Abstract
Haemophilus ducreyi 35000HP contains two genes, lspA1 and lspA2, whose predicted protein products have molecular weights of 456,000 and 543,000, respectively (C. K. Ward, S. R. Lumbley, J. L. Latimer, L. D. Cope, and E. J. Hansen, J. Bacteriol. 180:6013-6022, 1998). We have constructed three H. ducreyi 35000HP mutants containing antibiotic resistance cartridges in one or both of the lspA1 and lspA2 open reading frames. Western blot analysis using LspA1- and LspA2-specific monoclonal antibodies indicated that the wild-type parent strain 35000HP expressed LspA1 protein that was readily detectable in culture supernatant fluid together with a barely detectable amount of LspA2 protein. The lspA2 mutant 35000HP.2 expressed LspA1 protein that was detectable in culture supernatant fluid and no LspA2 protein. In contrast, the H. ducreyi lspA1 mutant 35000HP.1, which did not express the LspA1 protein, expressed a greater quantity of the LspA2 protein than did the wild-type parent strain. The lspA1 lspA2 double mutant 35000HP.12 expressed neither LspA1 nor LspA2. The three mutant strains adhered to human foreskin fibroblasts and to a human keratinocyte cell line in vitro at a level that was not significantly different from that of the wild-type strain 35000HP. Lack of expression of the LspA1 protein by both the lspA1 mutant and the lspA1 lspA2 double mutant was associated with an increased tendency to autoagglutinate. When evaluated in the temperature-dependent rabbit model for chancroid, the lspA1 lspA2 double mutant was substantially less virulent than the wild-type strain 35000HP. The results of these studies indicated that H. ducreyi requires both the LspA1 and LspA2 proteins to be fully virulent in this animal model for experimental chancroid.
Haemophilus ducreyi is the etiological agent of the sexually transmitted disease known as chancroid (58). Although the occurrence of this disease is rare in the United States, it is one of the leading causes of genital ulcer disease in some developing countries (58). The molecular mechanism(s) utilized by H. ducreyi to produce skin lesions has not been identified (52), although a number of putative virulence factors or mechanisms of this organism have been identified. These include several outer membrane proteins (23, 24, 53, 54), two toxins (5, 20, 42, 43), lipooligosaccharide (LOS) (14-16, 25, 28, 56), a copper-zinc superoxide dismutase (49, 50), resistance to phagocytosis (2, 62), and the ability to both attach to (4) and invade (57) human epithelial cells in vitro. To date, however, only H. ducreyi mutants lacking expression of the peptidoglycan-asso-ciated lipoprotein (26), the hemoglobin-binding outer membrane protein HgbA (7), and the DsrA outer membrane protein (12) have been shown to exhibit reduced virulence in the human challenge model for experimental chancroid.
We previously reported the identification of two extremely large open reading frames (ORFs), lspA1 and lspA2 (Lsp; large supernatant protein), whose predicted protein products have calculated masses of 456 and 543 kDa, respectively, and 86% identity (61). The H. ducreyi LspA1 and LspA2 proteins are 43% similar over their N-terminal half to the Bordetella pertussis filamentous hemagglutinin (FHA) (22, 46). The LspA1 and LspA2 proteins contain a central 260-amino-acid region with >70% identity to the Haemophilus somnus P76 protein, an immunoglobulin-binding protein (21) associated with the ability of this bovine pathogen to resist the complement-mediated bactericidal activity of bovine serum (17, 18). This same region of both LspA1 and LspA2 has some identity (36%) with the YopT cytotoxin of Yersinia enterocolitica (51, 63). The protein product of the lspA1 gene can be detected by Western blot analysis as a soluble antigen, with an apparent molecular weight greater than 250,000, that is present in concentrated culture supernatant fluid (CCS) from H. ducreyi 35000 as well as several other virulent H. ducreyi strains (61). In contrast, we were previously unable to detect reproducibly the LspA2 protein in CCS from several wild-type H. ducreyi strains, including strain 35000, even though the lspA2 gene of this latter strain is apparently transcribed both in vitro and in vivo (61).
To determine the relevance of the LspA1 and LspA2 proteins to the virulence potential of H. ducreyi, we constructed a set of H. ducreyi 35000HP strains with mutations in the lspA1 and lspA2 ORFs, and we examined the ability of these different mutants to attach to human cell lines in vitro, to resist the complement-mediated bactericidal activity of normal human serum, and to cause lesions in the temperature-dependent rabbit model for chancroid. We report here that an H. ducreyi mutant deficient in the production of both LspA1 and LspA2 was significantly less virulent than its wild-type parent strain in the temperature-dependent rabbit model for chancroid.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The H. ducreyi strains and plasmids used in this study are listed in Table 1. The human-passaged variant (35000HP) of strain 35000 (8) was used as the wild-type parent strain in this study. Wild-type H. ducreyi was routinely cultivated on chocolate agar (CA) plates at 33°C in a humidified atmosphere containing 5% CO2 as described previously (45). Mutant H. ducreyi strains were grown either on CA plates containing chloramphenicol (1.5 μg/ml), GC-heme agar plates (37) containing kanamycin (30 μg/ml), or CA plates containing both chloramphenicol (0.5 μg/ml) and kanamycin (30 μg/ml) as necessary. For some experiments, H. ducreyi strains were grown in a modified version of a Columbia broth-based medium, previously described for growing H. somnus (32), at 33 to 34°C in a water bath with agitation at 140 rpm. This modified medium (sCB) consisted of 35 g of Columbia broth (Difco Laboratories, Detroit, Mich.)/liter, 0.1% (wt/vol) Trizma base (Sigma Chemical Co., St. Louis, Mo.), 25 μg of equine hemin (Sigma Chemical Co.)/ml, 1% (vol/vol) IsoVitaleX (Becton Dickinson Microbiology Systems, Cockeysville, Md.), and 2.5% (vol/vol) heat-inactivated fetal bovine serum (JRH BioSciences, Lenexa, Kans.) (61). H. ducreyi CCS fluids were prepared as described previously (61). H. ducreyi cells harvested in the early stationary phase of growth were used in the adherence, autoagglutination, and serum bactericidal assays described below because this growth phase correlated with detectable expression of LspA1 in CCS of the wild-type parent strain.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| H. ducreyi strains | ||
| 35000HP | Wild-type strain previously passaged in a human volunteer | 8 |
| 35000HP.1 | Isogenic lspA1 mutant; kanr | This study |
| 35000HP.2 | Isogenic lspA2 mutant; chlorr | This study |
| 35000HP.12 | Isogenic lspA1 lspA2 mutant; kanr chlorr | This study |
| A77 | Serum-sensitive strain | 24 |
| Plasmids | ||
| pBluescript II KS(+) | Cloning vector; Ampr | Stratagene |
| pCW104 | pBluescript II KS(+) containing a 3.1-kb HindIII-KpnI fragment from pDad-5 with the 5′ portion of the lspA1 ORF | This study |
| pCW105 | pCW104 with the 1.0-kb HpaI fragment of lspA1 replaced by the 0.85-kb SmaI fragment (kan cartridge) from pUC18K3 | This study |
| pCW113 | pBluescriptII KS(+) with a 6.0-kb HindIII chromosomal DNA insert containing a central portion of lspA2 | 61 |
| pCW116 | pCW113 with the 1.47-kb BglII fragment from lspA2 replaced by the 1.36-kb BamHI fragment (cat cartridge) from pUCΔEcat | This study |
| pDad-5 | pBR322 with an 8.1-kb PstI insert containing the 5′ half of lspA1 | 61 |
| pUC18K3 | pUC18 containing the promoterless aphA-3 (kan) cartridge | James B. Kaper |
| pUCΔEcat | pUC19 containing a 1.4-kb cat cartridge | Bruce A. Green |
Escherichia coli XL1-Blue (Stratagene Corp., La Jolla, Calif.) was used as the host for general cloning manipulations. E. coli strains were grown in Luria-Bertani medium (48) supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (30 μg/ml) when appropriate for maintenance of plasmids. All plasmid constructs used to create isogenic H. ducreyi mutants were transformed into and isolated from E. coli HB101 prior to being used in the mutagenesis protocol (29).
DNA methods.
Plasmid purification, phenol-chloroform extractions, restriction enzyme digests, fill-in reactions using the Klenow fragment of DNA polymerase I and deoxynucleoside triphosphates, agarose gel electrophoresis, ligations, transformation of chemically competent E. coli strains, and Southern blot analysis were performed as described elsewhere (48). Nucleotide sequence analysis was performed using Big Dye Terminator chemistry and a model 373A automated DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.).
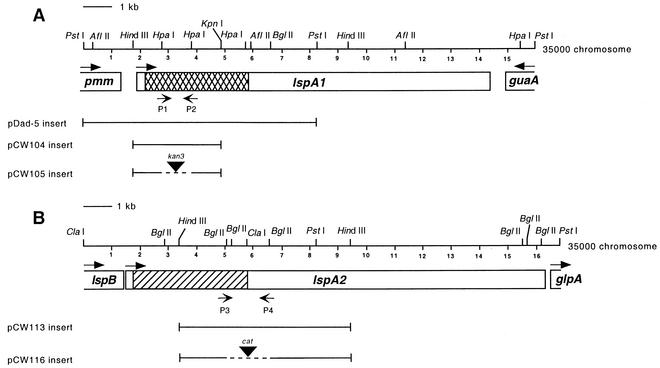
Construction of H. ducreyi mutants. (i) Construction of an lspA1 mutant.
A 3.1-kb HindIII-KpnI fragment of the H. ducreyi 35000 lspA1 gene (comprising nucleotides 1775 to 4836 of GenBank accession number AF057695) was subcloned from pDad-5 (Fig. 1A) (61) into pBluescript II KS(+) (Stratagene) that had been digested with HindIII and KpnI. The resultant plasmid, pCW104, was digested with HpaI to delete a 1.0-kb fragment of the lspA1 ORF and was blunt-end ligated to a 0.85-kb SmaI fragment of pUC18K3 containing a promoterless aphA-3 (kan) cartridge (39) to generate pCW105 (Fig. 1A). This particular kan cartridge (provided by James B. Kaper, University of Maryland) was designed for the construction of nonpolar insertion mutations (39). Nucleotide sequence analysis confirmed that this kan cartridge had been inserted properly into the lspA1 ORF. Plasmid pCW105 was propagated in E. coli HB101 and isolated as previously described (9). Plasmid pCW105 was linearized by digestion with PstI and purified by phenol-chloroform extraction followed by ethanol precipitation, and it was used to electroporate H. ducreyi 35000HP as previously described (29). H. ducreyi transformants were selected on GC-heme agar plates containing kanamycin (30 μg/ml), and one of these, 35000HP.1, was selected as the lspA1 mutant for use in this study.
FIG. 1.
Partial restriction endonuclease map of the H. ducreyi lspA1 (A) and lspA2 (B) loci and location of DNA inserts in recombinant plasmids used in this study. The cross-hatched boxes in the 5′ regions of the highly similar lspA1 and lspA2 ORFs indicate the only regions containing less than 60% identity. Unlabeled arrows above the boxes delineating the ORFs indicate the direction of transcription, whereas the labeled arrows below the boxes indicate the location of the PCR primers described in Materials and Methods. The dashed lines in pCW105 and pCW116 indicate DNA that had been deleted in these constructs. The lspA1 gene is flanked by genes encoding homologs of H. influenzae phosphomannomutase (pmm) and GMP synthase (guaA). The lspA2 gene is flanked by genes encoding homologs of B. pertussis fhaC (lspB) and H. influenzae anaerobic glycerol-3-phosphate dehydrogenase subunit A (glpA).
(ii) Construction of an lspA2 mutant.
Plasmid pCW113 (61) containing an internal 6-kb HindIII fragment of the lspA2 gene (nucleotides 3366 to 9329 of GenBank accession number AF057696) was digested with BglII to delete a 1.5-kb region of the lspA2 ORF (Fig. 1B). A 1.4-kb BamHI fragment containing the chloramphenicol acetyltransferase gene (cat) from pUCΔEcat (provided by Bruce A. Green, Wyeth-Lederle Vaccines and Pediatrics) was ligated into this site, and the resultant plasmid was designated pCW116. It is possible that insertion of this particular cat cartridge into an ORF may exert a polar effect. In this construct, the cat ORF was transcribed in the same direction as the lspA2 ORF. Plasmid pCW116 was linearized with SacI, purified, and used to electroporate strain 35000HP as described above. Transformants were selected on CA plates containing chloramphenicol (1.5 μg/ml), and one of these, 35000HP.2, was selected as the lspA2 mutant for use in this study.
(iii) Construction of an lspA1 lspA2 double mutant.
Plasmid pCW116 was linearized with SacI and was used to electroporate the H. ducreyi lspA1 mutant 35000HP.1 (described above). Transformants were selected on CA plates containing chloramphenicol (0.5 μg/ml) and kanamycin (30 μg/ml), and one of these, 35000HP.12, was selected as the lspA1 lspA2 mutant for use in this study.
PCR analysis.
Wild-type and mutant H. ducreyi strains were subjected to PCR analysis using Taq DNA polymerase (Promega Corp., Madison, Wis.) according to the manufacturer's recommendations. Templates for PCR consisted of either a small amount of bacterial growth scraped from a CA plate, suspended in 25 μl of water, and boiled for 5 min (30), or 200 ng of H. ducreyi chromosomal DNA purified as described previously (9). The lspA1-specific oligonucleotide primers P1 (5′-ATGCGAATATCCAGCTCC-3′) and P2 (5′-GGTTGTGTTACTATGAACTTCTAAG-3′) (Fig. 1A) yielded a 1.6-kb amplicon from H. ducreyi 35000HP and were used for preliminary identification of strains with a mutated lspA1 ORF. The lspA2-specific oligonucleotide primers P3 (5′-TTGAATTCGCATCGGTAAGATTTATGCAGGTAG-3′) and P4 (5′-TTTCTCGAGCCGGTCTGATTCACATCACCC-3′) (Fig. 1B) yielded a 2-kb amplicon from H. ducreyi 35000HP and were used for preliminary identification of strains with a mutated lspA2 ORF.
Characterization of outer membrane proteins and LOS.
Cell envelopes and Sarkosyl-insoluble preparations derived from these cell envelopes were prepared as previously described (36) from H. ducreyi strains grown overnight in sCB. H. ducreyi whole-cell lysates, Sarkosyl-insoluble preparations, and CCS were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as described elsewhere (40, 44, 61), except that Kodak BioMax film (Eastman Kodak, Rochester, N.Y.) was used for autoradiography. Monoclonal antibodies (MAbs) used in Western blot analysis included MAb 11B7, which binds to both LspA1 and LspA2, and MAbs 40A4 and 1H9, which are specific for the LspA1 and LspA2 proteins, respectively (61). For LOS analysis, H. ducreyi proteinase K-treated whole-cell lysates (44) were subjected to SDS-PAGE using a 15% (wt/vol) polyacrylamide separating gel and stained with silver (59). N-terminal amino acid sequence analysis of the 40-kDa outer membrane protein was accomplished as described previously (19).
Autoagglutination assay.
The ability of H. ducreyi strains to autoagglutinate was evaluated by suspending bacteria from an overnight culture grown in sCB in phosphate-buffered saline (PBS) and measuring the absorbance of this suspension held at ambient temperature over time as previously described (1).
Adherence assay.
Adherence of H. ducreyi to the human foreskin fibroblast (HFF) (3) and the human HaCaT keratinocyte cell lines (13) was evaluated using a modified version of a previously described attachment assay (1, 55). The HFF cell line was maintained in RPMI 1640 medium (Mediatech, Herndon, Va.) supplemented with 1 mM sodium pyruvate (Mediatech), 2 mM Gluta MAX-1 (Invitrogen, Grand Island, N.Y.), and 10% (vol/vol) heat-inactivated fetal bovine serum (HyClone, Logan, Utah). The HaCaT cell line was maintained in Dulbecco's modified Eagle's medium (Gibco BRL, Rockville, Md.) supplemented with 2 mM Gluta MAX-1 (Invitrogen), and 10% (vol/vol) heat-inactivated fetal bovine serum (HyClone).
For the adherence assay, approximately 6 × 104 HFF or HaCaT cells were seeded into each well of a 96-well tissue culture plate (Corning-Costar, Corning, N.Y.) and incubated at 37°C in a humidified atmosphere of 95% air-5% CO2 until confluent (approximately 60 h). H. ducreyi strains were grown overnight (14 to 16 h) in sCB, harvested by centrifugation at 6,500 × g, and suspended to an optical density at 600 nm (OD600) of approximately 1.0 (approximately 5 × 108 CFU/ml) in the supplemented tissue culture medium used to grow the cell line. Portions of this suspension (2.5 μl for HFF cells and 5 μl for HaCaT cells) were inoculated in duplicate into wells containing confluent monolayers of cells and 97.5 μl (HFF cells) or 95 μl (HaCaT cells) of the supplemented tissue culture medium. These plates were centrifuged at 165 × g for 5 min to facilitate contact between the bacteria and the eukaryotic cells and then incubated at 33°C in a humidified atmosphere of 95% air-5% CO2 for 2 h. Nonadherent bacteria were removed by gently washing each well four times with 200 μl of tissue culture medium per well. The washed monolayer in each well was then incubated for 5 to 15 min with 100 μl of 0.25% trypsin-0.1% EDTA (Mediatech) per ml diluted 1:5 in PBS. This cell suspension was then diluted and plated to enumerate the number of bacteria attached to the monolayer in each well. To account for bacterial growth during the assay, controls which contained the same amount of tissue culture medium and bacteria, but no eukaryotic cells, were incubated under the same conditions. For each bacterial strain, adherence was expressed as the percentage of bacteria present attached to the eukaryotic cells in duplicate wells relative to the number of bacteria present in the control (tissue culture medium in the absence of eukaryotic cells).
Serum bactericidal assay.
The serum bactericidal assay was performed as previously described (60) using 20% (vol/vol) normal human serum.
Virulence testing.
The temperature-dependent rabbit model for chancroid has been described elsewhere (45). Briefly, lesions were scored on days 2, 4, and 7 postinfection according to the following numerical scoring system: 0, no change; 1, erythema; 2, induration; 3, nodule; 4, pustule or necrosis. Statistical analysis of lesion scores using nonparametric methods was performed as described previously (45). On day 7 postinfection, the animals were euthanized, and the lesions which had been initially inoculated with 105 CFU were excised from each rabbit, bisected with a sterile scalpel blade, and rinsed with PBS to recover pustular material. PBS washes were spread onto CA plates to recover viable H. ducreyi cells.
RESULTS
Construction of isogenic H. ducreyi mutants.
Because of the remarkably high level of identity (∼75%) between the H. ducreyi lspA1 and lspA2 genes (61), we had to carefully introduce insertions into DNA fragments which contained regions unique to each of these genes in order to construct the H. ducreyi lspA1 mutant 35000HP.1, the lspA2 mutant 35000HP.2, and the lspA1 lspA2 double mutant 35000HP.12, as described in Materials and Methods. Each mutant was initially identified from a pool of antibiotic-resistant transformants by PCR analysis of boiled bacterial colony material using lspA1-specific or lspA2-specific primers (data not shown). Genomic DNA isolated from H. ducreyi mutants was subjected to Southern blot analysis to confirm that proper allelic exchange had occurred and that each mutant contained only a single copy of the appropriate antibiotic resistance cartridge inserted into its chromosome (data not shown).
Characterization of protein expression by wild-type and mutant H. ducreyi strains.
We previously reported that we could detect LspA1 but not LspA2 in CCS from the wild-type strain 35000 (61). Therefore, we examined culture supernatant fluids from the wild-type and mutant H. ducreyi strains described above for LspA1 and LspA2 expression by Western blot analysis. The LspA1- and LspA2-reactive MAb 11B7 bound to an antigen with an apparent molecular weight slightly in excess of 250,000 in CCS from the wild-type strain 35000HP (Fig. 2A, lane 1). Probing of the wild-type CCS with the LspA1-specific MAb 40A4 (Fig. 2B, lane 1) revealed two reactive antigens, one with an apparent molecular weight slightly greater than 250,000 and the other with an apparent molecular weight of less than 160,000. Interestingly, the wild-type CCS contained a very weakly reactive pair of antigens, with apparent molecular weights of about 160,000 and just greater than 250,000, that bound the LspA2-specific MAb 1H9 (Fig. 2C, lane 1). This was the first time that we had detected expression, albeit very modest, of the LspA2 protein by a wild-type H. ducreyi strain.
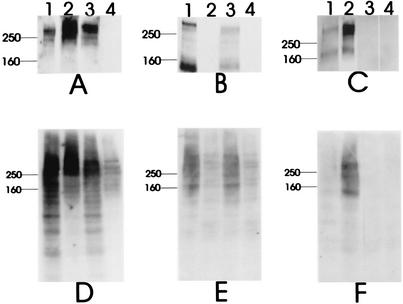
FIG. 2.
Western blot analysis of CCS and whole-cell lysates of wild-type and mutant H. ducreyi strains. Equivalent volumes of CCS from four cultures grown and processed simultaneously were used in panels A to C. Whole-cell lysates were prepared as described elsewhere (44). Proteins present in CCS (A to C) and whole-cell lysates (D to F) were probed with the LspA1- and LspA2 -reactive MAb 11B7 (A and D), the LspA1-specific MAb 40A4 (B and E), or the LspA2-specific MAb 1H9 (C and F). Lanes: 1, 35000HP; 2, lspA1 mutant 35000HP.1; 3, lspA2 mutant 35000HP.2; 4, lspA1 lspA2 mutant 35000HP.12.
The CCS from the lspA1 mutant 35000HP.1 contained a MAb 11B7-reactive antigen (Fig. 2A, lane 2) with an apparent molecular weight of slightly greater than 250,000 and which did not bind the LspA1-specific MAb 40A4 (Fig. 2B, lane 2). It is important to note that the CCS of this lspA1 mutant did contain two soluble antigens, with apparent molecular weights of approximately 250,000 and 160,000, which bound the LspA2-specific MAb 1H9 (Fig. 2C, lane 2). Therefore, this lspA1 mutant, which did not express LspA1, did express readily detectable LspA2 protein.
The CCS of the lspA2 mutant 35000HP.2 had a MAb 11B7-reactive antigen (Fig. 2A, lane 3) with an apparent molecular weight slightly greater than 250,000. The CCS from this mutant also had two soluble antigens, with apparent molecular weights of greater than 250,000 and less than 160,000, which bound the LspA1-specific MAb 40A4 (Fig. 2B, lane 3) in a pattern identical to that obtained with the wild-type CCS (Fig. 2B, lane 1). The CCS from this lspA2 mutant did not contain any antigens that bound the LspA2-specific MAb 1H9 (Fig. 2C, lane 3). Therefore, this lspA2 mutant expressed no detectable LspA2 in CCS but did express LspA1. Finally, the CCS of the lspA1 lspA2 double mutant 35000HP.12 did not react with any of these three MAbs (Fig. 2A to C, lane 4).
Whole-cell lysates of these four strains exhibited MAb reactivity patterns that were similar to those of the CCS, except that the number of MAb-reactive bands was greater and these bands were more diffuse in the whole-cell lysates (Fig. 2D to F). It should be noted that the whole-cell lysate of the lspA1 lspA2 double mutant exhibited weak reactivity with MAbs 11B7 and 40A4 (Fig. 2D and E, lane 4). To address this observation, a new lspA1 lspA2 double mutant was constructed with Ω antibiotic resistance cartridges inserted at the very beginning of both the lspA1 and lspA2 ORFs. The whole-cell lysate of this new double mutant lacked reactivity with MAb 11B7, which binds both LspA1 and LspA2 (data not shown).
Characterization of outer membrane proteins and LOS of the mutants.
The outer membrane protein profiles of the wild-type strain 35000HP and the three mutants, as represented by the proteins present in the Sarkosyl-insoluble fraction derived from cell envelopes, were virtually identical with the exception of a 40-kDa antigen that exhibited some variability in its expression among these mutants (data not shown). In several independently prepared Sarkosyl extracts, expression of this 40-kDa protein was usually reduced in the lspA1 and lspA1 lspA2 mutants and was sometimes reduced in the lspA2 mutant relative to the parent strain. N-terminal amino acid sequence analysis of this 40-kDa antigen revealed that its first 14 amino acids were VTLYEAEGTKIDLDG. When used to query the H. ducreyi genome database using the FASTA algorithm, this sequence was found to be identical to residues 21 to 34 of the predicted protein encoded by an ORF designated Hd1435 by Munson and colleagues (Robert S. Munson, Jr., personal communication). The predicted protein encoded by ORF Hd1435 has 32% identity with outer membrane protein P2 from Haemophilus influenzae (11). It should be noted that LspA1 and LspA2 were not detected by Coomassie blue staining of these Sarkosyl-insoluble preparations resolved by SDS-PAGE. No differences were observed in the migration patterns of the LOS of the three mutant strains, 35000HP.1, 35000HP.2, and 35000HP.12, compared to the migration pattern of the LOS of strain 35000HP (data not shown).
Characterization of growth and autoagglutination phenotypes of wild-type and mutant H. ducreyi strains.
To determine whether the lspA1 or lspA2 mutations exerted any significant growth defects which would complicate the interpretation of results derived from in vivo testing, we measured the growth rates (i.e., increase in the OD600) of the wild-type and mutant H. ducreyi strains in liquid broth culture. No substantial differences were noted in the growth rates of strains 35000HP, 35000HP.1, 35000HP.2, and 35000HP.12 in sCB over a 10-h time period (data not shown). However, the final density of broth cultures of the lspA1 mutant 35000HP.1 and the lspA1 lspA2 mutant 35000HP.12 was slightly but consistently lower than that of cultures of 35000HP and the lspA2 mutant 35000HP.2.
To investigate whether this latter result may have been due to an increased ability of the LspA1-deficient mutants to aggregate during growth, we measured the OD600 of suspensions of these strains over time. The two mutant strains which did not express the LspA1 protein (35000HP.1 and 35000HP.12) autoagglutinated much more rapidly than did strains 35000HP and 35000HP.2 (Fig. 3). In control experiments, the lspA1 lspA2 double mutant 35000HP.12 did not lose viability any more rapidly than did the wild-type parent strain over a 60-min time period in PBS (data not shown); this finding indicates that the relatively rapid decrease in the OD600 of suspensions of this double mutant was not the result of cell lysis.
FIG. 3.
Autoagglutination of wild-type and mutant H. ducreyi strains. Strain 35000HP (squares), the lspA1 mutant 35000HP.1 (triangles), the lspA2 mutant 35000HP.2 (circles), and the lspA1 lspA2 double mutant 35000HP.12 (diamonds) were grown overnight in sCB and then resuspended in PBS to an OD600 of approximately 1.0. The optical density of the undisturbed suspensions was measured over time. This graph is from a representative experiment.
Adherence of wild-type and mutant H. ducreyi strains to human cell lines.
Because the LspA1 and LspA2 proteins have some similarity to FHA, one of the major adhesins of B. pertussis (38), we evaluated the ability of the wild-type strain 35000HP and the lspA1 lspA2 mutant to adhere to two relevant human cell lines. The lspA1 lspA2 mutant attached to both HFF cells (Fig. 4A, column 3) and HaCaT keratinocytes (Fig. 4B, column 3) at levels similar to those obtained with the wild-type strain 35000HP (Fig. 4, column 1). In contrast, H. ducreyi strain A77, previously shown to be deficient in attachment to HFF cells (6) and used here as a negative control, attached significantly less to HFF cells (Fig. 4A, column 2) than did the wild-type strain 35000HP (Fig. 4A, column 1). These results indicated that the LspA1 and LspA2 proteins likely do not play a significant role in the attachment of H. ducreyi to these cell lines.
FIG. 4.
Adherence of wild-type and mutant H. ducreyi strains to HFF cells (A) and HaCaT keratinocytes (B). Adherence of the wild-type strain 35000HP (column 1), H. ducreyi strain A77 (column 2), and the lspA1 lspA2 double mutant 35000HP.12 (column 3) was evaluated after 2 h of incubation at 33°C and was expressed as the mean percentage of bacteria present attached to the eukaryotic cells in duplicate wells relative to controls containing bacteria but no eukaryotic cells. Each bar represents the mean of three independent experiments.
Effect of the lspA1 and lspA2 mutations on serum resistance of H. ducreyi.
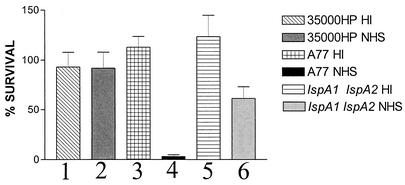
The LspA1 and LspA2 proteins each contain a 260-amino-acid region (amino acids 2763 to 3023 of LspA1 and amino acids 2892 to 3152 of LspA2) with >70% identity to the C-terminal portion of P76, a protein associated with serum resistance in H. somnus (17, 18). This observation prompted us to investigate whether the LspA1 and LspA2 proteins could be involved in the ability of H. ducreyi to resist the complement-mediated bactericidal activity of serum. H. ducreyi strain 35000HP (Fig. 5, column 2) was relatively resistant to killing by 20% normal human serum. The lspA1 lspA2 mutant 35000HP.12 (Fig. 5, column 6) exhibited only a modest reduction in viable numbers, relative to the wild-type parent strain, when incubated with normal human serum. In contrast, H. ducreyi strain A77 (Fig. 5, column 4), previously reported to be serum sensitive (31, 41), was readily killed by this normal human serum and was included in these experiments as a positive control for complement-mediated killing.
FIG. 5.
Susceptibility of wild-type and mutant strains of H. ducreyi to the complement-mediated bactericidal activity of 20% (vol/vol) normal human serum. H. ducreyi strain 35000HP (columns 1 and 2), strain A77 (columns 3 and 4), and the lspA1 lspA2 mutant 35000HP.12 (columns 5 and 6) were grown overnight in sCB, resuspended and diluted in PBS containing 0.15 mM CaCl2 and 0.5 mM MgCl2 (60), and incubated with normal human serum (NHS) (columns 2, 4, and 6) or heat-inactivated normal human serum (HI) (columns 1, 3, and 5) for 60 min at 33°C. Each bar represents the mean of four independent experiments.
Because this same region of the LspA proteins also has some identity with the YopT cytotoxin (46), we constructed a double deletion mutant of H. ducreyi that did not express either the cell-associated hemolysin (3, 39) or the cytolethal distending toxin (16). However, CCS from this double mutant did not kill either HFF or HeLa cells in vitro (data not shown).
Effect of the lspA1 and lspA2 mutations on the virulence of H. ducreyi.
These three mutants and the wild-type parent strain were tested for their ability to produce dermal lesions in the temperature-dependent rabbit model (45). In two independent experiments, the lspA1 mutant 35000HP.1 (which produced LspA2 but did not express LspA1) and the lspA2 mutant 35000HP.2 (which produced LspA1 but not LspA2) yielded lesion scores that were lower than those obtained with the wild-type parent strain 35000HP (Table 2). While these differences were very modest, they were nonetheless significant. The number of lesions which yielded viable organisms of each strain was similar (Table 2). In two other independent experiments, the lspA1 lspA2 mutant 35000HP.12 also produced significantly lower lesion scores than did the wild-type strain (Table 2). However, the lesion scores obtained with this double mutant were much lower than those derived from the use of the two single mutants (Table 2). Moreover, viable lspA1 lspA2 mutants were not recovered from any of the 16 lesions tested (Table 2).
TABLE 2.
Lesion formation by wild-type and mutant H. ducreyi strains in the temperature-dependent rabbit modela
| Experiment no. | Strain | Inoculum size (CFU) | Mean ± SD lesion score on day:
|
P valueb | Recoveryc | ||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 7 | |||||
| 1 | 35000HP | 105 | 4.00 ± 0 | 4.00 ± 0 | 4.00 ± 0 | 6/8 | |
| 35000HP.1 | 105 | 3.25 ± 0.46 | 3.38 ± 0.51 | 3.63 ± 0.51 | 0.0001 | 4/8 | |
| 35000HP.2 | 105 | 3.75 ± 0.46 | 3.88 ± 0.35 | 3.88 ± 0.35 | 0.0009 | 5/8 | |
| 35000HP | 104 | 3.38 ± 0.51 | 3.75 ± 0.46 | 3.75 ± 0.46 | |||
| 35000HP.1 | 104 | 2.88 ± 0.35 | 2.75 ± 0.46 | 3.00 ± 0.93 | |||
| 35000HP.2 | 104 | 3.00 ± 0 | 3.00 ± 0.76 | 3.25 ± 0.71 | |||
| 2 | 35000HP | 105 | 4.00 ± 0 | 4.00 ± 0 | 4.00 ± 0 | 5/7 | |
| 35000HP.1 | 105 | 3.14 ± 0.38 | 3.42 ± 0.53 | 3.86 ± 0.38 | 0.0001 | 2/7 | |
| 35000HP.2 | 105 | 3.71 ± 0.48 | 4.00 ± 0 | 4.00 ± 0 | 0.0110 | 5/7 | |
| 35000HP | 104 | 3.28 ± 0.49 | 3.71 ± 0.49 | 3.71 ± 0.49 | |||
| 35000HP.1 | 104 | 3.00 ± 0 | 3.00 ± 0 | 3.00 ± 0 | |||
| 35000HP.2 | 104 | 3.00 ± 0 | 3.28 ± 0.76 | 3.57 ± 0.53 | |||
| 3 | 35000HP | 105 | 4.00 ± 0 | 4.00 ± 0.00 | 4.00 ± 0.00 | 3/8 | |
| 35000HP.12 | 105 | 3.00 ± 0 | 2.63 ± 0.52 | 2.63 ± 0.52 | 0.0001 | 0/8 | |
| 35000HP | 104 | 3.38 ± 0.52 | 3.00 ± 0.53 | 3.13 ± 0.35 | |||
| 35000HP.12 | 104 | 2.63 ± 0.52 | 1.38 ± 0.74 | 0.75 ± 0.71 | |||
| 4 | 35000HP | 105 | 4.00 ± 0 | 4.00 ± 0.00 | 4.00 ± 0.00 | 7/8 | |
| 35000HP.12 | 105 | 3.13 ± 0.35 | 2.63 ± 0.52 | 2.63 ± 0.74 | 0.0001 | 0/8 | |
| 35000HP | 104 | 3.62 ± 0.52 | 4.00 ± 0 | 4.00 ± 0 | |||
| 35000HP.12 | 104 | 2.75 ± 0.46 | 2.00 ± 0 | 1.13 ± 1.25 | |||
Eight rabbits were used in each experiment, except in experiment 2 where seven rabbits were used.
P value calculated for the difference between wild-type and test strain lesion scores at both inoculum levels over the three scoring days.
Recovery of viable H. ducreyi from lesions, represented as follows: number of rabbits from which viable H. ducreyi were recovered/(total number of rabbits infected).
DISCUSSION
H. ducreyi 35000HP encodes two genes, lspA1 and lspA2, which are present in every H. ducreyi strain that we have examined to date (61). Despite containing lspA1 and lspA2 DNA sequences, several H. ducreyi strains previously determined to be avirulent in the temperature-dependent rabbit model (6) appeared not to release detectable LspA1 or LspA2 proteins into culture supernatant fluid (61), raising the possibility that these proteins could be involved in virulence expression in this animal model. To address this issue directly, we constructed H. ducreyi strains containing mutations in either or both of the lspA1 and lspA2 ORFs and examined several relevant phenotypic characteristics of these strains.
Consistent with our expectations, the lspA1 lspA2 double mutant 35000HP.12 did not produce LspA1 or LspA2 proteins detectable in CCS or whole-cell lysates. CCS from the lspA2 mutant 35000HP.2 contained LspA1 but not LspA2 and was, therefore, the same as CCS from the wild-type parent strain with regard to LspA1 expression. When we examined the lspA1 mutant strain 35000HP.1, we discovered that this mutant expressed LspA2 protein that was readily detectable in both CCS and whole-cell lysates. This finding was particularly noteworthy, as this result confirmed that the lspA2 gene had the potential to express its protein product. Our earlier inability to detect LspA2 protein in CCS from the wild-type strain 35000 (61) was likely related to very low level expression of this protein, because the use of a different type of autoradiography film in the present study permitted detection of this protein in wild-type CCS (Fig. 2). However, it must be noted that the apparent level of expression of LspA2 by the wild-type strain is clearly much lower than that of LspA1 (compare Fig. 2B and C, lane 1). It is also interesting that inactivation of the lspA1 gene resulted in greatly increased levels of expression of the LspA2 protein in this mutant (Fig. 2C, lane 2). The lspA1 and lspA2 genes are not physically adjacent to each other in the H. ducreyi chromosome, and it is not evident how a mutation in the former results in greatly increased expression of the latter gene product.
Both LspA1 and LspA2 can be detected in CCS and in whole-cell lysates by their reactivity in Western blot analysis with specific MAbs (Fig. 2). Efforts to purify LspA1 from CCS derived from the wild-type strain 35000HP have not been successful to date, thus preventing determination of the amino acid sequence of the N and C terminus of the soluble form of this protein. However, its apparent molecular weight in SDS-PAGE is considerably less than the calculated LspA1 molecular weight of 456,000, a finding which indicates that the soluble form of LspA1 is likely derived from a larger precursor by N- or C-terminal processing or both. We also found that our LspA1- and LspA2-specific MAbs bind at least two different forms of each antigen, with molecular weights of about 250,000 and 160,000 (Fig. 2). Additional, smaller MAb-reactive bands were even more apparent in Western blot analysis of the whole-cell lysates (Fig. 2D to F). The presence in SDS-PAGE of several different, smaller forms of a very large protein antigen is well documented, especially for the B. pertussis FHA protein (33). We have also found that LspA1 and LspA2 can be detected in Sarkosyl-insoluble fractions prepared from H. ducreyi cell envelopes (data not shown), suggesting that these two proteins exist in both a soluble state and in a cell-associated form.
Additional evidence for LspA1 and LspA2 being associated with the H. ducreyi cell surface was provided by the observation that the lspA1 mutant 35000HP.1 and the lspA1 lspA2 double mutant 35000HP.12 autoagglutinated much more readily than did the wild-type strain 35000HP or the lspA2 mutant strain 35000HP.2 (Fig. 3). One explanation for this phenomenon is that the LspA1 protein may be associated with the cell surface of H. ducreyi in such a manner as to mask another bacterial gene product that promotes aggregation or clumping of H. ducreyi cells. When the LspA1 protein is not expressed by H. ducreyi, this clumping factor may be relatively more surface exposed and allow intercellular aggregation. However, it appears that the LspA2 protein does not function in the same manner (i.e., preventing autoagglutination), because the lspA1 mutant (which expressed the LspA2 protein) readily autoagglutinated. It is also possible that this autoagglutination phenotype could have affected the outcome of the virulence tests by reducing the number of CFU of an lspA1 or lspA1 lspA2 mutant delivered by injection in the animal experiments. However, control experiments indicated that, even after 1 h, autoagglutination did not affect the number of CFU of the lspA1 lspA2 double mutant that were present in 0.1 ml of bacterial suspension expressed from the inoculating syringes (data not shown).
The results of tests in the temperature-dependent rabbit model (Table 2) indicate that expression of both LspA1 and LspA2 is required for full virulence of H. ducreyi 35000HP in this animal system. Inactivation of either lspA1 or lspA2 individually caused a slight but statistically significant reduction in lesion scores (Table 2), with the lspA1 mutant appearing to be slightly more attenuated than the lspA2 mutant. However, when both genes were inactivated, the resultant lspA1 lspA2 double mutant yielded lesion scores that were much lower than those obtained with either of the two strains carrying a single mutation. This latter result also provides evidence that the autoagglutination phenotype of the lspA1 lspA2 mutant, which was virtually identical to that of the lspA1 mutant (Fig. 3), was not solely responsible for the greatly decreased virulence of this double mutant. In view of the apparent involvement of the LspA proteins in virulence expression by H. ducreyi in this animal model, it should be noted that, in a recent study which used signature-tagged mutagenesis to identify Pasteurella multocida genes essential for virulence in a septicemic mouse model, two ORFs were identified whose encoded protein products had homology to LspA1 and LspA2 (27). The P. multocida PfhB1 and PfhB2 proteins have 45% similarity to LspA1 and LspA2, and the predicted PfhB2 protein (3,919 amino acids) is only slightly smaller than LspA1 (4,152 amino acids).
It is interesting that the three mutants constructed for the present study appeared to exhibit some variation in their expression of a 40-kDa outer membrane protein that has similarity to OmpP2 from H. influenzae. It is possible that this 40-kDa protein may interact in some manner with both LspA1 and LspA2 in the wild-type strain and that the absence of either one of these proteins somehow affects the stability or expression of the 40-kDa protein. However, the fact that the lspA1 lspA2 double mutant was substantially less virulent than either the lspA1 mutant or the lspA2 mutant, together with the fact that all three of these mutants tended to express reduced levels of the 40-kDa protein, would suggest that the lack of expression of the LspA1 and LspA2 proteins in this double mutant was the cause of its greatly reduced virulence. To date, efforts to clone either the lspA1 or lspA2 gene into a plasmid vector for use in complementation analysis have been unsuccessful, thus precluding efforts to determine whether restoration of the ability to express LspA1 or LspA2 would result in wild-type levels of expression of the 40-kDa protein.
Both LspA1 and LspA2 have been proposed to be members of the family of gram-negative bacterial proteins secreted by the two-partner secretion pathway (34). The lspB ORF, located immediately upstream from lspA2, encodes a predicted protein that most closely resembles the B. pertussis FhaC outer membrane protein, which has been proposed to form a β-barrel channel in the outer membrane through which FHA is translocated. By analogy with the FhaC/FHA system, it is expected that translocation of both LspA1 and LspA2 to the H. ducreyi cell surface would require expression of a functional LspB protein. Most recently, it was proposed that both LspA1 and LspA2 may contain β-helical domains similar to that described for B. pertussis FHA (35).
While the N-terminal region of both LspA1 and LspA2 shares a moderate level of similarity to proteins involved in the adhesion of B. pertussis (i.e., FHA) and H. influenzae (i.e., HMW1 and HMW2) to host cells (61), the H. ducreyi LspA1 and LspA2 proteins did not function as adhesins in the experiments described above. The apparent lack of adhesin activity attributable to LspA1 and LspA2 could be the result of the presence of other H. ducreyi adhesins that bind the cell types tested in this study. It is also possible that the LspA1 and LspA2 proteins may bind other cell types (57) or extracellular matrix components (10). The presence of alternative integrin-binding protein motifs (47) in the N-terminal halves of both LspA proteins increases the likelihood of the former possibility. Efforts to determine conclusively the role(s) of the LspA1 and LspA2 proteins in virulence expression by H. ducreyi are in progress.
Acknowledgments
This study was supported by U.S. Public Health Service grant AI32011 to E.J.H. and National Research Service award F32-AI09845 to C.K.W.
We are grateful to Bruce Green and James Kaper for providing plasmids containing antibiotic resistance cartridges, Michelle Alfa for providing H. ducreyi A77 and the HFF cell line, and Norbert E. Fusenig for providing the HaCaT keratinocyte cell line. We appreciate the assistance of Nikki Wagner and Yufan Zhu with nucleotide sequence analysis. We are especially grateful to Kate Fortney and Stanley Spinola for their scientific acumen.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. R. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis O35E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, H. J., C. Johansson, L. A. Svensson, K. Ahlman, M. Verdrengh, and T. Lagergard. 2002. In vitro and in vivo interactions of Haemophilus ducreyi with host phagocytes. Infect. Immun. 70:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfa, M. J. 1992. Cytopathic effect of Haemophilus ducreyi for human foreskin cell culture. J. Med. Microbiol. 37:43-50. [DOI] [PubMed] [Google Scholar]
- 4.Alfa, M. J., P. Degagne, and T. Hollyer. 1993. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect. Immun. 61:1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfa, M. J., P. Degagne, and P. A. Totten. 1996. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect. Immun. 64:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfa, M. J., M. K. Stevens, P. Degagne, J. Klesney-Tait, J. D. Radolf, and E. J. Hansen. 1995. Use of tissue culture and animal models to identify virulence-associated traits of Haemophilus ducreyi. Infect. Immun. 63:1754-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 9.Bauer, B. A., M. K. Stevens, and E. J. Hansen. 1998. Involvement of the Haemophilus ducreyi gmhA gene product in lipooligosaccharide expression and virulence. Infect. Immun. 66:4290-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, M. E., and S. M. Spinola. 1999. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect. Immun. 67:2649-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell, J., S. Grass, D. Jeanteur, and R. S. Munson, Jr. 1994. Diversity of the P2 protein among nontypeable Haemophilus influenzae isolates. Infect. Immun. 62:2639-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase. Identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 15.Campagnari, A. A., R. Karalus, M. A. Apicella, W. Melaugh, A. J. Lesse, and B. W. Gibson. 1994. Use of pyocin to select a Haemophilus ducreyi variant defective in lipooligosaccharide biosynthesis. Infect. Immun. 62:2379-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipopolysaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole, S. P., D. G. Guiney, and L. B. Corbeil. 1992. Two linked genes for outer membrane proteins are absent in four non-disease strains of Haemophilus somnus. Mol. Microbiol. 6:1895-1902. [DOI] [PubMed] [Google Scholar]
- 18.Cole, S. P., D. G. Guiney, and L. B. Corbeil. 1993. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J. Gen. Microbiol. 139:2135-2143. [DOI] [PubMed] [Google Scholar]
- 19.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, and G. H. McCracken, Jr. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cope, L. D., S. R. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbeil, L. B., F. D. Bastida-Corcuera, and T. J. Beveridge. 1997. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect. Immun. 65:4250-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domenighini, M., D. A. Relman, C. Capiau, S. Falkow, A. Prugnola, V. Scarlato, and R. Rappuoli. 1990. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol. Microbiol. 4:787-800. [DOI] [PubMed] [Google Scholar]
- 23.Elkins, C., C.-J. Chen, and C. E. Thomas. 1995. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect. Immun. 63:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkins, C., K. J. Morrow, Jr., and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filiatrault, M. J., R. S. Munson, Jr., and A. A. Campagnari. 2001. Genetic analysis of a pyocin-resistant lipooligosaccharide (LOS) mutant of Haemophilus ducreyi: restoration of full-length LOS restores pyocin sensitivity. J. Bacteriol. 183:5756-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller, T. E., M. J. Kennedy, and D. E. Lowery. 2000. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb. Pathog. 29:25-38. [DOI] [PubMed] [Google Scholar]
- 28.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J. Bacteriol. 179:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen, E. J., J. L. Latimer, S. E. Thomas, M. Helminen, W. L. Albritton, and J. D. Radolf. 1992. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J. Bacteriol. 174:5442-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennessy, K. J., J. J. Iandolo, and B. W. Fenwick. 1993. Serotype identification of Actinobacillus pleuropneumoniae by arbitrarily primed polymerase chain reaction. J. Clin. Microbiol. 31:1155-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiltke, T. J., M. E. Bauer, J. Klesney-Tait, E. J. Hansen, R. S. Munson, Jr., and S. M. Spinola. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93-102. [DOI] [PubMed] [Google Scholar]
- 32.Inzana, T. J., and L. B. Corbeil. 1987. Development of a defined medium for Haemophilus somnus isolated from cattle. Am. J. Vet. Res. 48:366-369. [PubMed] [Google Scholar]
- 33.Irons, L. I., L. A. E. Ashworth, and P. Wiltonsmith. 1983. Heterogeneity of the filamentous hemagglutinin of Bordetella pertussis studied with monoclonal antibodies. J. Gen. Microbiol. 129:2769-2778. [DOI] [PubMed] [Google Scholar]
- 34.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 35.Kajava, A. V., N. Cheng, R. Cleaver, M. Kessel, M. N. Simon, E. Willery, F. Jacob-Dubuisson, C. Locht, and A. C. Steven. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol. Microbiol. 42:279-292. [DOI] [PubMed] [Google Scholar]
- 36.Klesney-Tait, J., T. J. Hiltke, I. Maciver, S. M. Spinola, J. D. Radolf, and E. J. Hansen. 1997. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J. Bacteriol. 179:1764-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis, D. A., J. Klesney-Tait, S. R. Lumbley, C. K. Ward, J. L. Latimer, C. A. Ison, and E. J. Hansen. 1999. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect. Immun. 67:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locht, C., P. Bertin, F. D. Menozzi, and G. Renauld. 1993. The filamentous haemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol. Microbiol. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 39.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159-174. [DOI] [PubMed] [Google Scholar]
- 41.Odumeru, J. A., G. M. Wiseman, and A. R. Ronald. 1984. Virulence factors of Haemophilus ducreyi. Infect. Immun. 43:607-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer, K. L., W. E. Goldman, and R. S. Munson, Jr. 1996. An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol. Microbiol. 21:13-19. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 44.Patrick, C. C., A. Kimura, M. A. Jackson, L. Hermanstorfer, A. Hood, G. H. McCracken, Jr., and E. J. Hansen. 1987. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect. Immun. 55:2902-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell, B. K., J. A. Richardson, J. D. Radolf, and E. J. Hansen. 1991. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J. Infect. Dis. 164:359-367. [DOI] [PubMed] [Google Scholar]
- 46.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.San Mateo, L. R., M. M. Hobbs, and T. H. Kawula. 1998. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol. Microbiol. 27:391-404. [DOI] [PubMed] [Google Scholar]
- 50.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Neutropenia restores virulence to an attenuated Cu,Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect. Immun. 67:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorg, I., U. M. Goehring, K. Aktories, and G. Schmidt. 2001. Recombinant Yersinia YopT leads to uncoupling of RhoA-effector interaction. Infect. Immun. 69:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spinola, S. M., M. E. Bauer, and R. S. Munson, Jr. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinola, S. M., T. J. Hiltke, K. R. Fortney, and K. L. Shanks. 1996. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect. Immun. 64:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens, M. K., S. Porcella, J. Klesney-Tait, S. R. Lumbley, S. E. Thomas, M. V. Norgard, J. D. Radolf, and E. J. Hansen. 1996. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect. Immun. 64:1724-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St. Geme, J. W., III, and S. Falkow. 1990. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect. Immun. 58:4036-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson, Jr. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totten, P. A., J. C. Lara, D. V. Norn, and W. E. Stamm. 1994. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect. Immun. 62:5632-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 60.Ward, C. K., and T. J. Inzana. 1994. Resistance of Actinobacillus pleuropneumoniae to bactericidal antibody and complement is mediated by capsular polysaccharide and blocking antibody specific for lipopolysaccharide. J. Immunol. 153:2110-2121. [PubMed] [Google Scholar]
- 61.Ward, C. K., S. R. Lumbley, J. L. Latimer, L. D. Cope, and E. J. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood, G. E., S. M. Dutro, and P. A. Totten. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]