciência e Tecnologia—Portugal to M.C.
REFERENCES
- 1.Baker, S. M., C. E. Bronner, L. Zhang, A. W. Plug, M. Robatzek, G. Warren, E. A. Elliott, J. Yu, T. Ashley, N. Arnheim, R. A. Flavell, and R. M. Liskay. 1995. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell 82:309-319. [DOI] [PubMed] [Google Scholar]
- 2.Ban, C., and W. Yang. 1998. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell 95:541-552. [DOI] [PubMed] [Google Scholar]
- 3.Bellacosa, A. 2001. Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ. 8:1076-1092. [DOI] [PubMed] [Google Scholar]
- 4.Carmo-Fonseca, M. 2002. The contribution of nuclear compartmentalization to gene regulation. Cell 108:513-521. [DOI] [PubMed] [Google Scholar]
- 5.Cascalho, M., J. Wong, C. Steinberg, and M. Wabl. 1998. Mismatch repair co-opted by hypermutation. Science 279:1207-1210. [DOI] [PubMed] [Google Scholar]
- 6.Chang, D. K., L. Ricciardiello, A. Goel, C. L. Chang, and C. R. Boland. 2000. Steady-state regulation of the human DNA mismatch repair system. J. Biol. Chem. 275:18424-18431. [DOI] [PubMed] [Google Scholar]
- 7.Drotschmann, K., A. Aronshtam, H. J. Fritz, and M. G. Marinus. 1998. The Escherichia coli MutL protein stimulates binding of Vsr and MutS to heteroduplex DNA. Nucleic Acids Res. 26:948-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckett, D. R., J. T. Drummond, A. I. Murchie, J. T. Reardon, A. Sancar, D. M. Lilley, and P. Modrich. 1996. Human MutSα recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc. Natl. Acad. Sci. USA 93:6443-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelmann, W., P. E. Cohen, M. Kane, K. Lau, B. Morrow, S. Bennett, A. Umar, T. Kunkel, G. Cattoretti, R. Chaganti, J. W. Pollard, R. D. Kolodner, and R. Kucherlapati. 1996. Meiotic pachytene arrest in MLH1-deficient mice. Cell 85:1125-1134. [DOI] [PubMed] [Google Scholar]
- 10.Fink, D., S. Nebel, S. Aebi, H. Zheng, H. K. Kim, R. D. Christen, and S. B. Howell. 1997. Expression of the DNA mismatch repair proteins hMLH1 and hPMS2 in normal human tissues. Br. J. Cancer 76:890-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishel, R. 1999. Signaling mismatch repair in cancer. Nat. Med. 5:1239-1241. [DOI] [PubMed] [Google Scholar]
- 12.Frey, S., B. Bertocci, F. Delbos, L. Quint, J. C. Weill, and C. A. Reynaud. 1998. Mismatch repair deficiency interferes with the accumulation of mutations in chronically stimulated B cells and not with the hypermutation process. Immunity 9:127-134. [DOI] [PubMed] [Google Scholar]
- 13.Gong, J. G., A. Costanzo, H. Q. Yang, G. Melino, W. G. Kaelin, Jr., M. Levrero, and J. Y. Wang. 1999. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399:806-809. [DOI] [PubMed] [Google Scholar]
- 14.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradia, S., D. Subramanian, T. Wilson, S. Acharya, A. Makhov, J. Griffith, and R. Fishel. 1999. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol. Cell. 3:255-261. [DOI] [PubMed] [Google Scholar]
- 16.Guerrette, S., S. Acharya, and R. Fishel. 1999. The interaction of the human MutL homologues in hereditary nonpolyposis colon cancer. J. Biol. Chem. 274:6336-6341. [DOI] [PubMed] [Google Scholar]
- 17.Hangaishi, A., S. Ogawa, K. Mitani, N. Hosoya, S. Chiba, Y. Yazaki, and H. Hirai. 1997. Mutations and loss of expression of a mismatch repair gene, hMLH1, in leukemia and lymphoma cell lines. Blood 89:1740-1747. [PubMed] [Google Scholar]
- 18.Hawn, M. T., A. Umar, J. M. Carethers, G. Marra, T. A. Kunkel, C. R. Boland, and M. Koi. 1995. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 55:3721-3725. [PubMed] [Google Scholar]
- 19.Jager, A. C., M. Rasmussen, H. C. Bisgaard, K. K. Singh, F. C. Nielsen, and L. J. Rasmussen. 2001. HNPCC mutations in the human DNA mismatch repair gene hMLH1 influence assembly of hMutLα and hMLH1-hEXO1 complexes. Oncogene 20:3590-3595. [DOI] [PubMed] [Google Scholar]
- 20.Jans, D. A., C. Y. Xiao, and M. H. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? BioEssays 22:532-544. [DOI] [PubMed] [Google Scholar]
- 21.Kasper, L. H., P. K. Brindle, C. A. Schnabel, C. E. Pritchard, M. L. Cleary, and J. M. van Deursen. 1999. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol. Cell. Biol. 19:764-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, N., G. Bozek, J. C. Lo, and U. Storb. 1999. Different mismatch repair deficiencies all have the same effects on somatic hypermutation: intact primary mechanism accompanied by secondary modifications. J. Exp. Med. 190:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, N., and U. Storb. 1998. The role of DNA repair in somatic hypermutation of immunoglobulin genes. J. Exp. Med. 187:1729-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodner, R. D., and G. T. Marsischky. 1999. Eukaryotic DNA mismatch repair. Curr. Opin. Genet. Dev. 9:89-96. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, E., A. Horii, and S. Fukushige. 2001. The interacting domains of three MutL heterodimers in man: hMLH1 interacts with 36 homologous amino acid residues within hMLH3, hPMS1 and hPMS2. Nucleic Acids Res. 29:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung, W. K., J. J. Kim, L. Wu, J. L. Sepulveda, and A. R. Sepulveda. 2000. Identification of a second MutL DNA mismatch repair complex (hPMS1 and hMLH1) in human epithelial cells. J. Biol. Chem. 275:15728-15732. [DOI] [PubMed] [Google Scholar]
- 27.Li, G. M., and P. Modrich. 1995. Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc. Natl. Acad. Sci. USA 92:1950-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, S. H., and M. F. Clarke. 2001. Regulation of p53 localization. Eur. J. Biochem. 268:2779-2783. [DOI] [PubMed] [Google Scholar]
- 29.Love, D. C., T. D. Sweitzer, and J. A. Hanover. 1998. Reconstitution of HIV-1 rev nuclear export: independent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA 95:10608-10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattaj, I. W., and L. Englmeier. 1998. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67:265-306. [DOI] [PubMed] [Google Scholar]
- 31.Modrich, P., and R. Lahue. 1996. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 65:101-133. [DOI] [PubMed] [Google Scholar]
- 32.Moore, M. S., and G. Blobel. 1992. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell 69:939-950. [DOI] [PubMed] [Google Scholar]
- 33.Pang, Q., T. A. Prolla, and R. M. Liskay. 1997. Functional domains of the Saccharomyces cerevisiae Mlh1p and Pms1p DNA mismatch repair proteins and their relevance to human hereditary nonpolyposis colorectal cancer-associated mutations. Mol. Cell. Biol. 17:4465-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos, N., N. C. Nicolaides, Y. F. Wei, S. M. Ruben, K. C. Carter, C. A. Rosen, W. A. Haseltine, R. D. Fleischmann, C. M. Fraser, M. D. Adams, et al. 1994. Mutation of a mutL homolog in hereditary colon cancer. Science 263:1625-1629. [DOI] [PubMed] [Google Scholar]
- 35.Peltomaki, P. 2001. DNA mismatch repair and cancer. Mutat. Res. 488:77-85. [DOI] [PubMed] [Google Scholar]
- 36.Peltomaki, P., and H. F. Vasen. 1997. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 113:1146-1158. [DOI] [PubMed] [Google Scholar]
- 37.Rada, C., M. R. Ehrenstein, M. S. Neuberger, and C. Milstein. 1998. Hot spot focusing of somatic hypermutation in MSH2-deficient mice suggests two stages of mutational targeting. Immunity 9:135-141. [DOI] [PubMed] [Google Scholar]
- 38.Raschle, M., P. Dufner, G. Marra, and J. Jiricny. 2002. Mutations within the hMLH1 and hPMS2 subunits of the human MutLα mismatch repair factor affect its ATPase activity, but not its ability to interact with hMutSα. J. Biol. Chem. 277:21810-21820. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, C., E. Lipsius, and J. Kruppa. 1995. Nuclear and nucleolar targeting of human ribosomal protein S6. Mol. Biol. Cell. 6:1875-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran, P. T., and R. M. Liskay. 2000. Functional studies on the candidate ATPase domains of Saccharomyces cerevisiae MutLα. Mol. Cell. Biol. 20:6390-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vora, K. A., K. M. Tumas-Brundage, V. M. Lentz, A. Cranston, R. Fishel, and T. Manser. 1999. Severe attenuation of the B cell immune response in Msh2-deficient mice. J. Exp. Med. 189:471-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463-473. [DOI] [PubMed] [Google Scholar]
- 43.Wiesendanger, M., B. Kneitz, W. Edelmann, and M. D. Scharff. 2000. Somatic hypermutation in MutS homologue (MSH)3-, MSH6-, and MSH3/MSH6-deficient mice reveals a role for the MSH2-MSH6 heterodimer in modulating the base substitution pattern. J. Exp. Med. 191:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winter, D. B., Q. H. Phung, A. Umar, S. M. Baker, R. E. Tarone, K. Tanaka, R. M. Liskay, T. A. Kunkel, V. A. Bohr, and P. J. Gearhart. 1998. Altered spectra of hypermutation in antibodies from mice deficient for the DNA mismatch repair protein PMS2. Proc. Natl. Acad. Sci. USA 95:6953-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, X., L. H. Kasper, R. T. Mantcheva, G. T. Mantchev, M. J. Springett, and J. M. van Deursen. 2001. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc. Natl. Acad. Sci. USA 98:3191-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Y., and Y. Xiong. 2001. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292:1910-1915. [DOI] [PubMed] [Google Scholar]