Abstract
The reductive-oxidative status of tissues regulates the expression of many inflammatory genes that are induced during gram-negative bacterial infections. The cytokine gamma interferon (IFN-γ) is a potent stimulus for host inflammatory gene expression, and oxidative stress has been shown to inhibit its production in mice challenged with Escherichia coli bacteria. The objective of the present study was to characterize the cells that produced IFN-γ in a mouse bacterial peritonitis model and determine the effects of oxidative stress on their activation. The liver contained large numbers of IFN-γ-expressing lymphocytes following challenge with viable E. coli bacteria. The surface phenotypes of IFN-γ-expressing hepatic lymphocytes were those of natural killer (NK) cells (NK1.1+ CD3−), conventional T cells (NK1.1− CD3+), and NK T cells (NK1.1+ CD3+). Treating mice with diethyl maleate to deplete tissue thiols significantly impaired IFN-γ production by NK cells, conventional T cells, and CD1d-restricted NK T cells in response to E. coli challenge. However, IFN-γ expression by a subset of NK T cells, which did not bind α-galactosylceramide-CD1d tetramers, was resistant to the inhibitory effects of tissue oxidative stress. Stress-resistant IFN-γ-expressing cells were also predominantly CD8+ and bore γδ T-cell antigen receptors. The residual IFN-γ response by NK T cells may explain previous reports of hepatic gene expression following gram-negative bacterial challenge in thiol-depleted mice. The finding also demonstrates that innate immune cells differ significantly in their responses to altered tissue redox status.
Infection with gram-negative bacteria, such as Escherichia coli, results in a series of complex hemodynamic, metabolic, and innate immune responses that can culminate in the clinical condition referred to as sepsis. These responses are coordinated, in part, by the production of several key cytokines and the subsequent induction of a set of inflammatory genes. One such proinflammatory cytokine is gamma interferon (IFN-γ), which has been shown elsewhere to play a central role in the induction of endotoxicosis and gram-negative bacterial sepsis in several rodent models (9, 17, 19, 20, 22, 25, 38, 41). For example, injecting mice with recombinant IFN-γ stimulates the expression of intercellular adhesion molecule 1 (ICAM-1) and the inducible form of nitric oxide synthase (iNOS) (7, 11, 38). The expression of these genes contributes to changes in leukocyte migration and the production of highly reactive nitrogen radicals, respectively. Conversely, mice lacking IFN-γ or its receptor show decreased hepatic expression of iNOS and ICAM-1 following challenge with either live bacteria or endotoxic lipopolysaccharide (LPS) (38, 41). In response to microbial challenge, several cell types, including natural killer (NK) cells, conventional T cells, and NK T cells, synthesize IFN-γ (12, 33, 36, 42, 45).
Tissue oxidative stress is a natural consequence of infection and results, in part, from the combined effects of superoxide and nitric oxide produced by inflammatory cells. Endogenous intracellular antioxidants are important in maintaining the reductive-oxidative (redox) balance necessary for inflammatory responses (30, 31, 38, 50, 51) and are often depleted during infection (32, 37, 47). Thiol-reactive compounds have been used experimentally to create prooxidant tissue environments and have been reported to decrease significantly the expression of tumor necrosis factor alpha (TNF-α), iNOS, ICAM-1, and CD14 in rodent tissues in response to either LPS or E. coli challenge (31, 38, 50). Recent evidence has indicated that oxidative stress caused by thiol depletion also decreases E. coli-induced IFN-γ production and that this effect plays a major role in regulating inflammatory gene expression in the mouse liver (38).
The present study was undertaken with two objectives. First, we wanted to identify the anatomical site and the specific lymphocyte subsets involved in IFN-γ production in mice challenged with viable E. coli bacteria. Determining the effects of oxidative stress on IFN-γ production by these cells was a second important objective. Our results suggest that hepatic lymphocytes in the mouse are a major source of this cytokine following intraperitoneal (i.p.) challenge with viable bacteria and that multiple lymphocyte subsets participate in this response. The effect of oxidative stress on the IFN-γ response is not equivalent in each cell subset, suggesting that intracellular signaling for production of the cytokine is cell subset specific.
MATERIALS AND METHODS
Reagents.
E. coli O111:B4 LPS, diethyl maleate (DEM), sesame oil, glutathione reductase, lactate dehydrogenase, bovine serum albumin (BSA), and brefeldin A were purchased from Sigma Chemical Co. (St. Louis, Mo.). Fetal bovine serum was obtained from JRH Biosciences Co. (Lenexa, Kans.). ACK lysing buffer was purchased from Bio-Whittaker Co. (Walkersville, Md.). Normal rat immunoglobulin G (IgG) and normal hamster IgG were from Jackson ImmunoResearch Laboratories (West Grove, Pa.). Perm/Wash buffer, CytoFix/CytoPerm solution, streptavidin-Cy-Chrome conjugate, and streptavidin-fluorescein isothiocyanate (FITC) conjugate were obtained from BD PharMingen Inc. (San Diego, Calif.). LPS-free phosphate-buffered saline (PBS) and glucose were from Fisher Scientific (Pittsburgh, Pa.), RPMI 1640 medium was from GIBCO BRL (Rockville, Md.), Liberase RH and DNase I were purchased from Roche Diagnostics Corp. (Indianapolis, Ind.), and LPS-free Percoll and Ficoll-Paque were from Amersham Pharmacia Biotech (Piscataway, N.J.). Neutravidin was obtained from Molecular Probes (Eugene, Oreg.). The following antibodies were purchased from BD PharMingen: rat anti-mouse CD16/CD32 (clone 2.4G2), biotin-conjugated mouse anti-mouse NK1.1 (clone PK136), Cy-Chrome-conjugated hamster anti-mouse CD3ɛ (clone 145-2C11), FITC-conjugated hamster anti-mouse T-cell receptor γδ (TCRγδ; clone GL3), FITC-conjugated rat anti-mouse CD4 (clone GK1.5), FITC-conjugated rat anti-mouse CD8 (clone 53-6.7), phycoerythrin (PE)-conjugated rat anti-mouse IFN-γ (clone XMG1.2), Cy-Chrome-conjugated hamster IgG (clone G235-2356), and PE-conjugated rat IgG (clone R3-34). Tetramers comprised of mouse CD1d and β2-microglobulin were prepared as previously described (29), labeled with PE, and loaded with α-galactosylceramide (α-GalCer). Unloaded PE-conjugated CD1d-β2-microglobulin tetramers served as controls for measuring background staining.
Animals.
Female (8- to 12-week-old) C57BL/6J and CF-1 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and Charles River (Wilmington, Mass.), respectively. The animals were maintained in an American Association of Laboratory Animal Care-accredited facility on 12-h light-12-h dark cycles with food and water ad libitum. An institutional review committee at the University of Kansas Medical Center approved the animal care and use protocols used in this study.
Bacterial challenge.
E. coli O111:B4 bacteria were grown overnight from frozen stock in Trypticase soy broth at 37°C with shaking (100 rpm). An aliquot of the overnight culture was transferred into fresh Trypticase soy broth and grown to a density of approximately 5 × 108 bacteria/ml. The bacteria were then washed once with cold PBS, diluted, and injected by the i.p. route. The actual numbers of live bacteria injected were confirmed for each experiment by growing dilutions of the inoculums at 37°C overnight on Trypticase soy agar. The 50% lethal dose for this organism in CF-1 mice was approximately 107 CFU when injected by the i.p. route.
Preparation of cell suspensions.
Mice were euthanized, and various organs and tissues were removed under aseptic conditions. For liver perfusion, the portal vein was cannulated and 30 ml of warm Hanks' balanced salt solution (HBSS) containing 10 mM HEPES was infused at a rate of 1 ml/s. The perfusate was collected with a cannula placed into the superior vena cava. The liver was then removed and washed once with ice-cold HBSS containing 10 mM HEPES, minced with scissors, transferred to digestion buffer (HBSS, 10 mM HEPES, 25 μg of Liberase RH/ml, 40 μg of DNase I/ml, 1 μg of brefeldin A/ml), and incubated at 37°C for 30 min with shaking (60 cycles/min). The partially digested tissue was gently pressed through a 100-gauge stainless steel mesh, and the enzymes were inactivated by adding an equal volume of stop solution (Ca2+-Mg2+-free HBSS, 10 mM HEPES, 5% FBS). Following centrifugation (20 × g) at room temperature for 10 s to remove intact hepatocytes and debris, the suspension was carefully placed onto a layer of 28% isotonic Percoll (density = 1.063) and centrifuged at 300 × g at room temperature for 30 min. The supernatant and Percoll layers were carefully aspirated and discarded, and the cell pellet was recovered by centrifugation. Contaminating erythrocytes were then lysed by resuspending the cells in ACK lysing buffer and incubating the suspension at 37°C for 10 min. The cells were washed twice with warm RPMI 1640 supplemented with 10% FBS, 5 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and brefeldin A (1 μg/ml). To prepare lymphocytes from the spleen, lymph nodes, or thymi, the organs were gently teased and pressed through 100-gauge stainless steel mesh. Splenic erythrocytes were lysed with ACK lysing buffer. Lymphocytes from heparinized peripheral blood were prepared by centrifugation through Ficoll-Paque. Each lymphocyte preparation was washed twice with warm RPMI 1640 medium, resuspended in complete culture medium containing 3 to 7 μg of brefeldin A/ml, and incubated for 5 to 9 h at 37°C in an atmosphere of 5% CO2.
Assay for serum IFN-γ.
The concentrations of IFN-γ in mouse sera were determined by enzyme-linked immunosorbent assay (PharMingen) according to the manufacturer's instructions. The detection limit of this assay was 40 pg/ml.
Flow cytometry.
Brefeldin A-treated cells were washed twice with ice-cold phosphate glucose buffer (PBS, 0.1 M glucose, 0.5% BSA). To block nonspecific antibody binding, cells were sequentially incubated on ice for 15 min with rat anti-mouse CD16/CD32, normal rat IgG (3 μg/ml), and normal hamster IgG (3 μg/ml). After being washed in phosphate glucose buffer, the cells were treated on ice for 45 min with biotinylated anti-mouse NK1.1 followed by incubation on ice for 45 min with FITC-conjugated streptavidin. Control cells were incubated only with FITC-conjugated streptavidin. After additional washing, the cells were simultaneously fixed and permeabilized with CytoFix/CytoPerm solution on ice for 1 h. After four washes with Perm/Wash buffer, the cells were stained with Cy-Chrome-conjugated anti-mouse CD3ɛ. Control cells were incubated with Cy-Chrome-conjugated IgG. The cells were then washed and incubated with PE-conjugated anti-mouse IFN-γ on ice for 45 min. Control cells were stained with PE-conjugated IgG in a similar fashion. The cells were again washed with Perm/Wash buffer and analyzed on a Beckman Coulter (Hialeah, Fla.) model MCL-XL four-color flow cytometer equipped with a 488-nm diode laser. The lymphocyte populations were first gated based on forward and side light scatter. For three-color analysis, lymphocytes were further gated based on intracellular IFN-γ expression, and surface phenotypes were determined. The data were analyzed with System II data acquisition and analysis software (version 3.0; Beckman Coulter).
For the detection of NK T cells with specificity for α-GalCer-CD1d, brefeldin-treated cells were first washed twice with ice-cold fluorescence-activated cell sorting buffer (PBS, 0.5% BSA, 0.05% NaN3). Nonspecific binding sites were sequentially blocked with anti-mouse CD16/CD32, normal rat IgG, normal hamster IgG, and neutravidin on ice for 15 min. After being washed, the cells were stained with anti-NK1.1 as described above. Then they were incubated at room temperature for 20 min with either PE-conjugated α-GalCer-CD1d tetramer or PE-conjugated CD1d tetramer lacking α-GalCer. The cells were treated with CytoFix/CytoPerm for 1 h, washed four times, and stained with Cy-Chrome-conjugated anti-CD3ɛ on ice for 45 min.
Thiol depletion and measurement of tissue glutathione.
The use of DEM to deplete intracellular thiols in vivo has been described previously (38, 50). Briefly, mice were injected i.p. with either sesame oil or DEM (5.3 mmol/kg of body weight) prepared in sesame oil 2 h prior to bacterial challenge. At the time of sacrifice, a sample of liver tissue was frozen in liquid nitrogen and stored at −70°C until assay. Tissue digests were prepared with the aid of a Polytron (Brinkman Instruments, Westbury, N.Y.), and protein-free extracts were prepared as previously described (50). Total tissue glutathione concentrations (reduced plus oxidized) were measured by the kinetic recycling assay described by Tietze (48), and the results were expressed as nanomoles of glutathione per milligram of protein.
Statistical analysis.
Student's t test was used to determine whether there were significant differences between groups (P < 0.01).
RESULTS
IFN-γ responses in the mouse to E. coli challenge.
Mice were injected i.p. with various doses of viable E. coli O111:B4 bacteria, and their sera were collected 6 h later. Serum IFN-γ levels increased in a dose-dependent manner following bacterial challenge (Fig. 1A). A dose of 1 × 107 to 5 × 107 CFU of bacteria elicited serum IFN-γ responses comparable to those induced by 10 to 100 μg of E. coli LPS and was adopted as the standard dose range for all subsequent experiments. IFN-γ responses reached maximum levels between 6 and 12 h (Fig. 1B). Therefore, for all experiments reported here cellular responses were measured 6 h after bacterial challenge.
FIG. 1.
Dose- and time-dependent expression of IFN-γ following E. coli challenge. (A) Mice (two per group) were injected i.p. with the indicated quantities of E. coli O111:B4 bacteria or LPS, and serum IFN-γ concentrations were measured 6 h later. This experiment was repeated with similar results. (B) Mice (three per group) were challenged i.p. with 100 μg of LPS, and their sera were collected at the indicated times. The serum IFN-γ concentration was measured by enzyme-linked immunosorbent assay. A similar time course was obtained with mice challenged with viable E. coli bacteria.
We next determined the tissues in which IFN-γ production occurred by isolating lymphocytes from the livers, spleens, lymph nodes, thymi, blood, and peritoneal cavities of E. coli-challenged mice and enumerating cells expressing intracellular cytokines by flow cytometry. Cells were isolated as described in Materials and Methods and treated with brefeldin A to inhibit cytokine secretion. Intracellular IFN-γ was detected by using PE-conjugated rat anti-mouse IFN-γ, and the staining was compared to that for control cells treated with PE-conjugated rat IgG. As shown in Fig. 2A and B, lymphocytes from the liver contained the greatest percentage of IFN-γ-producing cells, with nearly 10% of the cells expressing intracellular cytokine. The typical liver yielded 5 × 106 to 10 × 106 lymphocytes, meaning that each liver contained approximately 5 × 105 to 10 × 105 IFN-γ-expressing cells 6 h after challenge. A much lower percentage of cytokine-expressing cells was found among splenic lymphocytes, although the total number of cytokine-producing cells in this organ (approximately 106) was comparable to that in the liver. Peritoneal exudates, lymph nodes, thymi, and peripheral blood contained relatively few IFN-γ+ cells.
FIG. 2.
Distribution of IFN-γ-expressing cells among various tissues following i.p. challenge with E. coli bacteria. Mice were injected i.p. with 5 × 107 CFU of E. coli and sacrificed 6 h later. Livers, lymph nodes (LN), spleens, and peritoneal exudate cells (PEC) were then recovered, and suspensions of lymphocytes were prepared as described in Materials and Methods. Intracellular IFN-γ expression was measured by flow cytometry. The results for a representative animal are shown in panel A. The data (means ± standard deviations) for three mice are shown in panel B. The IFN-γ responses of liver lymphocytes were significantly different from those of the cells from the other tissues (P < 0.01). The expression of CD3 by IFN-γ+ cells in the liver and spleen of a representative E. coli-challenged mouse is shown in panel C.
Several observations indicated that the IFN-γ-expressing cells recovered from the liver were not simply blood-borne cells circulating through the organ at the time of tissue recovery. Extensive perfusion of the liver through the portal vein did not change the number of recoverable hepatic lymphocytes or the proportion of cells staining positive for either intracellular IFN-γ (5.7% ± 1.9% without perfusion and 5.5% ± 4.0% with perfusion), surface CD3 (50.7% ± 4.4% without perfusion and 50.1% ± 10.7% with perfusion), or a pan-NK marker (data not shown). Conversely, blood lymphocytes either obtained by exsanguination or isolated from the liver perfusates of E. coli-challenged mice did not contain detectable IFN-γ+ cells. While 90% of blood lymphocytes were CD3+, only 50% of hepatic lymphocytes expressed CD3. For these reasons, we concluded that hepatic lymphocytes were distinct from blood lymphocytes, and for all subsequent experiments we isolated hepatic cells without prior perfusion of the livers.
Phenotypes of hepatic lymphocytes that produce IFN-γ in response to E. coli challenge.
Lymphocytes were isolated from the livers and spleens of E. coli-challenged mice, treated with brefeldin A to block cytokine secretion, fixed, and permeabilized. They were then stained with fluorochrome-conjugated antibodies to CD3 and IFN-γ and analyzed by flow cytometry. A comparison of the cells from these two organs indicated that nearly half of the IFN-γ+ cells from the spleen expressed CD3, whereas the majority of the hepatic IFN-γ response was mediated by CD3− cell subsets (Fig. 2C). These findings probably reflect the relative abundance of NK cells and NK T cells in the mouse liver compared to the spleen (13, 14, 18, 35).
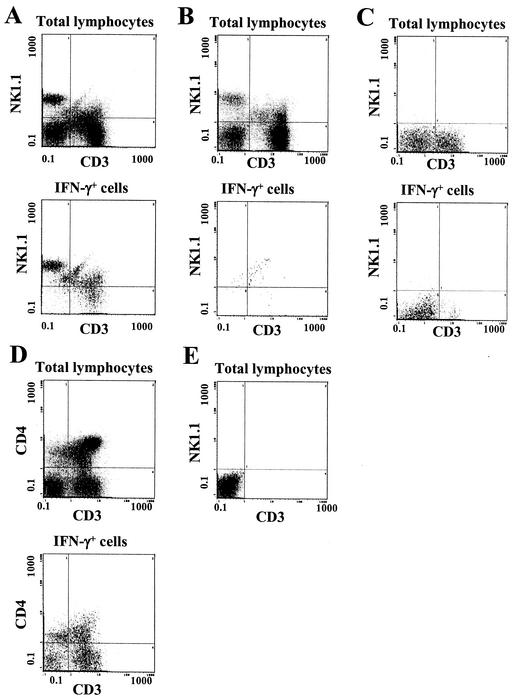
A further analysis of hepatic cell subsets was then undertaken. The entire lymphocyte population was first gated by light scatter parameters, and then two-color measurement of surface markers was performed (Fig. 3A, upper panel). Approximately 25% of the total cells had an NK1.1+ CD3− phenotype, while nearly 40% expressed a conventional T-cell phenotype (NK1.1− CD3+) (Fig. 4). A smaller group of cells bore both NK1.1 and CD3 and appeared to consist of two distinct subsets. When this analysis was performed on cells that had first been gated for the expression of intracellular IFN-γ, the majority of the cytokine-producing cells were found to be NK cells (NK1.1+ CD3−) (Fig. 3A, lower panel). However, 10 to 15% of the IFN-γ-positive cells had an NK T-cell phenotype (NK1.1+ CD3+) (Fig. 4). Again, this double-positive group of IFN-γ-expressing cells appeared to consist of two subsets based on the intensity of CD3 staining. Approximately half of the total NK cells and NK T cells expressed intracellular IFN-γ in response to E. coli challenge, whereas only a small percentage of the total T cells stained for intracellular cytokine. Of the NK1.1+ CD3−, NK1.1+ CD3+, and NK1.1− CD3+ lymphocyte subsets, 53.8 ± 14.8, 45.6 ± 13.7, and 5.0% ± 13.7%, respectively, expressed intracellular IFN-γ.
FIG. 3.
Phenotypes of hepatic lymphocytes that produce IFN-γ in response to E. coli challenge. Mice were injected i.p. with E. coli bacteria, and their hepatic lymphocytes were prepared 6 h later, treated with brefeldin A, and analyzed by flow cytometry. (A) Expression of CD3 and NK1.1 by various hepatic lymphocyte subsets of a representative E. coli-challenged B6 mouse. (Upper panel) Total hepatic lymphocytes after gating by light scatter; (lower panel) cells gated for the expression of intracellular IFN-γ. (B) Phenotypes of total or IFN-γ-expressing cells from a normal B6 mouse that had not been challenged with bacteria. (C) Analysis of the phenotypes of total and IFN-γ-expressing cells from a representative C3HeB/FeJ mouse, which lacks the NK1.1 allele. (D) Expression of CD3 and CD4 among total and IFN-γ-expressing hepatic lymphocytes of a representative B6 mouse. (E) Lack of staining of B6 hepatic lymphocytes in the absence of primary antibodies.
FIG. 4.
Percentages (means ± standard deviations) of total hepatic lymphocytes or hepatic IFN-γ-expressing lymphocytes with the indicated phenotypes (n = 13 mice from four experiments).
A number of control assays were performed to ensure that these results reflected specific antibody staining. For example, the data shown in Fig. 3B (lower panel) demonstrate that lymphocytes present in the livers of healthy, unchallenged B6 mice did not stain with anti-IFN-γ. Similarly, cells from strains of mice which did not express the NK1.1 allele (e.g., C3H) (Fig. 3C) did not evidence any NK1.1 staining when either the total hepatic lymphocytes (upper panel) or the IFN-γ+ lymphocytes (lower panel) were analyzed. Hepatic lymphocyte populations that were treated with anti-CD4 plus anti-CD3 (Fig. 3D) showed patterns of staining that were different from those of cells treated with anti-NK1.1 plus anti-CD3 (Fig. 3A). Cells not exposed to these primary antibodies showed no significant staining (Fig. 3E).
Effects of thiol depletion on hepatic lymphocyte IFN-γ responses to E. coli.
As a means of altering tissue redox status, B6 mice were injected i.p. with either vehicle (sesame oil) or various doses of the thiol-reactive compound DEM. Two hours later the mice were challenged i.p. with E. coli bacteria, and their livers were removed 6 h postchallenge. Total hepatic glutathione concentrations were determined, and the frequencies and surface phenotypes of hepatic IFN-γ+ lymphocytes were determined for each treatment group.
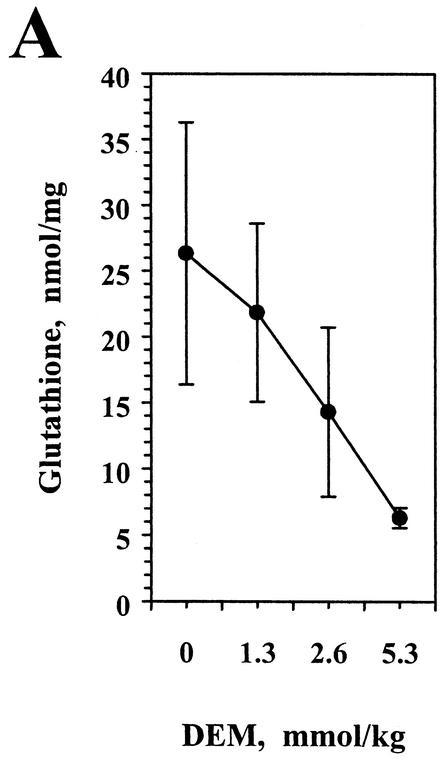
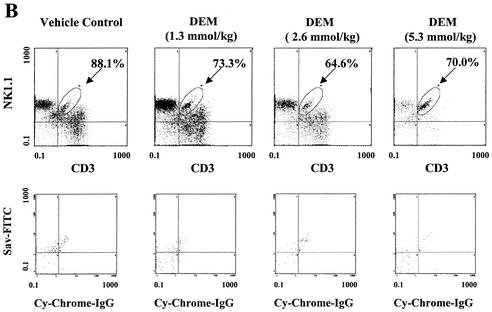
Tissue glutathione concentration is a sensitive indicator of overall intracellular thiol status. As reported previously (38), pretreatment with the thiol-reactive compound DEM resulted in decreased hepatic glutathione levels (Fig. 5A) in E. coli-challenged mice. Thiol depletion also significantly reduced the percentage of hepatic lymphocytes expressing intracellular IFN-γ (Fig. 5B, upper panels). Of interest, this effect was associated with a decrease in the expression of IFN-γ by NK cells, conventional T cells, and a portion of the NK T-cell population. However, a subset of hepatic NK T cells was relatively resistant to the effects of DEM treatment (circled populations, Fig. 5B). Thus, while the majority of the IFN-γ-expressing cells in the livers of control mice following E. coli challenge were NK cells, a subset of NK T cells constituted a large fraction of the hepatic IFN-γ+ cells in DEM-treated, E. coli-challenged mice. By analysis of the percentage of each lymphocyte subset that expressed IFN-γ (Fig. 5C), it was apparent that the CD3-dull (i.e., weakly staining) NK T-cell subset retained its ability to express the cytokine after treatment with DEM. When the effects of DEM treatment on IFN-γ expression by CD4+ NK1.1+ and CD8+ NK1.1+ cells were analyzed, thiol depletion significantly inhibited cytokine production only in the CD4 NK T-cell subset (32% positive versus 11% positive; P < 0.01). IFN-γ expression by CD8+ NK1.1+ cells was not significantly changed (64% positive versus 46% positive; P > 0.05). It should be noted that thiol depletion did not alter the number of lymphocytes recovered from the liver or the relative proportions of the total cells that expressed each of the surface phenotypes (data not shown).
FIG. 5.
Thiol depletion differentially inhibits IFN-γ production by hepatic lymphocyte subsets. Mice were injected i.p. with the indicated doses of DEM and 2 h later were challenged with E. coli bacteria. Liver lymphocytes were prepared 6 h later, treated with brefeldin A, and stained for intracellular IFN-γ and surface NK1.1 and CD3 expression. (A) Total glutathione content was measured for each liver 6 h postchallenge. The glutathione concentration for the group treated with the highest dose of DEM was significantly different from those for the other treatment groups (P < 0.01). (B) Three-color flow cytometry for a representative mouse in which IFN-γ+ cells were gated and analyzed for NK1.1 and CD3 expression (upper panels). The percentage of IFN-γ+ cells that fell within this gated area for the group receiving 5.3 mmol of DEM/kg was significantly different from those for the other treatment groups (P < 0.01). The double-positive subset expressing relatively low CD3 was then gated (circles), and the percentage of these cells expressing IFN-γ is indicated in each case. The lower panels represent staining in the absence of primary antibodies. (C) The percentage (mean ± standard deviation) of IFN-γ-expressing cells within each subset was determined for each treatment group (four mice per group). The data labeled NK1.1+ CD3+ represent only those double-positive cells that are circled in panel B. This experiment was performed twice with similar results.
Receptor specificity of IFN-γ-expressing NK T cells.
Many, but not all, NK T cells bear CD1d-restricted TCRs specific for the glycolipid α-GalCer (3-5, 40, 46). To determine whether the mouse hepatic NK T cells that produced IFN-γ in response to E. coli challenge expressed α-GalCer-CD1d-specific receptors, cells were stained with fluorochome-conjugated anti-NK1.1, anti-CD3, and α-GalCer-CD1d tetramers. Multicolor flow cytometry was used to identify cells from E. coli-challenged control mice that expressed both NK1.1 and CD3 (left panel, Fig. 6A), and the two double-positive subsets were gated and individually analyzed for α-GalCer-CD1d tetramer binding (center panels, Fig. 6A). Cells of subset A, which expressed relatively low levels of CD3, did not bind α-GalCer-CD1d tetramers any more than they bound control CD1d tetramers lacking α-GalCer. By contrast, many of the cells of subset B, which expressed relatively high levels of CD3, bore TCRs capable of binding α-GalCer-CD1d. Both subset A and subset B from these control animals expressed intracellular IFN-γ following bacterial challenge (right panel, Fig. 6A).
FIG. 6.
Restriction specificity of NK T cells activated for IFN-γ expression by E. coli challenge. Shown are hepatic lymphocytes from two representative mice of a single experiment. The mice were treated with either vehicle (A) or DEM (B) and then challenged with E. coli bacteria. (Left) Surface phenotypes of the total lymphocyte populations, including two subsets of double-positive cells (subsets A and B); (center) binding of α-GalCer-CD1d tetramers by these two NK T-cell subsets; (right) intracellular IFN-γ staining of the various subsets. This experiment was performed four times with similar results.
Hepatic lymphocytes isolated from mice that had been treated with DEM prior to E. coli challenge showed similar patterns of NK1.1 and CD3 staining (left panel, Fig. 6B). A comparison of α-GalCer-CD1d tetramer binding (center panels) and intracellular IFN-γ expression (right panel) again indicated that a population of double-positive cells existed that retained the ability to synthesize IFN-γ following thiol depletion and bacterial challenge. This subset did not bind α-GalCer-CD1d tetramers, indicating that it did not have a classical NK T-cell phenotype.
A similar analysis was performed to determine whether the redox-resistant subset of NK T cells expressed γδ T-cell antigen receptors. While NK1.1+ TCRγδ+ cells constituted a minor subset of the total liver lymphocytes, approximately 50% of these cells expressed IFN-γ in response to bacterial challenge (Fig. 7). While treating mice with DEM prior to challenge decreased IFN-γ expression by NK cells (NK1.1+ TCRγδ−), it did not significantly diminish the expression of IFN-γ by TCRγδ+ NK T cells. Likewise, TCRγδ+ conventional T cells retained the ability to express the cytokine despite thiol depletion.
FIG. 7.
IFN-γ production by cells bearing TCRγδ receptors is resistant to the effects of thiol depletion. B6 mice were treated with vehicle or DEM and then challenged with bacteria. Hepatic lymphocytes expressing intracellular IFN-γ were identified by flow cytometry as described in the Fig. 3 legend, and the surface expression of NK1.1 and TCRγδ was determined. Shown are the percentages of each hepatic lymphocyte subset from the two treatment groups that expressed intracellular IFN-γ. This experiment was performed twice with similar results. The responses of NK cells from DEM-treated mice were significantly different from those of NK cells from vehicle-treated mice (P < 0.01).
DISCUSSION
The present study was designed to characterize the phenotypes of lymphocytes within the mouse liver that produce IFN-γ in response to viable gram-negative bacteria and determine the effects of oxidative stress on the responses of each cell subset. The liver was of particular interest to us for a number of reasons. Numerous IFN-γ-expressing cells were found in this organ, and sufficient numbers of NK cells, NK T cells, and conventional T cells were present to permit characterization of the effects of redox imbalance on the responses of each of these subsets. The liver is especially susceptible to tissue redox imbalance during E. coli infection in this model (M. J. Parmely, M. Greenblatt, and G. Zhang, unpublished data) and redox-dependent changes in gene expression (38, 50). While the total number of IFN-γ-producing cells in the spleen following bacterial challenge was comparable to that found in the liver, the frequency of IFN-γ-producing cells that belonged to certain subsets (e.g., NK cells and NK T cells) was considerably lower, which precluded a similar analysis of the effects of oxidative stress on their activation.
The liver also plays a central role in innate immune responses to gram-negative bacterial infections. For example, the organ is recognized as a major source of early inflammatory cytokines, such as TNF-α (14, 24, 43). Recently, many of the innate immune functions of hepatic lymphocytes have also been characterized (18, 24, 43, 49). Both rodent and human livers contain significant numbers of T cells, NK cells, and NK T cells (13, 14, 18, 34, 35), which produce early inflammatory cytokines in response to infection or challenge with microbial products (16, 36, 39). The majority of the IFN-γ produced in mice challenged with bacterial LPS is synthesized by lymphocytes bearing NK cell markers (12, 33, 36, 42, 45), and the liver appears to be one of the richest sources of these cells.
Both the human and mouse livers are rich in NK T cells, which coexpress the TCR-CD3 complex and NK cell markers (4, 13, 18, 35). The NK T-cell population is itself heterogeneous (4, 15, 27, 29, 35). A large portion of mouse NK T cells express a common αβ TCR encoded by an invariant Vα14-Jα281 gene segment and a restricted pool of Vβ gene segments (6, 26, 45). These relatively invariant Vα14 NK T cells are thought to recognize primarily nonpeptide antigens presented by the nonpolymorphic major histocompatibility complex class I-like molecule CD1d. Although the physiological ligands for their receptors are not known, Vα14 NK T cells are often defined by their binding of tetramers composed of the glycolipid α-GalCer and CD1d (23, 46). In response to TCR ligation with antibodies, NK T cells can produce both IFN-γ and interleukin-4 (IL-4) (2, 3, 10, 13, 52), although some cell-activating agents have been reported to skew the IL-4-IFN-γ balance of cytokine secretion (8, 44).
The present study is the first to show that NK1.1+ cells, which include conventional NK cells and NK T cells, are the predominant IFN-γ-producing cells in the mouse liver following an acute challenge with viable E. coli bacteria. Approximately half of all hepatic NK cells and NK T cells were activated for IFN-γ expression by bacterial challenge, and over 90% of a subset of NK T cells, which did not bind α-GalCer-CD1d, expressed the cytokine. Recently, Baron et al. (3) described a similar subset of nonclassical hepatic NK T cells in mice challenged with hepatitis B virus envelope antigens that mediated acute viral hepatitis. In the present study, a CD3-dull NK T-cell subset was detected in the livers of E. coli-challenged mice that was not as apparent in the healthy mouse liver, suggesting that bacterial challenge may have led to the recruitment of these cells from an extrahepatic site(s). Of interest, this subset retained the ability to synthesize IFN-γ under prooxidant tissue conditions (i.e., after acute thiol depletion) and also appeared to express a CD8+ TCRγδ+ phenotype.
Hepatic NK and NK T cells have been shown elsewhere to mediate both protective immune responses and tissue damage in animal infection models (3, 21, 36, 39, 42). Seki et al. (43) showed that anti-NK1.1 treatment inhibited IFN-γ production by mouse hepatic lymphocytes in response to polymicrobial peritonitis. Ishigami et al. (21) reported that Jα281−/− mice lacking invariant NK T cells showed decreased liver injury in response to salmonella challenge compared to wild-type mice. A similar finding has recently been reported with a mouse virus hepatitis model (3).
An imbalance in the normal redox status of tissues is often observed during infections and acute inflammatory responses, and the mouse liver appears to be particularly sensitive to this effect. This probably reflects the high level of reactive oxygen and/or nitrogen species that are produced in the liver and the resulting depletion of antioxidants. Although the mechanism by which oxidative and nitrosative stress (28) affects gene expression in these models is not well characterized, redox control of transcription initiation is a likely target (1, 28). Among the most often reported effects of altered cellular redox balance on gene expression are modifications in the activities of the transcription factors NF-κB, AP-1, p53, and Stat, all of which play important roles in regulating the expression of early inflammatory genes.
To understand the effects of redox imbalance in infection models, a number of investigators have characterized responses to microbial challenge in thiol-depleted animals (30, 38, 50). Treating mice with either DEM or buthionine sulfoximine, an inhibitor of glutathione biosynthesis, inhibited the production of TNF-α and IFN-γ in response to either bacterial or LPS challenge. The LPS-stimulated animals also showed decreased expression of hepatic ICAM-1 and iNOS (38, 50), but exogenous IFN-γ restored these responses to LPS (38). These findings suggest that the inhibition of IFN-γ production by oxidative or nitrosative stress is a key event in regulating early inflammatory gene expression during gram-negative bacterial infections.
We do not believe that the effects of oxidative stress on IFN-γ production in the liver are due to the depression of IL-12 production. While serum IFN-γ responses were inhibited by approximately 90% in mice whose tissue glutathione was depleted prior to challenge (38), the serum IL-12 concentrations in the same animals were only diminished by half compared to control, challenged mice. Likewise, exogenous recombinant IL-12 did not restore IFN-γ responses in thiol-depleted, E. coli-challenged mice (G. Zhang and M. J. Parmely, unpublished data).
In the present study, treating mice with DEM significantly inhibited E. coli-induced IFN-γ production by three of the principal hepatic cell subsets, NK cells, T cells, and Vα14 NK T cells. However, a novel subset of NK T cells with an NK1.1+ CD3dim phenotype expressed the cytokine following bacterial challenge despite thiol depletion. This probably explains the relatively small but consistent IFN-γ responses reported previously for DEM-treated, E. coli-challenged animals (38). Because CD8+ NK1.1+ cells, but not CD4+ NK1.1+ cells, also retained the ability to express IFN-γ after thiol depletion, it is tempting to conclude that this novel NK T-cell population is also CD8+. The liver contains a range of heterogeneous overlapping lymphocyte subsets, including TCRγδ+ NK T cells (28, 35). Preliminary results reported here suggest that hepatic lymphocytes in thiol-depleted mice that respond to bacterial challenge bear γδ T-cell antigen receptors. Although the identity of each of the lymphocyte subsets responding to bacterial challenge has not been firmly established, it is clear from these findings that redox imbalance differentially regulates their activation during infection. This suggests that the signaling pathways utilized by hepatic lymphocytes are diverse and not coordinately regulated by the redox status of their tissues.
Acknowledgments
This work was supported by American Heart Association grant 51345Z and the Margaret Jane Harley Fund.
We thank Bill Justice for his assistance with flow cytometry.
Editor: J. T. Barbieri
REFERENCES
- 1.Allen, R. G., and M. Tresini. 2000. Oxidative stress and gene regulation. Free Radic. Biol. Med. 28:463-499. [DOI] [PubMed] [Google Scholar]
- 2.Arase, H., N. Arase, and T. Saito. 1996. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. [DOI] [PubMed] [Google Scholar]
- 4.Behar, S. M., and S. Cardell. 2000. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Immunology 12:551-560. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac, A., M. N. Rivera, S.-H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 6.Benlagha, K., and A. Bendelac. 2000. CD1d-restricted mouse Vα14 and human Vα24 T cells: lymphocytes of innate immunity. Semin. Immunol. 12:537-542. [DOI] [PubMed] [Google Scholar]
- 7.Bevilacqua, M. P. 1993. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 11:767-804. [DOI] [PubMed] [Google Scholar]
- 8.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 9.Car, B. C., V. M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon γ receptor deficient mice are resistant to endotoxin shock. J. Exp. Med. 179:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H., and W. E. Paul. 1997. Cultured NK1.1+CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J. Immunol. 159:2240-2249. [PubMed] [Google Scholar]
- 11.Cotran, R. S., and J. S. Pober. 1990. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J. Am. Soc. Nephrol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 12.Dobashi, H., S. Seki, Y. Habu, T. Ohkawa, S. Takeshita, H. Hiraide, and I. Sekine. 1999. Activation of mouse liver natural killer cells and NK1.1+ T cells by bacterial superantigen-primed Kupffer cells. Hepatology 30:430-436. [DOI] [PubMed] [Google Scholar]
- 13.Doherty, D. G., S. Norris, L. Madrigal-Estebas, G. McIntee, O. Traynor, J. E. Hegarty, and C. O'Farrelly. 1999. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2 and Th0 cytokine secretion patterns. J. Immunol. 163:2314-2321. [PubMed] [Google Scholar]
- 14.Doherty, D. G., and C. O'Farrelly. 2000. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174:5-20. [DOI] [PubMed] [Google Scholar]
- 15.Eberl, G., R. Lees, S. T. Smiolery, M. Taniguchi, M. J. Grusby, and H. R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410-6419. [PubMed] [Google Scholar]
- 16.Emoto, M., Y. Emoto, I. B. Buchwalow, and S. H. E. Kaufmann. 1999. Inductin of IFN-γ-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette-Guerin. Eur. J. Immunol. 29:650-659. [DOI] [PubMed] [Google Scholar]
- 17.Evans, T., A. Carpenter, A. Silva, and J. Cohen. 1992. Differential effects of monoclonal antibodies to tumor necrosis factor alpha and gamma interferon on induction of hepatic nitric oxide synthase in gram-negative sepsis. Infect. Immun. 60:4133-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata, K., X. R. Zhang, S. Iwatsuki, D. H. Van Thiel, R. B. Herberman, and T. L. Whiteside. 1990. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin. Immunol. Immunopathol. 56:401-419. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel, F. P. 1990. The role of IFN-γ in the pathology of experimental endotoxemia. J. Immunol. 145:2920-2924. [PubMed] [Google Scholar]
- 20.Heremans, H., J. Van Damme, C. Dillen, R. Dijkmans, and A. Billiau. 1990. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J. Exp. Med. 171:1853-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishigami, M., H. Nishimura, Y. Naiki, K. Yoshioka, T. Kawano, Y. Tanaka, M. Taniguchi, S. Kakumu, and Y. Yoshikai. 1999. The roles of intrahepatic Vα14+ NK1.1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology 29:1799-1808. [DOI] [PubMed] [Google Scholar]
- 22.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Tanaguchi. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 24.Kmiec, Z. 2001. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 161:1-151. [DOI] [PubMed] [Google Scholar]
- 25.Kohler, J., D. Heumann, G. Garotta, D. LeRoy, S. Bailat, C. Barras, J.-D. Baumgartner, and M. P. Glauser. 1993. IFN-γ involvement in the severity of Gram-negative infections in mice. J. Immunol. 151:916-921. [PubMed] [Google Scholar]
- 26.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees, R. K., I. Ferrero, and H. R. MacDonald. 2001. Tissue-specific segregation of TCRγδ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR γβ+ NKT cells. Eur. J. Immunol. 31:2901-2909. [DOI] [PubMed] [Google Scholar]
- 28.Marshall, H. E., K. Merchant, and J. S. Stamler. 2000. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 14:1889-1900. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda, J. L., O. V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.-R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matuschak, G. M., C. A. Johanns, Z. Chen, J. Gaynor, and A. J. Lechner. 1996. Brief hypoxic stress downregulates E. coli-induced IL-1α and IL-1β gene expression in perfused liver. Am. J. Physiol. 271:R1311-R1318. [DOI] [PubMed] [Google Scholar]
- 31.Nathens, A. B., R. Biaar, R. W. G. Watson, T. B. Issekutz, J. C. Marshall, A. P. B. Dackiw, and O. D. Rotstein. 1998. Thiol-mediated regulation of ICAM-1 expression in endotoxin-induced acute lung injury. J. Immunol. 160:2959-2966. [PubMed] [Google Scholar]
- 32.Nemoth, I., and D. Boda. 2001. Xanthine oxidase activity and blood glutathione redox ratio in infants and children with septic shock syndrome. Intensive Care Med. 27:216-221. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, K. B., and C. A. Biron. 1999. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-γ production. J. Immunol. 162:5238-5246. [PubMed] [Google Scholar]
- 34.Norris, S., C. Collins, D. G. Doherty, F. Smith, G. McEntee, O. Traynor, N. Nolan, J. Hegarty, and C. O'Farrelly. 1998. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J. Hepatol. 28:84-90. [DOI] [PubMed] [Google Scholar]
- 35.Norris, S., D. G. Doherty, C. Collins, G. McEntee, O. Traynor, J. E. Hegarty, and C. O'Farrelly. 1999. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Vα24-JαQ and γδ T cell receptor bearing cells. Hum. Immunol. 60:20-31. [DOI] [PubMed] [Google Scholar]
- 36.Ogasawara, K., K. Takeda, W. Hashimoto, M. Satoh., R. Ookuyama, N. Yanai, M. Obinata, K. Kumagai, H. Takada, H. Hiraide, and S. Seki. 1998. Involvement of NK1+ T cells and their IFN-γ production in the generalized Shwartzman reaction. J. Immunol. 160:3522-3527. [PubMed] [Google Scholar]
- 37.Pacht, E. R., A. P. Timerman, M. G. Lykens, and A. J. Merola. 1991. Deficiency of alveolar fluid glutathione in patients with sepsis and ARDS. Chest 100:1397-1403. [DOI] [PubMed] [Google Scholar]
- 38.Parmely, M. J., F. Wang, and D. Wright. 2001. Gamma interferon prevents the inhibitory effects of oxidative stress on host responses to Escherichia coli infection. Infect. Immun. 69:2621-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pien, G. C., and C. A. Biron. 2000. Compartmental differences in NK cell responsiveness to IL-12 during lymphocyte choriomeningitis virus infection. J. Immunol. 164:994-1001. [DOI] [PubMed] [Google Scholar]
- 40.Porcelli, S. A., and R. L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297-329. [DOI] [PubMed] [Google Scholar]
- 41.Salkowski, S. A., G. Detore, R. McNally, R. van Rooijen, and S. N. Vogel. 1997. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo. J. Immunol. 158:905-912. [PubMed] [Google Scholar]
- 42.Seki, S., S. I. Osada, S. Ono, S. Aosasa, Y. Habu, T. Nishikage, M. Homchizuki, and H. Hiraide. 1998. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect. Immun. 66:5286-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seki, S., Y. Habu, T. Kawamura, K. Takeda, H. Dobashi, T. Ohkawa, and H. Hiraide. 2000. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 174:35-46. [DOI] [PubMed] [Google Scholar]
- 44.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 45.Takahashi, M., K Ogasawara, K. Takeda, W. Hashimoto, H. Sakihara, K. Kumagai, R. Anzai, M. Satoh, and S. Seki. 1996. LPS induces NK-1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 156:2436-2442. [PubMed] [Google Scholar]
- 46.Taniguchi, M., and T. Nakayama. 2000. Recognition and function of Vα14 NKT cells. Semin. Immunol. 12:543-550. [DOI] [PubMed] [Google Scholar]
- 47.Taylor, D. E., A. J. Ghio, and C. A. Piantodosi. 1995. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch. Biochem. Biophys. 316:70-76. [DOI] [PubMed] [Google Scholar]
- 48.Tietze, F. 1969. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27:502-522. [DOI] [PubMed] [Google Scholar]
- 49.Trobonjaca, Z., F. Leithäuser, P. Möller, R. Schirmbeck, and J. Reinmann. Activating immunity in the liver. I. Liver dendritic cells (but no hepatocytes) are potent activators of IFN-γ release by liver NKT cells. J. Immunol. 167:1413-1422. [DOI] [PubMed]
- 50.Wang, F., L. Y. Wang, D. Wright, and M. J. Parmely. 1999. Redox imbalance differentially inhibits lipopolysaccharide-induced macrophage activation in the mouse liver. Infect. Immun. 67:5409-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wibbenmeyer, L. A., A. J. Lechner, C. F. Munoz, and G. M. Matuschak. 1995. Downregulation of E. coli-induced TNF-α expression in perfused liver by hypoxia-reoxygenation. Am. J. Physiol. 268:G311-G319. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W. E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science 270:1845-1847. [DOI] [PubMed] [Google Scholar]