Abstract
In this study we have shown that Eap (extracellular adherence protein) plays a role in the internalization process of Staphylococcus aureus into eukaryotic cells. Eap is a protein that is mostly extracellularly and to a lesser extent is bound to the bacterial surface as a result of rebinding. Eap is able to bind to several plasma proteins, such as fibronectin, fibrinogen, and prothrombin. It has the capacity to form oligomers and is able to agglutinate S. aureus. A mutant strain, Newman mAH12 (eap:: Eryr), with a deficient eap gene was used in the present study. We have demonstrated that (i) strain Newman mAH12 could adhere to and become internalized to a higher extent by eukaryotic cells than the isogenic mutant, (ii) strain Newman mAH12 complemented with the eap gene displayed restoration of the internalization level, (iii) externally added Eap enhanced the internalization of laboratory and clinical S. aureus strains as well as of S. carnosus (a coagulase-negative species devoid of proteins important for internalization), and (iv) antibodies against Eap were able to block the internalization process in strain Newman mAH12 and clinical isolates. Eap, with its broad binding capacity and its surface localization, thus seems to contribute to the internalization of S. aureus into eukaryotic cells. We therefore propose a novel internalization pathway for S. aureus in which Eap plays an enhancing role.
Staphylococcus aureus is a persistent pathogen that causes serious community-acquired and nosocomial infections. The range of disease caused by S. aureus is broad and includes endocarditis, osteomyelitis, and septic shock. The emergence of extended antibiotic resistance among S. aureus strains as a worldwide epidemic has necessitated the development of novel strategies to combat this microorganism.
The ability of S. aureus to establish a niche in the host is a crucial step in its pathogenesis. S. aureus produces a number of cell surface-localized binding proteins, including fibronectin binding proteins (FnBPs) (15, 44), a collagen binding protein (38), fibrinogen binding proteins (FgBP) (4, 30), a vitronectin binding protein (39) and an elastin binding protein (37). A recent suggestion is to term these proteins receptins (28). Receptins are proposed to contribute to the success of colonization and persistence at various sites of the host. Binding of S. aureus to fibrinogen (Fg) is due mainly to the cell-associated protein clumping factors (Clf A and B) (14, 30). In addition, four extracellular proteins with the ability to bind to fibrinogen are produced by S. aureus: coagulase (41), Efb (extracellular Fg binding protein) (35, 36), Eap (extracellular adherence protein) (23, 34), and Emp (22). Eap causes agglutination of the bacteria because of its ability to rebind to the surface of S. aureus and because of a strong tendency of Eap to form multimeric aggregates. Eap has a broad binding range for plasma proteins including Fn, Fg, and prothrombin. Exogenously added Eap significantly enhanced the adherence of S. aureus to fibroblasts and epithelial cells (23, 34) due to its dual affinity for plasma proteins on the cell surface and the bacterium itself. A putative target on the bacterial surface for Eap is a neutral phosphatase to which Eap has strong affinity (16).
If adherence of S. aureus to host components is the first step of infection, its ability to escape humoral immunity by internalization and intracellular survival might be the second most important function for long-term persistence. Internalization of S. aureus into nonprofessional phagocytic cells is well documented (3, 27, 32, 49). FnBPs were shown to be required for the internalization process into eukaryotic cells (13, 40, 46). It was proposed that the affinity of FnBP for fibronectin bound to β1 integrins would result in activation of host cell signal transduction pathways, which in turn would lead to actin-mediated phagocytosis of adherent bacteria (3, 13, 46). Although FnBP obviously plays a crucial part in the internalization process, bacteria lacking FnBPs could still be internalized at a lower rate. Furthermore, no correlation was found between adherence ability and the amount of FnBP produced by some S. aureus strains (20), and Fn binding capacity only partly correlated with the ability of various strains of S. aureus to be internalized (13, 40). This indicates that the internalization process for S. aureus is complex and probably involves more than one factor. Therefore, by analogy to Eap, some of these internalization mechanisms may critically depend on the presence of secreted molecules rather than or in addition to proteins covalently bound to the cell wall.
In addition to S. aureus, several other gram-positive bacteria, including Listeria monocytogenes, Streptococcus pyogenes, and Enterococcus faecalis, evade host immunity by internalization (25, 26, 43, 48). L. monocytogenes uses two invasion proteins for entry into mammalian cells, internalin A (InlA) and internalin B (InlB). InlA is a transmembrane cell adhesion protein (31) that promotes entry into the enterocyte-like epithelial cell line Caco-2 (17). InlB interacts with the mammalian protein gC1q-R (7) and is needed for entry into cultured hepatocytes and epithelial or fibroblast-like cell lines (12, 18, 19, 24, 29). Interestingly, InlB is not only cell associated but also found in culture supernatants of L. monocytogenes (29), analogous to Eap. It was also seen that InlB, when added to the bacteria, could rebind and enhance the internalization of L. monocytogenes into mammalian cells (6, 8). Thus, the internalization process of L. monocytogenes is a multifactorial event. Similarly, at least three proteins involved in the internalization process are known from Streptococcus pyogenes: F1, M1, and M6 (11, 26). E. faecalis aggregation substance is also expressed on the surface of the bacteria. It has been shown that aggregation substance aggregates bacteria and increases bacterial adherence to and internalization into epithelial cells from the colon and duodenum but not from the ileum (43, 48).
Therefore, the purpose of this study was to investigate the potential role of Eap in adherence and internalization of S. aureus. An S. aureus strain Newman mutant with an eap mutation (Newman mAH12) was used and found to have significantly reduced ability to adhere to and internalize fibroblasts and epithelial cells compared to the isogenic parental strain. Complementation of strain Newman mAH12 with eap could restore the internalization rate. Furthermore, anti-Eap antibodies were able to reduce the internalization of the wild-type strain. These data provide evidence for a role of Eap in the internalization process.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. aureus strain Newman and S. aureus strain Newman mAH12 (eap::Eryr) (23) were grown in Luria broth (LB) for 2 h or overnight (ON) at 37°C with shaking. The cells were washed with phosphate-buffered saline (PBS) and resuspended in PBS. S. aureus strains L12 (isolated from a patient with endocarditis) and U35 (a nasal colonizer) were cultured in the same way. Strain Newman mAH12 was also complemented with Eap, resulting in strain Newman mAH12(pCXEap) (23). This strain was grown in LB with 10 μg of chloramphenicol per ml. After 2 h of incubation, xylose (0.5% final concentration) was added to induce the expression of Eap. Strain TM300 of Staphylococcus carnosus is nonpathogenic and lacks most of the known S. aureus surface structures. TM300(pFNBA4) is a strain harboring a plasmid coding for FnBP A and was grown as described previously (45).
Purification of Eap.
One liter of S. aureus strain Newman was grown ON at 37°C in LB. The culture was centrifuged, and FgBPs from the supernatant were isolated by affinity chromatography on Fg-Sepharose (Pharmacia, Uppsala, Sweden) as described by Bodén and Flock (4, 5). Proteins were eluted with 0.7% acetic acid, dialyzed against 40 mM phosphate buffer (pH 6.5) (buffer A), and subjected to fast protein liquid chromatography on a Mono S column (Pharmacia), using a gradient of 0 to 100% buffer B (1 M NaCl in buffer A). Three peaks of proteins were eluted from strain Newman. The first eluted at 0.15 to 0.25 M NaCl (coagulase), the second eluted at 0.35 to 0.45 M NaCl (Efb), and the third eluted at 0.5 to 0.7 M NaCl (Eap). The eluate (third peak) was dialyzed against PBS.
Binding and internalization of S. aureus Newman, Newman mAH12, and Newman mAH12(pCXEap) to fibroblasts and epithelial cells.
Fibroblast (human fetal lung) cells were cultured in Eagle medium (Gibco BRL) supplemented with 10% fetal calf serum (HyClone), HEPES buffer, α-glutamine, penicillin (100 U/ml), and streptomycin (100 U/ml). Epithelial cells (HACAT keratinocytes) were cultured in Dulbecco's modified Eagle medium (with sodium pyruvate, glucose, and pyridoxine) supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 U/ml). Cells (fibroblasts and epithelial cells) were seeded (8 × 104 cells/ml) in 24-well culture plates (Costar) and incubated at 37°C under 5% CO2.
For the binding assay, the following standard procedure was followed. On reaching confluency (2.5 × 105 to 3.0 × 105 cells/well), the cells were washed with standard medium (Eagle medium without supplements), and 900 μl of the standard medium was added to the cells. The cells were inoculated with 100 μl of bacteria, consisting of a mixture of 50 μl of strain Newman and 50 μl of mAH12, to obtain a final concentration of 107 bacteria per well to produce a multiplicity of infection of 30 to 40. After incubation for 2 h at 37°C under 5% CO2, the wells were washed three times with PBS. A 200-μl volume of 10% trypsin was added to the wells to detach the cells, which were subsequently lysed by the addition of 800 μl of sterile water. The bacteria (both adherent and internalized) were serially diluted and plated on blood agar plates without antibiotic. After 24 h of incubation, at least 200 colonies were picked from the blood agar plates onto LB plates containing 4 μg of erythromycin per ml and incubated for 24 h at 37°C to determine the ratio between the two strains (only mAH12 is erythromycin resistant). The exact ratio between the two strains in the mixture before adherence was determined in the same way, giving an initial ratio of 50:50 between the strains
For the determination of internalization, lysostaphin at a final concentration of 20 μg/ml was added for 30 min to kill extracellular bacteria before the trypsin step was performed. Thus, only internalized bacteria are enumerated. The killing effect of lysostaphin was routinely checked in control wells at each experiment. The ratio between Newman and mAH12 was determined by picking at least 200 colonies onto erythromycin-containing plates as above.
For comparison of internalization of the complemented mutant mAH12(pCXEap) and mAH12 into fibroblasts, wells were incubated with each of the strains separately, not in a mixture. The coincubation strategy described above would require stability of the plasmid in the complemented mutant in order to allow proper assessment of the ratio between the strains. We found that lack of selective pressure during growth on antibiotic-free media resulted in plasmid loss, and reliable enumeration in the coincubation was not possible due to this instability. Bacteria were serially diluted and plated on blood agar plates to determine viable counts.
For different clinical isolates, adherence and internalization assays were performed in the same way. Strain Cowan 1 was included together with each strain and was given a relative value of 1 for adherence and internalization; the clinical isolates were evaluated with respect to this value (data not shown). High variation was observed among the clinical strains with regard to adherence and internalization, and for that reason two strains were chosen for further experiments, with strain U35 being internalized to the greatest extent and strain L12 being internalized to the least extent in comparison to strain Cowan 1.
Internalization of bacteria in the presence of Eap.
Fibroblast cells were cultured as described for the internalization assay. A 50-μl volume of strain Newman or Newman mAH12 ON culture (107 bacteria/ml) was preincubated for 30 min at 37°C with Eap protein (80 μg/ml). The bacteria were then added to the cells in the wells. Control wells were inoculated with bacteria and PBS. After incubation for 2 h at 37°C under 5% CO2, the same procedure as for the adherence and internalization assay was performed. Bacteria were serially diluted and plated on blood agar plates to determine viable counts. For strains L12, U35, S. carnosus TM300, and S. carnosus TM300(pFNBA4), a concentration of only 20 μg of Eap per ml was used since higher concentrations yielded similar results (data not shown).
Internalization in the presence of antibodies against Eap.
Sheep were immunized with Eap, clumping factor (Clf), or GST-D. GST-D is a fusion protein encompassing glutathione S-transferase (GST) and three binding domains from the FnBP from S. aureus (9). A, 150-μg portion of each antigen in Freund's complete adjuvant was given intramuscularly. Booster doses were given 2 and 4 weeks later using Freund's incomplete adjuvant. Blood samples were taken 2 weeks after the last booster. A protein G-Sepharose 4 Fast Flow apparatus (Pharmacia) was used to obtain immunoglobulin G using the procedure recommended.
Fibroblast cells were cultured as in the internalization assay. A 50-μl volume of strain Newman ON culture, strain L12, or strain U35 (107 bacteria/ml) was preincubated for 30 min at 37°C with 50 μl of antibodies against Eap (8 mg/ml). Control wells were inoculated with bacteria and preserum (7 mg/ml). The bacteria were then added to the cells in the wells. After incubation for 2 h at 37°C under 5% CO2, the internalization assay was performed. Bacteria were serially diluted and plated on blood agar plates to determine viable counts.
Immunohistochemical staining.
Uninfected fibroblasts and fibroblasts cocultivated with S. aureus strain Newman or Newman mAH12 (1.5 × 107 bacteria/ml) were incubated for 2 h at 37°C under 5% CO2. The cells were washed, detached by trypsination (10% for 10 min), transferred to adhesion glass slides (Erie Scientific, Portsmouth, N.H.), and fixed with freshly prepared 2% formaldehyde in PBS. Immunohistochemical staining was done as previously described (1, 2, 33), using specific antibodies against Clf at a concentration of 2 μg/ml. To detect both adherent (extracellular) and internalized bacteria, the staining procedure was performed in the presence of a cell-permeabilizing agent, 0.1% saponin (Sigma, St. Louis, Mo.). Biotinylated secondary antibody, rabbit anti-sheep immunoglobulin G (Chemicon International, Inc.), diluted 1:20,000 in balanced salt solution containing 0.1% saponin, was added, followed by an avidin-biotin-peroxidase solution (Vectastain-Elite kit; Vector Laboratories, Burlingame, Calif.), with 3,3-diaminobenzidine (Vector Laboratories) as a substrate. Triplicate fields were stained and evaluated for Eap and Clf by direct microscopy and in situ computerized imaging. Uninfected fibroblasts were included to control for nonspecific staining, and consistently thus staining was completely negative.
Statistical methods.
The unpaired Student t test or Wilcoxon signed rank test was used to determine the significance of the data.
RESULTS
Adherence and internalization of S. aureus strains Newman and Newman mAH12 to fibroblasts and epithelial cells.
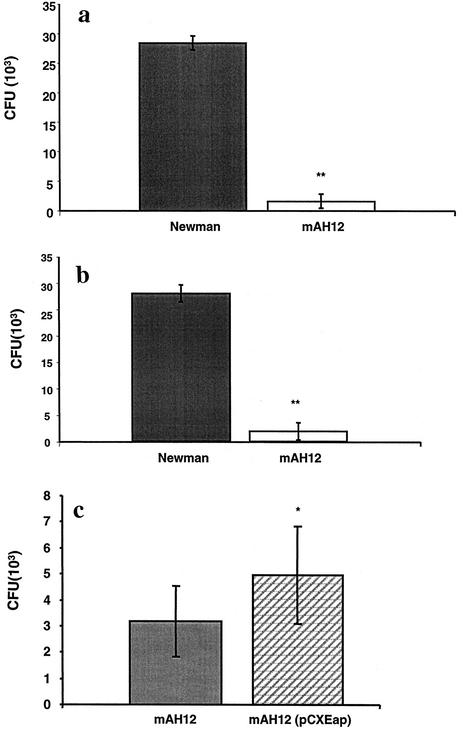
In an earlier study, it was shown that externally added Eap could enhance the binding of S. aureus Newman to fibroblasts and epithelial cells (23, 34). We also demonstrated that lack of the eap gene could reduce the adherence of S. aureus to fibroblasts (23). In the present experiment, we address the question whether internalization of the eap mutant was also reduced. A confluent layer of fibroblasts was inoculated with a mixture of S. aureus Newman and Newman mAH12 (eap::Eryr) and incubated for 2 hs. Overnight cultures of Newman and mAH12 were used since Eap is best expressed in a postexponential phase (4) and expression of FnBPs is low. Among the internalized bacteria in fibroblasts, the proportion of wild-type (WT) organisms clearly dominated compared to organisms of mutant strain mAH12, as shown in Fig. 1a (P < 0.01). The average number of internalized bacteria was 3 × 104 CFU for strain Newman and 2 × 103 for strain Newman mAH12.
FIG. 1.
Internalization of S. aureus strains Newman and Newman mAH12 into fibroblasts or epithelial cells. (a and b) Confluent layers of fibroblasts (a) or epithelial cells (b) were inoculated with a bacterial mixture of strains Newman and Newman mAH12 (ON cultures) and incubated at 37°C for 2 h. The wells were further incubated with lysostaphin to kill the extracellular bacteria. The cells were lysed, and the ratio between the two bacterial strains was determined based on the erythromycin resistance of mAH12. The data are presented as mean CFU in nine experiments. (c) Internalization of strains mAH12 and mAH12 (pCXEap) in fibroblast cells. Bacteria were used separately here because reliable quantification of mAH12 and mAH12 (pCXEap) in a mixture could not be done. The data are presented as mean number of internalized bacteria in eight experiments. Error bars indicate standard deviation. Statistical differences were determined by Student's t test. *, P < 0.05; **, P < 0.01.
To exclude the possibility of cell specificity epithelial cells were also subjected to the internalization assay. After incubation with the epithelial cells, a significant dominance of Newman over Newman mAH12 could again be seen in internalization (P < 0.01), as shown in Fig. 1b. In a typical experiment, approximately 104 CFU of strain Newman and 103 CFU of Newman mAH12 were found internalized when 107 CFU was added to the cells.
To demonstrate that these results were attributed to the lack of Eap and not to a secondary effect of the mutation, a complemented strain carrying a plasmid expressing Eap was employed. Figure 1c shows that using this complemented strain, the internalization level was significantly higher than that of mAH12 (P < 0.05), although it did not reach the same level as that of strain Newman. The expression level of Eap from this complemented strain was found not to be very high (23), explaining why the WT level of internalization was not obtained. In this experiment, strains mAH12 and mAH12(pCXEap) were added to separate wells since the growth of mAH12(pCXEap) on antibiotic-free medium led to loss of plasmid when cocultivated with mAH12, whereas WT and mAH12 (Figs. 1a and b) were coincubated.
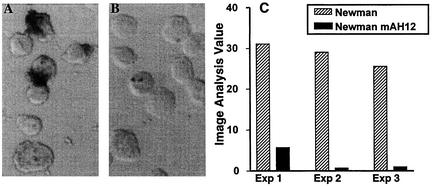
Immunostaining and in situ imaging verified the effect of Eap on adherence and internalization. In agreement with previous data, a dramatic reduction in the adherence of mAH12 to fibroblasts compared to that of strain Newman was observed, and in situ imaging analyses of the staining demonstrated a significant (P < 0.02) reduction in adherence (Fig. 2).
FIG. 2.
Immunohistochemical staining of adherent and internalized bacteria in fibroblasts. (A and B) Fibroblasts incubated with either strain Newman (A) or strain Newman mAH12 (B) at 37°C for 2 h were stained for expression of clumping factor by immunohistochemistry. The stainings were quantified by in situ imaging, and the data are presented as image analysis value corresponding to (frequency of positive area/total cell area) × mean intensity of positive area. (C) Imaging data from three separate experiments. Wilcoxon's rank sign test for paired samples revealed a significant difference between Newman and AH12 (P < 0.02).
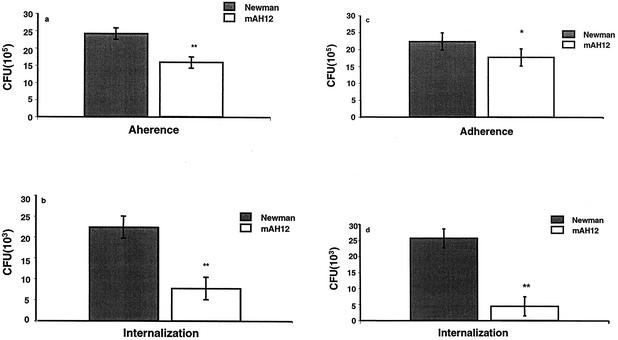
FnBPs promote the internalization of S. aureus into eukaryotic cells (13, 40, 46). FnBPs are cell surface-localized proteins that are expressed only in the very early exponential phase (42). To allow sufficient expression of FnBPs, 2-h cultures of strains Newman and mAH12 were also used in adherence and internalization assays on epithelial cells and fibroblasts. Figures 3a and b (on fibroblasts) and Fig. 3c and d (on epithelial cells) show that strain Newman again dominated in both adherence (P < 0.05 on epithelial cells and P < 0.01 on fibroblasts) and internalization (P < 0.01 on both cell types) over mAH12, although to a lesser extent than was the case with ON cultures. Thus, the effect of Eap in relation to FnBP was more prominent in ON cultures. In a 2-h culture, adherence and internalization were 2 × 106 CFU and 2.5 × 104 CFU, respectively, for strain Newman. The average adherence and internalization rate for strain mAH12 were 1.7 × 106 and 5 × 103, respectively.
FIG. 3.
Adherence and internalization of S. aureus strains Newman and Newman mAH12. Confluent layers of fibroblasts (a and b) or epithelial cells (c and d) were inoculated with bacterial mixture of strains Newman and Newman mAH12 (2-h culture for efficient expression of FnBPs) and incubated at 37°C for 2 h. For the internalization assay, wells were further incubated with lysostaphin to kill the extracellular bacteria. Cells were lysed, and the ratio between the two bacterial strains was determined. The bars show the mean CFU in six experiments. Error bars indicate standard deviation. Statistical differences were determined by Student's t test. *, P < 0.05; **, P < 0.01.
Internalization in the presence of externally added Eap.
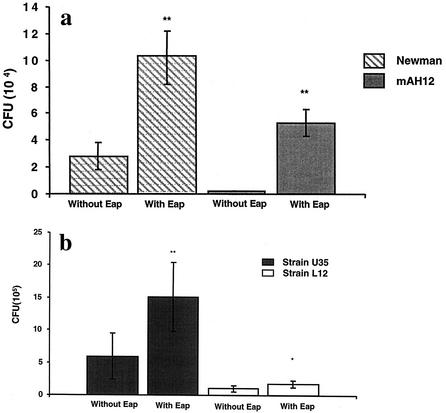
The lack of Eap reduces adherence and internalization. Consequently, we determined whether addition of external Eap could stimulate these events. Using the internalization assays on fibroblasts, bacteria were pretreated with Eap prior to addition to the cells. Figure 4a shows that internalization of both strains Newman and mAH12 was significantly enhanced (P < 0.01) by addition of Eap, agreeing with previous findings for adherence (34). Figure 4b shows that internalization of both clinical strains U35 and L12 was also significantly enhanced in the presence of Eap (P < 0.01 for U35 and P < 0.05 for L12).
FIG. 4.
Internalization in the presence of externally added Eap. (a) Confluent layers of fibroblast cells were inoculated with strains Newman and mAH12 with or without Eap present and incubated at 37°C for 2 h. To quantify internalization, wells were (after being washed) further incubated with lysostaphin to kill the extracellular bacteria. Cells were detached and lysed, and bacterial viable counts were determined. (b) Clinical isolates U35 and L12 in the presence or absence of Eap. The bars show the mean CFU in four experiments for Newman and mAH12 and six experiments for clinical isolates U35 and L12. Error bars indicate standard deviation. Statistical differences were determined by Student's t test, *, P < 0.05; **, P < 0.01.
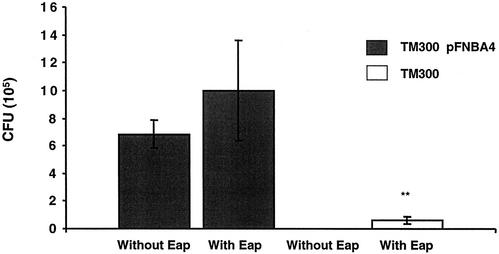
S. carnosus is distinguished from S. aureus by its lack of most of the adherence proteins. Thus, S. carnosus has a very low internalization rate. S. carnosus TM300 was previously complemented with a plasmid harboring FnBPA, and internalization was thereby restored due to the presence of FnBPA (45). External Eap was added to determine whether Eap could enhance the internalization rate in these strains. Figure 5 shows that the presence of Eap significantly enhanced the internalization of S. carnosus TM300 into fibroblasts (P < 0.01). Addition of external Eap did not increase the internalization of strain TM300(pFNBA4) significantly, probably due to the internalization-promoting effect of the FnBP complementation.
FIG. 5.
Internalization of strains TM300 and TM300(pFNBA4) in the presence of externally added Eap. Confluent layers of fibroblast cells were inoculated with strain TM300 with or without Eap or strain TM300(pFNBA4) with or without Eap and incubated at 37°C for 2 h. The wells were further incubated with lysostaphin to kill the extracellular bacteria. Cells were lysed, and viable counts of internalized bacteria were determined. The bars show the mean CFU in four experiments; error bars indicate standard deviation. P < 0.01 (determined by Student's t test) for TM300 with Eap (**).
Reduced internalization of S. aureus in the presence of antibodies against Eap.
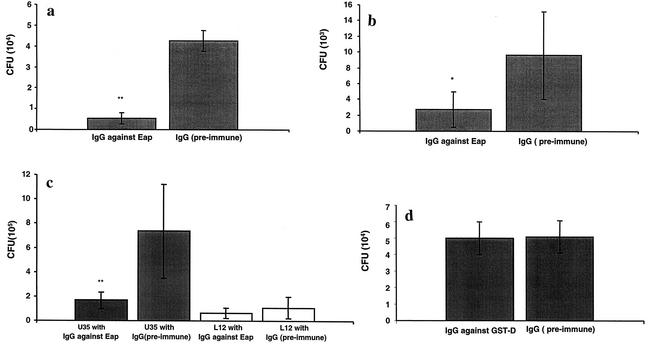
Addition of external Eap could enhance the adherence and internalization of both strains Newman and mAH12. In the next experiment, the aim was to see if antibodies against Eap could block the internalization process. Using the internalization assay on fibroblasts and epithelial cells, strain Newman was pretreated with antibodies against Eap prior to addition to the cells. Figures 6a and b show that these antibodies significantly reduced the internalization (P < 0.01 on fibroblasts and P < 0.05 on epithelial cells). Adherence of strain Newman to these cells was also reduced by the addition of antibodies against Eap (data not shown). Clinical isolate U35 was blocked significantly by antibodies against Eap, as shown in Fig. 6c (P < 0.01). A blocking effect could be observed with strain L12, which is a poorer internalizer, although it was not significant.
FIG. 6.
Internalization in the presence of antibodies against Eap. (a and b) Confluent layers of fibroblasts (a) or epithelial cells (b) were inoculated with strain Newman together with IgG against Eap or preimmune IgG and incubated at 37°C for 2 h. After being washed, the wells were further incubated with lysostaphin to kill the extracellular bacteria. Cells were detached and lysed, and viable counts were estimated. (c) Clinical isolates U35 and L12 in the presence of antibodies against Eap or preimmune IgG. The bars show the mean CFU in six experiments. Error bars indicate standard deviation. Statistical differences were determined by Student's t test. *, P < 0.05; **, P < 0.01. (d) Strain Newman in the presence of IgG against GST-D (the conserved functional D domain of FnBP) or preimmune antibodies. The bars show mean CFU in three experiments. No statistical difference was observed.
It has been convincingly shown that Fn binding is a major factor promoting the internalization of S. aureus into eukaryotic cells. We therefore tried the above experimental approach with antibodies against the D domain on FnBP. Surprisingly, these antibodies were unable to block the adherence or internalization of strain Newman into fibroblasts, as shown in Fig. 6d.
DISCUSSION
In this study, we could demonstrate that (i) strain Newman was internalized by eukaryotic cells to a greater extent than was strain Newman mAH12 (grown in overnight cultures for optimal expression of Eap); (ii) the complemented strain Newman mAH12(pCXEap) showed an increased internalization level compared with the mutant mAH12; (iii) externally added Eap enhanced the internalization of strain Newman, strain Newman mAH12, clinical strains, and even the distant strain S. carnosus TM300 into eukaryotic cells; and (iv) antibodies against Eap were able to block the internalization process in strain Newman and clinical isolates.
For S. aureus, FnBPs play an essential role in the internalization process by promoting an actin-mediated phagocytosis of the adherent bacteria (13, 40, 46). Affinity of FnBP for β1 integrins covered with Fn is proposed to be the main mechanism for internalization of S. aureus into eukaryotic cells (3, 13, 45, 46). In fact, by using FnbA and FnbB deletion mutants, complemented strains, D1-4 repeat peptide analogues, and heterologous complementation in S. carnosus, it was demonstrated that FnBPA and FnBPB are, to different extents, both necessary as well as sufficient for complete elicitation of the invasion process in a number of eukaryotic cells. However, these experiments were performed in an Eap-proficient background. It could therefore not be ruled out that Eap, or other factors still to be delineated, could be involved in the internalization process. The data presented here suggest that Eap may contribute to invasion, either independently or in concert with FnBPs. This suggestion is further supported by the fact that when 2-h cultures of strains Newman and mAH12 (a cultivation time resulting in enhanced expression of FnBPs) were used, a decrease in the adherence and internalization ratio between strains Newman and mAH12 was observed.
That FnBPs are not the only proteins involved in the internalization process is further implied by observations made by Dziewanowska et al. (13). A complete correlation between the efficiency in adherence of S. aureus to immobilized Fn and the level of internalization into Mac-T cells was not found, suggesting that other factors than Fn binding could affect the internalization in some isolates of S. aureus. Analogous to this finding, Jadoun et al. (26) also observed that protein F1, which binds to Fn and plays a leading role in the internalization process of Streptococcus pyogenes, did not have any effect on the internalization process of other streptococcal strains, suggesting that the observed effect might reflect a strain-specific mechanism of internalization (26). Previously, it was shown that the amount of expressed Eap varies between different strains (21), and there is obvious strain-to-strain variation in the dependence on Eap for internalization. This fact, however, does not necessarily mean that one pathway for internalization is more important than another. It should be further noted that the strain used here, Newman, has a lower internalization rate than other strains used (13, 46). The question whether the overall contribution to invasion is greatly or in part due to different expression of Eap in different clinical strains is currently under investigation in our laboratories.
In a study carried out in our laboratory, we have shown that antibodies against Eap are present in healthy blood donors and that patients with ongoing staphylococcal infections and patients in the convalescent stage after a staphylococcal infection have increased levels of antibody against Eap (unpublished data). This indicates that Eap is expressed in vivo and is able to challenge our immune system. Antibodies against Eap were therefore used to find if they could block the effects of Eap in the internalization process, and they were found to do so. When clinical isolates were exposed to antibodies against Eap, the blocking effect on internalization was highly variable (as exemplified by the results obtained with strains U35 and L12). Antibodies against Eap may have a greater blocking effect in strains where the role of Eap is more important than in strains where it is less important. On the other hand, antibodies against FnBP were unable to block internalization. This finding is analogous to the relatively poor ability of such antibodies to block the adherence of S. aureus to immobilized fibronectin (47). Instead, antibodies preferentially recognize ligand-induced binding sites formed on the docking of FnBP and Fn (10). On the other hand, when monolayers of Mac-T cells were pretreated with a synthetic peptide (D3) corresponding to a conserved functional region of the FnBPs, internalization could be blocked. It was shown that the D3 peptide interfered with internalization by competing with FnBP for its cellular receptor (13). Taken together, these findings indicate that, depending on culture-dependent gene expression FnBPs and Eap may constitute a concerted system of factors involved in staphylococcal invasion of eukaryotic cells.
In this study, we have shown that the Eap protein plays a contributory role in the internalization process of S. aureus. We therefore propose a novel internalization pathway for S. aureus, parallel or in addition to the pathway relying on FnBPs, These two parallel pathways presumably complement each other rather than being separate events along the same pathway. The experiments with S. carnosus show that internalization can occur without Eap or without FnBP, but one of these proteins need to be supplied to achieve internalization.
The clinical relevance of dual internalization pathways is to permit S. aureus to protect itself more efficiently against host defense. A further approach in this field would be to investigate the invasiveness of Eap in an in vivo model, which would allow a more profound understanding in the pathogenicity of invasiveness of S. aureus.
Acknowledgments
Grants were received from Biostapro AB, Swedish Medical Research Council (K2001-16X-12218-05C to J.-I.F. and K2001-16x-12610-04B to A.N.-T.) and from the Deutsche Forschungsgemeinschaft, Collaborative Research Centers 293 and 492 (to M.H).
Part of this work was performed in the institution of G. Peters, whose continuous input and support is gratefully acknowledged.
Editor: V. J. DiRita
REFERENCES
- 1.Andersson, J., and U. Andersson. 1993. Characterization of cytokine production in infectious mononucleosis studied at a single-cell level in tonsil and peripheral blood. Clin. Exp. Immunol. 92:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, J., S. Nagy, L. Björk, J. Abrams, S. Holm, and U. Andersson. 1992. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol. Rev. 127:69-96. [DOI] [PubMed] [Google Scholar]
- 3.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escape the endosome and induce apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodén, M., and J.-I. Flock. 1992. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb. Pathog. 12:289-298. [DOI] [PubMed] [Google Scholar]
- 5.Bodén, M., and J.-I. Flock. 1989. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect. Immun. 57:2358-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InIB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. [DOI] [PubMed] [Google Scholar]
- 7.Braun, L., B. Ghebrehiwet, and P. Cossart. 2000. gC1q-R/p32, a C1q-binding protein, is a receptor for the InIB invasion protein of Listeria monocytogenes. EMBO J. 19:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, L., H. Ohayon, and P. Cossart. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 9.Brennan, F. R., T. D. Jones, M. Longstaff, S. Chapman, T. Bellaby, H. Smith, F. Xu, W. D. O. Hamilton, and I.-J. Flock. 1999. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine 17:1846-1857. [DOI] [PubMed] [Google Scholar]
- 10.Casolini, F., L. Visai, D. Joh, P. G. Conaldi, A. Toniolo, M. Hook, and P. Speziale. 1998. Antibody response to fibronectin-binding adhesin FnbpA in patients with Staphylococcus aureus infections. Infect. Immun. 66:5433-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyognes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of L. monocytogenes into hepatocytes requires expression of InIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 13.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eidhin, D. N., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (Clf B), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 15.Flock, J.-I., G. Fröman, K. Jönsson, B. Guss, C. Signäs, B. Nilsson, M. Raucci, M. Höök, T. Wadström, and M. Lindberg. 1987. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flock, M., and J.-I. Flock. 2001. Rebinding of extracellular adherence protein Eap to Staphylococcus aureus can occur through a surface-bound neutral phosphatase. J. Bacteriol. 183:3999-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard, J.-L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 55:2822-2829. [DOI] [PubMed] [Google Scholar]
- 18.Gaillard, J.-L., F. Jaubert, and P. Berche. 1996. The inIAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med. 183:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Expression of the inIAB operon by Listeria monocytogenes is not required for entry into hepatic cells in vivo. Infect. Immun. 64:3983-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hienz, S. A., M. Palma, and J.-I. Flock. 1996. Insertional inactivation of the gene for collagen-binding protein has a pleiotropic effect on the phenotype of staphylococcus aureus. J. Bacteriol. 178:5327-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain, M., K. Becker, C. von Eiff, G. Peters, and M. Herrmann. 2001. Analogs of Eap protein are conserved and prevalent in clinical Staphylococcus aureus isolates. Clin. Diagn. Lab. Immunol. 8:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain, M., K. Becker, C. von Eiff, J. Schrenzel, G. Peters, and M. Herrmann. 2001. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J. Bacteriol. 183:6778-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain, M., A. Haggar, C. Heilmann, G. Peters, J. I. Flock, and M. Herrmann. 2002. Insertional inactivation of Eap in Staphylococcus aureus strain Newman confers reduced staphylococcal binding to fibroblasts. Infect. Immun. 70:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phospho-inositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 25.Isberg, R. R., and J. M. Leong. 1990. Multiple B1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 26.Jadoun, J., V. Ozeri, E. Burstein, E. Skutelsky, E. Hanski, and S. Sela. 1998. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J. Infect. Dis. 178:147-158. [DOI] [PubMed] [Google Scholar]
- 27.Jevon, M., C. Guo, B. Ma, N. Mordan, S. P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of intrnalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67:2677-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronvall, G., and K. Jonsson. 1999. Receptins: a novel term for an expanding spectrum of natural and engineered microbial proteins with binding properties for mammalian proteins. J. Mol. Recognit. 12:38-44. [DOI] [PubMed] [Google Scholar]
- 29.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inIA and inIB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 31.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mège, and P. Cosart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 32.Menzies, B. E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrby-Teglund, A., R. Lustig, and M. Kotb. 1997. Differential induction of Th1 versus Th2 cytokines by group A streptococcal toxic shock syndrome isolates. Infect. Immun. 65:5209-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palma, M., A. Haggar, and J.-I. Flock. 1999. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J. Bacteriol. 181:2840-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palma, M., O. Shannon, H. Concha Quezada, A. Berg, and J.-I. Flock. 2001. Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the alfa chain. J. Biol. Chem. 276:31691-31697. [DOI] [PubMed] [Google Scholar]
- 36.Palma, M., D. Wade, M. Flock, and J.-I. Flock. 1998. Multiple binding sites in the interaction between fibrinogen and an extracellular fibrinogen binding protein from Staphylococcus aureus. J. Biol. Chem. 273:13177-13181. [DOI] [PubMed] [Google Scholar]
- 37.Park, P. W., J. Rosenbloom, W. R. Abrams, J. Rosenbloom, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin binding protein (ebpS) in Stahylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 38.Patti, J. M., K. Jönsson, B. Guss, L. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1989. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesion. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 39.Paulsson, M., O. Liang, F. Ascencio, and T. Wadström. 1992. Vitronectin binding surface proteins of Staphylococcus aureus. Zentbl. Bakteriol. 277:54-64. [DOI] [PubMed] [Google Scholar]
- 40.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronetin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Pathog. Med. Microbiol. 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 41.Phonimdaeng, P., M. O'Reilly, P. Nowlan, A. J. Bramley, and T. J. Foster. 1990. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-dificient mutants. Mol. Microbiol. 4:393-404. [DOI] [PubMed] [Google Scholar]
- 42.Saravia-Otten, P., H. Muller, and S. Arvidson. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sartingen, S., E. Rozdzinski, A. Muscholl-Silberhorn, and R. Marre. 2000. Aggregation substance increases adherence and internalization, but not translocation, of Enterococcus faecalis through different intestinal epithelial cells in vitro. Infect. Immun. 68:6044-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Signäs, C., G. Raucci, K. Jönsson, P.-E. Lindgren, G. M. Anantharamaiah, H. Magnus, and L. Martin. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus and its use in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha, B., P. Francois, Y.-A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K.-H. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K.-H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 47.Sun, Q., G. M. Smith, C. Zahradka, and M. J. McGavin. 1997. Identification of D motif epitopes in Staphylococcus aureus fibronectin-binding protein for the production of antibody inhibitors of fibronectin binding. Infect. Immun. 65:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wells, C. L., E. A. Moore, J. Hoag, A. H. Hirt, G. M. Dunny, and S. L. Erlandsen. 2000. Inducible expression of Enterococcus faecalis aggregation substance surface protein facilitates bacterial internalization by cultured enterocytes. Infect. Immun. 68:7190-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wesson, C. A., L. E. Liou, K. M. Todd, G. A. Bohach, W. R. Trumble, and K. W. Bayles. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and inuction of apoptosis. Infect. Immun. 66:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]