Abstract
The peptide transporter PEPT2 mediates the cellular uptake of di- and tripeptides and selected drugs by proton-substrate cotransport across the plasma membrane. PEPT2 was functionally identified initially in the apical membrane of renal tubular cells but was later shown to be expressed in other tissues also. To investigate the physiological importance of PEPT2 and for a detailed analysis of the protein expression sites, we generated a Pept2 knockout mouse line in which the Pept2 gene was disrupted by insertion of a β-galactosidase gene under the control of the PEPT2 promoter. The Pept2−/− mice showed no obvious phenotypic abnormalities but also no adaptive upregulation in the expression level of related genes in the kidney. The importance of PEPT2 in the reabsorption of filtered dipeptides was demonstrated in knockout animals by significantly reduced renal accumulation of a fluorophore-labeled and a radiolabeled dipeptide after in vivo administration of the tracers. This indicates that PEPT2 is the main system responsible for tubular reabsorption of peptide-bound amino acids, although this does not lead to major changes in renal excretion of protein or free amino acids.
Cellular uptake of amino acids in peptide-bound form is a biological phenomenon found throughout nature. The membrane proteins responsible for uptake of di- and tripeptides have been grouped into the peptide transporter (PTR) family of proton-dependent peptide transporters (28). A common feature of this family is that the carriers couple substrate movement across the membrane to movement of protons down an inwardly directed electrochemical proton gradient, allowing transport of peptides against a substrate gradient.
The mammalian members of the PTR family are divided into two subfamilies, represented by the peptide transporters PEPT1 (SLC15A1) and PEPT2 (SLC15A2) and the peptide/histidine transporters PHT1 and PHT2. Not much is known about the latter except that they are able to transport di- and tripeptides and the amino acid histidine. PHT1 was exclusively found in brain and eye (30), although another study suggested its presence in other tissues, including the kidney (2). PHT2 has so far only been localized in the lymphatic system (22). Both proteins are presumably lysosomal transporters. Much more information is available on PEPT1 and PEPT2 (for reviews, see references 7, 17, and 20). These two peptide transporters possess the capability for sequence-independent but stereoselective transport of all possible di- and tripeptides, including the differently charged species.
Mammalian peptide transporters also have pharmacological importance, based on their ability to transport a large variety of drugs, including angiotensin-converting enzyme inhibitors and β-lactam antibiotics (for reviews, see references 13 and 20). PEPT1 is a low-affinity transporter type with apparent affinities in the millimolar range, and PEPT2 is a high-affinity carrier with apparent affinities in the micromolar range for the same substrates. Both are found mainly in apical membranes of epithelial cells. PEPT1 is highly expressed in the small intestine, with lower expression levels in the kidney and bile duct epithelium. PEPT2 has a wider distribution within the organism, with predominant expression in the kidney but also in the nervous system, lung, and mammary gland.
The main physiological role of the peptide transporter PEPT1 is the absorption of bulk quantities of amino acids in their peptide-bound form in the small intestine. The physiological importance of the peptide transporter PEPT2 is less well defined due to its wide distribution in the organism and the lack of knowledge on di- and tripeptide concentrations in the different compartments within the organism. In the kidney, the two peptide transporters display a regional distribution along the nephron (26). Both PEPT1 and PEPT2 could contribute to tubular peptide reabsorption and overall amino acid homeostasis along with several amino acid transporters also located in the apical membrane of tubular cells (for reviews, see references 1 and 18). PEPT1 is found in the proximal tubule in segment S1, whereas PEPT2 is located in the proximal tubule in segments S2 and S3 (26). This sequential localization of the two different transport systems probably allows efficient reabsorption of di- and tripeptides, avoiding their loss into urine and thereby contributing to amino acid conservation.
Based on our analysis of the mouse Pept2 gene structure and sequence (21), we generated a knockout mouse line that lacks a functional PEPT2 protein, which allows determination of the physiological role of this carrier in various tissues and verification of its proposed role in the kidney. The Pept2−/− mice are healthy and fertile, but after administration of tracer dipeptides in vivo, the knockout animals display a drastically reduced accumulation of the hydrolysis-resistant dipeptides in the kidney, confirming the predominant role of PEPT2 in renal reabsorption of peptide-bound amino acids.
MATERIALS AND METHODS
Animals.
Mice were housed and handled according to the German guidelines, and the studies were approved by the state ethics committee. The animals used in all experiments were Pept2−/−, Pept2+/−, or wild-type mice with the same mixed genetic background (C57BL/6 and 129/Sv), between 6 and 16 weeks old. Animals were kept in a temperature-controlled environment with a 12-h light/12-h dark cycle. They received a standard diet (1324; Altromin) and water ad libitum.
Construction of targeting vector.
The clone RCPIP711F17603Q3 was identified to contain the mouse Pept2 gene, as described previously (21). After digestion with SacI, a 4.6-kb fragment containing the first exon was subcloned into pCRII (Invitrogen). After insertion of a BamHI restriction site in the Pept2 ATG start site, the bacterial β-galactosidase gene was cloned in frame by ligating the lacZ cassette obtained after digestion of pGT4.5A (3). The 5′ arm comprised a 2.7-kb fragment of the promoter region and the noncoding part of exon 1. The pKSloxPNT vector created by W. Wurst (GSF, Neuherberg, Germany) contains a selectable thymidine kinase gene and a neomycin resistance gene flanked by loxP sites. A 1.8-kb fragment containing intron 1 was inserted in a modified pKSloxPNT vector (with an additional XhoI restriction site). Finally, the 5′ arm with the lacZ cassette was inserted in this plasmid. In all cases, the orientation and sequence were verified by restriction analysis and sequencing.
Targeting of Pept2 locus and generation of Pept2 knockout mice.
A total of 40 μg of the linearized targeting vector was electroporated into 7 × 106 129/Sv embryonic stem (ES) cells. Stable clones were grown under double selection by using 200 μg of G418 per ml (Gibco) and 2 μM ganciclovir (Cytovene) (29). Stable cell lines were tested for homologous recombination by Southern blot analysis by using a 5′ internal and a 3′ external probe. The positive clones were further tested by PCR analysis with the external 5′ primer M2g-3270 (5′-GTTCTGCTCCATTGATTGTC-3′) and the internal primer lacZ.1-R (5′-GTTCAACCACCGCACGATAG-3′). Three different ES cells from correctly targeted clones were injected into C57BL/6 blastocytes and implanted into pseudopregnant CD1 female mice. Chimeric males were mated to C57BL/6 females, and the offspring animals were genotyped by Southern blot analysis. Homozygous animals were generated by matings between heterozygotes. Homozygous males were mated with Cre recombinase-transgenic females (24).
Northern blot analysis.
Total RNA from the kidney was isolated with RNAwiz (Ambion) following the supplier's protocol. Ten micrograms of RNA was separated by electrophoresis on a 1% agarose gel and transferred to a positively charged membrane (Hybond N; AP Biotech). The blot was hybridized with a PEPT2 or PHT1 [α-32P]dATP (ICN)-radiolabeled cDNA for 1 h in Express Hyb solution (Clontech) at 68°C, followed by a high-stringency wash. After stripping for demonstration of RNA loading, the blot was hybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe.
Western blot analysis.
Brush border membrane vesicles from mouse and rat kidneys were prepared by the Mg2+/EGTA method as described previously (5). A total of 100 μg of membrane proteins were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE), followed by transfer to a polyvinylidene difluoride membrane with a semidry blotter (Bio-Rad). After blocking, the blot was immunostained with the PEPT2/CT (6) antibody diluted 1:2,000 or an anti-PEPT1 antibody (14) diluted 1:100. After washing with Tris-buffered saline, the blots were incubated with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (1:1,000; Dianova) and detected by 3-amine-9-ethylcarbazole staining.
β-Galactosidase staining.
Kidneys were removed from Pept2−/− mice, fixed, and subjected to β-galactosidase staining (9). Tissues were embedded in paraffin, and 5-μm sections were analyzed. The sections were observed under the microscope (Leica Microsystems). All images were generated with Adobe Photoshop 6.0.
Uptake studies.
Experiments were done in parallel for Pept2+/+ and Pept2−/− littermates of the same gender and approximately the same body weight. At 5 min after intravenous application of 100 μl of a 100 μM solution of the dipeptide conjugate d-alanyl-l-lysine-NΕ-7-amino-4-methylcoumarin-3-acetic acid (d-Ala-Lys-AMCA) (6), the mice were anesthetized and transcardially perfused for 15 min with 4% paraformaldehyde in buffer 1 (100 mM sucrose, 100 mM NaCl, 10 mM HEPES, pH 7.4) and washed for 5 min with buffer 1. The kidneys were processed for embedding in paraffin wax, and 10-μm sections were analyzed by confocal laser scanning microscopy (model TCS SP2; Leica).
Further uptake experiments were done by intraperitoneal administration of 0.5 μCi of the radiolabeled dipeptide d-[3H]Phe-Ala per g of body weight with a specific activity of 9 Ci/mmol (BioTrend). After 2 h, the animals were killed, and the kidneys were removed and weighed. The kidneys were homogenized by incubation overnight with 500 μl of Soluene-350 (Packard) at 50°C, and the radioactivity in the homogenates was measured by scintillation counting (MicroBetaTriLux; Perkin Elmer). The results are presented as the mean ± standard deviation of three experiments. As a reduction in accumulation was expected, a one-tailed unpaired Student's t test was used to analyze the significance of the differences of the means.
Analysis of urine samples.
For the collection of urine, 12- to 16-week-old mice were placed in metabolic cages for 24 h and deprived of food but given access to water. The containers for the collection of urine were cooled, and 10 μl of a 10% thymol solution in isopropanol was added as a preservative. For each mouse, three to five 24-h urine samples were collected, stored at −20°C, and pooled before analysis. For each sex and genotype, urine from 9 to 11 mice was collected and analyzed. Creatinine was determined by Sigma diagnostic kit procedure no. 555 according to the manufacturer's instructions. Urinary protein content was determined by the Bio-Rad protein assay, following the supplier's instructions, with bovine serum albumin as the standard. After deproteinization of the urine samples with sulfosalicylic acid, the amino acids were determined by ion-exchange chromatography on an Eppendorf Biotronic LC 3000 amino acid analyzer. All data are presented as means ± standard deviation. A two-tailed unpaired Student's t test was used to analyze the significance of the differences of the means. Statistical analysis was performed with SPSS 11.
RESULTS
Targeted disruption of Pept2 gene and phenotypic analysis.
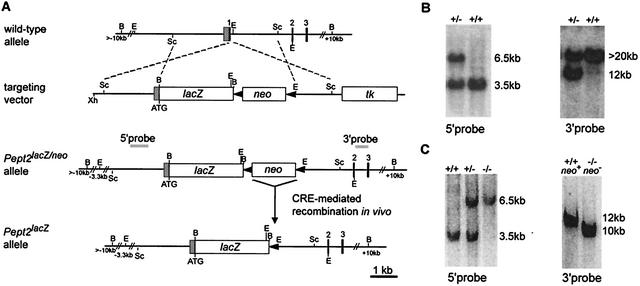
The mouse Pept2 gene was disrupted by replacing the coding region of exon 1 with the β-galactosidase gene via homologous recombination in ES cells (Fig. 1A). Correct targeting of the Pept2 locus was determined by Southern blot (Fig. 1B), and further PCR analysis confirmed the correct recombination in four ES clones. Chimeric mice were generated from ES cells and mated with C57BL/6 mice, resulting in germ line transmission, which was proven by Southern blot analysis (Fig. 1C). Homozygous mice, generated by mating heterozygote mice, were mated with Cre recombinase-transgenic mice to remove the neo cassette, which has been described to be a possible disturbing factor for the phenotype. The removal of the neo cassette was proven by Southern blot analysis (Fig. 1C).
FIG. 1.
Targeted disruption of the mouse Pept2 gene. (A) Schematic representation of the genomic Pept2 locus, the targeting construct, and the different mutated alleles. Homologous recombination of the targeting vector introduces the lacZ gene downstream of the Pept2 promoter, deleting the coding region of the first exon and the loxP-flanked neo gene. Cre-mediated recombination in vivo produces the excision of the neomycin resistance gene. Black boxes represent the coding exons, whereas the shaded box represents the noncoding region of exon 1. loxP sites are indicated as solid triangles. Dashed lines show regions of identity between the locus and the targeting vector. Grey bars indicate the 5′ internal and 3′ external probes used for the detection of the targeted allele by Southern blot analysis. tk, selectable thymidine kinase gene. B, BamHI; E, EcoRI; Sc, SacI; Xh, XhoI. (B) Southern blot analysis of genomic DNA from targeted ES cells. After digestion with EcoRI and probing with the 5′ internal probe, a 6.5-kb restriction fragment is diagnostic for the mutated allele (−/−), in contrast to the 3.5-kb fragment generated from the wild-type allele (+/+) (left). With the 3′ external probe and after digestion with BamHI, a 12-kb fragment indicates the mutated allele, whereas a fragment greater than 20 kb represents the wild-type allele (right). (C) Southern blot analysis of genomic DNA from mouse tail biopsy samples by digestion with EcoRI and hybridization with the 5′ probe (left). After in vivo Cre recombination digestion with BamHI and hybridization with the 3′ probe, a 10-kb instead of a 12-kb fragment indicates the excision of the neo gene in the mutant allele (right).
Deleted Pept2 locus is a null allele.
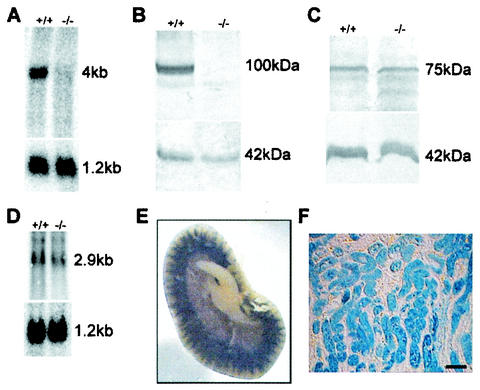
To verify that the targeting event resulted in a Pept2 null mutation, the lack of expression of PEPT2 mRNA and protein was determined. Northern blot and Western blot analysis showed that neither the PEPT2 mRNA (Fig. 2A) nor the PEPT2 protein was present in the mutant mice (Fig. 2B). We also investigated whether an upregulation in the expression level of the other mammalian PTR family members was detectable as a compensatory response to the Pept2 gene disruption. The peptide transporter PEPT1 showed the same protein expression level in the kidneys of knockout and wild-type mice (Fig. 2C). Similarly, no increase in the mRNA level of the peptide/histidine transporter PHT1 was detectable (Fig. 2D), and we were not able to detect the peptide/histidine transporter PHT2 mRNA in the kidney by Northern blot analysis (data not shown).
FIG. 2.
Mutated Pept2 allele is a null allele, and no compensatory upregulation is found in related genes. (A) Northern blot analysis with RNA isolated from the kidneys of wild-type (+/+) and Pept2 knockout (−/−) mice. The 4-kb fragment corresponding to the PEPT2 mRNA was not detectable in the RNA from the Pept2 knockout mice. The blot was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase probe as a loading and quality control (1.2-kb fragment). (B) Western blot analysis showing the absence of the PEPT2 protein in the kidney. The 100-kDa protein corresponding to the PEPT2 protein was not detectable in the knockout mice. The blot was probed with an antibody directed against actin as a quality and quantity control (42-kDa fragment). (C) Western blot analysis showing no increase in the PEPT1 protein level in the kidney. No differences were detected in the intensity of the 75-kDa band, which corresponds to the PEPT1 protein. The blot was probed with an antibody directed against actin as a quality and quantity control (42-kDa fragment). (D) Northern blot analysis showing no increase in the PHT1 mRNA level in the kidney. No differences were detected in the intensity of the 2.9-kb band which corresponds to the PHT1 RNA. The blot was striped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase probe as a loading and quality control (1.2-kb fragment). (E) Tissue overview after LacZ staining of kidney from adult Pept2−/− mice and slices (F). Bar, 80 μm.
Pept2 knockout mouse as a tool to study expression of PEPT2.
The insertion of the β-galactosidase gene in the open reading frame under the control of the Pept2 promoter allowed us to investigate the cellular expression of β-galactosidase which would translate into sites of PEPT2 expression in wild-type animals by lacZ staining. In the kidney, β-galactosidase staining showed the expression of PEPT2 in the outer but not the inner stripe of the outer medulla, which included the medullary rays protruding into the deeper cortical regions (Fig. 2E). This expression pattern correlates with findings from previous immunolocalization studies of PEPT2 in the kidney (26). Higher magnification demonstrated strong staining of the proximal tubule (Fig. 2F).
Accumulation of dipeptides in the kidney is reduced in Pept2−/− mice.
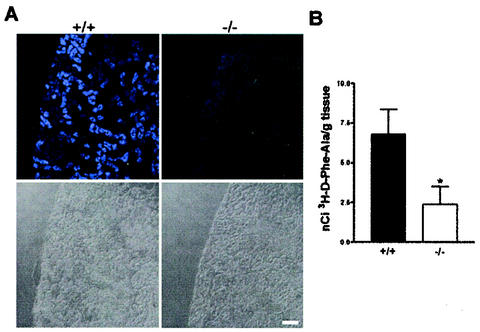
To validate the loss of function of PEPT2-mediated transport function in the kidneys of Pept2 knockout mice, we performed two sets of experiments. As shown in Fig. 3A, after intravenous administration of the fluorophore-conjugated dipeptide substrate d-Ala-Lys-AMCA, we detected a drastically reduced accumulation of fluorescence in the knockout animals compared to the wild-type mice. Only in the outer cortex was a weak fluorescence detectable. In the wild-type mice, the fluorescence was restricted to the outer medulla and some regions of the cortex but absent in the inner medulla (data not shown). This distribution correlates well with the presence of the peptide transporters in the proximal tubule, as found in immunolocalization studies. Moreover, in accordance with previous studies that have shown that the renal tubule is the only tissue capable of accumulating short-chain peptides after their intravenous administration (for reviews, see references 1 and 4), no fluorescence from d-Ala-Lys-AMCA could be detected in any of the other tissues examined (data not shown).
FIG. 3.
Reduced renal accumulation of a fluorophore-conjugated dipeptide (A) and a radiolabeled-dipeptide (B) in Pept2 knockout mice. (A) In kidney slices obtained from wild-type (+/+) mice after intravenous injection of d-Ala-Lys-AMCA, fluorescence was detectable in selected tubular structures of the cortex and outer medulla, whereas in slices from the Pept2 knockout (−/−) mice, only very weak fluorescence was detectable in the outer cortex. Bottom, light microscopy pictures of the corresponding kidney sections. Bar, 80 μm. (B) In kidney homogenates prepared from Pept2 knockout mice (−/−) and wild-type animals (+/+), accumulation of radioactivity after intraperitoneal administration of d-[3H]Phe-Ala was significantly (*, P < 0.05) reduced.
The second approach to determine the effects of the loss of PEPT2 function on renal peptide transport was based on the accumulation of the radiolabeled dipeptide d-[3H]Phe-Ala in the kidney after its intraperitoneal administration. As shown in Fig. 3B, the accumulation of this dipeptide in kidney tissues of the knockout animals was significantly reduced. Only 35% of the amount of radiolabeled dipeptide found in wild-type animals could be detected in the kidneys of the Pept2−/− mice.
No significant alteration in protein and free amino acid levels in urine after disruption of Pept2.
Urine samples were collected during a 24-h fasting period. As shown in Table 1, the volume of urine excreted per day by the Pept2−/− mice was not statistically different from that by the Pept2+/+ mice. Urine creatinine levels as well as the total protein-to-creatinine ratio did not differ significantly among the different genotypes. As PEPT2 is responsible for the tubular reabsorption of filtered di- and tripeptides, we also analyzed whether the impaired renal reabsorption in Pept2−/− mice causes changes in the excretion rate of free amino acids compared to those in wild-type mice. Table 1 gives values for all proteinogenic amino acids that could be quantified reliably by high-pressure liquid chromatography analysis. Although there was clearly a tendency towards higher values in the ratios of almost all amino acids to creatinine in Pept2−/− mice compared to wild-type animals, only the ratio for leucine reached the level of significance in the case of male Pept2−/− mice (P = 0.029).
TABLE 1.
Analysis of urine in 24-h urine samples of Pept2+/+, Pept2+/−, and Pept2−/− micea
| Gender | Genotype | Volume (ml/day) | Creatinine (mmol/liter) | Total protein (mg/mmol of creatinine) | Free amino acid (mmol/mol of creatinine)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycine | Alanine | Leucine | Isoleucine | Serine | Threonine | Methionine | Asn + Asp + Gln + Glu | |||||
| Male | +/+ | 1.3 ± 0.7 | 3.0 ± 0.6 | 189.7 ± 58.7 | 104.6 ± 43.8 | 26.4 ± 6.4 | 25.8 ± 3.2 | 18.9 ± 7.2 | 25.7 ± 6.6 | 28.8 ± 8.0 | 39.7 ± 11.6 | 125.1 ± 37.0 |
| +/− | 1.3 ± 0.4 | 3.1 ± 0.9 | 189.5 ± 37.3 | 95.9 ± 36.5 | 28.4 ± 12.5 | 23.9 ± 8.2 | 19.1 ± 1.4 | 26.6 ± 8.1 | 22.9 ± 2.6 | 34.8 ± 7.2 | 125.0 ± 61.2 | |
| −/− | 0.9 ± 0.3 | 3.4 ± 0.8 | 168.4 ± 20.4 | 141.0 ± 41.1 | 31.0 ± 6.7 | 33.9 ± 8.8b | 19.1 ± 4.1 | 28.4 ± 2.3 | 30.3 ± 10.1 | 37.3 ± 8.3 | 127.7 ± 30.6 | |
| Female | +/+ | 0.8 ± 0.2 | 2.8 ± 0.7 | 97.3 ± 35.3 | 101.9 ± 23.6 | 50.4 ± 20.0 | 30.1 ± 11.4 | 37.3 ± 10.3 | 35.8 ± 10.7 | 35.8 ± 10.1 | 38.9 ± 10.0 | 172.3 ± 64.8 |
| +/− | 0.9 ± 0.4 | 2.8 ± 0.9 | 104.4 ± 16.4 | 93.9 ± 13.5 | 44.7 ± 13.9 | 29.7 ± 13.2 | 37.0 ± 10.0 | 37.2 ± 11.6 | 39.4 ± 10.3 | 40.0 ± 7.0 | 162.1 ± 63.3 | |
| −/− | 1.3 ± 0.6 | 2.5 ± 0.6 | 112.3 ± 14.9 | 117.7 ± 25.7 | 53.2 ± 10.5 | 40.6 ± 3.3 | 41.9 ± 8.4 | 39.9 ± 8.6 | 43.7 ± 7.2 | 43.3 ± 8.3 | 144.4 ± 29.6 | |
Values are means ± standard deviations.
P < 0.05 versus wild type.
DISCUSSION
To assess the physiological role of the high-affinity-type peptide transporter PEPT2, we generated a Pept2 knockout mouse line, disrupting the Pept2 gene by replacing the coding region of the first exon with the β-galactosidase gene. These mice allowed us to study the phenotypic consequences of the lack of the PEPT2 protein and the expression of the Pept2 gene in tissues and cells by staining for lacZ. The validity of the lacZ-mediated detection of gene expression sites was demonstrated by the good correlation of the expression data obtained based on β-galactosidase gene activity and previously performed immunolocalization studies. Following the submission of our manuscript, a similar mouse line lacking the Pept2 gene was reported, showing that the lack of PEPT2 markedly reduced the transport of a model dipeptide in the choroid plexus epithelium without other physiological impairments (27).
In view of renal handling of peptides, it is generally accepted that the peptide transporters PEPT1 and PEPT2 contribute to amino acid homeostasis in the organism along with several classes of amino acid transporters that are also located in the apical membrane of tubular cells (for reviews, see references 1 and 18). Di- and tripeptides are delivered to PEPT1 and PEPT2 in renal epithelial cells either by surface hydrolysis of larger oligopeptides or by glomerular filtration. Data from animal studies suggest that up to 50% of circulating plasma amino acids might be peptide bound and that 25 to 50% could be di- and tripeptides (8, 25). However, the composition of this di- and tripeptide fraction circulating in plasma or provided in the filtrate is not known. Only a very few individual di- and tripeptides have been identified in plasma, and their concentrations appear to vary between 0.1 nM and around 50 μM (16, 19, 31). In this concentration range, PEPT2, based on its apparent high affinity, most probably plays the predominant role in the reabsorption of these peptides.
By the administration of two hydrolysis-resistant model dipeptides in vivo, we demonstrated that the lack of PEPT2 impairs their tubular uptake. The fluorophore-conjugated dipeptide d-Ala-Lys-AMCA has been shown to be transported by both mammalian peptide transporters (6, 11). Intravenous administration of this tracer with a calculated blood concentration of 5 μM showed that PEPT2 plays the predominant role in its renal reabsorption. In Pept2 knockout animals no fluorescence was detectable in the regions where PEPT2 is normally expressed. Moreover, almost no fluorescence could be detected in the outer cortex, known as the site of PEPT1 expression. The 3H-labeled dipeptide d-Phe-Ala is frequently used as a model substrate, with an apparent Km of 1.1 mM for PEPT1 and an apparent Km of 143 μM for PEPT2 (6). Although its affinity for PEPT2 is fairly low, PEPT2 is obviously more important than PEPT1, as the reduction in the renal accumulation of d-Phe-Ala after intraperitoneal administration was reduced to 35% of that in control animals. These observations demonstrate for the first time in vivo that PEPT2 plays the predominant role in renal peptide reabsorption, as suggested by studies with renal brush border membrane vesicles (15).
The renal zonation and the different affinities and transport capacities of the two transporters in concert may allow maximal reabsorption capacity and highest efficiency for conservation of peptide-bound amino acids. The low-affinity, high-capacity PEPT1 system may handle a higher peptide load, whereas the high-affinity, low-capacity PEPT2 system dominates at physiological substrate concentrations and was shown here to be the prime transport pathway for this concentration of tracer peptides. These findings are of importance for the metabolism of biologically active di- and tripeptides and the pharmacokinetics of drugs and prodrugs of which renal reabsorption by the peptide transporters alters their kinetics (for reviews, see references 13 and 20).
What are the consequences of impaired renal reabsorption of peptide-bound amino acids in animals lacking a PEPT2 protein? We analyzed the urinary excretion of amino acids, total protein, and creatinine in the knockout mice in comparison to the wild-type and heterozygous mice and observed no significant alterations between the genotypes. Although there was clearly a tendency for an increased amino acid-to-creatinine ratio for most of the free amino acids analyzed (for example, glycine and leucine excretion rates were 35% and 31% higher), significance was only reached in the case of leucine. Therefore, renal excretion of amino acids and amino acid nitrogen is only modestly altered by the lack of PEPT2. This may be due to further hydrolysis of the peptides not taken up by PEPT2 by brush border membrane-bound peptidases (12, 23) to free amino acids, which in turn could be taken up by the various amino acid transporters present in the brush border membranes of renal epithelial cells (for a review, see reference 18). By that route, enhanced absorption of free amino acids would compensate for the lack of peptide transport, and the amount of free amino acids found in urine would not necessarily be increased while maintaining renal amino nitrogen reabsorption.
In conclusion, the disruption of the peptide transporter PEPT2 in mice demonstrates in vivo its importance in the renal reabsorption processes involved in clearance of di- and tripeptides and pharmacologically relevant peptidomimetic drugs. Moreover, the insertion of the β-galactosidase gene under the control of the Pept2 promoter in the Pept2 knockout mouse line confirmed its zonal expression in the kidney. Although the contribution of PEPT2 to peptide transport in the choroid plexus epithelium has also recently been demonstrated in mice lacking the gene, the overall nutritional and pharmacological role of the PEPT2 carrier protein remains to be determined.
Acknowledgments
We thank Susanne Bourier, Heidi Krause, Bernhard Rey, and Verena Kaindl for technical assistance; Veronique Blanquet for providing the ES cells; Wolfgang Wurst for providing plasmids; and Klaus Pfeffer for providing Cre recombinase-transgenic mice.
This work was supported in part by a grant (F 2226) from the Bund der Freunde der Technischen Universität München e.V. and a grant (1512/282 72-5) from the Else-Kröner-Fresenius Stiftung.
REFERENCES
- 1.Adibi, S. A. 1997. Renal assimilation of oligopeptides: physiological mechanisms and metabolic importance. Am. J. Physiol. 272:E723-E736. [DOI] [PubMed] [Google Scholar]
- 2.Botka, C. W., T. W. Wittig, R. C. Graul, C. U. Nielsen, K. Higaka, G. L. Amidon, and W. Sadee. 2000. Human proton/oligopeptide transporter (POT) genes: identification of putative human genes with bioinformatics. AAPS Pharm Sci. 2:E16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broccoli, V., E. Boncinelli, and W. Wurst. 1999. The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401:164-168. [DOI] [PubMed] [Google Scholar]
- 4.Daniel, H., and M. Herget. 1997. Cellular and molecular mechanisms of renal peptide transport. Am. J. Physiol. 273:F1-F8. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, H., E. L. Morse, and S. A. Adibi. 1991. The high and low affinity transport systems for dipeptides in kidney brush border membrane respond differently to alterations in pH gradient and membrane potential. J. Biol. Chem. 266:19917-19924. [PubMed] [Google Scholar]
- 6.Doring, F., T. Michel, A. Rosel, M. Nickolaus, and H. Daniel. 1998. Expression of the mammalian renal peptide transporter PEPT2 in the yeast Pichia pastoris and applications of the yeast system for functional analysis. Mol. Membr. Biol. 15:79-88. [DOI] [PubMed] [Google Scholar]
- 7.Fei, Y. J., V. Ganapathy, and F. H. Leibach. 1998. Molecular and structural features of the proton-coupled oligopeptide transporter superfamily. Progress Nucleic Acid Res. Mol. Biol. 58:239-261. [DOI] [PubMed] [Google Scholar]
- 8.Gardner, M. L. G. 1994. Absorption of intact proteins and peptides, p. 1795-1820. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Lippincott-Raven, New York, N.Y.
- 9.Gossler, A., and J. Zachgo. 1993. Gene and enhancer trap screens in ES cells chimeras, p. 181-196. In A. L. Joyner (ed.), Gene targeting: a practical approach, Oxford University Press, New York, N.Y.
- 10.Groneberg, D. A., M. Nickolaus, J. Springer, F. Doring, H. Daniel, and A. Fischer. 2001. Localization of the peptide transporter PEPT2 in the lung. Am. J. Pathol. 158:707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groneberg, D. A., F. Doring, P. R. Eynott, A. Fischer, and H. Daniel. 2001. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. 281:G697-G704. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, N. M., J. N. Keen, and A. J. Turner. 1990. Characterization of the glycosyl-phosphatidylinositol-anchored human renal dipeptidase reveals that it is more extensively glycosylated than the pig enzyme. Biochem. J. 265:429-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inui, K. I., S. Masuda, and H. Saito. 2000. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 58:944-958. [DOI] [PubMed] [Google Scholar]
- 14.Knutter, I., I. Rubio-Aliaga, M. Boll, G. Hause, H. Daniel, K. Neubert, and M. Brandsch. 2002. H+-peptide cotransport in the human bile duct epithelium cell line SK-ChA-1. Am. J. Physiol. 283:G222-229. [DOI] [PubMed] [Google Scholar]
- 15.Lin, C. J., and D. E. Smith. 1999. Glycylsarcosine uptake in rabbit renal brush border membrane vesicles isolated from outer cortex or outer medulla: evidence for heterogeneous distribution of oligopeptide transporters. AAPS Pharm. Sci. 1:E1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsui, T., K. Tamaya, E. Seki, K. Osajima, K. Matsumo, and T. Kawasaki. 2002. Absorption of Val-Tyr with in vitro angiotensin I-converting enzyme inhibitory activity into the circulating blood system of mild hypertensive subjects. Biol. Pharm. Bull. 25:1228-1230. [DOI] [PubMed] [Google Scholar]
- 17.Meredith, D., and C. A. Boyd. 2000. Structure and function of eukaryotic peptide transporters. Cell. Mol. Life Sci. 57:754-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacin, M., R. Estevez, J. Bertran, and A. Zorzano. 1998. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 78:969-1054. [DOI] [PubMed] [Google Scholar]
- 19.Pastore, A., R. Massoud, C. Motti, A. Lo Russo, G. Fucci, C. Cortese, and G. Federici. 1998. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin. Chem. 44:825-832. [PubMed] [Google Scholar]
- 20.Rubio-Aliaga, I., and H. Daniel. 2002. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 23:434-440. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Aliaga, I., M. Boll, and H. Daniel. 2000. Cloning and characterization of the gene encoding the mouse peptide transporter PEPT2. Biochem. Biophys. Res. Commun. 276:734-741. [DOI] [PubMed] [Google Scholar]
- 22.Sakata, K., T. Yamashita, M. Maeda, Y. Moriyama, S. Shimada, and M. Tohyama. 2001. Cloning of a lymphatic peptide/histidine transporter. Biochem. J. 356:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh, S., Y. Keida, Y. Konta, M. Maeda, Y. Matsumoto, M. Niwa, and M. Kohsaka. 1993. Purification and molecular cloning of mouse renal dipeptidase. Biochim. Biophys. Acta 1163:234-242. [DOI] [PubMed] [Google Scholar]
- 24.Schwenk, F., U. Baron, and K. Rajewsky. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23:5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seal, C. J., and D. S. Parker. 1991. Isolation and characterization of circulating low molecular weight peptides in steer, sheep and rat portal and peripheral blood. Comp. Biochem. Physiol. B 99:679-685. [DOI] [PubMed] [Google Scholar]
- 26.Shen, H., D. E. Smith, T. Yang, Y. G. Huang, J. B. Schnermann, and F. C. Brosius 3rd. 1999. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. 276:F658-F665. [DOI] [PubMed] [Google Scholar]
- 27.Shen, H., D. E. Smith, R. F. Keep, J. Xiang, and F. C. Brosius III. 2003. Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J. Biol. Chem. 278:4786-4791. [DOI] [PubMed]
- 28.Steiner, H. Y., F. Naider, and J. M. Becker. 1995. The PTR family: a new group of peptide transporters. Mol. Microbiol. 16:825-834. [DOI] [PubMed] [Google Scholar]
- 29.Wurst, W., and A. L. Joyner. 1993. Production of targeted embryonic stem cell clones, p. 33-62. In A. L. Joyner (ed.), Gene targeting: a practical approach. Oxford University Press, New York, N.Y.
- 30.Yamashita, T., S. Shimada, W. Guo, K. Sato, E. Kohmura, T. Hayakawa, T. Takagi, and M. Tohyama. 1997. Cloning and functional expression of a brain peptide/histidine transporter. J. Biol. Chem. 272:10205-10211. [DOI] [PubMed] [Google Scholar]
- 31.Yang, X. D., J. Y. Ma, M. W. Barger, and J. K. Ma. 2002. Transport and utilization of arginine and arginine-containing peptides by rat alveolar macrophages. Pharm. Res. 19:825-831. [DOI] [PubMed] [Google Scholar]